Abstract
Unlike B cells, CD8-positive and CD4-positiveT cells of the adaptive immune system do not recognize intact foreign proteins but instead recognize polypeptide fragments of potential antigens. These antigenic peptides are expressed on the surface of antigen presenting cells bound to MHC class I and MHC class II proteins. Here, we review the basics of antigen acquisition by antigen presenting cells, antigen proteolysis into polypeptide fragments, antigenic peptide binding to MHC proteins, and surface display of both MHC class I-peptide and MHC class II-peptide complexes.
INTRODUCTION TO ANTIGEN PROCESSING
Major histocompatibility complex class I molecules (MHC-I) and class II molecules (MHC-II) are trans-membrane glycoproteins that share the property of binding short peptides that are produced by the cells that express them. The generation of peptides and their subsequent association with MHC molecules is referred to as antigen processing. Antigen processing by myeloid cells, particularly dendritic cells (DCs), and the presentation of antigen-derived peptides to CD4+ and CD8+ T cells by MHC-I and MHC-II expressed on these cells are critical steps for effective adaptive immune responses. However, the mechanisms involved in antigen processing for MHC-I and MHC-II are different (Fig. 1). For recognition by mature effector CD4+ T cells MHC-II-associated peptides are generated and bind within the endolysosomal system, while for recognition by mature CD8+ T cells MHC-I-associated peptides are generated in the cytosol from newly synthesized proteins and bind to MHC-I molecules in the endoplasmic reticulum (ER). For priming naive CD4+ T cells, the MHC-II processing pathway used by DCs also relies on peptide generation and binding in the endolysosomal system. However, priming CD8+ T cells requires endocytosis of antigens by the DCs followed by their transfer into the cytosol for proteolysis into peptides that ultimately bind to MHC-I molecules, a process known as cross-presentation or cross-priming. In this chapter we will discuss both general and myeloid-specific mechanisms of both MHC-I-and MHC-II-restricted antigen processing and presentation, phenomena that are intimately involved with the biosynthesis of the MHC glycoproteins.
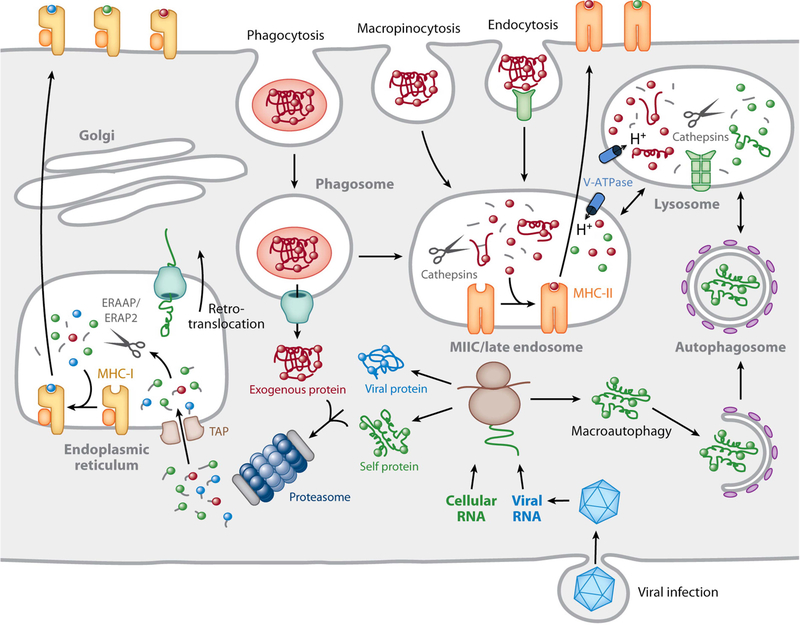
FIGURE 1.
Overview of MHC-peptide complex biogenesis. Cytosolic proteins are degraded by the proteasome into small peptides that are imported into the lumen of the ER by TAP, where they bind to nascent MHC-I molecules. ER peptides can be trimmed to 8 to 10 residues by the action of ERAAP/ERAP1 and ERAP2. Fully assembled MHC-I–peptide complexes leave the ER and are delivered to the plasma membrane by recognition by CD8+ T cells. Proteins internalized into endosomes by a variety of mechanisms are degraded into peptides in late endosomes rich in proteinases, classically called cathepsins, active at acidic pH. MHC-II molecules are transported to these compartments from the ER by virtue of its association with a chaperone termed the invariant chain (not shown). The MHC-II-positive compartment is indicated as MIIC/late endosome in the figure. Invariant chain is also proteolytically degraded in late endosomes, thereby making the MHC-II molecules available for peptide binding. Following a series of peptide-editing processes, immunodominant MHC-II–peptide complexes move to the plasma membrane for recognition by CD4+ T cells. In specialized APCs, particularly DCs, proteins that enter the cell by endocytosis/phagocytosis are retrotranslocated into the cytosol for subsequent proteasomal degradation and binding to MHC-I in a process termed cross-presentation. The retrotranslocation mechanism is currently undefined, but here it is depicted as a channel responsible for ERAD that may be recruited to the phagosome from the ER. This hypothesis remains unproven. Reprinted from reference 32, with permission.
OVERVIEW OF MHC-II-RESTRICTED ANTIGEN PROCESSING
MHC-II is constitutively expressed on a subset of cells termed professional antigen-presenting cells (APCs), which include most classes of DCs, B cells, and thymic epithelial cells. MHC-II expression is inducible, however, on most cell types, including monocytes and macrophages, most notably by gamma interferon (IFN-γ)-mediated activation. As discussed below, enhanced MHC-II biosyn-thesis or regulated degradation of MHC-II is an important way for APCs to focus their attention on pathogens that “alert” the immune system to an infection.
MHC-II binds peptides generated by proteolysis of antigens in endosomal/lysosomal “antigen-processing compartments.” Antigens gain access to these compartments by various mechanisms, including receptor-mediated endocytosis, macropinocytosis, phagocytosis, and autophagy. MHC-II molecules, which consist of a heterodimer of transmembrane α and β subunits, gain access to these same compartments by association with an accessory protein termed the invariant chain (Ii) shortly after biosynthesis in the ER (Fig. 2). Ii provides three distinct functions for MHC-II: (i) it acts as a molecular chaperone and promotes proper folding and movement of the MHC-II–Ii complex from the ER through the Golgi apparatus (1, 2); (ii) it prevents peptides and unfolded proteins present in the ER from binding to the peptide-binding site on the nascent MHC-II molecule (3, 4); and (iii) it contains targeting signals in its cytoplasmic domain that direct the MHCII–Ii complex to antigen-processing compartments (5, 6). The precise pathway taken by MHC-II–Ii complexes to access these compartments (i.e., whether the complexes are delivered directly into the endosomal pathway from the trans-Golgi network or whether they traffic to the plasma membrane and are then internalized) has been a matter of considerable debate (reviewed in 7). However, regardless of the pathway used, efficient movement of MHC-II into the late endocytic pathway depends on Ii association. The targeting signal in Ii consists of two dileucine-based internalization motifs (5, 6, 8). These motifs interact with clathrin-associated adaptor proteins to drive MHC-II–Ii complexes into the endocytic pathway (9, 10).
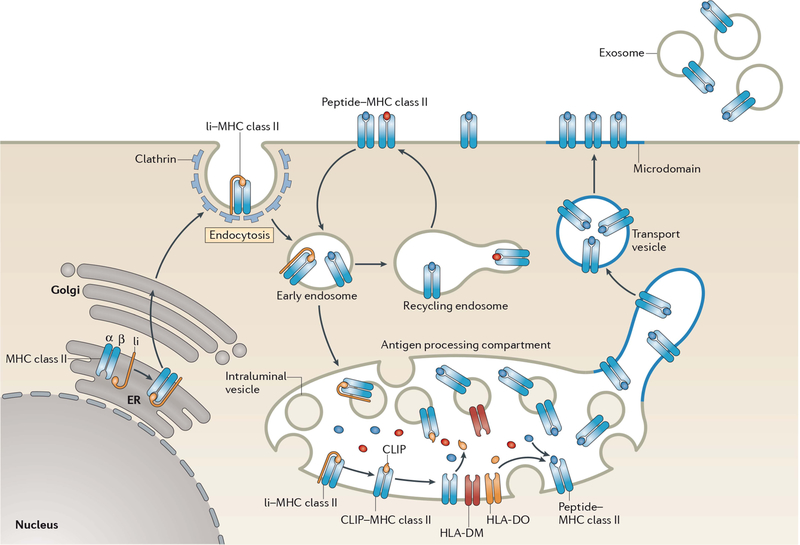
FIGURE 2.
