ABSTRACT
Distant metastasis accounts for the vast majority of deaths in patients with cancer. Breast cancer exhibits a distinct metastatic pattern commonly involving bone, liver, lung, and brain. Breast cancer can be divided into different subtypes based on gene expression profiles, and different breast cancer subtypes show preference to distinct organ sites of metastasis. Luminal breast tumors tend to metastasize to bone while basal-like breast cancer (BLBC) displays a lung tropism of metastasis. However, the mechanisms underlying this organ-specific pattern of metastasis still remain to be elucidated. In this review, we will summarize the recent advances regarding the molecular signaling pathways as well as the therapeutic strategies for treating breast cancer lung metastasis.
KEYWORDS: Breast cancer, cancer stem cell, chemokine, lung metastasis, microenvironment
Abbreviations
- BLBC
basal-like breast cancer
- TNBC
triple-negative breast cancer
- CSCs
cancer stem cells
- BCSCs
breast cancer stem cells
- EMT
epithelial-mesenchymal transition
- DKK1
Dickkopf1
- TGF-β
transforming growth factor-β
- DTCs
disseminated cancer cells
- ECM
extracellular matrix
- TAMs
tumor-associated macrophages
- CAFs
cancer-associated fibroblasts
- TNC
Tenascin-C
- POSTN
Periostin
- VCAN
Versica
- MSCs
mesenchymal stromal cells
- SCB
succinobucol
- VCAM-1
vascular cell adhesion molecule-1
- CXCR4
C-X-C motif chemokine receptor 4
- CXCL12
Chemokine (C-X-C motif) ligand 12
- GLI1
glioma-associated oncogene homolog 1
- TLR4
toll-like receptor 4
- NICD
Notch intracellular domain.
Introduction
Breast cancer is the most common malignant disease in women worldwide.1 It is a heterogeneous disease, and its pathogenesis remains unclear in most cases. Much progress has been made in early detection and better treatment of breast cancer, leading to improved survival. However, a considerable number of patients will relapse as a result of organ metastasis, especially those with triple-negative breast cancer (TNBC) which has the worst prognosis. Breast cancer cells are able to spread to distant sites, specifically lung, liver, bone, and brain2-4 There, they proliferate into macroscopic masses that lead to death of most patients.5,6 The 5-y survival rate of breast cancer patients who recurred with distant metastasis is less than 20%.7,8
The lung, bone, and liver, are the most common metastatic target sites for breast cancer. In fact, approximately 60% of metastatic breast cancer patients suffer lung or bone metastasis in their life.7 BLBC is specifically prone to metastasize to the lung. Life expectancy is low when this occurs, with median survival only 22 months after treatment for lung metastasis.9 In particular, 60–70% of metastatic breast cancer patients who eventually died were diagnosed with lung metastasis.10
Despite a variety of available approaches for the treatment of lung metastasis, such as chemotherapy, radiotherapy, and targeted therapy, the survival rate of breast cancer patients with lung metastasis remains very low. Elucidating and understanding the underlying mechanisms is crucial for developing new therapeutic strategies. Of note, BLBC markers such as EGFR and FOXC1 have been shown to control and correlate with lung metastasis.11-13 In this review, we seek to provide an overview of the recent advances in understanding the molecular basis of lung metastasis of breast cancer with a special emphasis on cancer stem cell pathways and microenvironment. In addition to presenting the clinical characteristics of breast cancer lung metastasis, we discuss the potential therapeutic approaches that may improve the prognosis of breast cancer patients with lung metastasis.
