Abstract
Background and aim
Treatment of hepatitis C with direct-acting antiviral agents (DAA) has few side effects. Although pivotal studies suggested that DAA were safe in patients with psychiatric diseases who could not be treated with previous antiviral therapies, their effects on anxiety and depression have not yet been analysed in clinical practice. The aim of our study was to analyse anxiety and depression in the setting of DAA treatment in a clinical practice series.
Methods
All patients starting DAA treatment between November 1, 2014 and October 31, 2015 were eligible. Patients completed the Hospital Anxiety and Depression scale at different times during treatment. The results were plotted on line graphs and evaluated using a linear regression model with repeated measures.
Results
One hundred and forty-five patients were included (11% with major psychiatric disorders; 32% on psychiatric treatment). Sustained virologic response (SVR) was achieved in 97.3% of cases. Anxiety and depression measures did not differ between time points. No differences between patients on psychiatric treatment or with advanced fibrosis or cirrhosis were found at any time point analysed.
Conclusion
DAA treatment had no impact on anxiety or depression during or after chronic hepatitis C infection treatment, even in high-risk patients with major psychiatric disorders.
Introduction
Hepatitis C is a major cause of chronic liver disease worldwide,[1–5] with an estimated 71 million people infected in 2015[6] and approximately 1.75 million new infections each year.(3) Chronic hepatitis C virus (HCV) infection is a leading cause of cirrhosis in the United States and Europe, and is associated with increased liver-related and overall mortality[4, 7, 8]. Antiviral treatment has the potential to improve long-term outcomes for patients with cirrhosis, and viral clearance is associated with histological improvement, decreased risk of hepatocellular carcinoma, reduced incidence of decompensation, and lower liver-related and non-liver-related mortality[9–11]. Van der Mer et al[12]. found an association between sustained virologic response (SVR) and a decrease in all-cause mortality among patients with chronic HCV infection and advanced hepatic fibrosis. In addition to physical symptoms, HCV-infected patients suffer fatigue, depression and anxiety that can impair health-related quality of life, lower work productivity and worsen patient-reported outcomes.[1]
Direct-acting antiviral agents (DAA) enable higher cure rates with fewer side effects and shorter treatment[1, 5]. DAAs were associated with reductions of over 30% in waiting lists for liver transplantation for HCV cases complicated by decompensated cirrhosis[13] Replacing pegylated interferon and ribavirin with DAAs has substantially improved health-related quality of life during treatment,[14, 15] and may result in better patient experience and higher treatment adherence.[3, 14] DAA treatment is associated with substantial improvements in patient-reported outcomes (PRO) during therapy that continues long after a sustained viral response (SVR) is achieved.[1, 16]
Patients with severe comorbid mental illness have historically been considered at high risk for HCV treatment, mainly because of the significant neuropsychiatric adverse effects associated with pegylated interferon.[2, 3, 15] As interferon-free regimens do not appear to be related to neuropsychiatric adverse effects, they can be administered to patients with severe mental illness and/or substance abuse.[2, 3, 15] However, pre-existing psychiatric issues may affect tolerability of therapy, and central nervous system drugs (mainly anti-psychotics and anti-depressants) can also interact with DAA therapy.[17–19] Nevertheless, these potential interactions can be well controlled, because the most common antidepressants or benzodiazepines administered have few interactions with DAA. Moreover, the different combinations of DAA allow one to be administered that does not interact with the patient’s psychiatric medication. Yek et al.[20] recently published their experience in clinical practice, where 38% of their patients presented with mental health disorders. The SVR in these patients was 89%, similar to that of the patients without mental illness.
Patients selected for inclusion in DAA clinical trials are often not representative of the complex variability seen in clinical practice, where patients are frequently older and/or sicker than those in clinical trials.[1, 5] In addition, clinical trials generally do not focus on measuring anxiety or depression in patients on DAA treatment, and mental disorders are generally an exclusion criterion. Real world evidence on these points is scant. Sundberg et al.[15] published a prospective study that was the first to specifically examine psychiatric symptoms during DAA treatment in real-world patients with substantial psychiatric morbidity (including a history of substance abuse). In this small study (n = 17), DAA treatment in patients with significant psychiatric comorbidity did not increase depressive symptoms or affect sleep quality. Treatment adherence was very high (> 95%), as was the treatment response (88%).
The primary aim of this study was to evaluate the change in anxiety and depression scores over the course of DAA treatment and after its completion in patients with chronic HCV infection in clinical practice. Secondary aims were to evaluate the tolerability, adherence and response in patients with severe mental disorders and advanced liver disease.
Methods
Patients
All HCV patients who initiated DAA treatment in our hepatology outpatient unit between November 1, 2014 and October 31, 2015 were included in the study. A signed informed consent was mandatory to be eligible. Exclusion criteria included: coinfection with HIV or hepatitis B virus (to avoid the possible effects of coinfection on anxiety and depression before and during DAA treatment), and inability to understand and complete the questionnaires.
The study was performed in the hepatology outpatient unit of Hospital Parc Taulí in Sabadell, a city 15 km from Barcelona.The hospital covers a population of 400 000 inhabitants.
Study design
This was a prospective observational study, conducted following approval by the hospital ethics committee (Comite Ètic d'Investigació Clínica de la Corporació Sanitària Universitària Parc Taulí, approval number 2010/612). The DAA treatment administered to the patients was determined by the physician according to guidelines, and administered as per standard clinical practice. Routine management of HCV infection in our hepatology unit at the time of the study involved a multidisciplinary team including pharmacists, psychiatrists, nurses, and hepatologists. The pharmacists dispensed medication and evaluated treatment adherence and the potential interactions of DAAs with other drugs; the nurses were responsible for patient education and follow-up, and reinforced the information given by the hepatologist and pharmacist; the hepatologists evaluated the patients, prescribed treatment according to both patient and infection characteristics, and coordinated care during and after treatment, thus overseeing the entire process; and the psychiatrists prospectively evaluated patients prior to DAA treatment and during follow-up when necessary.
