In light of highly prevalent resistance among previously unmonitored patients retained in care >2 years with virological failure in Malawi, differentiated care pathways with immediate switch to second-line antiretroviral therapy may be appropriate, maximizing clinical outcomes by avoiding treatment switch delays.
Keywords: antiretroviral therapy, human immunodeficiency virus (HIV), resistance, resource-limited settings, viral load monitoring
Abstract
We quantified resistance to first-line antiretroviral therapy among previously unmonitored patients in Malawi with viremia (≥1000 copies/mL). Ninety-five percent (n = 57/61) harbored nucleoside/tide reverse transcriptase inhibitor/non-nucleoside reverse transcriptase inhibitor resistance; resistance was more common comparing >2 (97%) versus ≤2 years (87%) on therapy. Immediate switch for persons retained in care may improve monitoring efficiency and maximize clinical outcomes.
Viral load (VL) testing is the preferred strategy for antiretroviral therapy (ART) monitoring in resource-limited settings [1]. Alternatives to plasma-based testing, including use of dried blood spots (DBSs), has facilitated expansion of VL testing to remote populations [2, 3]. As scale-up of VL testing proceeds, appropriate guidelines for regimen switching after viremia is identified require understanding human immunodeficiency virus (HIV) drug-resistance profiles among previously unmonitored patient populations.
Patients with treatment failure have repeatedly revealed high rates of first-line ART resistance. Failing patients in sub-Saharan Africa demonstrate resistance rates ranging from 57% to 97% with at least 1 major non-nucleoside reverse transcriptase inhibitor (NNRTI) or nucleoside/tide reverse transcriptase inhibitor (NRTI) mutation [4–6]. Current VL monitoring guidelines require confirmation of viremia after 3 months [7, 8]. Designed to preserve first-line options, this algorithm delays treatment switch for patients with first-line drug resistance, compromising outcomes and contributing to ongoing transmissions [9, 10]. Although adherence counseling must remain central to ART programs, adjustment of algorithms to address resistance among previously unmonitored patients in the context of expanding VL testing may maximize ART outcomes.
We sought to quantify the prevalence of resistance to first-line ART among previously unmonitored patients in rural Malawi identified with virological failure [2]. Malawi’s ART program includes approximately 500000 persons alive and retained on ART; many have been retained in care for >4 years [11]. As VL monitoring is rolled out, understanding resistance patterns is essential for devising appropriate, resource-efficient confirmatory testing and treatment switch algorithms.
METHODS
Remnant specimens from a public health evaluation of DBSs for VL monitoring in Malawi were used for resistance testing [2, 12]. The study enrolled HIV-infected adults (≥18 years) from district hospital–based ART clinics in Malawi. Patients were eligible for testing if they had been on first-line therapy for 6 months, 24 months, or any 24-month period thereafter +/− 3 months (routine monitoring) or if they met clinical failure criteria after 6 months on treatment. When a participant was enrolled under clinical failure criteria, time on ART was abstracted from health records rounded to the nearest 2-year time point for purposes of analysis. Persons with VL >5000 copies/mL received adherence counseling and confirmatory testing after 3 months. Patients were eligible for switch to second-line ART if confirmatory testing demonstrated continued viremia >5000 copies/mL.
Dried blood spot cards were collected using fingerstick or venous blood draws and underwent VL testing using Abbott RealTime HIV-1 Assay (Abbott Laboratories) [12]. Upon completion of the parent study, confirmatory cards with VLs ≥1000 copies/mL (or initial cards if no confirmatory specimen available) were shipped on dry ice to the United States for sequencing.
