Abstract
When drug-resistant epilepsy is poorly localized or surgical resection is contraindicated, current neurostimulation strategies such as deep brain stimulation and vagal nerve stimulation can palliate the frequency or severity of seizures. However, despite medical and neuromodulatory therapy, a significant proportion of patients continue to experience disabling seizures that impair awareness, causing disability and risking injury or sudden unexplained death. We propose a novel strategy in which neuromodulation is used not only to reduce seizures but also to ameliorate impaired consciousness when the patient is in the ictal and postictal states. Improving or preventing alterations in level of consciousness may have an effect on morbidity (e.g., accidents, drownings, falls), risk for death, and quality of life. Recent studies may have elucidated underlying networks and mechanisms of impaired consciousness and yield potential novel targets for neuromodulation. The feasibility, benefits, and pitfalls of potential deep brain stimulation targets are illustrated in human and animal studies involving minimally conscious/vegetative states, movement disorders, depth of anesthesia, sleep-wake regulation, and epilepsy. We review evidence that viable therapeutic targets for impaired consciousness associated with seizures may be provided by key nodes of the consciousness system in the brainstem reticular activating system, hypothalamus, basal ganglia, thalamus, and basal forebrain.
Keywords: level of consciousness, arousal, epilepsy, deep brain stimulation, DBS
OF the nearly 3 million patients with epilepsy, an estimated one quarter have medically refractory seizures (i.e., they continue to have disabling seizures). Refractory seizures significantly decrease safety, quality of life, and productivity, and they increase social stigma.73,93 The highest chance of cure lies with resection or ablation of epileptic foci; established Level I evidence exists only for open temporal lobe surgery for temporal lobe epilepsy.97 For a variety of reasons, however, only a small percentage of epilepsy patients undergo resection. Alternative approaches to ameliorate the effects of seizures could still profoundly benefit patients for whom resection is not feasible or not desirable. Such patients include the following: 1) those with generalized or poorly localized epilepsy; 2) those whose seizures are localized within deep or eloquent regions, posing higher risk for neurocognitive decline after resection; 3) those who do not remain free of seizures despite technically successful resection; and/or 4) those with significant other medical conditions that preclude resection.
Deep brain stimulation (DBS) is a mainstream therapy for several movement disorders and neuropsychiatric conditions. Likewise, neurostimulatory approaches are increasingly being recognized as safe and potentially effective for limiting seizure initiation or propagation. Multiple studies have shown constant (open-loop) and responsive (closed-loop) stimulation of deep and/or cortical brain sites to be modestly effective.27,29,41,47,49,60,71 Notably, recent pivotal trials demonstrated improved seizure control after DBS of the anterior nucleus of the thalamus (Stimulation of Anterior Nucleus of Thalamus for Epilepsy trial) and DBS of neocortical or mesial temporal structures (Responsive Neurostimulation trial).27,60 To date, however, such studies have not addressed whether neurostimulation might specifically restore an impaired level of consciousness when the patient is in the ictal or postictal states. Minimizing the effects of seizures on consciousness or enhancing cognition in the postictal brain presents unique challenges for scientific exploration and fertile opportunities for novel therapies, possibly by DBS or related neuromodulatory strategies.
For patients for whom seizure prevention is neither surgically nor medically feasible, mitigating or preventing the alterations in consciousness that occur during seizures would profoundly improve safety and lifestyle. The previously tested utility of DBS for treating disorders of consciousness makes it an exciting modality for approaching a broad range of therapeutic indications as well as for scientific discovery.
Plum and Posner’s bidimensional model of consciousness dichotomizes consciousness into level and content, of which level of consciousness includes arousal, attention, and awareness.70 These facets are correlated with an underlying anatomical consciousness network that includes the upper brainstem reticular activating system, medial thalami, basal forebrain, and frontoparietal association cortices.8 Recent advances in understanding the mechanisms by which different types of seizures impair consciousness suggest disruption of essential nodes in the consciousness network.8,20
Interacting with the networks from which level of consciousness emerges are absence, generalized tonic-clonic, and complex partial seizures. Absence seizures are associated with transient loss of awareness associated with a cortical electroencephalography (EEG) spike-and-wave pattern mediated by a thalamocortical circuit from the reticular nucleus to the medial frontal and parietal cortex.4,5,94 Partial seizures, although associated with focal onset, can impair awareness by disrupting wider frontotemporal networks and can maintain impaired levels of consciousness by activating deep inhibitory nuclei, such as the lateral septum and anterior hypothalamus, which in turn depress the reticular activating system—a tenet central to the network-inhibition hypothesis (Fig. 1).8,10,21,22,65 Primary or secondary generalized tonic-clonic seizures are thought to involve not only inhibition of subcortical arousal nuclei but also simultaneous disruption of widespread networks involving both hemispheres because of seizure propagation. Generalized seizures are most associated with profound consciousness deficits and a prolonged postictal state.8,9,23
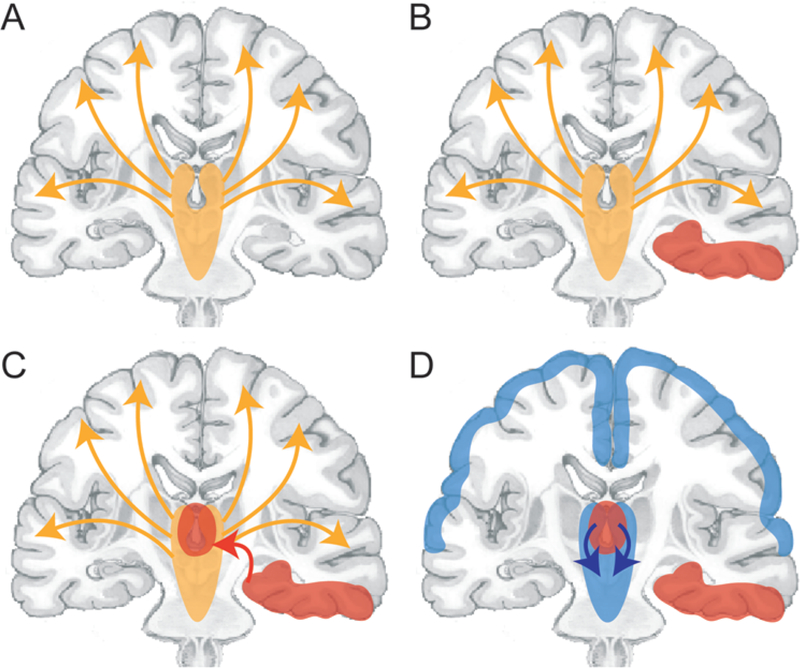
FIG. 1.
Network inhibition hypothesis for loss of consciousness in patients during partial seizures. A: Normal conditions. The upper brainstem–diencephalic activating systems interact with the cerebral cortex to maintain normal consciousness (orange arrows). B: A focal seizure involving the mesial temporal lobe unilaterally (red area). C: Propagation of seizure activity from the mesial temporal lobe to midline subcortical structures (red arrow pointing to midline red area). D: Disruption of the normal activating functions of the midline subcortical structures (blue arrows), together with the resulting depressed activity in bilateral regions of the frontoparietal association cortex (blue areas), which leads to loss of consciousness. Copyright Hal Blumenfeld. Published with permission.
The effect of electrical stimulation on the complex neuronal environment is not clearly understood. As the fundamental object of numerous technologies and therapies, such understanding is necessary for better network targeting, outcome efficacy, and side-effect control. The parameters that influence DBS results include location, frequency, pulse width, amplitude, temporal patterns (e.g., continuous, intermittent cycling, or responsive), electrode factors (e.g., size, shape, impedence), and stimulation field (e.g., monopolar, bipolar, multipolar).
