Abstract
Purpose:
No medication has been shown to be effective at preventing recurrence of calcium phosphate urinary stones. Potassium citrate may protect against calcium phosphate stones by enhancing urine citrate excretion and lowering urine calcium, but it raises urine pH which increases calcium phosphate saturation and may negate the beneficial effects. Citric acid can potentially raise urine citrate but not pH, and thus may be a useful countermeasure against calcium phosphate stones. We aimed to assess whether these two agents can significantly alter urine composition and reduce calcium phosphate saturation.
Materials and methods:
In a cross-over metabolic study, 13 recurrent calcium phosphate stone formers without hypercalciuria were evaluated at the end of three 1-week study phases during which they consumed a fixed metabolic diet and took assigned study medications (citric acid 30 mEq twice daily, potassium citrate 20 mEq twice daily, or matching placebo). 24-hour urine was collected for urine chemistry and calculation of phosphate saturation indices.
Results:
Urine parameters were not significantly different between the citric acid and placebo phases. Compared to citric acid and placebo, potassium citrate significantly increased urine pH, potassium and citrate (p<0.01), with a trend for lower urine calcium (p=0.062). Brushite saturation was increased by potassium citrate when calculated by relative supersaturation ratio, but not by saturation index.
Conclusions:
Citric acid at a dose of 60 mEq/day does not significantly alter urine composition in calcium phosphate stone formers. The long-term impact of potassium citrate on calcium phosphate stone recurrence needs to be studied further.
Patient Summary:
In this report we examined the impact of two medications on the propensity to form calcium phosphate urinary stones. At the doses evaluated, neither significantly altered surrogates of stone formation risk.
Keywords: Calcium Phosphate, Urolithiasis, Citrate, Potassium Citrate, Citric Acid
INTRODUCTION
Calcium phosphate (CaP) urinary stones account for around 15% of all stones analyzed, although their incidence has markedly increased in recent years.1-3 CaP and calcium oxalate (CaOx) stone formers (SFs) share several common metabolic risk factors including hypercalciuria (identified in 60-70% of CaP SFs), and hypocitraturia (30-50%).4-5 However, a notable distinction between these two stone types is the significantly higher urine pH (UpH) among CaP SFs.4-5 UpH above 6.0 is essential for CaP crystallization due to greater availability of divalent phosphate ion to form poorly soluble complexes with calcium.6
CaP SFs are usually treated with fluids, thiazides, and dietary protein and sodium restriction to reduce stone recurrence by lowering calciuria and CaP supersaturation.7 However, many CaP SFs experience stone recurrence despite compliance with these measures.4 Potassium citrate (K3Cit) has been shown to prevent recurrence of diverse stone types, but has not specifically been evaluated as therapy for CaP SFs.8-13 The alkali load provided by K3Cit lowers calciuria, thus reducing CaP supersaturation.14 Physicochemically, provision of alkali by K3Cit promotes the formation of soluble calcium citrate complexes, and further lowers urinary CaP supersaturation.15 In addition, citrate exerts a direct inhibitory role on CaP crystal agglomeration.16 However, the beneficial effects of K3Cit treatment may be negated by the concomitant rise in UpH that potentially increases CaP stone risk.17 At the same time, soluble calcium phosphate complexes other than brushite and hydroxyapatite could form in urine at higher UpH, potentially negating the adverse impact of higher UpH on CaP supersaturation, and alleviating the safety concerns regarding alkali therapy in CaP SFs.18-19
Another potential pharmacological countermeasure for CaP stones is citric acid (H3Cit), which has the potential to raise urine citrate but not pH.20,21 It is conceivable that the physiologic renal response of ingestion of a neutral substance such as H3Cit is the excretion of ammonium citrate in urine. The degree of citraturia may be modest in terms of acid-base balance, but sufficient to protect against stones. The potential role of H3Cit as a countermeasure to CaP stones has not been assessed previously.
