Abstract
Alcohol intake has been associated to breast cancer in pre and postmenopausal women; however results are inconclusive regarding tumor hormonal receptor status, and potential modifying factors like age at start drinking. Therefore, we investigated the relation between alcohol intake and the risk of breast cancer using prospective observational data from the European Prospective Investigation into Cancer and Nutrition (EPIC). Up to 334,850 women, aged 35-70 years at baseline, were recruited in ten European countries and followed up an average of 11 years. Alcohol intake at baseline and average lifetime alcohol intake were calculated from country-specific dietary and lifestyle questionnaires. The study outcomes were the Hazard ratios (HR) of developing breast cancer according to hormonal receptor status. During 3,670,439 person-years, 11,576 incident breast cancer cases were diagnosed. Alcohol intake was significantly related to breast cancer risk, for each 10g/day increase in alcohol intake the HR increased by 4.2% (95% CI: 2.7%-5.8%). Taking 0 to 5g/day as reference, alcohol intake of >5 to 15 g/day was related to a 5.9% increase in breast cancer risk (95% CI: 1%-11%). Significant increasing trends were observed between alcohol intake and ER+/PR+, ER-/PR-, HER2- and ER-/PR-/HER2- tumors. Breast cancer risk was stronger among women who started drinking prior to first full-time pregnancy. Overall, our results confirm the association between alcohol intake and both hormone receptor positive and hormone receptor negative breast tumors, suggesting that timing of exposure to alcohol drinking may affect the risk. Therefore, women should be advised to control their alcohol consumption.
Keywords: Alcohol consumption, Breast cancer, Prospective study
1. Introduction
A consistent association has been observed between alcohol intake and breast cancer (BC) among both pre and postmenopausal women,1 with a linear dose-response increase ranging from 2%2 to 12%3 for each additional drink per day (equivalent to about 10g/day). While the association is firmly established, some questions such as the association with specific tumor subtypes, the impact of the age at start drinking and a potential window of susceptibility, remain unanswered. Mechanistic evidences show that ethanol stimulates both cell proliferation and estrogen receptor (ER) signaling in the mammary gland.4–6 Most epidemiological studies report an impact of ethanol on ER+ tumors.7 However a recent meta-analysis showed an increased risk in both hormone receptor positive and negative tumors.8 The consumption of alcoholic beverages may interact with other BC risk factors such as hormonal status or first full-term pregnancy (FFTP),9,10 and thus differentially modulate breast cancer risk over a woman’s lifetime.11 Recent studies report that low to moderate alcohol intake between menarche and first pregnancy is associated with BC risk.12 It is therefore important to evaluate the association of alcohol intake and BC phenotypes in light of a potential modulating effect of age at start drinking.
2. Material and Methods
The European Prospective Investigation into Cancer and Nutrition (EPIC) cohort consists of approximately 370,000 women and 150,000 men, aged 35–69, recruited between 1992 and 1998 in 23 research centers across 10 Western European countries, Denmark (Aarhus and Copenhagen), France, Germany (Heidelberg and Potsdam), Greece, Italy (Florence, Varese, Ragusa, Turin, and Naples), Norway, Spain (Asturias, Granada, Murcia, Navarra, and San Sebastian), Sweden (Malmö and Umeå), the Netherlands (Bilthoven and Utrecht), and the United Kingdom (Cambridge and Oxford). The design and methodology has been published elsewhere.13 Eligible men and women were invited to participate; those who accepted gave informed consent and compiled questionnaires on diet, lifestyle, and medical history. EPIC recruited 367,993 women, aged 35-70 years. Women with prevalent cancers at any site at recruitment (n= 19,853) or with missing diagnosis or censoring date (n= 2,892) were excluded. A total of 3,339 subjects with missing dietary or lifestyle information, and 6,753 women in the top and bottom 1% of the ratio of energy intake to estimated energy requirement, calculated from age, sex, body weight and height, were excluded from the analysis. In addition, 217 non-first breast cancer cases were excluded. Thus, the analysis was performed in 334,850 EPIC women with complete exposure information. Within this group, 11,576 women with invasive breast cancer (including 1227 carcinoma in situ) were identified after a median follow-up of 11.0 years. Information on lifetime alcohol consumption was missing for Sweden, Norway, Naples and Bilthoven, 24.1% were then excluded from the sub-analyses on lifetime alcohol intake. The study was approved by IARC ethical committee and the local ethical committees of the participating centers.
Dietary assessment, lifestyle and alcohol consumption
Dietary and lifestyle questionnaires were completed by participants at enrolment when anthropometric measurements were taken.13 Past-year physical activity (PA) in occupational and recreational domains was assessed at baseline with a self-administered questionnaire. For occupational activity, both employment status as well as the level of physical activity done during work was recorded as: non-worker, sedentary, standing, manual, heavy manual, and unknown (for which duration and frequencies were not recorded). Recreational time physical activity included walking, cycling, and sport activities. The duration and frequency of recreational activity were multiplied by the intensity assigned by metabolic equivalent values (METs) for the different activities. A total PA index, the “Cambridge PA Index” was estimated by cross-tabulating occupational with recreational PA. This index is based on occupational, cycling, and sport activities.
Information on alcohol use at the time of enrolment into the study was based on a dietary assessment of usual consumption of alcoholic beverages and types of alcoholic beverage (i.e. wine, beer, spirits and liquors) during the past 12 month. In each country, intake was calculated based on the estimated average glass volume and ethanol content for each type of alcoholic beverage, using information collected in highly standardized 24-hour dietary recalls from a subset of the cohort.14 Information on past alcohol consumption (available for 75.9% of participants) was assessed as glasses of different beverages consumed per week at 20, 30, 40 and 50 years of age. Average lifetime alcohol intake was determined as a weighted average of intake at different ages, with weights equal to the time of individual exposure to alcohol at different ages. To determine which women had started drinking prior to FFTP, we used information on alcohol consumption at different ages and the age of FFTP reported by the women in the questionnaire.
