Abstract
Introduction:
MUC16 is overexpressed in multiple cancers and plays an important role in tumorigenicity and acquired resistance to therapy.
Area covered:
In this review, we describe the role of MUC16 under normal physiological conditions and during tumorigenesis. First, we provide a summary of research on MUC16 from its discovery as CA125 to present anti-MUC16 therapy trials that are currently in the initial phases of clinical testing. Finally, we discuss the reasons for the limited effectiveness of these therapies and discuss the direction and focus of future research.
Expert opinion:
Apart from its protective role in normal physiology, MUC16 contributes to disease progression and metastasis in several malignancies. Due to its aberrant overexpression, it is a promising target for diagnosis and therapy. Cleavage and shedding of the extracellular domain of MUC16 is the major barrier for efficient targeting of MUC16 expressing cancers. Concerted efforts should be undertaken to target the non-cleaved cell surface retained portion of MUC16. Such efforts should be accompanied by basic research to understand MUC16 cleavage and decipher the functioning of MUC16 cytoplasmic tail. While previous efforts to activate anti-MUC16 immune response using anti-CA125 idiotype antibodies have met with limited success, neo-antigen peptide vaccines show promise for MUC16 immunotherapy.
Keywords: MUC16, cancer Therapy, Mucins, Immunotherapy, Targeted Therapy
1. Introduction
MUC16 (previously known as CA125) has been extensively used as a biomarker for ovarian cancer, and its expression has been associated with disease progression. Key advances have revealed the structure and functions of this protein and the role it plays in fundamental processes, including protection of the epithelium and human carcinogenesis. The expression of mucins in resting, normal polarized cells is intricately controlled, with expression restricted on the apical membranes of exposed epithelia. Following loss of cell polarity during carcinogenesis, mucins are expressed all over the cell surface, and become available to interact with several growth factor receptors, that are typically restricted to the basolateral surface, and modulate their downstream signaling in various cancers [1, 2, 3]. Aberrant overexpression of MUC16 has been observed in several human malignancies, including ovarian, pancreatic, breast, and lung [4, 5, 6, 7]. Due to their aberrant overexpression and functional involvement, MUC16 and its ligands have emerged as potential targets for therapeutic intervention using monoclonal antibodies and immunotherapy.
2. History
CA125 was discovered in 1981 when Bast et al. [8] developed a monoclonal antibody OC125, that selectively recognized a high molecular weight protein expressed on the surface of ovarian cancer cells. The reactive antigen was subsequently found to be elevated in the sera of a majority of ovarian cancer patients which formed the basis of a radioimmunoassay test [5, 9, 10] (Figure 1). CA125 was found to be a sensitive biomarker for ovarian cancer patients in specific contexts [11, 12, 13]. Setting the benchmark cutoff of 35U/ml, it was found that 1% of normal women had elevated CA125, while 6% of those with benign disease and 28% of those with non-gynecological cancers had elevated antigen levels. In contrast, the antigen was elevated in more than 80% of women with non-mucinous ovarian cancer [9]. The levels of CA125 correlated with disease progression in initial trials [9, 11, 12]. However, it was soon apparent that CA125 was not a very sensitive and specific marker for ovarian cancer since many other non-gynecological malignancies and non-malignant conditions led to elevated serum CA125 levels [13, 14, 15, 16]. It was subsequently proposed that combination with other markers such as HE4 could potentially increase the specificity and sensitivity of detecting early ovarian cancer, but satisfying results have still not been obtained [17, 18, 19]. One of the possible reasons may be the rapid clearance of mucin fragments by the hepatic reticuloendothelial system that may potentially skew the serum levels of these markers leading to decreased sensitivity and specificity [20]. Moreover, the exact biochemical identity of CA125 was not understood until 2001, when Yin et al. [21, 22] sequenced the first CA125 cDNA clone and found that it corresponded to a new mucin protein, which they named MUC16 (Figure 1).
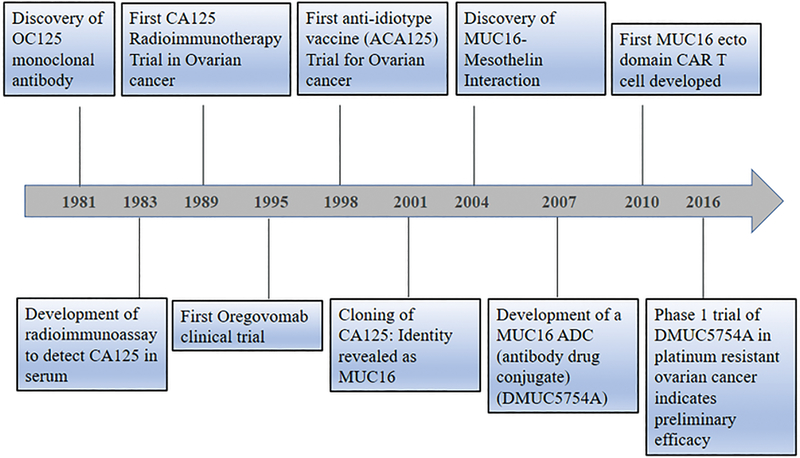
Figure 1:
Timeline depicting the significant discoveries related to MUC16 biology and therapy: MUC16 was first discovered as CA125 in Ovarian Cancer antigen in 1981. CA125 soon was found to be a biomarker for ovarian cancer and was capable of predicting recurrence and progression of the disease. Soon its utility as an immunotherapy agent was tested that resulted in the first radio immunotherapy trials in 1989. By 1995–1998, naked anti-CA125 antibodies and anti-idiotype antibodies (vaccines) were tested in ovarian cancer patients. Both resulted in the generation of potent idiotype network resulting in the robust anti-CA125 immune response. However, overall survival was not improved to a large extent. By 2001, cloning of CA125 structure revealed it to belong to mucin family and was named mucin MUC16. By 2004, a significant interaction of MUC16 with mesothelin was discovered that helped in the peritoneal metastasis of ovarian cancer cells to the abdomen. In 2007, the first antibody-drug conjugate and in 2010 the primary CAR-T cells were developed that target MUC16. In 2016, phase 1 clinical trial of the ADC showed preliminary efficacy.
3. Structure of MUC16
The first isolated CA125 cDNA clone displayed several unique characteristics. The 5,797 base pair sequence had an N-terminal region of nine tandem repeats and a characteristic C-terminal transmembrane domain. The tandem repeats were rich in serine, threonine, and proline residues and a tyrosine phosphorylation site was present downstream of transmembrane domain. They designated the newly discovered gene, MUC16, as a mucin. We now know that MUC16 is the largest mucin (Mol wt. ~ 3–5 million Da), and is the second-longest human protein after the muscle protein titin [23, 24]. MUC16 is encoded by ~179 kb gene present on the short arm of human chromosome 19 at 19p13.2. It is a type I transmembrane protein, comprising of the single membrane-spanning domain, a cytoplasmic tail and an extensively glycosylated (N- and O-glycosylation) N terminal domain consisting of tandem repeat sequence [23] (Figure 2).
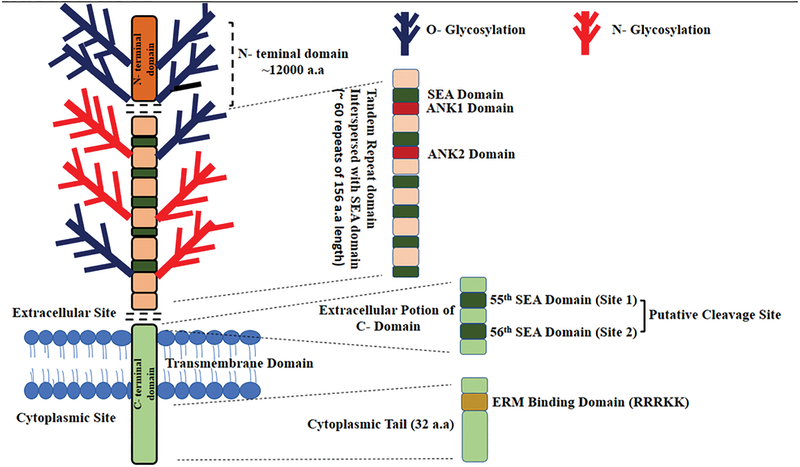
Figure 2:
Schematic representation of MUC16 structure: MUC16 contains three domains: The N-terminal domain (~12000 amino acids in length), tandem repeat domain which is interspersed with SEA domain and the C-terminal domain. The Tandem Repeat (TR) domain contains 18–60 repeats each with ~156 amino acids and has ankyrin (ANK) 1 and 2 sites along with the SEA domains. The C-terminal domain is further divided into an extracellular portion, which contains the putative cleavage site, a transmembrane domain and a cytoplasmic tail of 32 amino acid length. The cytoplasmic tail contains an ERM binding domain and a putative nuclear localization signal (RRRKK).
The N-terminal region is composed of approximately 12,000 amino acids. The tandem repeat region is composed of ~60 repeats; each repeat with 156 amino acids. The tandem repeat regions are frequently rich in serine and threonine residues that have served as potential sites for O-linked glycosylation [23, 25]. Extensive glycosylation at these sites provides clusters of carbohydrate moieties that can act as ligands for binding other molecules and are essential to MUC16 structure and function (Figure 2) [23]. Biochemical analysis of immunoaffinity purified CA125 has indicated that the carbohydrate content accounts for approximately 77% of the total weight of MUC16 and it is characterized by the presence of high amounts of carbohydrates commonly found in O-glycans, such as galactose and galactosamine [25]. The N-glycans in MUC16 are mainly associated with complex-type glycans such as bisecting type N glycans and high mannose type structures, while the O-glycans are predominantly of the core1 and core 2 types [26]. The tandem repeat domain is interspersed with sea urchin sperm, Enterokinase, and agrin (SEA) domains and contains a 14-leucine –rich repeats and two Ankyrin domains (Figure 2). The function of these 14-leucine-rich repeats and ANK domains is currently unknown. MUC16 has 56 SEA domains, where the second SEA domain has a conserved cleavage site that is similar to well characterized cleavage site in other mucins like MUC1 [27, 28]. The SEA domain of mouse MUC16 (which is homologous to membrane proximal human MUC16 SEA domain) is made up of 2 α helices and 4 β antiparallel strands, which together makes α/β sandwich [29].
