Abstract
Bisphenol A, (2,2-bis(4-hydroxyphenyl)propane, BPA)-free polycarbonate (PC) from six-membered di-cyclic carbonate, di-trimethylolpropane di-cyclic carbonate (DTMPC) was developed as a new type of PC by ring opening homo-polymerization. The polymerization was controlled by using metal-free organic-based catalyst systems. The results indicated that the conversion rate depends on the basicity of the catalyst in the order of 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), 4-dimethylaminopyridine (DMAP), triethylamine (TEA) from high to low. Over 99% conversion of DTMPC was obtained at 130°C within 15 min by TBD, DBU and DMAP. The resulting PC as a homo-polymer showed high optical transparency and hardness, low swelling property in organic solvents, and thermally stable at temperatures as high as 200 °C. High cell viability as the cyto-compatibility of C3H 10T1/2 cells seeded directly on the surface of PC films was obtained. This implied that PC is a viable material for biomedical and consumer products applications where safety is an important consideration.
Graphical abstract
BPA-free polycarbonate, a new type of high thermal stable, optical transparent and biocompatible material was prepared from di-cyclic carbonate.
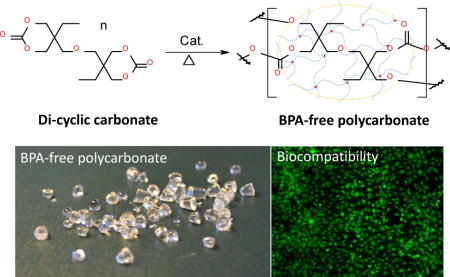
Introduction
Polycarbonates (PCs) have been used for numerous applications ranging from automotive parts to electronic appliances due to their excellent mechanical properties and durability. Recently developed biocompatible PCs have been incorporated in a variety of biomedical devices such as dental sealants and tooth coatings.1–4 Conventionally, PC is obtained from the polymerization of 2,2-bis (4-hydroxyphenyl) propane (bisphenol A, BPA) with highly toxic phosgene or diphenylcarbonate, which is also commonly derived from dimethylcarbonate or phosgene.5 The final product requires an additional purification step to eliminate the presence of chlorinated impurities (Scheme 1A).6
Scheme 1.
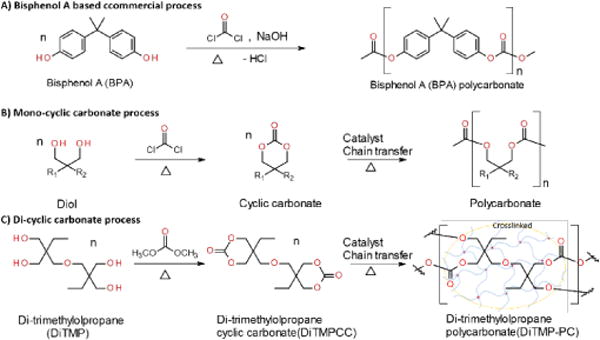
Polymerization pathways for the production of polycarbonates. A) Commercial process from bisphenol A by reaction with phosgene. B) Conventional process through mono-cyclic carbonate obtained by reaction of diol and highly hazard phosgene. C) Green and sustainable process through di-cyclic carbonate obtained by reaction of polyol and dimethylcarbonate.
In addition to the intrinsic toxicity of phosgene, the building block, BPA used in the production of PCs is an estrogen analog, which is suspected to be harmful during fatal and child development.2–4, 6 Due to the broad application of PCs in the food and drink packaging industry, the release of BPA by hydrolysis during their use has been investigated for exposure and risk assessments in many studies.2–4,7 It has been concluded that BPA will leak from PCs during their lifespan. Thus, there has been increasing attention and societal pressure to find BPA-free PC for applications in medical, food packaging industries, where toxicity to humans, especially children, is a great concern. Over the past decade, increasing attention has been paid to synthesize a biocompatible PC with a prominent role in the manufacturing of biomedical devices and biomaterials for tissue engineering and regenerative medicine.8–12
On the other hand, renewable and non-estriogenic alternatives to BPA and sustainable BPA free aliphatic PCs have been investigated extensively for the past decade as an alternative to conventional aromatic PCs.13,14 Additionally, their unique biocompatibility and biodegradability prompt them to be a great candidate for food and medical applications.1 Recently, the synthetic routes of poly(limonene carbonate) were successfully demonstrated with improvement of thermal and mechanical properties. The resulting bio-based poly(limonene carbonate) could be valorized with a double bond in the repeating unit.15–18 Also, ring opening polymerization (ROP) of cyclic carbonate monomers is one of the most promising synthesis pathways,9,19,20 while the ROP of cyclic carbonate with amines produced isocyanate-free polyurethanes with and without catalyst.21,22 Efforts have been put to study the effects of different initiators and catalysts on the reaction rates of ROP synthesis.19 As compared to conventional five-membered cyclic carbonates, six-membered alternatives are thermodynamically more suitable precursors, especially when different functional groups are introduced to impart advanced properties to the polymers, however their production has not been straightforward.19,23,24 Recently, we have developed a facile and green synthetic route of various six-membered functional cyclic carbonates.25–27 This provides an alternative synthesis pathway of PC to conventional methods involving toxic materials such as BPA and phosgene. However, with such an advantage, aliphatic PCs synthesized from ROP suffer significant shortcomings as compared to aromatic BPA-based PCs. For example, the aliphatic PCs have very poor thermal and chemical stabilities (Scheme 1B).1,28 Some variations can undergo hydrolysis in a phosphate buffer solution with pH 7.4 at 37°C, which resulted in complete degradation after 5 days.20 Meanwhile, much effort has been put in the development of metal-free catalytic systems to avoid issues revolving around residual metal trace in the final polymer. The state-of-the-art organometallic polymerization catalysis provides various proficient systems based on nontoxic metal centers, such as zinc, magnesium, calcium, or rare-earth metals, bearing suitable ancillary ligands.26,27 Recent advances in the ROP of cyclic esters have led to the emergence of a variety of mono- and bi-component organo-catalysts, as well as enzymes. Regarding the controlled ROP of trimethylene carbonate (TMC) (Scheme 1B), some organo-catalysts have been shown to lead to well defined poly(trimethylene carbonate)s (PTMCs) of high molar mass (up to MW. 72 000).29 Helou et al. have demonstrated that the commercially available organo-catalysts 4-N,N-dimethylaminopyridine (DMAP) and 1,5,7-triazabicyclo-[4.4.0] dec-5-ene (TBD) allow the controlled ROP of several six-membered monocyclic carbonates such as TMC, 3,3-dimethoxytrimethylene carbonate, and 3-benzyloxytrimethylenecarbonate under mild operating conditions (solvent free, 60–150 °C) by using an alcohol such as butanol, 1,3-propanediol and glycerol, as a co-initiator and chain-transfer agent.26 However, the resulting aliphatic polycarbonate PTMC has a low glass transition temperature of −17°C, making it unable to mold or cast to a desired device to be used at room temperature (Scheme 1B).1,28
In this paper, BPA-free aliphatic polycarbonate as a homo-polymer from a six-membered di-cyclic carbonate, di-trimethylolpropane di-cyclic carbonate (DTMPC) was synthesized with enhanced thermal stability and mechanical properties (Scheme 1C). The resulting new class of PC materials was evaluated on their thermal, physical and biocompatible properties. The direct homo-polymerization process was controlled by using metal-free organic-based catalyst systems, and employed to form films, casting of desired structures which can be used as advanced biomaterials for biomedical applications.
