Abstract
The present study was aimed to appraise the variations in biochemical, antioxidant and nutritional attributes of radish (Raphanus sativus L.) under foliar application of plant leaf aqueous extracts i.e., mulberry leaf extract (MLE), brassica leaf extract (BLE), sorghum leaf extract (SLE) and moringa leaf extract (MoLE) as natural growth regulators. Samples were collected after three sprays of extracts and analyzed. Total phenolic constituents were determined using Folin–Ciocalteu reagent method, whereas antioxidant potential was evaluated by 1,1 diphenyl-2-picrylhydrazyl radical radical scavenging and reducing power assays. Results revealed that application of MLE, BLE, SLE, and MoLE not only improved growth, but also enhanced biochemical and antioxidant activities. Foliar spray of MoLE furnished relatively three folds higher amounts of extractable bioactive compounds (37.65 ± 0.94%), phenolic constituents (54.51–182.71 mg GAE/g f.w). The radical scavenging capacity (RSC) and reducing potential were also enhanced considerably. Furthermore, the moisture, dietary fiber, crude protein, and carotenoids were also enhanced in response of foliar spray of plant extracts. From results, it is concluded that plant extracts are effective sources of natural growth regulars and might be useful for the production of vegetables with improved nutritional value and antioxidant activity.
Keywords: Radish, Antioxidant activities, Bioactive extracts, Foliar spray, Plant growth regulator
1. Introduction
Vegetables are potent sources of essential nutrient, dietary fiber and phytochemicals [1]. An inverse relation has been found between intake of vegetables and prevalence of various cardiac complications, especially coronary heart (CHD) and gastrointestinal disorders. These medicinal attributes of vegetables linked to the presence of various phytochemical constituents especially phenolics [2]. Radish (Raphanus sativus L.) commonly known as ‘moli’ in Pakistan, is a member of the Brassicaceae family and among the most frequently grown root vegetables worldwide. Radish contains plenty of potent phytochemicals, glucosinolates (GLSs), phenolics, vitamins and their metabolites having anti-carcinogenic activity along with other bio-activities [3]. Radish is rich in anthocyanins, fiber and minerals such as K, Ca and Mg [4] and is helpful in regulating blood pressure [5], coping with diabetics, beating cold and cough [6], recovering from jaundice, asthma, good for constipation and aging prevention [7]. Additionally, radish has vitamin C that acts as a powerful antioxidant to prevent the damaging effect of free radicals to the DNA and other biological tissues [8].
Application of plant extracts as growth promoters/regulator (PGRs) is one of the recently adopted approaches in agricultural, especially in developing countries. In this practice, the plant extracts enriched in bioactive compounds have been used as growth promoters since allele-chemicals are present in the leaves of plants like Azadirachta indica, Capsicum annuum, Brassica and Moringa oleifera, etc. Zeatin in moringa leaves, Brassinolides in Brassica leaves, and sorgoleone, 5-ethoxysorgoleone and 2,5-dimethoxysorgoleone in sorghum leaves have a potential along with other inorganic salts to boost up the growth and yield. The use of plant extract as growth regulator etc is cheap and environmentally friendly [9], [10], [11], [12], [13], [14], [15] versus chemical application to enhance the growth [16], biologically active compounds for medicinal [9], [17], [18], [19], [20], [21], [22] and food ingredient importance [21], [23]. In view of current environmental and soil pollution issues, eco-friendly agent should be used for sustained agricultural practices [14], [18], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52]. Nevertheless, the effect of plant extract as growth regulator have been tried regarding variation in nutritional and antioxidant attributes of radish in response to exogenous application of plant leaf extracts as PGRs. Hence, it would be of practical interest to analyze allelopathic effect of foliar spray of plant leaf extracts on biochemical, antioxidant activity and nutritional value of radish.
2. Materials and methods
2.1. Seed sowing
Radish seed was purchased from Ayub Agricultural Research Institute, Faisalabad. The healthy seeds were selected by hand picking, sown in soil filled pots (12 cm × 14.5 cm), and placed in green house at Department of Agronomy, University of Agriculture, Faisalabad. The trials were conducted under natural conditions during November to January 2014 i.e. average temperature 19.87 °C, rainfall 3.93 mm and 6.92 h sunshine, Agricultural Meteorology Cell, University of Agriculture Faisalabad, Pakistan.
2.2. Collection of plant leaves
Young fresh leaves and tender branches of four plants, Morus nigra L. (mulberry), Brassica napus L. (brassica), Sorghum bicolor L. (sorghum) and M. oleifera (moringa) were collected from Botanical Garden, University of Agriculture, Faisalabad. The plant leaves were washed 2–3 times with running tap water.
2.3. Preparation of leaf extracts
For the preparation of plant leaf extracts (PLEs), extraction was performed by grinding fresh leaves and the mixture was mixed with water (1 L/10 kg fresh material) and set for shaking for 24 h at room temperature. After filtration of crude mixture, extract was centrifuged at 8000 rpm for fifteen min and supernatant containing 100% leaf extract was collected. Different concentrations (3%, 5% and 10%) of plants leaf extract i.e. mulberry, brassica, sorghum and moringa were prepared from respective stocks by diluting with water and used for spray.
2.4. Foliar spray
Plant leaf extracts (PLEs), three concentrations (3%, 5% and 10%) were applied separately on radish Plant @ 160 L/ha in continual 15 days interval starting from the 15th day of emergence (DAE) [53]. Tap water was used as control under similar conditions.
2.5. Collection of samples
The radish samples were harvested at the end of experimental period (60 DAE). Samples were washed with tap water to remove dust and leaf and root portions were separated for response measurement. Leafy portion was used for chlorophyll and carotenoids content measurement, whereas root portion was used for antioxidant activity and proximate analysis.
