Abstract
Background
Satraplatin is an orally bioavailable platinum analog with preclinical activity in cisplatin resistant models and clinical activity in adults with refractory cancers. The cerebrospinal fluid (CSF) penetration of cisplatin and carboplatin in non-human primates (NHP) is limited (3.7 and 2.6%, respectively). We evaluated the plasma and CSF pharmacokinetics (PK) of satraplatin after an intravenous (IV) dose in NHP.
Methods
Satraplatin (120 mg/m2) was administered as 1 h IV infusion in DMSO (5%) and normal saline to 5 NHP. Serial blood and CSF samples were obtained over 48 h. Plasma ultrafiltrate (UF) was immediately prepared by centrifugation. Platinum was quantified in plasma UF and CSF using a validated atomic absorption spectroscopy assay with lower limit of quantification (LLQ) of 0.025 μM in UF and 0.006 μM after concentration in CSF. Pharmacokinetic parameters were estimated using non-compartmental analyses. CSF penetration was calculated from the CSF AUC0–48h : plasma UF AUC0–48h.
Results
Satraplatin was well tolerated. Median (range) PK parameters in plasma UF were: maximum concentration (Cmax) 8.3 μM (5.7–10.6), area under the curve (AUC0–48h) 29.2 μM h (22.6–33.2), clearance 0.36 l/h/kg (0.31–0.37), and t1/2 18.8 h (13.4–25). Satraplatin was detected in the CSF of all NHP. Median (range) PK parameters in CSF were: Cmax 0.07 μM (0.02–0.12), AUC0–48h 1.2 μM h (0.49–2.43). The median (range) CSF penetration of satraplatin was 4.3% (2.2–7.4).
Conclusions
Satraplatin penetration into CSF is similar to that of carboplatin and cisplatin, despite its greater lipophilicity. The development of a phase I trial of satraplatin for refractory childhood solid tumors including brain tumors is in progress.
Keywords: Pharmocology, Non-human primate, Satraplatin, Pharmacokinetics
Introduction
Three platinum analogues, cisplatin, carboplatin, and oxaliplatin, are approved by the Food and Drug administration (FDA) for clinical use in a variety of malignancies in adults including small cell lung cancer [1], ovarian cancer [2], and metastatic colorectal carcinoma [3]. While cisplatin and carboplatin are standard chemotherapy agents for the treatment of a variety of pediatric solid tumors including brain tumors, oxaliplatin did not demonstrate significant activity in refractory pediatric solid tumors [4, 5]. Each agent has a unique toxicity profile and can only be administered intravenously (IV). Cisplatin is associated with mild myelosuppression, and produces significant and potentially irreversible and cumulative nephrotoxicity, ototoxicity, and neurotoxicity [6–9]. The dose-limiting toxicity of carboplatin is myelosuppression, primarily thrombocytopenia. Allergic reactions to carboplatin are a concern, with the incidence of anaphylaxis being reported as high as 22% with carboplatin administration every 4 weeks, although this may be an outlier study [10]. Oxaliplatin caused reversible transient peripheral neuropathy in adult dose finding clinical trials [11] while cumulative neurotoxicity of unclear mechanism was seen in both children and adults, including pharyngolaryngeal dysesthesia, sensory neuropathy, and oxaliplatin-induced ataxia [12, 13]. Several mechanisms of resistance to platinum compounds have been described including decreased drug accumulation due to altered drug uptake or the presence of a membrane efflux pump, increased intracellular levels of thiol-containing species, and enhanced repair of platinum– DNA adducts by the nucleotide excision repair pathway [14–16].
Satraplatin ((OC-6–43)-bis(acetato)amminedichloro(cyclohexylamine)platinum, molecular weight 500.29 g/mol) is a novel orally bioavailable investigational platinum analog with preclinical activity in cisplatin sensitive and resistant models [17–23]. The IC50 for human prostate, ovarian, lung, cervical, colon, renal, CNS, leukemia and melanoma cell lines are 0.04–16 μM [24]. Satraplatin has a dichloro leaving group, a pair of acetato trans ligands, and a single amine and cyclohexamine group as its stable ligands. Its DNA adduct profile is altered by asymmetrical stable ligands. Satraplatin is less easily recognized by DNA mismatch repair proteins due to the formation of bulkier adducts. Aqueous stability in addition to greater lipophilicity allows for oral administration, and may overcome tumor resistance caused by reduced cellular uptake of cisplatin or carboplatin [25]. Clinically, satraplatin has activity in adults with refractory cancers including prostate cancer [26].