In this study, we examined whether H3Cit and K3Cit can reduce CaP saturation and the risk of recurrent nephrolithiasis in CaP SFs.
MATERIAL AND METHODS
Study Participants
The study was approved by the Institutional Review Board at the University of Texas Southwestern (UTSW) Medical Center, and all participants provided informed consent. Patients were recruited from the UTSW Mineral Metabolism and Urology clinics, and screened with a 24-hour urine and fasting blood studies prior to enrollment.
Inclusion criteria:
Participants were of either sex, any ethnicity, and age ⩾21 years. Diagnosis of CaP stones was based on the most recent available stone composition determined by X-ray crystallography, with CaP constituting >70% of stone components.
Exclusion criteria:
Urine Ca excretion ⩾ 300 mg/day in men and ⩾ 250 mg/day in women (as CaP SF with hypercalciuria were recruited into a separate study), history of struvite and/or ammonium urate on stone analysis, recurrent urinary tract infections, pregnancy, conditions altering acid-base balance such as chronic diarrhea, creatinine clearance < 60 mL/min/1.73m2, treatment with angiotensin 2 receptor blockers, angiotensin converting enzyme inhibitors, diuretics, antacids, alkali, or carbonic anhydrase inhibitors.
Study Protocol
In this double-blind, placebo-controlled, crossover study, each participant was evaluated during three phases, the order of which was randomized. The three phases were Placebo (PBO), Citric Acid (H3Cit), and Potassium Citrate (K3Cit). Each phase was one week in duration, during which participants took study medications, with a one-week washout between phases (Supplemental Figure 1). During the first four days of each phase, participants were instructed to adhere to a low-sodium diet at home with a calcium content of approximately 800 mg/day. On the last 3 days of each phase, participants were kept on a frozen metabolic diet prepared by the metabolic kitchen of the Clinical and Translational Research Center, and providing 55% of calories from carbohydrate, 30% from fat, and 15% from protein. The diet inorganic solute composition consisted of 800 mg (20 mmoles) calcium, 100 mEq sodium, 800 mg phosphorus (26 mmoles), 50 mEq potassium and 2 liters of distilled water daily. During the final two study days (days 6-7), participants collected two 24-hr urine, one under mineral oil to measure urine chemistries and acid-base parameters (bicarbonate, titratable acidity), the second for urine chemistries and crystallization studies. Fasting blood was obtained at the end of the second urine collection.
Study Medications:
Participants received three tablets with breakfast and dinner during each one-week phase: three 10 mEq tablets of H3Cit twice daily during the H3Citphase (60 mEq H3Cit/day), two 10 mEq K3Cit tablets and one placebo tablet twice daily during the K3Cit phase (40 mEq K3Cit/day), and 3 tablets twice daily of matching placebo during the PBO phase. Study tablets were prepared by a compounding pharmacy, with all tablets similar in appearance and size.