Experimental
Materials
Di-trimethylolpropane (DTMP) and dimethylcarbonate (DMC) were kindly provided by Perstorp AB (Sweden). 1,3-Propanediol (1.3-PDO, 98%), 1.2-propanediol (1,2-PDO, 99%), 1.6-hexanediol (1,6-HDO, 99%) trimethylamine (TEA, >99.5%), 4-Dimethylaminopyridine (DMAP, 98%), 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU, 99%), 1,5,7-Triazabicyclo [4.4.0] dec-5-ene (TBD, 98%), and molecular sieves (4Å) were purchased from Sigma-Aldrich. HPLC grade acetonitrile and toluene were purchased from MERCK (Germany). All chemicals were used without further treatment. Di-trimethylolpropane di-cyclic carbonate was prepared according to modified methods from previous report.25 DTMP (60 g) was reacted in DMC (0.9 L) with molecular sieves in a pressure reactor (2 L), which was heated in an oil bath at 120 °C. The reaction was monitored by gas chromatography. The crude DTMPC was obtained from the reaction mixture by simple filtration of solid particles and evaporation of excess DMC. Resulting solid residue was then recrystallized in 500 mL toluene at 4°C for 2 days. Purified DTMPC (45g, 98% purity) was then obtained by filtration and drying in a vacuum oven at 25°C.
Preparation of Polycarbonates (PCs) from DTMPC
The ROPs of DTMPC to PCs were evaluated on the conversion rate and yield using various catalysts and chain transfers at 110 °C, 130 °C, and 150 °C, respectively. As organic amine catalysts, TEA, DMAP and DBU, and TBD were investigated at various ratios to a monomer, DTMPC. As alcohol chain transfers, 1,3-PDO, 1,2-PDO and 1,6-HDO were evaluated on the conversion rate and yield at various ratios to a monomer, DTMPC. As a representative instance, 100 mg (0.33 mmol) DTMPC were placed and melted in a 4 mL vial at 110 °C and a 2.75 μL mixture of DBU (0.25 μL, 1.7 μmol) and 1,3-PDO (2.5 μL, 35 μmol) was added and immediately mixed. The mixture was continuously heated at a certain temperature (110 °C, 130 °C, or 150 °C) using heating blocks, and solidified to PC material. The resulting solid PC materials were collected, and used without further treatment for the structure, thermal and physical property analyses.
Quantitative Analysis of the Conversion Rate and Yield
Quantitative analysis of the reaction component (DiTMP) was performed using gas chromatography (GC, Varian 430-GC, Varian, USA) equipped with FactorFour Capillary column, VF-1ms (Varian, 15M × 0.25mm) and a flame ionization detector. The initial column temperature was increased from 50 °C to 250 °C at a rate of 20 °C/min. The samples, diluted with acetonitrile, to a concentration of 0.01–0.5 mg/mL, were injected in a split injection mode of 10% at 275 °C. About 10 mg of samples was taken from the reactant during time course, and was dissolved at 10 mg/mL concentration in acetonitrile at room temperature for 20 min. After the solution was centrifuged at 12,000 rpm for 1 min, the resulting supernatant was injected to measure the remaining monomer to GC (Fig. S2). The residual amount and conversion rate of DTMPC in the reaction were calculated by using standard curves of DTMPC concentration. All the data were obtained from two independent experiments and were provided as the average of the replicates with error bars.
FT-IR, Solid State NMR and UV-Vis Characterization
The ROPs of DTMPC were monitored based on transformation of functional groups such as hydroxyl, carbonyl, and carbonate by Fourier-transform infrared spectroscopy (FT-IR). The spectra of samples were obtained in a region of 500-4000 cm−1 using Nicolet-iS5 (Thermo Scientific, USA). An air background spectrum was collected before the analysis of the sample, and subtracted from each sample spectrum. Solid state 1H and 13C NMR data were recorded on an Agilent 400 MHz 54mm NMR DD2 spectrometer using Magic Angle spinning (MAS) and Cross Polarization Magic Angle Spinning (CP-MAS) technique, respectively. Optical absorbance was measured from 300 nm to 1000 nm using a UV-Vis spectrophotometer MULTISKAN GO (Thermo Scientific, USA). To prepare a film sample, DTMPCC was placed in a PDMS compartment covered by a glass coverslip, which was chemically modified by 3-(trimethoxysilyl)-propyl methacrylate, and mixed with 2.75 μL mixture of DBU (0.25 μL, 1.7 μmol) and 1,3-PDO (2.5 μL, 35 μmol). After covering with another PDMS compartment covered by a glass coverslip, the resulting mixture was continuously heated at 130 °C using heating blocks. The height between the two plates was held constant at 100 μm. Resulting PC scaffolds were placed in a 96-well polystyrene plate, and analyzed in ethanol or dry condition by UV-Vis spectrophotometer. Blank was used as a control.
Thermal Characterization
Differential scanning calorimeter (DSC) was carried out using a DSC Q1000 (TA Instrument) over a temperature range of 0 – 200 °C with a 10 °C/min heating rate under nitrogen. DSC was performed by 2 or 5 cycling of heating and cooling. Thermogravimetric analysis (TGA) curves were obtained by TGA Q500 (TA Instrument) with a heating rate of 5 °C/min under nitrogen with a flow rate of 35 cm3/min.
Mechanical Testing
Tensile mechanical test was carried out using Thermomechanical Analysis (TMA, Perkin Elmer) at room temperature. The samples were cut into 5mm × 10 mm × 0.5 mm strips. The test was conducted at 10 % strain failure with a strain rate of 1mm/min. The stress strain curve was obtained and analyzed to calculate the tensile modulus of the PC sample prepared with 0.01 % TBD and 1% 1,3-PDO at 130°C for 1 hour.