2.6. Chemicals and reagents
All the reagents including Folin–Ciocalteu reagent, 1,1 diphenyl-2-picrylhydrazyl radical (DPPH), butylated hydroxyl toluene (BHT) and gallic acids were purchased from Sigma chemicals (St. Louis, MO, USA). Chemicals and solvents comprising sodium phosphate, anhydrous sodium carbonate, sodium hydroxide, ferric chloride, methanol, ethanol, ferrous chloride, potassium ferricyanide, sulfuric acid, sodium phosphate, sodium bicarbonate, sulfo-salicylic acid, trichloroacetic acid, orthophosphoric acid, ninhydrin, toluene, glacial acetic acid and boric acid were of analytical grade and procured from Merck (Darmstadt, Germany). Ultra-pure water (Victor diagnostic laboratories, Pakistan) was used as solvent during in vitro assays.
3. Extract preparation of radish
3.1. Extraction of phenolics
The bioactive compounds in radish extracts were determined following reported method [54]. The chopped edible portion (5 g) of radish were mixed with aqueous methanol (methanol: water, 80:20 v/v) and shaken for 24 h at 120 rpm at room temperature in orbital shaker (Gallenkamp, UK). The residues were removed from extracts by filtration. The residues were re-extracted twice with the same solvent and extracts obtained thus were pooled. The pooled extracts were concentrated to dryness under reduced pressure at 45 °C, using a rotary evaporator (EYELA, N-N Series, Rikakikai Co. Ltd., Tokyo, Japan). The dry extracts were weighed to calculate the bioactive compound yield and stored in a refrigerator (−4 °C) until further analyses.
3.2. Total phenolic contents (TPC)
The TPC were assessed using Folin–Ciocalteu reagent [55]. Briefly, 50 mg crude extract of roots was mixed with 0.5 mL of Folin–Ciocalteu reagent and 7.5 mL of deionized water. The resulted mixture was kept at room temperature for 10 min and then, 1.5 mL of 20% sodium carbonate (w/v) was added. The obtained mixture was kept further at 40 °C for 20 min and cooled in ice bath and absorbance was measured at λmax 755 nm (U-2001, Hitachi Instrument Inc., Tokyo, Japan). The TPC was calculated versus Gallic acid reference standard and results were expressed as milligram Gallic acid equivalent (mg GAE/g) of fresh weight.
3.3. Reducing power determination
The reducing power of RBEs was determined according to the method reported elsewhere [56]. Different amounts of each extracts (2.5, 5, 7.5, 10 mg/mL) were mixed with sodium phosphate buffer (5 mL) followed by the addition of 5 mL potassium ferricyanide K3[Fe(CN)6] (1%w/v). The mixture was incubated at 50 °C for 20 min and 5 mL of trichloroacetic acid (10%w/v) was added. The resultant aliquot was centrifuged at 3000 rpm for 10 min and upper layer (5 mL) was collected which was further diluted with same volume of distilled water (5 mL) and one mL FeCl3 (0.1%w/v). Absorbance was measured at 700 nm and reducing power was expressed in comparison to vitamin C (positive control).
3.4. Radical scavenging capacity (RSC)
The RSC was assessed in term of their capacity to scavenge DPPH radical [57]. Five different concentrations 2.0, 1.5, 1.0, 0.5, 0.1 (mg/mL) in methanol were treated with freshly prepared DPPH (2.5 μM). After 15 min of incubation at room temperature, absorbance was measured at 517 nm and RSC was determined using Eq. (1):
| (1) |
where Δc indicates the absorbance of solution containing only DPPH°, whereas Δs is the absorbance of the sample. The minimal inhibitory concentration (IC50) was obtained from a plot of percentage inhibition verses concentration.
3.5. Proximate composition and nutritional profile
Moisture contents of radish roots were determined by heating fresh sample in vacuum oven at 105 °C until constant weight, whereas ash contents were assessed by incinerating sample at 750 ± 20 °C in a muffle furnace [58]. Percentage crude fiber was determined by using standard AOAC method [59]. The protein content was calculated by Kjeldahl’s method as g 100/g dry weight [60].
3.6. Chlorophyll and carotenoid contents
For chlorophyll and carotenoid contents measurements, radish leaves were extracted using acetone for 24 h at room temperature, and then, absorbance was measured at 663, 645, 505 and 453 nm. The chlorophyll (a and b) and carotenoid contents were calculated using relations shown in Eqs. (2), (3), (4), respectively [61] and [62], where, A is absorbance, V and W are presenting volume and weight, respectively.
| (2) |
| (3) |
| (4) |
3.7. Statistical analysis
All the experiments were conducted in triplicate and data reported as mean ± SD. The mean estimates of all parameters were compared by Duncan’s multiple range test. The results were considered statistically significantly different having P < 0.05.
4. Results and discussion
4.1. Growth and yield of radish bioactive extract (RBE)
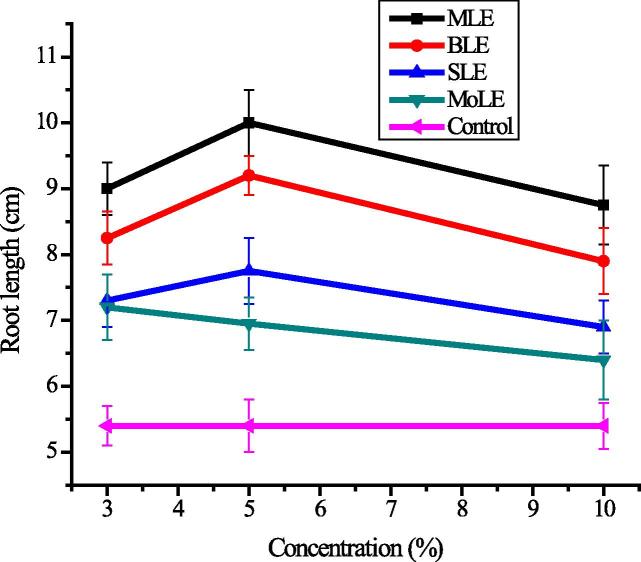
It is considered that allele-chemicals promote growth of plant through various physiological and biochemical transformations under their exogenous applications at very low concentration [53]. In the present work, different plant extracts were evaluated for their effect on radish biological characteristics. As a result of PLE foliar application a significant increase in root growth was observed (Fig. 1). The maximum increase in root length was recorded 84.13% in comparison to control in MoLE treated radish and 5% concentration showed promising response, which indicates that very minute concentration of allele-chemicals has considerable effect on root growth, which has been correlated with higher moisture availability and temperature regulation as a result of PLE application [63]. Sorghum and mulberry leaf extracts also showed better performance versus control. It was observed that a higher concentration of PLEs declined the root growth and might be dangerous to plant metabolic physiological pathways. Results revealed that the radish growth under applied PLEs is attributed to the phytochemicals present in extracts [64] and these results are in agreement with Jhangeer [65] who reported the increase in maize yield up to 52%, 42% and 42% by the foliar application of moringa, sorghum and brassica leaf extracts, respectively as PGRs. Bioactive constituents of plants are considered unique sources for natural food supplements, pharmaceuticals and other industrial applications [66]. Application of PGRs affects the growth to improve vegetable yield and thus indirectly increases the bioactive contents [67], [68].
