Abstract
Introduction:
The current seasonal influenza vaccine confers only limited protection due to waning antibodies or the antigenic shift and drift of major influenza surface antigens. A universal influenza vaccine which induces broad cross-protection against divergent influenza viruses with a comparable or better efficacy to seasonal influenza vaccines against matched strains will negate the need for an annual update of vaccine strains and protect against possible influenza pandemics.
Areas covered:
In this review, we summarize the recent progress in nanoparticle-based universal influenza vaccine development. We compared the most potent nanoparticle categories, focusing on how they encapsulate conserved influenza epitopes, stimulate the innate and adaptive immune systems, exhibit antigen depot effect, extend the period for antigen-processing and presentation, and exert an intrinsic adjuvant effect on inducing robust immune responses.
Expert commentary:
The development of an effective universal influenza vaccine is an urgent task. Traditional influenza vaccine approaches are not sufficient for preventing recurrent epidemics or occasional pandemics. Nanoparticles are compatible with different immunogens and immune stimulators and can overcome the intrinsically low immunogenicity of conserved influenza virus antigens. We foresee that an affordable universal influenza vaccine will be available within ten years by integrating nanoparticles with other targeted delivery and controlled release technology.
Keywords: HA stalk antigen, influenza virus, microneedle, nanoparticle, seasonal influenza vaccine, universal influenza vaccine
Introduction
Influenza continuously poses a serious public health risk. Both influenza A and B cause epidemics. Occasionally, influenza A can also cause pandemics. Another influenza pandemic could occur sometime in the future [1, 2]. Current seasonal influenza vaccines can effectively protect healthy adults against well-matched strains, but mismatches frequently occur because of the high rate of antigenic variation in influenza surface antigens. During the 2014–2015 influenza season, for instance, the overall vaccine effectiveness was only 19% in the US because the major circulating H3N2 viruses drifted from the vaccine strain. The 2017–2018 influenza season was the severest outbreak of an influenza epidemic since the 2009 pandemic due to a weak 25% vaccine effectiveness versus the prevalent H3N2 strain [3–5]. Influenza B virus infection is also causing more hospitalization of patients in western countries in the 2017–2018 influenza season than in the past [6]. Beyond epidemics, a non-human influenza virus could acquire mutations gaining the capacity for effective transmission in humans and result in an influenza pandemic [7, 8]. The outcome of such a pandemic could be tremendous panic to the public because humans have no historical immunity to such viruses.
A universal influenza vaccine will negate the need for yearly update of seasonal influenza vaccines and serve as a countermeasure against the emergence of novel pandemic strains by offering universal protection. Multiple approaches have been investigated — the promising ones utilize epitopes that are conserved across different influenza virus strains as the vaccine immunogens [9–11].
Conserved antigens (such as the ectodomain of influenza M2 protein, M2e, or HA stalk domains) generally induce weak immune responses and require adjuvants to boost their potency. Delivery of protein antigens is also a challenge because of their fast degradation and diffusion upon introduction to the body. Nanoparticle vaccine delivery systems can potentially solve both issues for a variety of diseases, including influenza. However, nanoparticle vaccines have their own technical issues. Common problems for nanoparticle vaccines include difficulty loading a sufficient dose of immunogens into the particle and denaturation of immunogens during the formulation process [12–14].
In recent years, our laboratory has studied solid protein nanoparticles (nanoclusters) as a universal influenza vaccine platform. Solid protein nanoparticles are comprised almost entirely of conserved antigenic proteins and an extremely small amount of a reducible cross-linker (Fig. 1A). A foreign self-assembling sequence is not required for antigenic proteins to assemble into particles. The absence of the self-assembling sequence avoids off-target immune responses that may limit a boost immunization or the utilization of other vaccines with the same self-assembling sequence. This nanoparticle design contains the maximum possible immunogen content. Different immunogens assembled in different layers of the particles provide potent differentiated antigen-processing and presentation. These features of the new nanoparticle universal influenza vaccine maximize a broad cross-protection [15–17].
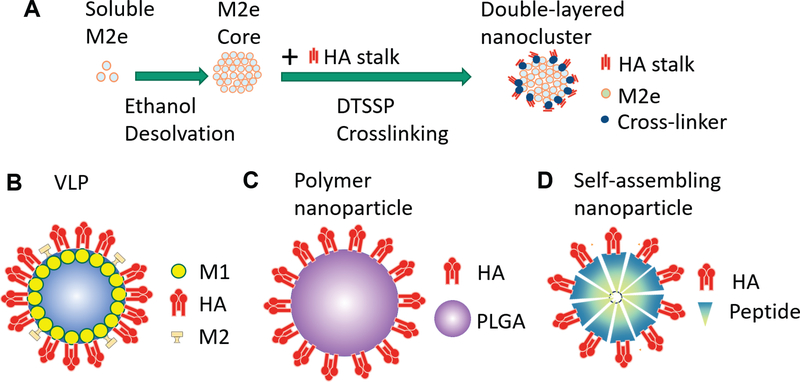
Fig.1. Four types of influenza nanoparticles.
A, Schematic diagram of double-layered protein nanoparticle generation. Soluble M2e fusion proteins are assembled into a nanoparticle (core) by ethanol desolvation. HA stalk antigens are coated on the surfaces of the core by DTSSP crosslinking. B, VLP: The antigen cDNA (HA, NA, M2) with M1 encoding genes in expression vectors are transfected into host cells. Host cells produce VLPs by budding. C, Polymer-based nanoparticle. Antigen like HA is adsorbed on the surface of PLGA. D, Self-assembling nanoparticle. Self-assembling fusion proteins which combine antigens like HA or M2e with a self-assembling sequence assemble into the nanoparticle spontaneously.
In this review article, we introduce our nanocluster universal influenza vaccine studies [16–20] and summarize the advances in nanoparticle-based universal influenza vaccine development. We compare the most important nanoparticle vaccine types, their strengths and weaknesses, and how they overcome the low immunogenicity of conserved influenza epitopes. We have also discussed how the current nanoparticle systems are being further refined and developed in search of an effective and affordable universal influenza vaccine with high accessibility and acceptability.
1. Major particulate platforms of universal influenza vaccines
The development of universal influenza vaccines has focused on four major categories of nanoparticles: virus-like particles (VLPs); polymer-based nanoparticles; self-assembling protein nanoparticles; and desolvation-driven nanoparticles. The four types of nanoparticles are schematically diagramed in Fig. 1.
2.1. Virus-Like-Particles (VLPs)
VLPs are nanoparticles that range in size from several to hundreds of nm in diameter and have been produced in a variety of different cell types by the expression of the viral envelope or capsid proteins. VLPs are easily produced in insect [21], mammalian [22], or plants cells [23], and can be designed to carry a wide variety of immunogens and adjuvants. The similar morphological features of VLPs as the viruses have led to their wide adoption in universal influenza vaccine development.
VLPs are an advantageous vaccine delivery system partly because of their natural characteristic of immune enhancement. The production of VLPs mimics the natural assembly of viruses, which gives VLPs the structure and morphology of viruses that the human immune system has evolved to combat. These important features improve the efficacy of the interface of vaccines to the immune system.
