Atrial fibrillation (AF) presents a serious health risk, but its mechanisms remain unclear due to the limited resolution, coverage, and surface-only recordings of multi-electrode mapping.1, 2 The advent of near-infrared optical mapping (NIOM) brought exciting opportunities for recording electrophysiological signals within the intramural heart wall in ex-vivo animal studies, and even blood-perfused hearts.3 Further application of high-resolution transmural NIOM in ex-vivo human atria has been able to resolve intramural conduction to a depth of a few millimeters within the 3D human atrial wall during AF and circumvent the limitations of surface-only multi-electrode mapping and has provided a better understanding of AF.2, 4 Thus, in-vivo NIOM may aid in defining patient-specific arrhythmia mechanisms, thereby potentially enhancing targeted treatment. However, NIOM has not been performed in large animal models in-vivo, a necessary first step.
For the first time, in a canine model, this study successfully conducted in-vivo high-resolution NIOM, integrated with a multi-electrode array (MEA), to visualize atrial conduction during sinus rhythm (SR), atrial pacing, and AF. The canine right atrium (RA, n=1) was simultaneously mapped with NIOM and MEA in-vivo, and subsequently ex-vivo, in accordance with The Ohio State University Institutional Animal Care and Use Committee. In-vivo NIOM was performed via a right-sided thoracotomy. Excitation lights (655±20nm) and a high-resolution CMOS camera (emission filter 720±20nm, 100×100 pixels, 0.32×0.32mm2, MiCAM Ultima-L, SciMedia) were arranged around the thoracic window and focused on the lateral RA (Figure [A]). A customized MEA (8×8 electrodes with 3mm distance, Abbott) with a transparent flex circuit, was placed on the RA epicardial surface (Figure [A]). Unipolar signals were acquired by an Ensite Velocity system (Abbott), and activation was marked at the point of maximum negative slope. An Octopus tissue stabilizer (Medtronic) was used to hold the MEA and decrease motion. A guide wire introduced via the right femoral artery guided a luminal catheter to the right coronary artery (Figure [A]), and 10mL of warm saline-diluted near-infrared voltage-sensitive dye (di-4-ANBDQBS, 1.5mg, University of Connecticut Health Center) was delivered during 20s. No myocardial ischemia or acute cardiotoxic effects were observed after dye injection. Staining was repeated after 40 minutes to counter washout. Right vagal nerve stimulation (VNS), at 10–30Hz (10–20s), was used to temporarily slow heart rate, produce AV block, and diminish contraction. In-vivo NIOM and MEA recordings were taken during SR, 300ms cycle length (CL) RA pacing, and SAN recovery from pacing, with and without VNS, and during AF, which was induced by burst pacing at 130ms CL during VNS. Following in-vivo NIOM, the intact canine atria was mapped ex-vivo with integrated NIOM and MEA. The data related to this study are available from the corresponding author upon reasonable request as well as in Supplement Material.
Figure: In-vivo high-resolution near-infrared optical mapping (NIOM) of the canine heart during right atrial appendage (RAA) pacing, sinus rhythm (SR), and atrial fibrillation (AF).