Biosynthesis of MHC-II–peptide complexes. MHC-II αβ dimers associate with Ii in the ER, and the assembled MHC-II–Ii complexes traffic through the Golgi apparatus and are delivered to the plasma membrane. The complexes are internalized by clathrin-mediated endocytosis and are transported to late endosomal multivesicular antigen-processing compartments. Some of these complexes sort onto the ILVs of these compartments, where sequential Ii proteolysis leads to persistence of a derived fragment (termed CLIP) in the MHC-II peptide-binding groove. CLIP is removed from CLIP–MHC-II complexes by DM molecules that are present on the ILV and limiting membrane of antigen-processing compartments, thereby allowing peptide binding onto nascent MHC-II. The activity of DM is regulated by DO; however, the mechanism of regulation remains unknown. It is likely that ILV-associated MHC-II is transferred to the limiting membrane and endo/lysosomal tubules that either directly fuse, or give rise to transport vesicles that fuse, with the plasma membrane. MHC-II–peptide association with lipid microdomains first occurs in antigen-processing compartments and allows clustering of MHC-II–peptide complexes on the cell surface. If an entire antigen-processing compartment fuses with the plasma membrane, the ILV can be released from the cell in the form of exosomes. Surface-expressed MHC-II–peptide complexes can internalize using a clathrin-independent endocytosis pathway and are targeted for lysosomal degradation or may be recycled back to the plasma membrane. Reprinted from reference 103, with permission.
In principle, any endo/lysosomal compartment that generates antigenic peptides capable of binding to MHCII can be considered an antigen-processing compartment, and MHC-II–peptide complexes can indeed be generated throughout the endocytic pathway (11). The findings that the MHC-II–Ii complex can enter the earliest of endosomes by endocytosis from the cell surface (12) and that all endosomes contain at least some proteinase activity (13) are consistent with the idea that MHC-II is available throughout the endocytic pathway for peptide loading.
MHC-II is not able to bind antigenic peptides until Ii is proteolytically degraded and dissociates from the MHC-II–Ii complex (3). The degradation of MHC-II-associated Ii occurs in a series of discrete steps catalyzed by different proteinases (14–16), leaving an Ii-derived polypeptide, termed CLIP (class II-associated invariant chain peptides) (17), associated with the MHC-II peptide-binding groove. CLIP is catalytically removed to make room for lysosomally generated peptides, including those derived from internalized antigens, by a homolog of MHC-II, termed HLA-DM in humans and H2-M or DM in mice (18). Newly synthesized DM traffics to antigen-processing compartments by clathrin-mediated endocytosis after arrival at the plasma membrane. Unlike Ii, however, the internalization motif on DM is tyrosine based and preferentially sorts DM to mature endosomal antigen-processing compartments (19). DM not only catalyzes CLIP release but also promotes the dissociation of MHC-II-bound peptides that possess an intrinsically fast off rate (20), thereby serving as a “peptide editor” for MHC-II to foster the generation of high-affinity immunodominant epitopes (21). Recent data have revealed that DM interacts with the MHC-II–CLIP complex near the P1 peptide-binding pocket on MHC-II and stabilizes an intermediate conformation of MHC-II that permits dissociation of weakly bound peptides (22, 23).
A second MHC-II homolog, called HLA-DO in humans and H2-O in mice (referred to here as DO), regulates the peptide-editing function of DM. DM binds tightly to DO in the ER and serves to escort DO to lysosome-like antigen-processing compartments (24). DO is expressed in both human and mouse B cells, thymic epithelial cells, and Langerhans cells and is present in all CD11c+ spleen DC subsets in the mouse (25, 26). DO expression is suppressed during DC maturation (25, 27), while DM expression changes are modest (25, 26). Most published studies show that DO association suppresses DM activity (28). in vitro peptide-binding assays have demonstrated that DO inhibition of DM activity is pH dependent: at pH of >5.5, DO completely abrogates DM activity, but at the pH of most antigen-processing compartments (4.5 to 5.0), DO does not inhibit DM function (29). Whether this is due to pH-dependent dissociation of DO from DM or conformational alterations in the DO/DM complex remains undetermined.
OVERVIEW OF MHC-I RESTRICTED ANTIGEN PROCESSING
The pathways of MHC-I-restricted antigen processing are indicated in detail in Fig. 3. MHC-I presentation to effector CD8+ T cells, or cytotoxic T lymphocytes, involves the generation of peptides from newly synthesized cytosolic proteins, including, for example, viral proteins produced during infection of a cell. These proteins are degraded by the proteasome into peptides that, potentially after further processing by cytosolic aminopeptidases, are translocated into the ER by a dedicated ATP-dependent transporter, the transporter associated with antigen processing (TAP). TAP is composed of two MHC-encoded subunits, TAP1 and TAP2, and is a member of the ATP-binding cassette family of transporters (30). Once in the ER, the peptides can be further trimmed by ER-resident aminopeptidases, called ERAP1 (ERAAP in the mouse) and ERAP2 (absent from the mouse), to a length of 8 to 10 amino acids suitable for binding to newly synthesized MHC-I molecules (31).
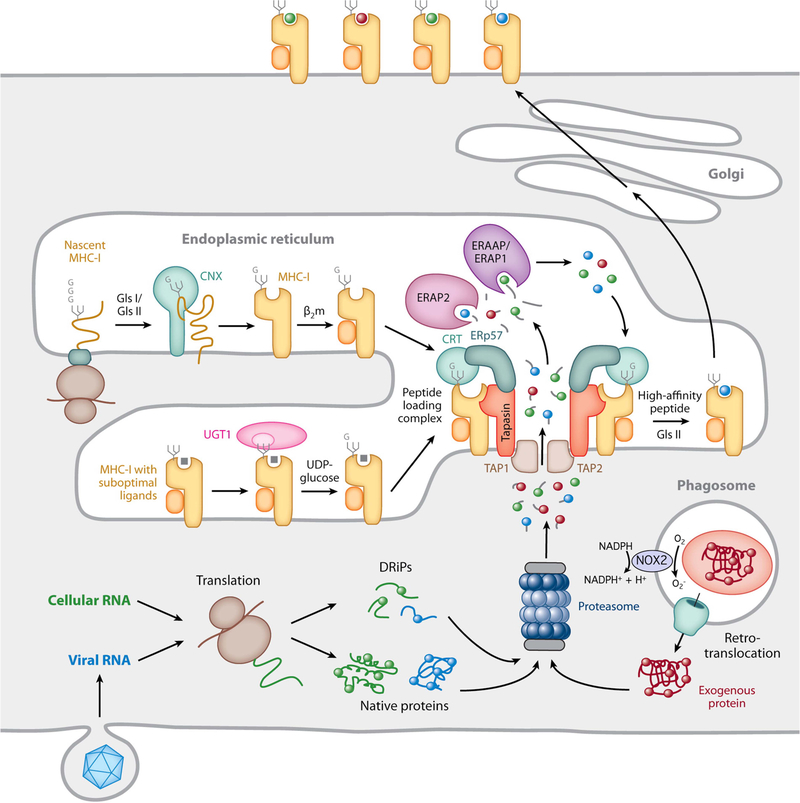
FIGURE 3.