Competing hypotheses about the mechanistic effects of DBS include axonal activation, local inhibition, network oscillation disruption, and effects on astrocytes.2 Physiological studies have shown that axons are the earliest elements to depolarize, which leads to action potentials.16 To test stimulation effects of axonal activation versus local in hibitory circuitry, one study measured cortical activation after a DBS target injection of ibotenic acid, which selectively destroys cell bodies without affecting passing axons, and muscimol, a gamma-aminobutyric acid (GABA)–A receptor agonist.37 At low stimulation frequencies, use of muscimol mimicked the DBS effect, suggesting activation of local GABAergic networks leading to inhibitory effect of DBS. At high stimulation frequencies, use of ibotenic acid did not impair distant cortical effects of DBS despite loss of target area cell bodies.37 Additionally, axonal excitation probably proceeds in both orthodromic and antidromic directions.45 Other studies have suggested that disturbance of network oscillations, rather than excitation or inhibition, plays a role as the putative mechanism based on dissociation of input and output signals.16 Astroglia might also play a significant role in the mechanism of DBS by modulating and amplifying the effect of stimulation on local principal and interneuron populations.24 The uncertainty surrounding the DBS mechanism of action argues for a dual approach in which putative therapeutic targets are continually pursued in human patients while electrical and optogenetic stimulation in animal models help to refine DBS parameters.
Here we review the brain structures involved in networks that modulate or maintain level of consciousness and discuss the clinical and translational literature pertaining to potential sites of stimulation in the brainstem, hypothalamus, basal ganglia, thalamus, and basal fore-brain. Incorporating studies of DBS for consequences of traumatic brain injury, movement disorders, epilepsy, and others disorders, we suggest that related targets may prove to be beneficial in the context of impaired consciousness in patients with epilepsy (Table 1). The mechanisms underlying how electrical stimulation via a wide spectrum of parameters can act on specific brain circuits to produce clinical effects are complex, incompletely understood, and ultimately beyond the scope of this review.
TABLE 1.
Selected stimulation experiments, organized by brain region, that show improved level of consciousness
| Authors & Year | Target | Species | No. of Patients | Stim Type | Uni-vs Bilateral | HFS/LFS | Freq | Amp | Pulse Width | Duration | State | Findings & Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Upper brainstem | ||||||||||||
| Arnulf etal., 2010 | PPT | Human | 2 | Electrical | Uni- + bilat | LFS/HFS | 10–25 Hz/80 Hz | 2.7–2.8 V/0.8–1.5 V | 60 μsec | 5 mins on, 3 mins off | Awake | During LFS, patients reported increased alertness. HFS caused acute-onset sleepiness & episodes of REM sleep. |
| Peppe et al., 2012 | PPT | Human | 5 | Electrical | Unilat | LFS | 25 Hz | 1.8–2.2 V | 60 usec | Continuous vs nighttime | Awake & sleep | PPT + STN stimulation improved daytime sleepiness, nocturnal restlessness, & nocturnal psychosis compared w/ STN stimulation alone. |
| Furman et al., 2013 | PPT | Rat | NR | Optoge-netic | Unilat | LFS* | 5–40 Hz | 20–60 mW/mm2 | 1–60 msec | 10 sec | Ictal | ChR2 stimulation of PPT efferents caused cortical desynchronization during seizure. |
| Solt et al., 2014 | VTA | Rat | 5 | Electrical | Unilat | HFS | 100 Hz | 30–120 μAmp | NR | 30 sec on, 30 sec off, 3 mins | Anesthesia (isoflurane) | Stimulation of VTA caused increase in beta (12–30 Hz) EEG & induced reanimation from anesthesia. |
| Marzo et al., 2014 | LC | Rat | 6 | Electrical | Bilat | LFS | 20–50 Hz | 30–50 μAmp | 400 μsec | 50–200 msec | Anesthesia (urethane) | Pulse train stimulations caused mPFC desynchronization, increased power in bands >20 Hz, & decreased delta band power (0–4 Hz). |
| Pillay et al., 2014 | PnO | Rat | 9 | Electrical | Unilat | HFS | 300 Hz | 5–7 V | 100 μsec | 3 sec on, 57 sec off | Anesthesia (isoflu-rane) | PnO stimulation caused cortical desynchronization & decreased delta band power. Increase in FC was seen w/ NBM, central medial thalamus, retrosplenium, caudate, & putamen stimulation. |
| A. Kundishora, personal communication, 2015 | PnO | Rat | 9 | Electrical | Bilat | LFS | 50 Hz | 30–75 μAmp | 500 μsec | 120 sec | Light anesthesia (ket-amine) ictal + postictal | Stimulation in PnO caused cortical desynchronization during deep anesthesia & during & after electrically triggered seizures (under light anesthesia) w/ decrease in delta band power. |
| Hypothalamus | ||||||||||||
| Nishida et al., 2007 | TMN | Rat | 6–12 | Electrical | Uni- + bilat | HFS | 100 Hz | 80–150 μAmp | 300 μsec | 30 mins; 10 sec on, 10 sec off | Anesthesia (ure-thane) | Protected against seizure occurrence in a histamine-1-receptor-dependent manner in a PTZ epilepsy model. Stimulation was at 150 μAmp for unilat & 80 μAmp for bilat. |
| Wu et al., 2008 | TMN | Rat | 8–10 | Electrical | Uni- + bilat | LFS/HFS | 1 Hz/100 Hz | 100–200 μAmp | 100 μsec | Continuous/3–15 mins | Awake | LSF facilitated amygdaloid-kindling-induced epileptogenesis. Findings for HFS were similar. |
| Blik et al., 2015 | TMN | Rat | 5 | Electrical | Bilat | HFS | 100 Hz | 68–88 μAmp | 300 μsec | Continuous | Sleep & awake | In WAG/Rij rat epileptic model, open-loop DBS at 70% of threshold stimulation reduced number of spike-wave discharges & altered animals’ sleep cycles by increasing wakefulness. |
| Franzini et al., 2008 | PH | Human | 2 | Electrical | Bilat | HFS | 100–185 Hz | 1.5–3.0 V | 90 μsec | Continuous | Awake | Decreased seizure activity & pathologically disruptive behavior in 2 patients w/ refractory multifocal epilepsy. |
| Franzini et al., 2013 | PH | Human | 7 | Electrical | Bilat | HFS | 185 Hz | 1–3 V | 60–90 μsec | Continuous | Awake | Treatment was effective for aggressive behavior & possibly had simultaneous antiseizure effects. |
| Whiting et al., 2013 | LH | Human | 3 | Electrical | Bilat | HFS | 185 Hz | 1–7 V | 90 μsec | 12–14 hrs, daily | Awake | All 3 patients reported increased arousal & activity when contact 3 was stimulated (most superior/superficial aspect of LH). |
| Basal ganglia | ||||||||||||
| Moll et al., 2009 | GPi | Human | 1 | Electrical | Unilat | HFS | 130 Hz | 0.5–0.7 V | 60 μsec | 2–3 mins | Anesthesia (propofol) | Unilat stimulation of GPi in an anesthetized patient w/ cervical dystonia induced a state of wakeful unawareness. |
| Fimm et al., 2009 | STN | Human | 13 | Electrical | Bilat | HFS | 130–185 Hz | 1.4–4.2 V | 60–90 μsec | Continuous | Awake | Reaction time was decreased in Parkinson disease patients after bilat STN stimulation. |
| Thalamus | ||||||||||||
| Fisher et al., 1992 | CM | Human | 7 | Electrical | Bilat | HFS | 65 Hz | Variable | 95 msec | 2 hrs/day; 1 min on, 4 mins off | Awake | Nonstatistically significant decrease in seizure frequency (30% vs 8%); however, after 24 hrs of continuous DBS, decrease in seizure frequency rose to 50%. |