While cisplatin and carboplatin have clinical activity in central nervous system malignancies, their cerebrospinal fluid (CSF) penetration in a non-human primate (NHP) model was limited at 3.7 and 2.6%, respectively [27]. We evaluated the plasma and CSF pharmacokinetics of satraplatin after an intravenous dose in the same NHP model, which is predictive of CSF pharmacokinetics in a variety of anticancer drugs in humans [28].
Materials and methods
Drug
Satraplatin was provided by Agennix AG (Princeton, NJ) in pure bulk compound. A dose of 6 mg/kg was solubilized in 5% DMSO and 0.9% saline for infusion and administered intravenously (IV) over 1 h. This dose corresponds to a human equivalent dose of 120 mg/m2, which is similar to the adult recommended phase II oral dose. Animals receiving satraplatin were NPO for at least 6 h prior and 2 h after drug administration, and they received IV Ondansetron (0.1 mg/kg, not to exceed 2 mg) prior to the dose of satraplatin to prevent nausea and vomiting. All drugs were infused through a central venous catheter. Animals also received IV fluid hydration during and after satraplatin.
Animals
Five adult male rhesus monkeys (Macaca mulatta) ranging in weight from 7.3 to 14.9 kg were used in this study. The experimental protocol was approved by the National Cancer Institute Animal Care and Use Committee. All animals were fed NIH Open Formula Extruded Nonhuman Primate Diet twice daily and group housed in accordance with the Guide for the Care and Use of Laboratory Animals [29, 30]. Blood samples were drawn through a temporary saphenous vein catheter placed contralateral to the site of drug administration. CSF was drawn from a temporary lumbar catheter (n = 1) or from a chronically indwelling Pudenz catheter implanted in the fourth ventricle, attached to a subcutaneously implanted Ommaya reservoir (n = 4).
Experiments
Blood (3 mL) and CSF (0.3 ml) samples were obtained before infusion, at 30 and 60 min after the start of infusion, and then 5, 15 and 30 min and 1, 2, 3, 4, 6, 8, 24 and 48 h after the end of infusion. Plasma was immediately separated by centrifugation, and the inactive protein bound platinum in plasma subsequently immediately separated from low molecular weight platinum species by ultrafiltration through a Microcon 10 K MWCO filter (Millipore Co., Bedford, MA) at 14,000 rpm for 30 min at 5°C. The plasma ultrafiltrate (UF) and CSF samples were immediately frozen at −20°C.
Sample analysis
Elemental platinum in plasma UF and CSF was quantified with a Perkin-Elmer AAnalyst 800 Atomic Absorption Spectrometer (AAS) (Perkin–Elmer Co., Norwalk, CT) with an AS autosampler and HGA-800 graphite furnace. Ten to twenty microliters of plasma UF or CSF were injected and the furnace was heated slowly to 2,550C. The absorbance of atomized platinum was measured at 265.7 nM. The validation of the assay for satraplatin was conducted according to the FDA bioanalytical method validation guidelines [30]. Satraplatin spiked plasma was stable at room temperature for at least 2 h. The standard curves were linear over a range 0.03–2.5 μM for plasma UF and 0.03–1 μM for CSF. Standards used were 0.03, 0.05, 0.1, 0.5, 1.0, 2.5 μM satraplatin for plasma UF and quality control (qc) samples 0.75, 2.0 μM; 0.03, 0.05, 0.08, 0.1, 0.5, 1.0 μM aqueous for CSF and qc samples 0.1, 0.5 μM. The lower limit of quantification in plasma UF was 0.025 μM and for CSF was 0.006 μM. Samples exceeding the highest concentration of the standard curve were diluted to fall within the standard curve, and samples that were lower than the lowest point on the standard curve were concentrated to fall within the standard curve. CSF samples were reliably concentrated up to 5-fold. For example, ten microliters of sample was injected onto the AA platform and heated to 100C and dried for 25 s, and then heated to 120C and dried for 25 s. Additional ten microliter aliquots were sequentially added and dried prior to heating the furnace to 2,550C. The total platinum concentration in the sample was corrected by dividing the measured concentration by the number of aliquots applied to the furnace. The intraday and interday coefficients of variation for plasma UF and aqueous quality control standards were<20%.
