Abstract
Background
Excessive extracellular matrix (ECM) remodeling and a reactive stroma can affect T-cell infiltration and T-cell activity in the tumor and hereby influence response to immune checkpoint inhibitors (ICI). In the pursuit of finding biomarkers that predict treatment response, we evaluated the association between serum biomarkers of collagen and vimentin turnover and outcomes in metastatic melanoma patients treated with the anti-CTLA-4 antibody ipilimumab (IPI).
Methods
Type III collagen formation (PRO-C3), MMP-degraded type I, type III and type IV collagens (C1M, C3M and C4M), and citrullinated and MMP-degraded vimentin (VICM) were measured with ELISAs in serum from metastatic melanoma patients before (n = 66) and 3 weeks after (n = 52) initiation of IPI treatment. Biomarker levels were associated with Disease Control Rate (DCR) and survival outcomes.
Results
We found that baseline levels of PRO-C3 (p = 0.011), C1M (p = 0.003), C3M (p = 0.013) and C4M (p = 0.027) were significantly elevated in patients with progressive disease (PD). Univariate Cox regression analysis identified high PRO-C3 (p = 0.021) and C4M (p = 0.008) as predictors of poor overall survival (OS) and the biomarkers remained significant when evaluated with other covariates (PRO-C3 (p = 0.049) and C4M (p = 0.046)). Multivariate analysis identified VICM as a predictor of longer OS (p = 0.026). Similarly, a high C3M/PRO-C3 ratio predicted for increased OS (p = 0.034). Only C3M (p = 0.003) and VICM (p < 0.0001) increased 3 weeks after treatment.
Conclusions
ECM and tissue remodeling quantified in pre-treatment serum were associated with response and survival outcomes in metastatic melanoma patients treated with IPI. This highlights the importance of addressing the ECM and stromal component non-invasively in future ICI studies.
Electronic supplementary material
The online version of this article (10.1186/s40425-018-0474-z) contains supplementary material, which is available to authorized users.
Keywords: Immunotherapy, Immune checkpoint inhibitors, Liquid biopsy, Biomarkers, Extracellular matrix, Collagen, Stroma
Background
Immune checkpoint blockade with monoclonal antibodies against cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1) or their ligands, such as PD1 ligand 1 (PD-L1) has revolutionized the treatment of metastatic melanoma patients with the possibility of durable and long-lasting responses [1]. However, only a proportion of patients benefit from immune checkpoint inhibitor (ICI) treatment [1, 2]. Given the frequency of adverse events, high costs and the lack of reliable biomarkers, novel predictive biomarkers of treatment efficacy represent an unmet need [3]. Identifying biomarkers associated with clinical response to ICIs is essential for selection of patients and for identifying potential targets for the development of combination therapies.
Emerging evidence suggests that the extracellular matrix (ECM), the non-cellular component of all tissues, and proteolytic ECM remodeling products have a crucial role in resistance to immunotherapy by regulating the cancer-immunity cycle [4]. ECM remodeling, collagen deposition and mechanical forces have been shown to regulate immune cell migration and activation [5]. Some patients have an immune-excluded phenotype, in which a dense stroma (desmoplasia) in the tumor microenvironment restricts the access of tumor infiltrating lymphocytes (TILs) into the tumor, resulting in poor clinical responses to ICIs [6–8]. Desmoplasia is characterized by excessive deposition of ECM, which are constantly undergoing a remodeling [9]. In healthy tissue, a balanced ratio between ECM degradation and formation maintains tissue function whereas altered ECM composition plays a vital role in cancer progression and invasion [10]. Melanoma is one of the most aggressive human cancers with a highly reactive stroma [11]. This results in increased cleavage of collagen proteins by matrix-remodeling enzymes (such as matrix metalloproteinases (MMPs)), and the products may act as chemokines, cytokines and immune regulating agents [12, 13]. These small protein fragments containing specific protease-generated neo-epitopes, or ‘protein fingerprints’, are released into the circulation where they can be used as serological biomarkers directly reflecting disease pathogenesis [14].
Specific protein fingerprint biomarkers reflect the formation or degradation of various ECM proteins such as collagens. Collagen is the major structural protein of the skin primarily composed of interstitial matrix collagens type I and III and the basement membrane collagen type IV [15, 16]. The PRO-C3 biomarker is a protein-fragment with a fingerprint of type III collagen formation which can be used to assess excessive collagen formation (desmoplasia) in a liquid biopsy [17]. Elevated PRO-C3 levels have been detected in metastatic colorectal cancer patients and furthermore been associated with shorter overall survival (OS) in metastatic breast cancer patients [18, 19]. Specific MMP-generated protein-fragments derived from type I, III and IV collagen can be measured with the protein fingerprint biomarkers C1M, C3M and C4M, respectively. They reflect degradation of the interstitial matrix (C1M and C3M) and basement membrane (C4M), and are elevated in patients with various tumors and have been linked to tumor activity (a reactive stroma) [18, 20].
Another interesting stromal biomarker is citrullinated and MMP-degraded vimentin (VICM) that is released from activated macrophages and has been found elevated in lung cancer [21, 22]. Macrophages are known to play a role in the tumor microenvironment and biomarkers measuring macrophage activity may be relevant for ICIs [23].
The hypothesis of the current study was that elevated levels of biomarkers originating from a dense and reactive stroma were associated with outcome in metastatic melanoma patients treated with ICIs. To our knowledge, this is the first study to examine the biomarker potential of quantifying altered collagen turnover in serum for predicting outcome to ICIs.
In this study, we evaluated PRO-C3, C1M, C3M, C4M and VICM in serum samples drawn prior to treatment and after 3 weeks of therapy, for associations with clinical response to ipilimumab (IPI) in patients with metastatic melanoma.
Methods
Patient samples
Serum samples were collected from 66 stage IV malignant melanoma patients treated with IPI as standard of care at Herlev Hospital (n = 32), and Aarhus University Hospital (n = 34), Denmark subsequent to informed consent. Patient inclusion and exclusion criteria are described elsewhere [24]. Patients were included between October 2012 and June 2014. The study was approved by the Ethics Committee for The Capital Region of Denmark (H-2-2012-058) in compliance with the Helsinki Declaration of 1975. Treatment with IPI was given as a total of four treatments with 3 weeks apart, at a fixed dose of 3 mg/kg body weight. All patients received at least two doses of IPI. Serum samples were collected at baseline (pre-treatment) and 3 weeks after the first treatment (before the 2nd dose of treatment). Sample analyses were performed blindly. Clinical response was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) (v. One.1.1). Patients were assessed within 4 weeks before the first treatment and every third months thereafter until progression. The median follow-up period was 473 days (range, 40–1258 days), defined as days to death or to last follow-up.