Study endpoints
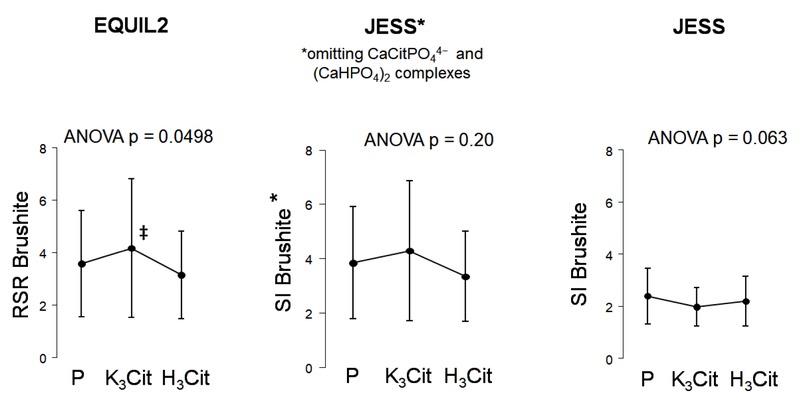
Urine analytes were assessed using established and previously described methods.5 Values for urine electrolytes assessed on days 6 and 7 were averaged for each phase, and urine saturation calculated from these parameters. Supersaturation index of Brushite (SIBr) was determined by the Joint Expert Speciation System (JESS) software, version 6.5,22 whereas Relative Supersaturation Ratio (RSRBr) was calculated by EQUIL2.23 These two programs estimate brushite saturation differently due to inclusion in JESS of additional soluble CaP complexes that are not included in EQUIL2. In addition, the JESS program was modified to recalculate SIBr after omitting phosphocitrate (CaCitPO4)4− and dicalcium-dihydrogen phosphate [Ca2H2(PO4)2] complexes, two soluble CaP complexes not recognized by EQUIL2.19
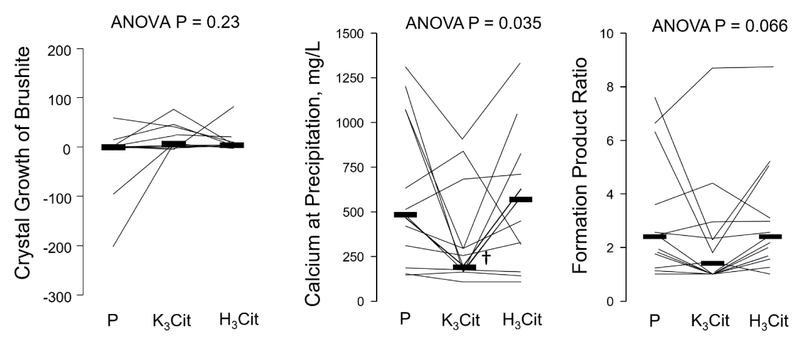
Based on methods previously used for the study of brushite crystallization in whole urine, we assessed brushite crystal growth (CGBr, representing growth of brushite crystals when a small quantity of brushite crystals is added to urine).24,25 Brushite is a precursor phase of hydroxyapatite, justifying its examination. A fresh aliquot of whole urine was obtained at the end of the second 24-hour urine collection. Urine was centrifuged, the supernatant was filtered (0.22 μm filter), and 0.25 mg/mL of brushite was added. Urine was then incubated at 37°C for 3 hours under constant stirring, with UpH checked hourly and maintained constant at voided UpH during the experiments. After 3 hours, 2 mL of suspension were filtered, with calcium and phosphate concentration analyzed in the filtrate. A decrement in [Ca]×[P] after seeding with brushite signified crystal growth, while an increment in [Ca]×[P] represented crystal dissolution.
We also measured overall activity of urinary promoters and inhibitors to brushite crystallization as formation product (FP). The FP represents the limit of metastability of brushite which is the initiation of spontaneous precipitation. FP was obtained by adding increasing amount of Ca as calcium chloride to a series of aliquots of urine at constant pH (equivalent to voided UpH) for 2hrs, and was identified by the point at which visible brushite precipitation is elicited. Calculated SI using [Ca] and [P] at that point represented FP brushite.25 The formation product ratio (FPR) was calculated as the ratio of the activity product at precipitation to the activity product of the urine.
Statistical Analyses
All data are presented as mean and standard deviation. To assess the effect of K3Cit and H3Cit on stone risk parameters, we compared results between the three phases of study using repeated measures ANOVA. Pairwise comparisons between phases were further conducted when ANOVA test showed significant differences across phases. All tests were performed 2-sided with a 0.05 significance level using SAS® (version 9.0 for Windows).
RESULTS
Demographics
Records on 286 patients with CaP stones were reviewed for inclusion/exclusion criteria. Of these, 41 met inclusion criteria and contacted for enrollment. A total of 13 subjects agreed to participate and were enrolled. They were 2 men and 11 women, with a mean age of 41±15 years and BMI of 26.7±6.4 kg/m2. Baseline characteristics of participants are listed in Table 1.