Evaluation of anxiety and depression
Before starting DAA treatment, all patients were evaluated in our hepatology outpatient unit using a standardized form (S1 Annex) to determine their social and psychiatric status, and whether they were able to sign the informed consent form. To measure their anxiety and depression status, patients completed the validated Spanish version of the Hospital Anxiety and Depression Scale (HADS)[21–23]. This is a self-administered 14-item questionnaire [21, 22], divided into two 7-item scales designed to screen and rate depression (HADS-D) and anxiety (HADS-A) in medical patients.[21, 22, 24]. Each item is scored from 0 to 3 points, with a higher score indicating more depression or anxiety. A score greater than 7 in one of the scales (depression or anxiety) indicates susceptibility to depression or anxiety. HADS was administered at the start of treatment (baseline) by the hepatologist. If patients scored more than 7 in depression or anxiety, they were evaluated by the psychiatrist to assess the need for psychiatric treatment, re-evaluate current psychiatric treatment or to consider special psychiatric follow-up during DAA treatment. HADS was administered by pharmacists in week 4 of treatment, week 12 of treatment, and/or at the end of treatment, before refilling patients’ prescriptions for DAA. The pharmacist also administered HADS during a follow-up visit 12 weeks after the end of treatment. Patients did not know the results of treatment before completing the questionnaires. Patients who did not complete at least three out of five HADS assessments were excluded from the final analyses of anxiety and depression. The questionnaires were completed voluntarily by the patients. Patients with psychiatric disorders were defined according to the WHO classification for major psychiatric disorders, which was the system generally used by the psychiatrist [25]. Those with at least one diagnosis in the ICD-10 classification of mental and behavioural disorders [25] were classified as having a severe mental disorder.
Measurable outcomes
Demographic data, alcohol consumption and medical history (including mental illnesses and psychiatric treatment) before and during DAA treatment, HCV characteristics (genotype and viral load), fibrosis stage, treatment adherence and response to DAA treatment were recorded. To evaluate the response to treatment, viral load (HCV-RNA) was measured 12 weeks after the end of treatment. which has a lower limit of detection of < 15 IU/ml. Sustained viral response (SVR) was defined as undetectable HCV RNA 12 weeks after the end of therapy [26]. Most patients were evaluated by elastography to evaluate the fibrosis stage. Values of ≥9.6 and ≥14 were considered as advanced fibrosis and cirrhosis, respectively. To evaluate treatment adherence, all patients answered the Spanish version of the Morisky-Green test, a validated questionnaire translated and validated into the Spanish language [27, 28].This test has the advantage of providing information on the causes of non-adherence. Affirmative answers were scored 0 points and negative answers 1 point. Patients who answered yes to all questions (total score 0 points) were considered adherent.
The primary endpoint was to assess the change in anxiety and depression scores during DAA treatment and until 12 weeks after the end of treatment. The secondary endpoint was the SVR rate 12 weeks after the end of treatment. We also analysed prognostic factors for anxiety or depression during treatment.
Antiviral treatment and dosage
Individual treatment was based on European and Spanish guidelines.[29, 30] The length of treatment ranged from 8–24 weeks depending on viral genotype, previous treatment and fibrosis stage. Patients were visited by the hepatologist at baseline, week 4, week 12,week 24 or at the end of treatment, and 12 weeks after the end of treatment. During the visits, the hepatologist evaluated symptoms, reinforced adherence and evaluated blood tests or other additional examinations such as abdominal ultrasound.
Statistical analysis
Descriptive statistics included frequencies and percentages for categorical variables and means and standard deviations for quantitative variables. Bivariate analyses were used to compare baseline characteristics between patients who completed at least three HADS questionnaires and those who completed two or less. The Chi-square test was used for categorical variables, and t-tests/Wilcoxon tests for quantitative variables.
A regression model with repeated measures was performed to analyse HADS-A and HADS-D over time.[31]Two linear regression mixed models were used to evaluate differences in HADS-A and HADS-D over time. Time was considered as a fixed effect and patient as a random effect to account for repeated measures. Additional linear mixed models were used to analyse the interactions between time and the variables treatment duration, psychiatric treatment, and fibrosis stage. Data was graphically represented using boxplots. Statistical significance was set at 0.05 for all tests. The analysis was performed with software SAS v9.4 (SAS Institute Inc. Cary, NC, USA).
Results
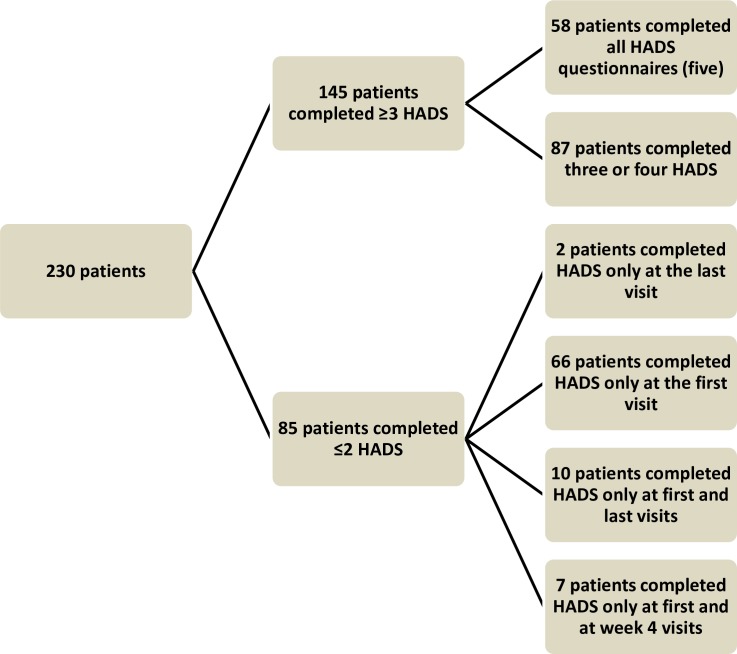
During the one-year inclusion period, 230 patients started DAA treatment; of these, 85 were excluded from the analyses because they did not complete at least three HADS questionnaires. Thus, 145 patients were eventually analysed. Reasons for not completing questionnaires were diverse and not recorded in our database, but included leaving the questionnaire at home and lack of interest since the treatment was well-tolerated.