Anthropometric measurements
Weight and height were measured at baseline, while the subjects were not wearing shoes, to the nearest 0.1 kg, or to the nearest 0.1, 0.5, or 1.0 cm, depending on the center.15 In France, Norway, and Oxford, height and weight were self-reported on a questionnaire. The procedures used to account for procedural differences between centers in the collection of anthropometric measurements are described elsewhere.16
Perspective ascertainment of breast cancer cases, coding of receptor status and determination of menopausal status
Incident BC cases were identified through population cancer registries (Denmark, Italy, the Netherlands, Norway, Spain, Sweden and United Kingdom) or by active follow-up (France, Germany, Naples and Greece). The active follow-up procedure used a combination of methods, including health insurance records, cancer and pathology registries and contacts with participants and their next-of-kin. Subjects were followed up from study entry and until cancer diagnosis (except for non-melanoma skin cancer cases), death and emigration or until the end of the follow-up period, whichever occurred first. The end of follow-up period was: December 2004 (Asturias), December 2006 (Florence, Varese, Ragusa, Granada and San Sebastian), December 2007 (Murcia, Navarra, Oxford, Bilthoven, Utrecht and Denmark), June 2008 (Cambridge), December 2008 (Turin, Malmo, Umea and Norway). For study centers with active follow-up, the last follow-up contact was: December 2006 for France, December 2009 for Greece, June 2010 for Heidelberg, December 2008 for Potsdam, and December 2006 for Naples. Cancer incidence data were classified according to the International Classification of Diseases for Oncology, 2th Revision (ICDO-2).
Information on tumor receptor status, on the available laboratory methods and on quantification descriptions used to determine receptor status, were collected by 20 centers. Information on ER, progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) was provided by each center based on pathology reports. To standardize the quantification of receptor status among the EPIC centers, the following criteria for a positive receptor status were used: ≥10% cells stained, any 'plus-system' description, ≥20 fmol/mg, an Allred score of ≥3, an IRS ≥2, or an H-score ≥10.17–21
Women were considered as pre-menopausal when reporting regular menses over the past 12 months, or when aged less than 46 years at recruitment. Women were considered as post-menopausal when not reporting any menses over the past 12 months, or having received bilateral ovariectomy. Women with missing or incomplete questionnaire data or with previous hysterectomy, were considered post-menopausal only if older than 55 years of age. Women were considered with unknown menopausal status when aged between 46 and 55 years and had missing or incomplete questionnaire data, or reported previous hysterectomy (without ovariectomy).22,23
Statistical analysis
Cox proportional hazards regression models were used to quantify the association between alcohol consumption and breast cancer risk. Age was the primary time variable and the Breslow method was adopted for handling ties.24 Time at entry was age at recruitment; time at exit was age at cancer diagnosis, death, loss to follow-up, or end of follow-up, whichever came first. Models were stratified by center to control for differences in questionnaire design, follow-up procedures, and other center effects. Further stratification by age at recruitment (1-year categories) was used. Systematic adjustments were made for menopausal status (dichotomized as postmenopausal or women that underwent an ovariectomy versus other), weight and height (all continuous), smoking (never, former, and current), educational attainment (five categories of schooling) as a proxy variable for socioeconomic status, physical activity (inactive, moderately inactive, moderately active, active). In addition, the following variables were included in the models: age at menarche (≤12, 12–14, >14 years), age at birth of first child (nulliparous, ≤21, 21–30, >30 years), and age at menopause (≤50, >50 years), ever use of contraceptive pill and ever use of replacement hormones, energy intake without alcohol consumption and adjustment for interaction ‘menopause, weight’.
Alcohol consumption was modeled as both continuous and categorical variable (none, 0.1-5, 5.1-15, 15.1-30, >30 g/day). Both baseline consumption and lifetime consumption were studied. Correlation between both estimations was high (r=0.80). P-trend values were obtained by modeling a score variable (from 1 to 5) category-specific level of alcohol at baseline. In addition, the shape of the dose-response curve between alcohol consumption and breast cancer risk was evaluated with fractional polynomials of order two,25 using 3g/day as reference value and after exclusion of former consumers at baseline. Non-linearity was tested comparing the difference in log-likelihood of a model with fractional polynomials with a model with a linear term only to a chis-square distribution with three degrees of freedom.25 For all models, the proportional hazards assumption was satisfied, evaluated via inclusion into the disease model of interaction terms between exposure and attained age (data not shown). Statistical heterogeneity of associations across countries or receptor status, was based on a chi-square statistics, computed comparing country-specific coefficients to an overall coefficient. Stratified analyses were conducted according to the time at start drinking (prior of after FFTP) and interaction term was tested using alcohol intake as continuous variable in multivariate models. Models were run with the exclusion of the first two years of follow-up, but the results did not differ from those including the entire cohort (data not shown).
Statistical tests were two sided, and p-values <0.05 were considered significant. All analyses were performed using SAS version 9.2 (SAS Institute, 1999) and STATA (Stata Statistical Software: Release 12 (2011) StataCorp.,College Station, TX: StataCorp LP).
3. Results
During an average of 11.0 years of follow-up (3,670,43940 person-years) of 334,850 study participants, the EPIC study documented 11,576 incident BC cases (e-Table 1). The overall percentage of women drinking over 15 g/day at baseline was 16.3% (e-Table 1).
The mean age at recruitment was 50.8 years, and the mean age at BC diagnosis was 59.4 years. TABLE 1 presents the baseline alcohol intake according to the distribution of major baseline demographic and lifestyle characteristics. At baseline, 35.2% of women were premenopausal and 43.1% postmenopausal (the menopausal status of 18.8% of women was not defined, and 2.9% reported bilateral ovariectomy) (Table 1). No drinkers at baseline were less likely to ever have used exogenous hormones and less likely to have ever smoked, were more moderately active and attained less education at baseline than drinkers at baseline (Table 1).
