Abstract
Purpose:
To evaluate the association between colony-stimulating factor (CSF) use and the risk of developing myelodysplastic syndromes or acute myeloid leukemia (t-MDS/AML) among a large cohort of elderly non-Hodgkin’s lymphoma (NHL) patients treated with chemotherapy.
Methods:
We identified 13,203 NHL patients from the Surveillance, Epidemiology, and End Results (SEER)-Medicare database diagnosed from 1992 to 2002. Patients were followed from their initial chemotherapy date until the date of developing t-MDS/AML, death or last follow-up (October 31, 2006).
Results:
Overall, 40% (n=5,266) of patients received CSF. During the follow-up period (median follow-up=2.9 years, ranging 1–14.7 years), 272 (5.2%) of patients receiving CSF developed t-MDS/AML compared to 230 (2.9%) of patients who did not (log-rank test p<0.0001). Five-year incidence of t-MDS/AML for patients receiving CSF was 14.1/1,000 person-years compared to 8.3/1,000 person-years for patients not receiving CSF. In a multivariable Cox regression analysis adjusting for gender, histology, stage, comorbidities, radiation and chemotherapy agent, CSF use was independently associated with a 53% increased risk of t-MDS/AML (hazard ratio=1.53; 95% CI=1.26–1.84). The observed association between CSF use and t-MDS/AML persisted across histologic subgroups (i.e. diffuse large B cell lymphoma, follicular lymphoma, and others). Patients who received both CSF and antimetabolite chemotherapy had a 2.5 fold increased risk of t-MDS/AML (hazard ratio=2.49; 95% CI=1.91–3.26) compared to patients with who received neither agents.
Conclusions:
This first large population-based study showed that the administration of CSF among elderly NHL patients receiving chemotherapy was associated with an increased risk of t-MDS/AML, even though the absolute risk was low.
Keywords: non-Hodgkin’s lymphoma, colony-stimulating factor, chemotherapy, myelodysplastic syndromes, acute myeloid leukemia
Condensed abstract
The administration of colony-stimulating factor among elderly non-Hodgkin’s lymphoma patients receiving chemotherapy was associated with an increased risk of myelodysplastic syndromes or acute myeloid leukemia even though the absolute risk was low.
Introduction
Therapy-related myelodysplastic syndromes and acute myeloid leukemia (t-MDS/AML), defined as MDS or AML occurring after chemotherapy and/or radiation therapy, are devastating long-term complications of cancer therapy. Initially recognized more than 30 years ago in multiple myeloma patients treated with melphalan, these complications have been reported subsequent to the treatment for many cancers.(1, 2) The general use of chemotherapy and/or radiotherapy has improved cancer survival; however, it has been reported that 1% to 15% of long-term cancer survivors treated with combination chemotherapy and radiotherapy develop t-MDS/AML.(3) Among non-Hodgkin’s lymphoma (NHL) patients treated with chemotherapy, most studies have reported a 10-year cumulative risk ranging from 4.6% to 10%.(4–10) Prognosis following a diagnosis of t-MDS/AML is bleak, with a median survival of less than 2 years.(11)
Granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) are supportive care agents intended to minimize the risk of febrile neutropenia in patients receiving intensive chemotherapy. There has been speculation suggesting that G-CSF and GM-CSF (collectively referred to as CSFs) may be associated with an increased risk of t-MDS/AML. Brodsky et al. (12), as early as 1996, suggested that the increased risk of AML in patients enrolled in clinical trials may be at least partially attributed to CSF administration among patients receiving intensive chemotherapy. Numerous clinical trials have monitored for t-MDS/AML as adverse events (13–20) following chemotherapy, and some studies have addressed the question specifically.(21–23) Recently, two studies utilizing the Surveillance, Epidemiology, and End Results (SEER)-Medicare data have explored this relationship in a population-based cohort of breast cancer patients,(21, 22) in which one study found a positive association (21) while the other study found no association.(22) The only other population-based study, conducted also among breast cancer patients, found a positive association.(23) In the only published study among patients with a hematologic malignancy, Relling et al (13) found that pediatric leukemia patients receiving G-CSF had an increased risk of t-MDS/AML.
While NHL is known to be very responsive to intensive chemotherapy, elderly patients are particularly susceptible to the detrimental myelosuppressive effects of chemotherapy.(24) Therefore, the potential benefit of CSF use is likely to be significant among elderly NHL patients due to the possibility of allowing for more dose-intense and dose-dense therapies while decreasing the likelihood of neutropenia. In fact, recently updated American Society of Clinical Oncology (ASCO) recommendations consider age as “one of the conditions for which prophylactic use of growth factors may be indicated irrespective of the threshold risk of neutropenia”.(25)
Incidence of t-MDS/AML among NHL patients is among the highest of any cancer type (10), possibly due to the underlying susceptibility of this patient population to hematologic malignancies. We therefore posit that while it is plausible that NHL patients, especially elderly patients, may benefit from the therapeutic effects of CSFs, they may also be particularly susceptible to the potential long-term leukemogenic effects of these agents. The purpose of this study, therefore, was to explore the relationship between CSF use and the incidence of t-MDS/AML among a large nationwide and population-based cohort of elderly NHL patients receiving chemotherapy with up to 15 years of follow-up.