Some conserved influenza immunogens have been modified to assemble into VLP vaccines for inducing broad cross-protection. M2e is one such candidate immunogen [24]. In an earlier study, M2e was fused to the hepatitis B virus core (HBc) protein and assembled into VLPs (H2HBc particles) in an E. coli protein expression system. In HBc VLPs, M2e epitopes are exposed on the particle surfaces, enabling detection by the immune system and stimulation of broad-spectrum, long-lasting protection against influenza A infections [25].
In our laboratory, we replaced the highly immunogenic variable domain of flagellin with four tandem versions of M2e. The retention of the TLR5 ligand domains of flagellin in the fusion protein boosted a robust M2e-specific immune response by initiating innate immune responses and orchestrating subsequent adaptive immunity. With the addition of a membrane-anchoring sequence, the fusion protein assembled into influenza M1-formed VLPs. Our mouse studies demonstrated the enhancement of immune response by this VLP design. Strong M2e-specific immune response conferred heterologous and heterosubtypic protection in mice [26, 27].
Although M2e is highly conserved among human influenza strains, greater variation exists amongst strains from different zoological backgrounds (e.g., swine and avian). If only human virus consensus M2e sequence is included in a universal influenza vaccine, the protection against other, possibly pandemic strains from zoological backgrounds might not be sufficient. M2e sequence variants conjugated into VLP universal influenza vaccines address this possible shortcoming [28–32]. Experiments in mice demonstrated that M2e variants in VLPs induced better protection against human influenza strains and avian influenza viruses, revealing the capacity of the M2e VLP vaccines to protect against influenza pandemics [31, 32].
Research on VLPs has also included modified influenza HA in search of broad cross-protection. To induce broadly protective immune responses, an important modification to HA is to remove its highly variable, immunodominant head domain but retain the conserved HA stalk region. An endeavor truncated HA by removing most of the head region and assembled the stalks into Gag-derived VLPs produced in transfected mammalian cells [33]. These VLPs induced broadly neutralizing antibody responses towards the conserved HA stalk regions.
A computationally optimized, broadly reactive antigen (COBRA) H1 HA incorporated into VLPs elicited broadly reactive antibody responses in mice and protected them from a lethal dose of pandemic H1N1 A/California/07/2009 [34]. Immunization with a cocktail of three COBRA HA VLPs and stable oil-in-water emulsion adjuvant elicited a broadly-reactive antibody response against various strains including H5N1 subtype viruses [35, 36].
Co-incorporation of molecular adjuvants into influenza VLPs is an effective approach for improving VLP immunogenicity. We have generated full-length HA VLPs which induced cross protection by including a potent adjuvant [37]. We also generated a chimeric VLP containing influenza HA and GPI-anchored CCL28 as an adjuvant. The GPI-anchored CCL28 attracted IgA antibody-secreting cells to the mucosal vaccination sites and elicited higher IgA levels in the lungs, tracheas, and intestines of immunized mice. The long-lasting antibody response protected mice from a viral challenge at eight months after boost vaccination [38]. Another study showed chimeric VLPs containing H5 HA, NA, GM-CSF, and flagellin, induced strong T helper type 1 (Th1) and Th2 cellular responses and protected mice from lethal 20× LD50 H5N1 challenges [39].
These universal influenza VLP vaccine studies show that broad cross-protection can be induced by immunogens displayed in highly immunogenic forms or co-displayed with immune stimulators. By adopting the VLP format, vaccines benefit from multiple VLP features such as the virion morphology and structure, repetitive antigen surface patterns, antigen depot effect, and delayed diffusion or degradation compared with soluble protein antigens. VLP vaccine design also benefits from the co-incorporation of immune stimulators like flagellin into VLPs as molecular adjuvants [26, 27, 37], and flagellin has been proved to be safe when applied with a lower dose below 3 μg in clinical study [40]. As well, VLPs are relatively safe because they are empty shells without influenza viral genome and cannot thus replicate or recombine in the vaccinated animals [41]. Because most VLPs display immunogens on their surfaces, the surface area may limit this application in pursuing multiple conserved influenza antigen-induced immune responses. And further studies are needed to elucidate the detailed immunological mechanisms underlying the adjuvant effect of VLPs.
2.2. Polymer nanoparticles
Polymer nanoparticles have been widely used in drug delivery and vaccine development because they can accommodate different drugs or antigens. Newly developed polymers are biodegradable and biocompatible[42, 43]. These features attract researchers to explore the possibility of developing a polymer nanoparticle-based universal influenza virus vaccine. Several research teams have targeted polymers such as polylactic-co-glycolic acid (PLGA) or modified chitosan with influenza epitopes. Generally, polymer nanoparticle influenza vaccines are formulated by mixing polymers and influenza antigens in a solvent under conditions optimized for the formation of immunogenic particles [45, 46]. Due to the protein feature of most antigens, the solvent selection is limited to those will retain the natural structures of the antigenic proteins.
PLGA particles are potent carriers that can absorb or encapsulate antigens in different conditions [47]. PLGA nanoparticles with HA and TLR ligands increased the induction of pro-inflammatory cytokines from dendritic cells and enhanced the B cell and antigen-specific T cell memory [48]. Trivalent influenza vaccine (TIV) loaded into cylindrical PLGA nanoparticles induced higher levels of HA-specific antibody responses than soluble TIV [45]. PLGA nanoparticles conjugated with T-cell epitopes enhanced IFN-γ secreting CD4 and CD8 T cell responses and controlled viral replication [49, 50]. M2e loaded PLGA particles increased M2e-specific Th1 immune responses and conferred long-lasting protection against lethal A/Puerto Rico/8/1934 (H1N1, PR8) challenges in old age mice [51]. These results show that PLGA formed polymer nanoparticles loaded with influenza immunogens trigger useful inflammatory responses, facilitate the maturation of antigen-presenting cells, and induce robust, broadly cross-protective antibody and T cell responses.
Natural and biocompatible polymeric materials are safe. The biosafety of these natural polymeric materials allows polymer nanoparticles to be delivered through non-traditional routes, like intranasally, to induce targeted immune responses in these sites. For instance, a major safety concern with artificial polymer nanoparticles with the intranasal route is the possible translocation of the materials to the central nervous system through the olfactory connection and the possible unknown consequences.
Intranasally-delivered, modified-chitosan nanoparticles carrying influenza vaccines have induced strong mucosal IgA antibody response [52]. A fusion protein of M2, HA2, and cholera toxin subunit A1 (an adjuvant) loaded into poly- g -glutamic acid (g -PGA)-chitosan nanoparticles induced robust immune responses [53]. Inactivated swine H1N2 antigen has been encapsulated into chitosan-based nanoparticles to immunize pigs with the intranasal route [54]. These chitosan-based nanoparticles significantly elicited mucosal IgA in the respiratory tract and protected immunized animals against challenges by homologous and heterologous influenza viruses.
2.3. Self-assembling protein nanoparticles.
Protein nanoparticles are a large category of solid nanostructures fabricated from proteins. Formation of protein nanoparticles can be self-assembling or desolvation-driven. Their most important feature is that protein nanoparticles are directly formed by entire protein molecules. Used as vaccine platforms, protein nanoparticles have high antigen loads. By modification, many proteins can be designed to be assembled into protein nanoparticles [17, 55].