Table 1. Demographic and lifestyle characteristics according to breast cancer status and alcohol intake at baseline.
| Demographic and lifestyle characteristics a | Breast cancer cases | Non-cases | Average daily alcohol intake (g/day) | ||||
|---|---|---|---|---|---|---|---|
| 0 | 0.1-5 | 5.1-15 | 15.1-30 | >30 | |||
| Participants (N) | 11,576 | 323,274 | 54,907 | 135,599 | 89,694 | 35,460 | 19,190 |
| Age at recruitment (years) (mean, SD) | 52.2 (8.8) | 50.8 (10.2) | 52.2 (9.0) | 50.9 (9.8) | 50.1 (9.5) | 50.2 (9.0) | 50.2 (9.0) |
| Breast cancer cases (N, %) | 11,576 | --- | 1,626 (2.96) | 4,280 (3.16) | 3,261 (3.64) | 1,475 (4.16) | 934 (4.87) |
| Receptor status (N, %) | |||||||
| ER+/PR+ | 3,653 (31.6) | --- | 527 (0.98) | 1,367 (1.03) | 970 (1.11) | 472 (1.37) | 317 (1.71) |
| ER+/PR- | 1,133 (9.8) | --- | 177 (0.33) | 404 (0.31) | 303 (0.35) | 162 (0.47) | 87 (0.42) |
| ER-/PR- | 1,050 (9.1) | --- | 131 (0.25) | 441 (0.33) | 252 (0.29) | 132 (0.39) | 94 (0.51) |
| HER- | 1,764 (75.6) | --- | 246 (0.46) | 662 (0.50) | 457 (0.53) | 238 (0.70) | 161 (0.87) |
| HER+ | 570 (24.4) | --- | 88 (0.16) | 231 (0.18) | 132 (0.15) | 81 (0.24) | 38 (0.21) |
| ER-/PR-/HER- | 226 (2.0) | --- | 25 (0.05) | 84 (0.06) | 62 (0.07) | 29 (0.09) | 26 (0.14) |
| Menopausal status (%) | |||||||
| Premenopausal | 24.4 | 35.2 | 31.4 | 37.2 | 35.5 | 32.5 | 29.1 |
| Perimenopausal | 22.0 | 18.8 | 17.3 | 18.8 | 19.2 | 20.3 | 20.6 |
| Postmenopausal | 50.7 | 43.1 | 47.3 | 41.4 | 42.7 | 44.5 | 47.1 |
| Surgical Postmenopausal | 2.8 | 2.9 | 4.0 | 2.6 | 2.6 | 2.7 | 3.2 |
| Reproductive factors | |||||||
| Age at menarche (years) (mean, SD) | 12.95 (1.51) | 13.01 (1.75) | 13.03 (1.57) | 12.98 (1.68) | 13.01 (1.65) | 13.02 (1.55) | 12.99 (1.56) |
| Age at menopause (years) (mean, SD) b | 49.52 (4.72) | 49.03 (5.85) | 48.80 (4.96) | 49.09 (5.29) | 49.20 (5.27) | 49.17 (4.89) | 49.07 (4.91) |
| Nulliparous (%) | 13.7 | 15.2 | 10.8 | 14.5 | 17.3 | 17.2 | 18.7 |
| N of full-term pregnancies (mean, SD, range) | 1.90 (1.17) (0-9) |
1.99 (1.37) (0-17) |
2.05 (1.21) (0-17) |
2.01 (1.30) (0-14) |
1.94 (1.28) (0-13) |
1.90 (1.20) (0-12) |
1.86 (1.20) (0-11) |
| Ever breastfed (%) | 71.7 | 72.2 | 75.3 | 73.4 | 71.1 | 68.8 | 67.1 |
| Exogenous hormone use (%) | |||||||
| Ever-used HRTb | 54.1 | 42.2 | 28.6 | 42.4 | 48.0 | 48.8 | 50.9 |
| Ever-used OC | 58.8 | 58.7 | 40.7 | 58.5 | 65.2 | 65.0 | 68.9 |
| Duration of OC use | 6.56 (6.95) | 6.48 (9.15) | 6.11 (7.08) | 6.32 (8.18) | 6.66 (8.06) | 6.82 (7.27) | 7.23 (7.21) |
| Anthropometric factors | |||||||
| Height (cm) (mean, SD) | 161.62 (5.89) | 161.08 (6.82) | 160.48 (6.06) | 160.94 (6.58) | 161.42 (6.39) | 161.70 (6.02) | 162.02 (6.03) |
| Weight (kg) (mean, SD) | 66.20 (11.22) | 65.70 (13.01) | 66.60 (11.57) | 65.96 (12.56) | 64.99 (12.19) | 64.88 (11.49) | 65.38 (11.51) |
| Waist-to-hip ratio (mean, SD) | 0.80 (0.06) | 0.80 (0.08) | 0.80 (0.07) | 0.80 (0.07) | 0.79 (0.07) | 0.80 (0.07) | 0.80 (0.07) |
| BMI (mean, SD) c | 25.40 (4.08) | 25.37 (4.73) | 25.91 (4.20) | 25.50 (4.56) | 25.00 (4.42) | 24.87 (4.17) | 24.97 (4.18) |
| Obese (BMI ≥30 kg/m2) (%) | 11.4 | 12.7 | 22.3 | 13.1 | 8.9 | 7.8 | 8.1 |
| Smoking status (%) | |||||||
| Never smoker | 56.0 | 57.0 | 67.2 | 58.8 | 54.8 | 49.5 | 39.2 |
| Former smoker | 25.0 | 23.0 | 14.8 | 22.3 | 26.3 | 27.5 | 29.3 |
| Current smoker | 19.0 | 20.0 | 18.1 | 19.0 | 18.9 | 23.0 | 31.5 |
| Total physical activity (%) | |||||||
| Inactive | 17.8 | 16.0 | 9.0 | 15.5 | 20.0 | 20.1 | 22.1 |
| Moderately inactive | 41.3 | 37.1 | 32.3 | 37.3 | 39.0 | 39.7 | 41.8 |
| Moderately active | 34.4 | 39.1 | 51.4 | 38.8 | 33.6 | 33.2 | 29.5 |
| Active | 6.6 | 7.8 | 7.3 | 8.4 | 7.4 | 7.0 | 6.6 |
| Highest education level (%) | |||||||
| None or primary school | 26.1 | 29.7 | 52.5 | 28.8 | 22.1 | 21.8 | 17.4 |
| Secondary/Technical/Professional school | 48.6 | 46.8 | 34.7 | 50.1 | 49.2 | 47.1 | 49.5 |
| University | 25.3 | 23.5 | 12.9 | 21.2 | 28.7 | 31.1 | 33.1 |
| Dietary intake (mean, SD) | |||||||
| Total energy intake (kcal/day) | 1,976 (512) | 1962 (594) | 1,868 (522) | 1,926 (567) | 1,993 (550) | 2,086 (519) | 2206 (520) |
| Total energy without alcohol (kcal/day) | 1,918 (505) | 1909 (586) | 1,868 (521) | 1,913 (566) | 1,928 (549) | 1,939 (518) | 1902 (519) |
| Total dietary fiber (g/day) | 22.1 (7.1) | 22.1 (8.2) | 22.1 (7.3) | 22.4 (8.0) | 22.2 (7.7) | 21.6 (7.3) | 20.6 (7.3) |
Note: Unknown values were excluded from the calculations. HRT: hormone replacement therapy; OC: oral contraceptives; SD: standard deviation; BMI: body mass index; All p values <.0001, except for age at menarche (not significant); Trend test for continuous variables; Cochran-Armitage test for trend for categorical variables and global Chi-square test.