MHC-I biosynthesis and peptide binding. The proteasome generates short antigenic peptides capable of binding to MHC-I molecules. These peptides are derived from native cytosolic proteins, defective ribosomal products (DRiPs), or, in the case of cross-presentation, exogenous proteins that enter the cell by phagocytosis and are translocated into the cytosol, either intact or as large proteolytic fragments. In cross-presenting mouse CD8+ DCs, the presence of NOX2 on the phagosomal membrane neutralizes acidification and reduces proteolytic activity, preserving protein integrity. Nascent MHC-I heavy chains initially interact with the molecular chaperone calnexin (CNX) and, after binding β2m, are recruited to the PLC by simultaneous noncovalent CRT interactions with a monoglucosylated N-linked glycan on the heavy chain and ERp57 disulfide linked to tapasin in the PLC. Peptide-free MHC-I molecules and those possessing suboptimal ligands are subject to a series of “editing” steps mediated by interaction with tapasin within the PLC as well as maintenance of the monoglucosylated N-linked glycan by the opposing actions of the enzymes glucosidase 2 (GlsII), which removes the terminal glucose residue, and UGT1, which adds back glucose to preserve the CRT interaction. MHC-I molecules containing high-affinity peptides ultimately leave the ER and are transported to the plasma membrane. Reprinted from reference 32, with permission.
MHC-I molecules are heterodimers consisting of a glycosylated transmembrane heavy chain of ~45 kDa, which is the polymorphic MHC-I gene product, and a small subunit of ~12 kDa called β2-microglobulin (β2m). The heavy chain-β2m dimers fold and assemble in the ER with the assistance of a number of chaperones, but peptide binding occurs after incorporation of the assembled dimers into the peptide loading complex (PLC). The PLC consists of TAP, tapasin (a transmembrane glycoprotein also encoded in the MHC), a protein disulfide isomerase homolog called ERp57, and the soluble chaperone calreticulin (CRT). Stoichiometric analysis indicates that there are two tapasin molecules per PLC, each of which is permanently disulfide linked to an ERp57 molecule. MHC-I molecules interact directly with tapasin and also, via their N-linked glycans, with CRT (reviewed in 32).
CRT is a lectin with specificity for a single terminal glucose residue transiently present on the glycans of newly synthesized glycoproteins. Such glycoproteins are subjected to a folding cycle in which CRT (or the related chaperone calnexin) also cooperates with ERp57 via a glycan-independent, noncovalent interaction to facilitate their correct folding and disulfide bond formation (33). After dissociation of glycoproteins from CRT, the glycan is enzymatically deglucosylated. However, if the glycoprotein remains improperly folded, it can be reglucosylated by the enzyme UDP-glucose glycoprotein transferase-1 (UGT-1), allowing reentry into the folding cycle (34). The covalent association of ERp57 with tapasin in the PLC provides a secondary anchor via CRT to cooperatively maintain the association of newly synthesized MHC-I with the PLC in an adaptation of the normal glycoprotein folding cycle. UGT-1 is used to maintain monoglucosylation of MHC-I molecules that lack associated high-affinity peptides. CRT, ERp57, and UGT-1 are all required for optimal MHC-I peptide loading (35–37). This is even more dependent on tapasin, which has a similar peptide-editing role for MHC-I that DM has for MHC-II, promoting the association of high-affinity peptides at the expense of low-affinity ones (38–40). Our molecular understanding of how tapasin does this is less advanced, but when peptides of sufficiently high affinity are bound, the completed MHC-I–peptide complexes permanently dissociate from the PLC and are transported to the cell surface.
Cross-presentation, or cross-priming, involves the binding of peptides derived from extracellular antigens with MHC-I and the recognition of these complexes by naive CD8+ T cells. Most data are consistent with a role for components of the conventional MHC-I processing pathway in cross-presentation (reviewed in 41); however, the precise cell biological mechanisms regulating this process are still not well understood. The most favored mechanism involves antigen internalization into endosomes, translocation of the antigens (or large fragments of them) from the endocytic pathway into the cytosol by an undetermined mechanism, and finally antigen proteolysis by proteasomal degradation. Cytosolically generated peptides are then translocated into either the ER, where they bind MHC-I molecules in a PLC-mediated fashion as in conventional MHC-I processing, or back into an endocytic or phagocytic compartment. Here they bind either to MHC-I molecules recycling between the plasma membrane and this compartment or to MHC-I molecules recruited to that compartment from the ER, along with PLC components. Some data in the literature argue that cross-presented peptides are generated by lysosomal proteolysis, much as they are for MHC-II. Data showing that DCs lacking the lysosomal enzyme cathepsin S are deficient in cross-presentation of certain antigens support this model (42). However, the mechanism underlying this observation is undefined, and the principle that cross-presented antigens undergo proteasomal processing in the cytosol prior to transport into MHC-I-containing compartments is generally accepted.
Although a number of cell types have been shown to be capable of cross-presentation in vitro, DCs are the major cell type that primes CD8+ T-cell responses in vivo (41). Considerable evidence indicates that in mice a particular subset of DCs, characterized by expression of the surface molecule CD8α, is the dominant cross-priming cell (43). Curiously, surface expression of CD8α is not believed to have any functional significance in this process. Whether a dominant cross-priming DC subset exists in humans is less clear. Human DCs expressing the marker CD141, or BDCA3, have been suggested to be the homolog of CD8+ mouse DCs (44, 45), but a recent study of human tonsillar DCs found that all subsets, identifiable by expression of a variety of surface markers, were competent to cross-present exogenous antigens via MHC-I (46).
DELIVERY OF ANTIGENS INTO ANTIGEN-PROCESSING COMPARTMENTS
Cross-presentation by MHC-I and successful antigen presentation by MHC-II share the requirement that protein antigens must gain access to the endocytic pathway. Here we discuss the various mechanisms used by DCs to mediate this process, illustrated in Fig. 4.
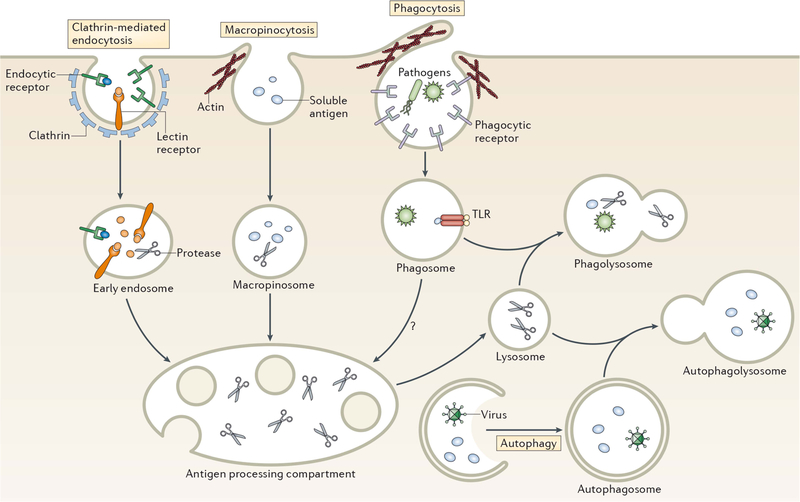
FIGURE 4.
Pathways of antigen entry into the processing compartments of myeloid cells. Pathogens as well as soluble and particulate antigens access the endolysosomal pathway of antigen-processing cells by a variety of mechanisms. Clathrin-mediated endocytosis generally involves the binding of ligands to one of a variety of endocytic receptors that deliver endocytosed cargo to early endosomes. Macropinocytosis is a nonspecific form of endocytosis that involves actin-dependent membrane ruffling that leads to solute encapsulation in structures that give rise to macropinosomes. Like early endosomes, macropinosomes are not highly proteolytic and antigen degradation only occurs following their fusion with acidic late endosomal/lysosomal compartments containing lysosomal proteinases. Pathogens and large particles that possess specific binding sites for surface receptors are internalized by phagocytosis, an endocytic process that combines the features of macropinocytosis and receptor-mediated endocytosis. Phagosomes are not acidic nor proteinase rich; however, maturation of phagosomes by fusion with late endosomes or lysosomes gives rise to proteolytic phagolysosomes that degrade phagocytosed material. Autophagy also provides material for endolysosomal degradation by sequestering cytosol into a double-membrane encapsulated autophagosome that, like a conventional phagosome, undergoes maturation upon fusion with lysosomes to generate proteolytic autophagolysosomes. Reprinted from reference 103, with permission.