Self-assembling protein nanoparticles are a type of protein structure aggregated together through noncovalent interaction: hundreds of identical peptides or small proteins subunit assemble to a symmetrical spatial structure [56, 57]. Some natural proteins intrinsically assemble into nanostructures. By fusing antigenic protein to self-assembling proteins or domains, targeted antigens can be embedded into the particles as well.
Ferritin is a self-assembling protein which can form a scaffold capable of carrying immunogens from various viruses like HIV or influenza [58, 59]. A Helicobacter pylori ferritin capsule consists of 24 ferritin molecules.
Kanekiyo et al. [59] employed the ferritin structure by creating a fusion protein of the original ferritin with the ectodomain of A/New Caledonia/20/1999 (1999 NC) HA. The HA spikes maintained an octahedral symmetry on the surface of the ferritin shell and demonstrated greater immunogenicity than TIV by inducing a significantly higher hemagglutination-inhibition (HAI) titer against highly divergent H1N1 strains. Ferrets immunized by these HA-ferritin nanoparticles generated neutralizing antibody responses against the HA stem and global head.
Yassine et al. generated a fusion protein of H1 HA stem and ferritin and assembled it into a nanoparticle (H1-SS-NP). Mice and ferrets immunized with H1-SS-NP produced protective antibody titers against the HA stem [60]. The H1-SS-NP vaccine or passive transfer of vaccine-induced immunoglobulin protected mice against heterozygous H5N1 viral challenge at a lethal dose.
Because most self-assembling sequence are derived from non-human species, one concern of self-assembling protein nanoparticles as a universal influenza vaccine is that the off-target immune responses could limit the subsequent boost immunizations or other vaccinations with vaccines containing the same self-assembling domains.
2.4. Desolvation-driven protein nanoparticles
An alteration in the physical or chemical condition of a protein solution can drive the aggregation of protein molecules into nanoscale structures. Most proteins in solution can be desolvated by adding desolvation reagents such as neutral salts or organic solvents (methanol, ethanol, etc.) to assemble into nanostructures [61]. The particulate structures can be fixed by using crosslinkers such as 3,3’-Dithiobis(sulfosuccinimidyl propionate) (DTSSP) or Bis(sulfosuccinimidyl) (BS3) [16, 19, 20]. Desolvation system can be amplified to gram-scale batches in a laboratory with stable nanoparticle yields and size distributions. The cross-linking effectiveness and nanoparticle size distribution can be controlled by cross-linker concentration [20, 62].
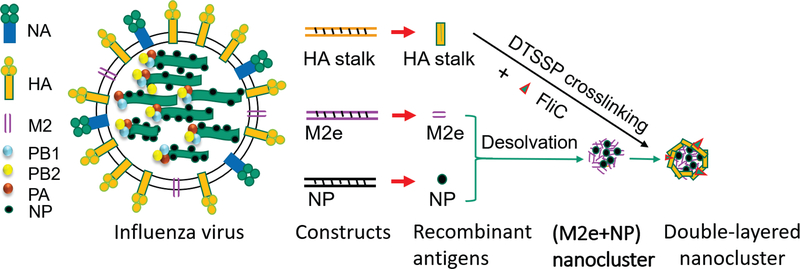
Because these desolvated protein nanoparticles can be further crosslinked with different proteins on their surfaces, protein nanoparticles with multiple layers can be fabricated to accommodate multiple immunogens for differential antigen-processing and presentation for a synergistic immune response (Fig. 1A). These nanoparticles are solid structures composed exclusively of protein immunogens, which gives them a very high immunogen load when compared with other forms of nanoparticle vaccines. While the particles are fixed by reversible crosslinkers, they can release free antigenic proteins for an extended period after uptake by APCs. This feature enables the layered protein nanoparticles to induce robust, long-lasting protective immunity. An immune stimulator can be co-crosslinked into coatings or different layers to generate chimeric solid layered protein nanoclusters for increased immunogenicity (Fig. 2).
Fig. 2. New double-layered protein nanoparticles from influenza conserved antigens.
The soluble recombinant conserved influenza antigens are purified from baculovirus-based insect cell protein expression system. Multivalent antigenic nanoparticles are generated by coating conserved influenza surface antigens and/or adjuvant proteins onto desolvated (M2e+NP) nanoparticle cores as an outer layer.
We have employed desolvation to fabricate nanoparticles from full-length HA protein, HA stalk antigens, immune stimulating protein molecules, and influenza internal nucleoprotein (NP) or polypeptide of known T cell epitopes of NP [16–20]. We have created a double-layered protein nanoparticle composed of desolvated M2e cores and crosslinked HA stalk coatings. The resulting double-layered protein nanoparticles induced cross-protection against viruses of the same HA phylogenetic groups [20]. The protection is conferred partially by antibody-dependent cell-mediated cytotoxicity (ADCC) and is long-lasting up to 6 months. A cocktail of two representative layered HA stalk nanoparticles from both HA phylogenetic groups induced protection against divergent viruses spanning the whole spectrum of type A influenza.
Desolvation can also be employed to fabricate polypeptide nanoparticle universal influenza vaccines. Using known peptide epitopes as vaccine immunogens can exclude off-target immune responses. However, compared with protein molecules, peptide antigens are taken up less by antigen-presenting cells and are more susceptible to diffusion or degradation. We have desolvated a polypeptide containing known T cell epitopes from influenza NP into nanoparticles as cores and coated these cores with an M2e immunogen [63]. The resulting double-layered peptide nanoparticles can maximize the immunological roles of different antigens in the cores and coatings for inducing broad cross-protection.
3. Conserved influenza epitopes for universal influenza vaccines
The influenza virion has three membrane proteins. There are two highly glycosylated proteins, HA and NA, and a third ionic channel protein, M2. Researchers focus on the conserved structures from HA, M2, and NP in developing a universal influenza vaccine [64].
3.1. Conserved sequences from HA
HA is the most abundant protein on the influenza virion surface. HA mediates receptor binding and membrane fusion during virus entry [65]. HA is a trimeric protein composed of a globular head demain and a stalk domain. The globular head domain binds to sialic acid in the host cell membrane and mediates virus entry. Current seasonal influenza vaccines target HA head domain and elicit antibody responses that predominantly direct to the head domain [66, 67]. However, targeted vaccines only confer efficacy to matched viruses because of the very high variability and mutability of the head domain [68]. HA stalk domain mediates influenza virion fusion with the host cell membrane and contains highly conserved domains [69]. These conserved domains are immunogens of interest and have been used to induce cross-protective antibodies that block the conformational rearrangements of HA [70] [71, 72].
Although the HA stalk domain is structurally unstable and tends to adopt the post-fusion conformation, several groups (including ours) have generated stabilized HA stalk antigens [20, 60, 73, 74]. When formulated into nanoparticles, these HA antigens induced variable levels of cross-protection, demonstrating the viability and value in developing this approach as a universal influenza vaccine.
Notably, HA stalk nanoparticles have induced non-neutralizing antibody responses. Natural influenza infections induce broadly neutralizing antibodies targeting the conserved HA stalk antigens, although these responses are rarely abundant [70, 75]. The detailed immunological mechanisms underlying the induction of the broadly neutralizing antibodies needs to be elucidated before an appropriate immunization approach can be designed to mimic this natural antibody production with HA stalk nanoparticle vaccines.