The carboxy-terminus of MUC16 is divided into three major regions, the juxtamembrane domain, the transmembrane domain, and the cytoplasmic tail (CT). The CT domain is composed of 32 amino acids and contains one serine, two threonine, and three tyrosine residues that can be potential phosphorylation sites. Studies show that phosphorylation of the CT domain leads to cleavage of extracellular portion of MUC16 [30]. The MUC16 CT domains also contain a polybasic sequence of amino acids (RRRKK) predicted to bind to the ezrin/radixin/moesin (ERM) family of proteins, and that can facilitate interaction of MUC16 with numerous membrane- associated proteins and with actin-cytoskeleton [31]. It is assumed that the polybasic amino acid sequence may also serve as a nuclear localization signal (NLS), but a recent study from our lab showed transfected MUC16 domain constructs go to the nucleus independent of its NLS sequence [27].
4. Monoclonal antibodies to MUC16
Traditionally, antibodies have played a key role in deciphering biochemical knowledge of mucin structure and functions. The fine specificity of these antibodies becomes crucial especially considering the expression of highly immunogenic tandem repeat epitopes and O-linked and N-linked glycosylation. Monoclonal antibodies (mAbs) have the extremely fine specificity that sometimes becomes a limitation given that individual mAbs can detect only a sub-population of molecules that present the desired conformation. In contrast, polyclonal antibodies mostly have broader specificities, and in fact, the first cDNA of MUC16 was cloned using a rabbit polyclonal antibody that was generated against affinity-purified CA125 [22]. Most of the times different literature reports have used different tandem repeat mAbs to study MUC16 in various benign and pathological conditions. This has led to variable results acquired between different groups and being against tandemly repeated portions, these reagents often demonstrate enhanced sensitivity that is often not reflective of absolute amounts of this protein. Because most of these antibodies detect cleaved CA125 in the serum, they cannot detect the proximal residual MUC16 protein fragment remaining on the surface of the cell after cleavage. One way to solve this problem is to make monoclonal reagents to non-tandem repeat domains that are not heavily glycosylated. Several groups have recently developed monoclonal antibodies to the cleaved proximal MUC16 fragment [32, 33]. A list of available anti-MUC16 antibodies is given in Table 1 [8, 25, 33, 34, 35, 36]. Broadly, there are two distinct classes of mAbs against MUC16; one group is directed against the N-terminal tandem repeat epitopes that are themselves sub-classified into three different families, OC125 type, M11 type, OV197 type. The other group comprises the non-tandem repeat antibodies developed very recently that target the cleaved cytoplasmic tail [32, 33]. The first diagnostic immunoassay for CA125 used only OC125 antibody for both capture and detection [37]. However, second-generation assays using different monoclonal antibodies (such as OC125 as the conjugate antibody and M11 as the capture antibody) were soon developed [38, 39]. OC125 and other related monoclonal antibodies bind to glycosylation-dependent epitopes present specifically on the tandem repeat region of the molecule [35, 40]. O’Brien et al. [41] have demonstrated that OC125 and M11 bind to CA125 at the 21- amino acid loop of the tandem repeats formed by cysteine disulfide bridges. However, another study showed that neither OC125 nor M11 bound to the same synthetic 21-mer peptide sequence [42, 43]. A more recent report showed that binding of CA125 mAbs (OC125 and M11) to tandem repeat regions is not significantly affected by either N or O-linked glycosylation [44].
Table1:
Antibodies to different domains of MUC16
| MUC16 regions | Antibody | Immunogen | Predicted binding regions |
References |
|---|---|---|---|---|
| N terminal Tandem Repeat | OC125 | Purified CA125/ Cancer Cells | Tandem Repeats | 8,42 |
| 35,42 | ||||
| M11 | ||||
| 35,42 | ||||
| OV197 | ||||
| 25 | ||||
| VK8 | ||||
| 5E11 | Tandem repeat | Tandem Repeats | 34 | |
| 3A5 | Purified CA125 | Tandem repeat | 134,135 | |
| MUC16 Carboxy terminus | 5E6, 3H1 | MUC16 carboxy terminus (114 amino acids) | Juxtamembrane domain | 32 |
| 2C6 | MUC16 Cytoplasmic tail | MUC16 Cytoplasmic tail | 33 | |
| LUM16–4 | MUC16 Cytoplasmic tail peptide | MUC16 Cytoplasmic Tail | 36 | |
| 11D10 | MUC16 extra cellular domain | 82-amino acid sequence C-terminal to the mucin repeat domain | 134,135 |
5. Role of MUC16 in Normal cells
MUC16 is normally expressed by the epithelial lining of several organs such as the ocular surface, tracheal surface, female reproductive tract, the mesothelium lining of the abdominal cavity, and cervical epithelium [23, 45, 46]. It functions as a barrier against external insults and helps in lubrication and maintenance of mucosa. The extracellular portion of MUC16 extends up to 700 nm above the cell surface and forms an important constituent of glycocalyx layer that covers the apical surfaces [46, 47]. The function of MUC16 in physiological context has been primarily studied in the ocular surface epithelia, where it provides lubrication that helps in movement such as blinking [48, 49, 50]. Sumiyoshi et al. [50] revealed that this phenomenon is mediated by the O-glycans attached to the extracellular domain of MUC16. Alterations in MUC16 expression or its O-glycosylation have been observed in non-Sjögren’s Dry Eye [48]. The barrier functions of MUC16 are also facilitated by its interaction with lectins, such as galectin-1 and galectin-3 [24, 51, 52]. MUC16 was found to be localized in exosome-like vesicles contained in the secretions of human tracheobronchial ciliated epithelium, with the capability to neutralize viral infections [49]. This neutralizing capability was attributed to α−2,6-linked sialic acid residues presented on molecules like MUC16. Another study found that bacteria like Streptococcus pneumoniae secrete metalloproteinases such as ZmpC (zinc metalloproteinase) that increased shedding of MUC16 and that weakens the glycocalyx barrier, allowing bacteria to invade the mucosal tissue [53].
MUC16 expression has been reported in normal endometrial tissue, specifically in glandular and epithelium cells and also in cervical mucus [54]. Tyler et al. [54] showed that MUC16 found in the female reproductive tract can bind to various immune cells (NK cells and monocytes) via Siglec-9 (an inhibitory immune cell surface receptor), leading to suppression of maternal immune response toward fetal tissue, that can potentially regulate fetal development. Cleaved MUC16 bound to Siglec-9 was believed to be an indicator of preeclampsia onset [54]. Loss of MUC16 from the apical surface of the uterodome during the receptive phase of the reproductive cycle facilitated implantation of the embryo into the uterus [55]. MUC16 has also been implicated in microplicae (membrane protrusions) formation in corneal and conjunctival epithelial cells via its cytoplasmic tail sequence (RRRKK) binding to the ERM (ezrin/radixin/moesin) family of proteins [31]
6. Role of MUC16 in Tumorigenesis
Several studies have shown that MUC16 is overexpressed in multiple cancer types. MUC16 was often found to be one of the top three frequently mutated genes [56] and was frequently shown to be associated with enhanced growth and metastasis of cancer cells [57, 58, 59, 60]. In addition, its overexpression has been linked to worse prognosis in multiple malignancies [61, 62, 63, 64]. One of the recent studies in bladder cancer has shown that a subset of patients with abnormal MUC16 O-glycome has a worse prognosis [65].
The first evidence for a direct role of MUC16 in cancer metastasis came from the discovery that it could bind to mesothelin (a protein that lines the mesothelial lining of peritoneal cavity) with high affinity [66]. This interaction leads to peritoneal metastasis of ovarian and pancreatic cancer cells by facilitating attachment of cancer cells to the mesothelial lining [62, 67]. This mechanism is believed to be regulated by elevated MMP-7 levels and increased phosphorylation of p38 Mitogen-activated protein kinase (MAPK). MUC16 was also found to be capable of binding to selectins in a similar manner and promote metastasis of pancreatic cancer cells [68]. It is still not known whether human MUC16 can interact with mouse mesothelin and/or vice versa. Ascertaining this fact is very important since success of anti-MUC16/mesothelin immunotherapy in preclinical mouse models depends on it. An indirect evidence for this interaction is seen because human ovarian cancer cells tends to metastasize to the peritoneal walls of mouse in xenograft studies [69, 70]. Also, murine mesothelin has been shown to be functionally equivalent to its human counterpart [71].
MUC16 was shown to modulate the innate immune response against ovarian cancer cells by directly inhibiting the function of Natural Killer (NK) cells, thus aiding cancer cells to escape the host immune response. The mechanism of action seems to be via down-regulation of CD16 on NK cells, its interaction with Siglec-9 on the surface of immune cells, and by inhibition of synapse formation between NK cells and ovarian cancer cells [72, 73, 74, 75].