Shore D Harness and Swelling Analysis Characterization
Six PC film samples were prepared at different ratios of catalytic systems to substrate, DTMPC (0.33 mmol) as 1) 1.7 μmol DBU + 3.5 μmol 1,3-PDO, 2) 1.7 μmol DBU + 35 μmol 1,3-PDO, 3) 1.7 μmol + 35 μmol 1,2-PDO, 4) 1.7 μmol + 24 μmol 1,6-HDO, 5) 0.09 μmol TBD + 14 μmol 1,3-PDO and 6) 0.18 μmol TBD + 14 μmol 1,3-PDO, respectively. The thickness was determined by the space between the two PDMS compartments, which was held constant at 1 mm. For the preparation of the PC films, DTMPC was polymerized in between the two PDMS compartments by heating at 130 °C for 1 hr. The hardness of the PC samples was measured at room temperature by using a digital hardness durometer (BGD 935/D, Biuged Laboratory Instruments).30 Results were averaged from a minimum of five tests conducted in several zones of the samples. Meanwhile, for swelling analysis, the thin films of BPA-free PCs were prepared as 100 μm thick from ROP of DTMP (0.33 mmol) using 1.7 μmol DBU and 35 μmol 1,3-PDO by heating at 130 °C for 1 hr. Resulting films were cut into 6 mm wide disks with biopsy punches. The disks were submerged in water, boiled water (95 °C), THF, ethanol, chloroform, DMSO and acetone for 20 min, removed, and quickly measured to determine swelling characteristics.
Cell Culture and Cell Viability Assay
The thin films of BPA-free PCs were prepared with a 100 μm thickness from ROP of DTMP at 130 °C under conditions of 1) PC-D1, 0.33mmol DTMPC + 1.7 μmol DBU + 3.5 μmol 1,3-PDO, 2) PC-D2, 0.33mmol DTMPC + 1.7 μmol DBU, 35 μmol 1,3-PDO, and 3) PC-T, 0.33mmol DTMPC + 0.09 μmol TBD + 14 μmol 1,3-PDO, respectively. Resulting films were used for the cell viability assay. For cell culture, C3H/10T1/2 cells (ATCC, Manassas, Virginia) were maintained in Dulbecco’s Modified Eagle Medium (DMEM, Gibco Life Technologies) supplemented with 10% v/v FBS (fetal bovine serum, Gibco Life Technologies), 1% penicillin/streptomycin solution (Penstrep) (Gibco Life Technologies).
To study the cell viability, 10T1/2 cells were obtained at passage 1 and used at passage 7. The PCs were cut into rectangular shapes with a surface area of 4 mm2, put into 24-well culture plates, and immersed in PBS with 1% Penstrep 2 days prior to seeding. The cells were seeded onto the PCs with a density of 500,000 cells/mL in a 500 μL growth media per well. After 1, 3 and 7 days of culturing, the samples were washed thoroughly with PBS, and a solution of calcein AM and ethidium homodimer-1 (EthD-1) (Biotium) in PBS was added to the sample per the manufacturer’s protocol. The samples were incubated for 30 min at room temperature on an orbital shaker, and cell viability was assessed by fluorescence microscopy. Cells seeded on the PCs were scanned, and the cells were counted using ImageJ.
Results and Discussion
ROP of di-cyclic carbonates by metal-free catalysis
In previous reports, DTMPC was used to avoid the deformation as a cross-linking agent (1 mol %) in the ring opening co-polymerization of two cyclic monomers, trimethylene carbonate and caprolactone with stannous octanoate as catalyst31. And a DBU-catalyzed ROP of neopentylglycol carbonate with DTMPC formed effectively networked polycarbonate films with good transparency and flexibility in THF at 60 °C for 12 hours32. Recently, a reprocessable acid-degradable vitrimer, hydroxylfunctionalized PC networks were prepared from DTMPC and 1,4-butanediol at ratio of 1:0.5 to 1:1 equivalent at elevated temperatures by transcarbonation in the presence of catalytic Ti(IV) alkoxides33.
In the present study, the ROP of di-cyclic carbonate, DTMPC as a single monomer was investigated to produce a highly crosslinked PC under solvent-free condition (Scheme 1C). Also as a green process to address the environmental concerns and safety issues from using potentially toxic and carcinogenic metal-based catalysts in conventional synthesis pathway of aliphatic PCs, the ROP was monitored and performed by some amine-based catalysts, TEA, DMAP, DBU, and TBD with alcohol such as 1,3-propanediol (1,3-PDO), 1,2-propanediol (1,2-PDO) and 1,6-hexanediol (1,6-HDO). The effects of catalysts and chain transfer agents at different concentrations have been investigated. These organocatalysts, regardless of whether in solution or in bulk, operate via an activated-monomer mechanism in the presence of alcohol (e.g., benzyl alcohol, 1,3-propanediol, glycerol; up to 20 equivalents). Alcohol acts as both a co-initiator and a chain transfer agent.29 The ROP was performed without using solvent as bulk polymerization by mixing catalyst and chain transfer under a heating condition (Fig. 1 and 2, and Table 1). The reaction rate and molar catalyst efficient depend on the basicity of the catalyst at 130 °C in the order of TBD (0.03 mol%) > DBU (0.5 mol%) > DMAP (2.5 mol%) > TEA (2.2 mol%) (Fig. 1A). Compared to TBD, TEA has a much lower conversion rate even at a 25 times higher concentration. The rapid reaction rate, however, does not guarantee a higher conversion rate, as shown in the comparison between TBD and DBU catalysts. The initial reaction rate was also dependent on the reaction temperature (Fig. 1B and 1C); over 99% conversion was obtained at 130 °C within 15 min by TBD, DBU and DMAP under given catalytic conditions (Fig. 1B and Table 1).
Fig. 1.