ELISA measurements and procedure
Each ECM fragment has a specific protease-generated neo-epitope that the monoclonal antibodies used in the enzyme-linked immunosorbent assays (ELISAs) are highly specific against. PRO-C3 is generated by the N-protease-mediated release of the N-terminal pro-peptide of type III collagen [17], C1M is from MMP-degraded type I collagen [25], C3M is from MMP-degraded type III collagen [26], C4M is from MMP-degraded type IV collagen [27] and VICM is from MMP-degraded citrullinated vimentin (VICM) [28]. Levels of these ECM biomarkers were assessed in serum samples using well characterized competitive ELISAs manufactured by Nordic Bioscience (Herlev, Denmark) and performed according to the manufacturer’s specifications. In brief, 96-well pre-coated streptavidin plates were coated with biotinylated peptides specific for the protein of interest and incubated for 30 min at 20 °C. A volume of 20 μl of standard peptide or pre-diluted serum sample were added followed by addition of peroxidase-conjugated monoclonal antibodies and incubated for 1 h at 20 °C (C3M and C4M) or overnight at 4 °C (PRO-C3, C1M and VICM). Next, tetramethylbenzinidine (TMB) (Kem-En-Tec Diagnostics, Denmark) was added and the plates were incubated for 15 min at 20 °C. All incubations included shaking of the plates at 300 rpm following by five times of wash (20 mM Tris, 50 mM NaCl, pH 7.2). The reaction of TMB was stopped by adding 1% sulfuric acid and the absorbance was measured at 450 nm with 650 nm as reference. A standard curve was plotted using a 4-parametric mathematical fit model and data were analyzed using the Softmax Pro v. 6.3 software. Levels of the biomarkers were measured in duplicates.
Statistical analyses
Data did not meet assumptions for parametric testing (D’Agostino & Pearson normality test) and therefore non-parametric tests were used to assess differences. Wilcoxon matched-pairs signed rank test was used to compare patients at baseline with week 3. Mann-Whitney test was used to compare patients with progressive disease (PD) to the combined group of patients with stable disease (SD), partial response (PR) and complete response (CR) at baseline. The odds ratio (OR) and the positive predictive value (PPV) were generated from a specific cut-off value, the 75th percentile for PRO-C3, C1M, C3M and C4M, which was based on a previous study [19], and the median for VICM, and analyzed using Fisher’s exact test.
Univariate Cox proportional-hazards regression models were used to calculate hazard ratios (HR) with 95%CI for prediction of OS and progression free survival (PFS) for the PRO-C3, C1M, C3M, C4M and VICM biomarkers as well as other relevant clinical covariates: age, lactate dehydrogenase (LDH) and prior systemic therapy. To assess any confounding effects, a multivariate Cox proportional-hazards regression model was used to calculate the independent HRs with 95%CI for OS and PFS for PRO-C3, C1M, C3M, C4M and VICM individually after adjusting for the above described covariates. For both univariate and multivariate analysis, baseline PRO-C3, C1M, C3M and C4M levels lower than the 75th percentile (Q1-Q3) were used as a reference to calculate the HRs for patients with baseline levels in the upper quartile (Q4). Baseline VICM levels in the lowest quartiles (Q1-Q2) were used as a reference to calculate the HRs for patients with baseline levels in the upper quartiles (Q3-Q4). LDH levels under 250 IU/L were used as a reference to calculate the HRs for patients with elevated LDH levels. Univariate analysis also included HRs for PRO-C3, C1M, C3M, C4M and VICM on a continuous scale. The OS for the patients in this study ranged from 40 days to 1258 days and the PFS ranged from 10 days to 1258 days from baseline. Kaplan-Meier (KM) survival curves were used to analyze PFS and OS in the same percentile groups as described for the Cox proportional-hazards regression models. KM survival curves were also used to analyze OS in patients with a low type III collagen degradation to formation (C3M/PRO-C3) ratio (Q1) compared to a high C3M/PRO-C3 ratio (Q2 + Q3 + Q4). A log-rank test was used to determine differences between KM curves. Statistical analysis was conducted using MedCalc (v16.8.4) and GraphPad Prism (v7.01) (GraphPad Software). A p-value of p < 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 66 metastatic melanoma patients were included in this study and biomarkers were measured in these patients at baseline and in 52 patients 3 weeks after IPI treatment. According to an assessment of the clinical response by RECIST, 41 patients had PD, 14 patients had SD, 9 patients had PR and 2 patients had CR. Of the 66 patients, 43 patients were deceased within the follow-up period. Baseline patient characteristics are shown in Table 1.
Table 1.
Patient characteristics
| Age at baseline (median with range) | 67 (35–83) |
| Gender (% females) | 36/66 (55%) |
| Prior systemic therapy: | |
| None | 36 |
| IL-2 | 12 |
| Temozolomide | 6 |
| Radiation therapy/IL-2 | 1 |
| Radiation therapy | 1 |
| Temozolomide/Vemorafenib | 1 |
| RT/Temozolomide | 1 |
| IFN/IL-2 | 6 |
| Vemorafenib | 2 |
| RECIST response | |
| PD | 41 |
| SD | 14 |
| PR | 9 |
| CR | 2 |
| Lactate dehydrogenase (LDH) | |
| > = 250 IU/L | 12 |
| < 250 IU/L | 53 |
PD Progressive disease, SD Stable disease, PR Partial response, CR Complete response
High pre-treatment PRO-C3, C1M, C3M and C4M levels are associated with progressive disease
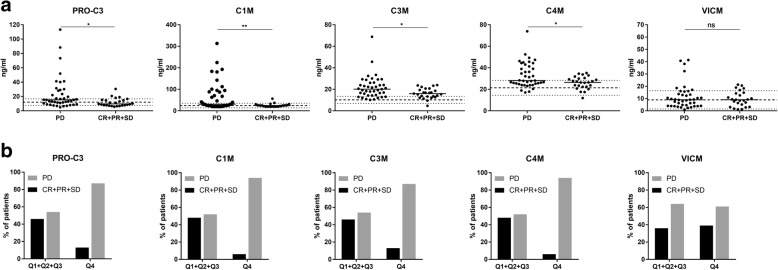
Serum concentrations of five different ECM biomarkers: PRO-C3, C1M, C3M, C4M and VICM, were measured at baseline. The majority of patients had biomarkers levels within the reference limits for healthy individuals, while a subgroup of patients had increased biomarker levels (Fig. 1a).