Macropinocytosis
One process used to capture extracellular material is macropinocytosis (47), an endocytic process that is responsible for the nonspecific uptake of extracellular material that can vary in size from small molecules to intact bacteria and protozoa. Macropinosomes are generated from plasma membrane ruffles that extend from the cell, fold back onto themselves, and then fuse with the plasma membrane. Macropinosomes ultimately fuse with early endosomes, delivering extracellular material to the endolysosomal pathway for antigen processing. Resting DCs are capable of internalizing large amounts of fluid by constitutive macropinocytosis (up to 2 fl/cell/min) (48), and this pathway is thought to represent a major mechanism of antigen acquisition by DCs.
Macropinocytosis is controlled by the Rho GTPase Cdc42 and Rac-mediated reorganization of the cortical actin cytoskeleton (49, 50). Activation of DCs in vitro, for example, by lipopolysaccharide, reduces active Cdc42 levels and profoundly suppresses macropinocytosis (49); however, some studies have shown that unlike their in vitro -activated counterparts, DCs activated in vivo retain the ability to internalize, process, and present soluble exogenous antigens to CD4 T cells (51–53). in vitro activation of macrophages does not alter their capacity for macropinocytosis (54); however, activation does reprogram the endocytic machinery from receptor-mediated phagocytosis to macropinocytosis (55), thereby increasing their ability to internalize and destroy infectious agents in an inflammatory environment.
Receptor-Mediated Endocytosis
APCs possess a variety of different surface receptors that mediate antigen internalization. Fcγ receptors on macrophages and DCs bind immune complexes and efficiently deliver them to antigen-processing compartments (52). DCs also possess lectin receptors, such as the mannose receptor and DEC-205, that recognize carbohydrate residues on self-proteins and some pathogens and target them for internalization via receptor-mediated phagocytosis. Conjugation of antigens to ligands for specific APC surface receptors can dramatically enhance the efficiency of processing and presentation to antigen-specific T cells (56). By following different endocytic routes, different receptors deliver their cargo to distinct classes of endosomes in DCs (57). For example, targeting antigens to the mannose receptor leads to their delivery to early endosomes (which can be useful for MHC-I cross-presentation), whereas targeting antigens to Fcγ receptors or DEC-205 leads to their delivery to late endosomes/prelysosomes for efficient antigen processing and presentation by MHC-II (52).
Phagocytosis
Perhaps the most important mechanism of antigen up-take in macrophages and DCs is phagocytosis. This process allows these cells to internalize a wide variety of insoluble particulate antigens including necrotic/apoptotic cells, bacteria, and viruses (58). Unlike nonspecific macropinocytosis, phagocytosis generally involves recognition of particles by specific phagocytic receptors on APCs. There are a wide variety of such receptors on DCs and macrophages, including diverse Fc receptors, complement receptors, and C-type lectin receptors. Al though in vitro activation suppresses phagocytosis in DCs, in vivo activation does not significantly alter the ability of DCs to capture antigens by phagocytosis and stimulate antigen-specific CD4 T cells (52). Sustained phagocytosis after maturation could be important to generate MHC-II complexes with pathogen-derived peptides and perhaps for prolonging cross-presentation by MHC-I.
Phagocytosis requires large amounts of membrane to generate a developing phagosome. Proteomic analysis of phagosomes has revealed the presence of ER proteins on phagosomes (59). This initially led to the suggestion that the ER is a major source of membrane during phagocytosis, although more-recent data suggest that the amount of ER recruitment to the phagosome, while significant, is actually quite small. This observation also led to considerable speculation that the mechanisms responsible for ER-associated degradation (ERAD), the process by which misfolded proteins in the ER are translocated into the cytosol, are adapted for transfer from the phagosome to mediate cross-presentation. Although some components, such as the AAA-ATPase p97, do appear to be involved in both, evidence that the ERAD retrotranslocation apparatus is involved has been difficult to come by. Curiously, components of the ER-associated MHC-I PLC (including TAP and tapasin) are present on phagosome membranes, allowing the phagosome to function as a “surrogate ER” for peptide loading onto phagosome-associated MHC-I during cross-presentation (60–62). Although recent studies have shown that recycling surface MHC-I enters phagosomes (63), it remains to be determined how significantly these MHC-I molecules contribute to phagosome-dependent cross-presentation.
Initially phagosomes are minimally proteolytic and therefore do not generate large amounts of antigenic peptides. Internalized cargo is only degraded during the process of phagosome maturation, in which phagosomes fuse with late endosomes/lysosomes to generate phagolysosomes (58). The comparative lack of proteolysis within early phagosomes makes them the organelle of choice for mediating cross-presentation because premature degradation of internalized proteins can actually destroy potential MHC-I epitopes prior to antigen entry into the cytosol. More extensive proteolysis is critical for MHC-II function, however, and the fusion of a phagosome with a late endosomal MHC-II-positive compartment leads to formation of a hybrid organelle that possesses all the components necessary to generate MHC-II complexes with peptides derived from phagocytosed cargo. Phagosome maturation is stimulated by Toll-like receptor (TLR) signaling in macrophages and DCs (64, 65), providing these cells with a mechanism to increase MHC-II-restricted antigen processing during phagocytosis of pathogens bearing TLR ligands. Given the importance of antigen integrity for translocation from phagosomes, cross-presentation by MHC-I is actually reduced during phagosome acidification. For this reason, DCs that are specialized in cross-presentation have adopted mechanisms to control the proteolytic activity of phagosomes. Cross-presenting CD8+ DCs recruit the NADPH oxidase NOX2 to the phagosomal membrane, a process that results in the alkalinization of the lumen of the phagosome by the reactive oxygen species generated by NOX2 (66, 67). This reduces the activity of cathep-sins, which have acidic pH optima, thereby suppressing antigen proteolysis in DC phagosomes.
Autophagy
Autophagy is a process in which cytosol is encapsulated in a double-membrane structure termed an autophago-some (68). Like conventional phagosomes, autophagosomes are not highly proteolytic; however, fusion with a lysosome-like, MHC-II-positive antigen-processing compartment forms a hybrid autophagolysosome that contains all of the machinery required to degrade antigens and generate MHC-II–peptide complexes (69). Since the protein precursors of MHC-I-associated peptides are already cytosolic, autophagy may not be important for conventional MHC-I-restricted antigen processing. However, ~25% of MHC-II-associated peptides in DCs are derived from cytosolic and/or nuclear proteins, highlighting the importance of this pathway for MHC-II function (70). Genetic disruption of the process of auto-phagy severely compromises positive and negative selection of CD4 T cells by thymic epithelial cells (71, 72), pointing to a prominent role for autophagy in the function of APCs in the thymus.
APCs also possess an alternative autophagy pathway termed chaperone-mediated autophagy (CMA) (73). CMA is distinct from macroautophagy in a number of ways. Whereas macroautophagy is induced rapidly upon cell stress (such as nutrient deprivation) and wanes within 24 h, CMA increases as macroautophagy decreases. Unlike macroautophagy, CMA does not generate double-membrane autophagosomes, but instead results in the formation of a macromolecular complex containing the late endosome/lysosome-associated membrane protein LAMP2A and the heat shock proteins Hsc70 and Hsp90. This molecular translocation complex results in the delivery of cytosolic proteins into the endosome/lysosome lumen for degradation.