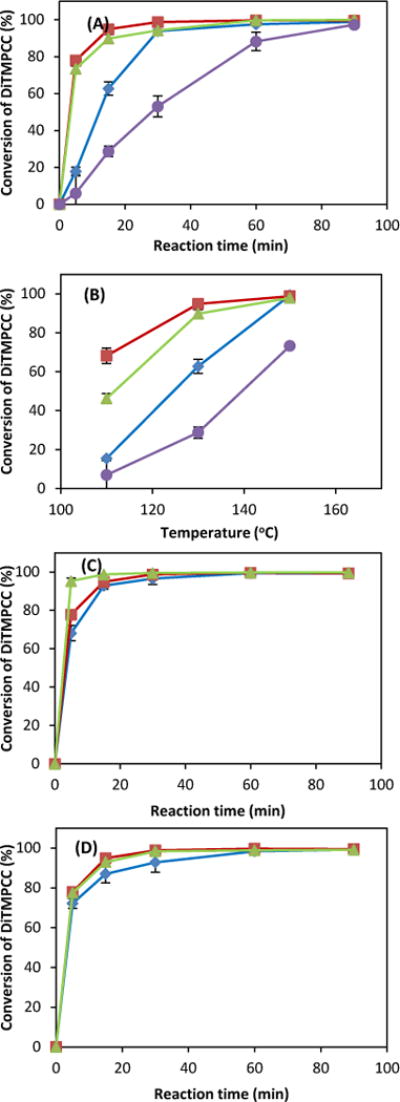
Synthesis of BPA-free PC from DTMPC (100 mg, 0.33 mmol) by ROP under various conditions. Effect of (A) catalyst system at 130°C and (B) temperature at 15 min in presence of 8.2 μmol DMAP and 35 μmol 1,3PDO (◆), 1.7 μmol DBU and 35 μmol 1,3PDO (■), 0.09 μmol TBD and 35 μmol 1,3PDO (▲), and 7.2 μmol TEA and 35 μmol 1,3PDO (●). C) Effect of temperature under condition of 1.7 μmol DBU and 35 μmol 1,3-PDO at 110°C (◆), 130°C (■) and 150°C (▲). D) Effect of catalyst amount with 0.67 μmol DBU and 35 μmol 1,3PDO (◆), 1.2 μmol DBU and 35 μmol 1,3PDO, (▲) 1.7 μmol DBU and 35 μmol 1,3PDO (■) at 130°C.
Fig. 2.
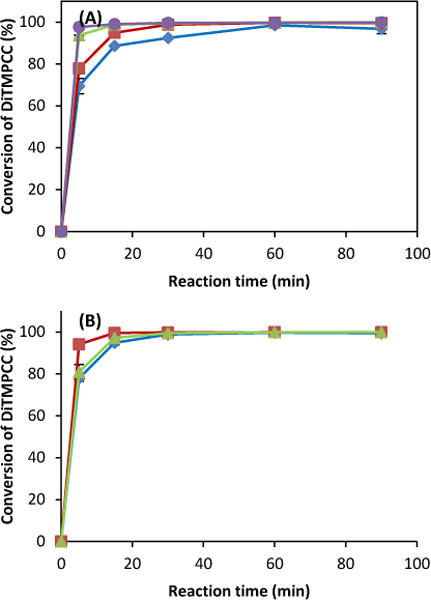
Synthesis of BPA-free PC from DTMPC (100 mg, 0.33 mmol) by ROP under various conditions. A) Effect of amount of chain transfer (1,3-PDO) using 1.7 μmol DBU with 3.5 μmol (◆), 35 μmol (■), 70 μmol (▲), and 140 μmol (●) of 1,3PDO at 130°C. (B) Effect of chain transfers using 1.7 μmol DBU with 35 μmol 1,3PDO (◆), 35 μmol 1,2PDO (■) and 35 μmol 1,6HDO (▲) at 130°C.
Table 1.
Summary of polymerization of DTMPC performed by various catalytic conditions. Molar ratio (%) to monomer (DTMPC, typically 100mg, 0.33 mmol). Conversion determined by GC.
| Run | Catalyst | Alcohol | Temp. (°C) | Time (min) | Conversion (%) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Catalyst | Ratio (%) | Alcohol | Ratio (%) | ||||
| 1 | DMAP | 2.5 | 13PDO | 10 | 110 | 90 | 55 |
| 2 | DMAP | 2.5 | 13PDO | 10 | 130 | 30 | 93.8 |
| 3 | DMAP | 2.5 | 13PDO | 10 | 130 | 90 | 98.8 |
| 4 | DMAP | 2.5 | 13PDO | 10 | 150 | 5 | 83.9 |
| 5 | DMAP | 2.5 | 13PDO | 10 | 150 | 15 | 99.1 |
| 6 | DBU | 0.5 | 13PDO | 10 | 110 | 15 | 92.9 |
| 7 | DBU | 0.5 | 13PDO | 10 | 110 | 60 | 99.4 |
| 8 | DBU | 0.5 | 13PDO | 10 | 130 | 15 | 94.9 |
| 9 | DBU | 0.5 | 13PDO | 10 | 130 | 30 | 98.8 |
| 10 | DBU | 0.5 | 13PDO | 10 | 150 | 5 | 95.3 |
| 11 | DBU | 0.5 | 13PDO | 10 | 150 | 30 | 99.5 |
| 12 | DBU | 0.5 | 13PDO | 1 | 130 | 30 | 92.5 |
| 13 | DBU | 0.5 | 13PDO | 1 | 130 | 60 | 98.5 |
| 14 | DBU | 0.5 | 13PDO | 20 | 130 | 30 | 99.5 |
| 15 | DBU | 0.5 | 12PDO | 10 | 130 | 15 | 99.5 |
| 16 | DBU | 0.5 | 16HDO | 10 | 130 | 30 | 99.4 |
| 17 | TBD | 0.03 | 13PDO | 4 | 110 | 30 | 91.4 |
| 18 | TBD | 0.03 | 13PDO | 4 | 110 | 90 | 96.6 |
| 19 | TBD | 0.03 | 13PDO | 4 | 130 | 30 | 94.7 |
| 20 | TBD | 0.03 | 13PDO | 4 | 130 | 60 | 96.1 |
| 21 | TBD | 0.03 | 13PDO | 4 | 150 | 5 | 96.2 |
| 22 | TBD | 0.03 | 13PDO | 4 | 150 | 30 | 99 |
| 23 | TEA | 2.2 | 13PDO | 10 | 110 | 90 | 7.2 |
| 24 | TEA | 2.2 | 13PDO | 10 | 130 | 30 | 53.1 |
| 25 | TEA | 2.2 | 13PDO | 10 | 130 | 90 | 97.3 |
| 26 | TEA | 2.2 | 13PDO | 10 | 130 | 30 | 82.7 |
| 27 | TEA | 2.2 | 13PDO | 10 | 130 | 90 | 96.7 |
The highest conversion rate was achieved at a combination of 1.7 μmol DBU and 35 μmol 1,3-PDO. The effect of temperature on the reaction rate was also studied using this catalyst combination. The results have shown an increase in the initial reaction rate at 150 °C as compared to the lower temperature cases (Fig. 1C). Additionally, the relationship between the conversion rate and initial catalyst concentration was investigated. As shown in Fig. 1D, the reaction rate increases with the initial DBU concentration. The reaction rate was also increased with an increasing amount of chain transfer, and affected by the type of transfers, which showed as an order of 1,2-PDO > 1,6-HDO ≥ 1,3-PDO (Fig. 2, Table 1). The basic trends and efficiency of organocatalysts were evaluated for the ROP of di-cyclic carbonate, and were in the similar line with those of mono-cyclic carbonate.29,34 ROP of a di-cyclic monomer was evaluated on the transformation of functional groups by FT-IR and solid state 1H-NMR. As a representative polymerization, the ROP of DTMPCC (0.33 mmol) was performed with 1.7 μmol DBU as a catalyst and 35 μmol 1,3-PDO as a chain transfer agent at 130 °C.
