Abstract
Introduction:
Magnetic resonance imaging (MRI) is of paramount importance for the early diagnosis of multiple sclerosis (MS) and MRI findings are part of the MS diagnostic criteria. There is a growing interest in the use of ultra-high-field strength −7 Tesla- (7T) MRI to investigate, in vivo, the pathological substrate of the disease.
Areas covered:
An overview of 7T MRI applications in MS focusing on increased sensitivity for lesion detection, specificity of the central vein sign and better understanding of MS pathophysiology and discuss the implications for disease diagnosis, monitoring and treatment planning.
Expert commentary:
7T MRI provides increased signal-to-noise and contrast-to-noise-ratio that allow higher spatial resolution and better detection of anatomical and pathological features. The high spatial resolution reachable at 7T has been a game changer for neuroimaging applications not only in multiple sclerosis but also in epilepsy, brain tumors, dementia, and neuro-psychiatric disorders. Furthermore, the first 7T device has recently been cleared for clinical use by the food and drug administration.
Keywords: multiple sclerosis, ultra-high field MRI, white matter lesions, central vein sign, gray matter lesions, FDA clearance, iron imaging, sodium imaging
1.0. Introduction
Multiple sclerosis (MS) is a neuroinflammatory/neurodegenerative disease of the central nervous system (CNS) that, according to estimates of the MS Foundation, affects more than two million people worldwide and represents the most common cause of non-traumatic disability in young adults. Although the disease etiology is still unknown, the complex interaction between environmental factors and the subject’s genetic background is believed to be play a crucial role for the disease pathogenesis. The pathological events starts with the priming of myelin-autoreactive T lymphocytes in the periphery that, then, cross the blood-brain-barrier (BBB) and induce an acute autoimmune reaction against myelin and activation of resident microglia and infiltrated macrophages [1]. About 80–85% of patients present with a first acute or subacute neurological deficit lasting at least 24 hours and reflecting focal or multifocal CNS inflammation, that, if the MS diagnosis is subsequently confirmed by fulfilling dissemination in space and time (DIS and DIT respectively), is referred to as clinically isolated syndrome-CIS. While in the initial stage the disease manifests with unpredictable clinical and radiological relapses (relapsing-remitting MS-RR MS), over time the recurrence of relapses tends to decrease and a gradual neurological worsening occurs (secondary progressive MS-SP MS) [2]. In the remaining 20–15% of cases, the disease is characterized by a gradual clinical worsening over time independent of relapses (primary progressive MS-PP MS) [1].
Magnetic resonance imaging (MRI) is of paramount importance for the early MS diagnosis and MRI parameters are part of the MS diagnostic criteria. MS diagnosis in patients with symptoms consistent with a CNS inflammatory demyelinating disease is based on the presence of clinical and MRI criteria of DIS and DIT, coupled with the exclusion of alternative diagnoses that could offer a better explanation for the patient’s clinical presentation [3]. Furthermore, MRI is of crucial importance for monitoring treatment response in clinical practice and experimental trials and for investigating the disease pathophysiology.
There is a growing interest in the use of MRI at ultra-high-field strength −7 Tesla- (7T) to investigate disease mechanisms in patients with MS. The main advantages of 7T MRI are the increased signal-to-noise ratio (SNR) and the contrast-to-noise-ratio (CNR) that allow the improvement of spatial resolution and the detection of anatomical and pathological features. Three major MR vendors currently provide 7 T MRI magnets for ethically approved clinical research. The number of installations in operation has increased to over 70 during the last several years. More recently, the food and drug administration (FDA) cleared the first 7T device (Magnetom Terra, Siemens Medical Solutions Inc.) for clinical use limited to the examination of head and extremities [4].
In this review we will present the main findings obtained by the application of 7T MRI in patients with MS in terms of increased detection of white and gray matter lesions, and potential increased specificity of the “central vein sign”. Then, we will discuss the implications of these findings for disease diagnosis, monitoring and treatment planning. Finally, we will review the relevance of 7T MRI applications for a better understanding of MS pathophysiology and elucidation of the underlying mechanisms.
2.0. Multiple Sclerosis diagnosis
MS diagnosis is based on the demonstration of lesion dissemination in space and time (DIS and DIT respectively), coupled with the exclusion of alternative diagnoses, in patients who present with a typical clinically isolated syndrome (CIS) suggestive of MS or symptoms consistent with a CNS inflammatory demyelinating disease [3].
Typical presentations include unilateral optic neuritis, focal supratentorial syndrome, focal brainstem or cerebellar syndrome, or partial myelopathy. In patients presenting CIS, a diagnosis of MS purely based on clinical symptom is theoretically possible when a second relapse occurs, in presence of objective clinical evidence of two lesions, when there is objective clinical evidence of one lesion with reasonable historical evidence of a prior attack, or if it is possible to demonstrate CSF-specific oligoclonal bands but access to imaging is always desirable to confirm DIS and DIT and, in particular, to exclude alternative diagnosis.
Radiological DIS is defined as one or more T2 hyperintense lesions within at least two of four anatomical locations characteristic for MS (periventricular, cortical or juxtacortical, infratentorial, spinal cord).
Radiological DIT is demonstrated by the presence of (i) gadolinium-enhancing and non-enhancing lesions on the same MRI or (ii) new T2 hyperintense and/or gadolinium-enhancing lesions on follow-up MRI compared to baseline, regardless of the time interval between the two scans.
Both symptomatic and asymptomatic MRI lesions can be considered to define DIS and DIT.
Cerebral spinal fluid (CSF) analysis can be used in support of the diagnosis of primary progressive MS that requires, in addition to one year of disease progression, two of the following criteria: (i) brain DIS based on at least one T2 lesion in at least one area characteristic for MS (periventricular, cortical or juxtacortical, infratentorial) (ii) spinal cord DIS based on at least two T2 lesions (iii) evidence of oligoclonal bands and/or elevated IgG index in the CSF.
In order to facilitate the application of these criteria in clinical settings, guidelines for the standardization of MR protocols utilized in MS diagnosis and follow-up have been published [5,6].
While the recent 2017 revision to McDonald criteria does not address directly the role of high field strength imaging in MS diagnosis, it identifies the issue as a high-priority area for research. Additionally, last year, the MAGNIMS study group concluded, in their consensus guidelines, that, although the current evidence does not support yet the application of 7T MRI scanners for an earlier diagnosis of MS, the increased sensitivity in lesion detection and the better characterization of lesion pathology at high- and ultra-high field MRI could lead to higher specificity, enhancing differentiation of MS from other disorders [7].