Patients and Methods
Data Source
This study used data from the merged Surveillance, Epidemiology, and End Results (SEER)-Medicare database. The SEER database program is a population-based registry sponsored by the National Cancer Institute that contains information on all newly diagnosed cancer cases. This study included the following geographic areas: Detroit, Atlanta, Seattle; and the states of California, Connecticut, Iowa, New Mexico, Utah, Hawaii, Kentucky, Louisiana, and New Jersey. SEER data are highly valid and complete with a completeness of case ascertainment of over 98%. (26)
The SEER registry collects information on patient demographics, tumor characteristics, stage at diagnosis, treatment within 4 months of diagnosis, and date and cause of death. This registry data is linked to claims data from Medicare, which is the primary insurer for 97% of the US population 65 years and older. All Medicare beneficiaries receive Part A coverage which covers inpatient care, skilled nursing, home health, and hospice care. Ninety-five percent of beneficiaries also subscribe to part B of Medicare to obtain benefits that cover physician services and outpatient care.(26) The Committee for the Protection of Human Subjects at the University of Texas Health Science Center at Houston approved this study.
Study Population and patient characterization
This retrospective cohort study included incident cases of NHL diagnosed from January 1, 1992 to December 13, 2002 who received chemotherapy within 12 months of diagnosis. Patients enrolled in a health maintenance organization during any period of the study time period were excluded because data were unavailable for these periods. Patients who did not participate in both Medicare parts A and B during any month were also excluded due to potential incomplete data.
Patients were characterized with respect to clinical and demographic variables available in the SEER-Medicare data as well as clinical and treatment characteristics that were abstracted from the Medicare claims data. Due to the reported reliability for differentiating certain subtypes in the SEER data (27), we specifically identified diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma, and grouped the remaining histologic subtypes as ‘other’ due relatively small number of cases. Chemotherapy and radiation therapy were characterized and quantified using International Classification of Diseases (ICD) diagnosis codes, ICD Procedural codes, Current Procedural Terminology (CPT) codes, Healthcare Common Procedural Coding System (HCPCS) codes, and revenue center codes.(26) The following codes were used for defining chemotherapy: ICD-9 CM procedure code 9925 for chemotherapy infusion/injection; CPT codes 96400–96549, J9000 – J9999 codes, and Q0083 – Q0085; revenue center codes 0331, 0332, and 0335; and ICD-9 V codes V58.1, V66.2, and V67.2. Chemotherapy use was stratified by type (e.g. alkylating agent, topoisomerase II inhibitors, anthracyclines, and antimetabolites) using CPT codes. The use of CSF was identified by the procedure codes of J1440 and J1441 (for G-CSF) and J2820 (for GM-CSF). Incidence of secondary MDS/AML was identified by two or more claims with a primary or secondary diagnosis of AML/MDS (ICD-9 codes: myeloid leukemia (205.xx); monocytic leukemia (206.xx); MDS (238.7)) occurring ≥ 30 days apart of each other with the initial diagnosis occurring 1 year or longer after the NHL diagnosis. Claims before diagnosis were used to identify pre-existing comorbidities. Comorbidities were aggregated to formulate the NCI comorbidity index, a revised version of the Charlson comorbidity index.(28)
Data Analysis
Patients were described with respect to demographic, clinical, and treatment characteristics overall and stratified by CSF use to identify potential underlying differences across exposure strata of the population. We used chi-square tests and t-tests to compare differences in patients by CSF status. Kaplan-Meier graphs and corresponding log-rank tests were used to compare the incidence of t-MDS/AML by CSF use. Follow-up time was defined as time from initial chemotherapy start date to the first development of t-MDS/AML. Patients who did not develop t-MDS/AML were censored at the date of death or end of study date (October 31, 2006). Cox proportional hazards modeling was used to estimate the association between CSF use and the development of t-MDS/AML after controlling for potential confounders. The proportionality assumption was confirmed using goodness of fit test developed by Harrell and Lee.(29) To further control for confounding, we calculated a propensity score and included this in a separate Cox regression model. Briefly, this score was calculated based on the probability of receiving CSF as calculated using logistic regression analysis in which CSF status (yes/no) was the dependent variable and patient demographic/clinical/treatment characteristics were considered as possible independent variables. Finally, because of plausible interactive effects between CSF use and specific chemotherapy agents on t-MDS/AML risk, we evaluated these interactions by including the product term of CSF and specific chemotherapy agent in separate Cox regression models.
Results
We identified 13,203 NHL patients from the SEER-Medicare database who received chemotherapy within 12 months of diagnosis and met the other eligibility criteria for this study. The overall median age at diagnosis was 74 years (range: 65–102 years). Fifty-three percent (n=7,051) of patients were female, and a large majority (n=11,776; 90%) were non-Hispanic white and lived in an urban setting (n=11,877; 90%). Forty-four percent (n=5,861) of patients had a diagnosis of diffuse large B-cell lymphoma; 18% (n=2,428) had follicular lymphoma; 28% (n=3,665) had other histologies; and 9% (n=1,249) had an unknown histology. Patient distribution by stage at diagnosis was 29% (n=3,564) stage I, 18% (n=2,214) stage II, 16% (n=1,971) stage III, and 37% (n=4,519) with stage IV. A large majority of patients (n=8,216; 62%) of patients had a low comorbidity burden (comorbidity index of ≤1).
Tables 1 and 2 show patient demographic, clinical, and treatment characteristics by CSF use. Overall, 40% (n=5,266) of patients with chemotherapy received CSF during the follow-up period. Most patients with CSF received only G-CSF (n=4,581; 87%), while 316 (6%) received only GM-CSF and 369 (7%) received both G-CSF and GM-CSF. Patients with CSF were more likely to have been diagnosed recently. While other statistically significant differences were observed by CSF status (urban residence, age at diagnosis, race/ethnicity, marital status, socioeconomic status, stage, and histology), the magnitude of these observed differences were relatively small (Table 1). With regard to patient treatment, those with CSF were more likely to be treated with agents known to be associated with t-MDS/AML development. For example, 92% (n=4,851) of patients receiving CSF were treated with alkylating agents compared to 77% (n=6,100) of patients not receiving CSF, and 33% (n=1,728) of patients receiving CSF were treated with topoisomerase II inhibitors compared to 20% (n=1,600) of those not receiving CSF. Furthermore, patients receiving CSF had a significantly higher number of chemotherapy administration services (mean 27 days vs. 13 days, p<0.0001).