Missing data in the total cohort were: 3.2% for age at menarche; 4.6% for parity; 2.5% for oral contraceptive; 2.3% for smoking status; 15.3% for physical activity; 7.7% for diabetes; 14.4% for hypertension; 28.4% for waist-to-hip ratio; 3.9% for education level; in postmenopausal women: 5.3% for HRT and 24.3% for age at menopause.
Continuous variables are presented as means and standard deviations (SD), adjusted by age at recruitment and center (except age, which is adjusted by center only).
Among postmenopausal women only.
Weight (kg)/height (m)2.
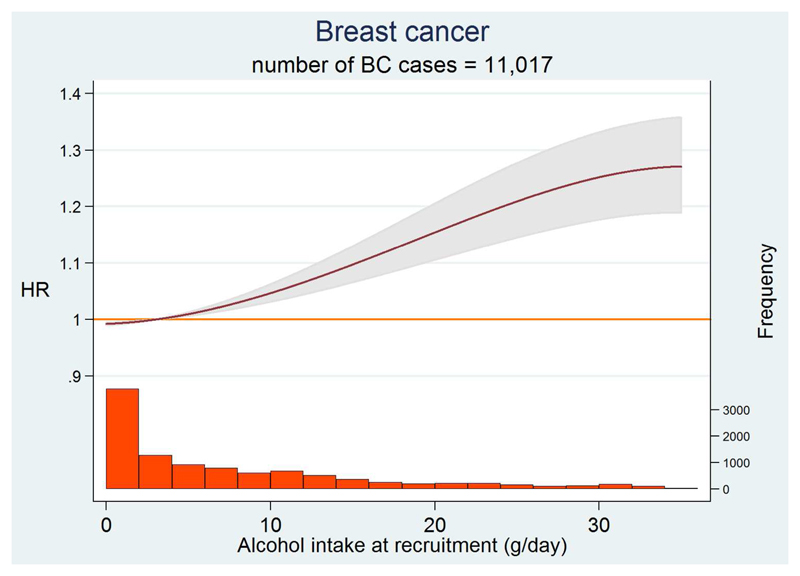
Alcohol intake showed a significant positive dose-response relation with BC (p<0.0001, TABLE 2). BC hazard ratio (HR) was increased by 6% (95% CI: 1%-11%), 12% (95% CI: 6%-19%) and 25% (95% CI: 17%-35%) for the consumption of 5-15 g/day, 15-30 g/day and >30 g/day, respectively, compared to the 0.1-5 g/day category of intake. For each additional 10g/day the HR increased by 4% (95% CI: 3%-6%). FIGURE 1 shows the relation between alcohol intake and BC risk, fractional polynomial of order 2 using 3g/day as reference. A statistically significant relation was observed (p<0.0001), while the test for non-linearity was compatible with a linear trend (p= 0.100).
Table 2. Breast cancer risk by hormonal subtypes according to alcohol consumption at baseline.
| Average daily alcohol intake at baseline (g/day) | ||||||||
|---|---|---|---|---|---|---|---|---|
| N cases/person-years | 0a | 0.1-5 | 5.1-15 | 15.1-30 | >30 | p-value (trend)b | HR (95%CI) per 10g/day | p-valuec |
| All cases | 1,626/605,217 | 4,280/1,488,055 | 3,261/983,711 | 1,475/387,280 | 934/206,177 | 11,576/3,670,440 | ||
| 1.04 (0.98-1.10) | 1.00 (ref) | 1.06 (1.01-1.11) | 1.12 (1.06-1.19) | 1.25 (1.17-1.35) | <.001 | 1.04 (1.03-1.06) | <.001 | |
| ER+/PR+ | 527/598,625 | 1,367/1,470,607 | 970/969,575 | 472/381,199 | 317/202,569 | 3,653/3,622,575 | ||
| 1.06 (0.95-1.18) | 1.00 (ref) | 1.09 (1.00-1.18) | 1.11 (0.99-1.23) | 1.30 (1.15-1.48) | 0.001 | 1.04 (1.01-1.06) | 0.003 | |
| ER+/PR- | 177/596,367 | 404/1,464,086 | 303/965,046 | 162/379,089 | 87/200,949 | 1,133/3,605,537 | ||
| 1.18 (0.98-1.42) | 1.00 (ref) | 1.13 (0.97-1.31) | 1.22 (1.01-1.47) | 1.13 (0.88-1.43) | 0.41 | 1.04 (0.99-1.09) | 0.09 | |
| ER-/PR+ | 27/595,288 | 88/1,461,768 | 53/963,248 | 35/378,194 | 14/200,458 | 217/3,598,956 | ||
| 0.93 (0.59-1.45) | 1.00 (ref) | 1.06 (0.74-1.51) | 1.37 (0.90-2.07) | 1.03 (0.57-1.86) | 0.26 | 1.05 (0.95-1.17) | 0.34 | |
| ER-/PR- | 131/596,019 | 441/1,464,103 | 252/964,537 | 132/378,867 | 94/200,964 | 1,050/3,604,490 | ||
| 0.89 (0.73-1.10) | 1.00 (ref) | 0.92 (0.78-1.08) | 1.03 (0.84-1.26) | 1.28 (1.01-1.61) | 0.06 | 1.05 (1.00-1.10) | 0.03 | |
| HER2- | 246/597,134 | 662/1,466,665 | 457/966,684 | 238/379,950 | 161/201,697 | 1,764/3,612,130 | ||
| 1.11 (0.95-1.29) | 1.00 (ref) | 1.09 (0.96-1.23) | 1.14 (0.98-1.34) | 1.41 (1.17-1.68) | 0.007 | 1.05 (1.02-1.09) | 0.004 | |
| HER2+ | 88/595,882 | 231/1,463,254 | 132/964,054 | 81/378,655 | 38/200,712 | 570/3,602,557 | ||
| 1.14 (0.87-1.49) | 1.00 (ref) | 0.89 (0.72-1.12) | 1.18 (0.90-1.54) | 0.97 (0.68-1.39) | 0.83 | 0.98 (0.92-1.06) | 0.68 | |
| ER-/PR-/HER2- | 25/595,363 | 84/1,461,973 | 62/963,514 | 29/378,261 | 26/200,594 | 226/3,599,705 | ||
| 1.09 (0.68-1.74) | 1.00 (ref) | 1.18 (0.84-1.66) | 1.20 (0.77-1.86) | 1.97 (1.23-3.16) | 0.03 | 1.12 (1.03-1.23) | 0.01 | |
Includes both never and former drinkers
test for a trend in HRs by categories of alcohol intake were computed by assigning consecutive scores (1, 2, 3, 4, 5) to the categories.
