Abstract
Context
The Early vs Late Intervention Trial with Estradiol showed that hormone therapy (HT) reduced progression of atherosclerosis when initiated in early but not in late postmenopause.
Objective
This posttrial analysis examined the association between plasma estradiol (E2) levels and atherosclerosis determined by rate of change in carotid artery intima-media thickness (CIMT) and tested whether this association is equally evident in early (<6 years) vs late (≥10 years) postmenopause.
Design
Randomized controlled trial stratified by time since menopause (ClinicalTrials.gov no. NCT00114517). Mixed-effects linear models tested the association of E2 levels with CIMT rate of change.
Setting
Los Angeles, California.
Participants
Healthy women in postmenopause.
Intervention
Oral E2 with/without cyclic vaginal progesterone.
Main Outcome Measures
Plasma E2 levels and CIMT assessed every 6 months over an average of 4.8 years.
Results
Among 596 women in postmenopause, higher E2 level was inversely associated with CIMT progression in those in early postmenopause (P = 0.041) and positively associated with CIMT progression in those in late postmenopause (P = 0.006) (P for interaction <0.001). CIMT progression rates for the lowest vs highest quartile of E2 levels among women in early postmenopause were 8.5 and 7.2 μm/y, respectively , whereas among women in late postmenopause they were 9.8 and 11.7 μm/y, respectively.
Conclusion
E2 levels were differentially associated with atherosclerosis progression according to timing of HT initiation. With higher E2 levels, CIMT progression rate was decreased among women in early postmenopause but increased among women in late postmenopause. These results support the timing hypothesis of HT initiation on cardiovascular benefit, with reduced atherosclerosis progression for initiation during early postmenopause.
In ELITE, higher plasma estradiol levels were associated with decreased atherosclerosis progression among women in early postmenopause but with increased progression among women in late postmenopause.
Meta-analyses of randomized controlled trials suggest that women who initiate hormone therapy (HT) when younger than 60 years and/or within 10 years of menopause have reduced risk of coronary heart disease and all-cause mortality compared with women who take placebo, whereas women who initiate HT when older than 60 years and/or more than 10 years since menopause have a null risk (1–3). The HT timing hypothesis posits that women respond to HT differentially according to the timing of therapy initiation relative to age and/or time since menopause (4).
In a direct evaluation of the timing hypothesis in healthy women in postmenopause, the Early vs Late Intervention Trial with Estradiol (ELITE) showed that when initiated within 6 years of menopause, HT significantly reduced the progression of subclinical atherosclerosis measured by carotid artery intima-media thickness (CIMT). In contrast, HT initiation in women 10 or more years since menopause had no effect on the progression of atherosclerosis (4). Although the effect of plasma estradiol (E2) on the progression of subclinical atherosclerosis has been well described (5), the effect of plasma E2 levels on atherosclerosis progression according to time of HT initiation in relation to time since menopause is unknown. In this secondary analysis of the ELITE data, we evaluated whether there is a differential association between plasma E2 levels and progression of subclinical atherosclerosis based on when HT was initiated in relation to time since menopause.
Materials and Methods
ELITE study design and outcome
ELITE (ClinicalTrials.gov no. NCT00114517) was a single-center, randomized, double-blinded, placebo-controlled trial of HT administered to women stratified within 6 years of menopause (early postmenopause) and 10 years or more after menopause (late postmenopause) (6). The trial was conducted from July 2005 to February 2013. ELITE was specifically designed to test the HT timing hypothesis (i.e., whether the effects of HT vary according to the timing of initiation in relation to menopause). Healthy women in postmenopause were randomly assigned to receive either HT or placebo according to time since menopause strata using a 1:1 ratio of stratified blocked randomization. The HT regimen for women with an intact uterus was daily oral micronized 17-beta-estradiol 1 mg/d with 4% vaginal micronized progesterone gel 45 mg/d for 10 days each month. Women without an intact uterus were given 1 mg/d of oral micronized 17-beta-estradiol alone. The trial was approved by the institutional review board of the University of Southern California. In the primary outcome analysis, HT was associated with less progression of subclinical atherosclerosis (measured as rate of change in CIMT) than placebo when the therapy was initiated in women in early postmenopause, but not in women in late postmenopause (4). The ELITE study was funded by the National Institute on Aging, National Institutes of Health (R01AG-024154).