Table 1.
Patient demographic and clinical characteristics by colony-stimulating factor (CSF) use
| CSF Use | ||||
|---|---|---|---|---|
| Factor | Overall (N=13,203) |
Yes (N=5,266) |
No (N=7,937) |
P-value |
| N (%) | N (%) | N (%) | ||
| Year of Diagnosis | ||||
| 1992–1993 | 1,759 (13.3) | 318 (6.0) | 1,441 (18.2) | |
| 1994–1995 | 1,837 (13.9) | 673 (12.8) | 1,164 (14.7) | |
| 1996–1997 | 1,912 (14.5) | 827 (15.7) | 1,085 (13.7) | |
| 1998–1999 | 1,852 (14.0) | 800 (15.2) | 1,052 (13.3) | |
| 2000–2001 | 3,778 (28.6) | 1,841 (35.0) | 1,937 (24.4) | |
| 2002 | 2,065 (15.6) | 807 (15.3) | 1,258 (15.8) | <0.001 |
| Urban Residence | ||||
| No | 1,326 (10.0) | 407 (7.7) | 919 (11.6) | |
| Yes | 11,877 (90.0) | 4,859 (92.3) | 7,018 (88.4) | <0.001 |
| Age at Diagnosis | ||||
| Mean (SD) | 74.94 (6.35) | 74.38 (5.92) | 75.32 (6.59) | <0.001 |
| Median (Range) | 74 (65 – 102) | 74 (65 – 98) | 75 (65 – 102) | |
| 65–69 | 3,090 (23.4) | 1,276 (24.2) | 1,814 (22.9) | |
| 70–74 | 3,598 (27.3) | 1,548 (29.4) | 2,050 (25.8) | |
| 75–79 | 3,324 (25.2) | 1,389 (26.4) | 1,935 (24.4) | |
| 80–84 | 2,107 (16.0) | 744 (14.1) | 1,363 (17.2) | <0.001 |
| 85+ | 1,084 (8.2) | 309 (5.9) | 775 (9.8) | |
| Gender | ||||
| Male | 6,152 (46.6) | 2,469 (46.9) | 3,683 (46.4) | |
| Female | 7,051 (53.4) | 1,797 (53.1) | 4,254 (53.6) | 0.57 |
| Race/Ethnicity | ||||
| Non-Hispanic White | 11,776 (89.2) | 4,716 (89.6) | 7,060 (89.0) | |
| Hispanic | 236 (1.8) | 100 (1.9) | 136 (1.7) | |
| Black | 441 (3.3) | 155 (2.9) | 286 (3.6) | |
| Asian | 404 (3.1) | 176 (3.3) | 228 (2.9) | |
| Other | 304 (2.3) | 104 (2.0) | 200 (2.5) | |
| Unknown | 42 (0.3) | 15 (0.3) | 27 (0.3) | 0.04 |
| Marital Status | ||||
| Yes | 7,797 (59.1) | 3,291 (62.5) | 4,506 (56.8) | |
| No | 4,838 (36.6) | 1,778 (33.8) | 3,060 (38.6) | |
| Unknown | 568 (4.3) | 197 (3.7) | 371 (4.7) | <0.001 |
| SES Quartiles | ||||
| 1 (High) | 3,376 (25.6) | 1,396 (26.5) | 1,980 (24.9) | |
| 2 | 3,251 (24.6) | 1,349 (25.6) | 1,902 (24.0) | |
| 3 | 3,305 (25.0) | 1,249 (23.7) | 2,056 (25.9) | |
| 4 (Low) | 3,065 (23.2) | 1,189 (22.6) | 1,876 (23.6) | |
| Missing | 206 (1.6) | 83 (1.6) | 123 (1.5) | 0.007 |
| Histology | ||||
| Diffuse Large B-cell | 5,861 (44.4) | 2,538 (48.2) | 3,323 (41.9) | |
| Follicular | 2,428 (18.4) | 922 (17.5) | 1,506 (19.0) | |
| Other | 3,665 (27.8) | 1,384 (26.3) | 2,281 (28.7) | |
| Unknown | 1,249 (9.5) | 422 (8.0) | 827 (10.4) | <0.001 |
| Stage at Diagnosis | ||||
| I | 3,564 (27.0) | 1,336 (25.4) | 2,228 (28.1) | |
| II | 2,214 (16.8) | 888 (16.9) | 1,326 (16.7) | |
| III | 1,971 (14.9) | 888 (16.9) | 1,083 (13.6) | |
| IV | 4,519 (34.2) | 1,835 (34.8) | 2,684 (33.8) | |
| Unknown | 935 (7.1) | 319 (6.1) | 616 (7.8) | <0.001 |
| Comorbidity Score | ||||
| 0 | 8,216 (62.2) | 3,308 (62.8) | 4,908 (61.8) | |
| 1 | 3,148 (23.8) | 1,257 (23.8) | 1,891 (23.8) | |
| ≥2 | 1,839 (13.9) | 701 (13.3) | 1,138 (14.3) | 0.24 |
Table 2.