Figure 1.
Variation in root growth in response of foliar application of plant growth regulators; MLE, mulberry leaf extracts; BLE, brassica leaf extracts; SLE, sorghum leaf extracts; MoLE, Moringa oleifera leaf extracts.
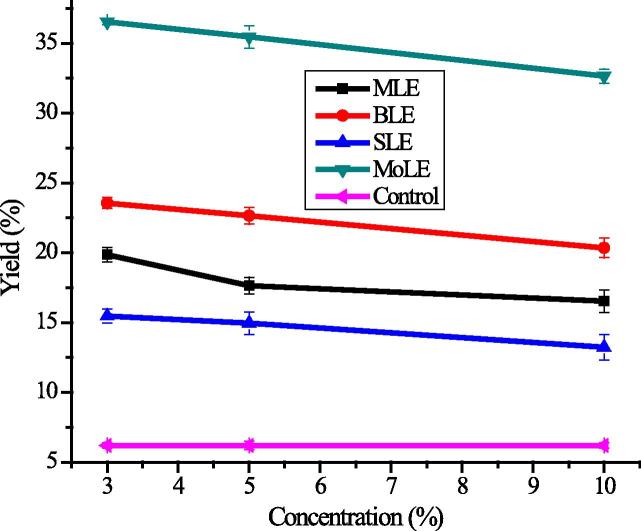
The findings revealed that application of plant leaf extracts as PGRs significantly (P < 0.05) improved extractable bioactive constituents (Fig. 2). The significant variation was observed in RBs in response to different concentrations of applied extracts. Maximum yield (37.65 ± 0.94%) of the RBs was obtained where MoLE was applied, which was almost six fold higher in comparison to control (6.21 ± 0.16%). This increase in yield is attributed to stimulation of cell division under the influence of applied MoLE due to the presence of high Zeatin contents in extract that acts as efficient plant growth regulators [53]. Sorghum leaf extract showed less efficiency (15.46 ± 0.39%) as a PGR source. It was found that the optimum concentration of all applied PLEs was 3% since the increase in concentration of PLEs as plant growth regulator (above 5%) was less effective to improve bioactive compound yield as compared to a lower concentration. This behavior indicates that a higher concentration may exert negative impact on respiration and photosynthetic processes, which in turn changes the amount of extractable phenolic bioactive compounds [69].
Figure 2.
Variation in yield in response of foliar application of plant growth regulators (explanations as given in Fig. 1).
4.2. Total phenolic content (TPC)
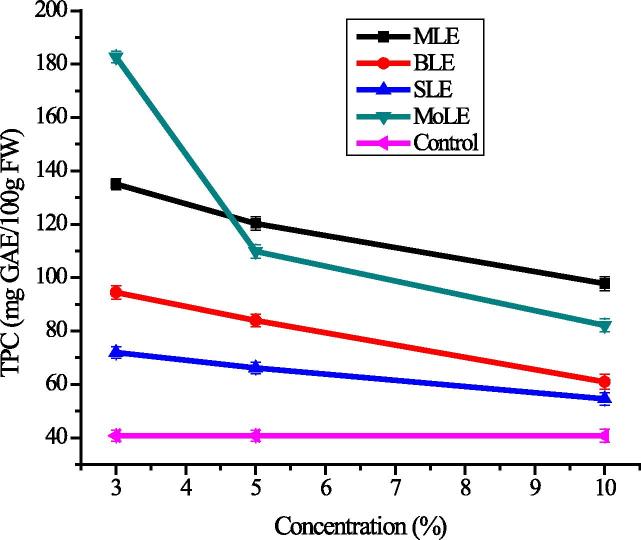
Phenolic compounds have gained much importance due to their health promoting and disease preventing properties and vegetables are a rich source of these phenolics [2]. Plant growth regulators create a certain stress condition with a change in genotype and metabolic pathways to enhance the production of such phytochemicals [70]. The TPC in radish was in the range of 54.51 ± 1.36–182.71 ± 4.57 mg GAE/100 gm FW. The maximum amount of phenolics (182.71 ± 4.57 mg GAE/100gm) was found in the radish samples, spraying with 3% MoLE, which might be ascribed to improved respiration rate and enhanced formation of phytochemicals. TPC varied significantly (P < 0.05) among samples which were sprayed with a lower concentration of PLEs, however higher concentrations of extracts showed insignificant effect on TPC. The lowest level of TPC was found in samples which were treated with 10% SLE extract, showing adverse effect of higher concentration because allele-chemicals favor the plant growth only at specified concentrations [71]. Results shown in Fig. 3, depicts that all PLEs enhanced TPC as compared to control and previous findings also support these results that phenolic contents were significantly enhanced in response of Verbesina encelioides application allelopathic in radish [72], sweet basil [73]. Lentil plant [74] and in Coriandrum sativum under the exogenous applications of plant growth regulators also revealed an enhancing effect on TPC [75].
Figure 3.
Variation in total phenolic contents (TPC) (mg GAE/100 g FW) in response of foliar application of plant growth regulators (explanations as given in Fig. 1).