MUC16 has been implicated in cancer cell signaling. Knockdown of MUC16 expression inhibited the growth of ovarian and breast cancer cell lines by induction of caspase-dependent or independent apoptosis. Further, MUC16 knockdown suppressed colony-forming ability, adhesion migration and invasive ability of cancer cells [76]. A study by Boivin et al. [77] demonstrated that MUC16 can contribute to drug resistance and decreased apoptosis in ovarian cancer cell lines. MUC16 knockdown induced epithelial-mesenchymal transition in OVCAR3 [78]. The same study showed that MUC16 knockdown also activated EGFR signaling with activation of AKT and ERK1/2. Studies from our lab demonstrated that MUC16 expression increases with increasing stages of pancreatic intraepithelial neoplasia, along with its further enhancement in pancreatic tumors and metastasis [4]. It has been suggested that MUC16 expression is stronger in moderately and poorly differentiated pancreatic cancer compared to well-differentiated pancreatic cancer [4, 79]. Recently much work has been carried out on the MUC16 C-terminal domain, and multiple studies have shown that the C terminal (CT) fragment of MUC16 has oncogenic properties [57, 58, 59, 80].
Previous reports from our lab have shown that MUC16 is cleaved at a location close to the transmembrane domain. This cleavage was shown to be independent of any proteases and primary sequence [27, 81]. MUC16 CT was also shown to interact with JAK2, leading to upregulation of the expression of the stem cell genes LMO2 and NANOG in pancreatic cancer cells [57]. Giannakouros et al. [58] transfected 283 amino acid MUC16 CT in NIH3T3, demonstrating that it promoted anchorage-dependent and independent colony formation and tumorigenesis in nude mice. Further studies showed that ectopic expression of MUC16 CT confers resistance to cytotoxic drugs such as cisplatin, and that it represses apoptosis [57, 77]. The exact mechanism is not known as yet, but studies reveal ERK and AKT signaling may be involved [77, 78]. The MUC16 C-terminal interacts with the Src family of kinases and leads to deregulation of ß-catenin and E-cadherin at junctional complexes, thus aiding cancer cells in making the epithelial-mesenchymal transition [60]. Our studies also demonstrated that MUC16 interacts with JAK2 (an ezrin/radixin/moesin domain-containing protein), induce phosphorylation of STAT3 and subsequent activation of c-Jun to facilitate breast cancer cell proliferation [6]. Further, elevated expression of MUC-16 upregulated TSYLP3 expression in lung cancer cells and enhanced cancer cell survival, proliferation, and induced drug resistance [7]. One of the recent studies in Human papillomavirus-16 oropharyngeal cancer (HPVOPC) revealed that its progression is associated with high expression of mutated MUC16. The study also demonstrated that HPVOPC cells release exosomes rich in MUC16 protein, which induces EMT and imparts invasive properties to non-tumorigenic mammary epithelial cells [82]. An interesting study showed that microRNAs could affect expression of MUC16 in pancreatic cancer [83]. Finally, in an in vivo model, transgenic MUC16CT mice, when crossed with a mutant p53 background, spontaneously developed more tumors (sarcomas) compared to mutant p53 alone [59]. MUC16CT expression alone was not sufficient to induce tumors, probably requiring a second insult, like p53 mutation. It is possible that MUC16 CT signaling may get more enhanced in the presence of mutant p53.
7. Autoantibodies to MUC16
In tumors, aberrant overexpression and/or modifications to biomolecules often breaks the host immunologic tolerance and leads to formation of autoantibodies. Human autoantibodies elicited against CA125 were reported in various pathological conditions, especially ovarian cancer, shortly after its discovery [84, 85, 86, 87]. Such antibodies are stable and occur early in disease [88, 89, 90], suggesting immunogenicity of the target antigen that can be exploited for immunotherapy. Of note, the presence of anti-CA125 autoantibodies has been tentatively correlated with better survival, although not statistically significant [90]. Whether this indicates neutralization of circulating CA125 by autoantibodies or better anti-tumor immune response is not known, but it was interesting that puerperal mastitis was associated with a significantly decreased risk for ovarian cancer and also with elevated levels of autoantibodies to MUC16 [91].
8. Targeted immunotherapy
8.1. Targeting MUC16-Mesothelin Interactions
MUC16 interacts with mesothelin expressed on the walls of the peritoneal cavity to promote the metastasis of ovarian cancer cells. MUC16-mesothelin interaction is of unusually high affinity (akin to antigen-antibody interactions) [67]. Exploiting this, the MUC16 binding domain of mesothelin was fused to a human antibody Fc region to create a targeting construct that can bind MUC16 expressed on tumor cells [92]. The resulting fusion gene exhibited high affinity and specificity for MUC16 expressing cancers and could also disrupt mesothelin-MUC16 interactions in vitro. It also exhibited antibody dependent cellular cytotoxicity (ADCC) against MUC16 expressing human ovarian cancer cells in vitro. In another study, mesothelin was conjugated to a TRAIL-based drug, TR3. This Meso-TR3 combination efficiently bound and killed MUC16 expressing tumors in vitro and in vivo [93], and the combination was further improved by reducing the mesothelin part to a 64-amino acid MUC16 binding domain. The resulting truncated construct showed enhanced ability to kill tumors compared to the previous full-length construct [94]. Such approaches have the advantage that the therapeutic protein (in this case mesothelin) is completely human and will not elicit any undesired immune response like murine or chimeric antibodies. But these therapeutic approaches have still not been translated into clinical practice.
8.2. Immunotherapy of CA125
When murine anti-CA125 antibody mAb-B43.13 was administered as an immunoscintigraphic agent to monitor recurrence of ovarian cancer in patients, it demonstrated surprising improvement in survival for these patients. The improved survival was found to be directly correlated to the human anti-murine antibody (HAMA) response. Hence, very early on it was discovered that a murine CA125 mAb had the capability of eliciting anti-idiotype antibodies that resulted in favorable patient responses. On administration of B43.13, it was observed that both the anti-B43.13 (Ab2) and anti-anti-B43.13 antibodies could be induced in a selected group of patients, and that such a generation of an idiotype network correlated with better survival [95, 96, 97, 98]. Furthermore, within minutes of administration, circulating CA125 formed complexes with mAb, leading to possible antigen cross-presentation and enhanced T cell and B cell responses to CA125. This was further confirmed in a human-PBL-SCID/BG mouse model (recapitulating the human immune system) using human CA125-positive ovarian cancer cells, and again showed the positive impact of murine mAb in CA125-positive cancers [99]. It is interesting to note that the mAb B43.13 (Oregovomab) used in these studies had an unusually high affinity for CA125 (1.16X1010 M−1). Whether this property is essential for development of an anti-idiotypic network or for cellular and humoral response to CA125-antibody complex is currently not known. However, there were some disappointments as well. A randomized, double-blind, study reported no survival benefit with Oregovomab compared to placebo controls in recurrent ovarian cancer after first-line therapy [100]. Of note, however, in a subset of the population that had been designated more amenable to immunotherapy, the Oregovomab treated group had greater disease-free survival compared to placebo controls. In that particular subset, anti-idiotype responders fared better than both non-responders and placebo controls [100]. In a more recent study, administration of Oregovomab as a maintenance therapy after first-line therapy also had no effect on clinical outcomes as compared to placebo controls [101]. An open-label Phase 2 study tested the effects of Oregovomab in heavily pretreated ovarian cancer patients, and demonstrated partial stabilization in some patients that coincided with development of the anti-CA125 immune response [102]. When Oregovomab was combined with chemotherapy, however, robust immune responses to CA125 were again seen, and improved survival benefit was observed in immunogenic responders compared to non-responders [103]. It seems that anti-CA125 antibodies elicits robust anti-idiotype response but fails to achieve significant clinical benefit.
8.3. Anti-idiotype vaccine
It is normally challenging to make a vaccine against a self-antigen, but the observation that anti-idiotype CA125 antibodies could improve survival encouraged these lines of studies, especially following the concept of immunogenic network development given by Jerne [104, 105]. According to this hypothesis, the variable regions of antigen binding sites are immunogenic and induce the formation of a set of antibodies traditionally called Ab2. Some of these Ab2 antibodies are structurally similar to the original antigen. Hence, Ab2 mimics the original antigen and, in fact, induces formation of a 3rd set of antibodies (Ab3) similar to those derived by immunization of the original antigen. The Ab3 antibodies against CA125 are in fact a polyclonal feature, also binding to different antigenic sites on CA125 (in contrast to the original monoclonal antibody injected), and inducing the robust immunogenic response against CA125 expressing tumors.
An anti-idiotype antibody against CA125 (ACA125) was developed [106] that imitates the antigenicity of the original CA125 antigen and induces T and B cell responses in animal models [106] and cancer patients [107]. It was further found that this vaccine was well tolerated, and that in approximately 61–67% of patients the vaccine could induce Ab3 responses. Those with robust Ab3 responses fared better in survival compared to those showing little Ab3 response [108, 109, 110, 111]. A single-chain fragment of ACA125 was also tried in a rat model, and although this could not improve specificity by increasing Ab3 response, it rather unexpectedly increased undesirable RAMA (rat anti-mouse IgG response) [112]. This could have been because the engineered scFv could be recognized as more “foreign” compared to native ACA125 full-length antibody, leading to higher RAMA response in rats. Further, a fusion protein consisting of chimeric ACA125 linked to IL-6 showed better Ab3 response compared to chimeric ACA125 alone [113]. A more pivotal but disappointing study was the MIMOSA Phase III trial [114] that studied 888 randomized ovarian cancer patients in remission, all with measurable CA125, to observe whether abagovomab (ACA125) used as maintenance therapy improves overall survival. Even though there were vaccine-induced robust immune responses, there was no statistically significant improvement in overall survival. It should be noted, however, that a subsequent study found that the inefficacy of the MIMOSA trial may have been due to the poor immune status of patients, since those with better CD8+ T cell counts had better progression-free survival compared to those with lower counts [115] (the fraction of the former was quite low, and therefore they were not statistically detected in the original study). In a sub-study of the MIMOSA trial, it was found that abagovomab administration could not induce any cytotoxic T lymphocytes against CA125, but that patients having Ab3 response still had better relapse-free survival compared to non-responders [116]. It would be of interest to evaluate ACA125 in conjunction with checkpoint blockade agents. More recently, it was found that MUC16 displays a high number of neoantigens in long term survivors of pancreatic ductal adenocarcinoma and this neoantigen expression correlated with better survival [117]. Despite the lack of clinical success, the use of anti-idiotype antibodies and/or neoantigen peptides provide attractive option for making specific vaccines and should be explored further, especially with use in combination with other immunomodulators.