3.2. The ectodomain of M2 protein (M2e)
Although much less abundant than HA, M2 is an indispensable protein that functions in viral assembly and morphogenesis. During entry into host cells, M2 is activated by the acidification of endosomes and brings more protons through its ion-channel. The M2 extracellular domain (M2e) contains a highly conserved amino acid sequence, SLLTEVET, which is over 99% conserved among all subtype A influenza viruses [76, 77].
Compared with HA, the natural M2e is weakly immunogenic. To improve this weak immunogenicity, researchers usually combine this epitope with more immunogenic components like HA or increase the copy number of the highly conserved sequence in the tetrameric structure [26, 31]. Nanoparticles are another option to improve the immunogenicity of M2e because the conserved epitope can be loaded at high density and co-loaded with immune stimulators. M2e nanoparticles have induced cross-protection against different subtypes of influenza viruses.
Karch et al. created a fusion protein compromised of M2e, helix C of the hemagglutinin stalk domain (from H1N1 PR8 or H5N2 Penn), flagellin, and a T cell epitope which strongly bind to the MHC II in various haplotypes. Under the right chemical conditions, the fusion protein self-assembled into 24-mer nanoparticles which carried the antigens above in their native conformations [78]. Antibody titers induced in chickens by the flagellin-adjuvanted H5N2 HA stalk domain nanoparticles conferred broad neutralization against various type of influenza viruses. The H1N1 PR8 nanoparticles protected mice from a lethal dose challenge, while the mice in an inactivated virus vaccine-immunized group showed limited protection.
Qi et al. adapted the ferritin cage structure to carry three tandem repeats of M2e. This 3M2e-ferritin nanoparticle induced balanced Th1/Th2 immune responses, a long-term humoral response, and long-lived M2e-specific lymphocytes [79]. Intranasally administered 3M2e-ferritin nanoparticles induced a predominantly secretory IgA at the mucosal surfaces and fully protected the immunized mice against challenges by lethal homosubtypic human H1N1 and heterosubtypic H9N2 avian influenza viruses.
Flagellin TLR5 ligand domains included in fusion proteins have improved the immunogenicity of different influenza epitopes including M2e. In one of our studies, the variable domain of full-length flagellin was replaced by four tandem M2e repeats, H1 HA2 domain, or H3 HA2 domain [19]. These flagellin-M2e or flagellin-HA2 were directly crosslinked by DTSSP and desolvated into <100nm nanoparticles. These nanoparticles retained the activity of the TLR 5 ligand and induced strong M2e or HA stalk-specific antibodies.
We have also fabricated nanoclusters with M2e and CpG adjuvant by desolvation. Mice intranasally immunized with these M2e-CpG nanoclusters had significantly higher IgA and IgG titers in the nasal and lung mucosa than the soluble protein group. This M2e-CpG nanocluster also induced higher IFN-γ and IL-4 secreting cells in the spleen. Immunized mice were protected against lethal challenge with H1N1 or H3N2 influenza A virus [17].
3.3. T cell epitopes in influenza internal nucleoprotein (NP)
Nucleoprotein (NP) is a vaccine target of interest because it induces cross-protection against diverse influenza A virus challenges [80]. Natural infection can induce NP-specific CD8+ cytotoxic T cell responses that are resilient to antigenic drift but inactivated influenza vaccines have inefficiently induced these responses [81, 82]. Cross-reactive and virus-specific CD8+ cytotoxic T cell responses to conserved influenza epitopes correlate to less severe and shorter illnesses in humans [83].
Recent clinical trials proved that NP-based universal influenza vaccines are safe and immunogenic in humans [84–86]. Universal influenza vaccines co-administered with seasonal influenza vaccines in older adults increased strain-specific antibody responses and boosted cross-reactive memory T cells responses [85]. Although these vaccines do not prevent infection, they significantly reduced morbidity, mortality, and viral titers in the respiratory tract [87].
Our recent studies have demonstrated that multiple conserved antigens in the internal and outer layers of nanoclusters synergized each other’s protective efficacy [63]. We desolvated NP or a polypeptide containing T cell epitopes from NP into particulate cores and then coated M2e epitope repeat proteins into the NP epitope core particles to fabricate layered nanocluster. Vaccination of mice with the resulting NP-M2e nanoclusters induced CD4+ and CD8+ T cell responses. This study demonstrated the potential for layered nanoclusters containing NP epitope cores to induce broad cross-protection against a wide range of influenza A viruses with different subtypes.
4. Integrated approaches combining targeted delivery and controlled release
Influenza nanoparticle vaccines have displayed numerous advantages and conferred better protection than traditional influenza vaccines against challenges by heterologous viruses. The immunogenicity of protein nanoparticles can be further synergized by a combination with other recently developed technology of vaccine delivery.
Microneedles are a new delivery platform for administration of drugs and vaccines into the skin. This technology delivers immunogens directly into the epidermis and dermis where dendritic cells and epidermal Langerhans cells are densely populated [88]. After absorbing immunogens, dendritic cells in the dermis migrate to draining lymph nodes and activate the expansion of T cells [89]. Langerhans cells in the dermis cross-present immunogens and have anti-tumor and anti-viral activities [90]. Researchers have studied how to encapsulate PNs or VLPs in microneedles in attempts of enhancing the induced protection.
VLPs have been stabilized in microneedles by adding a sugar molecule of trehalose disaccharide. The trehalose disaccharide-stabilized microneedles with coated influenza vaccines induced high titers of IgG2a and 100% protection against a lethal viral challenge, which was superior to intramuscular immunization or unstabilized microneedles [91]. As well, microneedle-delivered PR8 VLPs induced long-lasting (14 months after a single immunization) lung IgG and IgA responses, increased antibody-secreting splenocytes, and conferred protection to aged mice [92].
Kim et al. constructed a quintuple tandem M2e repeat (M2e5x) VLP which elicited IgG2a and IFN-γ secreting cell responses and protected mice against heterosubtypic H1N1, H3N2, and H5N1 influenza virus challenges via a microneedle delivery route. The M2e5x VLP microneedle was stable for eight weeks at room temperature [93]. In one of our recent studies, dissolvable microneedle patches encapsulated with NP polypeptide or NP protein nanoparticles demonstrated robust CD8+ T cell responses [63].
Skin vaccination by microneedle patches has displayed many advantages over traditional intramuscular vaccination and is an improved delivery method. Skin vaccination focuses on dermal antigen-presenting cells which effectively deliver vaccine antigens to near draining lymph nodes and stimulate T cells or B cells. Compared to intramuscular vaccination, microneedle patches improve the contact between immunogens and immune cells (leading to more efficient induction of immune responses), are less painful than intramuscular vaccination [94], and can be easily handled by healthcare providers or patients themselves [95].
5. . Conclusion
Development of an affordable universal vaccine is an urgent task. Nanoparticle technology can contribute to this important advance to improve public health worldwide. Other new technology developed for drug delivery and controlled releases, like dissolvable microneedle patches, can be integrated with nanoparticle universal influenza approaches to fulfill this unprecedented task. A few universal influenza vaccines are in their early phase clinical trials. It is expected that some candidates can be tested for later phase efficacy trials in five years and that a commercial universal influenza vaccine is likely to be available within 8 to 10 years.
6. Expert Commentary
Threat of influenza pandemics.