Pharmacokinetic analysis
The plasma UF and CSF pharmacokinetics of unbound satraplatin were analyzed by non-compartmental methods. The peak satraplatin concentration (Cmax) and time to peak concentration (Tmax) were determined from the timeconcentration data for each NHP. Area under the concentration × time curve (AUC) was calculated using the linear trapezoidal method and extrapolated to infinity (AUC0–inf). The terminal half-life was calculated by dividing 0.693 by the terminal rate constant. Clearance (Cl) was calculated by dividing the satraplatin dose by the AUC0–inf. The CSF penetration was calculated from the CSFAUC 0–48h: plasma UFAUC 0–48h ratio.
Results
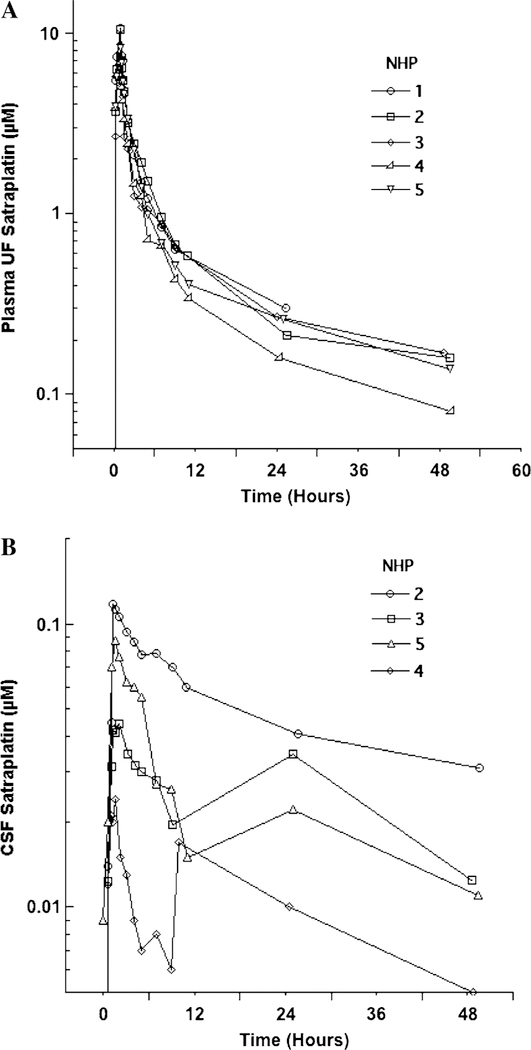
Satraplatin plasma UF (Table 1A) and CSF (Table 1B) model-independent pharmacokinetic parameters are described below. The plasma UF and CSF concentration x time profile for each NHP is shown in Fig. 1a, b. There was little variability for the plasma UF pharmacokinetic parameters following a single IV dose. The median (range) peak plasma UF platinum concentration was 8.3 μM (5.7–10.6) and median time to peak was 1.0 h (0.5–1.1) after the start of infusion. The median (range) AUC0–48 of satraplatin in plasma UF was 29.2 μM h (22.6–33.2). Comparing the AUC0–inf to the AUC0–48h, (with the exception of NHP#1 which was extrapolated from AUC0–24h) approximately 14% (median) of the drug exposure was extrapolated in the plasma. T ½was 18.8 h (13.4–25) in plasma UF but was not calculated in CSF as we could not determine the terminal rate constant. The median clearance of plasma UF was 0.36 l/h/kg (0.31–0.37). Median (range) peak CSF concentration was 0.09 lμM (0.02–0.62) and thus substantially lower than plasma UF concentrations. Platinum concentrations peaked in CSF within 1.5 h of the start of IV infusion. The median (range) AUC0–48 for CSF was 1.2 μM (0.49–2.43). Due to variability in CSF satraplatin concentrations obtained from late time points, the terminal rate constant of satraplatin from CSF could not be determined, and AUC was therefore not extrapolated to infinity. The median (range) CSF penetration (CSF AUC0–48h : plasma UF AUC0–48h) was 4.3% (2.2–7.4).
Table 1.