FT-IR spectra showed the peak shifts of the functional groups in the reaction. A peak shifted 10 cm−1 from cyclic carbonyl group of DTMPC at 1731 cm−1 (Fig. 3a) to linear carbonate linkages in the PC at 1741 cm−1 (Fig. 3d). Additionally, a peak of C-O-C asymmetric stretching band typically appears at 1290-1180 cm−1. The functionality changes of the C-O-C group from cyclic carbonate to linear polycarbonate provided a strong new peak at 1233 cm−1 (Fig. 3d). The hydroxyl group at 3400 cm−1 did not appear and indicated that the polymerization and crosslinking was quickly achieved after opening of the cyclic carbonate ring.
Fig. 3.
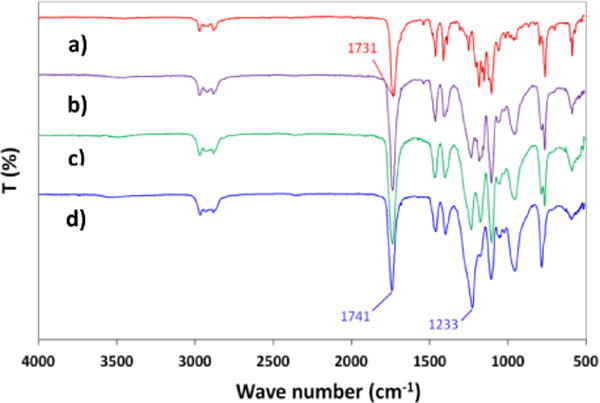
FT-IR spectra of the reaction components and polycarbonate product formed during ROP of DTMPC at 130 °C. (a) DTMPC, (b) product in 15 min of ROP, (c) product in 30 min in ROP, and (d) product in 120 min in ROP.
The resulting PC materials, which wasn’t dissolved in general organic solvents, was elucidated by solid state NMR spectroscopy (Fig. S3). The observed 1H NMR peaks were assigned to the methyl, methylene, and ether groups, confirming the expected polymer bond. 13C NMR spectrum of DTMPC presents six characteristic carbon peaks. The peaks near 157 ppm were assigned to carbonyl carbons in carbonate bonds of polymer. The broad peak at 70-72 ppm indicates carbons adjacent to carbonate group, while the peak at 45 ppm corresponds to carbons adjacent to ether group. A small peak at 38 ppm was assigned to quaternary carbons. The peaks of carbon in methylene and methyl groups appear at 25 ppm and 10 ppm, respectively. The NMR results demonstrated the formation of the PC product.
Optical, thermal and mechanical properties of the BPA-free PCs
The BPA-free PC films prepared from the ROP by organocatalytic systems indicated high transparency and good mechanical properties. Optical transparency of the PC variants was measured by UV-Vis spectrometry (Fig. 4).
Fig. 4.
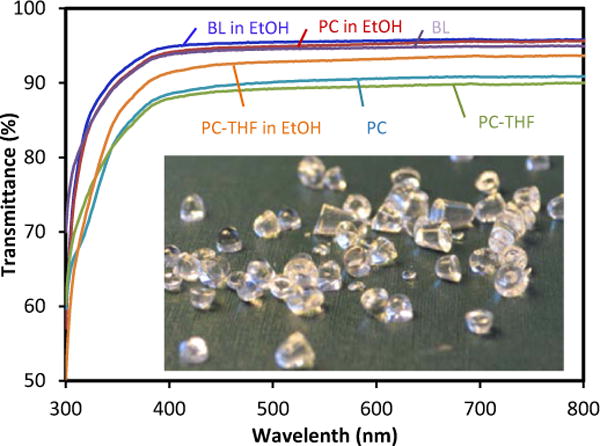
Optical (transmittance) properties of the BPA-free PCs prepared from DTMPC (0.33 mmol) with 1.7 μmol DBU and 35 μmol 13PDO at 130°C for 1hr. BL in EtOH; Blank in ethanol, PC in EtOH; PC in ethanol, BL; blank, PC-THF in EtOH; PC (after submerged in THF for 15 min) in ethanol, PC; PC, PC-THF; PC after submerged in THF for 15 min. Insert shows PC pellets prepared.
The transmittance of PC variants was above 85%, and the excellent optical transparency of PC submerged in ethanol solution was obtained as above 95% within the wavelength range of 300-800 nm, which is comparable to those of commercial polycarbonate sheets and blank.
The thermal behavior of the PCs was evaluated by repeated differential scanning calorimetry (DSC) cycles, and showed similar energy flow in response to temperature change. The smooth energy flow during both heating and cooling processes indicated the lack of internal crystallinity in all PC variants (Fig. 5A).
Fig. 5.
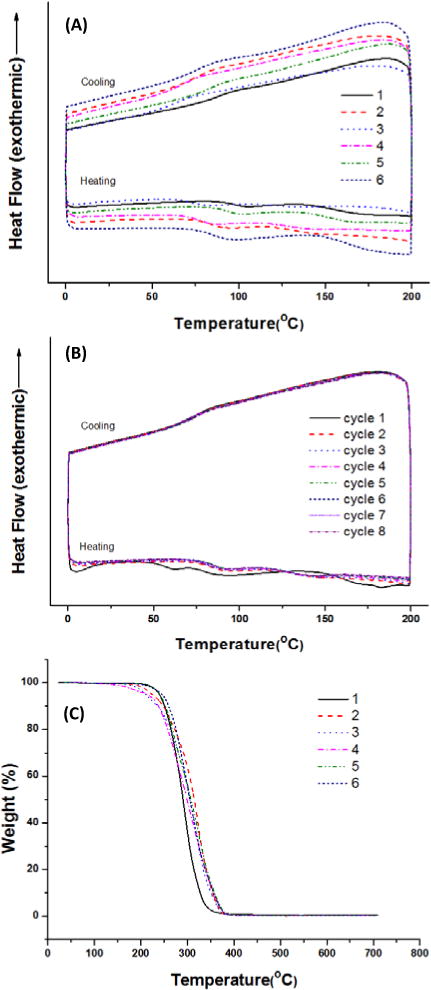
Thermal properties of the BPA-free PCs prepared from DTMPC (0.33 mmol) by different conditions. (A) DSC graphs for BPA-free PC samples prepared using (1) 1.7 μmol DBU and 3.5 μmol 1,3PDO, (2) 1.7 μmol DBU and 35 μmol 1,3PDO, (3) 1.7 μmol DBU and 70 μmol 1,3PDO, (4) 1.7 μmol DBU and 35 μmol 1,2PDO, (5) 1.7 μmol DBU and 24 μmol 1,6HDO, (6) 1.7 μmol DBU and 140 μmol 1,3PDO at 130°C. (B) DSC cycling graph of BPA-free PC prepared by 1.7 μmol DBU and 35 μmol 1,3-PDO at 130°C. (C) TGA graphs for PC samples prepared by the same condition with (A) DSC samples.