Fig. 1.
Relationship between PRO-C3, C1M, C3M, C4M and VICM levels at baseline and response. a Biomarker levels at baseline in serum from patients with progressive disease (PD) (n = 41) were compared to levels in patients with complete response (CR), partial response (PR) and stable disease (SD) (CR + PR + SD) (n = 25) with Mann-Whitney test. The black horizontal lines represent the median value of the patients. The reference limits for healthy individuals are illustrated with dotted lines (7.5–16.7 ng/ml, 14.0–35.6 ng/ml, 7–13.4 ng/ml, 14.5–28.3 ng/ml and 1.5–16.3 ng/ml for PRO-C3, C1M, C3M, C4M and VICM, respectively.) Asterisks indicate the following: *, p < 0.05 and **, p < 0.01. b Serum levels were dichotomized by the 75th percentile for PRO-C3, C1M, C3M and C4M (Q4, n = 16) and by the median for VICM (Q3 + Q4, n = 33). Next, quantity of patients achieving disease control (CR + PR + SD) where compared to non-responders (PD) in each group
We investigated if the observed inter-patient variations in biomarker levels at baseline were associated with Disease Control Rate (DCR). When comparing the serum levels in patients progressing despite treatment (PD) to patients achieving disease control (CR + PR + SD), PRO-C3 (p = 0.011), C1M (p = 0.003), C3M (p = 0.013) and C4M levels (p = 0.027) at baseline were significantly elevated in patients with PD compared to the combined groups of patients with disease control (Fig. 1a). No significant difference was detected in VICM levels (p = 0.834). To investigate the association with DCR further, the biomarker levels were dichotomized by the 75th percentile cut-point (Q4) for PRO-C3, C1M, C3M and C4M and by the median for VICM and followed by a comparison of percentage of patients achieving disease control (CR + PR + SD) with non-responders (PD) in each group. Of the patients with PRO-C3, C1M, C3M and C4M levels in the upper quartile (Q4), only 13, 6, 13 and 6% obtained disease control (CR + PR + SD) compared to 46, 48, 46 and 48% in the group of patients with lower biomarker levels (Q1 + Q2 + Q3) (Fig. 1b). Of the patients with VICM levels in the upper quartiles (Q3 + Q4), 39% obtained disease control compared to 36% in the group with lower levels (Q1 + Q2).
ORs were subsequently used to calculate the odds of being diagnosed with PD compared to CR + PR + SD. High levels (Q4) of PRO-C3, C1M, C3M and C4M resulted in ORs for being in the PD group of 6 (95%CI = 1.2–28, p = 0.019), 14 (95%CI = 2.2–151.6, p = 0.003), 6 (95%CI = 1.2–28, p = 0.019) and 14 (95%CI = 2.2–151.6, p = 0.003), respectively. In line with this, PPVs for having PD were 0.88, 0.94, 0.88 and 0.94, respectively. No association between VICM and DCR was observed (OR = 0.88, 95%CI = 0.3–2.5, p > 0.999).
PRO-C3, C4M and VICM are predictors of outcome
The individual ability of PRO-C3, C1M, C3M, C4M, VICM and clinical covariates at baseline to predict OS is shown in Table 2, which summarizes HRs with 95%CIs calculated from univariate Cox proportional-hazard models. When evaluated on a continuous scale, PRO-C3, C1M, C3M, C4M and LDH, but not VICM, were predictive of poor OS. When evaluated by the dichotomous cut-point (75th percentile cut-point, Q4), high pre-treatment levels of PRO-C3 (HR = 2.13, 95%CI = 1.12–4.04, p = 0.021) and C4M (HR = 2.43, 95%CI = 1.26–4.70, p = 0.008) were predictive of poor OS while a trend was seen for C1M (HR = 1.70, 95%CI = 0.85–3.38), p = 0.131) and C3M (HR = 1.60, 95%CI = 0.82–3.13, p = 0.167). In comparison, high VICM (Q3 + Q4) at baseline was predictive of a survival benefit (HR = 0.54, 95%CI = 0.29–0.99, p = 0.044). High LDH (> 250 IU/L) was the only covariate associated with poor OS (HR = 2.02, 95%CI = 0.99–4.12, p = 0.052). To determine the potential independent predictive value of PRO-C3, C1M, C3M, C4M and VICM for OS, multivariate Cox proportional-hazard models were used to calculate HRs for the biomarkers (dichotomized) adjusted for the covariates age, LDH and prior systemic treatment (Table 2). Of the collagen markers, only high PRO-C3 and C4M (Q4) were independently predicting poor OS (HR = 2.04, 95%CI = 1.00–4.16, p = 0.049 and HR = 2.18, 95%CI = 1.01–4.70, p = 0.046, for PRO-C3 and C4M, respectively). In contrast to PRO-C3 and C4M which predicted a poor outcome, high VICM (Q3 + Q4) was independently predictive of a survival benefit (HR = 0.49, 95%CI = 0.26–0.92, p = 0.026).
Table 2.