| Velasco et al., 1995 | CM | Human | 5 | Electrical | Bilat | HFS | 60 Hz | 440–790 μAmp | 0.09–1 msec | 1 min for 2 hr/day | Awake | CM stimulation dramatically reduced seizure frequency; was most effective for generalized tonic-clonic seizures but did not change frequency of complex-partial seizures. |
| Yamamoto et al., 2005 | CM-Pf | Human | 21 VS; 5 MCS | Electrical | Unilat | LFS | 25 Hz | Variable | NR | 30 mins every 2–3 hrs | VS/MCS | 8/21 VS patients were able to follow verbal commands, 4/5 MCS patients were no longer bedridden, according to 10 yrs of follow-up. |
| Valentin et al., 2012 | CM | Human | 1 | Electrical | Bilat | LFS | 6 Hz | 6 V | 90 μsec | Variable | Status epilepticus | Reduced myoclonic jerks w/ resolution of EEG epileptiform discharges in patient w/ refractory SE. |
| Schiff et al., 2007 | CL | Human | 1 | Electrical | Bilat | HFS | 100 Hz | 4 V | 90 μsec | Continuous | MCS | Central thalamic stimulation improved ratings in revised Coma Recovery Scale for patient in minimally conscious state 6 yrs after a traumatic brain injury. |
| Shirvalkar et al., 2006 | CL | Rat | 5–10 | Electrical | Unilat | HFS | 100 Hz | 1.5 mAmp | 50 μsec | 30 mins | Awake | Increased object recognition memory, arousal-related exploratory motor behavior, & goal-directed seeking behaviors in rats w/ CL stimulation. |
| Gummadavelli et al., 2014 | CL | Rat | 8–12 (terminal group) 3 (chronic group) | Electrical | Bilat | HFS | 100 Hz | 400 μAmp | 500 μsec | 20 sec | Postictal anesthesia (ketamine) sleep | Stimulation after electrically induced seizure, ketamine-xyla-zine anesthesia, or spontaneous sleep decreased low-frequency power & increased spontaneous exploratory behaviors. |
| Mair & Hembrook, 2008 | rILN | Rat | 20 | Electrical | Bilat | HFS | 120 Hz | Variable | 200 μsec | 2 sec | Awake | Low-current stimulation improved delayed matching-to-position working memory task when applied in memory delay or choice phases, indicating role in memory retrieval. |
| Basal forebrain | ||||||||||||
| Freund et al., 2009 | NBM | Human | 1 | Electrical | Bilat | LFS | 20 Hz | 1 V | 120 μsec | Continuous | Awake | Bilat electrical stimulation of the NBM caused significant improvement in cognitive tasks. STN stimulation alone showed no improvement. Patient underwent cognitive decline 24 hrs after cessation of NBM stimulation. |
| Han et al., 2014 | Ch-BF | Mouse | 5 | Optogenetic | Unilat | LFS* | 10 & 20 Hz | 0.5–1.5 mW | 30 msec | 1–15 sec every 1 min for 1 hr | Slow-wave sleep | Photostimulation induced a decrease in slow-wave & increase in theta-wave EEG activity & increased total time spent in the awake state. |
Amp = amplitude; Ch-BF = cholinergic basal forebrain; ChR2 = channelrhodopsin; CL = central lateral thalamus; CM = centromedian thalamus; CM-Pf = centromedian–parafascicular thalamus; FC = functional connectivity; freq = frequency; GPi = globus pallidus interna; HFS = high-frequency stimulation; LC = locus coeruleus; LH = lateral hypothalamus; LFS = low-frequency stimulation; MCS = minimally conscious state; mPFC = medial prefrontal cortex; NBM = nucleus basalis of Meynert; NR = not reported; PH = posterior hypothalamus; PnO = pontine nucleus oralis; PPT = pedunculopontine tegmental nucleus; PTZ = pentylenetetrazol; rILN = rostral intralaminar thalamus; SE = status epilepticus; stim = stimulation; STN = subthalamic nucleus; TMN = tuberomamillary nucleus; VS = vegetative state; VTA = ventral tegmental area.
Mechanism of optogenetic stimulation ensures neuronal depolarization, regardless of frequency.
Upper Brainstem
Notable brainstem nuclei associated with level of arousal, particularly those in the reticular activating system, have been the subject of study since the early 1950s. Stimulation in the reticular activating system was found to desynchronize cortical EEG patterns and abolish high-amplitude slow waves in anesthetized patients.61 However, the relative contributions of subsequently discovered nuclei in the networks underpinning the level of consciousness remain uncertain. Among the best studied, stimulation in the region of the pedunculopontine tegmental nucleus has been shown to play a dual role in postural stability, probably resulting from glutamatergic efferents46 and cognitive functions including rapid eye movement (REM) sleep, resulting from cholinergic efferents.66,72,81 Its consideration and subsequent exploration as a DBS target in patients with Parkinson disease has yielded mixed results; however, for some patients, improvements in attentiveness have been reported.81 Improvement in cognitive, rather than motor, domains may result in part from the regulatory effects of pedunculopontine tegmental nucleus DBS on sleep architecture (Table 1).68 Nevertheless, low-frequency stimulation may enhance patient alertness and high-frequency stimulation may promote sleep. Each phenomenon may represent overlapping but distinct network effects (Table 1).3,25 Interpatient targeting variability and the uncertainty of DBS mechanisms might limit the interpretation of such preliminary findings.55 However, animal experiments that used optogenetic stimulation of cholinergic efferents from the pedunculopontine tegmental nucleus have confirmed its role in arousal on the basis of cortical desynchronization during and after temporal lobe seizures (Table 1).33 Improved human pedunculopontine tegmental nucleus electrode targeting and stimulation techniques may improve its potential as a target for arousal.59
Stimulation experiments point to a number of other brainstem nuclei as having the capacity to produce alertness, including the ventral tegmental area, the locus coeruleus, and the pontine reticular nucleus oralis. In rats, electrical stimulation of the ventral tegmental area induces reanimation during continuous anesthetization with propofol (Table 1).80 Similarly, in anesthetized rats, unilateral stimulation of the locus coeruleus showed bilateral desynchronization in the medial prefrontal cortex (Table 1).53 This study measured responses in the medial prefrontal cortex on the order of 1 second, indicating a norepinephrine-dependent switch in cortical state toward information processing.53 Optogenetic stimulation of the locus coeruleus indicated that temporal characteristics of stimulation altered the resultant arousal effects.13 Vagus nerve stimulation, a widely available neuromodulatory device that modestly reduces seizure frequency in appropriately selected patients, putatively acts via activation of the locus coeruleus and/or nucleus tractus solitarius, and the device has been noted to improve arousal in patients with epilepsy.44
Stimulation of the pontine reticular nucleus oralis in a lightly anesthetized patient has also been shown to increase cortical desynchronization with increased poststimulation functional connectivity to basal forebrain-paralimbic structures such as the nucleus basalis of Meynert, central-medial nucleus of the thalamus, retrosplenium, and cau-date/putamen (Table 1).69 Preliminary data from studies of rats show robust cortical desynchronization after bilateral electrical stimulation of the pontine reticular nucleus oralis during and after partial seizures(Table 1) (A. Kundishora, personal communication, 2015). These diverse brainstem regions provide but a few potential DBS targets for enhancing consciousness in patients with epilepsy.