Clinical features of metastasis in breast cancer
With improvements in earlier diagnosis of breast cancer, only 5–10% of patients have distant metastasis at the time of diagnosis.14,15 However, the risk of recurrent metastatic disease following standard treatment is still high. More than 30% of breast cancer patients suffer recurrence, and the occurrence of lung or bone metastasis can reach greater than 60% in metastatic breast cancer patients.7 More than half a million women worldwide still suffer from metastatic breast cancer annually, and 90% of the deaths can be attributed to metastasis from breast cancer.16,17
Bone, liver, lung, and brain are the most common sites of distant metastasis in breast cancer, which are associated with the patients' poor survival outcome.18,19 Furthermore, the preference of metastatic organ has also shown to differ between subtypes of breast cancer.9,17,20,21 Bone metastasis preferentially occurs in luminal breast cancer patients, while lung metastasis is commonly diagnosed in TNBC.9,17,20,21 The incidence of lung metastasis can reach up to 40% in TNBC compared with only 20% in non-TNBC.22 Gene expression analysis showed that lung relapse patients were most abundant in the luminal B and basal subtypes, whereas bone relapse was less frequent in BLBC. Strikingly, the absence of lung relapse was observed in the luminal A subtype, while brain metastasis was predominantly found in patients with BLBC and HER2+ breast cancer.9 Of note, Yhim et al. analyzed the survival record of patients with lung metastasis and found that hormone receptor-positive breast cancers had the best clinical outcome, while HER2+ cancers and TNBC had the worst prognosis.23 The HER2+ subtype was also found to display a higher risk of developing liver metastasis.21,24
Most recently, a SEER database analysis indicated that patients with TNBC, especially BLBC, primarily presented with lung metastasis. However, there was no difference in the total probability of lung metastasis across all subtypes.25 Furthermore, the study revealed that all breast cancers regardless of subtype, were prone to metastasize to bone over other locations. Specifically the incidience of bone metastasis is highest in luminal cancers. Although there are discrepancies among reports regarding the preferred metastatic sites of breast cancer subtypes, it is widely accepted that different subtypes exhibit distinctive behavior with regards to the sites of distant metastasis.
In addition to the poor prognosis associated with metastatic breast cancer, the clinical presentations and consequences of lung metastasis are extremely serious. Pain, cough, hemoptysis, pleural effusion, and pulmonary dysfunction are common clinical symptoms which profoundly affect quality of life and survival. The prognosis of breast cancer patients with lung metastasis is still poor despite receiving chemotherapy, targeted therapy, and endocrine therapy based on molecular receptor profiles. Currently the best and only method to prevent breast cancer lung metastasis is an earlier diagnosis. Therefore, we must fully understand the mechanism of lung metastasis of breast cancer to create better treatment strategies. In this review, we summarize the reported cancer cell- and its surrounding microenvironment-based mechanisms of breast cancer lung metastasis and present the challenges we are facing.
Cancer stem cells and associated signaling pathways
Cancer stem cells (CSCs), also named tumor-initiating cells or stem-like cells from solid tumors of different organs (ie. breast, lung, thyroid, etc.), have the ability of self-renewal and differentiation. As such, CSCs can differentiate sufficiently to recapitulate the heterogeneity of tumors.26-28 It has now been established that breast cancer stem cells (BCSCs) are responsible for metastatic growth in breast cancer which contributes to the majority of breast cancer related mortality.29,30
A large body of evidence now suggests that the presence of BCSCs is highly associated with specific subtypes.31,32 Chekhun et al. demonstrated that BCSCs are not significantly associated with breast cancer of luminal and HER2-positive subtypes.33 Honeth et al. reported that the CSC phenotypes are enriched in BLBC compared with other breast cancer subtypes.34 Studies suggest that CSCs may play a role in breast cancer lung metastasis, although whether this is the primary mechanism underlying the organ tropism of BLBC metastasis is unclear. A subset of CD44+ CSCs in primary breast tumors may possess the ability to promote distant metastasis.35,36 Yae et al. found that the lung colonization potential of CD44v+ 4T1 mouse mammary tumor cells is much higher than that of CD44v- cells due to the increased activity of the cystine transporter xCT induced by CD44v. This transporter activity is in turn regulated by the gene epithelial splicing regulatory protein 1.37 In another related study involving clinical samples, Hu et al. further demonstrated the heterogeneity of BCSCs in lung metastasis capacities38 and found that CD44v expression both denotes a subset of BCSCs and promotes lung metastasis by interacting with osteopontin in the lung microenvironment. Of note, CD44 is not sufficient to identify all BCSCs. Whether CD44-negative human BCSCs also dictate lung metastasis awaits to be determined.
In summary, preclinical and clinical studies have shown that enrichment of BCSCs may result in increased invasiveness and a worse prognosis. The development of CSC properties is known to depend on an intricate signaling network. These signaling pathways play an important role in balancing self-renewal with differentiation of cancer stem cells.39,40 In the following sections, we summarize the potential roles of common CSC-associated signaling pathways in breast cancer lung metastasis.