Furthermore, the data from DSC also indicates that these new PCs are thermally stable at temperatures as high as 200 °C during 8-time cycles (Fig. 5B). Such unique thermal stability enables these PCs for applications involving an elevated temperature environment, such as autoclaving, which is commonly used to sterilize biomedical devices and surgical instruments for in vivo implantation or in vitro cell culture.
The TGA curves of aliphatic polycarbonate from the DBU catalyst system are shown in Fig. 5C. PCs show no weight loss up to 220 °C and decomposition occurs in a clean single stage with the maximum rate of degradation around 275 °C at a heating rate of 20 °C/min. The decomposition temperature is observed to be around 275 °C (50% weight loss) with no observable residue after 375 °C. As compared to common PCs, which have a decomposition temperature of about 500 °C, the aliphatic PCs are less stable at such a high temperature. But, this will not be an issue for common biomedical and consumer product applications using our PCs at body or room temperature. Future characterization such as thermal expansion and thermal transition coefficient will be needed for comprehensive analysis of the thermal properties of these new PCs. The stress vs. strain curve of the tensile test on PC-T is shown in Figure 6. The tensile modulus of the sample was calculated as 58 MPa from the tangent of the linear region of the curve. The elastic mechanical property indicated crosslinking is the mechanism for networked molecular structure formation.
Fig. 6.
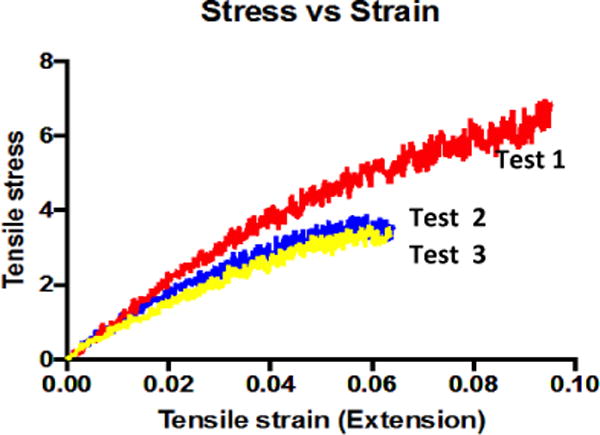
Tensile mechanical properties of BPA-free PC (PC-T) prepared from 0.33 mmol DTMPC, 0.09 μmol TBD and 14 μmol 1,3-PDO at 130°C for 1 hr.
The shore D hardness of the PCs prepared by different catalytic systems was measured at room temperature by using a digital hardness durometer, following ASTM D 2240 standard procedure (Fig. 7A). The mechanical measurement of PCs reveals a similar range of hardness from 83 to 87 of shore D hardness, while a PC sample from Nalgen showed 85 as an extra hard level of hardness. Furthermore, the swelling properties of PCs in various solvents were evaluated for their potential applications as containers for chemicals. As shown in Fig. 7B, after submerging in the solvents for 20 min, the rate of swelling was very low at a range of ±1% for all PC films prepared, and there was no apparent change and corrosion on the surface. The highest swelling rates with 0.7% were obtained in boiling water and DMSO. This swelling data reinforces the concept that a space-filling phenomenon with crosslinking is creating denser and more restrictive networks, and showed high solvent compatibility and resistant property, which didn’t allow the measurement of molecular weight of the networked PC in solvent by Gel permeation chromatography (GPC)-HPLC.
Fig. 7.
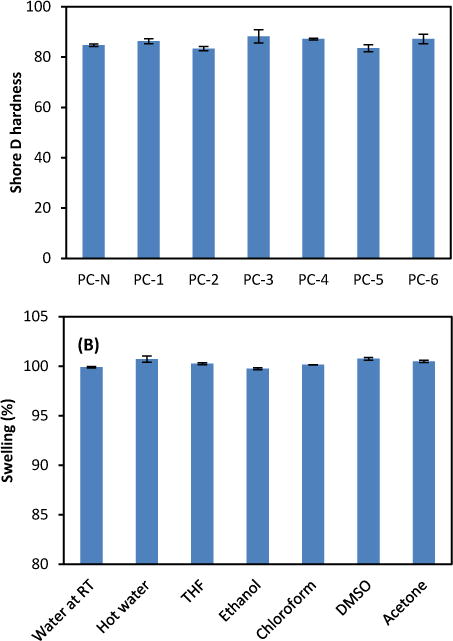
(A) Shore D hardness of BPA-free PCs prepared from DTMPC (0.33 mmol) by various catalytic conditions at 130°C. PC-N was purchased from Nalgen as a PC control. PC-1; 1.7 μmol DBU and 3.5 μmol 1,3PDO, PC-2; 1.7 μmol DBU and 35 μmol 1,3PDO, PC-3; 1.7 μmol DBU and 35 μmol 1,2PDO, PC-4; 1.7 μmol DBU and 24 μmol 1,6HDO, PC-5; 0.09 μmol TBD and 14 μmol 1,3PDO, and PC-6; 0.18 μmol TBD and 28 μmol 1,3PDO. (B) Swelling properties of BPA-free PCs prepared by using various solvents and conditions. BPA-free PC was prepared from DTMPC (0.33 mmol) by 1.7 μmol DBU and 35 μmol 1,3PDO at 130°C. The swelling was compared from 100% as the original size.