Hypothalamus
Since the early twentieth century, when naturally occurring hypothalamic lesions were paradoxically associated with either narcolepsy or insomnia, the hypothalamus has been theorized to be a key effector of arousal and sleep regulation.74 Since then, the antagonistic relationship between the sleep-promoting anterior hypothalamus and arousal centers in the posterior hypothalamus, basal forebrain, and brainstem has been elucidated.1,74 It has been hypothesized that mutual inhibition among distinct neuronal populations in the hypothalamus acts as a neural switch that transitions the brain between aroused and sedated states.74,75 Sleep-promoting neurons in the ventrolateral preoptic nucleus have inhibitory GABAergic and galanergic projections to the wake-promoting orexin/hypocretin neurons of the lateral hypothalamus and the histaminergic neurons of the tuberomammillary nucleus in the posterior hypothalamus. In turn, orexin/hypocretin and histamine have inhibitory effects on the ventrolateral preoptic nucleus; the tuberomammillary nucleus sends direct projections to the ventrolateral preoptic nucleus in addition to its diffuse cortical targets.17,74 Chemical inhibition of the ventrolateral preoptic nucleus with dexmedetomidine induces electrophysiological desynchronization and behavioral arousal from isoflurane anesthesia.56 DBS targeting the lateral hypothalamus has been tried as a treatment for refractory obesity in 3 patients; in addition to augmenting the resting metabolic rate, 1 of the stimulated contacts consistently increased arousal (Table 1).96
As the major seat of histaminergic neurons in the brain, the tuberomammillary nucleus is involved in regulation of sleep/wakefulness and states of consciousness and, as such, is a putative site for stimulation.36,51,67 Furthermore, different frequencies of tuberomammillary nucleus stimulation in animal experiments have shown either pro- or antiseizure effects, suggesting a complex relationship between the tuberomammillary nucleus and seizures. High-frequency (100 Hz) stimulation of the tuberomammillary nucleus in a pentylenetetrazol model of epilepsy protected against seizure occurrence in a histamine-1 receptor–dependent manner (Table 1).63 A subsequent study found that low-frequency (1 Hz) stimulation of the tuberomammillary nucleus and bilateral tuberomammillary nucleus lesions facilitated amygdaloid kindling–induced epileptogenesis, although low-frequency stimulation had no effect on pentylenetetrazol-kindled seizures (Table 1).98 In conflict with prior results, that study concluded that high-frequency stimulation also facilitated progression of amygdaloid-kindled seizures, indicating that differences in animal model preparations and high-frequency stimulation parameters (pulse width and stimulation duration) may have led to disparate results.98 Most recently, tuberomammillary nucleus stimulation in a WAG/Rij rat model of absence epilepsy concluded that open-loop 100-Hz stimulation reduced the number of spike-wave discharges and altered the animals’ sleep cycle by increasing active wakefulness (Table 1).7
Few human studies document stimulation of the posterior hypothalamus, of which the tuberomammillary nucleus is a subnucleus. For 2 patients, posterior hypothalamic DBS successfully treated refractory multifocal epilepsy by decreasing seizure activity as well as pathologically disruptive behavior (Table 1)31; this location has since been stimulated to treat aggressive behavior disorders in 5 additional patients and possibly had simultaneous antiseizure effects (Table 1).30 These data are complementary to data from recent human trials of posterior hypothalamic stimulation for the treatment of cluster headaches that report prolonged periods of wakefulness and disrupted sleep after DBS.48
Viewed in the context of these studies, the posterior and lateral hypothalamus could have a therapeutic role in epilepsy by affecting seizure frequency and impaired consciousness. Further data are needed regarding the effect of tuberomammillary nucleus DBS on histamine release.15 DBS to these locations may alter vegetative functions such as sleep and appetite. Such side effects could conceivably be mitigated by varying stimulation parameters over the day-night cycle.
Basal Ganglia
The role of basal ganglia stimulation (e.g., stimulation of the globus pallidus interna or subthalamic nucleus) is well recognized as a treatment for Parkinson disease and other movement disorders. However, evidence analyzing the association of these regions with DBS-driven alertness and conscious states is sparse. Many studies of subthalamic nucleus DBS in patients with Parkinson disease debate postsurgical decline in cognition and verbal fluency.14,39,42,101,102 Among these studies, alertness was not directly evaluated by use of consistent measures. When alertness was measured by use of a reaction-time task, reaction time was decreased in Parkinson disease patients who had undergone bilateral subthalamic nucleus stimulation versus those who had undergone sham stimulation (Table 1).26 Results of studies that have stimulated both the subthalamic nucleus and another arousal site indicate that the observed improvement in reaction time may be a motor phenomenon rather than improved attentiveness.32,68
The globus pallidus interna has functional connections to the arousal systems of the basal forebrain. Unilateral stimulation of the globus pallidus interna in an anesthetized patient with cervical dystonia induced a state of wakeful unawareness (Table 1).58 This report by Moll et al. exemplifies that measuring changes in alertness, arousal, or consciousness across DBS implantation locations and indications could contribute to understanding the role of the basal ganglia in arousal networks.
Thalamus
The intralaminar nuclei have long been the predominant thalamic nuclei implicated in arousal and attention for patients in various physiological (sleep) and pathological (posttrauma/stroke coma, vegetative, minimally conscious) states.85 Numerous historical experiments with electrical stimulation of the “nonspecific” intralaminar thalamus noted behavioral and electrophysiological markers of arousal from sleep95 and trauma-induced coma.40,57 Studies, however, were limited by low patient sample sizes and imprecise stimulation-site targeting. In 1 patient experiencing “subcoma” from medial midbrain and upper brainstem infarction, for whom electrode location was verified via postmortem histology, electrical stimulation of the “thalamic unspecific activating system” (left nucleus reticularis polaris thalami) induced immediate signs of arousal for prolonged periods.82 These findings, however, have not since been investigated in animal or human studies.
More recent trials of stimulation in the centromedian and rostral intralaminar thalamic nuclei yielded optimistic results in the context of vegetative and minimally conscious states; however, application was limited. Initial studies suggested improvement of nearly 50% of patients from a vegetative state after chronic centromedian–pars fascicularis stimulation83; later studies with increased sample sizes found greater recovery from a minimally conscious state (Table 1).100 A major limitation of these studies was absence of an effective control group to show that the behavioral improvements were not the result of spontaneous improvement, which is known to occur at varying rates for patients in vegetative and minimally conscious states.50 In an attempt to address this issue, analysis of electrophysiological features between a larger cohort of patients who did and did not receive chronic centromedian–pars fascicularis DBS was performed.99 However, a complete lack of any recovery among those who did not receive chronic stimulation suggested the possibility of selection bias, whereby patients for whom expected clinical outcome was better were chosen for the procedure.99
In contrast to the numerous centromedian–pars fascicularis DBS trials, few human or animal studies have experimentally stimulated the rostral intralaminar nuclei. In rats, electrical stimulation of the rostral intralaminar nuclei has led to improved visual object recognition, arousal behaviors, and memory retrieval (Table 1).52,79 According to a report of a case for which multiple internal controls were used, a patient who had been in a minimally conscious state for 6 years showed functional improvement in numerous components of the revised Coma Recovery Scale, including arousal, after bilateral high-frequency central thalamic stimulation targeting the central lateral nucleus (Table 1).76,77 It has been hypothesized that anterior forebrain network integrity was essential to the results noted in this case.78 Additional work with this approach is forthcoming and should be pursued in future studies.
Although studies investigating intralaminar thalamic DBS and level of consciousness in patients with epilepsy are rare, this procedure shows promise. Numerous early studies of humans suggested mixed results of chronic centromedian nucleus stimulation to reduce seizure frequency (Table 1).28,86,87,90,91 In some cases, increased alertness as a side effect of long-term stimulation was noted.89 Of note, low-frequency stimulation of the centromedian nucleus increased cortical slowing and spike-and-wave activity and was associated behaviorally with lip smacking and unresponsiveness, but high-frequency stimulation was associated with cortical EEG desynchronization.88 For 1 patient, bilateral centromedian nucleus stimulation interrupted refractory status epilepticus (Table 1).84 However, this patient remained in a persistent vegetative state despite stimulation, suggesting anatomical or functional disruption to the arousal network, or refuting prior evidence that centromedian–pars fascicularis DBS has a beneficial effect on level of consciousness.
Rostral intralaminar thalamic stimulation has not been attempted in human patients with epilepsy. However, in an animal model of temporal lobe seizure, neuroimaging, electrophysiological, and neurochemical data demonstrated decreased intralaminar thalamic functional MRI signal during and after complex partial seizures, decreased firing of cholinergic neurons in the pedunculopontine tegmental nucleus and basal forebrain, and decreased concentrations of choline in the intralaminar thalamus and cortex.62 Another study that used this model provided evidence that bilateral high-frequency central lateral thalamic stimulation improved electrophysiological markers and spontaneous exploratory behaviors during the postictal period (Table 1).35 Noting the importance of specific neuromodulatory systems, optogenetic excitatory stimulation of efferents from the pedunculopontine tegmental nucleus to the intralaminar thalamus dramatically reduced cortical slowing during complex partial seizures.33 Combined, these preclinical studies support activation of the intralaminar thalamus as a promising neurosurgical target for improving level of consciousness during and after seizures.