REGULATION OF PROTEOLYSIS IN ANTIGEN-PROCESSING COMPARTMENTS
Optimal MHC-II function requires proteolytic digestion of antigens in late endosomal/lysosomal antigen-processing compartments. However, a delicate balance must be maintained in APCs that allows the generation of immunodominant, antigenic peptides but does not result in their complete destruction (74). Lysosomal enzyme activity in DCs is ~50 times lower than it is in macrophages (75), and this leads (in part) to prolonged antigen retention and MHC-II stability in DCs compared to macrophages. Lysosomes are less acidic in DCs than in macrophages, in part because of reduced accumulation of the vacuolar ATPase (V-ATPase) that pumps protons into these compartments (76), thereby reducing their proteolytic activity. Similarly, phagosomes of DCs are less acidic (and less proteolytic) than phagosomes in macrophages. Recent work has shown that a major difference in the lysosomal and phagosomal properties of DCs and macrophages results from their differential expression and activation of transcription factor EB (TFEB), which is a master regulator of lysosomal function (77, 78). Transcription of a number of cathepsin genes, as well as genes encoding the subunits of V-ATPase, is regulated by TFEB, and DCs express significantly less TFEB than macrophages (104). Notably, CD8+ DCs in the spleen, which are the primary mediators of cross-presentation, express significantly less TFEB than other DC subsets in the mouse. It is therefore not surprising that overexpressing TFEB in DCs results in a reduction of cross-presentation, while suppressing TFEB expression with a short hairpin RNA in macrophages allows them to effectively mediate cross-presentation. Reciprocal effects were observed on MHC-II function: MHC-II-restricted antigen processing was increased in DCs overexpressing TFEB while it was decreased in macrophages with reduced TFEB. As noted above, the selective association of the ROS-generating enzyme NOX2 with phagosomal membranes in CD8+ DCs also increases the pH of developing phagosomes, thereby limiting antigen degradation and prolonging cross-presentation by MHC-I (66).
Immature DCs can retain intracellular antigens for extended periods of time, and acute stimulation of antigen-loaded DCs leads to rapid antigen degradation, the formation of MHC-II–peptide complexes, and their accumulation on the surface of the now activated DCs (79, 80). The DC activation process leads to increased association of the ATP-dependent vacuolar proton pump with antigen-processing compartments (76), increasing their acidification, and also induces the redistribution of cathepsins from conventional lysosomes into antigen-processing compartments (81). Taken together, these activation-induced changes promote the generation of MHC-II–peptide complexes in activating DCs that are required for effective antigen processing and presentation to CD4 T cells.
MOVEMENT OF MHC MOLECULES TO THE PLASMA MEMBRANE
Like most cargo internalized from the plasma membrane, internalized MHC-II–Ii complexes enter early endosomes and eventually sort into late endosomal antigen-processing compartments that have the properties of multivesicular bodies (MVBs). MHC-II–Ii complexes reside primarily on the intraluminal vesicles (ILVs) of MVBs in DCs (82); however, the signals present on the MHC-II–Ii complex that are required for sorting into these vesicles remains to be determined. It is likely that peptide loading onto MHC-II occurs when MHC-II is present on these ILVs, since MHC-II bound to the Ii degradation product CLIP (83) as well as other MHCII–peptide complexes are readily observed on these internal membranes by immunoelectron microscopy (84). MHC-II–Ii, MHC-II–CLIP, and peptide-loaded MHC-II molecules are also found on the peripheral, limiting, membrane of MVBs, but it remains to be determined whether or not MHC-II–CLIP or peptide-loaded MHCII are actually generated on these membranes. To be competent for insertion into the PM, the MHC-II must leave the ILV and be deposited into the limiting membrane of the MVB in a process that has been termed “back-fusion” (84). Whether back-fusion actually occurs remains unknown, and it is also unknown how MHC-II moves to the limiting membrane of the MVB for eventual transport to the plasma membrane.
When an intact MVB directly fuses with the plasma membrane, the MHC-II-bearing ILVs are released from the cell and these cell-free vesicles are termed exosomes (85). Exosomes are secreted from most cell types in the body, and DC-derived exosomes contain antigenic MHCII–peptide complexes, as well as costimulatory and adhesion molecules that allow exosomes to function as “mini-APCs” that are capable of directly activating T cells or indirectly activating T cells (after acquisition by other APCs) (86, 87). While the physiological role of DC-derived exosomes remains unknown, data showing that engagement of DCs with CD4+ T cells promotes exosome release (88) has led to the speculation that exosomes are able to help propagate T-cell activation.
The membrane transport pathways and molecular mechanisms that allow newly generated MHC-II–peptide complexes to move from intracellular antigen-processing compartments to the APC surface are poorly understood. Activation of DCs with TLR ligands (89) or interaction of antigen-loaded DCs with antigen-specific CD4+ T cells (90) results in the formation of elongated tubules that emanate from antigen-processing compartments toward the DC plasma membrane (90). Whether tubules or vesicles derived from tubules are responsible for the direct delivery of MHC-II to the cell surface remains to be conclusively demonstrated. MHC-II-containing vesicles have been observed to fuse with the surface of MHC-II-expressing melanoma cells (91), and even in professional APCs, these vesicles travel in a stop-and-go pattern along microtubule tracks in an actin-dependent manner from antigen-processing compartments to the plasma membrane (92). More-recent studies have identified a variety of actin-based molecular motors and GTPases that regulate MHC-II transport to the plasma membrane in DCs; however, the mechanisms used by these proteins to regulate vesicle movement are unknown. Once on the plasma membrane, MHC-II–peptide complexes are present in small microclusters (93). This has been attributed to the association of MHC-II–peptide complexes with lipid raft membrane microdo-mains (94), thereby locally concentrating small numbers of specific MHC-II–peptide complexes for efficient activation of CD4+ T cells.
ROLE OF MHC-II BIOSYNTHESIS/TURNOVER FOR APC FUNCTION
While all APCs can ultimately stimulate antigen-specific CD4+ T cells, expression of MHC-II in resting and activated states differs among different APC subtypes. For example, MHC-II mRNA is expressed in resting B cells, thymic epithelial cells, and DCs, whereas, particularly in the mouse, monocytes and macrophages do not constitutively express MHC-II. However, treatment with IFN-γ promotes the expression of the class II trans-activator (CIITA) that induces MHC-II transcription and protein expression in monocytes, macrophages, and other IFN-γ-responsive cells (95). Activation of DCs leads to a burst in MHC-II transcription and protein synthesis; however, this increase is short-lived and DC activation eventually leads to a profound reduction in MHC-II biosynthesis that has been observed both in vitro and in vivo (96, 97). Activation of either DCs or IFN-γ-treated macrophages with TLR ligands (such as lipopolysaccharide or CpG DNA) ultimately terminates CIITA expression and MHC-II synthesis (98, 99). Indeed, injection of the TLR ligand CpG into mice results in a near complete cessation of MHC-II biosynthesis within 16 h (97). This increase in MHC-II synthesis followed by a rapid decline serves to enhance the surface expression of MHC-II complexes with pathogen-derived peptides.
Under steady-state conditions, the continuous input of newly generated MHC-II–peptide complexes on the surface of DCs is accompanied by their rapid turnover. Without a mechanism to protect MHC-II from degradation, termination of MHC-II synthesis upon DC activation would be accompanied by a reduction in total MHC-II on the cell surface. The rapid turnover of MHC-II in immature DCs is mediated by ubiquitination of MHC-II by the E3 ubiquitin ligase March-I (100, 101). March-I is only expressed in immature DCs, and termination of March-I expression upon DC activation results in long-lived MHC-II–peptide complexes on the surface of activated DCs (100, 101). Taken together with the data showing regulated synthesis of MHC-II upon DC activation (96, 102), these findings have led to a widely accepted model in which DCs respond to activating pathogens by transiently increasing MHC-II synthesis and generating pathogen-derived MHC-II–peptide complexes that have enhanced stability on the surface of the pathogen-activated DC.
CONCLUDING REMARKS
As is clear from the foregoing narrative, antigen processing requires dedicated accessory components, such as tapasin and TAP for MHC-I and Ii and DM for MHC-II, that interact in sophisticated ways with evolutionarily ancient “housekeeping” functions that exist in all eukaryotic cells. These include proteasomal proteolysis in the cytosol (for MHC-I) and cathepsin-mediated proteolysis and pH control in the endocytic pathway (for MHC-II), as well as chaperone-mediated glycoprotein folding and assembly processes that are required to produce functional MHC molecules. These general housekeeping functions are adopted and modified in myeloid cells to work in concert with specific modulators of antigen processing to produce an optimal outcome for the immune system, namely, the efficient and appropriate generation of MHC-peptide complexes that result in effective T-cell immunity.