Notch signaling pathway
Notch signaling is a pathway that relies on cell-cell contact.41 The ligands of Notch bind to the receptors on adjacent cells leading to activation of the signaling pathway.42 In breast cancer, the activation of the Notch pathway could allow BCSCs to undergo uncontrolled proliferation.43-45 Studies demonstrated that Notch-1, a Notch signaling pathway receptor, could regulate epithelial-mesenchymal transition (EMT) in breast cancer and BCSCs, where it plays a critical role in self-renewal, proliferation, and apoptosis of BCSCs.46,47
As stated above, the abnormal activation of the Notch signaling pathway participates in breast cancer metastasis by primarily modulating EMT and angiogenesis.48 In addition, BCSCs that disseminate from primary sites to distant microenvironments establish lung niches that are associated with Notch.48 A study by Chen et al. focusing on the role of Notch in salivary adenoid cystic carcinoma cells, found that knockdown of Notch-1 significantly inhibited the formation of metastatic lung nodules induced by EMT.49 While it is unclear how the Notch signaling pathway regulates primary tumor cells disseminating to the lung, we speculate that it may play a critical role in the adaptation of breast cancer cells to metastatic niches. Notch signaling pathway may also interact with other signaling pathways to dictate the function and fate of breast cancer cells during the metastatic process.
Wnt/β-catenin signaling pathway
Wnt/β-catenin signaling also plays an important role in embryonic induction and tumorigenesis of the mammary gland.50,51 The β-catenin nuclear localization and overexpression is an indicator of Wnt/β-catenin signaling activation. Many clinical and laboratory studies demonstrated that aberrant activation of Wnt/β-catenin signaling is associated with poorer prognosis in breast cancer patients and is enriched in the subgroup of TNBC.52-54 Moreover, the Wnt co-receptor LRP6 is often overexpressed in a subtype of aggressive invasive breast cancer like triple-negative breast cancer.55,56
In addition, BCSCs have increased activation of Wnt/β-catenin signaling when compared to normal stem-like cells. Greater signaling could maintain BCSCs in a self-renewing state and induce the formation of metastatic niches.57 Suppression of GSK3β (a negative regulator of the Wnt pathway) was sufficient to diminish the stem cell features of breast cancer cells.58 Wnt/β-catenin signaling also contributes to EMT and metastasis in breast cancer. Deyet al. found that the patients identified by the Wnt/β-catenin classifier had a greater risk of lung metastasis in TNBC.59 Studies using xenograft models demonstrated that Wnt signaling may link cancer cell self-renewal and expression of EMT transcription factors with tumor seeding and lung metastasis in BLBC.60 Other studies suggested that Wnt/β-catenin can also regulate breast cancer cell proliferation.61,62 The proteins of the Wnt family are functionally separated into two classes: those activating the canonical Wnt/β-catenin pathway and those activating the planar cell polarity and Wnt/calcium pathways, which do not involve β-catenin.63,64 Recently, it was reported that the inhibitor of Wnt Dickkopf-related protein 1 (DKK1) suppresses macrophage and neutrophil recruitment in breast cancer lung metastases in part by antagonizing cancer cell non-canonical Wnt-JNK signaling.65 Lung metastasis inhibition by DKK1 is also mediated by reduced Wnt-NF-κB signaling in breast cancer cells.
It is well-documented that canonical Wnt signaling components are commonly up-regulated in breast cancer cells relative to normal mammary epithelial cells. In contrast, there are conflicting reports regarding the expression and role of non-canonical Wnt.66,67 Jiang et al. found that enhanced non-canonical Wnt (Wnt5a) expression in breast cancer cells can inhibit lung metastasis through downregulating multiple cell motility-related pathways by regulating transcription and splicing of some key pathway-associated genes.68 Concordantly, other studies showed that Wnt5a may suppress breast cancer progression and loss of its expression is associated with poor prognosis.67-74 On the contrary, reports also demonstrated a positive role of Wnt5a in promoting tumor growth and migration in TNBC.75-77 The observed paradoxical effects of Wnt5a may be dependent upon its signaling context, leading to controversy over its role in breast cancer tumorigenesis and metastasis. In addition, Wnt5a can elicit both canonical and non-canonical Wnt pathways. More work is needed to elucidate the mechanisms of specific Wnt members and pathways in breast cancer development and metastasis.