3.0. Advantages and challenges of 7 Tesla MRI
Since SNR scales with the field, 7T MRI provides images with higher spatial resolution within reasonable acquisition times and with finer anatomical details (Figure 1). In addition, 7T allows increased detection of subtle brain abnormalities that may improve disease diagnosis. The effects of small variations in tissue susceptibility are increased at ultra-high field MR leading to greater changes in image contrast especially in proximity of tissue with high density of small venous structures and high iron deposits [8]. Therefore, the combined effect of increased spatial resolution and susceptibility-based contrast has enabled the study of venous vasculature and the assessment of its relationship with perivenular infiltrates and inflammation in MS lesions [9].
Figure 1.
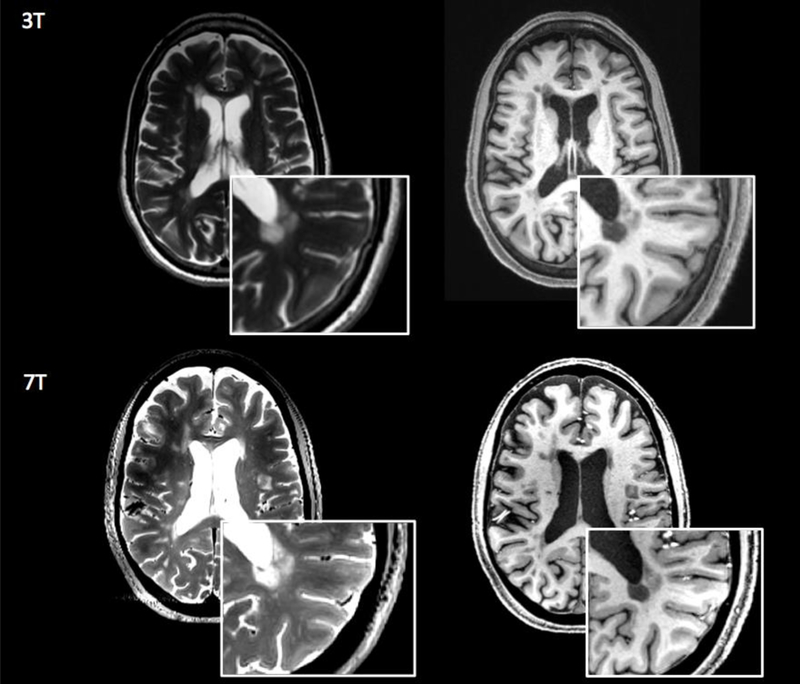
White matter lesionn detection at 3T and 7T. 3T and 7T axial images obtained for a patient with multiple slcerosis. White matter demyelinating lesions are visualized in greater detail in the 7T T2-weighted (first column) and T1-weighted image (second column). Image resolution: 3T T2-weighted=0.5×0.5×3 mm3,T1-weighted= 0.8×0.8×0.8 mm3; 7T T2-weighted=0.7×0.7×0.7 mm3, T1-weighted=0.7×0.7×0.7 mm3.
The gain in SNR and CNR, however, occurs at the expense of increased radiofrequency (B1) and static field (B0) inhomogeneity, which result in a variation of intensity across the image and distortions at structural boundaries that, in turn, can limit the interpretation of findings [10]. The direct scaling of B0 inhomogeneity with field strength results in distortions of image geometry and intensity especially in rapid acquisition schemes such as echo-planar imaging that are more prone to distortions due to susceptibility effects. With the increase of B0 field at 7T, B1 strength decreases in the brain periphery compared with the center resulting in signal drop-out and changes in image contrast. Nonetheless, the ongoing technical development of customized RF pulse and pulse sequence designs as well as the development of multiple transmit coils for RF signal transmission will help overcome the physical limitations of performing 7T imaging in vivo within recommended safety limits and reasonable acquisition times.
Furthermore, although risks at 7T are similar to those at lower field strength, there are additional concerns related to patients’ safety. Noise levels, RF energy deposition, and peripheral nerve stimulation are minimized by the adherence to the FDA safety guidelines. The limited numbers of implantable devices tested so far at 7T can limit the target population. However, several implants are currently under testing and will allow 7T imaging in a wider number of patients [11].
3.1. White matter lesions detection
The initial 7T MRI studies of patients with MS focused on investigating the relationship between WM lesions and small parenchymal blood vessels using susceptibility sensitive imaging [12,13]. The presence of a close relationship between demyelination and blood vessels had already been reported at post-mortem examination of MS samples [14,15] and T2-weighted MR images at 1.5T with subsequent venography had previously demonstrated an MS lesion and a blood vessel in close proximity [16]. However, only the application of 7T T2*-weighted MRI was able to demonstrate both structures simultaneously [12,13]. Periventricular lesions were defined as those with a border within 4 mm of the ventricular surface while all other lesions were defined as peripheral. Vessels appeared hypointense and were only counted if they could be visualized in at least two perpendicular planes. In line with histologic reports, a central vessel could be identified in 73 lesions (82%) in 8 MS patients supporting the good sensitivity of the technique[13]. Periventricular lesions were more likely to display a central vessel than peripheral lesions due, potentially, to a different mechanism of lesion development depending on localization or due to the general smaller size of peripheral lesions and the higher likelihood for them to be associated with smaller vessels.
These first 7T T2*-weighted MRI studies raised questions about the potential higher sensitivity of 7T MRI for detection of WM lesions compared to lower field MRI, about the clinical implication of an early MS diagnosis and about the potential high specificity of the “central vein sign” in the differential diagnosis of MS and other diseases mimicking MS.
In terms of higher sensitivity, the first comparative study between WM lesion detection at 7T versus 1.5T [17] did not show the expected gain due to the high specific absorption rate of radiofrequency energy that makes difficult to implement some of the clinical sequences at ultra-high field.
While the optimization of inversion recovery spin echo sequences such as FLAIR remains challenging at 7T, 3D MPRAGE is less affected by RF inhomogeneity, and therefore, one of the first comparative study between lesion detection at 7T and 3T focused on the comparison of standard clinical 3T FLAIR and MPRAGE sequences with 7T MPRAGE [18]. Although the majority of lesions were detected on both FLAIR and MPRAGE images, a substantial proportion were only detected using 3T MPRAGE (19% of the total number of lesions detected) and 7T MPRAGE (22% of the total number of lesions detected). Moreover, significantly more lesions were detected using 7T MPRAGE compared with 3T MPRAGE (p=0.012). 7T SNR resulted suboptimal in the lower half of the brain thus explaining why some lesions were missed on 7T MPRAGE compared with 3T FLAIR/MPRAGE. Admittedly, this improved lesion detection was limited by the presence of 3T FLAIR lesions not seen on 7T, mostly in the infra-tentorium, where there was signal dropout at 7T. There is encouraging evidence, however, that this issue might be resolved through the use of the advanced magnetization-prepared 2 rapid acquisition gradient echoes (MP2RAGE) technique, which corrects the image intensity variations due to large spatial B1 field inhomogeneities and thus can provide homogeneous T1 contrast across the entire brain at 7T [19]. Importantly, many of the lesions only detected on 7T were within what would have been interpreted as normal-appearing WM at 3T, supporting the notion that focal MS lesions contribute to the abnormalities known to exist in the normal-appearing WM.