Study population
Participants were recruited from the general population. Inclusion criteria were healthy women in postmenopause without clinical evidence of cardiovascular disease, with a serum E2 level lower than 25 pg/mL, and with cessation of menses for a minimum of 6 months. Exclusion criteria included inability to determine time since menopause; fasting plasma triglyceride levels >500 mg/dL; diabetes mellitus or fasting serum glucose levels >140 mg/dL; serum creatinine level >2.0 mg/L; uncontrolled hypertension; untreated thyroid disease; life-threatening disease with prognosis <5 years; a history of deep vein thrombosis, pulmonary embolism, or breast cancer; and current use of menopausal HT within 1 month of screening. On the basis of time since menopause at study enrollment, women were characterized as early postmenopause (<6 years) and late postmenopause (≥10 years).
Follow-up
After randomization, women attended study clinic visits every month for the first 6 months and every other month thereafter until trial completion. The median duration of follow-up was 4.8 years (range, 0.5 to 6.7 years).
CIMT assessment
The rate of change in intima-media thickness of the far wall of the right distal common carotid artery was assessed by means of computer image processing of B-mode ultrasonograms obtained at the baseline examination and every 6 months during the follow-up period. Serial imaging and measurement methodologies were specifically developed for longitudinal measurements of change in atherosclerosis; the coefficient of variation for baseline CIMT measurement was 0.69% (7–9).
E2 assay
Fasting blood samples for measurement of plasma total E2 were obtained every 6 months while the women were in the trial. The plasma level of total E2 was quantified by radioimmunoassay with preceding organic solvent extraction and Celite column partition chromatography (10) of the samples obtained at baseline and every 6 months during the follow-up period (6). The assay sensitivity is 2 pg/mL, and the interassay coefficients of variation are 11%, 13%, and 12% at 15, 36, and 101 pg/mL, respectively.
Statistical methods
Baseline characteristics were reported separately for each time since menopause stratum: early and late postmenopause. Continuous variables, including age, baseline CIMT, baseline E2 level, mean E2 level during the trial, and change in E2 level from baseline were reported as mean (SD) and were compared between time since menopause strata using two-sample t tests. Categorical variables, including race, education, randomized treatment, and hysterectomy status, were reported as frequency (percent) and were compared between strata with χ2 tests.
Per-participant rate of change in CIMT was analyzed with mixed-effects linear models; mean E2 level (per-participant average E2) was included as a main independent variable along with time (years) since randomization. Random effects were specified for participant-specific intercept (baseline CIMT) and slope (rate of change in CIMT). The analysis was first performed separately for women in early vs late postmenopause; an interaction of E2 level with time since randomization tested whether the rate of change in CIMT (association of CIMT with time) varied by E2 level. In a combined analysis using participants from the total cohort, time since menopause stratum was added into the model. The difference in the association of rate of change in CIMT with E2 by early/late postmenopause was tested by including a product term between time since menopause stratum, E2 level, and time since randomization. This analysis was repeated, including only women randomly assigned to HT. Analyses were further adjusted for body mass index, race, education, and hysterectomy status. Model-based estimates of the annual CIMT progression rates and 95% CIs were calculated for each quartile cut point (at the 25th, 50th, and 75th percentiles) of E2 levels separately for early and late postmenopause in the total cohort and among women randomly assigned to HT.
Results
A total of 643 eligible women in postmenopause (271 early postmenopause and 372 late postmenopause) were randomly assigned in the ELITE study; 596 women (248 early postmenopause and 348 late postmenopause) with follow-up data on E2 levels and CIMT were included in this analysis. Among these women, 297 were randomly assigned to receive HT (125 early postmenopause and 172 late postmenopause). Table 1 shows baseline characteristics of the study participants by time since menopause stratum. As expected, the mean age (±SD) for women in early postmenopause (54.7 ± 4.2 years) was lower than that for women in late postmenopause (63.6 ± 6.1 years). Women in late postmenopause had thicker CIMT at baseline than women in early postmenopause. Women in late postmenopause were more likely to be white, to have a lower education level, and to have a prior hysterectomy than those in early postmenopause.
Table 1.