Association between biomarkers at baseline, clinical covariates and overall survival for metastatic melanoma patients
| Variable | HR | 95%Cl | p-value | |
|---|---|---|---|---|
| PRO-C3 | Continuous | 1.03 | 1.02–1.05 | 0.0004 |
| 5.0–19.2 ng/ml, Q1-Q3 | 1.00 | – | – | |
| Univariate | 19.6–113.3 ng/ml, Q4 | 2.13 | 1.12–4.04 | 0.021 |
| 5.0–19.2 ng/ml, Q1-Q3* | 1.00 | – | – | |
| Multivariate | 19.6–113.3 ng/ml, Q4* | 2.04 | 1.00–4.16 | 0.049 |
| C1M | Continuous | 1.01 | 1.00–1.01 | 0.005 |
| 20–46.6 ng/ml, Q1-Q3 | 1.00 | – | – | |
| Univariate | 56.7–313.1 ng/ml, Q4 | 1.70 | 0.85–3.38 | 0.131 |
| 20–46.6 ng/ml, Q1-Q3* | 1.00 | – | – | |
| Multivariate | 56.7–313.1 ng/ml, Q4* | 1.17 | 0.54–2.51 | 0.693 |
| C3M | Continuous | 1.03 | 1.00–1.07 | 0.037 |
| 4.7–23.4, Q1-Q3 | 1.00 | – | – | |
| Univariate | 23.6–68.8, Q4 | 1.60 | 0.82–3.13 | 0.167 |
| 4.7–23.4, Q1-Q3* | 1.00 | – | – | |
| Multivariate | 23.6–68.8, Q4* | 1.06 | 0.48–2.33 | 0.886 |
| C4M | Continuous | 1.04 | 1.01–1.08 | 0.026 |
| 11.9–34.7 ng/ml, Q1-Q3 | 1.00 | – | – | |
| Univariate | 35.1–73.9 ng/ml, Q4 | 2.43 | 1.26–4.70 | 0.008 |
| 11.9–34.7 ng/ml, Q1-Q3* | 1.00 | – | – | |
| Multivariate | 35.1–73.9 ng/ml, Q4* | 2.18 | 1.01–4.70 | 0.046 |
| VICM | Continuous | 0.99 | 0.95–1.02 | 0.413 |
| 1.0–9.1 ng/ml, Q1-Q2 | 1.00 | – | – | |
| Univariate | 9.1–41.3 ng/ml, Q3-Q4 | 0.54 | 0.29–0.99 | 0.044 |
| 1.0–9.1 ng/ml, Q1-Q2* | 1.00 | – | – | |
| Multivariate | 9.1–41.3 ng/ml, Q3-Q4* | 0.49 | 0.26–0.92 | 0.026 |
| Age at baseline | 1.02 | 0.99–1.04 | 0.254 | |
| LDH at sampling | Continuous | 1.00 | 1.00–1.00 | 0.009 |
| (> = 250 IU/L) | 2.02 | 0.99–4.12 | 0.052 | |
| Prior systemic therapy | 1.32 | 0.73–2.40 | 0.363 | |
Hazard ratios (HR) were calculated by univariate and multivariate analysis (indicated by stars). By the univariate analysis, biomarkers were analyzed on both a continuous scale and divided into quartiles with the lower quartiles (Q1-Q3) or (Q1-Q2) used as a reference to calculate the HR for patients in the upper quartiles (Q4) or (Q3-Q4). All covariates were analyzed on a continuous scale and LDH was furthermore analyzed on a binominal scale. By the multivariate analysis, the individual biomarkers were adjusted for the covariates age, LDH and prior systemic treatment. LDH Lactate dehydrogenase
The biomarker levels at baseline were also assessed for their prediction of PFS. In brief, similar results as for OS were observed, with the exception of C1M levels (Additional file 1: Table S1). Interestingly for C1M, although high C1M (Q4) levels were not significantly predictive of OS (Table 2), high C1M levels were predictive of PFS by the univariate analysis (HR = 2.13, 95%CI = 1.17–3.88, p = 0.013) and borderline significant with the multivariate analysis (HR = 1.84, 95%CI = 0.97–3.51, p = 0.064) (Additional file 1: Table S1).
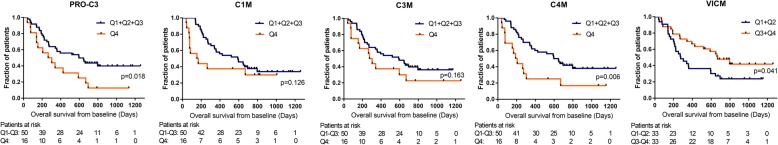
PRO-C3, C4M and VICM at baseline are associated with survival over time
KM survival plots show the OS within the follow-up period according to baseline levels for the five analyzed biomarkers (Fig. 2). High baseline levels (Q4) of PRO-C3 (p = 0.018) and C4M (p = 0.006) were significantly associated with shorter OS while a trend was seen for C1M (p = 0.126) and C3M (p = 0.163). The median OS was 285, 161, 290 or 198 days in biomarker high patients versus 596, 592, 592 or 621 days in biomarker low patients for PRO-C3, C1M, C3M and C4M, respectively. In contrast, high levels of VICM (Q3 + Q4) were associated with longer OS (p = 0.041) with a median OS of 669 days versus 275 days in VICM low patients. When assessing PFS curves comparable findings were observed (Additional file 1: Figure S1). Interestingly, when evaluating OS after 1 year, high baseline levels of C1M (Q4) were significantly associated with poor OS (p = 0.016) (Additional file 1: Figure S2).
Fig. 2.
Kaplan-Meier analysis of overall survival in ipilimumab treated melanoma patients. Overall survival for patients with biomarker levels in the upper quartile (Q4) vs the lower quartiles (Q1 + Q2 + Q3) for PRO-C3, C1M, C3M and C4M, while for VICM it is the upper quartiles (Q3 + Q4) vs the lower quartiles (Q1 + Q2). A log-rank test was used to determine differences between the survival curves where a p-value of p < 0.05 was considered statistically significant
High C3M/PRO-C3 ratio at baseline associates with increased overall survival
Next, we examined if the ratio of type III collagen degradation to formation (C3M/PRO-C3) at baseline could give additional information when looking on DCR and OS. We dichotomized the C3M/PRO-C3 serum levels by the 25th percentile (Q1 vs Q2 + Q3 + Q4) based on the observation that high PRO-C3 (Q4) was significantly associated with shorter OS compared to low PRO-C3 (Q1 + Q2 + Q3) suggesting that a low C3M/PRO-C3 ratio likewise was associated with decreased OS. Low levels of C3M/PRO-C3 (Q1) were able to differentiate PD from CR + PR + SD with an OR of 3.8 (95%CI = 1.0–13.5, p = 0.080) and a PPV of 0.82 (Fig. 3a). When looking at survival outcome, a high C3M/PRO-C3 ratio at baseline (Q2 + Q3 + Q4) was significantly associated with longer OS when assessed by a log-rank test (p = 0.015) (Fig. 3b) and the multivariate Cox proportional-hazard analysis (HR = 0.47, 95%CI = 0.24–0.95, p = 0.034). We assessed the correlation between the C3M/PRO-C3 ratio and PRO-C3 or C3M. It was demonstrated that a low level of PRO-C3 and an intermediate level of C3M drive a high C3M/PRO-C3 ratio (Additional file 1: Figure S3).
Fig. 3.