The 1918 influenza pandemic alone was estimated having claimed around 50 million lives. Influenza continues to pose a serious health risk worldwide. The 2009 pandemic illustrated the unpreparedness of the world for another influenza pandemic. The outbreak of another influenza pandemic is just a matter of time. A universal influenza vaccine will overcome the epidemic and pandemic threats of influenza virus.
Conserved influenza antigens for universal influenza vaccines.
The “holy grail” of a universal influenza vaccine is the induction of broadly reactive protective immunity. Protein structures conserved across influenza A and/or B are ideal immunogens for inducing such immune responses. Influenza surface antigens (HA stalk and M2e) and T cell epitopes from internal proteins (NP and M1) are attracting increasing attention from the universal influenza vaccine research community.
HA stalk antigens.
By removing the highly immunogenic HA globular head (Fig. 2), strong antibody responses can be focused on the conserved HA stalk regions. However, due to its metastable conformation, HA stalks expressed apart from the head subunit will spontaneously adopt the post-fusion conformation. A recombinant HA stalk antigen must be stabilized for proper use as an antigen [96, 97]. We optimized the design of HA stalk constructs by blocking the formation of a superhelix involved in virus-cell membrane fusion. This unique design retains conserved conformational and compositional components, ensuring the immunogenicity of the resulting HA stalk recombinant proteins [20].
The highly conserved M2e.
The ectodomain of the membrane protein M2 (M2e) is conserved among many influenza viruses. M2e is a potential component of a broadly protective influenza vaccine. However, results from our studies and others demonstrate that M2e may not be suitable as a standalone universal influenza vaccine. Instead, M2e based immunogens should be a synergistic part of the immunogenic portfolio of a universal influenza vaccine. The M2e sequence is more conserved within species-specific strains. This intra-species conservation means a human M2e consensus sequence vaccine protects less against influenza strains that emerge from avian or swine backgrounds. An approach to overcome this challenge is to construct an M2e fusion protein that includes conserved M2e sequences from different species-specific strains [20, 28].
Conserved T cell epitopes from nucleoprotein (NP).
NP is an internal influenza protein (Fig. 2). Multiple sequences of NP are highly conserved amongst influenza A strains and can trigger broad protective immunity in animal models [98, 99]. We generated a polypeptide of T cell epitopes from NP and fabricated layered nanoparticles by desolvating the NP polypeptide into cores and crosslinking M2e onto the particle surface. These nanoparticles induced broadly reactive immune responses which protected mice from avian-derived influenza challenges [63].
Nanoparticle universal influenza vaccines.
Conserved antigens tend to elicit weak immune responses and require adjuvants to boost their potency. Delivery of protein antigens is a challenge because of their fast degradation or diffusion upon introduction to the body. Nanoparticle vaccine delivery systems can solve both issues for a variety of diseases, including influenza. Desolvation nanoparticles are made solely of conserved antigenic proteins and trace amounts of a reducible cross-linker. This nanoparticle design contains the maximum possible antigen content and avoids any possible off-target immune responses [15–17]. This nanoparticle design can be further modified and optimized to combine multiple conserved antigens into a universal flu vaccine [16–20].
Impact.
Universal influenza vaccines with a novel format of layered protein nanoparticles contain only antigenic proteins of interest and are highly immunogenic, inducing robust and long-lasting immunity. The nanoparticles are approximately the size of influenza virions and have a core of M2e or NP displaying a shell of conserved influenza surface antigens. We have observed the synergistic roles of HA stalk antigens and M2e or M2e and NP in inducing broad protection against influenza A viruses. Newly designed layered nanoparticles by combining both M2e and NP in the cores and crossing HA stalk antigens as coatings must be able to maximize the immune responses (Fig. 2). The nanoparticle fabrication avoids the risk of the instability of VLPs or other vesicle particles under osmotic stresses or changes in salt concentration and prevents off-target immune responses against self-assembly motifs found in other protein nanoparticle designs. The reducibility of the crosslinker DTSSP by intracellular thiols provides a slow release of free antigenic proteins after uptake by APCs. Meanwhile, the abiotic nature of the protein nanoparticles also enhances their amenability to a cold chain-independent storage. Currently, several universal influenza vaccines are in their early phases of clinical trials. We can foresee some candidates can be tested for a later phase of efficacy trials in five years. Integrated with novel approaches for drug delivery and controlled release, like dissolvable microneedle patch-based skin vaccination, a convenient, syringe-free and painless, self-administrated affordable universal influenza vaccine can be available in eight to ten years.
7. Five-year View
The conserved influenza antigens HA stalk domain and M2e have been the major immunogens explored in universal influenza vaccine research. DNA or replicating vector-expressing nucleoprotein (NP) have raised attentions as well. One common feature of these candidate vaccines is the non-sterile immunity they induce. While this non-sterile immunity significantly reduced mortality rates, sickness and body weight loss were still observed. In the next five years, we expect that this challenge will be overcome in pre-clinical studies. Recent advancements in vaccine design and strategy means a potent candidate universal influenza vaccine could be ready for extensive clinical trials in this time frame.
The addition of previously underestimated conserved influenza antigens into universal influenza vaccine formulations has resulted in progress towards sterile-immunity. One such conserved antigen is the other influenza surface glycoprotein, neuraminidase (NA). NA contains highly conserved epitopes that have elicited universal anti-NA antibodies against all subtypes of influenza A [100]. In humans, high NAI titers reduce influenza-related symptoms and have more robust protective effects than HAI titers [101]. NA does not induce strong antibody responses because of its low concentration and the immune shielding effect of the immunodominant HA in seasonal influenza vaccines. The antibody response to NA can be improved by novel vaccine designs, for example, the layered protein nanoclusters. In this design, antigens in different layers of the particles are released with a tiny time gap after uptake by antigen-presenting cells to avoid immunodominance by any one component.
Another advancement is the integration of vaccine platform and vaccine delivery research. Because of the tremendous challenge in realizing a universal influenza vaccine, advancement in a single aspect of vaccine improvement will not be sufficient to fulfill this significant task. It requires researchers to integrate different aspects of antigen design, vaccine platform design, and vaccine delivery. An optimal, integrated approach can accommodate, protect, and release antigens in such a way as to trigger the innate immunity and activate the maturation of early responders to set up a suitable immunological environment and program the development of a potent protective immunity. To this end, dissolvable microneedle patches containing layered protein nanoparticles of conserved influenza antigens have demonstrated these powerful features [63]. We foresee an integrated universal influenza vaccine approach achieving the necessary preclinical milestones to move into clinical trials within the next 5-year time frame.
8. Key Issues.
Development of a universal influenza vaccine is an urgent need to prevent influenza pandemics and epidemics worldwide.
Highly conserved structures from HA stalk, M2, and internal protein NP are ideal immunogens for developing a universal influenza vaccine.
Nanoparticles comprised of various conserved influenza antigens and immune stimulators are powerful techniques to be developed into universal influenza vaccines.
Novel layered protein nanoparticles have demonstrated long-lasting immune responses and cross-protection in animal models.
Nanoparticle approaches integrated with new drug delivery and controlled release technology could result in an affordable universal influenza vaccine.