3.2. White matter lesions and the “central vein sign”
Although the diagnosis of MS is based on the identification of multifocal demyelinating lesions, which are disseminated in their time of onset, focal T2 lesions are not specific to MS, occurring in several other neurological disorders [20]. The finding that a central vessel could be identified in 45% of visible lesions using 3T T2* and 87% of visible lesions using 7T T2* (P = 0.0001) in MS patients [21] and in only 8% of the discrete WM lesions of healthy volunteers prompted further investigation as this would represent a clinically useful application.
Based on the demonstration that at T2* weighted imaging at 7T most MS lesions are centered on small parenchymal veins, Tallantyre et al. compared the appearance of brain lesions between patients with MS and patients with asymptomatic WM lesions to assess whether the central vein sign has a diagnostic value and differentiates between MS lesions and microangiopathic lesions [9]. WM lesions were identified on 7T T2* weighted MRI from 28 MS patients and 17 patients with cerebral WM lesions who did not have MS and analyzed for volume, location, and perivenous appearance. In MS patients, 80% of the 901 identified lesions were perivenous, while in non-MS patients only 19% of 428 identified lesions were perivenous. In addition, 7T T2*-weighted MRI reliably distinguished all patients with clinically definite MS (>40% lesions appeared perivenous) from those without clinical MS (<40% lesions appeared perivenous). Finally, perivenous lesion appearance was more predictive of MS (p <0.001) than subcortical or periventricular lesion location.
Over the past few years, various research groups have used 3T and 7T T2*-weighted MRI to evaluate the presence of central veins inside WM lesions associated with various neurological diseases, including neuromyelitis optica spectrum disorder (NMOSD), systemic autoimmune diseases (SAD), cerebral small vessel disease (CSVD), Susac syndrome, and migraine supporting the concept that the central vein sign may contribute to the differential diagnosis (Figure 2) [22].
Figure 2.
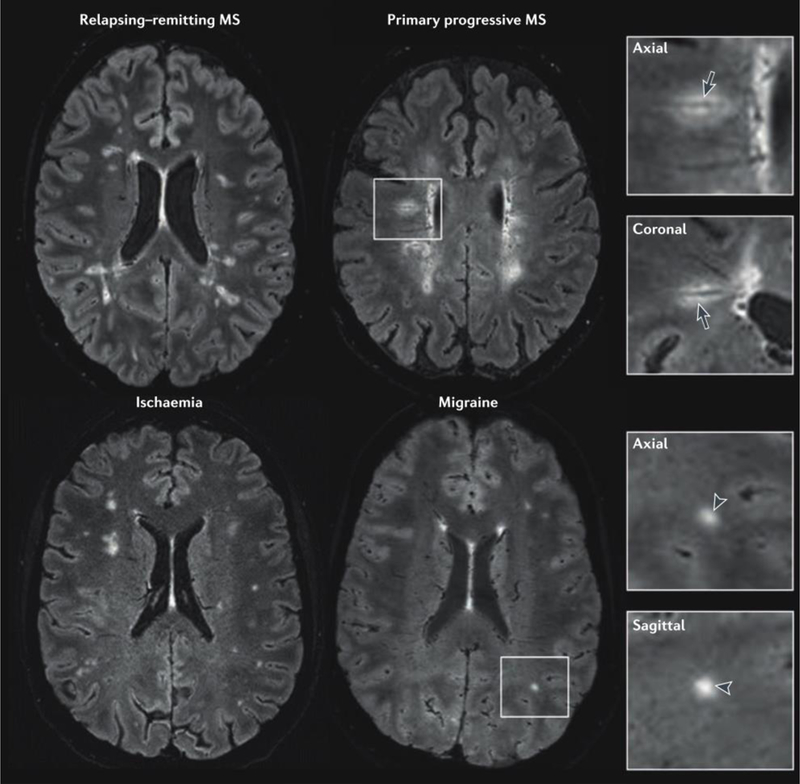
Perivenous distribution of multiple sclerosis lesions. 3 T FLAIR* (combined T2*-weighted MRI and FLAIR) images from four individuals with a variety of neurological conditions. In the patients with relapsing–remitting or primary progressive multiple sclerosis (MS), a central vessel is visible in most hyperintense lesions (arrows in magnified boxes). On the other hand, a central vein is absent from most of the lesions (arrowheads in magnified boxes) in the patient with migraine and the patient with ischaemic small vessel disease. Reproduced from Sati et al. [21] by permission of Nature Publishing Group (work is licensed under a Creative Commons Attribution 4.0 International License).
Therefore, the central vein sign (CVS) has recently been proposed as a novel MRI biomarker to improve the accuracy and speed of MS diagnosis. Evidence indicates that the presence of the CVS in individual lesions can accurately differentiate MS from other diseases that mimic this condition.
However, further studies are needed to establish the predictive value of the CVS for the development of clinical MS in patients with suspected demyelinating disease. Guidelines for the assessment of the CVS are nicely outlined in an expert consensus document recently published by the North American imaging in MS Cooperative [22]. As highlighted in this consensus statement, although T2* imaging is most sensitive at 7T, a high detection rate can still be achieved at clinical field strengths (1.5T and 3T) when applying optimized sequences.
3.3. Cortical and subcortical gray matter lesion detection
The increased spatial resolution and better tissue contrast of 7T imaging has been of crucial importance for the detection of cortical and subcortical GM lesions [17,23–30]. Cortical lesions are very frequent at histopathological examination, especially in the progressive forms of the disease [31]; however, due to the small size and lower amount of inflammation it is very difficult to detect them with in vivo MRI. Since the first ex-vivo and in vivo applications, it was clear that 7T MRI (e.g. T2*-weighted gradient echo and MPRAGE) improves not only detection of cortical lesions compared to 3T MRI but it also improves lesions localization and classification (Figure 3) [24]. To date, most of the 7T MRI study have used and compared several sequences with controversial results explained by the fact that each sequence has advantages and limitations and there is ongoing effort towards their optimization. For instance, one study evaluated the agreement between FLAIR, DIR and MPRAGE in 11 MS patients and 8 healthy controls that underwent FLAIR and DIR at 3T and MPRAGE at 7T [26]. While the agreement for cortical lesions was good between images, the agreement for intracortical lesions was less satisfactory and 25% of lesions could only be visualized on a single MRI sequence. Each sequence gave a complementary contribution to lesion detection with 7T MPRAGE facilitating the localization of lesions at the cortical boundary. A recent post-mortem study [32] comparing T2-, T1-, T2*-weighted sequences, FLAIR and DIR at 3T and 7T showed that none of the five sequences detected significant more lesions than any other sequence and that subpial cortical lesions were more extensive than what is revealed by the 7T. Continuous optimization of MRI sequences and coil technology at 7T is likely to lead to a better detection and classification of gray matter whose recognized clinical relevance could benefit MS patients’ management and treatment.