Characteristics of included and excluded patients
Fig 1 shows the breakdown of the number of surveys that patients completed. To check for inclusion bias, we analysed the differences between the 145 patients who completed at least three of the four HADS questionnaires and the 66 excluded patients who completed the initial survey only. There were no significant differences in age (58.6 ± 10.5 vs. 61.5 ± 11.1, P = 0.08), genotypes (P = 0.16), values for the controlled attenuation parameter on elastography (17.6 ± 11.5 vs. 21.3 ± 15, P = 0.101), or receiving previous treatment (26.4% vs. 73.6%, P = 0.1) in the group of excluded patients. Moreover, no differences were detected in the number of patients with previous history of mental illness (27 excluded patients vs. 37 included, p = ns). In addition, the baseline scores for depression (4.55±4.13 vs. 4.07±3.82) and anxiety (6.20±4.15 vs. 6.25±4.81) were similar for included and excluded patients. The only significant difference between included and excluded patients was found in those patients born outside Spain, who were less likely to complete the questionnaires, probably due to difficulties with language.
Fig 1. Flow chart showing the number of surveys completed by patients.
Demographic and clinical characteristics of included patients
Of the 145 patients included (mean age, 61.5±11.1 years); 81 (55.9%) were men; 94 (64.5%) were married; 108 (85.7%) had children; 142 (98.4%) were born in Spain; and 84 (57.9%) were retired. Genotype 1b was the most frequent; it was present in 99 (68.3%) patients. Elastography indicated cirrhosis in 85 (62%) patients (Table 1). Mean Child Pugh index score was 5.4±0.8 points and mean MELD score was 9.5±2.7 points.
Table 1. Demographic, clinical and virologic patient characteristics.
| Variable | |
|---|---|
| Age in years, (mean ± *SD) | 62 ± 11.11 |
| Men (n = 145) | 81 (55.9%) |
| Genotype: (n = 145) | |
| 1a | 17 (11.7%) |
| 1b | 99 (68.3%) |
| 2 | 5 (3.4%) |
| 3 | 12 (8.3%) |
| 4 | 10 (6.9%) |
| ¼ | 1 (0.7%) |
| Undetermined | 1 (0.4%) |
| Transient elastography (mean ± *SD) | 16.9 ± 15.07 kPA |
| F4 (> 14 KPa) (n = 137) | 85 (62%) |
| Endoscopy: (n = 104) | 97 (66.9%) |
| Esophageal varices | 53 (51%) |
| Portal gastropathy | 18 (17.5%) |
| Previous antiviral treatment (pegylated interferon and ribavirin) (n = 145) | 81 (73,6%) |
| Null responder | 37 (45.7%) |
| Relapsed | 23 (28.4%) |
| Treatment discontinued due to intolerance | 16 (19.7%) |
| Partial response | 5 (6.2%) |
| Mental diseases: (n = 145) | 46 (31.7%) |
| Depression | 19(51.4%) |
| Anxiety | 12 (32.4%) |
| Psychotic | 7 (18.9%) |
| Drug abuse | 6 16.2%) |
| Personality disorder | 1 (2.7%) |
| Current severe mental disorder | 16(11%) |
| Psychiatric medication (n = 145) | 42 (29.4%) |
| Social characteristics: | |
| Married / living with partner (n = 121) | 80 (64.5%) |
| Children, mean ± *SD (n = 126) | 2 ± 1.34 |
| Born in Spain (n = 126) | 124 (98.4%) |
| Completed primary school (n = 125) High school and University (n = 125) |
74 (59.2%) 34 (27.2%) |
| Employment status: (n = 126) | |
| Retired | 73 (57.9%) |
| Active | 34 (27%) |
| Unemployed | 12 (9.5%) |
| Homemaker | 7 (5.6%) |
*SD, Standard deviation
DAA treatment administered
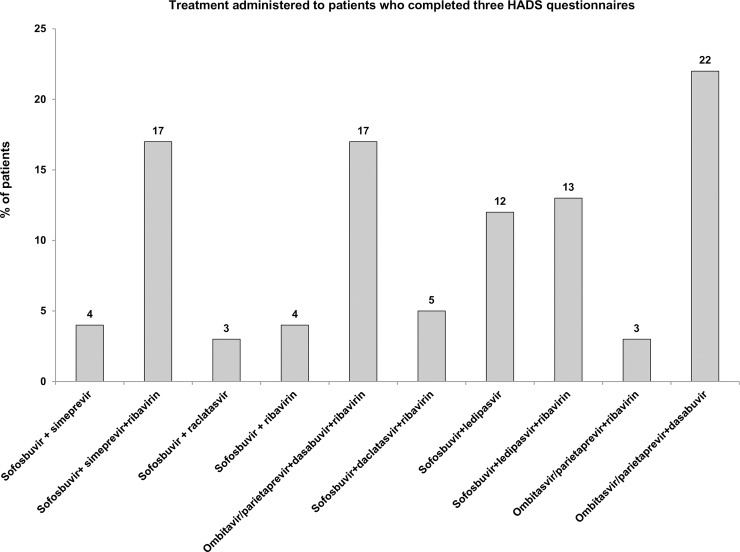
Data on treatment are shown in Fig 2. A total of 105 (72.4%) patients received 12 weeks of treatment and 40 patients (27.6%) received 24 weeks. No patient received treatment for 8 weeks. Eighty-four (58%) patients received ribavirin adjusted according to weight (ribavirin 1000mg per day in two separate doses if less than 75 kg or 1200mg if equal to or greater than 75 kg). Ribavirin dose was reduced to 600 mg per day in the case of severe anemia or other adverse events. All the patients in the study were 100% adherent to DAA treatment, as assessed using the Morisky-Green test.