Table 1.
Baseline demographic characteristics.
| Number of participants | 13 |
| Sex, men/women | 2 / 11 |
| Race, white/black | 11 / 2 |
| Ethnicity, Non-Hispanic/Hispanic | 8 / 5 |
| Age, years (Mean ± SD) | 41 ± 15 |
| Body mass index, kg/m2 (Mean ± SD) | 26.7 ± 6.4 |
| Stone Analysis*, % Hydroxypatite / % Brushite / % Calcium Oxalate | 69% / 21% / 9% |
| 24-hr urine calcium, mg/day | 175 ± 72 |
| 24-hr urine citrate, mg/day | 248 ± 155 |
| 24-hr urine pH | 6.2 ± 0.4 |
1 patient presented with pure brushite stones, 2 with pure hydroxyapatite stones, 6 with mixed hydroxyapatite / calcium oxalate stones, and 4 with mixed hydroxyapatite / brushite stones.
24-h urine parameters and saturation indices
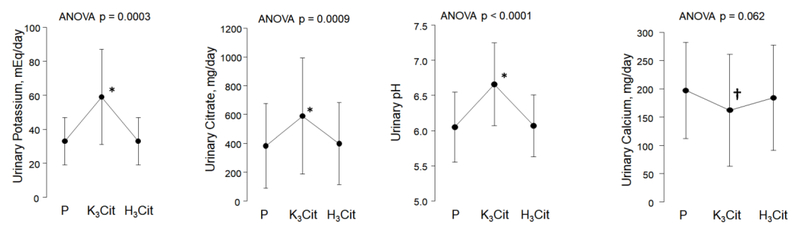
Compared with placebo and H3Cit, K3Cit led to an increase in urine potassium, citrate and pH (Figures 1A-1C, Table 2), and a decline in urine ammonium (Table 2) (p<0.01 for all). There was a trend toward lower urine calcium during the K3Cit phase (162 ± 99 mg/day for K3Cit, 197 ± 85 mg/day for placebo, and 184 ± 93 mg/day in H3Cit, p= 0.062, Figure 1D). H3Cit did not significantly alter citraturia, calciuria, pH, or ammonium compared with placebo (Figures 1B, 1C and Table 2). There were no significant differences in urine volume, sodium, sulfate, or any of the remaining measured urinary parameters between the three phases (Table 2).
Figure 1.
Changes in urinary parameters according to study phase
P: Placebo; K3Cit: Potassium Citrate; H3Cit: Citric Acid
P-value above graph refers to analysis of variance across phases.
* indicates p < 0.005 for pairwise comparison for K3Cit vs. Placebo phase and K3Cit vs. H3Cit phase
† indicates p < 0.05 for pairwise comparison for K3Cit vs. Placebo phase
Table 2.
24-hour urine biochemistry.