Figure 3.
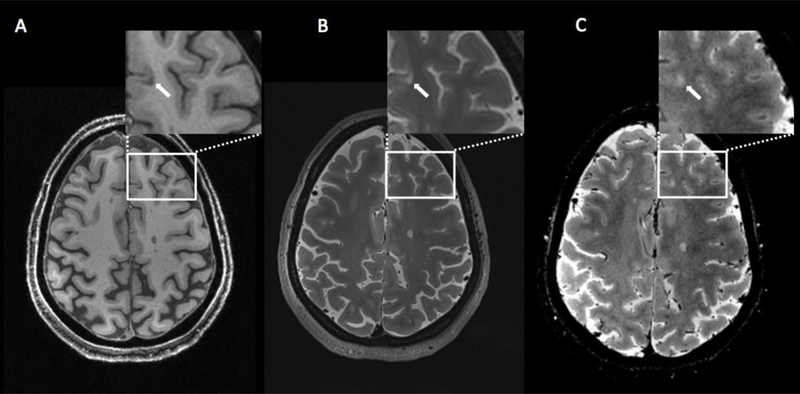
7T axial images obtained for a patient with multiple slcerosis. Cortical lesion (arrow) is visualized on T1-weigthed sequence (A), T2-weighted sequence (B) and T2*-weighted sequence (C).
3.4. Small CNS anatomical regions
The increased SNR and higher spatial resolution of 7T imaging could be also beneficial to the study of small anatomical regions of the central nervous system and the related pathological abnormalities (Figure 4).
Figure 4.
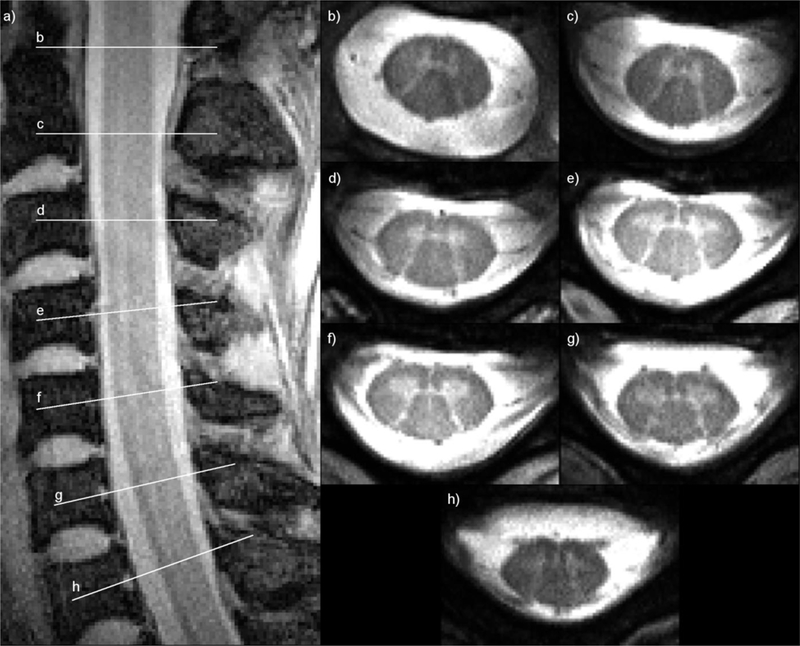
A sagittal GRE image of the cervical spinal cord at 0.78 × 0.78 x 3 mm3 resolution (a) shows the positions of seven axial high-resolution (0.3 × 0.3 × 3 mm3) multi-echo image slices at vertebral levels C1 through C7 (b–h) where grey and white matter are clearly discernible. Images are courtesy of Dr. Junqian Xu, Ichan School of Medicine at Mount Sinai. Reproduced from Zhang et al. [66] by permission of John Wiley & Sons, Ltd.
For instance, imaging of the spinal cord has always been challenging at lower MR field strengths due to the small size, surrounding bone-tissue interfaces and artifacts associated to respiratory- and cardiac-induced artifacts that limit the assessment of demyelinating lesions. A small comparative study of spinal cord imaging of MS patients at 3T and 7T has shown that not only 7T provides a better visualization of fine anatomical details such as the nerve roots and a better demarcation of gray and white matter, but it also improves by 50% spinal cord MS lesion detection [33]. Although there is a need for further technical development, these results are very promising and may be of great relevance since the spinal cord involvement accounts for most of patients’ clinical disability.
3.5. Quantitative imaging
3.5.1. Iron imaging
T2*-weighted gradient-echo sequence and susceptibility weighted imaging have proved useful not only for the visualization of the central vein but also for the quantification of iron deposits. After the finding that approximately 8% of MS lesions detected at 7T with gradient echo sequence show a rim on phase image [34], radiological-histopathological correlation studies have demonstrated the presence of iron within phagocytic macrophages and/or microglia in phase-rim MS lesions, providing a histologic validation for the radiological finding [35,36]. It has also been suggested that chronic phase-rim lesions are characterized by greater tissue destruction compared with non–phase-rim lesions, and therefore their identification and their evolution might be relevant for the understanding of disease progression and for patients’ treatment plans.
Although it is important to bear in mind that iron-sensitive sequences are also sensitive to changes in tissue density, water and fiber orientation, recent studies have applied an alternative technique to process gradient echo data, namely quantitative susceptibility mapping (QSM), that, by removing some of the contributors that complicate the interpretation of phase images, seems to improve our insights on the changes occurring at the periphery of MS lesions [37,38].
3.5.2. Multinuclear imaging
The greater SNR at 7T provides a signal boost to nuclei such as sodium (23Na) and phosphorus (31P) whose brain concentration is much lower than that of proton nuclei. In this review we will focus on sodium imaging since there are a few studies that have investigated brain sodium concentration in patients with MS. For more details about multinuclear imaging at ultra-high field MRI see Balchandani et al [39].
When demyelination occurs in MS, 23Na channels present a widespread re-distribution from the Ranvier nodes to long segments of demyelinated membrane. While this represents an adaptive mechanism to preserve action potential conduction and facilitate recovery of neurological deficits, it imposes a huge burden on the axonal metabolism thus increasing the risk of axonal damage secondary to energy deprivation [40,41]. In MS, however, in presence of mitochondrial dysfunction and ATP deficit, 23Na influx leads to the axonal accumulation of toxic levels of calcium [42,43] and neurodegeneration. Interestingly, it has been shown that 23Na channels blockers are able to protect axons from anoxic-ischemic injury in vitro [43–47] and in animal models of MS [48–52], at concentrations that do not compromise the conduction of action potentials.