Fig 2. Treatment administered to patients who completed three HADS questionnaires.
Percentage of patients are specified for every treatment. HADS, Hospital Anxiety and Depression Scale score.
Psychiatric characteristics
At the start of DAA therapy, 46 (31.7%) patients had a previous history of psychiatric disease, as reported in the patient treatment form (S1 Annex). A further 16 (11%) patients had a current severe mental disorder according to WHO international classification of mental and behavioural disorders[25] (5 schizophrenia, 1 schizoaffective disorder, 3 major depressive disorder, 1 bipolar disorder, 2 adaptive disorder, 1 obsessive-compulsive disorder, 1 somatoform disorder, and 2 generalized anxiety disorder), as evaluated by the psychiatrist. At the start of DAA therapy, 42 (29.4%) patients were receiving psychiatric medication (antidepressants [n = 20; 54.1%], antipsychotics [n = 7; 18.9%], benzodiazepines [n = 10; 27%], or others [n = 5; 13.5%]). Twenty (16.3%) patients reported they had consumed >120g of alcohol per day for > 5 years. None of them were actively consuming alcohol at the start of treatment (Table 1).
Laboratory test results and treatment adherence
Table 2 summarizes laboratory findings and treatment adherence over time (baseline, week 4, week 12, end of treatment, and 12 weeks after the end of treatment).
Table 2. Laboratory test results and treatment adherence.
| Variable | Baseline | Week 4 | Week 12 | End of treatment | 12 weeks after the end of treatment |
|---|---|---|---|---|---|
| Hemoglobin g/L (mean ± *SD) | 140.76 ± 19.79 | 128.61 ± 22.54 | 127.33 ±17.97 | 126.98 ± 21.58 | 141.23 ± 19.72 |
| Platelets ×10⁹/L (mean ± *SD) | 146.15 ± 69.59 | 163.47 ± 77.16 | 136.92 ± 71.82 | 162.95 ± 75.21 | 156.78 ± 70.27 |
| Viral load (RNA Log10 IU/ml) (mean ± *SD) | 6.05 ± 0.75 | 1.37 ± 0.29 | Undetectable | Undetectable | Undetectable |
| Albumin g/L (mean ± *SD) | 42.09 ± 5.41 | 42.21 ± 4.81 | 41.46 ± 4.94 | 41.91 ± 5.18 | 43.68 ± 4.09 |
| **INR (mean ± *SD) | 1.25 ± 0.42 | 1.23 ± 0.35 | 1.41 ± 0.54 | 1.29 ± 0.52 | 1.21 ± 0.28 |
| Bilirubin mg/dL (median****(IQR)) | 0.7 (0.7) | 0.9 (0.9) | 0.85 (1.05) | 0.7 (0.9) | 0.6 (0.45) |
| ***ALT U/L (median****(IQR)) | 61 (55) | 19 (10) | 18 (11) | 16 (9) | 17.5 (10) |
| Neutrophils ×10⁹/L (mean ± *SD) | 3.71(4.4) | 3.58 (1.6) | 3.36 (1.58) | 3.48(1.68) | 3.58(1.6) |
| Adherence | - | 100% | 100% | 100% | - |
*SD, Standard deviation
**INR, International Normalized Ratio
***ALT, Alanine aminotransferase
****IQR (interquartile range)
HADS evolution
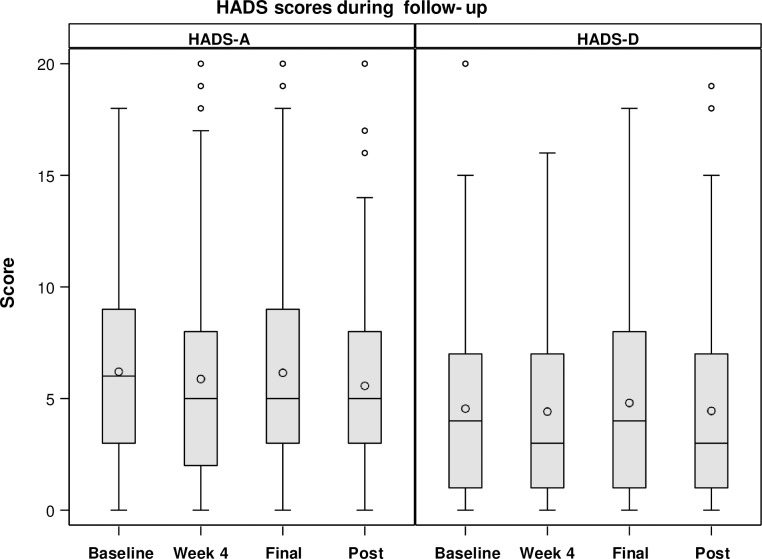
HADS-A values did not change significantly during follow-up (p = 0.19 and p = 0.62, respectively) (Fig 3). Values were: (baseline 6.20 ± 4.15; week 4, 5.88 ± 4.40; end of treatment, 6.14 ± 4.51; and 12 weeks after finishing treatment 5.57 ± 4.34) and HADS-D scores (baseline 4.55 ± 4.13; week 4, 4.42 ± 4.12; end of treatment, 4.80 ± 4.46; and 12 weeks after finishing treatment 4.45 ± 4.59). The results of patients who scored more or less or equal than 7 are reflected in Table 3.