8.4. MUC16 Radioimmunotherapy and imaging
MUC16 has been used as an agent for radioimmunoscintigraphy to detect residual disease during ovarian cancer surgery, and was also explored as a therapeutic option since radiation can also kill rapidly dividing cells. Imaging results were often compared with traditional CT scans and MRI for sensitivity to detect lesions (either primary or metastatic) [118, 119, 120]. I-131 labeled OC125 mAb was injected once in 20 recurrent ovarian cancer patients, of which 3 had a partial response [121]. In another study, I-131 radiolabeled OC125 F(ab)2 fragment was injected 5–10 days after surgical treatment and chemotherapy to 6 ovarian cancer patients. No significant therapeutic benefit was observed in any of these patients [122]. However, administration of radiolabeled OC125 (F(ab)2 fragments exhibited high sensitivity in detecting lesions but with low specificities, since non-ovarian cancer conditions also led to accumulation of the radiotracer [123, 124, 125, 126, 127, 128]. In another study, a direct comparison was made between OC125 radioimmunoscintigraphy and CT scans to detect primary tumors and metastasis in patients after chemotherapy and just before second surgery [119]. The former was more sensitive in detecting primary tumors within the pelvis, while CT scans showed superiority in detecting liver metastasis. In another study, radioimmunoscintigraphy with OC125 meant originally for diagnostic purposes was shown to have a statistically favorable outcome for ovarian cancer patients compared to those not receiving the treatment [129]. Using a different mAb to CA125 that binds to a different epitope than OC125, tumors could be delineated in a more sensitive way compared to CT or sonography [130]. In an interesting experiment, OC125 mAb was also tested as an agent for photodynamic therapy. OC125 mAb was labeled with a chlorine-based photosensitizer [131] and upon injection localized to the tumor of a NIH:OVCAR-3 xenograft model. Upon excitation with a particular wavelength of light, the sensitizer released a set of toxic chemicals such as singlet oxygen (that can have deleterious oxidative effects) that caused tumor cell killing. Animals receiving this treatment had a statistically significant greater survival compared to control mice [132]. In spite of modest success, this approach still doesn’t lead to level of favorable outcomes (either detection or therapy) that are expected in the clinic. Since murine antibody was used for these trials, the problem of HAMA generation in patients as mentioned before, may again be one reason for limited success seen with these immunoconjugates) [133]. MUC16 radioimmunotherapy seems to be hampered by sequestration of radiolabeled antibodies by circulating CA125, cleavage of CA125 from the cancer cell surface and elevated levels in non-cancerous conditions. Future efforts should be directed towards targeting those non-tandemly repeated epitopes that are retained on the cancer cell surface and are unaffected by cleavage.
8.5. MUC16 Antibody Drug Conjugates (ADC’s)
MUC16 is seen as a potential target to develop ADCs for ovarian cancer. The reasons are manifold: it has a sufficiently high expression on the surface of ovarian cancer cells, it has a large highly immunogenic ectodomain for making antibodies, and it has a limited expression in normal tissues. Importantly, it seems not to be vital for the human body since Muc16 knockout mice display no abnormal phenotype. With this in mind, Genentech designed two different classes of mAbs to MUC16, one directed against tandem repeats (mAb 3A5), and the other to the non-tandem repeating epitope located at the C-terminal end of CA125 (mAb 11D10) [134, 135]. Sandwich ELISA indicated that both 11D10 and 3A5 epitopes are located in the shed CA125 portion. Neither 3A5 nor 11D10 antibodies affected the growth of ovarian cancer cells, both in vitro and in vivo, presumably establishing that MUC16 is not a driver oncogene but may simply be a facilitator or a bystander. Alternatively, these antibodies bind to MUC16 but are incapable of interfering with the functions that MUC16 facilitates (i.e. these are not neutralizing antibodies).
One limitation of using MUC16 as an ADC target is that MUC16 is endocytosed very slowly from the cell surface [135]. This was further confirmed when the authors noted that both 3A5 and 11D10 mAbs were internalized into cells only after overnight incubation under constant exposure. MAb 3A5 fared better compared to 11D10 in terms of binding to the cell surface, with rates of internalization most probably due to tandemly repeating epitopes that led to binding of multiple antibodies per MUC16 molecule. Both were conjugated to the tubulin depolymerizing drug Monomethyl auristatin E (MMAE). The 3A5-MMAE conjugate (DMUC5754A) exhibited better efficacy than 11D10-MMAE in in vitro and xenograft models of MUC16 expressing ovarian cancer cells. Hence, DMUC5754A was evaluated in a Phase 1 dose escalation study in platinum-resistant ovarian cancer (66 patients) and unresectable pancreatic cancer (11 patients) [136]. The ADC was well tolerated, except for few cases of the more usual side effects such as nausea, diarrhea, and neutropenia. It was surprising that no toxicity was observed at sites where MUC16 is normally expressed in high amounts, such as the ocular surface. Patients having high MUC16 positivity in the tumors by IHC analysis performed better, as compared to patients with low MUC16 tumors. One complete response and 6 partial responses were recorded among these ovarian cancer patients. Two patients also had unconfirmed partial response and 6 had stable disease. None of the enrolled pancreatic cancer patients had a substantial radiographic response. The results of the Phase 1 trial appear to be positive, and future trials would be interesting using this agent. MUC16 ADC based approaches have the advantages of high selectivity and low toxicity and have shown clinical benefit. However, further improvements may still be needed to improve the efficacy of treatment.
8.6. MUC16 ecto domain Chimeric Antigen Receptor (CAR) T cells
An emerging field of adoptive transfer immunotherapy is the CAR T-cell therapy that has shown efficacy especially in hematological malignancies [137, 138]. This involves engineering patient’s own T cells to express an artificial chimeric T cell receptor containing the antigen recognizing scFv fragment fused to a T cell receptor containing the required intracellular signaling domains. The scFv fragment provides the antigen specificity while CARs targeted by the MUC16 ectodomain were generated by using a hybridoma against the MUC16 ectodomain (4H11). Both first generation (utilizing CD8 sequence) and second generation (using CD28 sequence) CARs were created by fusing with the CD3ζ signaling domain. The resulting CAR T cells specifically lysed MUC16 ectodomain-transfected cells both in vitro and in vivo, but failed to lyze native MUC16 expressing cells like OVCAR-3. However, they were able to kill fresh ovarian cancer cells isolated from ascites samples in vitro and xenograft-transfected MUC16 ectodomain OVCAR-3 cells in a SCID-Beige mouse model [139]. A Phase 1 trial using IL-12 transfected MUC16 ectodomain CAR T cells for recurrent ovarian cancer is in progress [140]. The IL-12 gene was added to mitigate effects of harsh tumor microenvironment, making it immunologically more conducive for killing cancer cells. To counter any undesired toxicity, a safety switch was added in the form of a truncated EGFR that could be safely eliminated using cetuximab. Even though these approaches are still in initial stages, CAR T-cell based immunotherapy holds promise as an alternative avenue for MUC16 based immunotherapy.
9. Expert Opinion
MUC16 is a large cell surface molecule that plays a protective role at the apical surface of normal epithelia. Although its increased expression should improve mucosal barrier and protective functions against infection, MUC16 is aberrantly overexpressed during progression of several malignancies. Several studies have demonstrated that MUC16 or CA125 is not only a biomarker for ovarian cancer, but also plays role in tumor progression and metastasis. Due to its involvement in cancer aggressiveness and progression, MUC16 has emerged as a promising candidate for the development of targeted therapies.
Several studies have investigated the potential of MUC16 as a therapeutic target. In many epithelial ovarian cancer patients, higher serum levels of MUC16 with cancer-associated glycoforms (Tn and STn) are detected, making MUC16 suitable for early detection, diagnostic staging and determining treatment success [141, 142, 143, 144, 145]. Recently some targeted therapies have been reported against MUC16, such as monoclonal antibodies, antidrug conjugates and antibody targeting MUC16-Mesothelin interaction. Although the in vitro and in vivo studies have noticeably revealed the potential and anti-tumor effect of these agents for targeting MUC16 in preclinical studies, clinical trials have shown limited benefits. This may be because the MUC16 extracellular domain undergoes cleavage, reducing the binding of these monoclonal antibodies to the tumor cells. Besides, it is also possible that, due to shedding, MUC16 targeting agents bind to the circulating MUC16 in the serum, thereby, limiting the fraction of the agent that actually accumulates in the tumors. Therefore, targeting the carboxyl-terminal domain of MUC16, which is retained by the tumor cells after cleavage, can expand the present immunotherapy. In context to this, there are more recent reports of monoclonal antibodies targeting the retained carboxy terminus that have been developed [32, 33, 146, 147]. Another hurdle in the field is the incomplete understanding of the structural and functional diversity of different MUC16 domains along with the aberrant glycoforms and possible splice variants, which are seen during disease condition. Further, the nature of MUC16 downstream signaling molecules and the associated signaling pathways remain uncharacterized and poorly understood. Its knowledge is essential because the deregulation of downstream signaling molecules might also play an important role in the success of therapeutic approaches. Concerted efforts are needed to develop more effective targeting agents and suitable animal models to evaluate MUC16-based therapies.