23Na yields the second strongest nuclear magnetic resonance (NMR) signal among biologically relevant NMR-active nuclei. 23Na concentration is strictly controlled by the ATP-driven Na/K pump to ensure the maintenance of tissue homeostasis and the preservation of intracellular structures and processes. Therefore, pathological changes such as tissue injury, edema or necrosis or functional impairment of the Na/K pump are expected to result in an increased tissue 23Na concentration [53–56].
Single quantum (SQ) 23Na MRI is an imaging technique that exploits the magnetic resonance properties of 23Na atomic nuclei, allowing the metabolic characterization of brain tissue in vivo. Unfortunately, the sodium signal is 30,000 times lower than that of protons due to the low brain concentration, smaller gyromagnetic ration and rapid bio-exponential decay [57]. Moreover, in the brain 23Na has a bi-compartimental distribution with higher concentration (140 mmol/L) in the extracellular space and a lower concentration (ranging from 10 to 15 mmol/L) in the intracellular space. All these features of sodium signal make high- and ultra-high field magnets highly desirable to improve sensitivity of in vivo 23Na MRI.
Several 3T sodium MRI studies from different laboratories around the world [58–64] have showed a widespread brain increase of total tissue sodium concentration (TSC) in patients with MS suggesting that this might reflect changes in cellular and metabolic integrity of both lesions and normal-appearing brain tissue. While in the early disease stage TSC increase is prevalent in macroscopic lesions [60], it spreads to normal-appearing white matter, cortical and deep gray matter as the disease progresses [58–61]. TSC increase in lesions might be explained by gliosis, tissue disruption and replacement with extracellular fluid, whereas TSC increase in normal-appearing brain tissue is related not only to increased extracellular space, caused by demyelination and axonal loss, but also to intra-axonal 23Na increase.
Although TSC can help monitoring the occurrence of tissue injury and disability, it is not useful in discriminating metabolic dysfunction from the irreversible cellular damage. Metabolic changes that affect 23Na exchange across cells membrane influence the intracellular sodium concentration (ISC) [53], that could therefore be considered as a pure functional marker. More recently, we have combined SQ and triple-quantum filtered 23Na MRI at 7T [65,66] and applied this technique to a small number of MS patients to quantify ISC and intracellular 23Na volume fraction, an indirect measure of extracellular 23Na concentration [67] (Figure 5). Our preliminary findings suggest that the ISC increase detected in the brain WM of patients compared to healthy controls might indicate axonal dysfunction, offering insights in axonal metabolism before the generation of stable, irreversible, axonal damage. Since the ISC accumulation is a reversible phenomenon, occurring spontaneously in a number of neurons in physiological conditions [68], it could be a putative target for therapeutic interventions.
Figure 5.
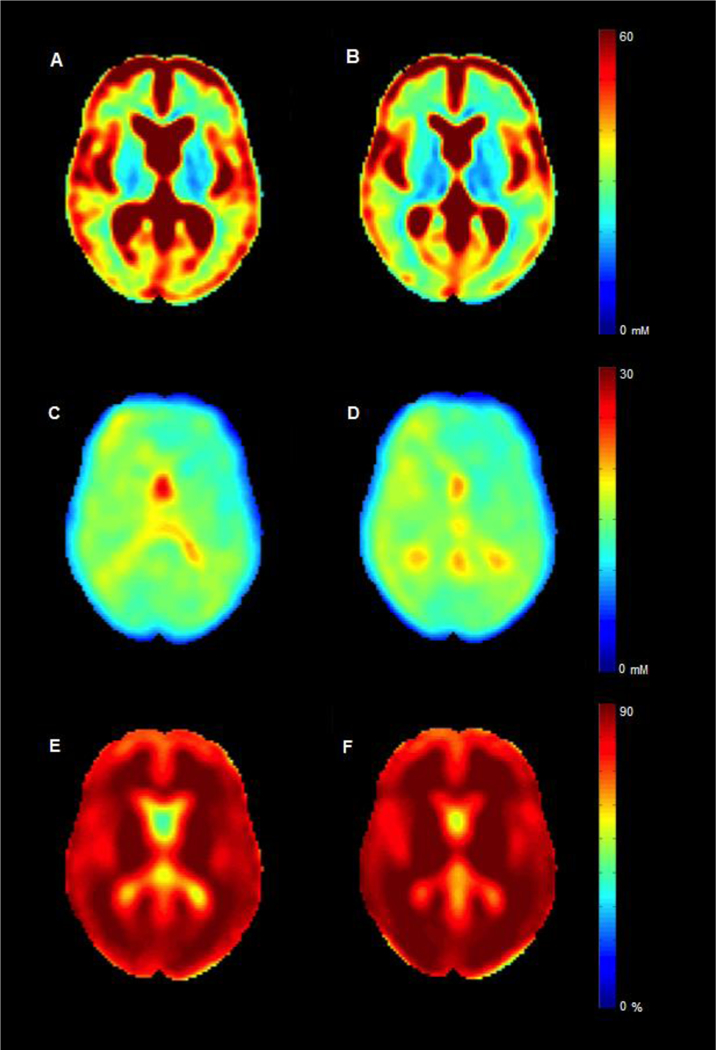
23Na group maps. Mean to total sodium concentration (TSC), (intracellular sodium concentration) ISC and (intra- cellular sodium volume fraction) ISVF maps for patients with multiple sclerosis (respectively, A, C and E) and controls (respectively, B, D and F). In both groups, TSC appears higher while ISC and ISVF are lower in grey matter than in white matter. As intracellular molar content and cell volume are equal to zero in extracellular tissue, ISVF and ISC measurements in CSF are meaningless. Reproduced by Petracca et al. [67] by permission of Oxford University Press.
4.0. Conclusion
7T MRI has improved the ability to detect ever smaller brain lesions, in both WM and GM, and to improve their localization in MS. Further technical implementation is needed to overcome the current limitations, especially in detection of cortical lesions. The “central vein sign” (CVS) might serve as a novel MRI biomarker to improve the accuracy and speed of MS diagnosis. However, further studies are needed to establish the predictive value of the CVS for the development of clinical MS in patient with suspected demyelinating disease. Metabolic and functional imaging as well as imaging of nuclei other than protons are ready for applications and can provide unique information to elucidate the mechanisms underlying the disease. Continued technical development of new signal transmission and readout methods is needed to overcome the limitations of performing 7T MRI within comfortable times and accepted safety limits. Additional clinical studies are needed to demonstrate the value of 7T for disease diagnosis, prognosis, treatment, and management.