Basal Forebrain
Anatomically, the basal forebrain includes cholinergic subcortical structures such as the substantia innominata, the vertical and horizontal limbs of the diagonal band of Broca, the medial septum and nucleus basalis of Meynert, and the dopaminergic ventral pallidum–nucleus accumbens nuclei. In parallel with the central thalamus, the basal forebrain also receives input from nuclei of the reticular activating system.19 A substantial amount of literature about cognition in dementia and sleep physiology reports that the basal forebrain plays an integral role in arousal. For example, in a dog model of narcolepsy, cholinergic activation altered the level of arousal as well as muscle tone opposite to that of control dogs, suggesting a close link between REM sleep and arousal.64 Similarly, chemical stimulation of the cholinergic basal forebrain with neurotensin increased EEG desynchronization, which correlated behaviorally with increased wakefulness, paradoxical/REM sleep, and loss of slow-wave sleep.11 Optogenetic activation of cholinergic basal forebrain neurons was sufficient to alter the cortical state from slow-wave sleep, but not REM sleep, to wakefulness and prolonged poststimulation arousal (Table 1).38,43 DBS of the cholinergic basal fore-brain nucleus basalis of Meynert has been proposed for treatment of dementia because of the cellular loss noted in the basal forebrain and subsequent decline in cortical acetylcholine.34 In a patient with Parkinson-dementia syndrome, stimulation of the nucleus basalis and subthalamic nucleus induced improvements in concentration, alertness, and motivation as well as motor symptoms; stimulation of the subthalamic nucleus alone improved motor symptoms only (Table 1).32
Further evidence shows that impaired arousal of patients in the ictal and postictal states involves decreased activity in the basal forebrain caused by lack of multiple neuromodulatory drives. The output of the nucleus basalis is influenced by noradrenergic and cholinergic inputs to result in cortical desynchronization.6,19,33 In a temporal lobe seizure model of epilepsy, decreased firing of cholinergic neurons in the nucleus basalis during seizures impaired consciousness in the same fashion as cholinergic neurons in the pedunculopontine tegmental area.62 This finding parallels findings of decreased serotonergic neuronal firing in the raphe, which also innervates the nucleus basalis, in the same animal model of partial seizures.103
Summary and Conclusions
We reviewed DBS targets that might have therapeutic potential for patients who experience deficits in consciousness after seizures (Table 1). There is evidence that in the upper brainstem, stimulation of the pedunculopontine tegmental nucleus leads to improvements in attentiveness and changes in sleep architecture. Stimulation studies of anesthetized patients indicate that alertness might be regulated by the ventral tegmental area, locus coeruleus, and upper brainstem reticular formation including the pontine nucleus oralis. Another area of interest is the tuberomammillary nucleus of the posterior hypothalamus; its stimulation protected against seizure occurrence and increased active wakefulness. In addition, increased wakefulness has also been reported after tuberomammillary nucleus DBS in humans. It should be noted, however, that stimulation of the orexin efferents from the lateral hypothalamus may also contribute to the observed wakefulness. In the basal ganglia, DBS of the subthalamic nucleus and the globus pallidus interna is already widely used to treat movement disorders; however, data regarding effects on level of consciousness are limited, given the broad clinical use of these procedures, suggesting avenues for future inquiry. The intralaminar thalamic nuclei are attractive targets because of their surgical accessibility and prior applications in patients with epilepsy and a minimally conscious state; bilateral central thalamic DBS in a minimally conscious patient improved features of arousal. In an animal model, bilateral central lateral thalamic stimulation reversed postictal cortical slowing and behavioral freezing.35 Last, the cholinergic basal forebrain nuclei are clinically feasible and novel targets for improving level of consciousness. Stimulation increases arousal in animal models of disordered sleep and has improved alertness in a patient with dementia, while also proving to be safe. However, no prior studies have been conducted with regard to seizures or epilepsy.
The many anatomical regions implicated in level of consciousness imply parallel networks with a currently unknown degree of integration. The interaction of these targets also highlights the convergence and cross-communication among 5 major neuromodulatory systems that are involved in supporting the level of consciousness. DBS target selection for patients with impaired consciousness during seizures should be approached as a complex problem; optimal targeting for any particular patient will probably vary according to epilepsy and seizure classification. The understanding of how seizure types interact with the consciousness network may inform the targeting decisions.
DBS in arousal-related nuclei may decrease the risk for sudden unexplained death in patients with epilepsy.103 Recent studies have indicated that dual deficits in dysregulated consciousness and breathing while in the ictal and postictal states may underlie this risk.54,103 For example, chemical stimulation of the region within the medullary raphe, one of the serotonergic nuclei known to participate in arousal and respiratory control, produced prolonged apnea.92 The medullary raphe was found to have multiple afferents from thermoregulatory, cardiovascular, nociceptive, and respiratory centers, indicating its integrative role in airway protection.92 A recent study in humans suggested that stimulation of the unilateral amygdala, which receives input from a major chemosensory nuclei (nucleus tractus solitarius), also produced asymptomatic apnea.18 Although directly modulating respiratory centers may pose challenges for investigating the mechanisms of sudden death during epilepsy and its treatments, the communication between respiratory circuitry and arousal circuitry provides potential therapeutic targets.
We have proposed numerous nodes within the consciousness network that may be investigated to modulate level of consciousness in the context of epilepsy (Fig. 2). Many of these areas have been targeted for indications other than improving consciousness; therefore, levels of arousal and cognitive function have been only secondarily evaluated (Table 1). The ethical aspect of DBS use for improving consciousness is prevalent in the literature about traumatic brain injury; although outside the scope of this review, we recognize the importance of such considerations when potentially restoring awareness during a seizure. Some of these areas have been investigated in animal models only; although objective data about safety in humans for DBS in these nuclei are scarce, the anatomical locations of these areas are in close proximity to more frequently targeted areas. In addition to the dramatic improvement in quality of life that could be gained by maintaining consciousness during seizures, some data indirectly suggest that improvement in level of consciousness decreases seizure frequency in patients with multiple seizure types.89 It is our opinion that use of DBS in these areas should be explored further with the aim of measuring level of consciousness by use of specific assessments such as the modified Coma Recovery Scale or comprehensive neuropsychiatric testing.
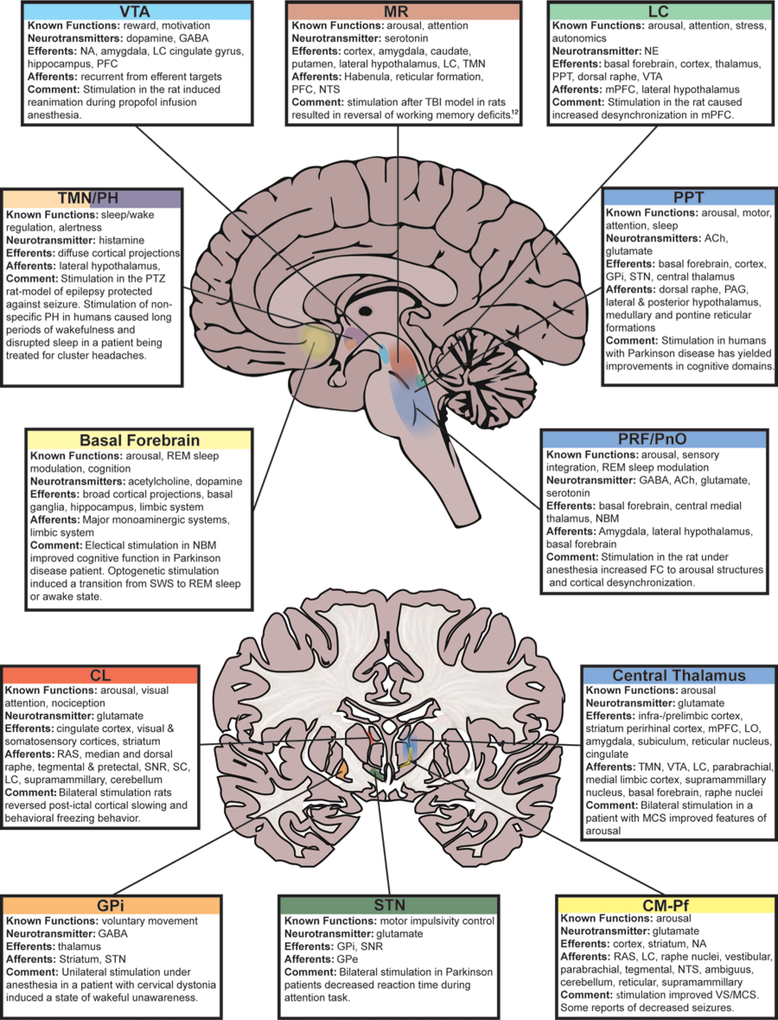
FIG. 2.