| Placebo | Potassium Citrate | Citric Acid | p value (by ANOVA) | |
|---|---|---|---|---|
| Total volume, liters/day | 1.02 ± 0.44 | 1.03 ± 0.49 | 1.04 ± 0.45 | 0.96 |
| Creatinine, mg/day | 1037 ± 231 | 1013 ± 514 | 1031 ± 259 | 0.96 |
| pH | 6.05 ± 0.50 | 6.66 ± 0.59*,‡ | 6.07 ± 0.44 | 0.0001 |
| Calcium, mg/day | 197 ± 85 | 162 ± 99* | 184 ± 93 | 0.062 |
| Citrate, mg/day | 383 ± 293 | 589 ± 403*,‡ | 399 ± 285 | 0.0009 |
| Potassium, mEq/day | 33 ± 14 | 59 ± 28*,‡ | 33 ± 14 | 0.0003 |
| Phosphorus, mg/day | 525 ± 175 | 484 ± 270 | 495 ± 186 | 0.73 |
| Oxalate, mg/day | 15.7 ± 7.8 | 15.8 ± 8.0 | 14.3 ± 6.1 | 0.72 |
| Sodium, mEq/day | 75 ± 46 | 64 ± 33 | 67 ± 30 | 0.47 |
| Magnesium, mg/day | 77 ± 26 | 71 ± 33 | 77 ± 28 | 0.55 |
| Titrable Acidity, mEq/day | 26.2 ± 22.1 | 23.9 ± 21.6 | 23.8 ± 14.8 | 0.90 |
| Ammonium, mEq/day | 27.6 ± 8.6 | 16.2 ± 9.0*,‡ | 26.4 ± 9.1 | < 0.0001 |
| Sulfate, mmol/day | 14.5 ± 4.8 | 15.5 ± 7.4 | 14.9 ± 5.6 | 0.81 |
Statistical significance by pairwise comparison for Potassium Citrate vs. Placebo phase (p < 0.05)
Statistical significance by pairwise comparison for Potassium Citrate vs. Citric Acid phase (p < 0.05)
Brushite saturation calculated by EQUIL2 as RSRBr was significantly higher with K3Cit vs. H3Cit (p<0.05), with no difference in RSRBr between H3Cit and placebo (Figure 2A). On the other hand, there was a trend toward lower brushite saturation with K3Cit when calculated by JESS as SIBr. When the two calcium phosphate complexes calcium phosphocitrate and dicalcium-dihydrogen phosphate were omitted from JESS, the pattern of saturation became similar to that of RSRBr (Figure 2A and 2B).
Figure 2.
Changes in urinary saturation parameters according to study phase.
P: Placebo; K3Cit: Potassium Citrate; H3Cit: Citric Acid
P-value above graph refers to analysis of variance across phases. ‡ indicates p < 0.05 for pairwise comparison for K3Cit vs. H3Cit phase
Crystallization Studies
There was no significant difference in CGBr at 3 hours after the addition of a 0.25 mg/mL brushite seed between the placebo, K3Cit and H3Cit phases (Figure 3A). In experiments testing the formation product of brushite, the concentration of calcium at the point of precipitation was significantly lower with K3Cit compared to placebo and H3Cit, p=0.035 (Figure 3B). FPRBr was not significantly different between the three phases (Figure 3C).
Figure 3.
Changes in urinary crystallization parameters according to study phase.
P: Placebo; K3Cit: Potassium Citrate; H3Cit: Citric Acid
P-value above graph refers to analysis of variance across phases. † indicates p < 0.05 for pairwise comparison for K3Cit vs. Placebo and H3Cit phases
DISCUSSION
This is the first metabolic study conducted to determine whether H3Cit or K3Cit can reduce CaP saturation and presumably the risk of recurrent CaP stones. Using a cross-over design, we compared effects of H3Cit and K3Cit versus placebo on urinary stone risk factors in normocalciuric CaP SFs consuming a controlled metabolic diet. We found that H3Cit did not significantly alter urinary citrate, other urinary parameters, or brushite saturation and crystallization compared to placebo. On the other hand, K3Cit significantly increased citraturia and UpH, and marginally lowered calciuria. Urine saturation with respect to brushite was higher with K3Cit compared to H3Cit when estimated as RSRBr (by EQUIL2), whereas it tended to be lower when assessed as SIBr (by JESS). This discrepancy is largely explained by the inclusion in JESS of two soluble calcium phosphate complexes that are not included in EQUIL2. In crystallization experiments done on urines collected during the metabolic study, no difference in CGBr or FPRBr were found.