Fig 3. HADS scores during follow-up.
Table 3. Results of patients with scores less than or equal to seven, or higher than seven.
| *HADS-A | *HADS-A | **HADS-D | **HADS-D | |
|---|---|---|---|---|
| Variable (Row Pct) | ≤ 7 | > 7 | ≤ 7 | >7 |
|
Baseline N (%) |
93 (68.06) |
44 (31.94) |
104 (77.08) |
33 (22.92) |
|
Week 4 N (%) |
97 (68.06) |
43 (31.94) |
105 (73.61) |
39 (26.39) |
|
End of treatment N (%) |
98 (68.28) |
46 (31.72) |
105 (74.48) |
39 (25.52) |
|
Week 12 after the end of treatment N (%) |
51 (68.31) |
21 (31.69) |
58 (73.24) |
16 (26.76) |
*HADS-A, Hospital Anxiety and Depression Scale-Anxiety score
**HADS-D, Hospital Anxiety and Depression Scale-Depression score
Results of HADS-A and HADS-D did not change when different patient subgroups were analysed. Thus, when considering treatment duration (12 vs. 24 weeks), scores did not change significantly over time (P = 0.55and P = 0.88 for HAD-A and HAD-D, respectively). Moreover, no differences were detected between patients with significant fibrosis or cirrhosis versus absence of or mild fibrosis either (P = 0.80 and P = 0.64 for HADS-A and HADS-D, respectively) or when considering sex and age (P = 0.20 and P = 0.62 for HADS-A and HADS-D, respectively). The change in HADS scores was also analysed depending on ribavirin intake. No significant differences were reported in this regard (P = 0.18) (Table 4). Only one patient of those on ribavirin treatment had anemia at week 4 of treatment.
Table 4. Change in Hospital Anxiety and Depression Scale scores according to ribavirin treatment.
| Time treatment | n | Ribavirin |
**HADS-A (Mean ± *SD) |
***HADS-D (Mean ± *SD) |
|---|---|---|---|---|
| Baseline | 82 | Yes | 6.18 ± 4.27 | 4.44 ± 4.10 |
| 55 | No | 6.24 ± 4.00 | 4.71 ± 4.21 | |
| Week 4 | 80 | Yes | 6.10 ± 4.67 | 4.49 ± 4.03 |
| 60 | No | 5.58 ± 4.02 | 4.32 ± 4.27 | |
| End of treatment | 84 | Yes | 6.18 ± 4.95 | 5.15 ± 4.64 |
| 60 | No | 6.08 ± 3.84 | 4.30 ± 4.17 | |
| 12 weeks after the end of treatment | 34 | Yes | 5.60 ± 5.16 | 4.66 ± 5.35 |
| 38 | No | 5.54 ± 3.51 | 4.26 ± 3.85 |
*SD Standard deviation
** HADS-A Hospital Anxiety and Depresion Scale -Anxiety score
***HADS-D Hospital Anxiety and Depresion Scale- Depression score
HADS evolution in psychiatric patients
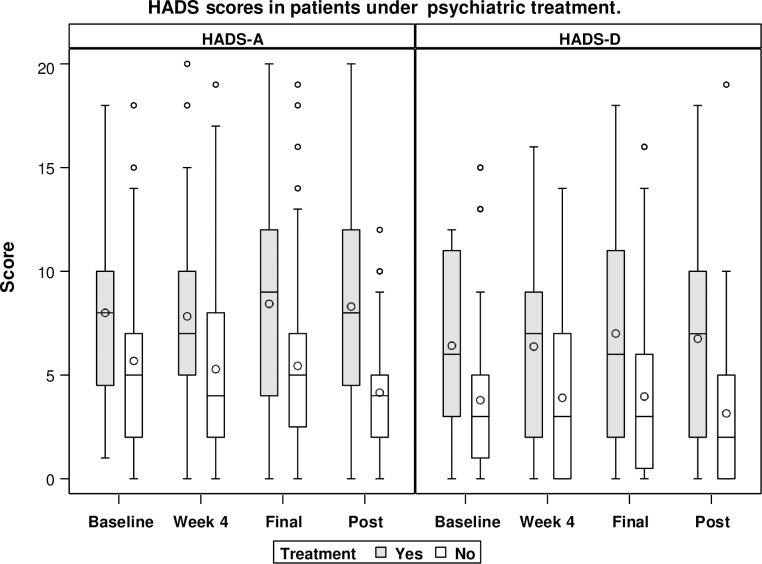
In patients with a history of psychiatric disorders (n = 37), HADS-A scores were: baseline, 8 ± 3.99; week 4, 7.83 ± 4.50; end of treatment, 8.43 ± 4.92; and 12 weeks after finishing treatment, 8.30 ± 5.33; P = 0.92. HADS-D scores were: baseline, 6.42 ± 4.25; week 4, 6.37 ± 4.29; end of treatment, 7 ± 4.94; and 12 weeks after finishing treatment, 6.75 ± 5.23; P = 0.89). Moreover, scores in the subgroup of patients on psychiatric treatment (n = 42) did not change significantly during follow-up (P = 0.93 and 0.68, respectively) (Fig 4). The HAD did not change in patients with severe mental disorders (n = 16) during follow-up. HADS-A scores were: baseline, 7.8 ± 4.3; week 4, 6.8 ± 4.9; end of treatment, 7.1± 4.2; and 12 weeks after finishing treatment, 6.6 ± 4.0; P = 0.92. HADS-D scores were: baseline, 7.9 ± 5.1; week 5.4 ± 4.2; end of treatment, 6.3 ± 4.8; and 12 weeks after finishing treatment, 6.6 ± 4; P = 0.89)
Fig 4. HADS scores in patients on psychiatric treatment.