Acknowledgments
Funding
This work is supported by the National Institutes of Health (NIH) under grants R01AI101047 and R01AI116835.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Chandra S, Deaths associated with influenza pandemic of 1918–19, Japan. Emerg Infect Dis, 2013. 19(4): p. 616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen PJ, Avian influenza pandemic: not if, but when. Pediatr Nurs, 2006. 32(1): p. 76–81. [PubMed] [Google Scholar]
- 3.CDC, Seasonal Influenza Vaccine Effectiveness, 2005–2017. https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm, 2018.
- 4.Update: Influenza Activity-United States, O., 2017-February 3, 2018, Morbidity and Mortality Weekly Reprot (MMWR). 2018. 67(6): p. 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC, Transcript for CDC Update on Flu Activity. https://www.cdc.gov/media/releases/2018/t0202-flu-update-activity.html, February 2, 2018.
- 6.WHO, Influenza undate-308. 2018.
- 7.Mei L, et al. , Changes in and shortcomings of control strategies, drug stockpiles, and vaccine development during outbreaks of avian influenza A H5N1, H1N1, and H7N9 among humans. Biosci Trends, 2013. 7(2): p. 64–76. [PubMed] [Google Scholar]
- 8.Gao R, et al. , Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med, 2013. 368(20): p. 1888–97. [DOI] [PubMed] [Google Scholar]
- 9.Sautto GA, Kirchenbaum GA, and Ross TM, Towards a universal influenza vaccine: different approaches for one goal. Virol J, 2018. 15(1): p. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eliasson DG, et al. , M2e-tetramer-specific memory CD4 T cells are broadly protective against influenza infection. Mucosal Immunol, 2018. 11(1): p. 273–289. [DOI] [PubMed] [Google Scholar]
- 11.Wu NC and Wilson IA, A Perspective on the Structural and Functional Constraints for Immune Evasion: Insights from Influenza Virus. J Mol Biol, 2017. 429(17): p. 2694–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng X, Liu R, and He Y, A simple method for the preparation of monodisperse protein-loaded microspheres with high encapsulation efficiencies. Eur J Pharm Biopharm, 2010. 76(3): p. 336–41. [DOI] [PubMed] [Google Scholar]
- 13.Jin T, et al. , Preparing polymer-based sustained-release systems without exposing proteins to water-oil or water-air interfaces and cross-linking reagents. J Control Release, 2008. 128(1): p. 50–9. [DOI] [PubMed] [Google Scholar]
- 14.Park TG, Lu W, and Crotts G, Importance of in vitro experimental conditions on protein release kinetics, stability and polymer degradation in protein encapsulated poly (d,l-lactic acid-co-glycolic acid) microspheres. Journal of Controlled Release, 1995. 33(2): p. 211–222. [Google Scholar]
- 15.Chang TZ, et al. , Effects of ovalbumin protein nanoparticle vaccine size and coating on dendritic cell processing. Biomater Sci, 2017. 5(2): p. 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, et al. , Coated protein nanoclusters from influenza H7N9 HA are highly immunogenic and induce robust protective immunity. Nanomedicine, 2017. 13(1): p. 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, et al. , Nanoclusters self-assembled from conformation-stabilized influenza M2e as broadly cross-protective influenza vaccines. Nanomedicine, 2014. 10(2): p. 473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang TZ, et al. , Host- and pathogen-derived adjuvant coatings on protein nanoparticle vaccines. Bioeng Transl Med, 2017. 2(1): p. 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng L, et al. , Protein nanoparticle vaccine based on flagellin carrier fused to influenza conserved epitopes confers full protection against influenza A virus challenge. Virology, 2017. 509: p. 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. **.Deng L, et al. , Double-layered protein nanoparticles induce broad protection against divergent influenza A viruses. Nat Commun, 2018. 9(1): p. 359 This research includes a novel combination of two conserved influenza surface antigens HA-stalks and M2e into layered solid protein nanoparticles to induce comprehensive broadly reactive influenza immunity, granting heterosubtypic cross-protection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandes F, et al. , Insect cells as a production platform of complex virus-like particles. Expert Rev Vaccines, 2013. 12(2): p. 225–36. [DOI] [PubMed] [Google Scholar]
- 22.Quan FS, et al. , Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J Virol, 2007. 81(7): p. 3514–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scotti N and Rybicki EP, Virus-like particles produced in plants as potential vaccines. Expert Rev Vaccines, 2013. 12(2): p. 211–24. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, et al. , Universal influenza vaccines, a dream to be realized soon. Viruses, 2014. 6(5): p. 1974–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neirynck S, et al. , A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med, 1999. 5(10): p. 1157–63. [DOI] [PubMed] [Google Scholar]
- 26.Wang BZ, et al. , Enhanced influenza virus-like particle vaccines containing the extracellular domain of matrix protein 2 and a Toll-like receptor ligand. Clin Vaccine Immunol, 2012. 19(8): p. 1119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, et al. , Virus-like particles containing the tetrameric ectodomain of influenza matrix protein 2 and flagellin induce heterosubtypic protection in mice. Biomed Res Int, 2013. 2013: p. 686549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim MC, et al. , Multiple heterologous M2 extracellular domains presented on virus-like particles confer broader and stronger M2 immunity than live influenza A virus infection. Antiviral Res, 2013. 99(3): p. 328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim MC, et al. , Influenza M2 virus-like particles confer a broader range of cross protection to the strain-specific pre-existing immunity. Vaccine, 2014. 32(44): p. 5824–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim MC, et al. , Immunogenicity and efficacy of replication-competent recombinant influenza virus carrying multimeric M2 extracellular domains in a chimeric hemagglutinin conjugate. Antiviral Res, 2017. 148: p. 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim MC, et al. , Virus-like particles containing multiple M2 extracellular domains confer improved cross-protection against various subtypes of influenza virus. Mol Ther, 2013. 21(2): p. 485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YJ, et al. , Cross-Protective Efficacy of Influenza Virus M2e Containing Virus-Like Particles Is Superior to Hemagglutinin Vaccines and Variable Depending on the Genetic Backgrounds of Mice. Front Immunol, 2017. 8: p. 1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steel J, et al. , Influenza virus vaccine based on the conserved hemagglutinin stalk domain. MBio, 2010. 1(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carter DM, et al. , Design and Characterization of a Computationally Optimized Broadly Reactive Hemagglutinin Vaccine for H1N1 Influenza Viruses. J Virol, 2016. 90(9): p. 4720–4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. *.Crevar CJ, et al. , Cocktail of H5N1 COBRA HA vaccines elicit protective antibodies against H5N1 viruses from multiple clades. Hum Vaccin Immunother, 2015. 11(3): p. 572–83. An interesting study which predicted the possible mutated inflluenza strain by using the technique of Computationally Optimized Broadly Reactive (COBRA). The predicted HA-containing influenza strain can be used for developing a “subtype universal” vaccine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen JD, et al. , Broadened immunity and protective responses with emulsion-adjuvanted H5 COBRA-VLP vaccines. Vaccine, 2017. 35(38): p. 5209–5216. [DOI] [PubMed] [Google Scholar]