Patient Treatment Characteristics by colony-stimulating factor (CSF) Use
| CSF Use | ||||
|---|---|---|---|---|
| Factor | Overall (N=13,203) | Yes (N=5,266) | No (N=7,937) | P |
| N (%) | N (%) | N (%) | ||
| CSF Use | ||||
| No | 7,937 (60.1%) | - | ||
| Yes | 5,266 (39.9) | 5,266 (100%) | ||
| G-CSF* | 4,581 (34.7) | 4,581 (87.0) | ||
| GM-CSF* | 316 (2.4) | 316 (6.0) | ||
| Both | 369 (2.8) | 369 (7.0) | ||
| Chemotherapy Agent | ||||
| Alkylating Agents | 10,951 (82.9) | 4,851 (92.1) | 6,100 (76.9%) | <0.0001 |
| Topo-isomerase II inhibitors | 3,328 (25.2) | 1,728 (32.8) | 1,600 (20.2) | <0.0001 |
| Anthracyclines | 7,068 (53.5) | 3,518 (66.8) | 3,550 (44.7) | <0.0001 |
| Antimetabolites | 3,148 (23.8) | 1,567 (29.8) | 1,581 (19.9) | <0.0001 |
| Platinums | 520 (3.9) | 358 (6.8) | 162 (2.0) | <0.0001 |
| Taxanes | 132 (1.0) | 85 (1.6) | 47 (0.6) | <0.0001 |
| Vinca Alkaloids | 10,718 (81.2) | 4,754 (90.3) | 5,964 (75.1) | <0.0001 |
| Targeted therapy | 6,937 (52.5) | 3,006 (57.1) | 3,931 (49.5) | <0.0001 |
| Other | 1,941 (14.7) | 797 (15.1) | 1,144 (14.4) | 0.25 |
| Number of Chemotherapy claims | ||||
| Mean (SD) | 18.9 (23.1) | 27.7 (29.6) | 13.1 (14.9) | |
| Median (Range) | 11 (1 – 394) | 18 (1 – 394) | 8 (1 – 212) | <0.0001 |
| Radiation Therapy | ||||
| No | 8,194 (62.1) | 3,125 (59.3) | 5,069 (63.9) | |
| Yes | 5,009 (37.9) | 2,141 (40.7) | 2,868 (36.1) | <0.0001 |
G-CSF denotes granulocyte colony-stimulating factor, and GM-CSF denotes granulocyte-macrophage colony-stimulating factor.
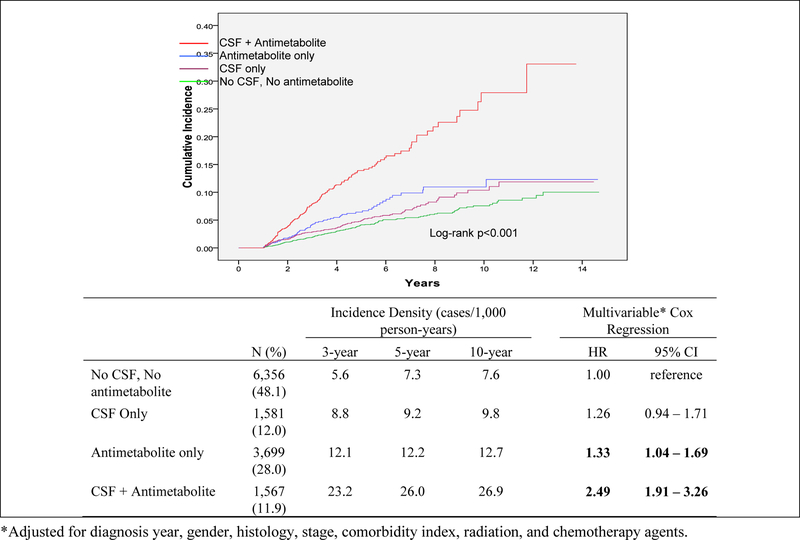
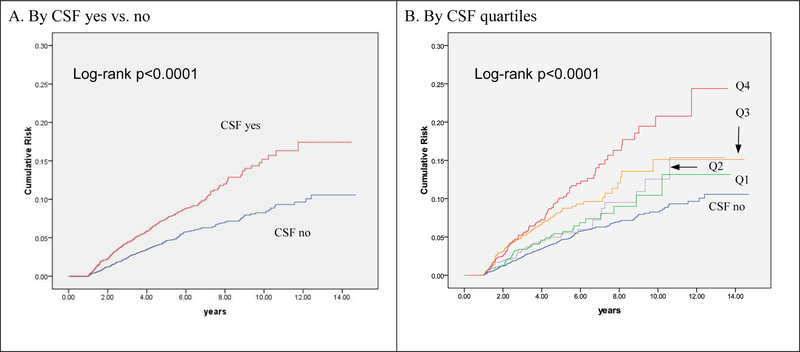
Figure 2 show the cumulative incidence of t-MDS/AML by the use of CSF and by number of CSF claims (quartiles). After a median follow-up time of 2.9 years (range: 1–14.7 years), 502 (3.8%) patients developed t-MDS/AML. Of patients who did not develop t-MDS/AML (n=12,701), 68% (n=8,589) died while 32% (n=4,112) were alive without t-MDS/AML at the end of the follow-up period. The median time to t-MDS/AML development was 3 years (range: 1–12 years). Over the follow-up period, a total of 272 (5.2%) of patients receiving CSF developed t-MDS/AML compared to 230 (2.9%) of patients who did not receive CSF (log-rank test p<0.0001). Three-year incidence of t-MDS/AML for patients receiving CSF was 13.2/1,000 person-years compared to 6.9/1,000 person-years for patients not receiving CSF (95% CI for rate difference= 3.7–8.8 cases/1,000 person-years). Five-year incidence of t-MDS/AML for patients receiving CSF was 14.1/1,000 person-years compared to 8.3/1,000 person-years for patients not receiving CSF (95% CI for rate difference= 3.6–8.1 cases/1,000 person-years). When we evaluated cumulative incidence of t-MDS/AML by number of CSF claims (categorized by quartile) (Figure 1), we found a significant dose-response effect (log-rank p<0.0001) with incidence increasing by quartile. Those in the highest quartile (23+ claims) had a 10 year cumulative incidence of 21%, followed by the 3rd quartile (10–22 claims; 15%), 2nd quartile (4–9 administrations; 13%), and 1st quartile (1–3 claims; 12%). The lowest incidence (8%) was among patients that received no CSF.