5.0. Expert Commentary
Offering a substantial increase in signal-to-noise ratio (SNR) and the contrast-to-noise-ratio (CNR) 7T imaging grants an anatomical definition not achievable with lower field strength, thus providing a fundamental tool for the exploration, in vivo, of physiological mechanisms and pathological substrate of a variety of neurological disorders. The key finding of the research done in this field so far is the successful overcoming of many technical challenges for structural and functional 7T imaging enabling the visualization of cortical layers and fine structure of small nuclei in the human brain with unprecedented detail. The high spatial resolution reachable at 7T has been a game changer for neuroimaging applications not only in multiple sclerosis but also in epilepsy, brain tumors, dementia, and neuro-psychiatric disorders. Specifically, in MS the higher white matter lesion detection provided by 7T and the potential higher specificity provided by the central vein sign in white matter lesions could significantly impact the diagnosis and the differential diagnosis of MS.
Additional clinical studies are needed to demonstrate the value of 7T for disease diagnosis, treatment, and management. This goal can be achieved with the larger availability of 7T scanners allowing for multi-site studies and with lowering the cost of such magnets. The recent FDA clearance of the first 7T device (Magnetom Terra, Siemens Medical Solutions Inc.) for clinical use will boost the production of 7T scanners that will be available in an increasing number of research and clinical Institution thus reducing the unit cost. Moreover, since most of the newer scanner are “zero boil-off” there is very little helium leak with time further reducing helium cost after the initial magnet installation. Therefore, clinical 7T applications, especially in neurological and musculoskeletal MRI applications, are expected to full bloom in the near future.
Besides clinical applications, 7T holds a huge potential for research applications made possible by the related improvement in several imaging modalities. Vascular and functional imaging takes advantage of the increased susceptibility at ultra-high field and enables a more effective visualization of smaller blood vessels, microbleeds and iron deposits through susceptibility weighted imaging and angiography. Magnetic resonance spectroscopy is benefited not only by the increased SNR but also by the increased spectral resolution that improves the quantification of the most commons metabolites and allows the visualization of low concentration metabolites not detectable at lower MR field strength. Another interesting field of research made possible by 7T is that of multinuclear MRI characterized by the imaging of nuclei other than protons (sodium-23, phosphorus-31, and chlorine-35) whose signals is much lower than the signal from protons and, therefore is boosted at 7T MRI and provides important information about cellular processes and ion homeostasis.
Several technical challenges and a few patients’ concerns remain, however, to be addressed. The major issues are represented by the inhomogeneity of B0 and B1 fields with consequent image artifacts and limited brain spatial coverage and by the increased deposition of RF power within subjects. Therefore, it is necessary to find technical solutions to overcome the physical challenges of 7T imaging and improve the safety issues. Indeed, brain imaging at 7T is booming with rapid developments in customized RF pulse, pulse sequence design, reconstruction techniques, improved gradient performance and specialized hardware such as new multiple transmit coils for RF signal transmission.
This continued innovative research in terms of technical development of new hardware, pulse sequences and readout methods will further mitigate the challenges of 7T thus enabling imaging within clinically reasonable times and in respect of safety limits.
6.0. Five-year view
With the FDA clearance of the first 7T device for clinical use in the brain and with the ever- increasing number of 7T magnets installed worldwide we expect a growing application of this method in neurological diseases. Continued technical implementation and optimization will allow to overcome present limitations and will move forward clinical applications. Future effort will focus on the performance of larger clinical studies to demonstrate the value of 7T for MS diagnosis, prognosis, treatment, and management of MS. Similarly, the access to finer anatomical and pathological details and the opportunity to study disease mechanisms of cortical layers demyelination, lesion development and evolution, meningeal inflammation, iron deposits and sodium dyshomeostasis will enable us to make the next steps forward in understanding MS pathophysiology and will provide better tools to investigate the effects of physical and pharmacological therapies.
7.0 Key issues.
MS diagnosis is based on the presence of the two criteria of dissemination in space and in time coupled with the exclusion of alternative diagnoses that could offer a better explanation for the patient’s clinical presentation in patients who present symptoms consistent with a CNS inflammatory demyelinating disease.
The main advantages of 7T MRI are the increased SNR and CNR that allow higher spatial resolution of fined anatomical and pathological details within reasonable acquisition times. The gain in SNR and CNR, however, occurs at the expense of increased radiofrequency (B1) and static field (B0) inhomogeneity which result in a variation of intensity across the image and distortions at structural boundaries that, in turn, can limit the interpretation of findings.
7T MRI provides higher sensitivity in WM and GM lesion detection compared to lower MRI fields. However, the gain is less than expected, especially with regard to GM lesions. Ongoing MRI sequence and RF coil development and optimization will hopefully overcome the limitations that prevent an optimal lesion detection at 7T.
The central vein sign (CVS) has recently been proposed as a novel MRI biomarker to improve the accuracy and speed of MS diagnosis. Evidence indicates that the presence of the CVS in individual lesions can accurately differentiate MS from other diseases that mimic this condition. However, further studies are needed to establish the predictive value of the CVS for the development of clinical MS in patients with suspected demyelinating disease.
Single quantum (SQ) 23Na MRI is an imaging technique that exploits the magnetic resonance properties of 23Na atomic nuclei. By combining SQ and triple-quantum filtered 23Na MRI at 7T is possible to quantify intra- and extracellular 23Na concentration. Preliminary findings suggest that the intra-cellular 23Na increase in MS patients compared might indicate axonal dysfunction, offering insights in axonal metabolism before the generation of irreversible, axonal damage.
Continued technical development of new signal transmission and readout methods is needed to overcome the limitations of performing 7T imaging in vivo. Additional clinical studies are needed to demonstrate the value of 7T for disease diagnosis, prognosis, treatment, and management.