- 37.Wang BZ, et al. , Incorporation of membrane-anchored flagellin into influenza virus-like particles enhances the breadth of immune responses. J Virol, 2008. 82(23): p. 11813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohan T, et al. , Chimeric virus-like particles containing influenza HA antigen and GPI-CCL28 induce long-lasting mucosal immunity against H3N2 viruses. Sci Rep, 2017. 7: p. 40226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu WC, et al. , Multi-subtype influenza virus-like particles incorporated with flagellin and granulocyte-macrophage colony-stimulating factor for vaccine design. Antiviral Res, 2016. 133: p. 110–8. [DOI] [PubMed] [Google Scholar]
- 40.Turley CB, et al. , Safety and immunogenicity of a recombinant M2e-flagellin influenza vaccine (STF2.4xM2e) in healthy adults. Vaccine, 2011. 29(32): p. 5145–52. [DOI] [PubMed] [Google Scholar]
- 41.Pandey A, et al. , Egg- independent vaccine strategies for highly pathogenic H5N1 influenza viruses. Hum Vaccin, 2010. 6(2): p. 178–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sadat Tabatabaei Mirakabad F, et al. , PLGA-based nanoparticles as cancer drug delivery systems. Asian Pac J Cancer Prev, 2014. 15(2): p. 517–35. [DOI] [PubMed] [Google Scholar]
- 43.Vela-Ramirez JE, et al. , Safety and biocompatibility of carbohydrate-functionalized polyanhydride nanoparticles. AAPS J, 2015. 17(1): p. 256–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Astete CE and Sabliov CM, Synthesis and characterization of PLGA nanoparticles. J Biomater Sci Polym Ed, 2006. 17(3): p. 247–89. [DOI] [PubMed] [Google Scholar]
- 45.Galloway AL, et al. , Development of a nanoparticle-based influenza vaccine using the PRINT technology. Nanomedicine, 2013. 9(4): p. 523–31. [DOI] [PubMed] [Google Scholar]
- 46.Mohamed SH, et al. , Preparation and immunological evaluation of inactivated avian influenza virus vaccine encapsulated in chitosan nanoparticles. Biologicals, 2018. 51: p. 46–53. [DOI] [PubMed] [Google Scholar]
- 47.Allahyari M and Mohit E, Peptide/protein vaccine delivery system based on PLGA particles. Hum Vaccin Immunother, 2016. 12(3): p. 806–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kasturi SP, et al. , Programming the magnitude and persistence of antibody responses with innate immunity. Nature, 2011. 470(7335): p. 543–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hiremath J, et al. , Entrapment of H1N1 Influenza Virus Derived Conserved Peptides in PLGA Nanoparticles Enhances T Cell Response and Vaccine Efficacy in Pigs. PLoS One, 2016. 11(4): p. e0151922. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Herrmann VL, et al. , Cytotoxic T cell vaccination with PLGA microspheres interferes with influenza A virus replication in the lung and suppresses the infectious disease. J Control Release, 2015. 216: p. 121–31. [DOI] [PubMed] [Google Scholar]
- 51.Watkins HC, et al. , A single dose and long lasting vaccine against pandemic influenza through the controlled release of a heterospecies tandem M2 sequence embedded within detoxified bacterial outer membrane vesicles. Vaccine, 2017. 35(40): p. 5373–5380. [DOI] [PubMed] [Google Scholar]
- 52.Amidi M, et al. , N-trimethyl chitosan (TMC) nanoparticles loaded with influenza subunit antigen for intranasal vaccination: biological properties and immunogenicity in a mouse model. Vaccine, 2007. 25(1): p. 144–53. [DOI] [PubMed] [Google Scholar]
- 53.Chowdhury MYE, et al. , Mucosal vaccination of conserved sM2, HA2 and cholera toxin subunit A1 (CTA1) fusion protein with poly gamma-glutamate/chitosan nanoparticles (PC NPs) induces protection against divergent influenza subtypes. Vet Microbiol, 2017. 201: p. 240–251. [DOI] [PubMed] [Google Scholar]
- 54.Dhakal S, et al. , Mucosal Immunity and Protective Efficacy of Intranasal Inactivated Influenza Vaccine Is Improved by Chitosan Nanoparticle Delivery in Pigs. Front Immunol, 2018. 9: p. 934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fletcher JM, et al. , Self-assembling cages from coiled-coil peptide modules. Science, 2013. 340(6132): p. 595–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lopez-Sagaseta J, et al. , Self-assembling protein nanoparticles in the design of vaccines. Comput Struct Biotechnol J, 2016. 14: p. 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pieters BJ, et al. , Natural supramolecular protein assemblies. Chem Soc Rev, 2016. 45(1): p. 24–39. [DOI] [PubMed] [Google Scholar]
- 58.Vzorov AN, et al. , Effects of modification of the HIV-1 Env cytoplasmic tail on immunogenicity of VLP vaccines. Virology, 2016. 489: p. 141–50. [DOI] [PubMed] [Google Scholar]
- 59. **.Kanekiyo M, et al. , Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature, 2013. 499(7456): p. 102–6. The paper first demonstrated a ferritin to stabilize influena HA stalks and assemble the stalk antigens into nanoparticles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yassine HM, et al. , Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat Med, 2015. 21(9): p. 1065–70. [DOI] [PubMed] [Google Scholar]
- 61.Verma D, et al. , Protein Based Nanostructures for Drug Delivery. J Pharm (Cairo), 2018. 2018: p. 9285854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. **.Geh KJ, Hubert M, and Winter G, Optimisation of one-step desolvation and scale-up of gelatine nanoparticle production. J Microencapsul, 2016. 33(7): p. 595–604. The paper first demonstrated a ferritin to stabilize influena HA stalks and assemble the stalk antigens into nanoparticles. [DOI] [PubMed] [Google Scholar]
- 63.Deng L, et al. , Heterosubtypic influenza protection elicited by double-layered polypeptide nanoparticles in mice. Proc Natl Acad Sci U S A, 2018. 115(33): p. E7758–E7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaminski DA and Lee FE, Antibodies against conserved antigens provide opportunities for reform in influenza vaccine design. Front Immunol, 2011. 2: p. 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skehel JJ and Wiley DC, Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem, 2000. 69: p. 531–69. [DOI] [PubMed] [Google Scholar]
- 66.Van Reeth K, et al. , Heterologous prime-boost vaccination with H3N2 influenza viruses of swine favors cross-clade antibody responses and protection. NPJ Vaccines, 2017. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peng B, et al. , Comparison of the Protective Efficacy of Neutralizing Epitopes of 2009 Pandemic H1N1 Influenza Hemagglutinin. Front Immunol, 2017. 8: p. 1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boni MF, Vaccination and antigenic drift in influenza. Vaccine, 2008. 26 Suppl 3: p. C8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wharton SA, Skehel JJ, and Wiley DC, Studies of influenza haemagglutinin-mediated membrane fusion. Virology, 1986. 149(1): p. 27–35. [DOI] [PubMed] [Google Scholar]
- 70.Ekiert DC, et al. , Antibody recognition of a highly conserved influenza virus epitope. Science, 2009. 324(5924): p. 246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krammer F, et al. , Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J Virol, 2013. 87(12): p. 6542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mallajosyula VV, et al. , Influenza hemagglutinin stem-fragment immunogen elicits broadly neutralizing antibodies and confers heterologous protection. Proc Natl Acad Sci U S A, 2014. 111(25): p. E2514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Impagliazzo A, et al. , A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science, 2015. 349(6254): p. 1301–6. [DOI] [PubMed] [Google Scholar]