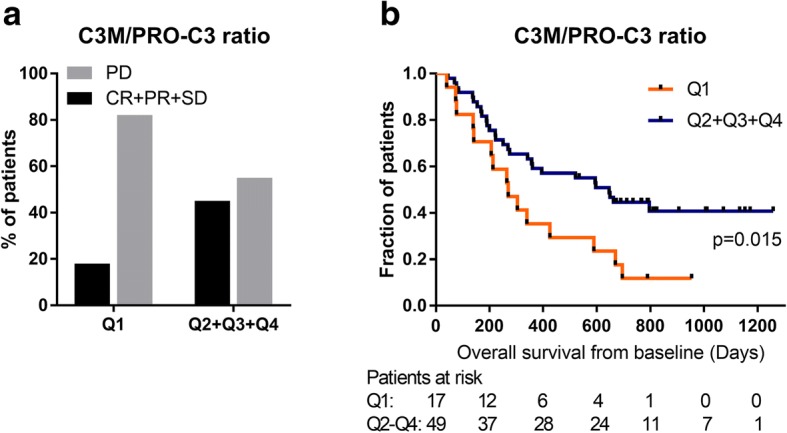
Relationship between C3M/PRO-C3 ratio at baseline and clinical outcome. C3M/PRO-C3 serum levels were dichotomized by the 25th percentile dividing the patients into a group with low C3M/PRO-C3 (Q1) and high C3M/PRO-C3 (Q2 + Q3 + Q4). a Quantity of patients achieving disease control (CR + PR + SD) compared to non-responders (PD) in each group. b Kaplan-Meier survival curve illustrate the overall survival for the two groups. A log-rank test was used to determine differences between the survival curves where a p-value of p < 0.05 was considered statistically significant
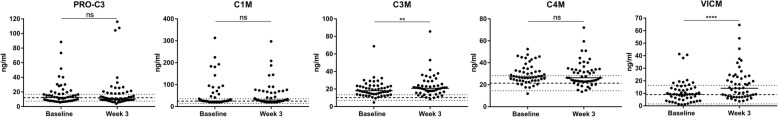
Changes in C3M and VICM levels after 3 weeks of treatment are not associated with overall survival
Serum concentrations of PRO-C3, C1M, C3M, C4M and VICM at baseline and at week 3 are shown in Fig. 4 (Raw data are shown in Additional file 1: Table S2). When the biomarker levels were paired, neither PRO-C3 (p = 0.735), C1M (p = 0.811) nor C4M (p = 0.642) showed significant changes during 3 weeks of treatment whereas significantly elevated levels of C3M (p = 0.003) and VICM (p < 0.0001) were observed at the 3 week time-point.
Fig. 4.
Biomarker levels in serum during treatment. PRO-C3, C1M, C3M, C4M and VICM levels in serum at baseline (n = 52) and 3 weeks after ipilimumab treatment (n = 52). The black horizontal lines represent the median value of the patients. Serum levels were compared using Wilcoxon matched-pairs rank test. Asterisks indicate the following: **, p < 0.01 and ****, p < 0.0001
Based on the observation of significantly elevated C3M and VICM levels during treatment, the OS was assessed according to percentage decrease or increase in biomarker levels from baseline to 3 weeks of treatment. The change (increase vs. decrease) in these biomarkers did not associate with OS: C3M (HR = 1.07, 95%CI = 0.52–2.18, p = 0.861) and VICM (HR = 2.17, 95%CI = 0.89–5.28, p = 0.089), albeit a trend was seen for VICM.
Discussion
In the present study, we measured a panel of five ECM biomarkers in serum from patients with stage IV malignant melanoma treated with IPI. The main findings showed that high baseline levels (Q4) of the collagen biomarkers PRO-C3, C1M, C3M and C4M were associated with poor response (according to RECIST) to IPI. In addition, high PRO-C3 and C4M (Q4) were associated with shorter OS. In contrast, high VICM (Q3 + Q4) at baseline was linked to a survival benefit. Application of the ratio of type III collagen degradation to formation (C3M/PRO-C3) added additional information with the observation that a high C3M/PRO-C3 ratio (Q2 + Q3 + Q4) was associated with longer OS.
ICIs induce only durable responses in a minority of patients, and is associated with significant risk of inflammatory toxicity and high costs. Therefore, biomarkers for prediction of response and resistance represent unmet needs. Factors as mutational load [29], PD-L1 expression [30], cytokines [31], blood immune cells [24, 32] and TILs [33] have been identified to correlate with treatment response to ICIs. However, tumor heterogeneity, technical issues and the complexity of the immune response limit the applicability of these biomarkers. There is increasing interest in exploring the stromal matrix components for its role in regulating anti-tumor immune responses [4]. To our knowledge, this is the first study to show the biomarker potential of quantifying altered ECM remodeling in a liquid biopsy (serum) for predicting outcome to ICIs. The ECM biomarkers in this study are measured by technical robust ELISAs, a relative simple and inexpensive technology, which accurately measure the analytes of interest [17, 25–28]. Peripheral blood biomarkers have an advantage due to the ease of accessing blood versus tumor tissue. Furthermore, it is presumed that ECM fragments are more stable in the circulation than for instance cytokines, which depend on the immune status of the patient.
Elevated levels of collagen-derived fragments reflecting an imbalance in ECM have previously been linked to cancer progression [18, 20, 22]. C1M and C3M reflect interstitial matrix degradation, which may pave the way for tumor cell migration and progression. In this study, high baseline levels of C1M and C3M were associated with PD and a trend was seen for an association to poor OS. However, when adjusting for covariates as LDH, high C1M and C3M levels were not independently linked to poor OS. Elevated serum LDH indicates tissue damage, which triggers inflammation and degradation of the ECM, which may account for the association. High LDH levels are an indicator of poor response to IPI in stage IV melanoma patients [34, 35].
It has previously been shown that ICI treatment can lead to immune-related adverse events with clinical manifestations similar to rheumatic disease [36]. In this study, an increase in C3M levels after IPI treatment was demonstrated, which may be associated with increased inflammation. In line with this, it has been shown that C3M levels are higher in rheumatoid arthritis patients than in healthy controls [37]. This link between potential IPI-induced C3M levels and rheumatic arthritis should be investigated in further studies.