Baseline Characteristics of Women by Time Since Menopause Stratum
| Variable | Total Participants | Early Postmenopause (<6 y) | Late Postmenopause (≥10 y) |
|---|---|---|---|
| Number of participants | N = 596 | N = 248 | N = 348 |
| Age,a y | 59.9 ± 6.9 | 54.7 ± 4.2 | 63.6 ± 6.1 |
| E2 level at baseline, pg/mL | 8.3 ± 5.3 | 7.9 ± 4.8 | 8.5 ± 5.7 |
| CIMT at baseline,a μm | 770.3 ± 105.5 | 747.1 ± 95.5 | 786.9 ± 109.2 |
| Racea | |||
| White non-Hispanic | 415 (69.6) | 161 (64.9) | 254 (72.9) |
| Black non-Hispanic | 52 (8.7) | 21 (8.5) | 31 (8.9) |
| Hispanic | 79 (13.3) | 36 (14.5) | 43 (12.4) |
| Asian/Pacific Islander | 50 (8.4) | 30 (12.1) | 20 (5.8) |
| Educationa | |||
| High school graduate or less | 22 (3.7) | 6 (2.4) | 16 (4.6) |
| Trade/business school/some college | 173 (29.0) | 60 (24.2) | 113 (32.5) |
| Bachelor’s degree/graduate/professional | 401 (67.3) | 182 (73.4) | 219 (62.9) |
| Treatment | |||
| Placebo | 299 (50.2) | 123 (49.6) | 176 (50.6) |
| Active hormone | 297 (49.8) | 125 (50.4) | 172 (49.4) |
| Hysterectomya | |||
| No | 487 (81.7) | 237 (95.6) | 250 (71.8) |
| Yes | 109 (18.3) | 11 (4.4) | 98 (28.2) |
Continuous variables: mean ± SD and P value from t test. Categorical variables: frequency (percent) and P value from χ2 test or Fisher’s exact test.
P value <0.05 from the comparison between early and late postmenopause.
The mean E2 level at baseline was 8.3 ± 5.3 pg/mL, and it did not differ by time since menopause stratum (Table 1). Mean E2 levels during the trial also did not differ significantly between early and late postmenopause in the total cohort combining HT- and placebo-treated women (Table 2). Among women in the HT group, mean E2 level during the trial and change in E2 level from baseline were significantly higher in the early postmenopause group than in the late postmenopause group despite similar adherence (measured by percentage of ideal pill count) between the two groups. Among women in the placebo group, both mean E2 levels and change in E2 levels from baseline were equivalent in early and late postmenopause. The HT effect on change in E2 levels differed between the two time since menopause strata (treatment * time since menopause stratum interaction, adjusted for baseline E2 level; analysis of covariance P = 0.02).
Table 2.
Mean E2 Level During the Trial and Change in E2 Level From Baseline Among Total Sample and Participants in HT Group by Time Since Menopause Stratum
| Variable | Early Postmenopause (<6 y) | Late Postmenopause (≥10 y) | P Value |
|---|---|---|---|
| Total Cohort | |||
| N = 248 | N = 348 | ||
| Mean E2 level during the trial, pg/mL | 29.7 ± 31.8 | 25.5 ± 22.5 | 0.06 |
| Change of E2 level from baseline, pg/mL | 21.7 ± 31.6 | 17.0 ± 22.7 | 0.03 |
| Participants in HT Group | |||
|---|---|---|---|
| N = 125 | N = 172 | ||
| Mean E2 level during the trial, pg/mL | 48.2 ± 35.8 | 40.2 ± 23.6 | 0.02 |
| Change of E2 level from baseline, pg/mL | 40.4 ± 35.4 | 31.6 ± 24.0 | 0.01 |
| Participants in Placebo Group | |||
|---|---|---|---|
| N = 123 | N = 176 | ||
| Mean E2 level during the trial, pg/mL | 10.9 ± 5.9 | 11.0 ± 6.0 | 0.85 |
| Change of E2 level from baseline, pg/mL | 2.8 ± 5.9 | 2.6 ± 6.8 | 0.88 |
Continuous variables: mean ± SD and P value from t test. Among total cohort, test for interaction of treatment * menopausal strata adjusting for baseline E2 level P value = 0.02.
Combining HT- and placebo-treated women, the mixed-effects analysis of the CIMT progression rate based on the mean E2 level during the trial showed that a higher level of E2 was inversely associated with CIMT progression rate in women in early postmenopause [β coefficient = −0.04 (95% CI: −0.09, −0.001) μm CIMT per year per 1 pg/mL E2; P = 0.04] but was positively associated with CIMT progression rate in women in late postmenopause [β coefficient = 0.063 (95% CI: 0.018, 0.107) μm CIMT per year per 1 pg/mL E2; P = 0.006] (Table 3).