REFERENCES
- 1.Anderson MS, Miller J. 1992. Invariant chain can function as a chaperone protein for class II major histocompatibility complex molecules. Proc Natl Acad Sci U S A 89:2282–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elliott EA, Drake JR, Amigorena S, Elsemore J, Webster P, Mellman I, Flavell RA. 1994. The invariant chain is required for intracellular transport and function of major histocompatibility complex class II molecules. J Exp Med 179:681–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roche PA, Cresswell P. 1990. Invariant chain association with HLA-DR molecules inhibits immunogenic peptide binding. Nature 345:615–618. [DOI] [PubMed] [Google Scholar]
- 4.Teyton L, O’Sullivan D, Dickson PW, Lotteau V, Sette A, Fink P, Peterson PA. 1990. Invariant chain distinguishes between the exogenous and endogenous antigen presentation pathways. Nature 348:39–44. [DOI] [PubMed] [Google Scholar]
- 5.Bakke O, Dobberstein B. 1990. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell 63:707–716. [DOI] [PubMed] [Google Scholar]
- 6.Lotteau V, Teyton L, Peleraux A, Nilsson T, Karlsson L, Schmid SL, Quaranta V, Peterson PA. 1990. Intracellular transport of class II MHC molecules directed by invariant chain. Nature 348:600–605. [DOI] [PubMed] [Google Scholar]
- 7.Hiltbold EM, Roche PA. 2002. Trafficking of MHC class II molecules in the late secretory pathway. Curr Opin Immunol 14:30–35. [DOI] [PubMed] [Google Scholar]
- 8.Pieters J, Bakke O, Dobberstein B. 1993. The MHC class II-associated invariant chain contains two endosomal targeting signals within its cytoplasmic tail. J Cell Sci 106:831–846. [DOI] [PubMed] [Google Scholar]
- 9.Dugast M, Toussaint H, Dousset C, Benaroch P. 2005. AP2 clathrin adaptor complex, but not AP1, controls the access of the major histo-compatibility complex (MHC) class II to endosomes. J Biol Chem 280: 19656–19664. [DOI] [PubMed] [Google Scholar]
- 10.McCormick PJ, Martina JA, Bonifacino JS. 2005. Involvement of clathrin and AP-2 in the trafficking of MHC class II molecules to antigen-processing compartments. Proc Natl Acad Sci U S A 102:7910–7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castellino F, Germain RN. 1995. Extensive trafficking of MHC class II-invariant chain complexes in the endocytic pathway and appearance of peptide-loaded class II in multiple compartments. Immunity 2:73–88. [DOI] [PubMed] [Google Scholar]
- 12.Roche PA, Teletski CL, Stang E, Bakke O, Long EO. 1993. Cell surface HLA-DR-invariant chain complexes are targeted to endosomes by rapid internalization. Proc Natl Acad Sci U S A 90:8581–8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tjelle TE, Brech A, Juvet LK, Griffiths G, Berg T. 1996. Isolation and characterization of early endosomes, late endosomes and terminal lysosomes: their role in protein degradation. J Cell Sci 109(Pt 12):2905–2914. [DOI] [PubMed] [Google Scholar]
- 14.Shi GP, Villadangos JA, Dranoff G, Small C, Gu L, Haley KJ, Riese R, Ploegh HL, Chapman HA. 1999. Cathepsin S required for normal MHC class II peptide loading and germinal center development. Immunity 10: 197–206. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa TY, Brissette WH, Lira PD, Griffiths RJ, Petrushova N, Stock J, McNeish JD, Eastman SE, Howard ED, Clarke SR, Rosloniec EF, Elliott EA, Rudensky AY. 1999. Impaired invariant chain degradation and antigen presentation and diminished collagen-induced arthritis in cathepsin S null mice. Immunity 10:207–217. [DOI] [PubMed] [Google Scholar]
- 16.Manoury B, Mazzeo D, Li DN, Billson J, Loak K, Benaroch P, Watts C. 2003. Asparagine endopeptidase can initiate the removal of the MHC class II invariant chain chaperone. Immunity 18:489–498. [DOI] [PubMed] [Google Scholar]
- 17.Riberdy JM, Newcomb JR, Surman MJ, Barbosa JA, Cresswell P. 1992. HLA-DR molecules from an antigen-processing mutant cell line are associated with invariant chain peptides. Nature 360:474–477. [DOI] [PubMed] [Google Scholar]
- 18.Denzin LK, Cresswell P. 1995. HLA-DM induces CLIP dissociation from MHC class II αβ dimers and facilitates peptide loading. Cell 82:155–165. [DOI] [PubMed] [Google Scholar]
- 19.Marks MS, Roche PA, van Donselaar E, Woodruff L, Peters PJ, Bonifacino JS. 1995. A lysosomal targeting signal in the cytoplasmic tail of the β chain directs HLA-DM to MHC class II compartments. J Cell Biol 131:351–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kropshofer H, Vogt AB, Moldenhauer G, Hammer J, Blum JS, Hammerling GJ. 1996. Editing of the HLA-DR-peptide repertoire by HLA-DM. EMBO J 15:6144–6154. [PMC free article] [PubMed] [Google Scholar]
- 21.Sant AJ, Chaves FA, Jenks SA, Richards KA, Menges P, Weaver JM, Lazarski CA. 2005. The relationship between immunodominance, DM editing, and the kinetic stability of MHC class II:peptide complexes. Immunol Rev 207:261–278. [DOI] [PubMed] [Google Scholar]
- 22.Yin L, Stern LJ. 2013. HLA-DM focuses on conformational flexibility around P1 pocket to catalyze peptide exchange. Front Immunol 4:336. doi: 10.3389/fimmu.2013.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pos W, Sethi DK, Call MJ, Schulze MS, Anders AK, Pyrdol J, Wucherpfennig KW. 2012. Crystal structure of the HLA-DM-HLA-DR1 complex defines mechanisms for rapid peptide selection. Cell 151:1557–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liljedahl M, Kuwana T, Fung-Leung WP, Jackson MR, Peterson PA, Karlsson L. 1996. HLA-DO is a lysosomal resident which requires association with HLA-DM for efficient intracellular transport. EMBO J 15: 4817–4824. [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Reed-Loisel LM, Karlsson L, Jensen PE. 2006. H2-O expression in primary dendritic cells. J Immunol 176:3548–3556. [DOI] [PubMed] [Google Scholar]
- 26.Fallas JL, Yi W, Draghi NA, O’Rourke HM, Denzin LK. 2007. Expression patterns of H2-O in mouse B cells and dendritic cells correlate with cell function. J Immunol 178:1488–1497. [DOI] [PubMed] [Google Scholar]
- 27.Hornell TM, Burster T, Jahnsen FL, Pashine A, Ochoa MT, Harding JJ, Macaubas C, Lee AW, Modlin RL, Mellins ED. 2006. Human dendritic cell expression of HLA-DO is subset specific and regulated by maturation. J Immunol 176:3536–3547. [DOI] [PubMed] [Google Scholar]
- 28.Denzin LK, Fallas JL, Prendes M, Yi W. 2005. Right place, right time, right peptide: DO keeps DM focused. Immunol Rev 207:279–292. [DOI] [PubMed] [Google Scholar]
- 29.Liljedahl M, Winqvist O, Surh CD, Wong P, Ngo K, Teyton L, Peterson PA, Brunmark A, Rudensky AY, Fung-Leung WP, Karlsson L. 1998. Altered antigen presentation in mice lacking H2-O. Immunity 8:233–243. [DOI] [PubMed] [Google Scholar]
- 30.Hinz A, Tampe R. 2012. ABC transporters and immunity: mechanism of self-defense. Biochemistry 51:4981–4989. [DOI] [PubMed] [Google Scholar]
- 31.Saveanu L, Carroll O, Lindo V, Del Val M, Lopez D, Lepelletier Y, Greer F, Schomburg L, Fruci D, Niedermann G, van Endert PM. 2005. Concerted peptide trimming by human ERAP1 and ERAP2 aminopeptidase complexes in the endoplasmic reticulum. Nat Immunol 6:689–697. [DOI] [PubMed] [Google Scholar]
- 32.Blum JS, Wearsch PA, Cresswell P. 2013. Pathways of antigen processing. Annu Rev Immunol 31:443–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hebert DN, Garman SC, Molinari M. 2005. The glycan code of the endoplasmic reticulum: asparagine-linked carbohydrates as protein maturation and quality-control tags. Trends Cell Biol 15:364–370. [DOI] [PubMed] [Google Scholar]