Hedgehog signaling pathway
Hedgehog (Hh) signaling plays an essential role in ductal development in the mammary gland. It also regulates BCSCs and plays a crucial role in carcinogenesis.78-81 Several recent studies have provided evidence that paracrine Hh signaling appears to be an important mechanism in breast cancer growth.82,83 Moreover, Hh has been shown to regulate breast cancer cell migration.84 We also found such paracrine signaling is associated with poor prognosis and the basal-like phenotype.85
Inaquma et al. demonstrated that the glioma-associated oncogene homolog 1 (GLI1) transcription factor enhances lung metastasis of breast cancer cells in a mouse model via its interaction of CXCL12-CXCR4 axis.86 FOXC1, a transcription factor that is normally overexpressed in BLBC, could directly induce CXCR4 expression by activating its promoter in endothelial cells thereby controlling angiogenesis in breast cancer.87 Furthermore, FOXC1 controls the cancer stem cell (CSC) properties enriched in BLBC cells via activation of Smoothened (SMO)-independent Gli2 activation. This activation leads to enhanced lung metastasis.88 Zuo et al. used mouse models to demonstrate that FOXC1 overexpression has more tumorigenicity and pulmonary metastatic ability in BLBC.89 It is possible the secretion of Hh ligand by breast tumor cells mediates a crosstalk with the lung environment in a paracrine manner.
In breast cancer, dysregulated Hh signaling also exerts its function through its interaction with other signaling pathways. In a hepatocellular carcinoma study, activation of Hh signaling and the transforming growth factor-β (TGF-β) was shown to promote liver cancer lung metastasis in mouse models.90 Whether the same mechanism is involved in lung metastasis of breast cancer remains to be determined. It is noted that concomitant dysregulation of Hh, Notch, and Wnt signaling pathways has been observed in cancer, suggesting their potential cooperation in promoting tumor development and metastasis.58,91-94 Co-activation of both Hh and Wnt pathways in clinical TNBC samples is associated with shorter recurrence-free and overall survival.93 Consistent with this finding, nuclear β-catenin has been shown to increase Gli1 transcriptional activity in other cancer types.95 To date, mechanisms that coordinate Hh, Notch, and Wnt activity in cell function remain poorly understood. Using HEK293T, human embryonic kidney cells, and gene knockout mouse models, Kikuchi et al. demonstrated that parafibromin, a PAF complex component, binds to β-catenin, Gli1, and Notch, thereby enabling concerted activation of Hh-, Wnt-, and Notch-target genes.96 How these pathways crosstalk in breast cancer is unclear. In short, further understanding of their role, regulation, and potential interaction in breast cancer metastasis may facilitate the development of more effective therapeutic strategies (Fig. 1).
Figure 1.
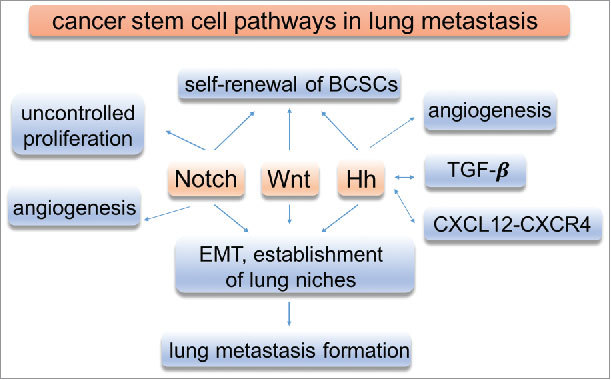
Functions of signaling pathways in breast cancer. Signaling pathways play important roles in breast cancer development and lung metastasis.
Chemokines
Nearly every tissue expresses chemokines and chemokine receptors. Chemokines are small proteins that govern the directed migration of leukocytes under homeostatic conditions and during specific immune responses.97-99 They are grouped into four families: C, CC, CXC and CX3C.100,101 Currently, more than fifty chemokines and twenty chemokine receptors have been discovered.102 Apart from their major function in leukocyte recruitment and inflammation, chemokines have been implicated in the progression of many cancers including breast cancer.