Table 3.
Mixed Model Linear Regression Analysis of the Association of Mean E2 Level During the Trial With CIMT Progression Rate (µm/y): by Time Since Menopause Stratum
| Variables | Early Postmenopause (<6 y) | P Value | Late postmenopause (≥10 y) | P Value |
|---|---|---|---|---|
| Number of participants | N = 248 | N = 348 | ||
| Number of CIMT data points | N = 2435 | N = 3173 | ||
| Intercept | 751.0 (734.6, 767.4) | <0.001 | 792.0 (774.5, 809.5) | <0.001 |
| Time since randomization, y | 7.28 (5.43, 9.13) | <0.001 | 7.67 (6.15, 9.19) | <0.001 |
| Mean E2 level, pg/mL | −0.10 (−0.47, 0.28) | 0.62 | −0.18 (−0.70, 0.33) | 0.49 |
| Time since randomization, y, × mean E2 level, pg/mL | −0.040 (−0.090, -0.001) | 0.041 | 0.063 (0.018, 0.107) | 0.006 |
Numbers in table are β coefficients (95% CI); P value from the Wald test.
The effect of E2 levels on CIMT progression rate during the trial differed significantly between the time since menopause strata (time since randomization * mean E2 level * time since menopause strata interaction; P < 0.001 when analyzed among the total cohort, and P = 0.004 when analyzed only among HT participants) adjusted for body mass index, race, education, and hysterectomy status (Table 4). The association of E2 levels on CIMT progression rate was not significantly modified by level of baseline CIMT.
Table 4.
Evaluation of the Differential E2 Association With CIMT Progression Rate (µm/y) by Menopause Stratum From Mixed Model Linear Regression Analysis: Total Cohort and Participants in HT Group
| Variables | Total Cohort | P Value | HT Group | P Value |
|---|---|---|---|---|
| Number of participants | N = 596 | N = 297 | ||
| Number of data points | N = 5608 | N = 2784 | ||
| Intercept | 692.8 (624.1, 761.6) | <0.001 | 706.9 (596.6, 817.1) | <0.001 |
| Time since randomization, y | 9.2 (4.9, 13.6) | <0.001 | 7.8 (1.4, 14.2) | 0.017 |
| Mean E2 level, pg/mL | −0.28 (−0.77, 0.21) | 0.27 | −0.29 (−0.96, 0.37) | 0.39 |
| Time since randomization, y, × mean E2 level, pg/mL | 0.06 (0.02, 0.11) | 0.007 | 0.10 (0.04, 0.17) | 0.002 |
| Time since menopause strata | −42.4 (−67.2, -17.6) | <0.001 | −38.7 (−81.9, 4.5) | 0.08 |
| Time since randomization, y, × time since menopause strata | −0.39 (−2.75, 1.95) | 0.74 | −0.50 (−4.81, 3.80) | 0.82 |
| Mean E2 level, pg/mL, × time since menopause strata | 0.16 (−2.7, 2.0) | 0.62 | 0.10 (−0.73, 0.92) | 0.82 |
| Time since randomization, y, × mean E2 level, pg/mL, × time since menopause strata | −0.11 (−0.17, −0.05) | <0.001 | −0.12 (−0.20, -0.04) | 0.004 |
Numbers in table are β coefficients (95% CI); P values from the Wald test. The interaction term of the E2 association with CIMT rate by time since menopause stratum interaction terms represents the early menopause group (using late menopause as the referent group).