- 34.D’Alessio C, Caramelo JJ, Parodi AJ. 2010. UDP-GlC:glycoprotein glucosyltransferase-glucosidase II, the ying-yang of the ER quality control. Seminars Cell Dev Biol 21:491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao B, Adhikari R, Howarth M, Nakamura K, Gold MC, Hill AB, Knee R, Michalak M, Elliott T. 2002. Assembly and antigen-presenting function of MHC class I molecules in cells lacking the ER chaperone calreticulin. Immunity 16:99–109. [DOI] [PubMed] [Google Scholar]
- 36.Garbi N, Tanaka S, Momburg F, Hammerling GJ. 2006. Impaired assembly of the major histocompatibility complex class I peptide-loading complex in mice deficient in the oxidoreductase ERp57. Nat Immunol 7:93–102. [DOI] [PubMed] [Google Scholar]
- 37.Zhang W, Wearsch PA, Zhu Y, Leonhardt RM, Cresswell P. 2011. A role for UDP-glucose glycoprotein glucosyltransferase in expression and quality control of MHC class I molecules. Proc Natl Acad Sci U S A 108:4956–4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams AP, Peh CA, Purcell AW, McCluskey J, Elliott T. 2002. Optimization of the MHC class I peptide cargo is dependent on tapasin. Immunity 16:509–520. [DOI] [PubMed] [Google Scholar]
- 39.Howarth M, Williams A, Tolstrup AB, Elliott T. 2004. Tapasin enhances MHC class I peptide presentation according to peptide half-life. Proc Natl Acad Sci U S A 101:11737–11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wearsch PA, Cresswell P. 2007. Selective loading of high-affinity peptides onto major histocompatibility complex class I molecules by the tapasin-ERp57 heterodimer. Nat Immunol 8:873–881. [DOI] [PubMed] [Google Scholar]
- 41.Joffre OP, Segura E, Savina A, Amigorena S. 2012. Cross-presentation by dendritic cells. Nat Rev Immunol 12:557–569. [DOI] [PubMed] [Google Scholar]
- 42.Shen L, Sigal LJ, Boes M, Rock KL. 2004. Important role of cathepsin S in generating peptides for TAP-independent MHC class I crosspresentation in vivo. Immunity 21:155–165. [DOI] [PubMed] [Google Scholar]
- 43.den Haan JM, Lehar SM, Bevan MJ. 2000. CD8+ but not CD8– dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med 192:1685–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, Chen CJ, Dunbar PR, Wadley RB, Jeet V, Vulink AJ, Hart DN, Radford KJ. 2010. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med 207:1247–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poulin LF, Salio M, Griessinger E, Anjos-Afonso F, Craciun L, Chen JL, Keller AM, Joffre O, Zelenay S, Nye E, Le Moine A, Faure F, Donckier V, Sancho D, Cerundolo V, Bonnet D, Reis e Sousa C. 2010. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8α+ dendritic cells. J Exp Med 207:1261–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Segura E, Durand M, Amigorena S. 2013. Similar antigen cross-presentation capacity and phagocytic functions in all freshly isolated human lymphoid organ-resident dendritic cells. J Exp Med 210:1035–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim JP, Gleeson PA. 2011. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol Cell Biol 89:836–843. [DOI] [PubMed] [Google Scholar]
- 48.Norbury CC, Chambers BJ, Prescott AR, Ljunggren HG, Watts C. 1997. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur J Immunol 27:280–288. [DOI] [PubMed] [Google Scholar]
- 49.Garrett WS, Chen LM, Kroschewski R, Ebersold M, Turley S, Trombetta S, Galan JE, Mellman I. 2000. Developmental control of endocytosis in dendritic cells by Cdc42. Cell 102:325–334. [DOI] [PubMed] [Google Scholar]
- 50.West MA, Prescott AR, Eskelinen EL, Ridley AJ, Watts C. 2000. Rac is required for constitutive macropinocytosis by dendritic cells but does not control its downregulation. Curr Biol 10:839–848. [DOI] [PubMed] [Google Scholar]
- 51.Ruedl C, Koebel P, Karjalainen K. 2001. in vivo -matured Langerhans cells continue to take up and process native proteins unlike in vitro -matured counterparts. J Immunol 166:7178–7182. [DOI] [PubMed] [Google Scholar]
- 52.Platt CD, Ma JK, Chalouni C, Ebersold M, Bou-Reslan H, Carano RA, Mellman I, Delamarre L. 2010. Mature dendritic cells use endocytic receptors to capture and present antigens. Proc Natl Acad Sci U S A 107: 4287–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drutman SB, Trombetta ES. 2010. Dendritic cells continue to capture and present antigens after maturation in vivo. J Immunol 185:2140–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jayachandran R, Sundaramurthy V, Combaluzier B, Mueller P, Korf H, Huygen K, Miyazaki T, Albrecht I, Massner J, Pieters J. 2007. Survival of mycobacteria in macrophages is mediated by coronin 1-dependent activation of calcineurin. Cell 130:37–50. [DOI] [PubMed] [Google Scholar]
- 55.Bosedasgupta S, Pieters J. 2014. Inflammatory stimuli reprogram macrophage phagocytosis to macropinocytosis for the rapid elimination of pathogens. PLoS Pathog 10:e1003879. doi: 10.1371/journal.ppat.1003879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. 2004. in vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med 199:815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chatterjee B, Smed-Sörensen A, Cohn L, Chalouni C, Vandlen R, Lee BC, Widger J, Keler T, Delamarre L, Mellman I. 2012. Internalization and endosomal degradation of receptor-bound antigens regulate the efficiency of cross presentation by human dendritic cells. Blood 120:2011–2020. [DOI] [PubMed] [Google Scholar]
- 58.Stuart LM, Ezekowitz RA. 2005. Phagocytosis: elegant complexity. Immunity 22:539–550. [DOI] [PubMed] [Google Scholar]
- 59.Gagnon E, Duclos S, Rondeau C, Chevet E, Cameron PH, Steele-Mortimer O, Paiement J, Bergeron JJ, Desjardins M. 2002. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell 110:119–131. [DOI] [PubMed] [Google Scholar]
- 60.Ackerman AL, Kyritsis C, Tampe R, Cresswell P. 2003. Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens. Proc Natl Acad Sci U S A 100:12889–12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, Van Endert P, Amigorena S. 2003. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 425:397–402. [DOI] [PubMed] [Google Scholar]
- 62.Houde M, Bertholet S, Gagnon E, Brunet S, Goyette G, Laplante A, Princiotta MF, Thibault P, Sacks D, Desjardins M. 2003. Phagosomes are competent organelles for antigen cross-presentation. Nature 425:402–406. [DOI] [PubMed] [Google Scholar]
- 63.Nair-Gupta P, Baccarini A, Tung N, Seyffer F, Florey O, Huang Y, Banerjee M, Overholtzer M, Roche PA, Tampe R, Brown BD, Amsen D, Whiteheart SW, Blander JM. 2014. TLR signals induce phagosomal MHC-I delivery from the endosomal recycling compartment to allow cross-presentation. Cell 158:506–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blander JM, Medzhitov R. 2004. Regulation of phagosome maturation by signals from Toll-like receptors. Science 304:1014–1018. [DOI] [PubMed] [Google Scholar]
- 65.Blander JM, Medzhitov R. 2006. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 440:808–812. [DOI] [PubMed] [Google Scholar]
- 66.Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC, Lennon-Duménil AM, Seabra MC, Raposo G, Amigorena S. 2006. NOX2 controls phagosomal pH to regulate antigen processing during cross-presentation by dendritic cells. Cell 126:205–218. [DOI] [PubMed] [Google Scholar]
- 67.Savina A, Peres A, Cebrian I, Carmo N, Moita C, Hacohen N, Moita LF, Amigorena S. 2009. The small GTPase Rac2 controls phagosomal alkalinization and antigen crosspresentation selectively in CD8+ dendritic cells. Immunity 30:544–555. [DOI] [PubMed] [Google Scholar]
- 68.Crotzer VL, Blum JS. 2010. Autophagy and adaptive immunity. Immunology 131:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmid D, Pypaert M, Munz C. 2007. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity 26:79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adamopoulou E, Tenzer S, Hillen N, Klug P, Rota IA, Tietz S, Gebhardt M, Stevanovic S, Schild H, Tolosa E, Melms A, Stoeckle C. 2013. Exploring the MHC-peptide matrix of central tolerance in the human thymus. Nat Commun 4:2039. doi: 10.1038/ncomms3039. [DOI] [PubMed] [Google Scholar]