Satraplatin pharmacokinetic parameters for plasma (A) and cerebrospinal fluid (B)
| Animals | Tmax (h) | Cmax (μM) | AUC0–48h (μM h) | AUC0–inf (μM h) | T½(h) | Cl (l/h/kg) |
|---|---|---|---|---|---|---|
| A: Plasma UF | ||||||
| 1 | 1.1 | 10.6 | 29.3# | 35.1 | 13.4 | 0.34 |
| 2 | 1.0 | 10.4 | 33.2 | 38.2 | 19.5 | 0.31 |
| 3 | 0.5 | 5.7 | 27.4 | 32.1 | 18.8 | 0.37 |
| 4 | 1.0 | 6.9 | 22.6 | 32.1 | 17.4 | 0.37 |
| 5 | 1.0 | 8.3 | 29.2 | 24.7 | 25 | 0.36 |
| Median (range) | 1.0 (0.5–1.1) | 8.3 (5.7–10.6) | 29.2 (22.6–33.2) | 32.1 (24.7–38.2) | 18.8 (13.4–25) | 0.36 (0.31–0.37) |
| Animals | Tmax (h) | Cmax (μM) | AUC0–48h (μM h) | Ratio AUC0–48h CSF: AUC0–48h plasma UF (%) |
|---|---|---|---|---|
| B: CSF | ||||
| 1* | 1.1 | 0.62 | – | – |
| 2 | 1.3 | 0.12 | 2.43 | 7.4 |
| 3 | 2.1 | 0.05 | 1.26 | 4.6 |
| 4 | 1.5 | 0.02 | 0.49 | 2.2 |
| 5 | 1.5 | 0.09 | 1.14 | 3.9 |
| Median (range) | 1.5 (1.1–2.1) | 0.09 (0.02–0.62) | 1.2 (0.49–2.43) | 4.3 (2.2–7.4) |
UF Ultrafiltrate, CSF cerebrospinal Fluid, PK pharmacokinetics, Tmax time of maximum concentration after start of infusion, Cmax maximum concentration, AUC0–48h area under the concentration × time curve from 0 to 48 h, AUC0–inf from 0 to infinity, T1/2 half life, Cl Clearance, CSF: plasma, ratio of AUC 0–48h in CSF to AUC0–48h in plasma
Intralumbar catheter
AUC0–24h
Fig. 1.
Plasma ultrafiltrate (a) and cerebrospinal fluid (b) Concentration × time curves of satraplatin in non-human primates
Discussion
Despite their common side effects and limited CNS penetration, cisplatin and carboplatin are important treatment components for a variety of childhood solid tumors including brain tumors [27, 31–33]. We studied the plasma UF pharmacokinetics and CSF penetration of satraplatin, a novel oral platinum analog, because it has a favorable side effect profile compared to carboplatin and cisplatin, and is lipophilic with a potentially greater CSF penetration.
One of the potential advantages of satraplatin is oral administration. However, for our studies we elected to administer satraplatin IV to NHP due to the technical challenges in giving oral compounds to the NHP. In addition, IV administration reduces the variability in time to peak concentration in plasma and CSF, and results in higher plasma concentrations, which in turn facilitates accurate measurement of plasma and CSF concentrations at later time points. Satraplatin was readily solubilized in 5% DMSO in normal saline, and infused as a single dose over 1 h at a dose equivalent to the adult recommended dose of 120 mg/m2. The NHP tolerated this dose well and had no adverse clinical or laboratory sequelae.
We were able to measure drug in both plasma UF and CSF, and satraplatin was detected or quantified out to 48 h. The presence of satraplatin after IV administration was analyzed by measuring total platinum in plasma UF and CSF using AAS. Satraplatin has at least 6 metabolites, but the primary metabolite detected in human plasma UF is JM-118. JM-118 is the most potent inhibitor of tumor cell growth in vitro, and has been up to 16-fold more potent than parent drug satraplatin [24, 34]. Due to the limits of AAS, we were not able to quantify satraplatin metabolites.
The plasma UF concentration x time profiles of satraplatin in NHP after IV administration were consistent and showed little inter animal variability (Fig. 1a). The median maximum concentration (Cmax) for plasma UF was 8.3 μM (range 5.7–10.6) and thus within the IC50 for human cell lines (0.04–16 μM) [24]. Although administered as a single dose IV, satraplatin resulted in prolonged drug exposure with a median terminal elimination half-life of 18.8 h (range 13.4–25), which exceeds the reported half-life in human phase I trials after oral dosing (mean 7 h) [35, 36].