Excessive collagen deposition is important for tumor progression and has been linked to a blocking of the T-cell recruitment, which is important for efficient ICI therapy [38, 39]. In the present study, high levels of PRO-C3 (which measures true formation of type III collagen [17]) at baseline were associated with PD and poor OS, thereby suggesting that a dense fibrotic network blocks T-cell infiltration which would contribute to the resistance to anti-CTLA-4 treatment. In a recent study, the lack of response to anti-PD-L1 treatment was associated with transforming growth factor-β (TGF-β) signaling in fibroblasts, and this occurred particularly in patients with T-cell excluded tumors where T cells got trapped in the surrounding collagen [8]. TGF-β promotes production of ECM components and it was furthermore shown that the co-administration of TGF-β-blocking and anti-PD-L1 antibodies to an immune-excluded mouse model facilitated T-cell penetration into tumors and tumor regression [8]. This highlights the prominent role of ECM deposition in reducing anti-tumor immunity and furthermore the potential for combination therapies with stroma targeting agents.
The role of altered collagen degradation to formation for response to IPI was investigated in our study by looking at the C3M/PRO-C3 ratio at baseline. Interestingly, a high C3M/PRO-C3 ratio predicts good outcome, highlighting that the balance between ECM degradation to formation is important and indicating that the ratio/balance is clinically informative for outcome measures in malignant melanoma patients treated with IPI. This suggest that patients with less net fibrosis/collagen deposition (PRO-C3) are more likely to respond to ICI treatment. In support, Wang et al. showed that the C3M/PRO-C3 ratio provided true predictive value for response to PEGPH20, a stromal modifier, in a phase II trial of patients with metastatic pancreatic cancer [40].
The collagen biomarker C4M reflects basement membrane remodeling, which is associated with malignant progression and metastatic dissemination [41]. The association between high baseline levels of C4M and poor outcome supports that basement membrane remodeling contributes to PD. The collagen turnover biomarkers in this study have high PPVs for identifying patients with PD, which support their applicability for excluding a subpopulation of patients with poor chance of responding.
Several studies have indicated that collagen fragments have biological activity in which fragments of collagens binds to integrins or ITIM-bearing receptors resulting in pro- or anti-tumorigenic responses [14, 42]. LAIR-1 is an ITIM receptor expressed by PBMCs that binds collagens, which negatively regulate the immune response [43]. A mechanism that could maintain infiltrating immune cells in a suppressive pro-tumorigenic state. ECM bioactive fragments can also be chemotactic for myeloid cells, like neutrophils, which are associated with poor prognostic outcome and short OS in melanoma patients undergoing immunotherapy [44–46]. Protease-derived collagen fragments from type I and IV collagen have previously been shown to promote neutrophil chemotaxis suggesting that elevated C1M and C4M might contribute to an immunosuppressive tumor microenvironment, which leads to poor outcome [47].
We demonstrated that high VICM levels at baseline were associated with longer OS indicating a role of macrophages in response to IPI. In support of this, it has been shown that melanoma patients responding to IPI display higher frequencies of tumor-infiltrating macrophages at baseline compared with non-responding patients [48].
Although we discovered a change in C3M and VICM levels after 3 weeks of treatment, only a trend towards an association with OS was seen for VICM. It is possible that 3 weeks might not be enough time for inducing sufficient change in biomarker levels associated with a difference in outcome and further analyses are warranted.
The present study is limited by that half of the patients were initially treated with systemic therapy before receiving IPI. However, when we adjusted for prior systemic therapy in the multivariate analysis, high levels of PRO-C3 and C4M were independently predictive of poor OS while high levels of VICM were independently predictive for longer OS suggesting that prior systemic therapy does not affect the potential of these biomarkers.
This study was a retrospective analysis, which needs to be validated using larger patient cohorts and different types of cancer and ICI therapies. These neo-epitope biomarkers reflecting changes of the ECM can probably not be used to guide decisions about immunotherapy straight away but they have indeed potential for being promising biomarkers. Furthermore, these preliminary results can improve the scientific understanding of ECM as being a vital factor that influence response to ICIs.
Conclusions
ICIs induce only durable responses in a subset of metastatic melanoma patients suggesting that the clinical response is indeed complex and multifactorial. Emerging evidence suggests that the ECM and proteolytic ECM remodeling products have a crucial role in regulating the cancer-immunity cycle. We show that stromal matrix components can be used as biomarkers in an ICI setting. High PRO-C3, C1M, C3M and C4M levels at baseline are associated with poor response to IPI while high VICM is associated with longer OS. This explorative study provides knowledge about ECM as a main player in anti-tumor immune responses and suggests that ECM-derived biomarkers have the potential of improving patient stratification for ICI treatment.
Additional file
Table S1. Association between biomarkers at baseline, clinical covariates and progression free survival for metastatic melanoma patients. Figure S1. Kaplan-Meier analysis of progression free survival in ipilimumab treated melanoma patients. Figure S2. Kaplan-Meier analysis of overall survival in ipilimumab treated melanoma patients. Figure S3. Correlation between the C3M/PRO-C3 ratio and levels of C3M or PRO-C3 in the individual melanoma patients. Table S2. Biomarker levels in serum at baseline and 3 weeks after ipilimumab treatment. (DOCX 238 kb)
Acknowledgements
We acknowledge the Danish Cancer Society for supporting DHM.
Funding
We acknowledge the Danish Research Foundation for supporting the extracellular matrix biomarker measurement in this study.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- C1M
MMP-degraded type I collagen
- C3M
MMP-degraded type III collagen
- C3M/PRO-C3
Type III collagen degradation to formation
- C4M
MMP-degraded type IV collagen
- CR
Complete response
- CTLA-4
Cytotoxic T lymphocyte antigen 4
- DCR
Disease control rate
- ECM
Extracellular matrix
- ELISA
Enzyme-linked immunosorbent assay
- HR
Hazard ratio
- ICI
Immune checkpoint inhibitor
- IPI
Ipilimumab
- KM
Kaplan-Meier
- LDH
Lactate dehydrogenase
- MMP
Matrix metalloproteinase
- OR
Odds ratio
- OS
Overall survival
- PD
Progressive disease
- PD-1
Programmed cell death protein 1
- PD-L1
PD1 ligand 1
- PFS
Progression free survival
- PPV
Positive predictive value
- PR
Partial response
- PRO-C3
Type III collagen formation
- RECIST
Response Evaluation Criteria in Solid Tumors
- SD
Stable disease
- TGF-β
Transforming growth factor-β
- TIL
Tumor infiltrating lymphocyte
- VICM
Citrullinated and MMP-degraded vimentin
Authors’ contributions
CJ and NW carried out the measurements. CJ carried out data analysis and statistical analysis in discussion with MAK and NW. CJ wrote the first draft of the manuscript. DHM, MH, HS and IMS provided the samples and patient information. All authors critical reviewed and approved the final version of the manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee for The Capital Region of Denmark (H-2-2012-058) in compliance with the Helsinki Declaration of 1975. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
MAK and NW are employed at Nordic Bioscience A/S, which is a company involved in discovery and development of biochemical biomarkers. MAK owns stocks at Nordic Bioscience.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Christina Jensen, Email: chj@nordicbio.com.