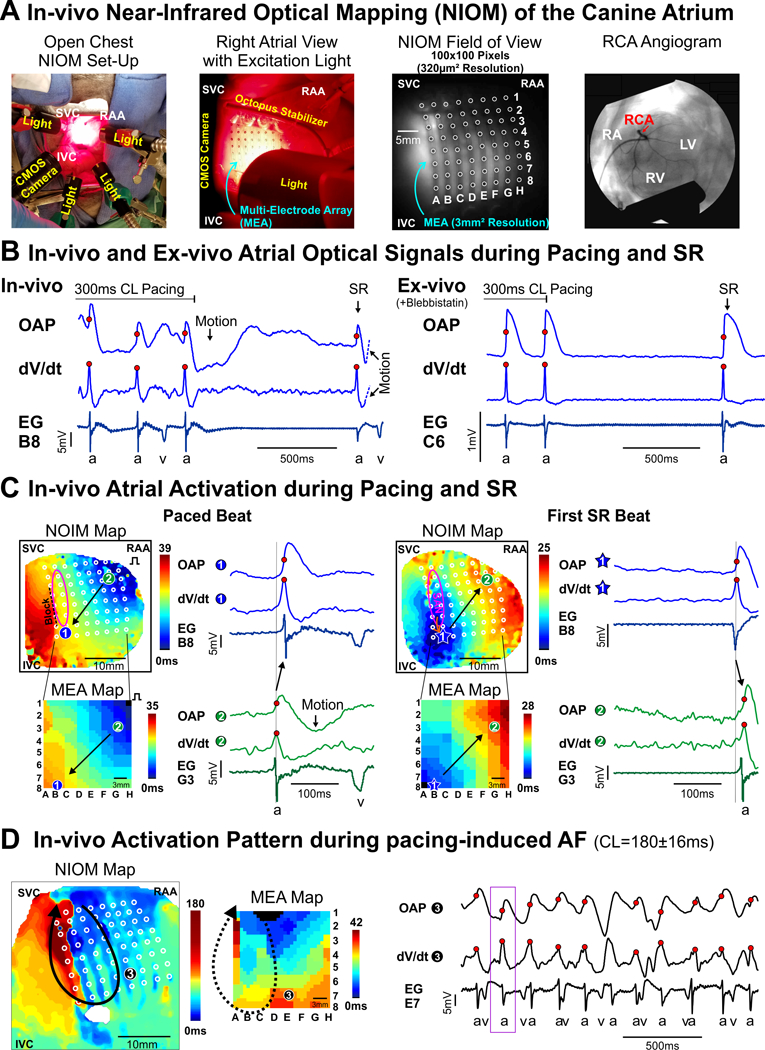
A. Left to right: photograph of in-vivo NIOM camera and lights set-up; close-up photograph of atrial surface with multi-electrode array (MEA) and octopus tissue stabilizer; in-vivo optical field of view from NIOM camera showing MEA (white circles); fluoroscopy angiogram showing the right coronary artery (RCA) where near-infrared voltage-sensitive dye (di-4-ANBDQBS) was delivered. B. In-vivo (Left) and ex-vivo (Right) optical action potentials (OAPs), their derivatives (dV/dt), and unipolar electrograms (EG) from nearest electrode (a- atrial beat; v- ventricular beat) during the end of RAA pacing and the first spontaneous sinus rhythm (SR) beat. C. In-vivo NIOM maps (Top) and simultaneous MEA maps (Bottom) showing activation during RAA pacing (Left) and the first SR beat (Right). Pink oval indicates sinoatrial node (SAN) region. Black arrow represents direction of atrial conduction. Star indicates earliest atrial activation. D. In-vivo NIOM and MEA maps during atrial fibrillation (AF) demonstrate the feasibility of in-vivo NIOM to study arrhythmia mechanisms. Black arrow represents reentry circuit. Purple box indicates mapped time interval. Abbreviations: CL - cycle lengths; IVC and SVC - inferior and superior vena cava; RA - right atrium; RV and LV -right and left ventricles.