4.3. Radical scavenging activity (RSC)
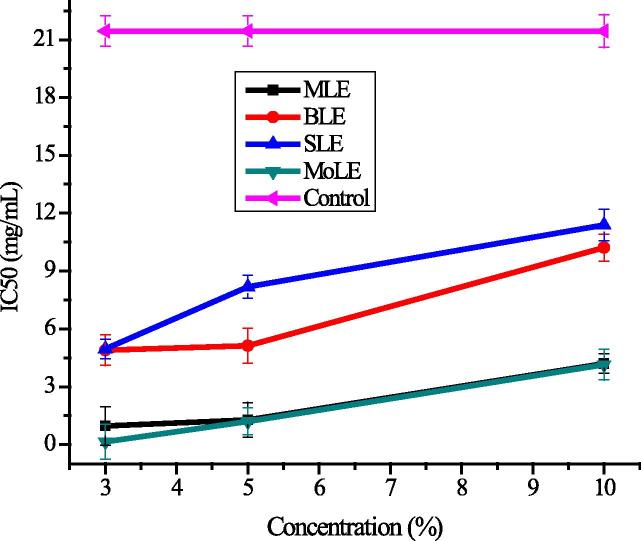
Green leafy vegetables are the excellent sources of antioxidant, which act as radical scavenger and previously exogenous application of PGRs have been practiced to alleviate adverse effect of abiotic stress, which were due to enhanced production of radical scavenging potential [76]. In this study, influence of foliar application of PLEs on the radical scavenging capacity of radish was investigated and results are shown in Fig. 4. DPPH radical scavenging is an important determinant to evaluate the antioxidant potential and findings demonstrate that foliar application of PLEs as growth regulators significantly (P < 0.05) affected radical scavenging capacity of the radish. It was found to be higher (smaller IC50) in the sample that was treated with a relatively lower concentration of selected PLEs. Application of mulberry (MLE), brassica (BLE), sorghum (SLE) and moringa (MoLE) leaf extracts enhanced the radical scavenging capacity significantly of radish as compared to control (Fig. 4). Samples under foliar spray of MoLE leaf extracts at a lower concentration (3%) were found out to be superior radical scavenger (0.15 ± 0.04 mg/mL IC50), whereas control showed the least radical scavenging capacity (IC50 21.45 ± 0.04 mg/mL). Previously, it is reported that the improvement in scavenging effect of plants by reducing the stressed conditions and PGRs were the best elicitors of the antioxidant potential by reducing the stress effect [77]. Results of present investigation are strongly correlated with the already reported findings regarding enhanced antioxidant capacity in Andrographis paniculata [78], Orthosiphon stamineus B [79] and lettuce by the foliar application of different PGRs [77].
Figure 4.
Variation in radical scavenging capacity (IC50, mg/mL) in response of foliar application of plant growth regulators (explanations as given in Fig. 1).
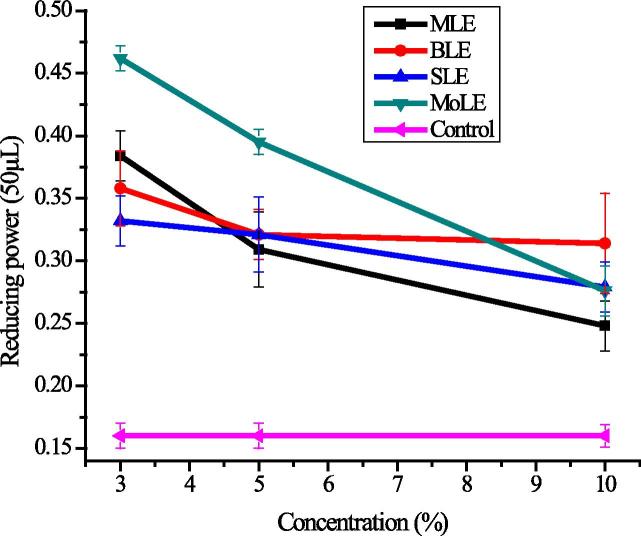
4.4. Reducing potential
Reducing power was measured at four different concentrations (2.5, 5.0, 7.5 and 10.0 mg/mL). An increasing concentration of extracts enhanced reducing potential. The higher concentration indicates more electrons to neutralize the oxidizing species and to cope with free radical to terminate chain reaction [80]. Foliar spray of PLEs significantly (P < 0.05) improved the reducing power as compared to control. The appreciable effect was observed by the application of 3% MoLE followed by 5% MoLE and 3% MLE (Fig. 5). This enhancement in reducing power might be due to the presence of different types of phytochemicals i.e., flavonoid pigments, glucosinolates, isothiocyanates and zeatin that promoted the formation of phytochemical compounds in radish extracts that results in more antioxidant activity [81], [82]. Similar finding has also been reported by Shabir [83] that reducing power of gold mohar leaf extracts at concentration of 10 mg/mL was enhanced considerably.
Figure 5.
Variation in reducing power of extracts in response of foliar application of plant growth regulators (explanations as given in Fig. 1).
4.5. Proximate analysis
In proximate composition ash is the component of biological material, which contains non aqueous residues, salts and mineral constituents. Minerals provided by the vegetables have immense metabolic importance to maintain a better osmoregulation [84]. Application of PLEs increased the radish growth and values of proximate components. Variation in the ash contents of radish plant under the application of PLEs is given in Table 1. Results revealed that the ash contents increased under all applied concentrations of MoLE. However, a lower concentration (3%) was found to be more effective in enhancing the ash contents (Table 1). Mulberry leaf extracts at a lower concentration (3%) also showed a promising effect regarding ash contents (28.34 ± 0.38%). Observed ash contents of radish (29.67%) in response to PGRs were higher than A. hybrids having 17.8% ash contents [85]. Similarly, the enhancement in ash contents in Solanum lycopersicon by the application of PGRs has also been reported [86]. Green leafy vegetables have been recognized as a rich and the cheapest source of proteins because of their ability to synthesize amino acid from primary compounds [87]. Various methods have been practiced to improve the protein contents in vegetables that ameliorate beneficial characteristics. But the recent trend of foliar application of PGRs as protein elicitors in the vegetables showed more prominent effect [77]. The effect of exogenous application of PLEs on protein contents of radish is given in Table 1. The MoLE (3%) concentration showed most prominent effect on protein contents and protein contents were recorded to be 41.22 ± 1.03 g/100 g of DW, which were 14.12 ± 0.35 g/100 g DW in control. However, higher concentrations (5% and 10%) of the same extract showed less protein contents, but higher than control. The MoLE (3%) concentration also improved the protein contents up to 34.23 ± 0.86 g/100 g of DW. The SLE 10% extract showed 15.25 ± 0.35 g/100 g DW protein content. The decline in protein contents and green pigments of radish with increasing concentrations of salicylic acid has also been reported [88]. The findings of present study are in line with studied that PGR application effect on proximate parameters was positive [89], [90]. Crude fiber is the fibrous food residue having pivotal effect on digestive system and cope different diseases [91]. These fiber contents can be enhanced by the exogenous application of PGRs [86]. Results revealed that application of PLEs as growth regulators also improved the fiber contents of radish. Overall, 3% MoLE extract remarkably increased the fiber contents, which was 29.31 ± 0.57 g/100 g of DW and control showed 17.00 ± 0.35 g/100 g DW fiber content. Other concentrations of MoLE i.e., 5% and 10% showed the crude fiber up to 23.09 ± 0.39 g/100 g DW and 21.36 ± 0.35 g/100 g DW, respectively (Table 1). Trend showed an inverse relation between the concentration of the extract application and the response of fiber. These findings are in agreement with the previous study [92], who evaluated the effect of foliar application of 5-aminolevulinic acid on lettuce that reduced the fiber contents at a higher level of 5-aminolevulinic acid application, however, a low concentration was found to be promising in enhancing fiber contents.