Figure 2.
Joint Effect of Colony-stimulating Factor (CSF) and Antimetabolites on the risk of developing myelodysplastic syndromes or acute myeloid leukemia (MDS/AML)
Figure 1.
Cumulative Incidence of t-MDS/AML by CSF use yes vs. no and by quartiles of CSF claims
We used Cox Proportional hazards modeling to estimate the association between CSF use and time to the development of t-MDS/AML after adjusting for potential confounders (Table 3). CSF use remained significantly associated with t-MDS/AML risk (hazard ratio (HR)=1.53; 95% Confidence Interval: 1.26–1.84) after adjusting for gender, histology, stage, comorbidities, chemotherapy service claims, and chemotherapy agent. The dose-response effect remained significant, with increasing risk by quartile of more CSF claims. To further control for confounding, we included a propensity score to adjust for the baseline probability of receiving CSF in a separate model and found that CSF use remained significantly associated with a 42% increased risk of t-MDS/AML (1.42, 1.18–3.98). Again, patients receiving more doses of CSF had the higher risk of developing t-MDS/AML (1.83, 1.40–2.40).
Table 3.
Hazard ratio of risk of developing myelodysplastic syndromes or acute myeloid leukemia (MDS/AML) by colony-stimulating factor (CSF) use
| Univariable | Covariate-Adjusted | Propensity score-adjusted | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |||
| CSF Use | |||||||||||
| No | 1.00 | 1.00 | 1.00 | ||||||||
| Yes | 1.73 | 1.45 – 2.07 | <0.001 | 1.53 | 1.26 – 1.84 | <0.001 | 1.42 | 1.18 – 3.98 | <0.001 | ||
| Q1 (1–3 administrations) | 1.29 | 0.94 – 1.77 | 0.12 | 1.22 | 0.89 – 1.68 | 0.22 | 1.20 | 0.87 – 1.65 | 0.28 | ||
| Q2 (4–9 administrations) | 1.28 | 0.93 – 1.77 | 0.13 | 1.20 | 0.87 – 1.66 | 0.26 | 1.14 | 0.82 – 1.57 | 0.45 | ||
| Q3 (10–22 administrations) | 1.84 | 1.42 – 2.38 | <0.001 | 1.70 | 1.43 – 2.41 | <0.001 | 1.57 | 1.19 – 2.05 | 0.001 | ||
| Q4 (23+ administrations) | 2.35 | 1.86 – 2.97 | <0.001 | 1.75 | 1.75 – 2.82 | <0.001 | 1.83 | 1.40 – 2.40 | <0.001 | ||
| p-trend | <0.001 | <0.001 | <0.001 | ||||||||
| Year of Diagnosis | |||||||||||
| 1992–1995 | 1.00 | ||||||||||
| 1996–1999 | 0.99 | 0.80 – 1.25 | 0.98 | ||||||||
| 2000–2002 | 1.02 | 0.82 – 1.27 | 0.87 | ||||||||
| Age at diagnosis | 0.99 | 0.98 – 1.01 | 0.50 | ||||||||
| Gender | |||||||||||
| Female | 1.00 | 1.00 | |||||||||
| Male | 1.30 | 1.09 – 1.55 | 0.003 | 1.22 | 1.02 – 1.46 | 0.03 | |||||
| Race | |||||||||||
| Non-Hispanic White | 1.00 | ||||||||||
| Hispanic | 1.21 | 0.68 – 2.15 | 0.51 | ||||||||
| Black | 1.03 | 0.62 – 1.72 | 0.92 | ||||||||
| Asian | 0.95 | 0.58 – 1.57 | 0.84 | ||||||||
| Other | 0.64 | 0.28 – 1.42 | 0.27 | ||||||||
| SES Quartile | |||||||||||
| 1 (High) | 1.00 | ||||||||||
| 2 | 1.09 | 0.85 – 1.39 | 0.50 | ||||||||
| 3 | 1.05 | 0.82 – 1.35 | 0.69 | ||||||||
| 4 (Low) | 1.11 | 0.86 – 1.43 | 0.44 | ||||||||
| Histology | |||||||||||
| Diffuse Large B-cell | 1.00 | 1.00 | |||||||||
| Follicular | 1.23 | 0.96 – 1.58 | 0.10 | 0.94 | 0.73 – 1.22 | 0.65 | |||||
| Other | 1.95 | 1.58 – 2.41 | <0.001 | 1.28 | 1.01 – 1.62 | 0.04 | |||||
| Stage at Diagnosis | |||||||||||
| I | 1.00 | 1.00 | |||||||||
| II | 0.63 | 0.44 – 0.91 | 0.02 | 1.19 | 0.89 – 1.60 | 0.25 | |||||
| III | 0.76 | 0.51 – 1.12 | 0.17 | 1.58 | 1.19 – 2.11 | 0.002 | |||||
| IV | 1.18 | 0.81 – 1.73 | 0.40 | 1.50 | 1.18 – 1.81 | 0.001 | |||||
| Comorbidity Score | |||||||||||
| 0 | 1.00 | ||||||||||
| 1 | 1.24 | 1.01 – 1.53 | 0.004 | 1.24 | 1.00 – 1.53 | 0.050 | |||||
| >2 | 1.45 | 1.10 – 1.91 | 0.008 | 1.40 | 1.06 – 1.85 | 0.018 | |||||
| Chemotherapy | |||||||||||
| Duration (DOS) | 1.01 | 1.009 – 1.012 | <0.001 | 1.007 | 1.005 – 1.009 | <0.001 | |||||
| Chemotherapy Agent (yes vs. no) | |||||||||||
| Alkylating Agents | 0.68 | 0.55 – 0.85 | 0.001 | 1.04 | 0.73 – 1.47 | 0.83 | |||||
| Topo-isomerase II inhibitor | 1.31 | 1.08 – 1.59 | 0.007 | 0.89 | 0.71 – 1.11 | 0.30 | |||||
| Anthracyclines | 0.69 | 0.58 – 0.82 | <0.001 | 0.79 | 0.63 – 0.98 | 0.03 | |||||
| Antimetabolite | 2.43 | 2.03 – 2.90 | <0.001 | 1.58 | 1.28 – 1.95 | <0.001 | |||||
| Platinums | 1.71 | 1.19 – 2.48 | 0.004 | 1.11 | 0.74 – 1.65 | 0.62 | |||||
| Taxanes | 1.81 | 0.90 – 3.64 | 0.10 | 0.76 | 0.55 – 1.07 | 0.11 | |||||
| Vinca Alkaloids | 0.61 | 0.49 – 0.75 | <0.001 | 1.09 | 0.54 – 2.22 | 0.81 | |||||
| Targeted therapy | 1.37 | 1.14 – 1.65 | 0.001 | 1.11 | 0.92 – 1.34 | 0.29 | |||||
| Other | 1.37 | 1.10 – 1.70 | 0.005 | 1.17 | 0.93 – 1.46 | 0.18 | |||||
| Radiation Therapy | |||||||||||
| No | 1.00 | ||||||||||
| Yes | 1.06 | 0.89 – 1.26 | 0.53 | ||||||||