Dried blood spots are an alternative source to plasma for drug-resistance genotyping [13–15]. The mSample Preparation System was used for RNA extraction on the Abbott m2000sp (Abbott Laboratories). Depending on availability, 1–3 spots were eluted in 1.7 mL of RNA Lysis Buffer (Promega) and extracted using the Abbott 0.6-mL protocol. Previously described primers [16] did not produce amplicons; new primers for the reverse transcriptase (RT) gene were used. RNA was concentrated using RNA Clean and Concentrator columns (Zymo Research), eluting into 10 uL of water. Concentrated RNA was used in a single cDNA synthesis reaction with 2 downstream primers (RT1Adn [5’-TAGGTATGGTGAATGCAGTATACTTCCT] and RT2Adn [5’-TGTACTGTCCATTTGTCAGGATG]) and SuperScript III reverse transcriptase (Invitrogen). One-quarter of the reaction was used in nested polymerase chain reaction using the Expand High Fidelity PCR System (Roche Life Science). First-round (labeled A) upstream primers were RT1Aup (5’-GGCCATTGACAGAAGAAAAAATAAAAGC) and RT2Aup (5’-GAAGACTTCAGGAAGTATACTGC); second-round (labeled B) primers were RT1Bup (5’-TAAAAGCATTAACAGAAATTTGTACAGA), RT1Bdn (5’-TCCTGAAGTCTTCATCTAAAGGAACTGA), RT2Bup (5’-TATACTGCATTCACCATACCTAG), and RT2Bdn (5’-TTGTCAGGATGGAGTTCATATCC). Two regions were amplified and sequenced, encompassing RT codons 41–116 (region RT1) and 135–230 (region RT2). Sequences were analyzed using Sequencher 5.3 software and submitted to Stanford University Drug Resistance Database (http://hivdb.stanford.edu) to determine mutations. Resistance was only scored for the region(s) that amplified.
Statistical analyses were performed using Stata (version 13.0; StataCorp). The National Health Sciences Research Committee of Malawi and the Biomedical Institutional Review Board at the University of North Carolina, Chapel Hill approved this study. All participants provided written informed consent for the parent study.
RESULTS
Among 1498 patients enrolled in the parent study, 88 (5.8%) met protocol-defined failure (initial VL ≥5000 copies/mL); 90% (n = 78/88) had confirmatory testing. After adherence counseling, 24 of 78 (30.8%) participants had repeat VL <5000 copies/mL and 16.7% (n = 13) had VL <1000 copies/mL. Persons who resuppressed were more likely to have lower initial VL, but time on ART was similar (data not shown) [2]. We performed resistance testing on 75 specimens; 62 (83%) were confirmatory specimens. Both regions (RT codons 41–116 and 135–230) amplified for 43 of 75 (58%); 18 (24%) had partial amplification (41–115 [3] and 135–230 [14]). Neither region amplified for 14 of 75 (19%), consistent with historical sequencing success rates of fingerstick DBSs [13]. Most amplified specimens were obtained as confirmatory tests (n = 49/61); among initial specimens (n = 12) that amplified, 11 of 12 harbored resistance.
Among participants with full or partial amplification (n = 61), most (70.5%) were female; average age was 36, and average time on ART was 4.1 years (SD = 2.3). Median VL among amplified specimens was 46313 versus 5440 copies/mL among specimens without amplification (Mann-Whitney P < .05; 2-tailed).
Nearly 95% (n = 57/61) of amplified sequences demonstrated resistance to NRTI (n = 51) and/or NNRTI (n = 55) classes. Primary mutations within the NRTI class included M184M/V (n = 48/57), A62V (n = 7/47), D67N (n = 7/47), K65K/R (n = 9/47), and T215F/I/Y (n = 16/57). The most common NNRTI mutations included Y181C/Y/I/T (n = 27/57), G190A/G/R/S (n = 18/57), and K103K/N/S (n = 17/47). Most participants harbored high levels of resistance to lamivudine (3TC) (85.7%; n = 48/56); only a quarter (26.8%; n = 11/41) demonstrated high resistance to tenofovir. Among partially amplified specimens, 89% (n = 16/18) demonstrated resistance. Resistance tended to increase with duration of therapy: 87% (n = 20/23) of persons on therapy ≤2 years harbored resistance compared with 97% (n = 37/38) among those on therapy >2 years (P = .10). This trend continued comparing persons on therapy ≤4 years to those on ART >4 years (92% vs 96%; P = .60).