HADS-A, Hospital Anxiety and Depression Scale-Anxiety score; HADS-D, Hospital Anxiety and Depression Scale-Depression score.
Efficacy of DAA treatment
The intention-to-treat analysis found an SVR in 140 (97.2%) patients; 5 (2.8%) patients relapsed after treatment. The SVR was 100% in both the subgroup of patients with a history of psychiatric disorders (n = 46) and the subgroup with current severe mental disorder (n = 16).
Discussion
The present study confirms that DAA therapy does not affect anxiety or depression scores either during treatment or after achieving SVR, even in patients with a history of psychiatric disorder or current severe mental disorder. Some recent clinical series have reported improved quality of life in patients treated with DAAs, but these studies did not specifically measure anxiety or depression.[16, 32–34] Our results are new and in line with a more recent study that reported successful DAA treatment in psychiatric patients without increasing depressive symptoms.[15] The limitation of this study was the small sample size.
Another interesting finding in our study was the lack of changes in anxiety and depression scores in the patients on previous psychiatric treatment during DAA treatment. Before DAAs became available, these patients were unable to receive treatment for chronic HCV infection because of the neuropsychiatric adverse effects associated with pegylated interferon. In our study, one-third of our patients had a psychiatric disorder at the start of treatment, and many had been diagnosed with a severe psychiatric disorder and were on psychiatric medication. This high prevalence is in agreement with previous studies showing a higher percentage of psychiatric diseases in patients with chronic HCV infections.[18, 35, 36]Few studies have specifically reported their experience with DAAs and mental disease. In a systematic review of 11 studies before the introduction of DAAs, Sublette et al.[37] showed that patients with psychiatric disorders had comparable SVR rates to controls if they continued psychological therapy (mean 42%), although discontinuation rates for antiviral agents were high (14%– 48%). In a recent retrospective cohort study of 43 patients from five clinical trials who were diagnosed with mental health disease requiring antidepressants, antipsychotics, mood stabilizers, or other psychotropics and were treated with DAAs, the authors found not only a high SVR, but also an improvement in the Beck Depression Inventory score. Moreover, adherence was similar in psychiatric and non-psychiatric patients.[3] Our study corroborates these findings, with no worsening of anxiety or depression in patients with psychiatric disorders, and low treatment discontinuation rate.
Psychiatric symptoms are influenced by sex and age in many cases.[38, 39] In the present study, no significant differences were found in the HADS score when variables were analysed according to these parameters.
Advanced fibrosis or cirrhosis affects PROs, especially in patients with ascites, encephalopathy, and malnutrition,[40] and mental health is one of the variables measured in PRO questionnaires[5, 8]. We did not find changes in depression or anxiety during DAA treatment in this patient subgroup either, confirming psychiatric safety also in this high-risk population.
Our study also showed that the use of ribavirin (58% of our patients) did not play a role in anxiety or depression during the follow-up period. During the inclusion period of our study, ribavirin retained an important role in the optimal treatment of some patient subgroups, particularly those who have historically been considered more difficult to cure, such as genotype 3, decompensated cirrhosis or failure of previous treatment.[41] Ribavirin is known to cause anemia and other side effects that could impair quality of life, but the frequency and severity of these effects are considerably reduced and easy to manage when co-administered with DAAs in the absence of interferon[41]. Despite this, anemia can affect patients with psychiatric disorders,[42] and can cause cognitive function disorders and depression or exacerbate an existing psychiatric condition when left untreated.[43] Our study showed that the addition of ribavirin to DAA regimens had no effects on anxiety and/or depression during the treatment.
Our study has some limitations. We cannot rule out a potential bias due to physicians’ criteria for prescription, or to missing data resulting from incomplete patient records or data entry errors. Another limitation of our study is the lack of measure of quality of life or PRO. When the protocol was designed, we considered that additional questionnaires would represent an extra difficulty for patients. Consequently, and considering that our primary endpoint was the assessment of anxiety and depression, no additional questionnaires were included in the study. Another limitation is that, although our study suggests that a multidisciplinary team allows excellent adherence and cure rates to be obtained, this may not be generalizable to areas with fewer resources. Nursing care was very important in our experience to enhance patients’ knowledge and adherence. Furthermore, pharmacist monitoring of adherence is important for such expensive drugs. Finally, psychiatric evaluation of all patients with pathological HADS at the start of treatment could have affected the final HADS results by improving patient management. However, results of the study modified clinical practice in our unit, in that we were confident in reducing the intensity of psychiatric monitoring during treatment, limiting intervention to patients with newly detected or unstable psychiatric diseases. This study, therefore, reassures clinicians of the safety of DAAs, even in the special high-risk population.
In conclusion, our study shows that in clinical practice, DAA treatment had no impact on anxiety or depression in chronic HCV patients, even if they had previous or current psychiatric disorders or advanced fibrosis.
Supporting information
(DOCX)
Acknowledgments
Medical writing support was provided by Almudena Fuster-Matanzo, PhD from Medical Statistics Consulting S.L. (Valencia, Spain).