CC and CXC
CC chemokines are important determinants of macrophages and lymphocytes that infiltrate human carcinomas of the breast.103,104 In addition, CCL2 mediates the development of the cancer stem cell phenotype.105 In fact, CCL2 can induce lung overexpression of endogenous toll-like receptor 4 (TLR4) ligands like S100A8 and SAA3, which can enhance cancer cell survival.106,107 The endogenous TLR4-dependent innate immune system plays an important role in pre-metastatic niche formation in the lung, which is an essential procedure of process in lung metastasis.106
The CXCL12-CXCR4 axis is one of the most extensively studied CXC chemokine signals in metastasis. The expression of C-X-C motif chemokine receptor 4 (CXCR4) is higher in malignant breast tumors than in normal breast tissues. Chemokine (C-X-C motif) ligand 12 (CXCL12) is highly expressed in the lung, bone, liver and lymph nodes, locations where breast cancer cells prefer to metastasize.108-110 Chemokine and chemokine receptors have interactions with inflammatory microenvironments in metastatic sites.111 In a study using radiation-treatment mouse models of breast cancer, Gong et al. found that pulmonary injury from radiation-treatment induced CXCL12-CXCR4 overexpression, which resulted in increased number of metastatic nodules in the lungs.112 Recently, the expression of CXCR4 was reported to be higher in TNBC, which has a propensity for lung metastasis.113 Although how the CXCL12-CXCR4 axis induces lung metastasis remains unclear, some studies suggest that this may be due to increased macrophages and micro vessel density.114 In addition, many reports have demonstrated VEGF, estrogen, hypoxia and NF-κβ can upregulate CXCR4.115-117 Therefore, CXCR4 may serve as a key downstream effector or mediator for these cancer progression regulators.
Atypical chemokine
Atypical chemokine receptor proteins are predominantly expressed on non-leukocytic cell types and are unlikely to be directly involved in leukocyte migration. D6 and DARC are atypical receptors for most inflammatory CC chemokines including CCL2.118, 119 The overexpression of D6 or DARC in breast cancer was reported to downregulate CCL2 levels and to subsequently inhibit the proliferation and metastasis of breast cancer.119-122 However, there is limited research regarding atypical chemokines, and the invasive effects of D6 and DARC combined expression in breast cancer cells has not been demonstrated.
Microenvironment factors
It is well-established that the metastatic cascade is composed of numerous barriers that must be overcome in order for cancer cells to form distant metastasis. As discussed, when breast cancer cells spread from the primary tumor, they prefer to metastasize to specific tissues such as bone, lung, liver and brain. The communication between disseminated tumor cells (DTCs) and resident stromal cells in those colonized tissues is diverse. There are diverse components that create the microenvironment of tumors such as growth factors, immune cells, cytokines, chemokines, extracellular matrix (ECM), tumor-associated macrophages (TAMs), cancer-associated fibroblasts(CAFs) as well as other components that have not yet been confirmed.123,124 The metastatic microenvironment can be influenced by both organ-specific factors and the infiltration of different stromal cells.125
Extracellular matrix (ECM) proteins
ECM proteins like Tenascin-C (TNC), Periostin (POSTN) and Versican (VCAN) are important for the formation of metastasis and play a critical role during the earliest stage of breast cancer colonization of a metastatic site such as the lung.126 TNC, normally produced by fibroblasts, can also be expressed by BCSCs.127 This aberrant expression of TNC by BCSCs exerts a metastasis-initiating effect for niche formation for lung colonization.127,128 POSTN is also a stromal-derived factor capable of binding to Wnt ligands. It has been shown to promote cancer stem cell expansion in lung metastasis development.129 Similarly, the infiltrating bone marrow-derived CDllb+/Ly6Chigh myeloid cells secrete VCAN within metastatic niches in the lung to potentiate lung metastasis.130 In addition, ECM components may facilitate metastatic growth by providing a milieu for disseminated tumor cells to interact with other cells. Vascular cell adhesion molecule-1 (VCAM-1) is aberrantly expressed in breast cancer cells and binds to α4β1 integrin, which also interacts with fibronectin and is expressed in natural killer cells, monocytes, and other immune cells.131 It was shown that the pulmonary parenchyma containing collagen and elastin fibers acts as a preferable soil for the homing of VCAM-1-expressing breast cancer.132
Transforming growth factor β (TGF-β)
Numerous studies have demonstrated that abnormal expression of TGF-β promotes breast cancer progression by altering the microenvironment.133-136 Ye et al. used the 4T1 syngeneic mouse model to demonstrate that TGF-β participates in creating a lung pre-metastatic microenvironment by modulating certain inflammatory cytokines (S100A8/A9) and growth factors (VEGF, Angpt2).137 Park et al. found that IN-1130, a novel TGFβ−1 receptor kinase (ALK5) inhibitor, could suppress lung metastasis in the 4T1 breast cancer orthotopic xenograft mouse model.138 Another ALK-5 inhibitor, EW-7197, also blocked breast cancer metastasis to the lung.139 According to the results of these studies, we could presume that inhibition of TGF-β signaling alone or combined with immunotherapy may act as a promising therapy for breast cancer lung metastasis.