Anatomical localizations of key nuclei thought to play a role in level of consciousness. See text for references. ACh = acetylcholine; CL = central lateral; CM-Pf = centromedian-parafascicular thalamus; FC = functional connectivity; GPe = globus pallidus externa; GPi = globus pallidus interna; LC = locus coeruleus; LO = lateral orbitofrontal cortex; MCS minimally conscious state; mPFC = medial prefrontal cortex; MR = midbrain raphe; NA = nucleus accumbens; NBM = nucleus basalis of Meynert; NE = norepinephrine; NTS = nucleus tractus solitarius; PAG = periaqueductal gray matter; PFC = prefrontal cortex; PH = posterior hypothalamus; PnO = pontine nucleus oralis; PPT = pedunculopontine tegmental area; PRF = pontine reticular formation; PTZ = pentylenetetrazol; RAS = reticular activating system; SC = superior colliculus; SNR = substantia nigra reticularis; STN = subthalamic nucleus; SWS = slow-wave sleep; TBI = traumatic brain injury; TMN = tubulomammillary nucleus; VS = vegetative state; VTA = ventral tegmental area.
Acknowledgment
We thank Dr. George Richerson for graciously sharing work in review.
DISCLOSURE
This work was supported by Howard Hughes Medical Institutes—Citizens United for Research in Epilepsy Medical Student Fellowships (to A.G. and A.K), NIH R01 NS066974 (to H.B.), R21 NS083783 (to H.B.), the Loughridge-Williams Foundation, and by the Betsy and Jonathan Blattmachr Family. Dr. Willie is a consultant for Monteris Medical and MRI Interventions. Dr. Gerard is a consultant for Medtronic.
ABBREVIATIONS
- DBS
deep brain stimulation
- EEG
electroencephalography
- GABA
gamma-aminobutyric acid
- REM
rapid eye movement
References
- 1.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L: Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 450:420–424, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agnesi F, Johnson MD, Vitek JL: Deep brain stimulation: how does it work? Handb Clin Neurol 116:39–54, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Arnulf I, Ferraye M, Fraix V, Benabid AL, Chabardès S, Goetz L, et al. : Sleep induced by stimulation in the human pedunculopontine nucleus area. Ann Neurol 67:546–549, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Bai X, Vestal M, Berman R, Negishi M, Spann M, Vega C, et al. : Dynamic time course of typical childhood absence seizures: EEG, behavior, and functional magnetic resonance imaging. J Neurosci 30:5884–5893, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berman R, Negishi M, Vestal M, Spann M, Chung MH, Bai X, et al. : Simultaneous EEG, fMRI, and behavior in typical childhood absence seizures. Epilepsia 51:2011–2022, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berridge CW, Stellick RL, Schmeichel BE: Wake-promoting actions of medial basal forebrain beta2 receptor stimulation. Behav Neurosci 119:743–751, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Blik V: Electric stimulation of the tuberomamillary nucleus affects epileptic activity and sleep-wake cycle in a genetic absence epilepsy model. Epilepsy Res 109:119–125, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Blumenfeld H: Impaired consciousness in epilepsy. Lancet Neurol 11:814–826, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blumenfeld H, McNally KA, Vanderhill SD, Paige AL, Chung R, Davis K, et al. : Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex 14:892–902, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Blumenfeld H, Taylor J: Why do seizures cause loss of consciousness? Neuroscientist 9:301–310, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Cape EG, Manns ID, Alonso A, Beaudet A, Jones BE: Neurotensin-induced bursting of cholinergic basal forebrain neurons promotes gamma and theta cortical activity together with waking and paradoxical sleep. J Neurosci 20:8452–8461, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carballosa Gonzalez MM, Blaya MO, Alonso OF, Bramlett HM, Hentall ID: Midbrain raphe stimulation improves behavioral and anatomical recovery from fluid-percussion brain injury. J Neurotrauma 30:119–130, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, et al. : Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci 13:1526–1533, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castelli L, Rizzi L, Zibetti M, Angrisano S, Lanotte M, Lopiano L: Neuropsychological changes 1-year after subthalamic DBS in PD patients: A prospective controlled study. Parkinsonism Relat Disord 16:115–118, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Chang SY, Jay T, Muñoz J, Kim I, Lee KH: Wireless fast-scan cyclic voltammetry measurement of histamine using WINCS—a proof-of-principle study. Analyst (Lond) 137:2158–2165, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiken S, Nambu A: Disrupting neuronal transmission: mechanism of DBS? Front Syst Neurosci 8:33, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB: Afferents to the ventrolateral preoptic nucleus. J Neurosci 22:977–990, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dlouhy BJ, Gehlbach BK, Kreple CJ, Kawasaki H, Oya H, Buzza C, et al. : Mechanism for sudden unexpected deathin epilepsy: the amygdala as a pathway to seizure-induced apnea, respiratory agnosia and sudden death. Neurosurgery 61 (Suppl 1):223, 2014 [Google Scholar]
- 19.Dringenberg HC, Olmstead MC: Integrated contributions of basal forebrain and thalamus to neocortical activation elicited by pedunculopontine tegmental stimulation in urethane-anesthetized rats. Neuroscience 119:839–853, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Englot DJ, Blumenfeld H: Consciousness and epilepsy: why are complex-partial seizures complex? Prog Brain Res 177:147–170, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Englot DJ, Mishra AM, Mansuripur PK, Herman P, Hyder F, Blumenfeld H: Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci 28:9066–9081, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Englot DJ, Modi B, Mishra AM, DeSalvo M, Hyder F, Blumenfeld H: Cortical deactivation induced by subcortical network dysfunction in limbic seizures. J Neurosci 29:13006–13018, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Englot DJ, Yang L, Hamid H, Danielson N, Bai X, Marfeo A, et al. : Impaired consciousness in temporal lobe seizures: role of cortical slow activity. Brain 133:3764–3777, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fenoy AJ, Goetz L, Chabardès S, Xia Y: Deep brain stimulation: are astrocytes a key driver behind the scene? CNS Neurosci Ther 20:191–201, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferraye MU, Debû B, Fraix V, Krack P, Charbardès S, Seigneuret E, et al. : Subthalamic nucleus versus pedunculo pontine nucleus stimulation in Parkinson disease: synergy or antagonism? J Neural Transm 118:1469–1475, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Fimm B, Heber IA, Coenen VA, Fromm C, Noth J, Kronenbuerger M: Deep brain stimulation of the subthalamic nucleus improves intrinsic alertness in Parkinson’s disease. Mov Disord 24:1613–1620, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, et al. : Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 51:899–908, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Fisher RS, Uematsu S, Krauss GL, Cysyk BJ, McPherson R, Lesser RP, et al. : Placebo-controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia 33:841–851, 1992 [DOI] [PubMed] [Google Scholar]
- 29.Fisher RS, Velasco AL: Electrical brain stimulation for epilepsy. Nat Rev Neurol 10:261–270, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Franzini A, Broggi G, Cordella R, Dones I, Messina G: Deep-brain stimulation for aggressive and disruptive behavior. World Neurosurg 80:S29.e11–S29.e24, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Franzini A, Messina G, Marras C, Villani F, Cordella R, Broggi G: Deep brain stimulation of two unconventional targets in refractory non-resectable epilepsy. Stereotact Funct Neurosurg 86:373–381, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Freund HJ, Kuhn J, Lenartz D, Mai JK, Schnell T, Klosterkoetter J, et al. : Cognitive functions in a patient with Parkinson-dementia syndrome undergoing deep brain stimulation. Arch Neurol 66:781–785, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Furman M, Zhan Q, Lerner BA, Meng J, Motelow JE, Li W, et al. : Optogenetic stimulation of cholinergic mesopontine neurons for preventing cortical dys-function during seizures. Presented at the Society for Neuroscience 2013 Annual Meeting, San Diego, California, 2013 (Poster) (http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=c2f32be5-bb05-4432-88a0-f38b71f0101c&cKey=43681027-1963-43cd-9aa2-6baeb7b8f777&mKey=8d2a5bec-4825-4cd6-9439-b42bb151d1cf) [Google Scholar]