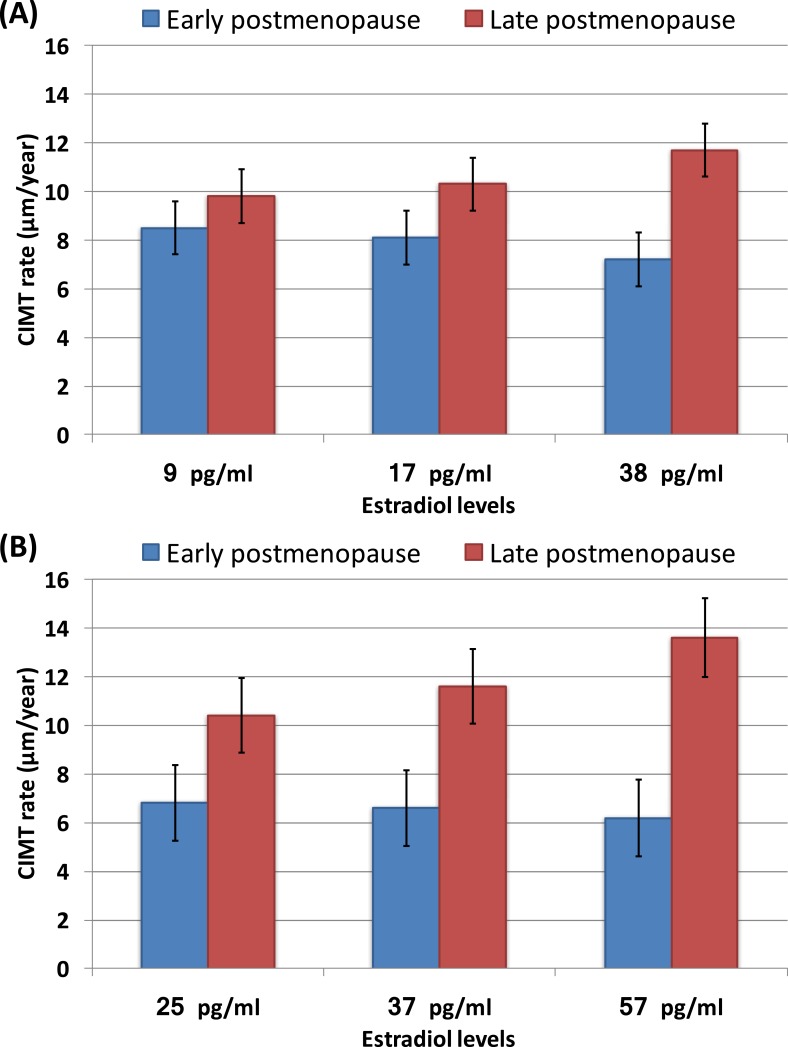
For the total cohort combining HT- and placebo-treated women, the model-estimated CIMT progression rates, estimated at quartile cut points of the on-trial E2 level: the 25th percentile (9 pg/mL), the 50th percentile (17 pg/mL), and the 75th percentile (38 pg/mL), are presented graphically in Fig. 1A.
Figure 1.
(A) Model-estimated CIMT progression rates at different quartiles of E2 level according to time since menopause strata among the total cohort. (B) Model-estimated CIMT progression rate at different quartiles of E2 level according to time since menopause strata among participants in the HT group. The lines represent standard error. Number of participants (%) in each quartile of E2: First-quartile HT group N = 10 (6.5%), placebo group N = 144 (93.5%); second-quartile HT group N = 21 (14.9%), placebo group N = 120 (85.1%); third-quartile HT group N = 122 (78.7%), placebo group N = 33 (21.39%); fourth-quartile HT group N = 144 (98.6%), placebo group N = 2 (1.4%). (A) Estimates of CIMT rate (with 95% CI) by E2 level: 25th percentile at 9 pg/mL: early postmenopause 8.5 (4.1, 12.8) μm/y, late postmenopause 9.8 (5.5, 14.1) μm/y (P = 0.18); 50th percentile at 17 pg/mL: early postmenopause 8.1 (3.8, 12.4) μm/y, late postmenopause 10.3 (6.1, 14.6) μm/y (P = 0.014); 75th percentile at 38 pg/mL: early postmenopause 7.2 (2.9, 11.5) μm/y, late postmenopause 11.7 (7.3, 16) μm/y (P < 0.0001). (B) Estimates of CIMT rate (with 95% CI) by E2 level: 25th percentile at 25 pg/mL: early postmenopause 6.8 (0.7, 12.9) μm/y, late postmenopause 10.4 (4.3, 16.4) μm/y (P = 0.0144); 50th percentile at 37 pg/mL: early postmenopause 6.6 (0.6, 12.6) μm/y, late postmenopause 11.6 (5.6, 17.6) μm/y (P < 0.0001); 75th percentile at 57 pg/mL: early postmenopause 6.2 (0.2, 12.2) μm/y, late postmenopause 13.6 (7.4, 20.0) μm/y (P < 0.0001).
For the analysis confined to women in the HT group, the model-estimated CIMT progression rates by quartiles of the on-trial E2 levels: the 25th percentile (25 pg/mL), the 50th percentile (37 pg/mL), and the 75th percentile (57 pg/mL), are shown in Fig. 1B.