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Younossi Z, Henry L. The impact of the new antiviral regimens on patient reported outcomes and health economics of patients with chronic hepatitis C. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2014;46 Suppl 5:S186–96. [DOI] [PubMed] [Google Scholar]
- 2.Sockalingam S, Sheehan K, Feld JJ, Shah H. Psychiatric care during hepatitis C treatment: the changing role of psychiatrists in the era of direct-acting antivirals. The American journal of psychiatry. 2015;172(6):512–6. 10.1176/appi.ajp.2015.14081041 [DOI] [PubMed] [Google Scholar]
- 3.Tang LS, Masur J, Sims Z, Nelson A, Osinusi A, Kohli A, et al. Safe and effective sofosbuvir-based therapy in patients with mental health disease on hepatitis C virus treatment. World journal of hepatology. 2016;8(31):1318–26. 10.4254/wjh.v8.i31.1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jimenez Galan R, Albacete Ramirez A, Monje Agudo P, Borrego Izquierdo Y, Morillo Verdugo R. [New drugs in the treatment of chronic hepatitis C]. Farm Hosp. 2014;38(3):231–47. 10.7399/fh.2014.38.3.7314 [DOI] [PubMed] [Google Scholar]
- 5.Younossi Z, Henry L. Systematic review: patient-reported outcomes in chronic hepatitis C—the impact of liver disease and new treatment regimens. Alimentary pharmacology & therapeutics. 2015;41(6):497–520. [DOI] [PubMed] [Google Scholar]
- 6.Organization WH. Hepatitis C. WHO fact Sheet 164 2017 [http://www.who.int/mediacentre/factsheets/fs164/en/].
- 7.Younossi ZM, Stepanova M, Marcellin P, Afdhal N, Kowdley KV, Zeuzem S, et al. Treatment with ledipasvir and sofosbuvir improves patient-reported outcomes: Results from the ION-1, -2, and -3 clinical trials. Hepatology. 2015;61(6):1798–808. 10.1002/hep.27724 [DOI] [PubMed] [Google Scholar]
- 8.Younossi ZM, Stepanova M, Nader F, Henry L. Patient-Reported Outcomes of Elderly Adults with Chronic Hepatitis C Treated with Interferon- and Ribavirin-Free Regimens. J Am Geriatr Soc. 2016;64(2):386–93. 10.1111/jgs.13928 [DOI] [PubMed] [Google Scholar]
- 9.Morgan TR, Ghany MG, Kim HY, Snow KK, Shiffman ML, De Santo JL, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010;52(3):833–44. 10.1002/hep.23744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Tilborg M, Maan R, van der Meer AJ, de Knegt RJ. Interferon-free antiviral therapy for chronic hepatitis C among patients in the liver transplant setting. Best Pract Res Clin Gastroenterol. 2017;31(2):219–25. 10.1016/j.bpg.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 11.van der Meer AJ, Feld JJ, Hofer H, Almasio PL, Calvaruso V, Fernandez-Rodriguez CM, et al. Risk of cirrhosis-related complications in patients with advanced fibrosis following hepatitis C virus eradication. J Hepatol. 2017;66(3):485–93. 10.1016/j.jhep.2016.10.017 [DOI] [PubMed] [Google Scholar]
- 12.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. Jama. 2012;308(24):2584–93. 10.1001/jama.2012.144878 [DOI] [PubMed] [Google Scholar]
- 13.Flemming JA, Kim WR, Brosgart CL, Terrault NA. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology (Baltimore, Md). 2017;65(3):804–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Younossi ZM, Stepanova M, Nader F, Lam B, Hunt S. The patient's journey with chronic hepatitis C from interferon plus ribavirin to interferon- and ribavirin-free regimens: a study of health-related quality of life. Alimentary pharmacology & therapeutics. 2015;42(3):286–95. [DOI] [PubMed] [Google Scholar]
- 15.Sundberg I, Lannergard A, Ramklint M, Cunningham JL. Direct-acting antiviral treatment in real world patients with hepatitis C not associated with psychiatric side effects: a prospective observational study. BMC Psychiatry. 2018;18(1):157 10.1186/s12888-018-1735-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Younossi ZM, Stepanova M, Jacobson IM, Asselah T, Gane EJ, Lawitz E, et al. Sofosbuvir and velpatasvir with or without voxilaprevir in direct-acting antiviral-naive chronic hepatitis C: patient-reported outcomes from POLARIS 2 and 3. Alimentary pharmacology & therapeutics. 2018;47(2):259–67. [DOI] [PubMed] [Google Scholar]
- 17.Chasser Y, Kim AY, Freudenreich O. Hepatitis C Treatment: Clinical Issues for Psychiatrists in the Post-Interferon Era. Psychosomatics. 2017;58(1):1–10. 10.1016/j.psym.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 18.Evon DM, Golin CE, Fried MW, Keefe FJ. Chronic hepatitis C and antiviral treatment regimens: where can psychology contribute? Journal of consulting and clinical psychology. 2013;81(2):361–74. 10.1037/a0029030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol. 2018. [DOI] [PubMed] [Google Scholar]
- 20.Yek C, de la Flor C, Marshall J, Zoellner C, Thompson G, Quirk L, et al. Effectiveness of direct-acting antiviral therapy for hepatitis C in difficult-to-treat patients in a safety-net health system: a retrospective cohort study. BMC Med. 2017;15(1):204 10.1186/s12916-017-0969-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golden J, Conroy RM, O'Dwyer AM. Reliability and validity of the Hospital Anxiety and Depression Scale and the Beck Depression Inventory (Full and FastScreen scales) in detecting depression in persons with hepatitis C. Journal of affective disorders. 2007;100(1–3):265–9. 10.1016/j.jad.2006.10.020 [DOI] [PubMed] [Google Scholar]