In previous in vitro studies, urinary citrate was shown to form soluble complexes with urinary calcium, preventing CaP precipitation, potentially leading to a reduction in stone formation.24 A randomized placebo-controlled trial reported that K3citrate reduced stone recurrence in CaOx and mixed CaOx/CaP stone formers likely by dual mechanisms of increasing citraturia and decreasing calciuria.11 Furthermore, in prospective observational studies, K3Cit significantly reduced stone recurrence in patients with incomplete distal renal tubular acidosis (dRTA) and medullary sponge kidneys, two conditions that frequently present with CaP stones.9,26 Despite these observations, the role of citrate in preventing CaP stone recurrence had not been specifically studied.
In our current study, K3Cit significantly increased UpH and citraturia, and decreased urinary ammonium as expected from an alkali load (Figure 1). However, it marginally lowered calciuria, which contrasts with the marked urinary calcium reduction in patients with incomplete dRTA given alkali.9 Brushite saturation with K3Cit was higher when estimated as RSR but tended to be lower when assessed as SI due to the inclusion in JESS of additional soluble CaP complexes (Figure 2). Based on a previous in vitro study in which SIBr was found to approximate empirically measured brushite saturation better than RSRBr,19 it is plausible that K3Cit could reduce brushite saturation. In our crystallization studies conducted at voided UpH, K3Cit had no significant impact on CGBr, although the metastability studies showed precipitation at a lower calcium concentration and a trend for lower FPR Brushite with K3Cit compared to H3Cit and placebo (Figure 3). In a recently report, K3Cit increased citraturia and UpH and lowered calciuria in genetic hypercalciuric stone forming rats,27 similar to our findings. Furthermore, CaP supersaturation measured by EQUIL2 (RSR) was higher with K3Cit compared to control. Both K3Cit-treated and control animals had similar numbers of exclusively CaP stones, suggesting no beneficial effect in preventing CaP stone formation. The long-term impact of K3Cit on clinical CaP stone events and progression remains to be determined in humans.
Unlike K3Cit which provides an alkali load and raises UpH, H3Cit does not alter acid-base balance, and should not modify UpH. Single dose H3Cit was previously found to modestly and transiently raise citraturia in healthy non-stone forming volunteers.20 However, the current study found no significant impact of H3Cit vs. placebo on 24-hour urine parameters and in crystallization studies. The discrepancy between the current results and the previously reported citraturic effect of H3Cit may be related to differences in the patient population studied (CaP SFs vs. non-stone formers), follow-up duration, and/or dose used (60 mEq/24 hours vs. 40 mEq/4 hours). The lack of citraturia with H3Cit ingestion may be due to the metabolism of orally ingested H3Cit in the liver prior to reaching the systemic circulation, with no significant impact on citraturia.
A clinical correlate to our results is the differential impact of orange juice vs. lemon juice/lemonade on urine citrate and pH: A previous study found that an alkali load is delivered by administration of orange juice but not lemonade despite equivalent citrate content.28 These prior results were ascribed to the cation accompanying citrate in orange juice vs. lemonade (potassium vs. hydrogen, equivalent to the provision of K3Cit vs. H3Cit),28 a finding highlighted by our study results.
Our study has several limitations. The number of patients included was relatively small, in part due to the restrictive inclusion criteria. We did not study hypercalciuric CaP SFs who may have benefited from thiazides for stone prevention. Participants were kept on a metabolic diet which may not reflect the environment in which they formed their CaP stones. However, such a diet controls for dietary variation that could have impacted comparisons between phases. In addition, we measured saturation and crystallization indices as a surrogate for stone formation. However, it has been shown that calculated urinary saturation indices are associated with stone formation, and a reduction in saturation is associated with lower stone formation.29 Finally, crystallization studies were performed in voided bladder urine, which may not be representative of the urinary environment in nephron sites at which CG and aggregation occurs.
CONCLUSIONS
Overall our findings suggest that in CaP SFs, there is no significant difference between citric acid and placebo with respect to urine chemistry and physicochemistry relevant to CaP stone prevention. The overall impact of K3Cit on stone recurrence in CaP SFs needs to be studied further in view of its variable effects on urinary saturation and lack of definite benefit in terms of crystallization parameters.