test of trend for alcohol intake continuous.
Note: Stratified by center and age at recruitment (1-year interval), and adjusted for menopausal status (pre/peri vs postmenopausal women), oral contraceptive use (yes/no/missing), hormone replacement therapy use (yes/no/missing), height (continuous), weight (continuous), interaction menopause and weight, smoking status (never, ex, current and missing), educational level (primary, no schooling, technical or professional or secondary, longer education, missing), physical activity (inactive, moderately active, moderately inactive, active and unknown), age at first menses (≤ 12, 13-14, 15+, missing), age at first full term pregnancy (nulliparous, ≤21, 22-30, >30, missing), age at menopause (<50, ≥50, missing) and energy intake without alcohol intake.
Figure 1. Dose-response curve of BC risk with alcohol intake at recruitment.
The dose-response curve is displayed up to 35 g/day, corresponding to the 99th percentile of the alcohol intake distribution.
When the associations were evaluated according to hormone receptor status, for each additional 10g/day the HR significantly increased by 4% (95% CI: 1%-6%) in ER+/PR+, by 5% (95% CI: 0%-10%) in ER-/PR-, by 5% (95% CI: 2%-9%) in HER2- and by 12% (95% CI: 3%-23%) in ER-/PR-/HER2- breast tumors (TABLE 2). Test for heterogeneity between alcohol consumption and hormone receptor status was not significant (p=0.26). No significant association was observed for ER+/PR-, ER-/PR+ and HER2+. When using lifetime alcohol intake slightly lower estimates were observed (see eTable2). Similar results were observed for pre and postmenopausal women, although, given the smaller sample size among premenopausal women, statistically significance was reached only in the overall analysis. There was no heterogeneity in results between pre and postmenopausal women (p interaction= 0.48). No interaction was observed with body mass index (BMI) or use of exogenous hormones either. Since statistical adjustment for smoking can be difficult, analyses in non-smokers at baseline were carried out and results remained virtually similar (data not shown).
Age at start drinking according to FFTP, was positively related to BC risk among women who start drinking prior to FFTP. Stronger associations were observed for ER-, PR-, ER-/PR- and ER-/PR-/HER2- tumors (TABLE 3). In a multivariable model, an increase of 10g of alcohol/day was related to an 8% (95% CI: 2%-14%) increased risk of ER- tumors in women who start drinking prior to FFTP, while no association could be detected among women who start drinking after FFTP (p for interaction= 0.047), and a 9% (95% CI: 2%-16%) increased risk of ER-/PR- tumors in women who start drinking prior to FFTP (p for interaction= 0.10). When using lifetime alcohol intake slightly lower estimates were observed (see eTable3). We were not able to evaluate the amount of alcohol consumed prior to FFTP.
Table 3. Breast cancer risk among parous women with alcohol intake at baseline by age at start drinking before/after first full-term pregnancy.
| Age at start drinking | Average daily alcohol intake at baseline | ||||
|---|---|---|---|---|---|
| N cases/person-years | HR (95%CI) for 10g/day | p-value | Interaction p-value* | ||
| All cases | Before FFTP | 4,104/1,216,204 | 1.04 (1.02-1.06) | ≤.001 | 0.14 |
| After FFTP | 2,747/793,546 | 1.02 (0.99-1.05) | 0.26 | ||
| ER+ | Before FFTP | 2,221/1,205,111 | 1.04 (1.01-1.07) | 0.005 | 0.16 |
| After FFTP | 1,460/786,197 | 1.02 (0.98-1.07) | 0.32 | ||
| PR+ | Before FFTP | 1,375/1,199,890 | 1.04 (0.99-1.07) | 0.06 | 0.40 |
| After FFTP | 987/783,211 | 1.01 (0.96-1.07) | 0.60 | ||
| ER+/PR+ | Before FFTP | 1,286/1,199,505 | 1.04 (1.00-1.08) | 0.04 | 0.39 |
| After FFTP | 924/782,918 | 1.01 (0.96-1.07) | 0.65 | ||
| ER- | Before FFTP | 552/1,194,218 | 1.08 (1.02-1.14) | 0.009 | 0.05 |
| After FFTP | 371/778,873 | 0.97 (0.88-1.06) | 0.49 | ||
| PR- | Before FFTP | 776/1,196,034 | 1.06 (1.02-1.11) | 0.009 | 0.05 |
| After FFTP | 545/780,237 | 0.98 (0.91-1.06) | 0.66 | ||
| ER-/PR- | Before FFTP | 383/1,193,437 | 1.09 (1.02-1.16) | 0.01 | 0.10 |
| After FFTP | 261/778,358 | 0.97 (0.88-1.09) | 0.65 | ||
| ER-/PR-/HER2- | Before FFTP | 99/1,191,822 | 1.17 (1.04-1.31) | 0.007 | 0.24 |
| After FFTP | 50/777,139 | 0.97 (0.75-1.24) | 0.78 | ||
age start prior to first full-term pregnancy (FFTP), was defined based on the information on 'Age at start drinking alcohol' and 'Age at first full-term pregnancy'. Results of stratified analyses by age start prior/after FFTP are displayed. Significance of interaction term was tested including in a multivariate model using alcohol as continuous variable and age start prior/after FFTP as categorical variable.