Daniel Hargbøl Madsen, Email: daniel.hargboel.madsen@regionh.dk.
Morten Hansen, Email: morten.hansen.01@regionh.dk.
Henrik Schmidt, Email: henrschm@rm.dk.
Inge Marie Svane, Email: inge.marie.svane@regionh.dk.
Morten Asser Karsdal, Email: mk@nordicbio.com.
Nicholas Willumsen, Email: nwi@nordicbio.com.
References
- 1.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 2.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Velasco G, Je Y, Bossé D, Awad MM, Ott PA, Moreira RB, et al. Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in Cancer patients. Cancer Immunol Res. 2017;5(4):312–318. doi: 10.1158/2326-6066.CIR-16-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mushtaq MU, Papadas A, Pagenkopf A, Flietner E, Morrow Z. Tumor matrix remodeling and novel immunotherapies: the promise of matrix-derived immune biomarkers. J Immunother Cancer. 2018;6:1–14. [DOI] [PMC free article] [PubMed]
- 5.Huse M. Mechanical forces in the immune system. Nat Rev Immunol. 2017;17:679–690. doi: 10.1038/nri.2017.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A, et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Investig. 2012;122:899–910. doi: 10.1172/JCI45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen DS, Mellman I. Elements of cancer immunity and the cancer–immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 8.Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. Nature. 2018. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells; pp. 544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willumsen N, Thomsen LB, Bager CL, Jensen C, Karsdal MA. Quantification of altered tissue turnover in a liquid biopsy: a proposed precision medicine tool to assess chronic inflammation and desmoplasia associated with a pro-cancerous niche and response to immuno-therapeutic anti-tumor modalities. Cancer Immunol Immunother. 2017;67:1–12. [DOI] [PMC free article] [PubMed]
- 10.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziani L, Safta-Saadoun TB, Gourbeix J, Cavalcanti A, Robert C, Favre G, et al. Melanoma-associated fibroblasts decrease tumor cell susceptibility to NK cell-mediated killing through matrix-metalloproteinases secretion. Oncotarget. 2017;8:19780–94. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5386721/. [DOI] [PMC free article] [PubMed]
- 12.Rygiel TP, Stolte EH, de Ruiter T, van de Weijer ML, Meyaard L. Tumor-expressed collagens can modulate immune cell function through the inhibitory collagen receptor LAIR-1. Mol Immunol. 2011;49:402–406. doi: 10.1016/j.molimm.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Thomas AH, Edelman ER, Stultz CM. Collagen fragments modulate innate immunity. Experimental biology and medicine (Maywood, NJ) 2007. [PubMed] [Google Scholar]
- 14.Karsdal MA, Nielsen SH, Leeming DJ, Langholm LL, Nielsen MJ, Manon-Jensen T, et al. The good and the bad collagens of fibrosis - their role in signaling and organ function. Adv Drug Deliv Rev. 2017;121:43–56. doi: 10.1016/j.addr.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Cheng W, Yan-hua R, Fang-gang N, Guo-an Z. The content and ratio of type I and III collagen in skin differ with age and injury. Afr J Biotechnol. 2011;10:2524–2529. [Google Scholar]
- 16.Vázquez F, Palacios S, Alemañ N, Guerrero F. Changes of the basement membrane and type IV collagen in human skin during aging. Maturitas. 1996;25:209–215. doi: 10.1016/S0378-5122(96)01066-3. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen MJ, Nedergaard AF, Sun S, Veidal SS, Larsen L, Zheng Q, et al. The neo-epitope specific PRO-C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am J Transl Res. 2013;5:303–315. [PMC free article] [PubMed] [Google Scholar]
- 18.Kehlet SN, Sanz-Pamplona R, Brix S, Leeming DJ, Karsdal MA, Moreno V. Excessive collagen turnover products are released during colorectal cancer progression and elevated in serum from metastatic colorectal cancer patients. Sci Rep. 2016;6:30599. doi: 10.1038/srep30599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipton A, Leitzel K, Ali SM, Polimera HV, Nagabhairu V, Marks E, et al. High turnover of extracellular matrix reflected by specific protein fragments measured in serum is associated with poor outcomes in two metastatic breast cancer cohorts. Int J Cancer. 2018;143(11):3027–3034. doi: 10.1002/ijc.31627. [DOI] [PubMed] [Google Scholar]
- 20.Bager CL, Willumsen N, Leeming DJ, Smith V, Karsdal MA, Dornan D, et al. Collagen degradation products measured in serum can separate ovarian and breast cancer patients from healthy controls: a preliminary study. Cancer Biomarkers. 2015;15:783–788. doi: 10.3233/CBM-150520. [DOI] [PubMed] [Google Scholar]
- 21.Bay-jensen AC, Guo X, Mortensen JH, Karsdal MA. VICM is a novel biomarker of macrophage activity evaluated in a phase IIb clinical trial of Mavrilimumab. Arthritis Rheumatol. 2015;67(suppl 10).