Supplementary Material
ACKNOWLEDGMENTS
Grants: The authors were supported by the National Institute of Diabetes and Digestive and Kidney Diseases DK097476, and the National Center for Advancing Translational Sciences under award Number UL1TR001105, the O’Brien Kidney Research Center (P30DK079328), and the Pak and Seldin Center of Metabolic Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Steeve Doizi was supported by the Association Frangaise d’Urologie. Francisco Blanco was supported by the Universitat Autonoma de Barcelona.
Footnotes
Disclosure Statement: All authors declare that they have no conflict of interest.
REFERENCES
- 1.Daudon M, Bouzidi H, Bazin D. Composition and morphology of phosphate stones and their relation with etiology. Urol Res. December 2010;38(6):459–467. [DOI] [PubMed] [Google Scholar]
- 2.Mandel N, Mandel I, Fryjoff K, Rejniak T, Mandel G. Conversion of calcium oxalate to calcium phosphate with recurrent stone episodes. J Urol. June 2003;169(6):2026–2029. [DOI] [PubMed] [Google Scholar]
- 3.Parks JH, Worcester EM, Coe FL, Evan AP, Lingeman JE. Clinical implications of abundant calcium phosphate in routinely analyzed kidney stones. Kidney Int. August 2004;66(2):777–785. [DOI] [PubMed] [Google Scholar]
- 4.Krambeck AE, Handa SE, Evan AP, Lingeman JE. Profile of the brushite stone former. J Urol. October 2010;184(4):1367–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pak CY, Poindexter JR, Peterson RD, Heller HJ. Biochemical and physicochemical presentations of patients with brushite stones. J Urol. March 2004;171(3):1046–1049. [DOI] [PubMed] [Google Scholar]
- 6.Pak CY, Eanes ED, Ruskin B. Spontaneous precipitation of brushite in urine: evidence that brushite is the nidus of renal stones originating as calcium phosphate. Proc Natl Acad Sci U S A. July 1971;68(7):1456–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coe FL, Evan A, Worcester E. Pathophysiology-based treatment of idiopathic calcium kidney stones. Clin J Am Soc Nephrol. August 2011;6(8):2083–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pak CY, Fuller C, Sakhaee K, Preminger GM, Britton F. Long-term treatment of calcium nephrolithiasis with potassium citrate. J Urol. July 1985;134(1):11–19. [DOI] [PubMed] [Google Scholar]
- 9.Preminger GM, Sakhaee K, Skurla C, Pak CY. Prevention of recurrent calcium stone formation with potassium citrate therapy in patients with distal renal tubular acidosis. J Urol. July 1985;134(1):20–23. [DOI] [PubMed] [Google Scholar]
- 10.Pak CY, Sakhaee K, Fuller C. Successful management of uric acid nephrolithiasis with potassium citrate. Kidney Int. September 1986;30(3):422–428. [DOI] [PubMed] [Google Scholar]
- 11.Barcelo P, Wuhl O, Servitge E, Rousaud A, Pak CY. Randomized double-blind study of potassium citrate in idiopathic hypocitraturic calcium nephrolithiasis. J Urol. December 1993;150(6):1761–1764. [DOI] [PubMed] [Google Scholar]
- 12.Whalley NA, Meyers AM, Martins M, Margolius LP. Long-term effects of potassium citrate therapy on the formation of new stones in groups of recurrent stone formers with hypocitraturia. Br J Urol. July 1996;78(1):10–14. [DOI] [PubMed] [Google Scholar]
- 13.Soygur T, Akbay A, Kupeli S. Effect of potassium citrate therapy on stone recurrence and residual fragments after shockwave lithotripsy in lower caliceal calcium oxalate urolithiasis: a randomized controlled trial. J Endourol. April 2002;16(3):149–152. [DOI] [PubMed] [Google Scholar]