Discussion
In this analysis from the ELITE randomized controlled trial, E2 levels were inversely associated with CIMT progression in women in early postmenopause (who were within 6 years of menopause) and were positively associated with CIMT progression in women in late postmenopause (who were at least 10 years postmenopause). The primary results of ELITE showed that oral E2 therapy reduced the progression of CIMT compared with placebo when therapy was initiated in women in early postmenopause but had no effect on CIMT progression when it was initiated in women in late postmenopause (4). The results of the current analysis showed that E2 levels achieved with oral E2 therapy were associated with CIMT progression in both early and late postmenopause, although in opposite directions. These opposite effects on CIMT progression occurred despite the fact that women in early and late postmenopause had achieved similar E2 levels from oral HT during the trial. These results not only support the HT timing hypothesis tested by the main trial but also add an explanatory mechanism consistent with the timing hypothesis.
Our findings demonstrate that the effect of HT-induced E2 level on atherosclerosis progression differed according to the timing of HT initiation relative to time since menopause. The timing of initiation could serve as a possible chronological indicator of underlying vascular health and hence as a determinant of whether E2 will reduce or accelerate the progression of atherosclerosis (4).
Human and animal data suggest that the atheroprotective effect of HT is limited to the endothelium without complicated atherosclerosis (4, 6). Estrogen inhibited atherosclerotic lesion formation in mice but did not alter the progression of established atherosclerotic lesions (11). In nonhuman primates, HT reduced coronary atherosclerosis when initiated immediately after surgical menopause but had no effect after a 2-year (equivalent to 6 years in humans) delay in initiating HT after surgical menopause (12, 13). ELITE extends these animal studies by suggesting that a healthy vasculature without complicated atherosclerosis (as inferred by chronological age or time since menopause) is required to respond beneficially to E2 levels with the reduction of atherosclerosis progression. Randomized controlled trials evaluating coronary artery atherosclerosis with coronary angiography showed that HT does not affect the progression of coronary artery atherosclerosis in women with established coronary artery disease (14). Favorable vascular effects of E2 appear to be limited to those women who are in early postmenopause (and possibly late postmenopause) but have not yet developed atherosclerotic vascular disease and resistance to the atheroprotective effects of estrogen (7, 15).
Although the mechanism(s) underlying the differential response of arteries to similar E2 levels is unknown, estrogens are known to regulate estrogen receptor expression levels, which are reduced after menopause and in the presence of atherosclerotic lesions (16–18). One possible mechanism may be related to the expression and/or signaling of estrogen receptors in healthy vs atherosclerosis-related tissues as a function of aging and/or time since menopause. Immunostaining of human postmortem coronary artery specimens has demonstrated that the number of estrogen receptors is reduced in women who are postmenopausal compared with women who are premenopausal. In premenopause, estrogen receptor differences are even more pronounced in women with atherosclerosis than in those without (16). In this regard, an age-related increase in methylation of the CpG islands in the promoter region of the estrogen receptor has been shown in vascular tissue as well as in coronary atherosclerotic plaques compared with normal vessels (17). Proliferating aortic smooth muscle cells that are characteristically found in atherosclerotic lesions selectively show increased estrogen receptor promoter methylation (18).
The strengths of this study are the randomized design testing the hormone timing hypothesis, the prospective data, and the specimen collection with simultaneous serial measurements of E2 and CIMT levels over 5 years. Repeated measurements reduce the variability of the exposure and outcome measurement, thus yielding more precise results. Because ELITE is the only trial with a priori stratification of early and late postmenopause, we had sufficient statistical power to identify the opposite directionality of the association between plasma E2 levels and atherosclerosis progression between the two strata of time since menopause.
Certain study limitations should be noted. The analysis was limited to evaluation of total E2 levels and did not account for other possibly related levels of free E2, estrone, and sex hormone‒binding globulin. This was a single-site study, which may limit the generalizability of the results. However, the uniform study conduct provided by a single site reduced variability in trial administration, participant follow-up, data collection, and longitudinal ultrasonographic imaging. This trial included predominantly healthy, well-educated women. Although perhaps limiting generalizability, education level and race were well balanced between HT and placebo groups. Although approximately 30% of the ELITE sample included other nonwhite women, the subgroup sample size of nonwhite women was not sufficient to evaluate possible effect modification, in particular within specific racial or ethnic groups. The contrasting association of E2 level and CIMT progression rate between early vs late menopause was equally evident in white and nonwhite women.