Acknowledgments
Funding
This study was supported in part by grants from National Institute of Health (R01NS099527), Teva Neuroscience (CNS-2014–221), and the Noto Foundation.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- [1].Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. [DOI] [PubMed] [Google Scholar]
- [2].Lublin FD, Reingold SC, Cohen J a, et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology. 2014;83:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2017. pii: S1474–4422(17)30470–2.* Current criteria for multiple sclerosis diagnosis
- [4].Voelker R Twice the Power in New MRI. JAMA. 2017;318:2017.** First report of FDA clearance for 7T clinical use
- [5].Traboulsee a L, Simon J, Stone L, et al. Revised Recommendations of the Consortium of MS Centers Task Force for a Standardized MRI Protocol and Clinical Guidelines for the Diagnosis and Follow-Up of Multiple Sclerosis. AJNR. Am. J. Neuroradiol. 2016;314:2034–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rovira Á, Wattjes MP, Tintoré M, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis - Clinical implementation in the diagnostic process. Nat. Rev. Neurol. 2015;11:471–482. [DOI] [PubMed] [Google Scholar]
- [7].Filippi M, Rocca MA, Ciccarelli O, et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol. 2016;15:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Duyn JH. The future of ultra-high field MRI and fMRI for study of the human brain. Neuroimage. 2012;62:1241–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76:534–539.* Pathological specificity of perivenous lesion location
- [10].Uǧurbil K, Adriany G, Andersen P, et al. Ultrahigh field magnetic resonance imaging and spectroscopy. Magn. Reson. Imaging. 2003;21:1263–1281. [DOI] [PubMed] [Google Scholar]
- [11].Trattnig S, Springer E, Bogner W, et al. Key clinical benefits of neuroimaging at 7T. Neuroimage. 2016;S1053–8119:30651–30656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ge Y, Zohrabian VM, Grossman RI. Seven-Tesla Magnetic Resonance Imaging. JAMA Neurol. 2009;65:812–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tallantyre EC, Brookes M, Dixon J, et al. Demonstrating the perivascular distribution of MS lesions in vivo with 7-Tesla MRI. Neurology. 2008;70:2076–2078. [DOI] [PubMed] [Google Scholar]
- [14].Fog T On the vessel-plaque relationships in the brain in multiple sclerosis. Acta Neurol Scand. 1964;40:9–15. [PubMed] [Google Scholar]
- [15].Adams C, Poston R, Bulk S. Pathology, histochemistry and immunocytochemistry of lesions in acute multiple sclerosis. J Neurol Sci. 1989;92:291–306. [DOI] [PubMed] [Google Scholar]
- [16].Tan IL, Van Schijndel RA, Pouwels PJW, et al. MR venography of multiple sclerosis. Am. J. Neuroradiol. 2000;21:1039–1042. [PMC free article] [PubMed] [Google Scholar]
- [17].Kollia K, Maderwald S, Putzki N, et al. First clinical study on ultra-high-field MR imaging in patients with multiple sclerosis: Comparison of 1.5T and 7T. Am. J. Neuroradiol. 2009;30:699–702.** Direct comparison of 1.5 and 7T imaging
- [18].Mistry N, Tallantyre EC, Dixon JE, et al. Focal multiple sclerosis lesions abound in “normal appearing white matter.” Mult. Scler. J. 2011;17:1313–1323. [DOI] [PubMed] [Google Scholar]
- [19].Marques JP, Kober T, Krueger G, et al. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage. 2010;49:1271–1281. [DOI] [PubMed] [Google Scholar]
- [20].Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tallantyre EC, Morgan PS, Dixon JE, et al. A Comparison of 3T and 7T in the Detection of Small Parenchymal Veins Within MS Lesions. Invest. Radiol. 2009;44:491–494. [DOI] [PubMed] [Google Scholar]
- [22].Sati P, Oh J, Todd Constable R, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: A consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat. Rev. Neurol. 2016;12:714–722.** The role of the central vein sign in the diagnosis of multiple sclerosis
- [23].Kangarlu A, Bourekas EC, Ray-Chaudhury a, et al. Cerebral cortical lesions in multiple sclerosis detected by MR imaging at 8 Tesla. AJNR Am J Neuroradiol. 2007. February;28(2):262–6. [PMC free article] [PubMed] [Google Scholar]
- [24].Mainero C, Radding A. In vivo imaging of cortical pathology in multiple sclerosis using ultra-high field MRI. Neurology. 2009;941–948.** Utility of 7T for in vivo classification of corticol lesions
- [25].Metcalf M, Xu D, Okuda DT, et al. High-resolution phased-array MRI of the human brain at 7 tesla: Initial experience in multiple sclerosis patients. J. Neuroimaging. 2010;20:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tallantyre EC, Morgan PS, Dixon JE, et al. 3 Tesla and 7 Tesla MRI of multiple sclerosis cortical lesions. J. Magn. Reson. Imaging. 2010;32:971–977. [DOI] [PubMed] [Google Scholar]
- [27].Pitt D, Boster A, Pei W, et al. Imaging Cortical Lesions in Multiple Sclerosis With Ultra–High-Field Magnetic Resonance Imaging. JAMA Neurol. 2015;67:812–818. [DOI] [PubMed] [Google Scholar]
- [28].Nielsen AS, Kinkel RP, Tinelli E, et al. Focal cortical lesion detection in multiple sclerosis: 3 tesla DIR versus 7 tesla FLASH-T2. J. Magn. Reson. Imaging. 2012;35:537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yao B, Hametner S, Van Gelderen P, et al. 7 Tesla magnetic resonance imaging to detect cortical pathology in multiple sclerosis. PLoS One. 2014;9:e108863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Harrison DM, Oh J, Roy S, et al. Thalamic lesions in multiple sclerosis by 7T MRI: clinical implications nad relationship to cortical pathology. Mult. Scler. 2015;21:1139–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–2712. [DOI] [PubMed] [Google Scholar]
- [32].Kilsdonk ID, Jonkman LE, Klaver R, et al. Increased cortical grey matter lesion detection in multiple sclerosis with 7 T MRI: A post-mortem verification study. Brain. 2016;139:1472–1481. [DOI] [PubMed] [Google Scholar]
- [33].Dula AN, Pawate S, Dortch RD, et al. Magnetic Resonance Imaging of the Cervical Spinal Cord in Multiple Sclerosis at 7T. Mult. Scler. 2017;22:320–328.** 7T application to the study of small structures
- [34].Hammond KE, Metcalf M, Carvajal L, et al. Quantitative in vivo magnetic resonance imaging of multiple sclerosis at 7 Tesla with sensitivity to iron. Ann. Neurol. 2008;64:707–713. [DOI] [PubMed] [Google Scholar]
- [35].Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: A combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3599–3612.** Combined imaging and pathological study demonstrating 7T sensitivity to iron identification