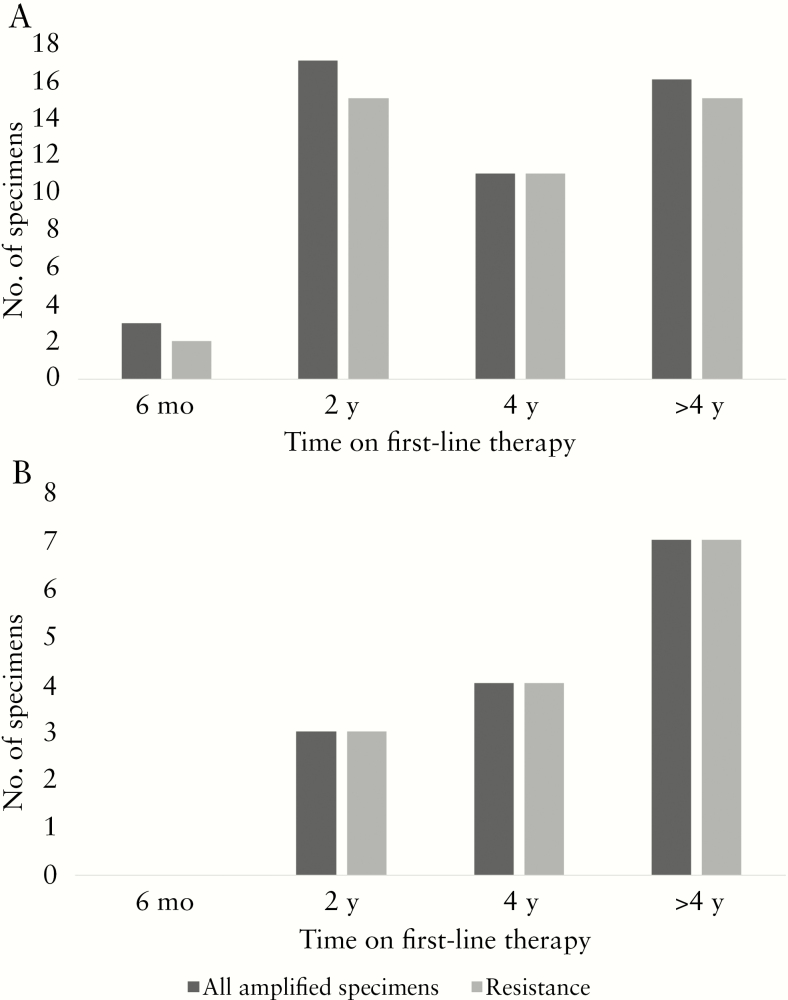
Participants with clinical failure had elevated VL compared with persons enrolled based on routine monitoring (11.4% vs 5.2%; P = .01) [2]. Resistance did not differ based on presence of clinical failure: 100% (n = 14/14) of clinical failure enrollees versus 91.5% (n = 43/47) among routine monitoring enrollees (P = .26) (Figure 1).
Figure 1.
Frequency of resistance among amplified specimens according to time on therapy by participants enrolled under routine monitoring eligibility criteria (A) and participants enrolled under targeted eligibility criteria (ie, those with clinical signs of failure at time of enrollment) (B). Persons enrolled using routine criteria were eligible for 6 (+/−1), 24, or 48 months or any 24-month increment (+/−3). Persons enrolled under clinical criteria who were not also eligible based on antiretroviral therapy exposure time points were categorized as follows: 0–11 months “6 months”; 12–35 months “2 years”; 35–51 months “4 years”; and >51 months “>4 years.”
Among participants who resuppressed (<5000 copies/mL), 54.2% (n = 13/24) had detectable viremia ≥ 1000 copies/mL. Of these, 85% (n = 11/13) were available for resistance testing, 5 of 11 (45.5%) were sequenced successfully, and all 5 showed intermediate or high resistance to NRTIs or NNRTIs.