Satraplatin was detected in all CSF samples. Values at later time points for CSF were variable as the LLQ of 0.006 μM was reached. Thus, the CSF terminal half-life was not calculated. Cmax for CSF was low, but was within the IC50 for cell lines listed above for plasma UF, with the exception of one NHP (#4). The median CSF penetration of satraplatin in the NHP model was 4.3% and similar to that of carboplatin (2.6%) and cisplatin (3.7%) in the same model [27]. This model has been shown to be highly predictive of human pharmacokinetics [28]. Previous studies with carboplatin, cisplatin, and oxaliplatin using brain microdialysis in the same NHP model predicted for low (<5%) CNS penetration of all three platinum analogues, and CSF measurements of carboplatin and cisplatin were identified as valid surrogates for blood–brain barrier penetration [37, 38]. Individuals with CNS tumors often have a disrupted blood brain barrier, and may thus have greater exposure to the agent under evaluation. There has been no investigation of CSF penetration of satraplatin or documented administration to patients with brain tumors to date. While the plasma UF drug exposure greatly exceeded the satraplatin plasma UF exposure in humans after oral administration, the CSF penetration after an oral dose would also be expected to be 4.3% of the plasma UF AUC. Satraplatin accumulation has been shown with daily dosing in clinical trials (accumulation factor as high as 4.1) [35], and with daily oral × 5 days dosing the drug exposure is thus expected to increase.
This is the first report of the pharmacokinetics of satraplatin after IV administration, and the absolute bioavailability of satraplatin is not known. In rats, bioavailability is estimated to be 15–20% based on drug exposure in plasma UF (personal communication, Agennix). A comparison of satraplatin exposure in humans at oral doses of satraplatin equivalent to drug doses in NHP after IV administration demonstrate a substantially greater Cmax and an approximately 20-fold greater AUC0–24h in NHP, suggesting low oral bioavailability of satraplatin.
Preclinical and clinical activity in adults with refractory cancers combined with its ease of oral administration and favorable side effect profile provide the rationale for further investigation of satraplatin in clinical trials. A phase I trial of satraplatin for children with recurrent or refractory solid tumors including brain tumors is in progress.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Conflicts of interest None
Contributor Information
Leigh Marcus, National Cancer Institute, Pediatric Oncology Branch, 10 Center Drive, Building 10-CRC, Room 1-5742, Bethesda, MD 20892-1101, USA, marculei@mail.nih.gov.
Robert Murphy, National Cancer Institute, Pediatric Oncology Branch, 10 Center Drive, Building 10-CRC, Room 1-5742, Bethesda, MD 20892-1101, USA.
Elizabeth Fox, Children’s Hospital of Philadelphia, 3615 Civic Center Blvd, Philadelphia, PA 19104, USA.
Cynthia McCully, National Cancer Institute, Pediatric Oncology Branch, 10 Center Drive, Building 10-CRC, Room 1-5742, Bethesda, MD 20892-1101, USA.
Raphael Cruz, National Cancer Institute, Pediatric Oncology Branch, 10 Center Drive, Building 10-CRC, Room 1-5742, Bethesda, MD 20892-1101, USA.
Katherine E. Warren, National Cancer Institute, Pediatric Oncology Branch, 10 Center Drive, Building 10-CRC, Room 1-5742, Bethesda, MD 20892-1101, USA
Thorsten Meyer, Agennix, Munich, Germany; Agennix, Princeton, NJ, USA.
Edward McNiff, Agennix, Munich, Germany; Agennix, Princeton, NJ, USA.