Despite these challenges, the data and rationale support MUC16 as a promising candidate for the development of targeted therapies. With recent developments in antibody engineering, antibody drug conjugates, CAR T cells, CRISPR-Cas9 technology, and novel genetically engineered mouse models, the stage has been set to develop and evaluate new targeted therapies. The recent example of Genentech’s MUC16 ADC effort shows how it is possible to target the mucin in the clinical context. Beyond this, it would also be interesting to combine MUC16 targeted therapy with other targeted or non-targeted therapeutic modalities, including chemotherapy, radiation, and immunotherapy to determine how they may combine to exert more efficacious therapeutic effects and improve the outcomes of MUC16 expressing cancer patients.
Article Highlights.
MUC16 is overexpressed in breast, ovarian and pancreatic tumors and plays an important role in progression and metastasis.
MUC16 is shed/cleaved from the cell surface and enters the circulation, and its serum levels are greatly elevated in multiple cancer patients. Circulating MUC16 levels play critical roles in the ability of cancers to evade host immune responses and hinders anti-MUC16 targeted agents to reach the tumor area.
Serum MUC16 levels serve as FDA approved biomarkers for predicting ovarian cancer recurrence but lacks sensitivity and specificity.
Anti-idiotype antibodies against MUC16 induce T and B cell response in patients and hence have shown promise as vaccine candidates, but significant clinical efficacy has not been observed. MUC16 neoantigen peptide vaccines show good promise and should be evaluated in future.
MUC16 carboxy terminus (CT) acts as an oncogene and induces signaling events that lead to tumor progression. Further research should be undertaken to understand MUC16 CT induced regulation of tumorigenesis.
MUC16 antibody-drug conjugates (ADC) have shown preliminary efficacy in ovarian cancer patients. MUC16 ectodomain specific CAR T-cell therapy is in the initial phase of development and holds promise as a potential future therapy for ovarian cancer patients.
Acknowledgements
We would like to thank Dr. Adrian E. Koesters, Research Editor at UNMC, for her editorial contribution to this manuscript.
Funding
The authors on this manuscript are, in parts, supported by the grants from the National Institutes of Health (PO1CA217798, RO1 CA183459, R01CA210637, R44 DK117472, UO1 CA200466, UO1 CA210240, and P50 CA127297), and the Nebraska Department of Health and Human Services LB595.
Footnotes
Declaration of Interests
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
12. References
- 1.Joshi S, Kumar S, Choudhury A, et al. Altered Mucins (MUC) trafficking in benign and malignant conditions. Oncotarget 2014;5(17):7272–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaturvedi P, Singh AP, Batra SK. Structure, evolution, and biology of the MUC4 mucin. Faseb j 2008;22(4):966–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer 2009;9(12):874–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haridas D, Chakraborty S, Ponnusamy MP, et al. Pathobiological implications of MUC16 expression in pancreatic cancer. PLoS One 2011;6(10):e26839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klug TL, Bast RC Jr., Niloff JM, et al. Monoclonal antibody immunoradiometric assay for an antigenic determinant (CA 125) associated with human epithelial ovarian carcinomas. Cancer Res 1984;44(3):1048–53. [PubMed] [Google Scholar]
- 6.Lakshmanan I, Ponnusamy MP, Das S, et al. MUC16 induced rapid G2/M transition via interactions with JAK2 for increased proliferation and anti-apoptosis in breast cancer cells. Oncogene 2012;31(7):805–17.* Implicated MUC16 in JAK2/STAT3 signaling in Breast cancer.
- 7.Lakshmanan I, Salfity S, Seshacharyulu P, et al. MUC16 Regulates TSPYL5 for Lung Cancer Cell Growth and Chemoresistance by Suppressing p53. Clin Cancer Res 2017;23(14):3906–3917.* Implicated MUC16 in JAK2/STAT3 signaling in lung cancer.
- 8.Bast RC Jr., Feeney M, Lazarus H, et al. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest 1981;68(5):1331–7.** First report that discovered the CA125 antigen using OC125 monoclonal antibody
- 9.Bast RC Jr., Klug TL, St John E, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med 1983;309(15):883–7** First report to describe a assay for measuring CA125 antigen in clinical samples. CA125 as a biomarker for ovarian cancer was proposed.
- 10.Kabawat SE, Bast RC, Welch WR, et al. Immunopathologic characterization of a monoclonal antibody that recognizes common surface antigens of human ovarian tumors of serous, endometrioid, and clear cell types. Am J Clin Pathol 1983;79(1):98–104. [DOI] [PubMed] [Google Scholar]
- 11.Kudlacek S, Schieder K, Kolbl H, et al. Use of CA 125 monoclonal antibody to monitor patients with ovarian cancer. Gynecol Oncol 1989;35(3):323–9. [DOI] [PubMed] [Google Scholar]
- 12.Li XG, Chen DX, Schwartz PE, et al. A study of the monoclonal antibody OC 125 to diagnose malignant ovarian tumors. Gynecol Oncol 1989;32(3):327–30. [DOI] [PubMed] [Google Scholar]
- 13.Wu JT, Miya T, Knight JA, et al. Improved specificity of the CA 125 enzyme immunoassay for ovarian carcinomas by use of the ratio of CA 125 to carcinoembryonic antigen. Clin Chem 1988;34(9):1853–7. [PubMed] [Google Scholar]
- 14.Haga Y, Sakamoto K, Egami H, et al. Evaluation of serum CA125 values in healthy individuals and pregnant women. Am J Med Sci 1986;292(1):25–9. [DOI] [PubMed] [Google Scholar]
- 15.Niloff JM, Knapp RC, Schaetzl E, et al. CA125 antigen levels in obstetric and gynecologic patients. Obstet Gynecol 1984;64(5):703–7. [PubMed] [Google Scholar]
- 16.Ricolleau G, Chatal JF, Fumoleau P, et al. Radioimmunoassay of the CA 12 5 antigen in ovarian carcinomas: advantages compared with CA 19–9 and CEA. Tumour Biol 1984;5(3–4):151–9. [PubMed] [Google Scholar]
- 17.Moore RG, Brown AK, Miller MC, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol 2008;108(2):402–8. [DOI] [PubMed] [Google Scholar]
- 18.Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol 2009;112(1):40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Gorp T, Cadron I, Despierre E, et al. HE4 and CA125 as a diagnostic test in ovarian cancer: prospective validation of the Risk of Ovarian Malignancy Algorithm. Br J Cancer 2011;104(5):863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wahrenbrock MG, Varki A. Multiple hepatic receptors cooperate to eliminate secretory mucins aberrantly entering the bloodstream: are circulating cancer mucins the “tip of the iceberg”? Cancer Res 2006;66(4):2433–41. [DOI] [PubMed] [Google Scholar]
- 21.Yin BW, Dnistrian A, Lloyd KO. Ovarian cancer antigen CA125 is encoded by the MUC16 mucin gene. Int J Cancer 2002;98(5):737–40.** CA125 discovered as MUC16
- 22.Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem 2001;276(29):27371–5.** CA125 discovered as MUC16
- 23.Haridas D, Ponnusamy MP, Chugh S, et al. MUC16: molecular analysis and its functional implications in benign and malignant conditions. Faseb j 2014;28(10):4183–99.* Comprehensive Review on MUC16 Structure and Functions
- 24.Taniguchi T, Woodward AM, Magnelli P, et al. N-Glycosylation affects the stability and barrier function of the MUC16 mucin. J Biol Chem 2017;292(26):11079–11090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lloyd KO, Yin BW, Kudryashov V. Isolation and characterization of ovarian cancer antigen CA 125 using a new monoclonal antibody (VK-8): identification as a mucin-type molecule. Int J Cancer 1997;71(5):842–50. [DOI] [PubMed] [Google Scholar]
- 26.Kui Wong N, Easton RL, Panico M, et al. Characterization of the oligosaccharides associated with the human ovarian tumor marker CA125. J Biol Chem 2003;278(31):28619–34.** Comprehensive characterization of glycosylation of MUC16
- 27.Das S, Majhi PD, Al-Mugotir MH, et al. Membrane proximal ectodomain cleavage of MUC16 occurs in the acidifying Golgi/post-Golgi compartments. Sci Rep 2015;5:9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macao B, Johansson DG, Hansson GC, et al. Autoproteolysis coupled to protein folding in the SEA domain of the membrane-bound MUC1 mucin. Nat Struct Mol Biol 2006;13(1):71–6. [DOI] [PubMed] [Google Scholar]
- 29.Maeda T, Inoue M, Koshiba S, et al. Solution structure of the SEA domain from the murine homologue of ovarian cancer antigen CA125 (MUC16). J Biol Chem 2004;279(13):13174–82. [DOI] [PubMed] [Google Scholar]
- 30.Fendrick JL, Staley KA, Gee MK, et al. Characterization of CA 125 synthesized by the human epithelial amnion WISH cell line. Tumour Biol 1993;14(5):310–8. [DOI] [PubMed] [Google Scholar]
- 31.Blalock TD, Spurr-Michaud SJ, Tisdale AS, et al. Functions of MUC16 in corneal epithelial cells. Invest Ophthalmol Vis Sci 2007;48(10):4509–18. [DOI] [PubMed] [Google Scholar]