DISCUSSION
We observed a high rate of ART resistance among persons with elevated VLs in a cohort of retained, previously unmonitored ART patients in Malawi. The 95% resistance rate is comparable with elsewhere in sub-Saharan Africa [5, 17] but is considerably higher than reports from other Malawian cohorts, likely because of the proportion of persons on therapy >2 years [18]. Among Malawian pregnant women initiated on ART and retained in care at 6 months, resistance was only 35% [19]. Our findings highlight the challenges that Malawi and other sub-Saharan African countries face in managing persons with newly identified virological failure in the context of extended, unmonitored ART.
Current guidelines require confirmatory testing for all patients, regardless of time on therapy [7]—a process that incurs additional costs and delays treatment switch to second-line therapy for persons with resistance. Among patients eligible for second-line therapy with confirmed elevated VL, average time from first elevated VL to initiation of second-line therapy was 181 days; this delay is even longer in other sub-Saharan African programs [2, 20, 21].
Differentiated care pathways may be 1 opportunity to improve efficiency and effectiveness of VL monitoring scale-up while maximizing ART outcomes. These pathways customize packages of services for patient subgroups. For example, VL-differentiated care is a cost-effective strategy where patients with suppressed viremia require fewer clinic visits [22]. Our results highlight another opportunity to differentiate HIV care pathways: the high rate of resistance among long-term patients with elevated VL represents a cadre of failing patients who could be switched immediately to second-line therapy, avoiding costs and delays of confirmatory testing. Indeed, many patients likely failed earlier, accumulating resistance mutations despite adequate adherence, with some estimates suggesting that median duration of ART prior to failure may be closer to 12 months [23]. For patients on ART for <24 months, adherence support should remain a mainstay of ART programs. But expedited switch pathways for long-term ART patients with high VL are needed to manage patients with viremia and resistance, which is fundamentally different from patients who may resuppress with improved adherence. Individualized pathways are critical in the context of VL monitoring scale-up.
Our results suggest that providers consider immediate switch for patients retained in care >2 years with VLs >5000 copies/mL, the failure threshold in Malawi guidelines at the time of the study [24]. Importantly, we considered persons to be retained if they engaged in care based on ART clinic visits with or without documented adherence. Persons may have had preceding periods of missed visits and gaps in care, but available data do not permit further characterization of care retention. Low-level viremia (<1000 copies/mL) may also harbor clinically relevant resistance [25–27]. Resistance among persons presenting with VL <5000 copies/mL is particularly important given the updated WHO guidelines that define treatment failure thresholds at 1000 copies/mL [7], and although all amplified specimens between 1000–5000 copies/mL demonstrated resistance, only 50% of specimens amplified in this range. Unsuccessful DBS amplification was a limitation of this study and could represent an over- or underestimation of resistance rates. We hypothesize that substandard long-term storage conditions and fingerstick specimen source compromised extraction efforts [13, 14, 28]. Further advancements in DBS-based amplification techniques will help elucidate implications for those with lower-level viremia and will be critical to expand on the small sample size presented here. We did not assess any pretreatment resistance because study eligibility criteria required a minimum of 6 months on ART, but pretreatment resistance is likely uncommon [29]. The parent study identified a low failure rate (5.9%), and loss to follow-up was rare [2]. The low failure rate is likely due to eligibility-driven selection of persons retained in care, but our resistance results should be verified in settings with higher failure rates. Retention within the study could result in a subpopulation for whom viremia is more likely secondary to resistance rather than inadequate adherence, using retention in care as a proxy for adherence. However, given that this real-world evaluation used existing Ministry of Health infrastructure without patient incentives, study-related retention bias is unlikely. Finally, considering the all-comer nature of our cohort and the widespread use of DBSs for VL monitoring in many resource-limited settings, our results are generalizable.