Note: Adjustments are the same as in Table 2. The statistical significance of interactions was assessed using likelihood ratio tests based on the models with and without the interaction terms formed by the product of age at start drinking alcohol before or after first pregnancy and the value of alcohol intake at recruitment.
BC hazard ratios, with data stratified according to the median period between menarche and FFTP (13 years) among women who start drinking prior to FFTP, was of 5.6% (95%CI: 2.6%-8.8%) among women with longer median period and of 2.6% (95%CI: 1.0%-6.2%) among their counterpart. These data suggest that a longer time between menarche and FFTP may modulate BC risk among women who start drinking prior to FFTP. However, the test for interaction was not significant (p=0.23) (data not shown).
4. Discussion
In this prospective study of 334,850 women and 11,576 incident BC cases, an increased intake of 10g of alcohol/day was related to a 4.2% increased BC risk (95% CI: 2.7%-5.8%). This was observed for both ER+/PR+ and ER-/PR- tumor subtypes with the largest risk observed for triple negative tumors (ER-/PR-/HER2-). No interaction was observed with BMI and use of hormones. Women who started drinking before their FFTP appeared to be at higher risk for BC than women who started drinking after their FFTP.
Most studies published to date have reported an increased BC risk with increasing alcohol intake.1 A previous analysis within the EPIC cohort on a smaller number of BC cases (n=4,285), reported a 3% increase in BC incidence for each additional 10g/day of alcohol.26 Our results, based on more than 11,000 incident BC cases, confirm our previous results and suggest a slightly stronger association. We did not observe strong differences in estimates across tumor receptor status (triple negative tumors showed the strongest risk, however the sample size in this category was small). Although most of prior studies have reported a higher risk for ER+ and/or PR+ tumors compared to ER- and/or PR- tumors in particular, for the highest versus the lowest alcohol intake group,9,27–33 an increased risk for hormone receptor negative tumors was also reported.8,34,35 This inconsistency of results across studies might be partially due to the smaller number of BC cases with negative hormone receptor status. The very large number of both hormone receptor positive and hormone receptor negative tumors in our study increased our power on the association. Non-hormonal pathways such as DNA damage are likely to be involved in the incidence of receptor negative tumors.8 The effect of alcohol appears linear, suggesting that there is no safe level of intake for BC risk.
A limited number of studies have investigated the presence of a window of susceptibility to alcohol carcinogenesis in the breast. Some epidemiological studies suggest that drinking alcohol during adolescence or early adulthood has a strong impact on BC risk.36 Results from the Nurses' Health Study II show that low to moderate alcohol intake during adolescence and early adulthood is dose-dependently associated with an increased risk of proliferative benign breast disease, which may lead to invasive BC later in life.37 More recent results support the effect of drinking alcohol between menarche and FFTP on BC risk (RR= 1.11 per 10g/day intake; 95%CI: 1.00-1.23) and on proliferative benign breast disease (RR= 1.16 per 10g/day intake; 95%CI: 1-1.02).11 In addition, the association between drinking before FFTP and development of breast neoplasia appeared to be stronger with longer menarche to first pregnancy intervals. These results are consistent with the hypothesis that alcohol carcinogens may preferentially act during mammary development.38 We observed a stronger effect of alcohol intake prior to FFTP, with a significant interaction for receptor negative tumors. Our findings suggest that starting drinking before FFTP might be a more sensitive period, even if we cannot exclude the possibility that the stronger association between alcohol intake and BC in women who started drinking before FFTP might be the consequence of longer duration and amount of drinking.
In our study, demographic characteristic, lifestyle and alcohol intake of women with available hormone receptor status could have differed from women with unavailable status. However, we did not observe such differences among cases with known and unknown ER status and sub analyses of these groups led to similar overall results. Similar strategies were adopted to inspect BC cases with and without available information on PR and HER2 status. In addition, a bias due to the influence of preclinical disease on alcohol intake is unlikely, given that similar results were obtained after exclusion of samples from the first two years of follow-up. However, we conducted multiple comparison analyses based on hormonal status and chance findings cannot be excluded.
Major strengths of our study include the prospective and population based design, the large sample size, detailed information on alcohol intake at different period of life, age at start drinking and types of beverage, data on hormone receptor status, excellent follow-up and large number of cases, which provided us with good power for subgroups analyses. Information on alcohol intake was self-reported and potential misclassification may have underestimated the effect of alcohol intake. Still, assessment of alcohol intake has been shown to be reliable in the EPIC cohort39,40 and the prospective setting of our study minimizes recall bias on age at start drinking and lifetime alcohol intake. We were unable to determine the amount of alcohol consumed before FTTP and while consumption both at baseline and over lifetime was associated with a stronger adverse effect among women who start drinking prior to FFTP than among their counterpart, our results should be interpreted with caution.
In conclusion, findings from the EPIC cohort confirm the carcinogenic effect of alcohol intake on both receptor positive and negative breast tumors. Starting to drink prior to FFTP appears to have a larger adverse effect than after FTTP. No interaction with body fatness and use of hormone was observed. Alcohol has been shown to act through the estrogen pathway, however our results suggest that non-hormonal pathways are likely to act and need to be further investigated.
Supplementary Material
Novelty and impact.
Recent studies suggested alcohol intake before first full-term pregnancy (FFTP) to be associated with breast cancer (BC) risk. Using a prospective study with 11,576 incident BC cases we confirmed that women who started drinking before their FFTP have higher risk of BC than women who started afterwards. Moreover, although alcohol has been shown to act through the estrogen pathway, our results suggest that non-hormonal pathways are likely to act and need to be further investigated.