Green perspective and cell viability of PC materials
PCs have been widely used in biomedical research due to their biocompatibility as well as their mechanical strength.35,36 Currently, the naturally derived materials such as polysaccharides (alginate, chitosan, starch, cellulose) and proteins (collagen, silk fibroin) have been frequently used in biomedical devices and applications due to their intrinsic compatibility with cells, insignificant toxicity, and low manufacture and disposal costs.37–41 However, the physical and mechanical properties of natural polymers do not always match the properties of tissues and devices. Additionally, the processing conditions and polymerization mechanism often do not allow us to fabricate desired scaffolds and devices. On the other hand, the advancement of polymer technology continues to create polymeric biomaterials that could fulfill the needs in medical research and clinical operations.31
Green Chemistry is defined as the “design of chemical products and processes to reduce or eliminate the use and generation of hazardous substances”.42,43 As a green chemistry process with replacing hazardous ingredients, the overall reaction does not include any cytotoxic materials and the product is potentially toxic-free. In the reported reaction, DTMPC was prepared from reaction of DTMP and DMC, which can be a potential biobased polyol producible from bio-butanol and methanol, and a well-known green solvent prepared from CO2 or CO with methanol, respectively.44,45 The overall process was performed without solvents and additives. The resulting aliphatic PCs can replace conventional PCs as a BPA-free PC, and showed desired physical and thermal properties for manufacturing (e.g. casting and molding) to produce designed devices and consumables. To further explore and verify the potential of using BPA-free PC for biomedical research, we investigated the cyto-compatibility of C3H 10T1/2 cells seeded directly on the surface of PC films without additional surface coating (Fig. 8).
Fig. 8.
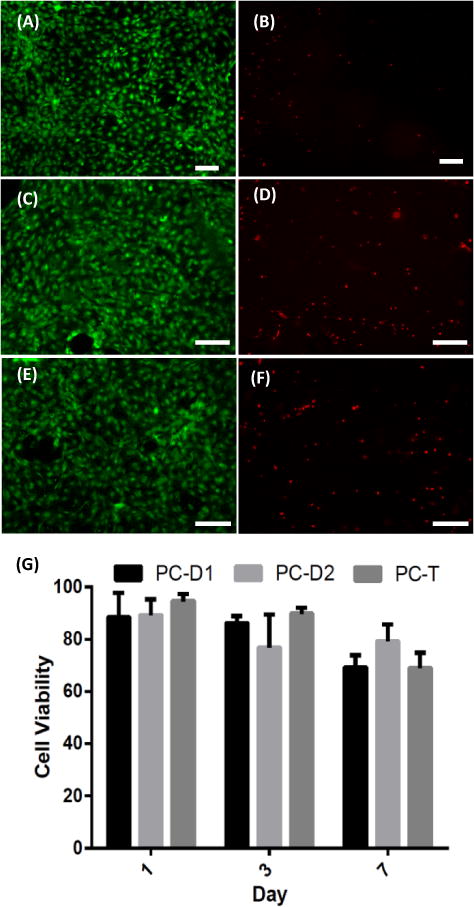
Representative immunostaining to examine cell viability after seeded on PC (PC-D2) prepared from DTMPC (0.33 mmol) by 1.7 μmol DBU and 35 μmol 1,3PDO at 130°C. (A) and (B) for day 1, (C) and (D) for day 4, (E) and (F) for day 7. (A), (C) and (E) for live cells, and (B), (D) and (F) for dead cells are showed. (G) Cells seeded on all 3 PCs have more than 75% and 65% cell viability after 3 days and 7 days’ post seeding, respectively. Scale bar = 200 μm.
This type of fibroblast cell is widely used as a model cell in many biology studies, especially toxicity screening and tissue engineering.46,47 The results are shown in Fig. 8, where the cells were seeded directly on the culturing plate. Definitely, these PCs are more biocompatible than conventional non-treated PCs which are hardly suitable for cell seeding. Both the morphology and the high viability of the cells suggested that PCs are non-toxic for the cells seeded and can be considered as a viable material choice for biomedical applications. Future studies will be carried out to systematically evaluate the biocompatibility and toxicity of the new material for particular biomedical applications.
Conclusions
As a BPA-free material, a new type of aliphatic PC from six-membered di-cyclic carbonate was prepared by thermal ROP. The mechanical and thermal properties of the new aliphatic PC materials were characterized. The high optical transparency and cell viability make these new PCs an excellent candidate for a variety of biological applications and food contacting materials in packaging. The technology can be used to tailor-make novel PC materials with specific properties, and could fulfill the demands to critical safety issues required in biomedical and consumer products applications. The materials could have broader applications such as mechanically strong and durable medical implants, tissue engineering scaffolds, micro-fluidic devices, diagnostic probes, lab-on-a-chip systems, and customized cell culture devices.
Supplementary Material
Acknowledgments
P. Wang and J.H. Park contributed equally to this work. The work was supported by the Swedish Research Council Formas for Environment, Agricultural Sciences and Spatial Planning (942-2016-63) to S-H.P., “Next Generation Carbon Upcycling Project” (Project no. NRF-2017M1A2A2043183) through the National Research Foundation (NRF) funded by the Ministry of Science and ICT, Republic of Korea to J.H.P., and grants from the California Institute for Regenerative Medicine (RT3-07899), National Institutes of Health (R01EB021857 and R21HD090662) and National Science Foundation (1547005 and 1644967) to S.C.
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/x0xx00000x
Conflicts of interest
There are no conflicts to declare.