- 34.Gratwicke J, Kahan J, Zrinzo L, Hariz M, Limousin P, Foltynie T, et al. : The nucleus basalis of Meynert: a new target for deep brain stimulation in dementia? Neurosci Biobehav Rev 37:2676–2688, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Gummadavelli A, Motelow JE, Smith N, Zhan Q, Schiff ND, Blumenfeld H: Thalamic stimulation to improve level of consciousness after seizures: evaluation of electrophysiology and behavior. Epilepsia 56:114–124, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haas H, Panula P: The role of histamine and the tuber-omamillary nucleus in the nervous system. Nat Rev Neurosci 4:121–130, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Hamani C, Nobrega JN: Preclinical studies modeling deep brain stimulation for depression. Biol Psychiatry 72:916–923, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han Y, Shi YF, Xi W, Zhou R, Tan ZB, Wang H, et al. : Selective activation of cholinergic basal forebrain neurons induces immediate sleep-wake transitions. Curr Biol 24:693–698, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Harati A, Müller T: Neuropsychological effects of deep brain stimulation for Parkinson’s disease. Surg Neurol Int 4 (Suppl 6):S443–S447, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hassler R, Ore GD, Dieckmann G, Bricolo A, Dolce G: Behavioural and EEG arousal induced by stimulationof unspecific projection systems in a patient with post-traumatic apallic syndrome. Electroencephalogr Clin Neurophysiol 27:306–310, 1969 [DOI] [PubMed] [Google Scholar]
- 41.Heck CN, King-Stephens D, Massey AD, Nair DR, Jobst BC, Barkley GL, et al. : Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia 55:432–441, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heo JH, Lee KM, Paek SH, Kim MJ, Lee JY, Kim JY, et al. : The effects of bilateral subthalamic nucleus deep brain stimulation (STN DBS) on cognition in Parkinson disease. J Neurol Sci 273:19–24, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Irmak SO, de Lecea L: Basal forebrain cholinergic modulation of sleep transitions. Sleep 37:1941–1951, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jain SV, Glauser TA: Effects of epilepsy treatments on sleep architecture and daytime sleepiness: an evidence-based review of objective sleep metrics. Epilepsia 55:26–37, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Kang G, Lowery MM: Effects of antidromic and orthodromic activation of STN afferent axons during DBS in Parkinson’s disease: a simulation study. Front Comput Neurosci 8:32, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karachi C, Grabli D, Bernard FA, Tandé D, Wattiez N, Belaid H, et al. : Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J Clin Invest 120:2745–2754, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kossoff EH, Ritzl EK, Politsky JM, Murro AM, Smith JR, Duckrow RB, et al. : Effect of an external responsive neurostimulator on seizures and electrographic discharges during subdural electrode monitoring. Epilepsia 45:1560–1567, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Kovac S, Wright MA, Eriksson SH, Zrinzo L, Matharu M, Walker MC: The effect of posterior hypothalamus region deep brain stimulation on sleep. Cephalalgia 34:219–223, 2014 [DOI] [PubMed] [Google Scholar]
- 49.Labar D, Dakov P, Kobylarz E, Nikolov B, Schwartz TH, Fisher S: Effects of responsive electrical brain stimulation on intracranial electroencephalogram spikes. Neuromodulation 16:355–362, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Lammi MH, Smith VH, Tate RL, Taylor CM: The minimally conscious state and recovery potential: a follow-up study 2 to 5 years after traumatic brain injury. Arch Phys Med Rehabil 86:746–754, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Lin JS, Sakai K, Jouvet M: Hypothalamo-preoptic histaminergic projections in sleep-wake control in the cat. Eur J Neurosci 6:618–625, 1994 [DOI] [PubMed] [Google Scholar]
- 52.Mair RG, Hembrook JR: Memory enhancement with event-related stimulation of the rostral intralaminar thalamic nuclei. J Neurosci 28:14293–14300, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marzo A, Totah NK, Neves RM, Logothetis NK, Eschenko O: Unilateral electrical stimulation of rat locus coeruleus elicits bilateral response of norepinephrine neurons and sustained activation of medial prefrontal cortex. J Neurophysiol 111:2570–2588, 2014 [DOI] [PubMed] [Google Scholar]
- 54.Massey CA, Sowers LP, Dlouhy BJ, Richerson GB: Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nat Rev Neurol 10:271–282, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazzone P, Sposato S, Insola A, Scarnati E: The clinical effects of deep brain stimulation of the pedunculopontine tegmental nucleus in movement disorders may not be related to the anatomical target, leads location, and setup of electrical stimulation. Neurosurgery 73:894–906, 2013 [DOI] [PubMed] [Google Scholar]
- 56.McCarren HS, Chalifoux MR, Han B, Moore JT, Meng QC, Baron-Hionis N, et al. : α2-Adrenergic stimulation of the ventrolateral preoptic nucleus destabilizes the anesthetic state. J Neurosci 34:16385–16396, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McLardy T, Ervin F, Mark V, Scoville W, Sweet W: Attempted inset-electrodes-arousal from traumatic coma: neuropathological findings. Trans Am Neurol Assoc 93:25–30, 1968 [PubMed] [Google Scholar]
- 58.Moll CK, Sharott A, Hamel W, Münchau A, Buhmann C, Hidding U, et al. : Waking up the brain: a case study of stimulation-induced wakeful unawareness during anaesthesia. Prog Brain Res 177:125–145, 2009 [DOI] [PubMed] [Google Scholar]
- 59.Morita H, Hass CJ, Moro E, Sudhyadhom A, Kumar R, Okun MS: Pedunculopontine nucleus stimulation: where are we now and what needs to be done to move the field forward? Front Neurol 5:243, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morrell MJ: Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 77:1295–1304, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Moruzzi G, Magoun HW: Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol 1:455–473, 1949 [PubMed] [Google Scholar]
- 62.Motelow JE, Li W, Zhan Q, Mishra AM, Sachdev RN, Liu G, et al. : Decreased subcortical cholinergic arousal in focal seizures. Neuron 85:561–572, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nishida N, Huang ZL, Mikuni N, Miura Y, Urade Y, Hashimoto N: Deep brain stimulation of the posterior hypothalamus activates the histaminergic system to exert anti-epileptic effect in rat pentylenetetrazol model. Exp Neurol 205:132–144, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Nishino S, Tafti M, Reid MS, Shelton J, Siegel JM, Dement WC, et al. : Muscle atonia is triggered by cholinergic stimulation of the basal forebrain: implication for the pathophysiology of canine narcolepsy. J Neurosci 15:4806–4814, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Norden AD, Blumenfeld H: The role of subcortical structures in human epilepsy. Epilepsy Behav 3:219–231, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Pahapill PA, Lozano AM: The pedunculopontine nucleus and Parkinson’s disease. Brain 123:1767–1783, 2000 [DOI] [PubMed] [Google Scholar]