Acknowledgements
This work was supported by the International Agency for Research on Cancer. The coordination of EPIC is financially supported by the European Commission (DG-SANCO) and the International Agency for Research on Cancer. The national cohorts are supported by Danish Cancer Society (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM) (France); Deutsche Krebshilfe, Deutsches Krebsforschungszentrum and Federal Ministry of Education and Research (Germany); Hellenic Health Foundation, the Stavros Niarchos Foundation, and the Hellenic Ministry of Health and Social Solidarity (Greece); Italian Association for Research on Cancer (AIRC) and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands (The Netherlands); ERC-2009-AdG 232997 and Nordforsk, Nordic Centre of Excellence programme on Food, Nutrition and Health. (Norway), Health Research Fund (FIS), Regional Governments of Andalucía, Asturias, Basque Country, Murcia (project number 6236) and Navarra, ISCIII RETIC (RD06/0020) (Spain); Swedish Cancer Society, Swedish Scientific Council and Regional Government of Skåne and Västerbotten (Sweden); Cancer Research UK, Medical Research Council, Stroke Association, British Heart Foundation, Department of Health, Food Standards Agency, and Wellcome Trust (United Kingdom).
Abbreviations
- BC
breast cancer
- EPIC
European Prospective Investigation into Cancer and Nutrition
- HR
hazard ratio
- CI
confidence interval
- FFQ
food-frequency questionnaire
- ER
estrogen receptor
- PR
progesterone receptor
- HER2
human epidermal growth factor receptor
- FFTP
first full-term pregnancy
- BMI
body mass index
References
- 1.IARC Monogr Eval Carcinog Risks Hum. Personal habits and indoor combustions. Volume 100 E. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. 2012;100:1–538. [PMC free article] [PubMed] [Google Scholar]
- 2.Allen NE, Beral V, Casabonne D, Kan SW, Reeves GK, Brown A, et al. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009;101:296–305. doi: 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- 3.Ellison RC, Zhang Y, McLennan CE, Rothman KJ. Exploring the relation of alcohol consumption to risk of breast cancer. Am J Epidemiol. 2001;154:740–7. doi: 10.1093/aje/154.8.740. [DOI] [PubMed] [Google Scholar]
- 4.Reichman ME, Judd JT, Longcope C, Schatzkin A, Clevidence BA, Nair PP, et al. Effects of alcohol consumption on plasma and urinary hormone concentrations in premenopausal women. J Natl Cancer Inst. 1993;85:722–7. doi: 10.1093/jnci/85.9.722. [DOI] [PubMed] [Google Scholar]
- 5.Fan S, Meng Q, Gao B, Grossman J, Yadegari M, Goldberg ID, et al. Alcohol stimulates estrogen receptor signaling in human breast cancer cell lines. Cancer Res. 2000;60:5635–9. [PubMed] [Google Scholar]
- 6.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–31. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 7.Coronado GD, Beasley J, Livaudais J. Alcohol consumption and risk of breast cancer. Salud Publica Mex. 2011;53:440–7. [PubMed] [Google Scholar]
- 8.Suzuki R, Orsini N, Mignone L, Saji S, Wolk A. Alcohol intake and risk of breast cancer defined by estrogen and progesterone receptor status--a meta-analysis of epidemiological studies. Int J Cancer. 2008;122:1832–41. doi: 10.1002/ijc.23184. [DOI] [PubMed] [Google Scholar]
- 9.Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011;306:1884–90. doi: 10.1001/jama.2011.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terry MB, Zhang FF, Kabat G, Britton JA, Teitelbaum SL, Neugut AI, et al. Lifetime alcohol intake and breast cancer risk. Ann Epidemiol. 2006;16:230–40. doi: 10.1016/j.annepidem.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 11.Onland-Moret NC, Peeters PH, van der Schouw YT, Grobbee DE, van Gils CH. Alcohol and endogenous sex steroid levels in postmenopausal women: a cross-sectional study. J Clin Endocrinol Metab. 2005;90:1414–9. doi: 10.1210/jc.2004-0614. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Colditz GA, Rosner B, Berkey CS, Collins LC, Schnitt SJ, et al. Alcohol Intake Between Menarche and First Pregnancy: A Prospective Study of Breast Cancer Risk. J Natl Cancer Inst. 2013;105:1571–8. doi: 10.1093/jnci/djt213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5:1113–24. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 14.Slimani N, Deharveng G, Charrondiere RU, van Kappel AL, Ocké MC, Welch A, et al. Structure of the standardized computerized 24-h diet recall interview used as reference method in the 22 centers participating in the EPIC project. European Prospective Investigation into Cancer and Nutrition. Comput Methods Programs Biomed. 1999;58:251–66. doi: 10.1016/s0169-2607(98)00088-1. [DOI] [PubMed] [Google Scholar]
- 15.Friedenreich C, Cust A, Lahmann PH, Steindorf K, Boutron-Ruault MC, Clavel-Chapelon F, et al. Anthropometric factors and risk of endometrial cancer: the European prospective investigation into cancer and nutrition. Cancer Causes Control. 2007;18:399–413. doi: 10.1007/s10552-006-0113-8. [DOI] [PubMed] [Google Scholar]
- 16.Haftenberger M, Lahmann PH, Panico S, Gonzalez CA, Seidell JC, Boeing H, et al. Overweight, obesity and fat distribution in 50- to 64-year-old participants in the European Prospective Investigation into Cancer and Nutrition (EPIC) Public Health Nutr. 2002;5:1147–62. doi: 10.1079/PHN2002396. [DOI] [PubMed] [Google Scholar]
- 17.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–81. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 18.McCann J. Better assays needed for hormone receptor status, experts say. J Natl Cancer Inst. 2001;93:579–80. doi: 10.1093/jnci/93.8.579. [DOI] [PubMed] [Google Scholar]