Frank M. Balis, Children’s Hospital of Philadelphia, 3615 Civic Center Blvd, Philadelphia, PA 19104, USA
Brigitte C. Widemann, National Cancer Institute, Pediatric Oncology Branch, 10 Center Drive, Building 10-CRC, Room 1-5742, Bethesda, MD 20892-1101, USA
References
- 1.Pallis AG et al. (2010) Treatment of small-cell lung cancer inelderly patients. Cancer 116(5):1192–1200 [DOI] [PubMed] [Google Scholar]
- 2.Steer CB (2009) Chemotherapy for ovarian cancer in the olderadult. Curr Treat Options Oncol 10(3–4):159–170 [DOI] [PubMed] [Google Scholar]
- 3.Braun AH et al. (2004) New systemic frontline treatment formetastatic colorectal carcinoma. Cancer 100(8):1558–1577 [DOI] [PubMed] [Google Scholar]
- 4.Huncharek M, Kupelnick B, Bishop D (1998) Platinum analoguesin the treatment of recurrent high grade astrocytoma. Cancer Treat Rev 24(5):307–316 [DOI] [PubMed] [Google Scholar]
- 5.Fouladi M et al. (2006) Phase II study of oxaliplatin in childrenwith recurrent or refractory medulloblastoma, supratentorial primitive neuroectodermal tumors, and atypical teratoid rhabdoid tumors: a pediatric brain tumor consortium study. Cancer 107(9): 2291–2297 [DOI] [PubMed] [Google Scholar]
- 6.Stohr W et al. (2007) Nephrotoxicity of cisplatin and carboplatinin sarcoma patients: a report from the late effects surveillance system. Pediatr Blood Cancer 48(2):140–147 [DOI] [PubMed] [Google Scholar]
- 7.Bertolini P et al. (2004) Platinum compound-related ototoxicity inchildren: long-term follow-up reveals continuous worsening of hearing loss. J Pediatr Hematol Oncol 26(10):649–655 [DOI] [PubMed] [Google Scholar]
- 8.Knight KR, Kraemer DF, Neuwelt EA (2005) Ototoxicity inchildren receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol 23(34):8588–8596 [DOI] [PubMed] [Google Scholar]
- 9.Lanvers-Kaminsky C et al. (2006) Continuous or repeated prolonged cisplatin infusions in children: a prospective study on ototoxicity, platinum concentrations, and standard serum parameters. Pediatr Blood Cancer 47(2):183–193 [DOI] [PubMed] [Google Scholar]
- 10.Walker DA, Taylor RE, Perilongo G et al. (2000) Vincristine(VCR) Carboplatin (CBDCA) in low grade glioma: an interim report of the International Consortium on Low Grade Glioma (ICLGG). ISPNO
- 11.Extra JM et al. (1990) Phase I study of oxaliplatin in patients withadvanced cancer. Cancer Chemother Pharmacol 25(4):299–303 [DOI] [PubMed] [Google Scholar]
- 12.Spunt SL et al. (2007) Phase I clinical trial of oxaliplatin inchildren and adolescents with refractory solid tumors. J Clin Oncol 25(16):2274–2280 [DOI] [PubMed] [Google Scholar]
- 13.Sweeney CW (2002) Understanding peripheral neuropathy inpatients with cancer: background and patient assessment. Clin J Oncol Nurs 6(3):163–166 [DOI] [PubMed] [Google Scholar]
- 14.Boulikas T, Vougiouka M (2003) Cisplatin and platinum drugs atthe molecular level (Review). Oncol Rep 10:1663–1682 [PubMed] [Google Scholar]
- 15.Bosken CH et al. (2002) An analysis of DNA repair as a determinant of survival in patients with non-small-cell lung cancer. J Natl Cancer Inst 94(14):1091–1099 [DOI] [PubMed] [Google Scholar]
- 16.Kelland LR (2000) Preclinical perspectives on platinum resistance. Drugs 59(Suppl 4):1–8 discussion 37–38 [DOI] [PubMed] [Google Scholar]
- 17.Kelland LR et al. (1992) Establishment and characterization of anin vitro model of acquired resistance to cisplatin in a human testicular nonseminomatous germ cell line. Cancer Res 52(7): 1710–1716 [PubMed] [Google Scholar]
- 18.Twentyman PR et al. (1992) Sensitivity to novel platinum compounds of panels of human lung cancer cell lines with acquired and inherent resistance to cisplatin. Cancer Res 52(20):5674–5680 [PubMed] [Google Scholar]