- [36].Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J. Clin. Invest. 2016;126:2597–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cronin MJ, Wharton S, Al-Radaideh A, et al. A comparison of phase imaging and quantitative susceptibility mapping in the imaging of multiple sclerosis lesions at ultrahigh field. Magn. Reson. Mater. Physics, Biol. Med. 2016;29:543–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang Y, Spincemaille P, Liu Z, et al. Clinical quantitative susceptibility mapping (QSM): Biometal imaging and its emerging roles in patient care. J. Magn. Reson. Imaging. 2017;46:951–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Balchandani P, Naidich TP. Ultra-high-field MR neuroimaging. Am. J. Neuroradiol. 2015;36:1204–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Craner MJ, Newcombe J, Black JA, et al. Molecular changes in neurons in multiple sclerosis : Altered axonal expression of Na v 1 . 2 and Na v 1 . 6 sodium channels and Na+/Ca2+ exchanger. PNAS. 2004;101:8168–8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].England JD, Gamboni F, Levinson SR. Increased numbers of sodium channels form along demyelinated axons. 1991;548:334–337. [DOI] [PubMed] [Google Scholar]
- [42].Waxman SG. Conduction in Myelinated, Unmyelinated, and Demyelinated Fibers. Arch. Neurol. 1977;34:585–589. [DOI] [PubMed] [Google Scholar]
- [43].Stys PK, Lesiuk H. Correlation between electrophysiological effects of mexiletine and ischemic protection in central nervous system white matter. Neuroscience. 1996;71:27–36. [DOI] [PubMed] [Google Scholar]
- [44].Stys PK, Waxman SG, Ransom BR. Ionic mechanisms of anoxic injury in mammalian CNS white matter: role of Na+ channels and Na(+)-Ca2+ exchanger. J. Neurosci. 1992;12:430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Stys K, Ransom R, Waxman G. Tertiary and Quatemary Local Anesthetics Protect CNS White Matter From Anoxic Injury at Concentrations That do not Block Excitability. 1992;67:1–5. [DOI] [PubMed] [Google Scholar]
- [46].Fern R, Ransom BR, Stys PK, et al. Pharmacological protection of CNS white matter during anoxia: actions of phenytoin, carbamazepine and diazepam. J. Pharmacol. Exp. Ther. 1993;266:1549–1555. [PubMed] [Google Scholar]
- [47].Stys PK. Protective Effects of Antiarrhythmic Agents Against Anoxic Injury in eNS White Matter. J. Cereb. blood flow Metab. 1995;15:425–432. [DOI] [PubMed] [Google Scholar]
- [48].Bechtold D a, Kapoor R, Smith KJ. Axonal protection using flecainide in experimental autoimmune encephalomyelitis. Ann. Neurol. 2004;55:607–616. [DOI] [PubMed] [Google Scholar]
- [49].Lo AC, Saab CY, Black J a, et al. Phenytoin protects spinal cord axons and preserves axonal conduction and neurological function in a model of neuroinflammation in vivo. J. Neurophysiol. 2003;90:3566–3571. [DOI] [PubMed] [Google Scholar]
- [50].Black J a, Liu S, Carrithers M, et al. Exacerbation of experimental autoimmune encephalomyelitis after withdrawal of phenytoin and carbamazepine. Ann. Neurol. 2007;62:21–33. [DOI] [PubMed] [Google Scholar]
- [51].Bechtold D a, Miller SJ, Dawson AC, et al. Axonal protection achieved in a model of multiple sclerosis using lamotrigine. J. Neurol. 2006;253:1542–1551. [DOI] [PubMed] [Google Scholar]
- [52].Al-Izki S, Pryce G, Hankey DJR, et al. Lesional-targeting of neuroprotection to the inflammatory penumbra in experimental multiple sclerosis. Brain. 2014;137:92–108. [DOI] [PubMed] [Google Scholar]
- [53].Jain RK. Transport of Molecules in the Tumor Interstitium : A Review Transport of. Cancer Res. 1987;47:3039–3051. [PubMed] [Google Scholar]
- [54].Camero IL, Smith NKR, Pool TB, et al. Intracellular Concentration of Sodium and Other Elements as Related to Mitogenesis and Oncogenesis in Vivo. Cancer Res. 1980;40:1493–1500. [PubMed] [Google Scholar]
- [55].Nagy IZ, Lustyik G, Lukács G, et al. Correlation of Malignancy with the Intracellular Na + : K + Ratio in Human Thyroid Tumors. Cancer Res. 1983;43:5395–5402. [PubMed] [Google Scholar]
- [56].Thulborn KR, Davis D, Snyder J, et al. Sodium MR Imaging of Acute and Subacute Stroke for Assessment of Tissue Viability. Neuroimaging Clin. N. Am. 2005;15:639–653. [DOI] [PubMed] [Google Scholar]
- [57].Maudsley AA, Hilal SK. Biological aspects of sodium-23 imaging. Br. Med. Bull. 1984;40:165–166. [DOI] [PubMed] [Google Scholar]
- [58].Inglese M, Madelin G, Oesingmann N, et al. Brain tissue sodium concentration in multiple sclerosis: a sodium imaging study at 3 tesla. Brain. 2010;133:847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Paling D, Solanky BS, Riemer F, et al. Sodium accumulation is associated with disability and a progressive course in multiple sclerosis. Brain. 2013;136:2305–2317. [DOI] [PubMed] [Google Scholar]
- [60].Zaaraoui W, Konstandin S, Audoin B, et al. Distribution of Brain Sodium Accumulation Correlates with Disability in Multiple Sclerosis : Radiology. 2012;264:859–867. [DOI] [PubMed] [Google Scholar]
- [61].Maarouf A, Audoin B, Konstandin S, et al. Topography of brain sodium accumulation in progressive multiple sclerosis. MAGMA. 2014;27:53–62. [DOI] [PubMed] [Google Scholar]
- [62].Maarouf A, Audoin B, Pariollaud F, et al. Increased total sodium concentration in gray matter better explains cognition than atrophy in MS. Neurology. 2017;88:289–295. [DOI] [PubMed] [Google Scholar]
- [63].Eisele P, Konstandin S, Szabo K, et al. Sodium MRI of T1 High Signal Intensity in the Dentate Nucleus due to Gadolinium Deposition in Multiple Sclerosis. J. Neuroimaging. 2017;27:372–375. [DOI] [PubMed] [Google Scholar]
- [64].Huhn K, Mennecke A, Linz P, et al. 23Na MRI reveals persistent sodium accumulation in tumefactive MS lesions. J. Neurol. Sci. 2017;379:163–166.28716233 [Google Scholar]
- [65].Fleysher L, Oesingmann N, Inglese M. B₀ inhomogeneity-insensitive triple-quantum-filtered sodium imaging using a 12-step phase-cycling scheme. NMR Biomed. 2010;23:1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Fleysher L, Oesingmann N, Brown R, et al. Noninvasive quantification of intracellular sodium in human brain using ultrahigh-field MRI. NMR Biomed. 2013;26:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Petracca M, Vancea RO, Fleysher L, et al. Brain intra- and extracellular sodium concentration in multiple sclerosis : a 7 T MRI study. Brain. 2016;1–12.** Characterization of intra- and extracellular sodium concentration in multiple sclerosis
- [68].Nikić I, Merkler D, Sorbara C, et al. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat. Med. 2011. [;17:495–499. [DOI] [PubMed] [Google Scholar]
- [69].Zhang B, Seifert A, Kim J, et al. 7 Tesla 22-channel wrap-around coil array for cervical spinal cord and brainstem imaging. MRM. 2017;78:1623–1634. [DOI] [PubMed] [Google Scholar]