Because participants in the ELITE study were healthy women without clinical evidence of cardiovascular disease or diabetes, future studies among populations with more heterogeneity in underlying atherosclerosis status are needed to test the hypothesis of differential response to E2 level based on relative vascular health. Unlike ELITE, the Kronos Early Estrogen Prevention Study (KEEPS) showed a null effect of HT on atherosclerosis progression in young women who were within 3 years after menopause when randomized. The hormone regimen in KEEPS (oral conjugated equine estrogens 0.45 mg/d or transdermal 17-beta-estradiol 50 mcg/d, each with 200 mg of oral progesterone for 12 days per month) differed from that in ELITE and was lower dose. It has been reported that the effect of estrogen on CIMT is dose-dependent (19). Although both ELITE and KEEPS excluded women with a history of cardiovascular disease, KEEPS additionally excluded women who had coronary artery calcium assessed on CT imaging. Compared with participants in ELITE, participants in KEEPS had lower levels of cardiovascular risk factors, such as lower glucose, total cholesterol, triglyceride, and LDL cholesterol levels and higher high-density lipoprotein cholesterol level. These differences may account for the null effect of HT on atherosclerosis progression in KEEPS. In addition, KEEPS did not include a group of women in late postmenopause and therefore cannot provide an analysis similar to that reported herein for ELITE (20).
In conclusion, plasma E2 levels achieved through oral E2 therapy had opposite effects on the progression of atherosclerosis among women in early and late postmenopause. With higher plasma E2 levels, CIMT progression was decreased among women in early postmenopause and was increased among women in late postmenopause. These effects were statistically demonstrated in both the total cohort and in women receiving HT.
Acknowledgments
Financial Support: This work was supported by the National Institute on Aging, National Institutes of Health (R01-AG024154 and R01-AG059690; to H.N.H.) and P50-AG047366 (to V.W.H.).
Clinical Trial Information: ClinicalTrials.gov no. NCT00114517 (registered 16 June 2005).
Disclosure Summary: I.S., H.N.H., and W.J.M. received an unrestricted research grant (year 2018) from TherapeuticsMD. F.Z.S. consults for TherapeuticsMD, Agile Therapeutics, and Mithra. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- CIMT
carotid artery intima-media thickness
- E2
estradiol
- ELITE
Early vs Late Intervention Trial with Estradiol
- HT
hormone therapy
- KEEPS
Kronos Early Estrogen Prevention Study
References
- 1. Boardman HMP, Hartley L, Eisinga A, Main C, Roqué i Figuls M, Bonfill Cosp X, Gabriel Sanchez R, Knight B. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2015;(3):CD002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Salpeter SR, Walsh JM, Greyber E, Salpeter EE. Brief report: coronary heart disease events associated with hormone therapy in younger and older women: a meta-analysis. J Gen Intern Med. 2006;21(4):363–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salpeter SR, Walsh JME, Greyber E, Ormiston TM, Salpeter EE. Mortality associated with hormone replacement therapy in younger and older women: a meta-analysis. J Gen Intern Med. 2004;19(7):791–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Hwang-Levine J, Li Y, Feng M, Dustin L, Kono N, Stanczyk FZ, Selzer RH, Azen SP; ELITE Research Group . Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karim R, Hodis HN, Stanczyk FZ, Lobo RA, Mack WJ. Relationship between serum levels of sex hormones and progression of subclinical atherosclerosis in postmenopausal women. J Clin Endocrinol Metab. 2008;93(1):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hodis HN, Mack WJ, Shoupe D, Azen SP, Stanczyk FZ, Hwang-Levine J, Budoff MJ, Henderson VW. Methods and baseline cardiovascular data from the Early versus Late Intervention Trial with Estradiol testing the menopausal hormone timing hypothesis. Menopause. 2015;22(4):391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hodis HN, Mack WJ, Lobo RA, Shoupe D, Sevanian A, Mahrer PR, Selzer RH, Liu Cr CR, Liu Ch CH, Azen SP; Estrogen in the Prevention of Atherosclerosis Trial Research Group . Estrogen in the prevention of atherosclerosis: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135(11):939–953. [DOI] [PubMed] [Google Scholar]