Table 1.
Variation in protein contents, crud fiber and ash contents (g/100 g DW) in radish roots in response of foliar application of plant growth regulators.
| Treatment concentration | Protein contents | Crud fiber | Ash contents | |
|---|---|---|---|---|
| Mulberry leaf extract | 3% | 34.23⁎ ± 0.86cD | 27.09 ± 0.48cD | 28.34 ± 0.38bD |
| 5% | 29.18 ± 0.73cC | 24.23 ± 0.51cC | 18.50 ± 0.34bB | |
| 10% | 21.24±.65bA | 21.71 ± 0.32cA | 15.81 ± 0.31bA | |
| Brassica leaf extract | 3% | 24.45 ± 0.65bcD | 25.50 ± 0.67cD | 26.60 ± 0.42bD |
| 5% | 22.35 ± 0.61bCD | 21.03 ± 0.52cB | 19.20 ± 0.36bB | |
| 10% | 16.25 ± 0.54aA | 19.38 ± 0.42cA | 16.51 ± 0.25bA | |
| Sorghum leaf extract | 3% | 18.33 ± 0.36abD | 29.00 ± 0.78cD | 18.08 ± 0.38bD |
| 5% | 16.98 ± 0.47aB | 23.06 ± 0.50cB | 16.71 ± 0.47bB | |
| 10% | 15.25 ± 0.35aA | 20.35 ± 0.28cA | 14.54 ± 0.34aA | |
| Moringa leaf extract | 3% | 41.22 ± 1.03eD | 29.31 ± 0.57cdD | 29.61 ± 0.47bD |
| 5% | 37.88 ± 0.95eCD | 23.09 ± 0.39cAB | 21.45 ± 0.41bB | |
| 10% | 31.65 ± 0.87dA | 21.36 ± 0.35cA | 18.75 ± 0.47bA | |
| Control | 14.12 ± 0.35a | 17.00 ± 0.35c | 11.2 ± 0.28a | |
Values are mean ± SD of triplicate for each treatment. Superscripted small alphabets indicate variation in response of plant growth regulator application, whereas subscripted capital letters elaborate the effect of plant growth regulator concentration at 95% confidence interval of mean.
4.6. Carotenoids
Carotenoids are the organic pigments present in chromoplasts and chloroplasts in living cells. In plants, it absorbs visible light (500–550 nm) for photosynthesis and prevents cell organelles from oxidative damage [93]. In animals, it absorbs certain blue and ultraviolet light to protect retinal damage. It also acts as vitamin A and antioxidant by scavenging free radical. Deficiency of β-carotenoids in plasma or serum may lead to lung cancer [94]. Greater amount of carotenoids in leaves is the indication of better photosynthetic activity. Application of plant growth regulators on the growing plant segments altered the carotenoid contents. Carotenoid content increased significantly in response of foliar application of PLEs, 3% MoLE improved the β carotenoids up to 1.25 ± 0.06 mg/g FW (Table 2). With the increase in concentration of applied extracts, a decreasing trend in carotenoid contents was observed and the level decreased to 0.66 ± 0.02 and 0.55 ± 0.01 mg/g FW with 5% and 10% of MoLE application, respectively. Similar to the present study, variation in carotenoids by the exogenous application of abscisic acid on the Lettuce was also reported in response of PGRs application [77] and two varieties of onion by application of GA, SA, 6BA, methionine and cysteine also enhanced carotenoid contents [95].
Table 2.
Variation in the chlorophyll “a”, “b” and β carotene (mg/100 g fresh weight) in response of foliar application of plant growth regulators.
| Treatments | Concentration | Chlorophyll b | Chlorophyll a | β carotene |
|---|---|---|---|---|
| Mulberry leaf extract | 3% | 0.62⁎ ± 0.01eD | 1.47 ± 0.07eD | 0.51 ± 0.01aA |
| 5% | 0.57 ± 0.02eC | 0.87 ± 0.04dAB | 1.18 ± 0.04eD | |
| 10% | 0.42 ± 0.02dA | 0.81 ± 0.04cdA | 0.78 ± 0.03cC | |
| Brassica leaf extract | 3% | 0.54 ± 0.2eD | 1.02 ± 0.01eD | 0.57 ± 0.04bAB |
| 5% | 0.26 ± 0.01bAB | 0.52 ± 0.02bAB | 0.94 ± 0.02dD | |
| 10% | 0.21 ± 0.01bA | 0.51 ± 0.06bA | 0.54 ± 0.03bA | |
| Sorghum leaf extract | 3% | 0.63 ± 0.02eD | 1.02 ± 0.08eD | 1.18 ± 0.04eD |
| 5% | 0.58 ± 0.01eC | 0.99 ± 0.05eAB | 0.73 ± 0.03cA | |
| 10% | 0.41 ± 0.01dA | 0.93 ± 0.05dA | 0.71 ± 0.03cA | |
| Moringa leaf extract | 3% | 0.69 ± 0.02eD | 1.11 ± 0.05eD | 1.25 ± 0.06eD |
| 5% | 0.44 ± 0.01dCD | 0.92 ± 0.05dB | 0.66 ± 0.02cAB | |
| 10% | 0.27 ± 0.01bA | 0.75 ± 0.04cA | 0.55 ± 0.01cA | |
| Control | 0.18 ± 01a | 0.53 ± 0.03b | 0.46 ± 0.02b | |
Values are mean ± SD of triplicate for each treatment. Superscripted small alphabets indicate variation in response of plant growth regulator application, whereas subscripted capital letters elaborate the effect of plant growth regulator concentration at 95% confidence interval of mean.