Notes and references
- 1.Feng FJ, Zhuo RX, Zhang XZ. Prog Polym Sci. 2012;37:211–236. [Google Scholar]
- 2.Gimeno P, Spinau C, Lassu N, Maggio AF, Brenier C, Lempereur L. J Sep Sci. 2015;38:3727–3734. doi: 10.1002/jssc.201500552. [DOI] [PubMed] [Google Scholar]
- 3.Huygh J, Clotman K, Malarvannan G, Covaci A, Schepens T, Verbrugghe W, Dirinck E, Van Gaal L, Jorens PG. Environ Int. 2015;81:64–72. doi: 10.1016/j.envint.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Franke AA. Anal Bioanal Chem. 2015;407:3869–3874. doi: 10.1007/s00216-015-8563-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woo BG, Choi KY, Song KH, Lee SH. J Appl Polym Sci. 2001;80:1253–1266. [Google Scholar]
- 6.Fukuoka S, Hachiya H, Matsuzaki K, Miyaji H. US2008/0041712 A1. 2008 [Google Scholar]
- 7.Chapin RE, Adams J, Boekelheide K, Gray LE, Jr, Hayward SW, Lees PS, McIntyre BS, Portier KM, Schnorr TM, Selevan SG, Vandenbergh JG, Woskie SR. Birth Defects Res, Part B: Dev Reprod Toxicol. 2008;83:157–395. doi: 10.1002/bdrb.20147. [DOI] [PubMed] [Google Scholar]
- 8.Chen FM, Liu X. Prog Polym Sci. 2016;53:86–168. doi: 10.1016/j.progpolymsci.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang LS, Cheng SX, Zhuo RX. Polym Sci, Ser B: Polym Phys. 2013;55:604–610. [Google Scholar]
- 10.Grogan SP, Chung PH, Soman P, Chen P, Lotz MK, Chen S, D’Lima DD. Acta Biomater. 2013;9:7218–7226. doi: 10.1016/j.actbio.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cha C, Soman P, Zhu W, Nikkhah M, Camci-Unal G, Chen S. Biomater Sci. 2014;2:703–709. doi: 10.1039/C3BM60210A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soman P, Lee JW, Phadke A, Varghese S, Chen S. Acta Biomater. 2012;8:2587–2594. doi: 10.1016/j.actbio.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trita AS, Over LC, Pollini J, Baader S, Riegsinger S, Meier MAR, Gooßen LJ. Green Chem. 2017;19:3051–3060. [Google Scholar]
- 14.Mutlu H, Ruiz J, Solleder SC, Meier MA. Green Chem. 2012;14:1728–1735. [Google Scholar]
- 15.Li C, van Berkel S, Sablong RJ, Koning CE. Eur Polym J. 2016;85:466–477. [Google Scholar]
- 16.Byrne CM, Allen SD, Lobkovsky EB, Coates GW. J Am Chem Soc. 2004;126:11404–11405. doi: 10.1021/ja0472580. [DOI] [PubMed] [Google Scholar]
- 17.Kindermann N, Cristòfol A, Kleij AW. ACS Catalysis. 2017;7:3860–3863. [Google Scholar]
- 18.Hauenstein O, Reiter M, Agarwal S, Rieger B, Greiner A. Green Chem. 2016;18:760–770. [Google Scholar]
- 19.Pyo SH, Persson P, Mollaahmad MA, Sörensen K, Lundmark S, Hatti-Kaul R. Pure Appl Chem. 2012;84:637–661. [Google Scholar]
- 20.Lonnecker AT, Lim YH, Wooley KL. ACS Macro Lett. 2017;6:748–753. doi: 10.1021/acsmacrolett.7b00362. [DOI] [PubMed] [Google Scholar]
- 21.Maisonneuve L, Wirotius AL, Alfos C, Grau E, Cramail H. Polym Chem. 2014;5:6142–6147. [Google Scholar]
- 22.Sardon H, Pascual A, Mecerreyes D, Taton D, Cramail H, Hedrick JL. Macromolecules. 2015;48:3153–3165. [Google Scholar]
- 23.Gimeno P, Spinau C, Lassu N, Maggio AF, Brenier C, Lempereur L. J Polym Sci A: Polym Chem. 2015;38:3727–3734. doi: 10.1002/jssc.201500552. [DOI] [PubMed] [Google Scholar]
- 24.Rokicki G. Prog Polym Sci. 2000;25:259–342. [Google Scholar]
- 25.Pyo SH, Hatti-Kaul R. Adv Synth Catal. 2016;358:834–839. [Google Scholar]
- 26.Pyo SH, Persson P, Lundmark S, Hatti-Kaul R. Green Chem. 2011;13:976–982. [Google Scholar]
- 27.Pyo SH, Hatti-Kaul R. Adv Synth Catal. 2012;354:797–802. [Google Scholar]
- 28.Pastusiak M, Dobrzynski P, Kasperczyk J, Smola A, Janeczek H. J Appl Polym Sci. 2014;131:40037. [Google Scholar]
- 29.Helou M, Miserque O, Brusson JM, Carpentier JF, Guillaume SM. Chem Eur J. 2010;16:13805–13813. doi: 10.1002/chem.201001111. [DOI] [PubMed] [Google Scholar]
- 30.Saralegi A, Rueda L, Fernández-d’Arlas B, Mondragon I, Eceiza A, Corcuera Ma. Polym Int. 2013;62:106–115. doi: 10.1016/j.carbpol.2012.09.093. [DOI] [PubMed] [Google Scholar]
- 31.Yang LQ, He B, Meng S, Zhang JZ, Li M, Guo J, Guan Y-M, Li JX, Gu ZW. Polymer. 2013;54:2668–2675. [Google Scholar]
- 32.Matsukizono H, Endo T. J Appl Polym Sci. 2015;132:41956. [Google Scholar]
- 33.Snyder RL, Fortman DJ, De Hoe GX, Hillmyer MA, Dichtel WR. Macromolecules. 2018;51:389–397. [Google Scholar]
- 34.Mespouille L, Coulembier O, Kawalec M, Dove AP, Dubois P. Prog Polym Sci. 2014;39:1144–1164. [Google Scholar]
- 35.Brannigan RP, Dove AP. Biomater Sci. 2017;5:9–21. doi: 10.1039/c6bm00584e. [DOI] [PubMed] [Google Scholar]
- 36.Ajiro H, Haramiishi Y, Chanthaset N, Akashi M. Polym J. 2016;48:751–60. [Google Scholar]
- 37.Kapoor S, Kundu SC. Acta biomaterialia. 2016;29:17–32. doi: 10.1016/j.actbio.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 38.Leite ÁJ, Mano JF. J Mater Chem B. 2017;5:4555–4568. doi: 10.1039/c7tb00404d. [DOI] [PubMed] [Google Scholar]
- 39.Kucinska-Lipka J, Gubanska I, Janik H, Sienkiewicz M. Mater Sci Eng C: Mater Biol Appl. 2015;46:166–176. doi: 10.1016/j.msec.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 40.Ravichandran R, Subramanian S, Jayarama RV, Shayanti M, Seera R. Macromol Biosci. 2012;12:286–311. doi: 10.1002/mabi.201100325. [DOI] [PubMed] [Google Scholar]
- 41.Zhu W, Ma X, Gou M, Mei D, Zhang K, Chen S. Curr Opin Biotechnol. 2016;40:103–112. doi: 10.1016/j.copbio.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 42.Anastas PT, Warner JC. Green Chemistry: Theory and Practice. Oxford University Press; New York: 2000. [Google Scholar]
- 43.Anastas P, Eghbali N. Chem Soc Rev. 2010;39:301–312. doi: 10.1039/b918763b. [DOI] [PubMed] [Google Scholar]
- 44.Pyo SH, Park JH, Chang TS, Hatti-Kaul R. Curr Opin Green Sus Chem. 2017;5:62–66. [Google Scholar]
- 45.Tundo P, Selva M. Acc Chem Res. 2002;35:706–716. doi: 10.1021/ar010076f. [DOI] [PubMed] [Google Scholar]
- 46.Noushad M, Kannan TP, Husein A, Abdullah H, Ismail AR. Toxicol Vitr. 2009;23:1145–1150. doi: 10.1016/j.tiv.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 47.Pyo SH, Pengrui W, Hwang HH, Zhu W, Warner J, Chen C. ACS Appl Mater Interfaces. 2017;9:836–844. doi: 10.1021/acsami.6b12500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