- 19.Loh SY et al. (1992) Reduced drug accumulation as a majormechanism of acquired resistance to cisplatin in a human ovarian carcinoma cell line: circumvention studies using novel platinum (II) and (IV) ammine/amine complexes. Br J Cancer 66(6):1109–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mellish KJ, Kelland LR, Harrap KR (1993) In vitro platinumdrug chemosensitivity of human cervical squamous cell carcinoma cell lines with intrinsic and acquired resistance to cisplatin. Br J Cancer 68(2):240–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orr RM et al. (1994) Evaluation of novel ammine/amine platinum(IV) dicarboxylates in L1210 murine leukaemia cells sensitive and resistant to cisplatin, tetraplatin or carboplatin. Br J Cancer 70(3):415–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fokkema E et al. (2002) JM216-, JM118-, and cisplatin-inducedcytotoxicity in relation to platinum-DNA adduct formation, glutathione levels and p53 status in human tumour cell lines with different sensitivities to cisplatin. Biochem Pharmacol 63(11):1989–1996 [DOI] [PubMed] [Google Scholar]
- 23.Martelli L et al. (2006) Different accumulation of cisplatin, oxaliplatin and JM216 in sensitive and cisplatin-resistant human cervical tumour cells. Biochem Pharmacol 72(6):693–700 [DOI] [PubMed] [Google Scholar]
- 24.Wosikowski K et al. (2007) Preclinical antitumor activity of theoral platinum analog satraplatin. Cancer Chemother Pharmacol 60(4):589–600 [DOI] [PubMed] [Google Scholar]
- 25.Choy H, Park C, Yao M (2008) Current status and future prospects for satraplatin, an oral platinum analogue. Clin Cancer Res 14(6):1633–1638 [DOI] [PubMed] [Google Scholar]
- 26.McKeage MJ (2007) Satraplatin in hormone-refractory prostatecancer and other tumour types: pharmacological properties and clinical evaluation. Drugs 67(6):859–869 [DOI] [PubMed] [Google Scholar]
- 27.Jacobs SS et al. (2005) Plasma and cerebrospinal fluid pharmacokinetics of intravenous oxaliplatin, cisplatin, and carboplatin in nonhuman primates. Clin Cancer Res 11(4):1669–1674 [DOI] [PubMed] [Google Scholar]
- 28.McCully CL et al. (1990) A rhesus monkey model for continuousinfusion of drugs into cerebrospinal fluid. Lab Anim Sci 40(5):520–525 [PubMed] [Google Scholar]
- 29.U.S., D.o.H.a.W.P., G.P. Office (1996) Guide for the care and useof laboratory animals
- 30.FDA (2001) Guidance for Industry Bioanalytical Method Validation 1–22
- 31.Packer RJ et al. (2005) Phase 1 study of concurrent RMP-7 andcarboplatin with radiotherapy for children with newly diagnosed brainstem gliomas. Cancer 104(6):1281–1287 [DOI] [PubMed] [Google Scholar]
- 32.Rutkowski S et al. (2005) Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med 352(10):978–986 [DOI] [PubMed] [Google Scholar]
- 33.MacDonald TJ et al. (2005) Phase II study of high-dose chemotherapy before radiation in children with newly diagnosed highgrade astrocytoma: final analysis of Children’s Cancer Group Study 9933. Cancer 104(12):2862–2871 [DOI] [PubMed] [Google Scholar]
- 34.Raynaud FI et al. (1996) Biotransformation of the platinum drugJM216 following oral administration to cancer patients. Cancer Chemother Pharmacol 38(2):155–162 [DOI] [PubMed] [Google Scholar]
- 35.McKeage MJ et al. (1997) Phase I and pharmacokinetic study ofan oral platinum complex given daily for 5 days in patients with cancer. J Clin Oncol 15(7):2691–2700 [DOI] [PubMed] [Google Scholar]
- 36.Kurata T et al. (2000) Pharmacokinetic and pharmacodynamicanalysis of bis-acetato-ammine-dichloro-cyclohexylamine-platinum(IV) (JM216) administered once a day for five consecutive days: a phase I study. Jpn J Clin Oncol 30(9):377–384 [DOI] [PubMed] [Google Scholar]
- 37.Jacobs S et al. (2005) Extracellular fluid concentrations of cisplatin, carboplatin, and oxaliplatin in brain, muscle, and blood measured using microdialysis in nonhuman primates. Cancer Chemother Pharmacol 65(5): 817–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox E et al. (2002) Zidovudine concentration in brain extracellularfluid measured by microdialysis: steady-state and transient results in rhesus monkey. J Pharmacol Exp Ther 301(3):1003–1011 [DOI] [PubMed] [Google Scholar]