4.7. Chlorophyll contents
Photosynthesis is the essential process for the synthesis of nutrients, which is then used for growth and the remaining is stored as food. The chlorophyll absorbs light for photosynthetic process and is the most crucial component of this process and PGRs showed promising effect on chlorophyll contents. Results shown in Table 2 revealed that PGRs significantly increased the chlorophyll (a and b) contents in radish leaves. Overall, chlorophyll (a) and chlorophyll (b) values were found in the range of 0.51 ± 0.06–1.47 ± 0.07 mg/100 g FW and 0.18 ± 01–0.69 ± 0.02 mg/100 g FW, respectively. All the growth regulators significantly (P < 0.05) influenced chlorophyll contents in comparison to the control. MoLE (3%) showed promising efficiency regarding both chlorophyll a and chlorophyll b enhancement followed by the 3% SLE application. It is reported that foliar application of leaf extracts prevented the pre-maturation of leaf senescence and results in more leaf area with higher photosynthetic pigments that ultimately improve the chlorophyll contents [96]. These findings are also in line with a previous report that PGRs have a positive effect on chlorophyll contents [97], [98]. In other studies, application of PGRs also significantly enhanced the chlorophyll contents in Hibiscus sabdariffa [99], H. sabdariffa L. [89], Capsicum Annuum L. [100] and Eruca sativa [101].
5. Conclusions
In the present investigation, effects of different plant leaf extracts were evaluated on biochemical and antioxidant activity of radish cultivar. It was observed that application of PLEs as plant growth regulator showed promising effect in enhancing the growth, biochemical and antioxidant activity of radish. Foliar spray of PLEs i.e., mulberry leaf extract, brassica leaf extract, sorghum leaf extract and moringa leaf extract not only enhanced growth rate, but also improved nutritional quality and antioxidant characteristics in a concentration dependent manner. Lower concentration of plant extract as PGRs was more promising versus higher concentration. Therefore, application of PLEs as growth regulator might be a more viable and green choice to improve crop productivity versus synthetic PGRs in order to produce healthier food.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
Contributor Information
Bushra Sultana, Email: bushrasultana2005@yahoo.com.
Munawar Iqbal, Email: bosalvee@yahoo.com.
References
- 1.Hervert-Hernandez D., Goni I. Food Rev. Int. 2011;27:154–169. [Google Scholar]
- 2.Kongkachuichai R., Charoensiri R., Yakoh K., Kringkasemsee A., Insung P. Food Chem. 2015;173:838–846. doi: 10.1016/j.foodchem.2014.10.123. [DOI] [PubMed] [Google Scholar]
- 3.Gutiérrez R.M.P., Perez R.L. Sci. World J. 2004;4:811–837. doi: 10.1100/tsw.2004.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goyeneche R., Roura S., Ponce A., Vega-Gálvez A., Quispe-Fuentes I., Uribe E., Di Scala K. J. Funct. Food. 2015;16:256–264. [Google Scholar]
- 5.Ghayur M.N., Gilani A.H. Fund. Clin. Pharmacol. 2006;20:57–63. doi: 10.1111/j.1472-8206.2005.00382.x. [DOI] [PubMed] [Google Scholar]
- 6.Atzl I., Helms R. Springer; 2009. Common Cold; pp. 1–21. [Google Scholar]
- 7.Beevi S.S., Mangamoori L.N., Subathra M., Edula J.R. Plant Food Human Nutrit. 2010;65:200–209. doi: 10.1007/s11130-010-0178-0. [DOI] [PubMed] [Google Scholar]
- 8.Lugasi A., Blázovics A., Hagymási K., Kocsis I., Kéry Á. Phytother. Res. 2005;19:587–591. doi: 10.1002/ptr.1655. [DOI] [PubMed] [Google Scholar]
- 9.Asif M. Curr. Sci. Perspect. 2015;1:1–11. [Google Scholar]
- 10.Asif M. Curr. Sci. Perspect. 2015;1:51–61. [Google Scholar]
- 11.Asif M. Curr. Sci. Perspect. 2015;1:77–90. [Google Scholar]
- 12.Mensah J.K., Golomeke D. Curr. Sci. Perspect. 2015;1:69–76. [Google Scholar]
- 13.Mensah J.K., Kwoseh C., Banahene N., Atuilik S.A., Oppong D., Borigu M. Curr. Sci. Perspect. 2015;1:96–101. [Google Scholar]
- 14.Ukpaka C.P., Collins I. Curr. Sci. Perspect. 2016;2:69–77. [Google Scholar]
- 15.Younis A., Riaz A., Javaid F., Ahsan M., Tariq U., Aslam S., Majeed N. Curr. Sci. Perspect. 2015;1:16–21. [Google Scholar]
- 16.Zodape S., Gupta A., Bhandari S., Rawat U., Chaudhary D., Eswaran K., Chikara J. J. Sci. Ind. Res. 2011;70:215–219. [Google Scholar]
- 17.Ali A., Khalil-ur-Rahman, Jamil A., Jahan N., Tahir A. Curr. Sci. Perspect. 2015;1:102–106. [Google Scholar]