In analyses stratified by histologic subtype, we found that CSF use remained a significant predictor of t-MDS/AML for patients within each of the major histologic subgroups. For patients with diffuse large B-cell lymphoma, CSF use was associated with a 36% increased risk of t-MDS/AML (1.36, 1.04–1.89); for patients with follicular lymphoma there was a 90% increased risk (1.90, 1.21–2.98); and for patients with other histologic types there was an 80% increased risk (1.80, 1.30–2.49).
We tested for interactions between CSF use and chemotherapy agents on the risk of t-MDS/AML. No significant interactions were observed between CSF and alkylating agents, topoisomerase II inhibitors, anthracyclines, or vinca alkaloids. We did, however, find a significant interaction between CSF and antimetabolite use (p-interaction = 0.04). Patients who were treated with both antimetabolites and CSF had a 2.5-fold increased risk of t-MDS/AML (HR=2.49; 95% CI: 1.91–3.26) compared to patients with who received neither agent (Figure 2).
Discussion
Results of this study support our hypothesis that CSF use among elderly NHL patients receiving chemotherapy was significantly associated with an increased risk of developing t-MDS/AML. We found that CSF use overall was associated with a 1.5-fold increased risk of developing t-MDS/AML, which increased with increasing number/dose of CSFs. This association persisted within histologic subtypes. In addition, we found a significant interaction between CSF use and antimetabolite chemotherapy. Patients who received both CSFs and antimetabolites had a 2.5-fold increased risk of t-MDS/AML. This finding has clinical significance considering that approximately 10% of the overall population received this therapeutic combination.
Our results are in line with previous studies of t-MDS/AML among NHL patients.(4–9) We found that the 10-year cumulative risk of t-MDS AML ranged from approximately 5% to 10% depending on CSF status, which is very similar to the range of 4.6% - 10% as reported in previous studies.(4–9) The main outlier was the subset of our population who received both antimetabolites and CSF, who had a 10-year cumulative incidence of approximately 25% (Figure 1).
The biological plausibility behind the hypothesis that CSFs may be leukemogenic is based on the observation that CSFs not only stimulate the proliferation and differentiation of hematopoietic stem cells, but also interfere with apoptosis.(12) This suppression of apoptotic cell regulation could contribute to a leukemogenic effect of growth factors either independently or by interacting with cytotoxic therapies. Lieschke et al. (30) reported that CSF induced the growth of AML blast cells in vitro in about 50% of cases; however, they were not found to be leukemogenic.
An important finding of this study was the interactive effect between CSF and antimetabolite therapy on t-MDS/AML risk. Several studies have demonstrated an increased risk of t-MDS/AML among patients receiving nucleoside analogue therapy, including antimetabolite therapy.(31) These agents incorporate themselves into the DNA, leading to DNA damage and interference with repair pathways.(31–36) It is therefore plausible that antimetabolites might interact synergistically with the proliferative and/or anti-apoptotic activity of CSFs to initiate and then facilitate leukemogenesis.
To the best of our knowledge, this is the first large nationwide and population-based study to evaluate the association between CSF use and t-MDS/AML risk among NHL patients. The strengths of this study include the use of SEER-Medicare data, which allows for the design of large population-based studies of long-term outcomes following cancer therapy. While many clinical trials of CSF use that monitored for t-MDS/AML as adverse events were hampered by insufficient follow-up time or smaller sample size, this study had a large population with a long follow-up time. In addition, while clinical trial data typically have strong internal validity, the application of sound study design to population-based data has the potential to yield results that are more generalizable to community-based care and may be more reflective of real-world outcomes. Furthermore, the focus on elderly NHL patients also has a clinical significance of this study, as this is a population for whom treatment choice is not straightforward and therefore identification of patient subpopulations that may be at increased risk for adverse events (including t-MDS/AML) could provide valuable guidance in delineating treatment options.