- 8. Selzer RH, Hodis HN, Kwong-Fu H, Mack WJ, Lee PL, Liu CR, Liu CH. Evaluation of computerized edge tracking for quantifying intima-media thickness of the common carotid artery from B-mode ultrasound images. Atherosclerosis. 1994;111(1):1–11. [DOI] [PubMed] [Google Scholar]
- 9. Selzer RH, Mack WJ, Lee PL, Kwong-Fu H, Hodis HN. Improved common carotid elasticity and intima-media thickness measurements from computer analysis of sequential ultrasound frames. Atherosclerosis. 2001;154(1):185–193. [DOI] [PubMed] [Google Scholar]
- 10. Probst-Hensch NM, Ingles SA, Diep AT, Haile RW, Stanczyk FZ, Kolonel LN, Henderson BE. Aromatase and breast cancer susceptibility. Endocr Relat Cancer. 1999;6(2):165–173. [DOI] [PubMed] [Google Scholar]
- 11. Rosenfeld ME, Kauser K, Martin-McNulty B, Polinsky P, Schwartz SM, Rubanyi GM. Estrogen inhibits the initiation of fatty streaks throughout the vasculature but does not inhibit intra-plaque hemorrhage and the progression of established lesions in apolipoprotein E deficient mice. Atherosclerosis. 2002;164(2):251–259. [DOI] [PubMed] [Google Scholar]
- 12. Clarkson TB. Estrogen effects on arteries vary with stage of reproductive life and extent of subclinical atherosclerosis progression. Menopause. 2007;14(3):373–384. [DOI] [PubMed] [Google Scholar]
- 13. Mikkola TS, Clarkson TB, Notelovitz M. Postmenopausal hormone therapy before and after the Women’s Health Initiative study: what consequences? Ann Med. 2004;36(6):402–413. [DOI] [PubMed] [Google Scholar]
- 14. Herrington DM, Espeland MA, Crouse JR III, Robertson J, Riley WA, McBurnie MA, Burke GL. Estrogen replacement and brachial artery flow-mediated vasodilation in older women. Arterioscler Thromb Vasc Biol. 2001;21(12):1955–1961. [DOI] [PubMed] [Google Scholar]
- 15. Hodis HN, Mack WJ, Azen SP, Lobo RA, Shoupe D, Mahrer PR, Faxon DP, Cashin-Hemphill L, Sanmarco ME, French WJ, Shook TL, Gaarder TD, Mehra AO, Rabbani R, Sevanian A, Shil AB, Torres M, Vogelbach KH, Selzer RH; Women’s Estrogen-Progestin Lipid-Lowering Hormone Atherosclerosis Regression Trial Research Group . Hormone therapy and the progression of coronary-artery atherosclerosis in postmenopausal women. N Engl J Med. 2003;349(6):535–545. [DOI] [PubMed] [Google Scholar]
- 16. Losordo DW, Kearney M, Kim EA, Jekanowski J, Isner JM. Variable expression of the estrogen receptor in normal and atherosclerotic coronary arteries of premenopausal women. Circulation. 1994;89(4):1501–1510. [DOI] [PubMed] [Google Scholar]
- 17. Post WS, Goldschmidt-Clermont PJ, Wilhide CC, Heldman AW, Sussman MS, Ouyang P, Milliken EE, Issa JP. Methylation of the estrogen receptor gene is associated with aging and atherosclerosis in the cardiovascular system. Cardiovasc Res. 1999;43(4):985–991. [DOI] [PubMed] [Google Scholar]
- 18. Ying AK, Hassanain HH, Roos CM, Smiraglia DJ, Issa JJ, Michler RE, Caligiuri M, Plass C, Goldschmidt-Clermont PJ. Methylation of the estrogen receptor-α gene promoter is selectively increased in proliferating human aortic smooth muscle cells. Cardiovasc Res. 2000;46(1):172–179. [DOI] [PubMed] [Google Scholar]
- 19. Ostberg JE, Storry C, Donald AE, Attar MJH, Halcox JPJ, Conway GS. A dose-response study of hormone replacement in young hypogonadal women: effects on intima media thickness and metabolism. Clin Endocrinol (Oxf). 2007;66(4):557–564. [DOI] [PubMed] [Google Scholar]
- 20. Harman SM, Black DM, Naftolin F, Brinton EA, Budoff MJ, Cedars MI, Hopkins PN, Lobo RA, Manson JE, Merriam GR, Miller VM, Neal-Perry G, Santoro N, Taylor HS, Vittinghoff E, Yan M, Hodis HN. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: a randomized trial. Ann Intern Med. 2014;161(4):249–260. [DOI] [PubMed] [Google Scholar]