Identifying efficient and effective means of scaling up VL testing in resource-limited settings requires innovative strategies. Point-of-care VL technologies will improve monitoring efficiency, but even inexpensive point of care assays incur treatment switch delays via mandatory 3-month confirmatory periods. We have identified a public health approach of differentiated care for previously unmonitored persons with extended ART exposure and newly identified virological failure. Although aggressive adherence counseling and confirmatory testing may be important early with ART, the resistance rate among persons on therapy >2 years demonstrates an opportunity to expedite second-line therapy transitions. Delaying second-line switch for persons harboring resistance mutations has both financial and public health implications. Next steps should include an evaluation of the cost-effectiveness of the proposed differentiated care pathway as well as further evaluation of resistance profiles among persons retained in care with low-level viremia.
Acknowledgments
We would like to thank the numerous ART patients and providers who made this research possible. Sequencing was done at the UNC-CH Genome Analysis Facility.
Financial support. This work was supported by the National Institute of Mental Health at the National Institutes of Health (F30 MH098731 to S. E. R.), the National Institutes of Health (T32 GM008719), as well as by the University of North Carolina at Chapel Hill Center for AIDS Research, a National Institutes of Health funded program (P30 AI50410).
Potential conflicts of interest. All authors: No reported conflicts of interest.All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Consolidated Guidelines on the Wse of Antiretroviral Drugs for Treating and Preventing HIV Infection. Geneva, Switzerland: WHO; 2013. [PubMed] [Google Scholar]
- 2. Rutstein SE, Hosseinipour MC, Kamwendo D, et al. Dried blood spots for viral load monitoring in Malawi: feasible and effective. PLoS One. 2015;10:e0124748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schmitz ME, Agolory S, Junghae M, et al. ; for VL-DBS Study Group Field evaluation of dried blood spots for HIV-1 viral load monitoring in adults and children receiving antiretroviral treatment in Kenya: implications for scale-up in resource-limited settings. J Acquir Immune Defic Syndr. 2017;74:399–406. [DOI] [PubMed] [Google Scholar]
- 4. Boyd MA, Moore CL, Molina JM, et al. ; SECOND-LINE study group Baseline HIV-1 resistance, virological outcomes, and emergent resistance in the SECOND-LINE trial: an exploratory analysis. Lancet HIV. 2015;2:e42–51. [DOI] [PubMed] [Google Scholar]
- 5. Boyd MA, Kumarasamy N, Moore CL, et al. Ritonavir-boosted lopinavir plus nucleoside or nucleotide reverse transcriptase inhibitors versus ritonavir-boosted lopinavir plus raltegravir for treatment of HIV-1 infection in adults with virological failure of a standard first-line ART regimen (SECOND-LINE): a randomised, open-label, non-inferiority study. Lancet. 2013;381:2091–99. [DOI] [PubMed] [Google Scholar]
- 6. Gregson J, Tang M, Ndembi N, et al. Global epidemiology of drug resistance after failure of WHO recommended first-line regimens for adult HIV-1 infection: a multicentre retrospective cohort study. Lancet Infect Dis. 2016;16:565–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. 2nd ed. Geneva, Switzerland: World Health Organization; 2016. [PubMed] [Google Scholar]
- 8. Malawi Ministry of Health. Clinical Management of HIV in Children and Adults. Lilongwe: Ministry of Health, Malawi; 2014. [Google Scholar]
- 9. Levison JH, Orrell C, Losina E, et al. Early outcomes and the virological effect of delayed treatment switching to second-line therapy in an antiretroviral roll-out programme in South Africa. Antivir Ther. 2011;16:853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Petersen ML, Tran L, Geng EH, et al. Delayed switch of antiretroviral therapy after virologic failure associated with elevated mortality among HIV-infected adults in Africa. AIDS. 2014;28:2097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malawi Ministry of Health. Integrated HIV Program Report July–September 2013. Lilongwe: Government of Malawi, Ministry of Health; 2013. [Google Scholar]