This study had its limitations. While the results are more generalizable to the elderly Medicare population, they may have limited application to younger populations and to populations covered under managed care or other private insurance. However, with the aging population of the US, it will become increasingly important to focus attention on this demographic cohort. Other limitations are related to the nature of the data used to conduct this study. Using claims data, one cannot fully quantify and control for chemotherapy dose and intensity, which are likely significant confounders. We did attempt to control for this confounding by including the number of chemotherapy administration as a covariate in the multivariable analyses. While this might be considered a reasonable proxy for dose and intensity, the possibility for residual confounding remains. In addition, the ‘other’ category of NHL included many types of NHL that may be heterogeneous in receiving chemotherapy, CSF and risk of t-MDS/AML. Small sample sizes of these subtypes of NHL limited the generation of meaningful results, thus requiring caution in interpreting the findings for these subtypes combined. Furthermore, due to the inability to determine exact dosing for CSF, it was not possible to categorize dosing according to clinically meaningful cut points; instead we had to categorize the number of CSF claims by quartile to evaluate dose-response effect. Due to the observational nature of the data used for this study, it is likely that a certain degree of selection bias was introduced. For example, many patients received CSF for primary prevention of febrile neutropenia and infection, while some received it for treatment after neutropenia and infection. There was no accurate information from this study data on marrow function at the time of CSF, so that it was difficult to quantify the risk of t-MDS/AML that was associated with disease itself or associated with the receipt of CSF. Also, although we studied only those patients who received chemotherapy within 12 months of diagnosis, the sequence of various chemotherapy agents (such as first or second line of antimetabolites) was not well ascertained, which might have confounded the study findings, even though we adjusted for the number of chemotherapy claims and the number of CSF claims. We attempted to thoroughly describe our treatment cohorts to the greatest extent possible in order to identify potential confounding factors that might be unbalanced between the two comparison groups, and we tried to evaluate this using separate models and propensity scores to control for selection bias. However, we cannot entirely rule out the possibility that there may be factors associated with CSF use that may increase the risk of t-MDS/AML. Therefore, it will become increasingly important in the future to incorporate more clinically-rich data (e.g. emergency room visits and lab data) into studies in order to explore the causal mechanisms underlying observed associations. Future studies should also incorporate markers of genetic susceptibility to fully evaluate the etiology of t-MDS/AML.
In conclusion, this population-based study documented that CSF use among elderly NHL patients treated with chemotherapy was associated with an increased risk of developing t-MDS/AML. Our findings suggesting an interactive effect between CSF and antimetabolites on t-MDS/AML risk highlight the potential clinical importance of exploring plausible interactions between therapeutic agents in the ‘real world’. Further studies, both observational and clinical, are necessary to verify these results in younger population and to determine the potential clinical implications of this observed interaction. These studies should evaluate CSF use relative to other clinical outcomes as well as cost-effectiveness to more fully explain the appropriate use of CSF in the care of elderly NHL patients.
Acknowledgments:
We acknowledge the efforts of the National Cancer Institute; Center for Medicare and Medicaid Services; Information Management Services, Inc.; and the Surveillance, Epidemiology, and End Results Program tumor registries in the creation of this database. The interpretation and reporting of these data are the sole responsibilities of the authors. This study was supported in part by a grant from the Agency for Healthcare Research and Quality (R01-HS016743).
References
- 1 ).Vega-Stromberg T Chemotherapy-induced secondary malignancies. J Infus Nurs 2003;26:353–61. [DOI] [PubMed] [Google Scholar]
- 2 ).Godley L, Larson R. The syndrome of therapy-related myelodysplasia and myeloid leukemia. In: Bennett J, ed. The Myelodysplastic Syndromes: Pathology and Clinical Management Marcel Dekker, Inc, 2002:139–76. [Google Scholar]
- 3 ).Leone G, Mele L, Pulsoni A, Equitani F, Pagano L. The incidence of secondary leukemias. Haematologica 1999;84:937–945. [PubMed] [Google Scholar]
- 4 ).Travis LB, Curtis RE, Stovall M, et al. Risk of leukemia following treatment for non-Hodgkin’s lymphoma. J Natl Cancer Inst 1994;86:1450–7. [DOI] [PubMed] [Google Scholar]
- 5 ).Greene MH, Wilson J. Second cancer following lymphatic and hematopoietic cancers in Connecticut, 1935–82. Natl Cancer Inst Monogr 1985;68:191–217. [PubMed] [Google Scholar]
- 6 ).Ingram L, Mott MG, Mann JR, et al. Second malignancies in children treated for non-Hodgkin’s lymphoma and T-cell leukaemia with the UKCCSG regimens. Br J Cancer 1987;55:463–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7 ).Lavey RS, Eby NL, Prosnitz LR. Impact on second malignancy risk of the combined use of radiation and chemotherapy for lymphomas. Cancer 1990;66:80–8. [DOI] [PubMed] [Google Scholar]