- 14.Sakhaee K, Nicar M, Hill K, Pak CY. Contrasting effects of potassium citrate and sodium citrate therapies on urinary chemistries and crystallization of stone-forming salts. Kidney Int. September 1983;24(3):348–352. [DOI] [PubMed] [Google Scholar]
- 15.Berg C, Tiselius HG. The effects of citrate on hydroxyapatite induced calcium oxalate crystallization and on the formation of calcium phosphate crystals. Urol Res. 1989;17(3):167–172 [DOI] [PubMed] [Google Scholar]
- 16.Højgaard I, Tiselius HG. The effects of citrate and urinary macromolecules on the aggregation of hydroxyapatite crystals in solutions with a composition similar to that in the distal tubule. Urol Res. 1998;26(2):89–95. [DOI] [PubMed] [Google Scholar]
- 17.Goldfarb DS. A woman with recurrent calcium phosphate kidney stones. Clin J Am Soc Nephrol. 2012. July;7(7):1172–8. [DOI] [PubMed] [Google Scholar]
- 18.Rodgers A, Allie-Hamdulay S, Jackson G. Therapeutic action of citrate in urolithiasis explained by chemical speciation: increase in pH is the determinant factor. Nephrol Dial Transplant. 2006. February;21(2):361–9 [DOI] [PubMed] [Google Scholar]
- 19.Pak CY, Moe OW, Maalouf NM, Zerwekh JE, Poindexter JR, Adams-Huet B. Comparison of semi-empirical and computer derived methods for estimating urinary saturation of brushite J Urol. 2009. March;181(3):1423–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakhaee K, Alpern R, Poindexter J, Pak CY. Citraturic response to oral citric acid load. J Urol. April 1992;147(4):975–976. [DOI] [PubMed] [Google Scholar]
- 21.Koff SG, Paquette EL, Cullen J, Gancarczyk KK, Tucciarone PR, Schenkman NS. Comparison between lemonade and potassium citrate and impact on urine pH and 24-hour urine parameters in patients with kidney stone formation. Urology. June 2007;69(6):1013–1016. [DOI] [PubMed] [Google Scholar]
- 22.May PM, Murray K.JESS, A joint expert speciation system-I. Raison d’être. Talanta. 1991. December;38(12):1409–17. [DOI] [PubMed] [Google Scholar]
- 23.Wilson JW, Werness PG, Smith LH. Inhibitors of crystal growth of hydroxyapatite: a constant composition approach. J Urol. December 1985;134(6):1255–1258. [DOI] [PubMed] [Google Scholar]
- 24.Pak CY, Rodgers K, Poindexter JR, Sakhaee K. New methods of assessing crystal growth and saturation of brushite in whole urine: effect of pH, calcium and citrate. J Urol. October 2008;180(4):1532–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pak CY, Holt K. Nucleation and growth of brushite and calcium oxalate in urine of stone-formers. Metabolism. June 1976;25(6):665–673. [DOI] [PubMed] [Google Scholar]
- 26.Fabris A, Lupo A, Bernich P, et al. Long-term treatment with potassium citrate and renal stones in medullary sponge kidney. Clin J Am Soc Nephrol. September 2010;5(9):1663–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krieger NS, Asplin JR, Frick KK, Granja I, Culbertson CD, Ng A, Grynpas MD, Bushinsky DA. Effect of Potassium Citrate on Calcium Phosphate Stones in a Model of Hypercalciuria. J Am Soc Nephrol. 2015. December;26(12):3001–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odvina CV. Comparative value of orange juice versus lemonade in reducing stoneforming risk. Clin J Am Soc Nephrol. 2006;1:1269–1274. [DOI] [PubMed] [Google Scholar]
- 29.Robertson WG, Peacock M, Marshall RW, Marshall DH, Nordin BE. Saturation-inhibition index as a measure of the risk of calcium oxalate stone formation in the urinary tract. N Engl J Med. 1976. January 29;294(5):249–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.