- 32.Aithal A, Junker WM, Kshirsagar P, et al. Development and characterization of carboxy-terminus specific monoclonal antibodies for understanding MUC16 cleavage in human ovarian cancer. PLoS One 2018;13(4):e0193907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gipson IK, Mandel U, Menon B, et al. Generation and characterization of a monoclonal antibody to the cytoplasmic tail of MUC16. Glycobiology 2017;27(10):920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcos-Silva L, Ricardo S, Chen K, et al. A novel monoclonal antibody to a defined peptide epitope in MUC16. Glycobiology 2015;25(11):1172–82. [DOI] [PubMed] [Google Scholar]
- 35.Nustad K, Bast RC Jr., Brien TJ, et al. Specificity and affinity of 26 monoclonal antibodies against the CA 125 antigen: first report from the ISOBM TD-1 workshop. International Society for Oncodevelopmental Biology and Medicine. Tumour Biol 1996;17(4):196–219. [DOI] [PubMed] [Google Scholar]
- 36.Davies JR, Kirkham S, Svitacheva N, et al. MUC16 is produced in tracheal surface epithelium and submucosal glands and is present in secretions from normal human airway and cultured bronchial epithelial cells. Int J Biochem Cell Biol 2007;39(10):1943–54. [DOI] [PubMed] [Google Scholar]
- 37.Bast RC Jr., Xu FJ, Yu YH, et al. CA 125: the past and the future. Int J Biol Markers 1998;13(4):179–87. [DOI] [PubMed] [Google Scholar]
- 38.Kenemans P, Verstraeten AA, van Kamp GJ, et al. The second generation CA 125 assays. Ann Med 1995;27(1):107–13. [DOI] [PubMed] [Google Scholar]
- 39.Kenemans P, van Kamp GJ, Oehr P, et al. Heterologous double-determinant immunoradiometric assay CA 125 II: reliable second-generation immunoassay for determining CA 125 in serum. Clin Chem 1993;39(12):2509–13. [PubMed] [Google Scholar]
- 40.Nap M, Vitali A, Nustad K, et al. Immunohistochemical characterization of 22 monoclonal antibodies against the CA125 antigen: 2nd report from the ISOBM TD-1 Workshop. Tumour Biol 1996;17(6):325–31. [PubMed] [Google Scholar]
- 41.O’Brien TJ, Beard JB, Underwood LJ, et al. The CA 125 gene: an extracellular superstructure dominated by repeat sequences. Tumour Biol 2001;22(6):348–66. [DOI] [PubMed] [Google Scholar]
- 42.Felder M, Kapur A, Gonzalez-Bosquet J, et al. MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress. Mol Cancer 2014;13:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bressan A, Bozzo F, Maggi CA, et al. OC125, M11 and OV197 epitopes are not uniformly distributed in the tandem-repeat region of CA125 and require the entire SEA domain. Dis Markers 2013;34(4):257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marcos-Silva L, Narimatsu Y, Halim A, et al. Characterization of binding epitopes of CA125 monoclonal antibodies. J Proteome Res 2014;13(7):3349–59. [DOI] [PubMed] [Google Scholar]
- 45.Gipson IK, Spurr-Michaud S, Tisdale A, et al. Comparison of the transmembrane mucins MUC1 and MUC16 in epithelial barrier function. PLoS One 2014;9(6):e100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Govindarajan B, Gipson IK. Membrane-tethered mucins have multiple functions on the ocular surface. Exp Eye Res 2010;90(6):655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frey A, Giannasca KT, Weltzin R, et al. Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: implications for microbial attachment and oral vaccine targeting. J Exp Med 1996;184(3):1045–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Argueso P, Spurr-Michaud S, Russo CL, et al. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest Ophthalmol Vis Sci 2003;44(6):2487–95. [DOI] [PubMed] [Google Scholar]
- 49.Kesimer M, Scull M, Brighton B, et al. Characterization of exosome-like vesicles released from human tracheobronchial ciliated epithelium: a possible role in innate defense. Faseb j 2009;23(6):1858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sumiyoshi M, Ricciuto J, Tisdale A, et al. Antiadhesive character of mucin O-glycans at the apical surface of corneal epithelial cells. Invest Ophthalmol Vis Sci 2008;49(1):197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Argueso P, Guzman-Aranguez A, Mantelli F, et al. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. J Biol Chem 2009;284(34):23037–45.** MUC16-galectin-3 interaction discovered.
- 52.Seelenmeyer C, Wegehingel S, Lechner J, et al. The cancer antigen CA125 represents a novel counter receptor for galectin-1. J Cell Sci 2003;116(Pt 7):1305–18.** MUC16-galectin-1 interaction discovered.
- 53.Govindarajan B, Menon BB, Spurr-Michaud S, et al. A metalloproteinase secreted by Streptococcus pneumoniae removes membrane mucin MUC16 from the epithelial glycocalyx barrier. PLoS One 2012;7(3):e32418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tyler C, Kapur A, Felder M, et al. The mucin MUC16 (CA125) binds to NK cells and monocytes from peripheral blood of women with healthy pregnancy and preeclampsia. Am J Reprod Immunol 2012;68(1):28–37.** MUC16 role in immunosuppression by down regulation of NK cells elucidated.
- 55.Gipson IK, Blalock T, Tisdale A, et al. MUC16 is lost from the uterodome (pinopode) surface of the receptive human endometrium: in vitro evidence that MUC16 is a barrier to trophoblast adherence. Biol Reprod 2008;78(1):134–42.* MUC16 role in blastocyst implantaion elucidated.
- 56.Kim N, Hong Y, Kwon D, et al. Somatic mutaome profile in human cancer tissues. Genomics Inform 2013;11(4):239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Das S, Rachagani S, Torres-Gonzalez MP, et al. Carboxyl-terminal domain of MUC16 imparts tumorigenic and metastatic functions through nuclear translocation of JAK2 to pancreatic cancer cells. Oncotarget 2015;6(8):5772–87.* Tumorigenic role of MUC16 carboxy terminus elucidated.
- 58.Giannakouros P, Matte I, Rancourt C, et al. Transformation of NIH3T3 mouse fibroblast cells by MUC16 mucin (CA125) is driven by its cytoplasmic tail. Int J Oncol 2015;46(1):91–8.* Tumorigenic role of MUC16 carboxy terminus elucidated.
- 59.Rao TD, Tian H, Ma X, et al. Expression of the Carboxy-Terminal Portion of MUC16/CA125 Induces Transformation and Tumor Invasion. PLoS One 2015;10(5):e0126633.* Tumorigenic role of MUC16 carboxy terminus elucidated.
- 60.Theriault C, Pinard M, Comamala M, et al. MUC16 (CA125) regulates epithelial ovarian cancer cell growth, tumorigenesis and metastasis. Gynecol Oncol 2011;121(3):434–43. [DOI] [PubMed] [Google Scholar]
- 61.Shimizu A, Hirono S, Tani M, et al. Coexpression of MUC16 and mesothelin is related to the invasion process in pancreatic ductal adenocarcinoma. Cancer Sci 2012;103(4):739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen SH, Hung WC, Wang P, et al. Mesothelin binding to CA125/MUC16 promotes pancreatic cancer cell motility and invasion via MMP-7 activation. Sci Rep 2013;3:1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Higashi M, Yamada N, Yokoyama S, et al. Pathobiological implications of MUC16/CA125 expression in intrahepatic cholangiocarcinoma-mass forming type. Pathobiology 2012;79(2):101–6. [DOI] [PubMed] [Google Scholar]
- 64.Liang C, Qin Y, Zhang B, et al. Oncogenic KRAS Targets MUC16/CA125 in Pancreatic Ductal Adenocarcinoma. Mol Cancer Res 2017;15(2):201–212. [DOI] [PubMed] [Google Scholar]
- 65.Cotton S, Azevedo R, Gaiteiro C, et al. Targeted O-glycoproteomics explored increased sialylation and identified MUC16 as a poor prognosis biomarker in advanced-stage bladder tumours. Mol Oncol 2017;11(8):895–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rump A, Morikawa Y, Tanaka M, et al. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem 2004;279(10):9190–8.** MUC16-mesothelin interaction discovered.
- 67.Gubbels JA, Belisle J, Onda M, et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer 2006;5(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen SH, Dallas MR, Balzer EM, et al. Mucin 16 is a functional selectin ligand on pancreatic cancer cells. Faseb j 2012;26(3):1349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bobbs AS, Cole JM, Cowden Dahl KD. Emerging and Evolving Ovarian Cancer Animal Models. Cancer Growth Metastasis 2015;8(Suppl 1):29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Connolly DC, Hensley HH. Xenograft and Transgenic Mouse Models of Epithelial Ovarian Cancer and Non Invasive Imaging Modalities to Monitor Ovarian Tumor Growth In situ -Applications in Evaluating Novel Therapeutic Agents. Curr Protoc Pharmacol 2009;45:14.12.1–14.12.26. [PMC free article] [PubMed] [Google Scholar]
- 71.Zervos E, Agle S, Freistaedter AG, et al. Murine mesothelin: characterization, expression, and inhibition of tumor growth in a murine model of pancreatic cancer. J Exp Clin Cancer Res 2016;35:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Belisle JA, Horibata S, Jennifer GA, et al. Identification of Siglec-9 as the receptor for MUC16 on human NK cells, B cells, and monocytes. Mol Cancer 2010;9:118.** Siglec-9 discovered as the receptor for MUC16 on NK cells.