- 74.Krammer F and Palese P, Advances in the development of influenza virus vaccines. Nat Rev Drug Discov, 2015. 14(3): p. 167–82. [DOI] [PubMed] [Google Scholar]
- 75.Ekiert DC, et al. , A highly conserved neutralizing epitope on group 2 influenza A viruses. Science, 2011. 333(6044): p. 843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu W, et al. , Sequence comparison between the extracellular domain of M2 protein human and avian influenza A virus provides new information for bivalent influenza vaccine design. Microbes Infect, 2005. 7(2): p. 171–7. [DOI] [PubMed] [Google Scholar]
- 77.Kang SM, Kim MC, and Compans RW, Virus-like particles as universal influenza vaccines. Expert Rev Vaccines, 2012. 11(8): p. 995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karch CP, et al. , Vaccination with self-adjuvanted protein nanoparticles provides protection against lethal influenza challenge. Nanomedicine, 2017. 13(1): p. 241–251. [DOI] [PubMed] [Google Scholar]
- 79.Qi M, et al. , Intranasal Nanovaccine Confers Homo- and Hetero-Subtypic Influenza Protection. Small, 2018. 14(13): p. e1703207. [DOI] [PubMed] [Google Scholar]
- 80.Zheng M, Luo J, and Chen Z, Development of universal influenza vaccines based on influenza virus M and NP genes. Infection, 2014. 42(2): p. 251–62. [DOI] [PubMed] [Google Scholar]
- 81.Altenburg AF, Rimmelzwaan GF, and de Vries RD, Virus-specific T cells as correlate of (cross-)protective immunity against influenza. Vaccine, 2015. 33(4): p. 500–6. [DOI] [PubMed] [Google Scholar]
- 82.Forrest BD, et al. , Correlation of cellular immune responses with protection against culture-confirmed influenza virus in young children. Clin Vaccine Immunol, 2008. 15(7): p. 1042–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sridhar S, et al. , Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med, 2013. 19(10): p. 1305–12. [DOI] [PubMed] [Google Scholar]
- 84.Antrobus RD, et al. , A T cell-inducing influenza vaccine for the elderly: safety and immunogenicity of MVA-NP+M1 in adults aged over 50 years. PLoS One, 2012. 7(10): p. e48322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Antrobus RD, et al. , Coadministration of seasonal influenza vaccine and MVA-NP+M1 simultaneously achieves potent humoral and cell-mediated responses. Mol Ther, 2014. 22(1): p. 233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lillie PJ, et al. , Preliminary assessment of the efficacy of a T-cell-based influenza vaccine, MVA-NP+M1, in humans. Clin Infect Dis, 2012. 55(1): p. 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Price GE, et al. , Single-dose mucosal immunization with a candidate universal influenza vaccine provides rapid protection from virulent H5N1, H3N2 and H1N1 viruses. PLoS One, 2010. 5(10): p. e13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heath WR and Carbone FR, The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nat Immunol, 2013. 14(10): p. 978–85. [DOI] [PubMed] [Google Scholar]
- 89.Chandrasekhar S, et al. , Microarrays and microneedle arrays for delivery of peptides, proteins, vaccines and other applications. Expert Opin Drug Deliv, 2013. 10(8): p. 1155–70. [DOI] [PubMed] [Google Scholar]
- 90.Zaric M, et al. , Dissolving microneedle delivery of nanoparticle-encapsulated antigen elicits efficient cross-priming and Th1 immune responses by murine Langerhans cells. J Invest Dermatol, 2015. 135(2): p. 425–434. [DOI] [PubMed] [Google Scholar]
- 91.Quan FS, et al. , Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J Virol, 2010. 84(15): p. 7760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Quan FS, et al. , Long-term protective immunity from an influenza virus-like particle vaccine administered with a microneedle patch. Clin Vaccine Immunol, 2013. 20(9): p. 1433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim MC, et al. , Microneedle patch delivery to the skin of virus-like particles containing heterologous M2e extracellular domains of influenza virus induces broad heterosubtypic cross-protection. J Control Release, 2015. 210: p. 208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gill HS, et al. , Effect of microneedle design on pain in human volunteers. Clin J Pain, 2008. 24(7): p. 585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Birchall JC, et al. , Microneedles in clinical practice--an exploratory study into the opinions of healthcare professionals and the public. Pharm Res, 2011. 28(1): p. 95–106. [DOI] [PubMed] [Google Scholar]
- 96.Bullough PA, et al. , Structure of influenza haemagglutinin at the pH of membrane fusion. Nature, 1994. 371(6492): p. 37–43. [DOI] [PubMed] [Google Scholar]
- 97.Wilson IA, Skehel JJ, and Wiley DC, Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature, 1981. 289(5796): p. 366–73. [DOI] [PubMed] [Google Scholar]
- 98.Chen W, Nucleoprotein of Influenza a Virus Is a Major Target of Immunodominant Cd8(+) T Cell Responses. Clinical and Experimental Pharmacology and Physiology, 2013. 40: p. 33–33. [DOI] [PubMed] [Google Scholar]
- 99.Potter P, et al. , Differential processing and presentation of the H-2D(b)-restricted epitope from two different strains of influenza virus nucleoprotein. Journal of General Virology, 2001. 82: p. 1069–1074. [DOI] [PubMed] [Google Scholar]
- 100. *.Doyle TM, et al. , Universal anti-neuraminidase antibody inhibiting all influenza A subtypes. Antiviral Res, 2013. 100(2): p. 567–74. This reserch provides evidence of a promising but previously underestimated neuraminidase epitope to be extensively studied in universal influenza vaccine development in the future. [DOI] [PubMed] [Google Scholar]
- 101.Memoli MJ, et al. , Evaluation of Antihemagglutinin and Antineuraminidase Antibodies as Correlates of Protection in an Influenza A/H1N1 Virus Healthy Human Challenge Model. MBio, 2016. 7(2): p. e00417–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schotsaert M, et al. , Long-Lasting Cross-Protection Against Influenza A by Neuraminidase and M2e-based immunization strategies. Sci Rep, 2016. 6: p. 24402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen YQ, et al. , Influenza Infection in Humans Induces Broadly Cross-Reactive and Protective Neuraminidase-Reactive Antibodies. Cell, 2018. 173(2): p. 417–429 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Krammer F, et al. , NAction! How Can Neuraminidase-Based Immunity Contribute to Better Influenza Virus Vaccines? MBio, 2018. 9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gschoesser C, et al. , CD4+ and CD8+ mediated cellular immune response to recombinant influenza nucleoprotein. Vaccine, 2002. 20(31–32): p. 3731–8. [DOI] [PubMed] [Google Scholar]
- 106.Ilyinskii PO, et al. , Prime-boost vaccination with a combination of proteosome-degradable and wild-type forms of two influenza proteins leads to augmented CTL response. Vaccine, 2008. 26(18): p. 2177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhu W, et al. , A boosting skin vaccination with dissolving microneedle patch encapsulating M2e vaccine broadens the protective efficacy of conventional influenza vaccines. J Control Release, 2017. 261: p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Littauer EQ, et al. , Stable incorporation of GM-CSF into dissolvable microneedle patch improves skin vaccination against influenza. J Control Release, 2018. 276: p. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]