- 18.Ashraf M.W., Bilal M., Iqbal M. Curr. Sci. Perspect. 2015;1:12–15. [Google Scholar]
- 19.Ali A., Khalil-Ur-Rahman N.J., Iqbal M., Abbas M. Curr. Sci. Perspect. 2015;1:91–95. [Google Scholar]
- 20.Hussain F., Shahid M., Javed K. Curr. Sci. Perspect. 2016;2:5–9. [Google Scholar]
- 21.Asif M. Chem. Int. 2015;1:134–163. [Google Scholar]
- 22.Asif M. Chem. Int. 2016;2:1–18. [Google Scholar]
- 23.Asif M. Chem. Int. 2015;1:174–183. [Google Scholar]
- 24.Adesola B., Ogundipe K., Sangosanya K.T., Akintola B.D., Oluwa A., Hassan E. Chem. Int. 2016;2:89–102. [Google Scholar]
- 25.Babarinde A., Onyiaocha G.O. Chem. Int. 2016;2:37–46. [Google Scholar]
- 26.Iqbal M. Chemosphere. 2016;144:785–802. doi: 10.1016/j.chemosphere.2015.09.048. [DOI] [PubMed] [Google Scholar]
- 27.Iqbal M., Bhatti I.A., Shahid M., Nisar J. Biocatal. Agric. Biotechnol. 2016;6:116–122. [Google Scholar]
- 28.Iqbal M., Iqbal N., Bhatti I.A., Ahmad N., Zahid M. Ecol. Eng. 2016;88:265–275. [Google Scholar]
- 29.Iqbal M., Nisar J. J. Environ. Chem. Eng. 2015;3:1912–1917. [Google Scholar]
- 30.Iqbal M., ul Haq Z., Jamil Y., Nisar J. Biocatal. Agric. Biotechnol. 2016;6:173–183. [Google Scholar]
- 31.Iqbal M., ul Haq Z., Malik A., Ayoub C.M., Jamil Y., Nisar J. Biocatal. Agric. Biotechnol. 2016;5:30–37. [Google Scholar]
- 32.Jamil Y., Iqbal M., Perveen T., Amin N. Int. Agrophys. 2012;26:375–380. [Google Scholar]
- 33.Mumtaz M.W., Mukhtar H., Dilawer U.A., Hussain S.M., Hussain M., Iqbal M. Biocat. Agric. Biotechnol. 2016;5:162–167. [Google Scholar]
- 34.Mushtaq M., Bhatti H.N., Iqbal M., Noreen S. J. Environ. Manage. 2016;176:21–33. doi: 10.1016/j.jenvman.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Nadeem R., Manzoor Q., Iqbal M., Nisar J. J. Ind. Eng. Chem. 2016;35:184–195. [Google Scholar]
- 36.Naz A., Jamil Y., Iqbal M., Ahmad M.R., Ashraf M.I., Ahmad R. Indian J. Biochem. Biophys. 2012;49:211–214. [PubMed] [Google Scholar]
- 37.Rashid A., Bhatti H.N., Iqbal M., Noreen S. Ecol. Eng. 2016;91:459–471. [Google Scholar]
- 38.ul Haq Zia, Jamil Y., Irum S., Randhawa M.A., Iqbal M., Amin N. Pol. J. Environ. Stud. 2012;21:369–374. [Google Scholar]
- 39.Iqbal M., Khera R.A. Chem. Int. 2015;1:157b–163b. [Google Scholar]
- 40.Jafarinejad S. Chem. Int. 2016;2:242–253. [Google Scholar]
- 41.Jamal M.A., Muneer M., Iqbal M. Chem. Int. 2015;1:12–16. [Google Scholar]
- 42.Majolagbe A.O., Adeyi A.A., Osibanjo O. Chem. Int. 2016;2:232–241. [Google Scholar]
- 43.Peter U.C., Chinedu U. Chem. Int. 2016;2:80–88. [Google Scholar]
- 44.Qureshi K., Ahmad M.Z., Bhatti I.A., Iqbal M., Khan A. Chem. Int. 2015;1:53–59. [Google Scholar]
- 45.Sayed M. Chem. Int. 2015;1:81–86. [Google Scholar]
- 46.Ukpaka C. Chem. Int. 2016;2:19–28. [Google Scholar]
- 47.Ukpaka C.P. Chem. Int. 2016;2:128–135. [Google Scholar]
- 48.Ukpaka C.P. Chem. Int. 2016;3:8–18. [Google Scholar]
- 49.Jafarinejad S. Curr. Sci. Perspect. 2016;2:78–82. [Google Scholar]
- 50.Pandey B.K., Vyas S., Pandey M., Gaur A. Curr. Sci. Perspect. 2016;2:52–56. [Google Scholar]
- 51.Pandey B.K., Vyas S., Pandey M., Gaur A. Curr. Sci. Perspect. 2016;2:39–46. [Google Scholar]
- 52.Ukpaka C., Wami E., Amadi S. Curr. Sci. Perspect. 2015;1:107–111. [Google Scholar]
- 53.El-Hamied S.A.A., El-Amary E.I. IOSR J. Agric. Vet. Sci. 2015;8:1–9. [Google Scholar]
- 54.Liu Q., Yao H. Food Chem. 2007;102:732–737. [Google Scholar]
- 55.Chaovanalikit A., Wrolstad R. J. Food Sci. 2004;69:FCT67–FCT72. [Google Scholar]
- 56.Yen G.-C., Duh P.-D., Chuang D.-Y. Food Chem. 2000;70:437–441. [Google Scholar]
- 57.Iqbal S., Bhanger M. Food Chem. 2007;100:246–254. [Google Scholar]
- 58.Ayuba V., Ojobe T., Ayuba S. J. Med. Plant. Res. 2011;5:2952–2955. [Google Scholar]
- 59.AOAC, 1995.
- 60.Andini R., Yoshida S., Ohsawa R. Agronomy. 2013;3:391–403. [Google Scholar]
- 61.Iqbal M., Haq Z., Jamil Y., Ahmad M. Int. Agrophys. 2012;26:25–31. [Google Scholar]
- 62.Nagata M., Dan K., Yamashita I. J. Jpn. Soc. Hortic. Sci. 1992;61:686–687. [Google Scholar]
- 63.Mackey A., Barber S. Agron. J. 1985;77:524–527. [Google Scholar]
- 64.Sahib N.G., Anwar F., Gilani A.H., Hamid A.A., Saari N., Alkharfy K.M. Phytother. Res. 2013;27:1439–1456. doi: 10.1002/ptr.4897. [DOI] [PubMed] [Google Scholar]
- 65.A. Jahangeer, (M.Sc. thesis), Department of Agronomy, University of Agriculture, Faisalabad, Pakistan, 2011.