- 73.Gubbels JA, Felder M, Horibata S, et al. MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells. Mol Cancer 2010;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Belisle JA, Gubbels JA, Raphael CA, et al. Peritoneal natural killer cells from epithelial ovarian cancer patients show an altered phenotype and bind to the tumour marker MUC16 (CA125). Immunology 2007;122(3):418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patankar MS, Jing Y, Morrison JC, et al. Potent suppression of natural killer cell response mediated by the ovarian tumor marker CA125. Gynecol Oncol 2005;99(3):704–13. [DOI] [PubMed] [Google Scholar]
- 76.Reinartz S, Failer S, Schuell T, et al. CA125 (MUC16) gene silencing suppresses growth properties of ovarian and breast cancer cells. Eur J Cancer 2012;48(10):1558–69. [DOI] [PubMed] [Google Scholar]
- 77.Boivin M, Lane D, Piche A, et al. CA125 (MUC16) tumor antigen selectively modulates the sensitivity of ovarian cancer cells to genotoxic drug-induced apoptosis. Gynecol Oncol 2009;115(3):407–13. [DOI] [PubMed] [Google Scholar]
- 78.Comamala M, Pinard M, Theriault C, et al. Downregulation of cell surface CA125/MUC16 induces epithelial-to-mesenchymal transition and restores EGFR signalling in NIH:OVCAR3 ovarian carcinoma cells. Br J Cancer 2011;104(6):989–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Muniyan S, Haridas D, Chugh S, et al. MUC16 contributes to the metastasis of pancreatic ductal adenocarcinoma through focal adhesion mediated signaling mechanism. Genes Cancer 2016;7(3–4):110–124.* MUC16 role in metastasis of pancreatic cancer elucidated.
- 80.Shukla SK, Gunda V, Abrego J, et al. MUC16-mediated activation of mTOR and c-Myc reprograms pancreatic cancer metabolism. Oncotarget 2015;6(22):19118–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Das S, Batra SK. Understanding the Unique Attributes of MUC16 (CA125): Potential Implications in Targeted Therapy. Cancer Res 2015;75(22):4669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kannan A, Hertweck KL, Philley JV, et al. Genetic Mutation and Exosome Signature of Human Papilloma Virus Associated Oropharyngeal Cancer. Sci Rep 2017;7:46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Radhakrishnan P, Mohr AM, Grandgenett PM, et al. MicroRNA-200c modulates the expression of MUC4 and MUC16 by directly targeting their coding sequences in human pancreatic cancer. PLoS One 2013;8(10):e73356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Budiu RA, Mantia-Smaldone G, Elishaev E, et al. Soluble MUC1 and serum MUC1-specific antibodies are potential prognostic biomarkers for platinum-resistant ovarian cancer. Cancer Immunol Immunother 2011;60(7):975–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fortner RT, Schock H, Le Cornet C, et al. Ovarian cancer early detection by circulating CA125 in the context of anti-CA125 autoantibody levels: Results from the EPIC cohort. Int J Cancer 2018;142(7):1355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kutteh WH, Miller DS, Mathis JM. Immunologic characterization of tumor markers in human ovarian cancer cell lines. J Soc Gynecol Investig 1996;3(4):216–22. [PubMed] [Google Scholar]
- 87.Taylor DD, Gercel-Taylor C, Parker LP. Patient-derived tumor-reactive antibodies as diagnostic markers for ovarian cancer. Gynecol Oncol 2009;115(1):112–20. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 88.Chatterjee M, Tainsky MA. Autoantibodies as biomarkers for ovarian cancer. Cancer Biomark 2010;8(4–5):187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Finn OJ. Immune response as a biomarker for cancer detection and a lot more. N Engl J Med 2005;353(12):1288–90. [DOI] [PubMed] [Google Scholar]
- 90.Frietze KM, Roden RB, Lee JH, et al. Identification of Anti-CA125 Antibody Responses in Ovarian Cancer Patients by a Novel Deep Sequence-Coupled Biopanning Platform. Cancer Immunol Res 2016;4(2):157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cramer DW, Williams K, Vitonis AF, et al. Puerperal mastitis: a reproductive event of importance affecting anti-mucin antibody levels and ovarian cancer risk. Cancer Causes Control 2013;24(11):1911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xiang X, Feng M, Felder M, et al. HN125: A Novel Immunoadhesin Targeting MUC16 with Potential for Cancer Therapy. J Cancer 2011;2:280–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Garg G, Gibbs J, Belt B, et al. Novel treatment option for MUC16-positive malignancies with the targeted TRAIL-based fusion protein Meso-TR3. BMC Cancer 2014;14:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Su Y, Tatzel K, Wang X, et al. Mesothelin’s minimal MUC16 binding moiety converts TR3 into a potent cancer therapeutic via hierarchical binding events at the plasma membrane. Oncotarget 2016;7(21):31534–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Madiyalakan R, Sykes TR, Dharampaul S, et al. Antiidiotype induction therapy: evidence for the induction of immune response through the idiotype network in patients with ovarian cancer after administration of anti-CA125 murine monoclonal antibody B43.13. Hybridoma 1995;14(2):199–203.** Anti-idiotype immune responses due to administartion of anti-CA125 antibody was described.
- 96.Mobus VJ, Baum RP, Bolle M, et al. Immune responses to murine monoclonal antibody-B43.13 correlate with prolonged survival of women with recurrent ovarian cancer. Am J Obstet Gynecol 2003;189(1):28–36. [DOI] [PubMed] [Google Scholar]
- 97.Noujaim AA, Schultes BC, Baum RP, et al. Induction of CA125-specific B and T cell responses in patients injected with MAb-B43.13--evidence for antibody-mediated antigen-processing and presentation of CA125 in vivo. Cancer Biother Radiopharm 2001;16(3):187–203. [DOI] [PubMed] [Google Scholar]
- 98.Schultes BC, Baum RP, Niesen A, et al. Anti-idiotype induction therapy: anti-CA125 antibodies (Ab3) mediated tumor killing in patients treated with Ovarex mAb B43.13 (Ab1). Cancer Immunol Immunother 1998;46(4):201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schultes BC, Zhang C, Xue LY, et al. Immunotherapy of human ovarian carcinoma with OvaRex MAb-B43.13 in a human-PBL-SCID/BG mouse model. Hybridoma 1999;18(1):47–55. [DOI] [PubMed] [Google Scholar]
- 100.Berek JS, Taylor PT, Gordon A, et al. Randomized, placebo-controlled study of oregovomab for consolidation of clinical remission in patients with advanced ovarian cancer. J Clin Oncol 2004;22(17):3507–16. [DOI] [PubMed] [Google Scholar]
- 101.Berek J, Taylor P, McGuire W, et al. Oregovomab maintenance monoimmunotherapy does not improve outcomes in advanced ovarian cancer. J Clin Oncol 2009;27(3):418–25. [DOI] [PubMed] [Google Scholar]
- 102.Ehlen TG, Hoskins PJ, Miller D, et al. A pilot phase 2 study of oregovomab murine monoclonal antibody to CA125 as an immunotherapeutic agent for recurrent ovarian cancer. Int J Gynecol Cancer 2005;15(6):1023–34 [DOI] [PubMed] [Google Scholar]
- 103.Gordon AN, Schultes BC, Gallion H, et al. CA125- and tumor-specific T-cell responses correlate with prolonged survival in oregovomab-treated recurrent ovarian cancer patients. Gynecol Oncol 2004;94(2):340–51. [DOI] [PubMed] [Google Scholar]
- 104.Jerne NK. Towards a network theory of the immune system. Ann Immunol (Paris) 1974;125c(1–2):373–89. [PubMed] [Google Scholar]
- 105.Jerne NK. Idiotypic networks and other preconceived ideas. Immunol Rev 1984;79:5–24. [DOI] [PubMed] [Google Scholar]
- 106.Schlebusch H, Wagner U, Grunn U, et al. A monoclonal antiidiotypic antibody ACA 125 mimicking the tumor-associated antigen CA 125 for immunotherapy of ovarian cancer. Hybridoma 1995;14(2):167–74.** Anti-idiotype antibody as a CA125 vaccine developed.
- 107.Reinartz S, Boerner H, Koehler S, et al. Evaluation of immunological responses in patients with ovarian cancer treated with the anti-idiotype vaccine ACA125 by determination of intracellular cytokines--a preliminary report. Hybridoma 1999;18(1):41–5. [DOI] [PubMed] [Google Scholar]
- 108.Reinartz S, Kohler S, Schlebusch H, et al. Vaccination of patients with advanced ovarian carcinoma with the anti-idiotype ACA125: immunological response and survival (phase Ib/II). Clin Cancer Res 2004;10(5):1580–7. [DOI] [PubMed] [Google Scholar]
- 109.Sabbatini P, Dupont J, Aghajanian C, et al. Phase I study of abagovomab in patients with epithelial ovarian, fallopian tube, or primary peritoneal cancer. Clin Cancer Res 2006;12(18):5503–10. [DOI] [PubMed] [Google Scholar]
- 110.Wagner U, Kohler S, Reinartz S, et al. Immunological consolidation of ovarian carcinoma recurrences with monoclonal anti-idiotype antibody ACA125: immune responses and survival in palliative treatment. See The biology behind: K. A. Foon and M. Bhattacharya-Chatterjee, Are solid tumor anti-idiotype vaccines ready for prime time? Clin. Cancer Res, 7:1112–1115, 2001. Clin Cancer Res. 2001;7(5):1154–62. [PubMed] [Google Scholar]
- 111.Wagner U, Schlebusch H, Kohler S, et al. Immunological responses to the tumor-associated antigen CA125 in patients with advanced ovarian cancer induced by the murine monoclonal anti-idiotype vaccine ACA125. Hybridoma 1997;16(1):33–40. [DOI] [PubMed] [Google Scholar]
- 112.Reinartz S, Wagner U, Giffels P, et al. Immunological properties of a single-chain fragment of the anti-idiotypic antibody ACA125. Cancer Immunol Immunother 2000;49(4–5):186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reinartz S, Hombach A, Kohler S, et al. Interleukin-6 fused to an anti-idiotype antibody in a vaccine increases the specific humoral immune response against CA125+ (MUC-16) ovarian cancer. Cancer Res 2003;63(12):3234–40. [PubMed] [Google Scholar]
- 114.Sabbatini P, Harter P, Scambia G, et al. Abagovomab as maintenance therapy in patients with epithelial ovarian cancer: a phase III trial of the AGO OVAR, COGI, GINECO, and GEICO--the MIMOSA study. J Clin Oncol 2013;31(12):1554–61.** A phase III MIMOSA trial demonstrating abagovomab not to prolong disease free survival.