- 67.Parmentier R, Ohtsu H, Djebbara-Hannas Z, Valatx JL, Watanabe T, Lin JS: Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: evidence for the role of brain histamine in behavioral and sleep-wake control. J Neurosci 22:7695–7711, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peppe A, Pierantozzi M, Baiamonte V, Moschella V, Caltagirone C, Stanzione P, et al. : Deep brain stimulation of pedunculopontine tegmental nucleus: role in sleep modulation in advanced Parkinson disease patients: one-year follow-up. Sleep 35:1637–1642, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pillay S, Liu X, Baracskay P, Hudetz AG: Brainstem stimulation increases functional connectivity of basal forebrainparalimbic network in isoflurane-anesthetized rats. Brain Connect 4:523–534, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Plum F, Posner JB: The Diagnosis of Stupor and Coma, ed 3. Philadelphia: FA Davis, 1982 [Google Scholar]
- 71.Rolston JD, Englot DJ, Wang DD, Shih T, Chang EF: Comparison of seizure control outcomes and the safety of vagus nerve, thalamic deep brain, and responsive neuro-stimulation: evidence from randomized controlled trials. Neurosurg Focus 32(3):E14, 2012 [DOI] [PubMed] [Google Scholar]
- 72.Rye DB: Contributions of the pedunculopontine region to normal and altered REM sleep. Sleep 20:757–788, 1997 [DOI] [PubMed] [Google Scholar]
- 73.Sander JW: The epidemiology of epilepsy revisited. Curr Opin Neurol 16:165–170, 2003 [DOI] [PubMed] [Google Scholar]
- 74.Saper CB, Chou TC, Scammell TE: The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci 24:726–731, 2001 [DOI] [PubMed] [Google Scholar]
- 75.Saper CB, Scammell TE, Lu J: Hypothalamic regulation of sleep and circadian rhythms. Nature 437:1257–1263, 2005 [DOI] [PubMed] [Google Scholar]
- 76.Schiff ND: Central thalamic deep brain stimulation for support of forebrain arousal regulation in the minimally conscious state. Handb Clin Neurol 116:295–306, 2013 [DOI] [PubMed] [Google Scholar]
- 77.Schiff ND, Giacino JT, Kalmar K, Victor JD, Baker K, Gerber M, et al. : Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature 448:600–603, 2007 [DOI] [PubMed] [Google Scholar]
- 78.Shah SA, Schiff ND: Central thalamic deep brain stimulation for cognitive neuromodulation: a review of proposed mechanisms and investigational studies. Eur J Neurosci 32:1135–1144, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shirvalkar P, Seth M, Schiff ND, Herrera DG: Cognitive enhancement with central thalamic electrical stimulation. Proc Natl Acad Sci U S A 103:17007–17012, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Solt K, Van Dort CJ, Chemali JJ, Taylor NE, Kenny JD, Brown EN: Electrical stimulation of the ventral tegmental area induces reanimation from general anesthesia. Anesthesiology 121:311–319, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stefani A, Peppe A, Galati S, Bassi MS, D’Angelo V, Pierantozzi M: The serendipity case of the pedunculopontine nucleus low-frequency brain stimulation: chasing a gait response, finding sleep, and cognition improvement. Front Neurol 4:68, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sturm V, Kühner A, Schmitt HP, Assmus H, Stock G: Chronic electrical stimulation of the thalamic unspecific activating system in a patient with coma due to midbrain and upper brain stem infarction. Acta Neurochir (Wien) 47:235–244, 1979 [DOI] [PubMed] [Google Scholar]
- 83.Tsubokawa T, Yamamoto T, Katayama Y, Hirayama T, Maejima S, Moriya T: Deep-brain stimulation in a persistent vegetative state: follow-up results and criteria for selection of candidates. Brain Inj 4:315–327, 1990 [DOI] [PubMed] [Google Scholar]
- 84.Valentín A, Nguyen HQ, Skupenova AM, Agirre-Arrizubieta Z, Jewell S, Mullatti N, et al. : Centromedian thalamic nuclei deep brain stimulation in refractory status epilepticus. Brain Stimulat 5:594–598, 2012 [DOI] [PubMed] [Google Scholar]
- 85.Van der Werf YD, Witter MP, Groenewegen HJ: The intra-laminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev 39:107–140, 2002 [DOI] [PubMed] [Google Scholar]
- 86.Velasco F, Velasco M, Ogarrio C, Fanghanel G: Electrical stimulation of the centromedian thalamic nucleus in the treatment of convulsive seizures: a preliminary report. Epilepsia 28:421–430, 1987 [DOI] [PubMed] [Google Scholar]
- 87.Velasco F, Velasco M, Velasco AL, Jimenez F, Marquez I, Rise M: Electrical stimulation of the centromedian thalamic nucleus in control of seizures: long-term studies. Epilepsia 36:63–71, 1995 [DOI] [PubMed] [Google Scholar]
- 88.Velasco M, Velasco F, Velasco AL, Brito F, Jiménez F, Marquez I, et al. : Electrocortical and behavioral responses produced by acute electrical stimulation of the human centromedian thalamic nucleus. Electroencephalogr Clin Neurophysiol 102:461–471, 1997 [DOI] [PubMed] [Google Scholar]
- 89.Velasco M, Velasco F, Velasco AL, Jiménez F, Brito F, Márquez I: Acute and chronic electrical stimulation of the centromedian thalamic nucleus: modulation of reticulo-cortical systems and predictor factors for generalized seizure control. Arch Med Res 31:304–315, 2000 [DOI] [PubMed] [Google Scholar]
- 90.Velasco M, Velasco F, Velasco AL, Luján M, Vázquezdel Mercado J: Epileptiform EEG activities of the centro-median thalamic nuclei in patients with intractable partial motor, complex partial, and generalized seizures. Epilepsia 30:295–306, 1989 [DOI] [PubMed] [Google Scholar]
- 91.Velasco M, Velasco F, Velasco AL, Velasco G, Jiménez F: Effect of chronic electrical stimulation of the centromedian thalamic nuclei on various intractable seizure patterns: II. Psychological performance and background EEG activity. Epilepsia 34:1065–1074, 1993 [DOI] [PubMed] [Google Scholar]
- 92.Verner TA, Pilowsky PM, Goodchild AK: Retrograde projections to a discrete apneic site in the midline medulla oblongata of the rat. Brain Res 1208:128–136, 2008 [DOI] [PubMed] [Google Scholar]
- 93.Vickrey BG, Berg AT, Sperling MR, Shinnar S, Langfitt JT, Bazil CW, et al. : Relationships between seizure severity and health-related quality of life in refractory localization-related epilepsy. Epilepsia 41:760–764, 2000 [DOI] [PubMed] [Google Scholar]
- 94.von Krosigk M, Bal T, McCormick DA: Cellular mechanisms of a synchronized oscillation in the thalamus. Science 261:361–364, 1993 [DOI] [PubMed] [Google Scholar]
- 95.Westmoreland BF, Groover RV, Klass DW: Spontaneous sleep and induced arousal. A depth-electroencephalographic study. J Neurol Sci 28:353–360, 1976 [DOI] [PubMed] [Google Scholar]
- 96.Whiting DM, Tomycz ND, Bailes J, de Jonge L, Lecoultre V, Wilent B, et al. : Lateral hypothalamic area deep brain stimulation for refractory obesity: a pilot study with preliminary data on safety, body weight, and energy metabolism. J Neurosurg 119:56–63, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wiebe S, Blume WT, Girvin JP, Eliasziw M: A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 345:311–318, 2001 [DOI] [PubMed] [Google Scholar]
- 98.Wu DC, Zhu-Ge ZB, Yu CY, Fang Q, Wang S, Jin CL, et al. : Low-frequency stimulation of the tuberomammillary nucleus facilitates electrical amygdaloid-kindling acquisition in Sprague-Dawley rats. Neurobiol Dis 32:151–156, 2008 [DOI] [PubMed] [Google Scholar]
- 99.Yamamoto T, Katayama Y, Obuchi T, Kobayashi K, Oshima H, Fukaya C: Deep brain stimulation and spinal cord stimulation for vegetative state and minimally conscious state. World Neurosurg 80:S30.e1–S30.e9, 2013 [DOI] [PubMed] [Google Scholar]
- 100.Yamamoto T, Kobayashi K, Kasai M, Oshima H, Fukaya C, Katayama Y: DBS therapy for the vegetative state and minimally conscious state. Acta Neurochir Suppl 93:101–104, 2005 [DOI] [PubMed] [Google Scholar]
- 101.York MK, Dulay M, Macias A, Levin HS, Grossman R, Simpson R, et al. : Cognitive declines following bilateral subthalamic nucleus deep brain stimulation for the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry 79:789–795, 2008 [DOI] [PubMed] [Google Scholar]
- 102.Zangaglia R, Pacchetti C, Pasotti C, Mancini F, Servello D, Sinforiani E, et al. : Deep brain stimulation and cognitive functions in Parkinson’s disease: A three-year controlled study. Mov Disord 24:1621–1628, 2009 [DOI] [PubMed] [Google Scholar]
- 103.Zhan Q, Buchanan G, Motelow J, Serout F, Chen W, Gummadavelli A, et al. : Peri-ictal impairment of brainstem 5-HT neurons: Insight into depressed arousal, reduced ventilation and sudden unexpected death in epilepsy (SUDEP), in American Epilepsy Society 2014 Annual Meeting Program Book. West Hartford, CT: AES, 2014, Poster #1.164 [Google Scholar]