- 12. Rutstein SE, Kamwendo D, Lugali L, et al. Measures of viral load using Abbott RealTime HIV-1 Assay on venous and fingerstick dried blood spots from provider-collected specimens in Malawian district hospitals. J Clin Virol. 2014;60:392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parry CM, Parkin N, Diallo K, et al. Field study of dried blood spot specimens for HIV-1 drug resistance genotyping. J Clin Microbiol. 2014;52:2868–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rottinghaus EK, Beard RS, Bile E, et al. Evaluation of dried blood spots collected on filter papers from three manufacturers stored at ambient temperature for application in HIV-1 drug resistance monitoring. PLoS One. 2014;9:e109060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kimaro J, Shao E, Nyombi B, et al. Using dried blood spots collected under field condition to determine HIV-1 diversity and drug resistance mutations in resource limited Tanzania. J Int AIDS Soc. 2014;17:19686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Farr SL, Nelson JA, Ng’ombe TJ, et al. ; BAN Study Team Addition of 7 days of zidovudine plus lamivudine to peripartum single-dose nevirapine effectively reduces nevirapine resistance postpartum in HIV-infected mothers in Malawi. J Acquir Immune Defic Syndr. 2010;54:515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Manasa J, Lessells RJ, Skingsley A, et al. ; Southern African Treatment and Resistance Network High-levels of acquired drug resistance in adult patients failing first-line antiretroviral therapy in a rural HIV treatment programme in KwaZulu-Natal, South Africa. PLoS One. 2013;8:e72152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hosseinipour MC, Gupta RK, Van Zyl G, et al. Emergence of HIV drug resistance during first- and second-line antiretroviral therapy in resource-limited settings. J Infect Dis. 2013;207(suppl 2):S49–S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hosseinipour M, Nelson JAE, Trapence C, et al. ; PURE Malawi Consortium Viral suppression and HIV drug resistance at 6 months among women in Malawi’s option B+ program: results from the PURE Malawi study. J Acquir Immune Defic Syndr. 2017;75(suppl 2):149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boulle A, Van Cutsem G, Hilderbrand K, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS. 2010;24:563–72. [DOI] [PubMed] [Google Scholar]
- 21. Fox MP, Cutsem GV, Giddy J, et al. ; IeDEA-SA collaboration Rates and predictors of failure of first-line antiretroviral therapy and switch to second-line ART in South Africa. J Acquir Immune Defic Syndr. 2012;60:428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Phillips A, Shroufi A, Vojnov L, et al. Sustainable HIV treatment in Africa through viral-load-informed differentiated care. Nature. 2015;528:S68–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boender TS, Sigaloff KC, McMahon JH, et al. Long-term virological outcomes of first-line antiretroviral therapy for HIV-1 in low- and middle-income countries: a systematic review and meta-analysis. Clin Infect Dis. 2015;61:1453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malawi Ministry of Health. Clinical Management of HIV in Children and Adults. Lilongwe: Ministry of Health, Malawi; 2011. [Google Scholar]
- 25. Mackie N, Dustan S, McClure MO, et al. Detection of HIV-1 antiretroviral resistance from patients with persistently low but detectable viraemia. J Virol Methods. 2004;119:73–8. [DOI] [PubMed] [Google Scholar]
- 26. Gonzalez-Serna A, Min JE, Woods C, et al. Performance of HIV-1 drug resistance testing at low-level viremia and its ability to predict future virologic outcomes and viral evolution in treatment-naive individuals. Clin Infect Dis. 2014;58:1165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Santoro MM, Fabeni L, Armenia D, et al. Reliability and clinical relevance of the HIV-1 drug resistance test in patients with low viremia levels. Clin Infect Dis. 2014;58:1156–64. [DOI] [PubMed] [Google Scholar]
- 28. Aitken SC, Wallis CL, Stevens W, et al. Stability of HIV-1 nucleic acids in dried blood spot samples for HIV-1 drug resistance genotyping. PLoS One. 2015;10:e0131541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boender TS, Hoenderboom BM, Sigaloff KC, et al. Pretreatment HIV drug resistance increases regimen switches in sub-Saharan Africa. Clin Infect Dis. 2015;61:1749–58. [DOI] [PubMed] [Google Scholar]