- 115.Battaglia A, Fossati M, Buzzonetti A, et al. A robust immune system conditions the response to abagovomab (anti-idiotypic monoclonal antibody mimicking the CA125 protein) vaccination in ovarian cancer patients. Immunol Lett 2017;191:35–39. [DOI] [PubMed] [Google Scholar]
- 116.Buzzonetti A, Fossati M, Catzola V, et al. Immunological response induced by abagovomab as a maintenance therapy in patients with epithelial ovarian cancer: relationship with survival-a substudy of the MIMOSA trial. Cancer Immunol Immunother 2014;63(10):1037–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Balachandran VP, Luksza M, Zhao JN, et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 2017;551(7681):512–516.** Expression of MUC16 neoantigens was found for the first time to be associated with long term survival in pancreatic cancer
- 118.Maughan TS, Haylock B, Hayward M, et al. OC125 immunoscintigraphy in ovarian carcinoma: a comparison with alternative methods of assessment. Clin Oncol (R Coll Radiol) 1990;2(4):199–205. [DOI] [PubMed] [Google Scholar]
- 119.Papazefkos V, Michalas S, Papantoniou V, et al. Comparative study of RIS with the 131I-OC 125 F(ab’)2 Mab and CT scan prior to second look operation for ovarian cancer. Eur J Obstet Gynecol Reprod Biol 1990;37(3):271–7. [DOI] [PubMed] [Google Scholar]
- 120.Perkins AC, Powell MC, Wastie ML, et al. A prospective evaluation of OC125 and magnetic resonance imaging in patients with ovarian carcinoma. Eur J Nucl Med 1990;16(4–6):311–6. [DOI] [PubMed] [Google Scholar]
- 121.Finkler NJ, Muto MG, Kassis AI, et al. Intraperitoneal radiolabeled OC 125 in patients with advanced ovarian cancer. Gynecol Oncol 1989;34(3):339–44. [DOI] [PubMed] [Google Scholar]
- 122.Mahe MA, Fumoleau P, Fabbro M, et al. A phase II study of intraperitoneal radioimmunotherapy with iodine-131-labeled monoclonal antibody OC-125 in patients with residual ovarian carcinoma. Clin Cancer Res 1999;5(10 Suppl):3249s–3253s. [PubMed] [Google Scholar]
- 123.Barzen G, Mayr AC, Langer M, et al. Radioimmunoscintigraphy of ovarian cancer with 131-iodine labeled OC-125 antibody fragments. Eur J Nucl Med 1989;15(1):42–8. [DOI] [PubMed] [Google Scholar]
- 124.Baum RP, Lorenz M, Hottenrott C, et al. Radioimmunoscintigraphy using monoclonal antibodies to CEA, CA 19–9 and CA 125. Int J Biol Markers 1988;3(3):177–84. [DOI] [PubMed] [Google Scholar]
- 125.Kalofonos HP, Giannakenas C, Kosmas C, et al. Radioimmunoscintigraphy in patients with ovarian cancer. Acta Oncol 1999;38(5):629–34. [DOI] [PubMed] [Google Scholar]
- 126.Kalofonos HP, Karamouzis MV, Epenetos AA. Radioimmunoscintigraphy in patients with ovarian cancer. Acta Oncol 2001;40(5):549–57. [DOI] [PubMed] [Google Scholar]
- 127.Peltier P, Dutin JP, Chatal JF, et al. Usefulness of imaging ovarian cancer recurrence with In-111-labeled monoclonal antibody (OC 125) specific for CA 125 antigen. The INSERM Research Network (Nantes, Rennes, Reims, Vuillejuif, Saclay. Ann Oncol 1993;4(4):307–11. [DOI] [PubMed] [Google Scholar]
- 128.Vuillez JP, Levrot E, Mousseau M, et al. [Evaluation of the diagnostic usefulness of CA125 immunoscintigraphy for ovarian carcinoma follow-up after treatment: contribution of this technique in Grenoble University Medical Center]. Bull Cancer 1997;84(11):1033–42. [PubMed] [Google Scholar]
- 129.Osmers RG, Rybicki T, Meden H, et al. Does an immunoscintigraphy with OC 125 affect the prognosis of ovarian cancer? Eur J Gynaecol Oncol 1997;18(3):177–82. [PubMed] [Google Scholar]
- 130.Chung JK, Kang SB, Lee HP, et al. Clinical immunoscintigraphy of ovarian carcinoma using iodine-131-labeled 145–9 monoclonal antibody. J Nucl Med 1993;34(10):1651–5. [PubMed] [Google Scholar]
- 131.Goff BA, Bamberg M, Hasan T. Photoimmunotherapy of human ovarian carcinoma cells ex vivo. Cancer Res 1991;51(18):4762–7. [PubMed] [Google Scholar]
- 132.Goff BA, Hermanto U, Rumbaugh J, et al. Photoimmunotherapy and biodistribution with an OC125-chlorin immunoconjugate in an in vivo murine ovarian cancer model. Br J Cancer 1994;70(3):474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Muto MG, Finkler NJ, Kassis AI, et al. Human anti-murine antibody responses in ovarian cancer patients undergoing radioimmunotherapy with the murine monoclonal antibody OC-125. Gynecol Oncol 1990;38(2):244–8. [DOI] [PubMed] [Google Scholar]
- 134.Chen Y, Clark S, Wong T, et al. Armed antibodies targeting the mucin repeats of the ovarian cancer antigen, MUC16, are highly efficacious in animal tumor models. Cancer Res 2007;67(10):4924–32.** First MUC16 antibody drug conjugates developed.
- 135.Leipold D, Mallet WG. Case Study: An Antibody–Drug Conjugate Targeting MUC16 for Ovarian Cancer. In: Phillips GL, editor. Antibody-Drug Conjugates and Immunotoxins: From Pre-Clinical Development to Therapeutic Applications New York, NY: Springer New York; 2013. p. 221–239. [Google Scholar]
- 136.Liu JF, Moore KN, Birrer MJ, et al. Phase I study of safety and pharmacokinetics of the anti-MUC16 antibody-drug conjugate DMUC5754A in patients with platinum-resistant ovarian cancer or unresectable pancreatic cancer. Ann Oncol 2016;27(11):2124–2130.** Phase 1 trial of MUC16 ADC demonstrates preliminary efficacy.
- 137.Almasbak H, Aarvak T, Vemuri MC. CAR T Cell Therapy: A Game Changer in Cancer Treatment. J Immunol Res 2016;2016:5474602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Maus MV, Grupp SA, Porter DL, et al. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood 2014;123(17):2625–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chekmasova AA, Rao TD, Nikhamin Y, et al. Successful eradication of established peritoneal ovarian tumors in SCID-Beige mice following adoptive transfer of T cells genetically targeted to the MUC16 antigen. Clin Cancer Res 2010;16(14):3594–606.** First MUC16 ectodomain specific CAR T-cells developed.
- 140.Koneru M, O’Cearbhaill R, Pendharkar S, et al. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16(ecto) directed chimeric antigen receptors for recurrent ovarian cancer. J Transl Med 2015;13:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Akita K, Yoshida S, Ikehara Y, et al. Different levels of sialyl-Tn antigen expressed on MUC16 in patients with endometriosis and ovarian cancer. Int J Gynecol Cancer 2012;22(4):531–8. [DOI] [PubMed] [Google Scholar]
- 142.Chen K, Gentry-Maharaj A, Burnell M, et al. Microarray Glycoprofiling of CA125 improves differential diagnosis of ovarian cancer. J Proteome Res 2013;12(3):1408–18. [DOI] [PubMed] [Google Scholar]
- 143.Jankovic MM, Milutinovic BS. Glycoforms of CA125 antigen as a possible cancer marker. Cancer Biomark 2008;4(1):35–42. [DOI] [PubMed] [Google Scholar]
- 144.Ricardo S, Marcos-Silva L, Pereira D, et al. Detection of glyco-mucin profiles improves specificity of MUC16 and MUC1 biomarkers in ovarian serous tumours. Mol Oncol 2015;9(2):503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saldova R, Struwe WB, Wynne K, et al. Exploring the glycosylation of serum CA125. Int J Mol Sci 2013;14(8):15636–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dharma Rao T, Park KJ, Smith-Jones P, et al. Novel monoclonal antibodies against the proximal (carboxy-terminal) portions of MUC16. Appl Immunohistochem Mol Morphol 2010;18(5):462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rao TD, Fernandez-Tejada A, Axelrod A, et al. Antibodies Against Specific MUC16 Glycosylation Sites Inhibit Ovarian Cancer Growth. ACS Chem Biol 2017;12(8):2085–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]