In-vivo optical action potentials (OAPs) showed a sharp upstroke that corresponded with atrial activation at a neighboring electrode (Figure [B] left). When comparing subsequent ex-vivo NIOM using excitation-contraction blocker, blebbistatin (10μM) (Figure [B] right), in-vivo OAPs were affected by motion artifact ~40–50ms after OAP upstrokes, which compromised repolarization pattern analysis. However, analyzing the OAP derivative (dV/dtmax) within the pre-motion time frame reliably reproduced the activation conduction pattern seen simultaneously by MEA (Figure [C]) and later in ex-vivo (Figures S1–3). During SR at baseline (124±11 bpm) and during VNS (59±9 bpm), integration of NIOM and MEA revealed atrial activation originating from the sinoatrial node (SAN) region (Figures [C], right). RA pacing led to a retrograde conduction pattern that propagated anisotropically around the intramural SAN-septal block zone, as previously described5 (Figure [C], left). The accuracy and reproducibility of in-vivo NIOM are further supported by beat to beat reproducibility of conduction pattern and timing, including the earliest atrial activation sites (exit points from SAN) during SR at baseline and after 0.2μM acetylcholine perfusion, used as an ex-vivo surrogate for VNS (Figures S1 & S2). Intramural SAN-septal block zone was also seen consistently by NIOM (Figure [C]), which was obscured on canine endo-epicardial MEA.1
During in-vivo AF episodes, the atrial contraction-induced motion artifact was decreased, and OAP upstrokes could be analyzed between ventricular contractions. Thus, NIOM was able to show reentrant activation around the SAN region as previously seen in canine,5 while MEA showed an incomplete portion of the same reentrant circuit due to its smaller coverage (Figure [C]). Importantly, ex-vivo AF had a similar RA activation pattern (Figure S3). Future studies to further develop this novel methodology should focus on minimizing the influence of motion artifact and validation in structurally remodeled hearts, where fibrosis and fat infiltration may affect OAP quality.
In conclusion, this study, for the first time provides evidence of successful in-vivo application of high-resolution NIOM to directly visualize detailed atrial conduction patterns during SR, atrial pacing, and AF. Thus, this study opens the door to a promising potential application of the NIOM method to overcome the limitations of clinical surface electrode mapping and resolve patient-specific arrhythmia mechanisms to improve treatment.
Supplementary Material
Acknowledgments:
We thank Abbott engineer, Mr. Brian Pederson, for his help in integrating multi-electrode array mapping.
Sources of Funding: This work was supported by NIH HL115580 and HL135109, and American Heart Association Grant in Aid #16GRNT31010036 (VVF). Lhota Family Fund for Cardiovascular Research.
Footnotes
Disclosures: Dr. Fedorov has received research support from Abbott Laboratories. Dr. Hummel is a Consultant to Abbott Laboratories.
References:
- 1.Schuessler RB, Kawamoto T, Hand DE, Mitsuno M, Bromberg BI, Cox JL, Boineau JP. Simultaneous epicardial and endocardial activation sequence mapping in the isolated canine right atrium. Circulation. 1993;88:250–263. [DOI] [PubMed] [Google Scholar]
- 2.Hansen BJ, Zhao J, Li N, Zolotarev A, Zakharkin SO, Wang Y, Atwal J, Kalyanasundaram A, Abudulwahed SH, Helfrich KM, Bratasz A, Powell KA, Whitson B, Mohler PJ, Janssen PML, Simonetti OP, Hummel JD, Fedorov VV. Human atrial fibrillation drivers correlated with integrated functional and structural imaging to benefit clinical mapping. J Am Coll Cardiol EP. 2018;DOI: 10.1016/j.jacep.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matiukas A, Mitrea BG, Qin M, Pertsov AM, Shvedko AG, Warren MD, Zaitsev AV, Wuskell JP, Wei MD, Watras J, Loew LM. Near-infrared voltage-sensitive fluorescent dyes optimized for optical mapping in blood-perfused myocardium. Heart Rhythm. 2007;4:1441–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen BJ, Zhao J, Csepe TA, Moore BT, Li N, Jayne LA, Kalyanasundaram A, Lim P, Bratasz A, Powell KA, Simonetti OP, Higgins RS, Kilic A, Mohler PJ, Janssen PM, Weiss R, Hummel JD, Fedorov VV. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur Heart J. 2015;36:2390–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lou Q, Hansen BJ, Fedorenko O, Csepe TA, Kalyanasundaram A, Li N, Hage LT, Glukhov AV, Billman GE, Weiss R, Mohler PJ, Gyorke S, Biesiadecki BJ, Carnes CA, Fedorov VV. Upregulation of adenosine A1 receptors facilitates sinoatrial node dysfunction in chronic canine heart failure by exacerbating nodal conduction abnormalities revealed by novel dual-sided intramural optical mapping. Circulation. 2014;130:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