- 8 ).Pedersen-Bjergaard J, Ersbøll J, et al. Risk of acute nonlymphocytic leukemia and preleukemia in patients treated with cyclophosphamide for non-Hodgkin’s lymphomas. Comparison with results obtained in patients treated for Hodgkin’s disease and ovarian carcinoma with other alkylating agents. Ann Intern Med 1985;103:195–200. [DOI] [PubMed] [Google Scholar]
- 9 ).Pui CH. Therapy-related myeloid leukaemia. Lancet 1990;336:1130–1. [DOI] [PubMed] [Google Scholar]
- 10.) Armitage JO, Carbone PP, Connors JM, et al. Treatment-related myelodysplasia and acute leukemia in non-Hodgkin’s lymphoma patients. J Clin Oncol 2003;21:897–906. [DOI] [PubMed] [Google Scholar]
- 11.) Park D, Koeffler H. Therapy-related myelodysplastic syndromes. Sem Hematol 1996; 33:256–273. [PubMed] [Google Scholar]
- 12.) Brodsky RA, Bedi A, Jones RJ. Are growth factors leukemogenic? Leukemia 1996;10:175–7. [PubMed] [Google Scholar]
- 13.) Relling MV, Boyett JM, Blanco JG, et al. Granulocyte colony-stimulating factor and the risk of secondary myeloid malignancy after etoposide treatment. Blood 2003;101:3862–7. [DOI] [PubMed] [Google Scholar]
- 14.) Smith RE, Bryant J, DeCillis A, Anderson S. National Surgical Adjuvant Breast and Bowel Project Experience. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: the National Surgical Adjuvant Breast and Bowel Project Experience. J Clin Oncol 2003;21:1195–204. [DOI] [PubMed] [Google Scholar]
- 15.) Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 2003;21:1431–9. [DOI] [PubMed] [Google Scholar]
- 16.) Papaldo P, Lopez M, Cortesi E, et al. Addition of either lonidamine or granulocyte colony-stimulating factor does not improve survival in early breast cancer patients treated with high-dose epirubicin and cyclophosphamide. J Clin Oncol 2003;21:3462–8. [DOI] [PubMed] [Google Scholar]
- 17.) Crump M, Tu D, Shepherd L, et al. Risk of acute leukemia following epirubicin-based adjuvant chemotherapy: a report from the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2003;21:3066–71. [DOI] [PubMed] [Google Scholar]
- 18.) Venturini M, Del Mastro L, Aitini E, et al. Dose-dense adjuvant chemotherapy in early breast cancer patients: results from a randomized trial. J Natl Cancer Inst 2005;97:1724–33. [DOI] [PubMed] [Google Scholar]
- 19.) Praga C, Bergh J, Bliss J, et al. Risk of acute myeloid leukemia and myelodysplastic syndrome in trials of adjuvant epirubicin for early breast cancer: correlation with doses of epirubicin and cyclophosphamide. J Clin Oncol 2005. June 20;23(18):4179–91. [DOI] [PubMed] [Google Scholar]
- 20.) Veyret C, Levy C, Chollet P, et al. Inflammatory breast cancer outcome with epirubicin-based induction and maintenance chemotherapy: ten-year results from the French Adjuvant Study Group GETIS 02 Trial. Cancer 2006;107:2535–44. [DOI] [PubMed] [Google Scholar]
- 21.) Hershman D, Neugut AI, Jacobson JS, et al. Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J Natl Cancer Inst 2007; 99:196–205. [DOI] [PubMed] [Google Scholar]
- 22.) Patt DA, Duan Z, Fang S, et al. Acute myeloid leukemia after adjuvant breast cancer therapy in older women: understanding risk. J Clin Oncol 2007;25:3871–6. [DOI] [PubMed] [Google Scholar]
- 23.) Le Deley MC, Suzan F, Cutuli B, et al. Anthracyclines, mitoxantrone, radiotherapy, and granulocyte colony-stimulating factor: risk factors for leukemia and myelodysplastic syndrome after breast cancer. J Clin Oncol 2007;25:292–300. [DOI] [PubMed] [Google Scholar]
- 24.) Repetto L, Balducci L. A case for geriatric oncology. Lancet Oncol 2002;3:289–297. [DOI] [PubMed] [Google Scholar]
- 25.) Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 2006;24:3187–205. [DOI] [PubMed] [Google Scholar]
- 26.) Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002;40(8 Suppl):IV-3–18. [DOI] [PubMed] [Google Scholar]
- 27.) Clarke CA, Undurraga DM, Harasty PJ, et al. Changes in cancer registry coding for lymphoma subtypes: reliability over time and relevance for surveillance and study. Cancer Epidemiol Biomarkers Prev 2006;15:630–8. [DOI] [PubMed] [Google Scholar]
- 28.) Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. D. Ann Epidemiol 2007;17:584–90. [DOI] [PubMed] [Google Scholar]
- 29.) Harrell F, Lee K. Verifying assumptions of the proportional hazards model. Proc 11th Annual SAS User’s Group Int 1986;11:823–828. [Google Scholar]
- 30.) Lieschke GJ, Burgess AW. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (2). N Engl J Med 1992;327:99–106. [DOI] [PubMed] [Google Scholar]
- 31.) Guillem V, Tormo M Influence of DNA damage and repair upon the risk of treatment related leukemia. Leuk Lymphoma 2008;49:204–17. [DOI] [PubMed] [Google Scholar]
- 32.) Kroft SH, Tallman MS, Shaw JM, et al. Myelodysplasia following treatment of chronic lymphocytic leukemia (CLL) with 2-chlorodeoxyadenosine (2-CdA). Leukemia 1997;11:170. [DOI] [PubMed] [Google Scholar]
- 33.) Van Den Neste E, Louviaux I, Michaux JL, et al. Myelodysplastic syndrome with monosomy 5 and/or 7 following therapy with 2-chloro-2’- deoxyadenosine. Br J Haematol 1999;105:268–270. [PubMed] [Google Scholar]
- 34.) Robak T, Blonski JZ, Gora-Tybor J, et al. Second malignancies and Richter’s syndrome in patients with chronic lymphocytic leukemia treated with cladribine. Eur. J. Cancer 2004;40:383–389. [DOI] [PubMed] [Google Scholar]
- 35.) Robak T Second malignancies and Richter’s syndrome in patients with chronic lymphocytic leukemia. Hematology 2004;9:387–400. [DOI] [PubMed] [Google Scholar]
- 36.) Cheson BD,Vena DA, Barrett J, Freidlin B. Second malignancies as a consequence of nucleoside analog therapy for chronic lymphoid leukemias. J Clin Oncol 1999;17:2454–60. [DOI] [PubMed] [Google Scholar]