- 22.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta psychiatrica Scandinavica. 1983;67(6):361–70. [DOI] [PubMed] [Google Scholar]
- 23.Quintana JM, Padierna A, Esteban C, Arostegui I, Bilbao A, Ruiz I. Evaluation of the psychometric characteristics of the Spanish version of the Hospital Anxiety and Depression Scale. Acta psychiatrica Scandinavica. 2003;107(3):216–21. [DOI] [PubMed] [Google Scholar]
- 24.Navines R, Castellvi P, Moreno-Espana J, Gimenez D, Udina M, Canizares S, et al. Depressive and anxiety disorders in chronic hepatitis C patients: reliability and validity of the Patient Health Questionnaire. Journal of affective disorders. 2012;138(3):343–51. 10.1016/j.jad.2012.01.018 [DOI] [PubMed] [Google Scholar]
- 25.Organization WH. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. Geneva: World Health Organization; 1992. [Google Scholar]
- 26.Rogers G, Campbell L. Hepatitis C virus: its prevalence, implications and management. Nurs Times. 2003;99(50):30–1. [PubMed] [Google Scholar]
- 27.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Medical care. 1986;24(1):67–74. [DOI] [PubMed] [Google Scholar]
- 28.Nogues Solan X, Sorli Redo ML, Villar Garcia J. [Tools to measure treatment adherence]. Anales de medicina interna (Madrid, Spain: 1984). 2007;24(3):138–41. [DOI] [PubMed] [Google Scholar]
- 29.EASL Recommendations on Treatment of Hepatitis C 2016. Journal of hepatology. 2017;66(1):153–94. 10.1016/j.jhep.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 30.Pineda Juan Antonio RA, Forns Xavier, Calleja José Luis, Berenguer Marina, Buti María, Carrión José antonio, Fernández Inmaculada, Crespo Manel,García Federico, Macías Juan, Montes María Luisa. Guías AEEH/SEIMC para el manejo de la Hepatitis C http://aeeh.es/wp-content/uploads/2017/03/documento-consenso.pdf2017
- 31.Littell RC, Milliken George A., Stroup Walter W., Wolfinger Russell D., and Oliver Schabenberger. SAS for mixed models, Second Edition Cary, NC: SAS Institute Inc; Second Edition ed2006. [Google Scholar]
- 32.Miller ER, McNally S, Wallace J, Schlichthorst M. The ongoing impacts of hepatitis c—a systematic narrative review of the literature. BMC public health. 2012;12:672 10.1186/1471-2458-12-672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golabi P, Elsheikh E, Karrar A, Estep JM, Younossi I, Stepanova M, et al. The levels of monoamine neurotransmitters and measures of mental and emotional health in HCV patients treated with ledipasvir (LDV) and sofosbuvir (SOF) with or without ribavirin (RBV). Medicine. 2016;95(46):e5066 10.1097/MD.0000000000005066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Younossi ZM, Stepanova M, Feld J, Zeuzem S, Jacobson I, Agarwal K, et al. Sofosbuvir/velpatasvir improves patient-reported outcomes in HCV patients: Results from ASTRAL-1 placebo-controlled trial. Journal of hepatology. 2016;65(1):33–9. 10.1016/j.jhep.2016.02.042 [DOI] [PubMed] [Google Scholar]
- 35.Schaefer M, Capuron L, Friebe A, Diez-Quevedo C, Robaeys G, Neri S, et al. Hepatitis C infection, antiviral treatment and mental health: a European expert consensus statement. Journal of hepatology. 2012;57(6):1379–90. 10.1016/j.jhep.2012.07.037 [DOI] [PubMed] [Google Scholar]
- 36.Mathew S, Faheem M, Ibrahim SM, Iqbal W, Rauff B, Fatima K, et al. Hepatitis C virus and neurological damage. World journal of hepatology. 2016;8(12):545–56. 10.4254/wjh.v8.i12.545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sublette VA, Douglas MW, McCaffery K, George J, Perry KN. Psychological, lifestyle and social predictors of hepatitis C treatment response: a systematic review. Liver international: official journal of the International Association for the Study of the Liver. 2013;33(6):894–903. [DOI] [PubMed] [Google Scholar]
- 38.Nolen-Hoeksema S, Larson J, Grayson C. Explaining the gender difference in depressive symptoms. Journal of personality and social psychology. 1999;77(5):1061–72. [DOI] [PubMed] [Google Scholar]
- 39.Compton MT, Berez C, Walker EF. The Relative Importance of Family History, Gender, Mode of Onset, and Age at Onsetin Predicting Clinical Features of First-Episode Psychotic Disorders. Clinical schizophrenia & related psychoses.11(3):143–50. [PubMed] [Google Scholar]
- 40.Bhanji RA, Carey EJ, Watt KD. Review article: maximising quality of life while aspiring for quantity of life in end-stage liver disease. Alimentary pharmacology & therapeutics. 2017;46(1):16–25. [DOI] [PubMed] [Google Scholar]
- 41.Feld JJ, Jacobson IM, Sulkowski MS, Poordad F, Tatsch F, Pawlotsky JM. Ribavirin revisited in the era of direct-acting antiviral therapy for hepatitis C virus infection. Liver Int. 2017;37(1):5–18. 10.1111/liv.13212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin P, Jensen DM. Ribavirin in the treatment of chronic hepatitis C. Journal of gastroenterology and hepatology. 2008;23(6):844–55. 10.1111/j.1440-1746.2008.05398.x [DOI] [PubMed] [Google Scholar]
- 43.Korkmaz S, Yildiz S, Korucu T, Gundogan B, Sunbul ZE, Korkmaz H, et al. Frequency of anemia in chronic psychiatry patients. Neuropsychiatr Dis Treat. 2015;11:2737–41. 10.2147/NDT.S91581 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.