- 22.Willumsen N, Bager CL, Leeming DJ, Smith V, Christiansen C, Karsdal MA, et al. Serum biomarkers reflecting specific tumor tissue remodeling processes are valuable diagnostic tools for lung cancer. Cancer Med. 2014;3:1136–1145. doi: 10.1002/cam4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers. 2014;6(3):1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjoern J, Juul Nitschke N, Zeeberg Iversen T, Schmidt H, Fode K, Svane IM. Immunological correlates of treatment and response in stage IV malignant melanoma patients treated with Ipilimumab. Oncoimmunology. 2016;5:e1100788. [DOI] [PMC free article] [PubMed]
- 25.Leeming D, He Y, Veidal S, Nguyen Q, Larsen D, Koizumi M, et al. A novel marker for assessment of liver matrix remodeling: An enzyme-linked immunosorbent assay (ELISA) detecting a MMP generated type I collagen neo-epitope (C1M). Biomarkers. 2011;16:616–28. Available from: https://www.tandfonline.com/doi/abs/10.3109/1354750X.2011.620628?journalCode=ibmk20. [DOI] [PubMed]
- 26.Barascuk N, Veidal SS, Larsen L, Larsen DV, Larsen MR, Wang J, et al. A novel assay for extracellular matrix remodeling associated with liver fibrosis: an enzyme-linked immunosorbent assay (ELISA) for a MMP-9 proteolytically revealed neo-epitope of type III collagen. Clin Biochem. 2010;43:899–904. doi: 10.1016/j.clinbiochem.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Sand JM, Larsen L, Hogaboam C, Martinez F, Han M, Larsen MR, et al. MMP mediated degradation of type IV collagen alpha 1 and alpha 3 chains reflects basement membrane remodeling in experimental and clinical fibrosis - validation of two novel biomarker assays. PLoS One. 2013;8(12):e84934. doi: 10.1371/journal.pone.0084934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vassiliadis E, Oliveira CP, Alvares-da-Silva MR, Zhang C, Carrilho FJ, Stefano JT, et al. Circulating levels of citrullinated and MMP-degraded vimentin (VICM) in liver fibrosis related pathology. Am J Transl Res. 2012;4:403–414. [PMC free article] [PubMed] [Google Scholar]
- 29.Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14:847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 31.Najjar YG, Ding F, Lin Y, VanderWeele R, Butterfield LH, Tarhini AA. Melanoma antigen-specific effector T cell cytokine secretion patterns in patients treated with ipilimumab. J Transl Med. 2017;15:39 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5319167/. [DOI] [PMC free article] [PubMed]
- 32.Jacquelot N, Roberti MP, Enot DP, Rusakiewicz S, Ternès N, Jegou S, et al. Predictors of responses to immune checkpoint blockade in advanced melanoma. Nat Commun. 2017;8(1):592. doi: 10.1038/s41467-017-00608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamid O, Schmidt H, Nissan A, Ridolfi L, Aamdal S, Hansson J, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011;9:204. Available from: https://translational-medicine.biomedcentral.com/articles/10.1186/1479-5876-9-204. [DOI] [PMC free article] [PubMed]
- 34.Deichmann M, Benner A, Bock M, Jäckel A, Uhl K, Waldmann V, et al. S100-beta, melanoma-inhibiting activity, and lactate dehydrogenase discriminate progressive from nonprogressive American joint committee on Cancer stage IV melanoma. J Clin Oncol. 1999;17:1891–1896. doi: 10.1200/JCO.1999.17.6.1891. [DOI] [PubMed] [Google Scholar]
- 35.Kelderman S, Heemskerk B, van Tinteren H, van den Brom RRH, Hospers GAP, van den Eertwegh AJM, et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immunother. 2014; Available from: http://link.springer.com/10.1007/s00262-014-1528-9. [DOI] [PMC free article] [PubMed]
- 36.Cappelli LC, Shah AA, Bingham CO. Cancer immunotherapy-induced rheumatic diseases emerge as new clinical entities. RMD Open. 2016;2:e000321. doi: 10.1136/rmdopen-2016-000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bay-Jensen AC, Wichuk S, Byrjalsen I, Leeming DJ, Morency N, Christiansen C, et al. Circulating protein fragments of cartilage and connective tissue degradation are diagnostic and prognostic markers of rheumatoid arthritis and ankylosing spondylitis. PLoS One. 2013;8(1):e54504. doi: 10.1371/journal.pone.0054504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 39.Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang S, Willumsen N, Bager C, Karsdal MA, Chondros D, Taverna D. Extracellular matrix (ECM) circulating peptide biomarkers as potential predictors of survival in patients (pts) with untreated metastatic pancreatic ductal adenocarcinoma (mPDA) receiving pegvorhyaluronidase alfa (PEGPH20), nab-paclitaxel (A), and gemcita. J Clin Oncol. 2018;36:12030. doi: 10.1200/JCO.2018.36.15_suppl.12030. [DOI] [Google Scholar]
- 41.Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4:165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ricard-Blum S, Vallet SD. Matricryptins network with matricellular receptors at the surface of endothelial and tumor cells. Front Pharmacol. 2016;7:11. doi: 10.3389/fphar.2016.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lebbink RJ, de Ruiter T, Adelmeijer J, Brenkman AB, van Helvoort JM, Koch M, et al. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J Exp Med. 2006;203:1419–1425. doi: 10.1084/jem.20052554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antonio N, Bonnelykke-Behrndtz ML, Ward LC, Collin J, Christensen IJ, Steiniche T, et al. The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. EMBO J. 2015;34:2219–2236. doi: 10.15252/embj.201490147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt H, Suciu S, Punt CJA, Gore M, Kruit W, Patel P, et al. Pretreatment levels of peripheral neutrophils and leukocytes as independent predictors of overall survival in patients with American joint committee on Cancer stage IV melanoma: results of the EORTC 18951 biochemotherapy trial. J Clin Oncol. 2007;25:1562–1569. doi: 10.1200/JCO.2006.09.0274. [DOI] [PubMed] [Google Scholar]
- 46.Gebhardt C, Sevko A, Jiang H, Lichtenberger R, Reith M, Tarnanidis K, et al. Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with Ipilimumab. Clin Cancer Res. 2015;21:5453–5459. doi: 10.1158/1078-0432.CCR-15-0676. [DOI] [PubMed] [Google Scholar]
- 47.Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol. 2008;40(6-7):1101–1110. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romano E, Kusio-Kobialka M, Foukas PG, Baumgaertner P, Meyer C, Ballabeni P, et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci U S A. 2015;112:6140–6145. doi: 10.1073/pnas.1417320112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Association between biomarkers at baseline, clinical covariates and progression free survival for metastatic melanoma patients. Figure S1. Kaplan-Meier analysis of progression free survival in ipilimumab treated melanoma patients. Figure S2. Kaplan-Meier analysis of overall survival in ipilimumab treated melanoma patients. Figure S3. Correlation between the C3M/PRO-C3 ratio and levels of C3M or PRO-C3 in the individual melanoma patients. Table S2. Biomarker levels in serum at baseline and 3 weeks after ipilimumab treatment. (DOCX 238 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.