- 66.Gutiérrez-Mañero F.J., García-Villaraco A., Lucas J.A., Gutiérrez E., Ramos-Solano B. Int. J. Curr. Microbiol. Appl. Sci. 2015;4:224–241. [Google Scholar]
- 67.Arthur G., Stirk W., Van Staden J., Scott P., Afr S. J. Bot. 2003;69:207–211. [Google Scholar]
- 68.Zodape S., Kawarkhe V., Patolia J., Warade A. J. Sci. Ind. Res. 2008;67:1115–1117. [Google Scholar]
- 69.Jaleel C.A., Manivannan P., Wahid A., Farooq M., Al-Juburi H.J., Somasundaram R., Panneerselvam R. Int. J. Agric. Biol. 2009;11:100–105. [Google Scholar]
- 70.Howladar S.M. Ecotoxicol. Environ. Saf. 2014;100:69–75. doi: 10.1016/j.ecoenv.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 71.Farooq M., Bajwa A.A., Cheema S.A., Cheema Z.A. Int. J. Agric. Biol. 2013;15:1367–1378. [Google Scholar]
- 72.Inderjit, Asakawa C., Dakshini K. Can. J. Bot. 2000;77:1419–1424. [Google Scholar]
- 73.Koca N., Karaman Ş. Food Chem. 2015;166:515–521. doi: 10.1016/j.foodchem.2014.06.065. [DOI] [PubMed] [Google Scholar]
- 74.Giannakoula A.E., Ilias I.F., Maksimović J.J.D., Maksimović V.M., Živanović B.D. J. Food Compos. Anal. 2012;28:46–53. [Google Scholar]
- 75.Aslam M., Sultana B., Ali S. Asian J. Chem. 2014;26 [Google Scholar]
- 76.Ashraf M., Foolad M. Environ. Experimen. Bot. 2007;59:206–216. [Google Scholar]
- 77.Li Z., Zhao X., Sandhu A.K., Gu L. J. Agric. Food Chem. 2010;58:6503–6509. doi: 10.1021/jf1006962. [DOI] [PubMed] [Google Scholar]
- 78.Anuradha V., Jaleel C.A., Salem M.A., Gomathinayagam M., Panneerselvam R. Pest. Biochem. Physiol. 2010;98:312–316. [Google Scholar]
- 79.Ibrahim M.H., Jaafar H.Z. Molecules. 2013;18:7957–7976. doi: 10.3390/molecules18077957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sultana B., Anwar F., Przybylski R. Food Chem. 2007;104:1106–1114. [Google Scholar]
- 81.Taha L.S., Taie H.A., Hussein M. J. Appl. Pharma. Sci. 2015;5:030–036. [Google Scholar]
- 82.Bose C.K. Medscape Gen. Med. 2007;9:26. [PMC free article] [PubMed] [Google Scholar]
- 83.Shabir G., Anwar F., Sultana B., Khalid Z.M., Afzal M., Khan Q.M., Ashrafuzzaman M. Molecules. 2011;16:7302–7319. doi: 10.3390/molecules16097302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.É. Daiuto, R. Vieites, D. Pigoli, L. de Carvalho, Nativa: Pesquisas Agrárias e Ambientais 3, 2015, pp. 102–108.
- 85.Iheanacho K.M., Udebuani A.C. J. Appl. Sci. Environ. Manage. 2009;13:35–38. [Google Scholar]
- 86.Olaiya C., Soetan K., Ogunkolade N. Afr. J. Food Sci. 2010;4:041–045. [Google Scholar]
- 87.García M., Puchalska P., Esteve C., Marina M. Talanta. 2013;106:328–349. doi: 10.1016/j.talanta.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 88.Çanakçi S. J. Biol. Sci. 2008;8:431–435. [Google Scholar]
- 89.Mukhtar F. Int. J. Pure Appl. Sci. 2008;2:70–75. [Google Scholar]
- 90.Manas D., Bandopadhyay P., Chakravarty A., Pal S., Bhattacharya A. Afr. J. Plant Sci. 2014;8:320–335. [Google Scholar]
- 91.Dawczynski C., Schubert R., Jahreis G. Food Chem. 2007;103:891–899. [Google Scholar]
- 92.Xu F., Wang W., Yu D. Afr. J. Biotechnol. 2012;11:11591–11594. [Google Scholar]
- 93.Tracewell C.A., Cua A., Stewart D.H., Bocian D.F., Brudvig G.W. Biochem. 2001;40:193–203. doi: 10.1021/bi001992o. [DOI] [PubMed] [Google Scholar]
- 94.Clevidence B., Paetau I., Smith J.C. HortScience. 2000;35:585–588. [Google Scholar]
- 95.Lokhande A.A., Gaikwad D.K. Indian J. Adv. Plant Res. 2014;1:15–18. [Google Scholar]
- 96.Yasmeen A., Basra S., Farooq M., ur Rehman H., Hussain N. Plant Growth Regul. 2013;69:225–233. [Google Scholar]
- 97.Jat N.K., Sharma V. Am. J. Plant Physiol. 2006;1:132–141. [Google Scholar]
- 98.Hörtensteiner S., Kräutler B. BBA Bioenerget. 2011;1807:977–988. doi: 10.1016/j.bbabio.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 99.Kadiri M., Mukhtar F., Agboola D. Rev. Biol. Trop. 1997;44:23–28. [PubMed] [Google Scholar]
- 100.Ouzounidou G., Ilias I., Giannakoula A., Papadopoulou P. Pak. J. Bot. 2010;42:805–814. [Google Scholar]
- 101.Al-Whaibi M.H., Siddiqui M.H., Al-Munqadhi B.M., Sakran A.M., Ali H.M., Basalah M.O. J. Med. Plant Res. 2012;6:1948–1954. [Google Scholar]