- 71.Nedjic J, Aichinger M, Emmerich J, Mizushima N, Klein L. 2008. Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature 455:396–400. [DOI] [PubMed] [Google Scholar]
- 72.Aichinger M, Wu C, Nedjic J, Klein L. 2013. Macroautophagy substrates are loaded onto MHC class II of medullary thymic epithelial cells for central tolerance. J Exp Med 210:287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaushik S, Cuervo AM. 2012. Chaperone-mediated autophagy: a unique way to enter the lysosome world. Trends Cell Biol 22:407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Manoury B, Mazzeo D, Fugger L, Viner N, Ponsford M, Streeter H, Mazza G, Wraith DC, Watts C. 2002. Destructive processing by asparagine endopeptidase limits presentation of a dominant T cell epitope in MBP. Nat Immunol 3:169–174. [DOI] [PubMed] [Google Scholar]
- 75.Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. 2005. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science 307:1630–1634. [DOI] [PubMed] [Google Scholar]
- 76.Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. 2003. Activation of lysosomal function during dendritic cell maturation. Science 299:1400–1403. [DOI] [PubMed] [Google Scholar]
- 77.Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, Di Malta C, Donaudy F, Embrione V, Polishchuk RS, Banfi S, Parenti G, Cattaneo E, Ballabio A. 2009. A gene network regulating lysosomal biogenesis and function. Science 325:473–477. [DOI] [PubMed] [Google Scholar]
- 78.Settembre C, Fraldi A, Medina DL, Ballabio A. 2013. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol 14:283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Inaba K, Turley S, Iyoda T, Yamaide F, Shimoyama S, Reis e Sousa C, Germain RN, Mellman I, Steinman RM. 2000. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J Exp Med 191:927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turley SJ, Inaba K, Garrett WS, Ebersold M, Unternaehrer J, Steinman RM, Mellman I. 2000. Transport of peptide-MHC class II complexes in developing dendritic cells. Science 288:522–527. [DOI] [PubMed] [Google Scholar]
- 81.Lautwein A, Burster T, Lennon-Duménil AM, Overkleeft HS, Weber E, Kalbacher H, Driessen C. 2002. Inflammatory stimuli recruit cathepsin activity to late endosomal compartments in human dendritic cells. Eur J Immunol 32:3348–3357. [DOI] [PubMed] [Google Scholar]
- 82.Kleijmeer MJ, Oorschot VM, Geuze HJ. 1994. Human resident Langerhans cells display a lysosomal compartment enriched in MHC classII. J Invest Dermatol 103:516–523. [DOI] [PubMed] [Google Scholar]
- 83.Stang E, Guerra CB, Amaya M, Paterson Y, Bakke O, Mellins ED. 1998. DR/CLIP (class II-associated invariant chain peptides) and DR/peptide complexes colocalize in prelysosomes in human B lymphoblastoid cells. J Immunol 160:4696–4707. [PubMed] [Google Scholar]
- 84.Kleijmeer M, Ramm G, Schuurhuis D, Griffith J, Rescigno M, Ricciardi-Castagnoli P, Rudensky AY, Ossendorp F, Melief CJ, Stoorvogel W, Geuze HJ. 2001. Reorganization of multivesicular bodies regulates MHC class II antigen presentation by dendritic cells. J Cell Biol 155:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. 2000. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci 113(Pt 19):3365–3374. [DOI] [PubMed] [Google Scholar]
- 86.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. 1998. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 4:594–600. [DOI] [PubMed] [Google Scholar]
- 87.Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. 2002. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol 3:1156–1162. [DOI] [PubMed] [Google Scholar]
- 88.Buschow SI, Nolte-’t Hoen EN, van Niel G, Pols MS, ten Broeke T, Lauwen M, Ossendorp F, Melief CJ, Raposo G, Wubbolts R, Wauben MH, Stoorvogel W. 2009. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic 10:1528–1542. [DOI] [PubMed] [Google Scholar]
- 89.Chow A, Toomre D, Garrett W, Mellman I. 2002. Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature 418:988–994. [DOI] [PubMed] [Google Scholar]
- 90.Boes M, Cerny J, Massol R, Op den Brouw M, Kirchhausen T, Chen J, Ploegh HL. 2002. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature 418:983–988. [DOI] [PubMed] [Google Scholar]
- 91.Wubbolts R, Fernandez-Borja M, Oomen L, Verwoerd D, Janssen H, Calafat J, Tulp A, Dusseljee S, Neefjes J. 1996. Direct vesicular transport of MHC class II molecules from lysosomal structures to the cell surface. J Cell Biol 135:611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rocha N, Neefjes J. 2008. MHC class II molecules on the move for successful antigen presentation. EMBO J 27:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bosch B, Heipertz EL, Drake JR, Roche PA. 2013. Major histocompatibility complex (MHC) class II-peptide complexes arrive at the plasma membrane in cholesterol-rich microclusters. J Biol Chem 288:13236–13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anderson HA, Roche PA. 2015. MHC class II association with lipid rafts on the antigen presenting cell surface. Biochim Biophys Acta 1853:775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reith W, LeibundGut-Landmann S, Waldburger JM. 2005. Regulation of MHC class II gene expression by the class II transactivator. Nat Rev Immunol 5:793–806. [DOI] [PubMed] [Google Scholar]
- 96.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. 1997. In-flammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature 388:782–787. [DOI] [PubMed] [Google Scholar]
- 97.Young LJ, Wilson NS, Schnorrer P, Mount A, Lundie RJ, La Gruta NL, Crabb BS, Belz GT, Heath WR, Villadangos JA. 2007. Dendritic cell preactivation impairs MHC class II presentation of vaccines and endogenous viral antigens. Proc Natl Acad Sci U S A 104:17753–17758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Landmann S, Muhlethaler-Mottet A, Bernasconi L, Suter T, Waldburger JM, Masternak K, Arrighi JF, Hauser C, Fontana A, Reith W. 2001. Maturation of dendritic cells is accompanied by rapid transcriptional silencing of class II transactivator (CIITA) expression. J Exp Med 194:379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yao Y, Xu Q, Kwon MJ, Matta R, Liu Y, Hong SC, Chang CH. 2006. ERK and p38 MAPK signaling pathways negatively regulate CIITA gene expression in dendritic cells and macrophages. J Immunol 177:70–76. [DOI] [PubMed] [Google Scholar]
- 100.De Gassart A, Camosseto V, Thibodeau J, Ceppi M, Catalan N, Pierre P, Gatti E. 2008. MHC class II stabilization at the surface of human dendritic cells is the result of maturation-dependent MARCH I down-regulation. Proc Natl Acad Sci U S A 105:3491–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Walseng E, Furuta K, Bosch B, Weih KA, Matsuki Y, Bakke O, Ishido S, Roche PA. 2010. Ubiquitination regulates MHC class II-peptide complex retention and degradation in dendritic cells. Proc Natl Acad Sci U S A 107:20465–20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pierre P, Turley SJ, Gatti E, Hull M, Meltzer J, Mirza A, Inaba K, Steinman RM, Mellman I. 1997. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature 388:787–792. [DOI] [PubMed] [Google Scholar]
- 103.Roche PA, Furuta K. 2015. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol 15:203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Samie M, Cresswell P. 2015. The transcription factor TFEB acts as a molecular switch that regulates exogenous antigen-presentation pathways. Nat Immunol 16:729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]