- 19.Layfield LJ, Gupta D, Mooney EE. Assessment of Tissue Estrogen and Progesterone Receptor Levels: A Survey of Current Practice, Techniques, and Quantitation Methods. Breast J. 2000;6:189–96. doi: 10.1046/j.1524-4741.2000.99097.x. [DOI] [PubMed] [Google Scholar]
- 20.Flowers JL, Burton GV, Cox EB, McCarty KS, Sr, Dent GA, Geisinger KR, et al. Use of monoclonal antiestrogen receptor antibody to evaluate estrogen receptor content in fine needle aspiration breast biopsies. Ann Surg. 1986;203:250–4. doi: 10.1097/00000658-198603000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathology. 1987;8:138–40. [PubMed] [Google Scholar]
- 22.Kaaks R, Rinaldi S, Key TJ, Berrino F, Peeters PH, Biessy C, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr Relat Cancer. 2005;12:1071–82. doi: 10.1677/erc.1.01038. [DOI] [PubMed] [Google Scholar]
- 23.Kaaks R, Berrino F, Key T, Rinaldi S, Dossus L, Biessy C, et al. Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2005;97:755–65. doi: 10.1093/jnci/dji132. [DOI] [PubMed] [Google Scholar]
- 24.Thiebaut AC, Benichou J. Choice of time-scale in Cox's model analysis of epidemiologic cohort data: a simulation study. Stat Med. 2004;23:3803–20. doi: 10.1002/sim.2098. [DOI] [PubMed] [Google Scholar]
- 25.Sauerbrei W, Royston P. Building multivariable prognostic and diagnostic models: transformation of the predictors by using fractional polynomials. J R Statist Soc A. 1999;162:71–94. [Google Scholar]
- 26.Tjonneland A, Christensen J, Olsen A, Stripp C, Thomsen BL, Overvad K, et al. Alcohol intake and breast cancer risk: the European Prospective Investigation into Cancer and Nutrition (EPIC) Cancer Causes Control. 2007;18:361–73. doi: 10.1007/s10552-006-0112-9. [DOI] [PubMed] [Google Scholar]
- 27.Zhang SM, Lee IM, Manson JE, Cook NR, Willett WC, Buring JE. Alcohol consumption and breast cancer risk in the Women's Health Study. Am J Epidemiol. 2007;165:667–76. doi: 10.1093/aje/kwk054. [DOI] [PubMed] [Google Scholar]
- 28.Lew JQ, Freedman ND, Leitzmann MF, Brinton LA, Hoover RN, Hollenbeck AR, et al. Alcohol and risk of breast cancer by histologic type and hormone receptor status in postmenopausal women: the NIH-AARP Diet and Health Study. Am J Epidemiol. 2009;170:308–17. doi: 10.1093/aje/kwp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawase T, Matsuo K, Hiraki A, Suzuki T, Watanabe M, Iwata H, et al. Interaction of the effects of alcohol drinking and polymorphisms in alcohol-metabolizing enzymes on the risk of female breast cancer in Japan. J Epidemiol. 2009;19:244–50. doi: 10.2188/jea.JE20081035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terry MB, Knight JA, Zablotska L, Wang Q, John EM, Andrulis IL, et al. Alcohol metabolism, alcohol intake, and breast cancer risk: a sister-set analysis using the Breast Cancer Family Registry. Breast Cancer Res Treat. 2007;106:281–8. doi: 10.1007/s10549-007-9498-7. [DOI] [PubMed] [Google Scholar]
- 31.Chlebowski RT, Anderson GL, Lane DS, Aragaki AK, Rohan T, Yasmeen S, et al. Predicting risk of breast cancer in postmenopausal women by hormone receptor status. J Natl Cancer Inst. 2007;99:1695–705. doi: 10.1093/jnci/djm224. [DOI] [PubMed] [Google Scholar]
- 32.Setiawan VW, Monroe KR, Wilkens LR, Kolonel LN, Pike MC, Henderson BE. Breast cancer risk factors defined by estrogen and progesterone receptor status: the multiethnic cohort study. Am J Epidemiol. 2009;169:1251–9. doi: 10.1093/aje/kwp036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li CI, Chlebowski RT, Freiberg M, Johnson KC, Kuller L, Lane D, et al. Alcohol consumption and risk of postmenopausal breast cancer by subtype: the women's health initiative observational study. J Natl Cancer Inst. 2010;102:1422–31. doi: 10.1093/jnci/djq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnes BB, Steindorf K, Hein R, Flesch-Janys D, Chang-Claude J. Population attributable risk of invasive postmenopausal breast cancer and breast cancer subtypes for modifiable and non-modifiable risk factors. Cancer Epidemiol. 2011;35:345–52. doi: 10.1016/j.canep.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Kabat GC, Kim M, Phipps AI, Li CI, Messina CR, Wactawski-Wende J, et al. Smoking and alcohol consumption in relation to risk of triple-negative breast cancer in a cohort of postmenopausal women. Cancer Causes Control. 2011;22:775–83. doi: 10.1007/s10552-011-9750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez SV. Estrogen, alcohol consumption, and breast cancer. Alcohol Clin Exp Res. 2011;35:389–91. doi: 10.1111/j.1530-0277.2010.01355.x. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Tamimi RM, Berkey CS, Willett WC, Collins LC, Schnitt SJ, et al. Intakes of alcohol and folate during adolescence and risk of proliferative benign breast disease. Pediatrics. 2012;129:1192–8. doi: 10.1542/peds.2011-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berkey CS, Willett WC, Frazier AL, Rosner B, Tamimi RM, Rockett HR, et al. Prospective study of adolescent alcohol consumption and risk of benign breast disease in young women. Pediatrics. 2010;125:1081–7. doi: 10.1542/peds.2009-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tjonneland A, Overvad K, Haraldsdottir J, Bang S, Ewertz M, Jensen OM. Validation of a semiquantitative food frequency questionnaire developed in Denmark. Int J Epidemiol. 1991;20:906–12. doi: 10.1093/ije/20.4.906. [DOI] [PubMed] [Google Scholar]
- 40.Kaaks R, Slimani N, Riboli E. Pilot phase studies on the accuracy of dietary intake measurements in the EPIC project: overall evaluation of results. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol. 1997;26(Suppl 1):S26–36. doi: 10.1093/ije/26.suppl_1.s26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.