Immune cells
The immune system can both suppress tumor growth and facilitate tumor progression. In addition to their role in response to infection, leukocytes are also involved in cancer progression and metastasis. Studies have shown that subclinical changes in leukocyte composition at distant sites of the primary tumor can induce metastasis.140-142 Neutrophils, one type of leukocyte, could act as mediators of metastatic initiation.143-145 However, how neutrophils affect metastasis is poorly understood and remains controversial. A lung metastasis model of murine breast cancer demonstrated that some special neutrophils like CXCR2+ neutrophils are responsible for the pro-metastatic effect of mesenchymal stromal cells (MSCs).146 Recently Wculek et al. defined the role of neutrophils as mediators of metastatic initiation by modifying the pre-metastatic lung microenvironment in breast cancer mouse models,147,148 suggesting that immune cells regulate the formation of metastatic niches.
Poolard et al. demonstrated that TAMs are essential for the formation of lung metastasis in breast cancer.149,150 This is partially due to CCL18, secreted by TAMs, which induces EMT in breast cancer cells.151,152 Several clinical studies demonstrated that macrophage infiltration could increase metastatic potential and correlates with poor prognosis in cancer.153 Some pre-clinical studies found that pulmonary macrophages play an important role in initiation of lung metastasis.154-156 One study demonstrated that binding of TAMs to receptor VCAM-1 could provide a survival advantage to breast cancer cells in the lung microenvironment.153
CAFs likely also play a major role in breast cancer metastasis. A report showed that CAFs express Tiam1 and osteopontin in human breast cancer and regulate metastasis of breast cancer.157 Moreover, expression of platelet-derived growth factor receptor β (PDGFRβ), a CAFs associated protein is significantly associated with lung metastasis in breast cancer.158 CAFs could also regulate TGF-β ligands, thereby promoting primary tumor growth. CAFs may also regulate the accumulation of fibrosis, which is associated with distant lung metastasis in breast cancer.159 Takai et al. demonstrated that Pirfenidone (PFD), a TGF-β inhibitor, could inhibit the tumor-fibrosis and TGF-β signaling. Its combination with doxorubicin could inhibit tumor growth and lung metastasis in TNBC patients.160
In conclusion, diverse resident and infiltrating cell types, along with secreted growth factors, chemokines, cytokines and the deposition of ECM components in the metastatic microenvironment (Fig. 2), create a fertile soil for the formation of organ-specific niches. Targeting the microenvironment may be an effective strategy to improve the outcome of breast cancer metastasis.
Figure 2.
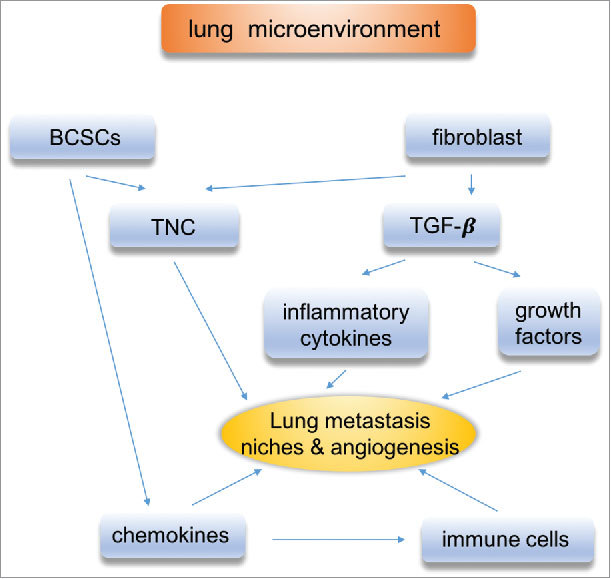
Lung microenvironment. The communication between disseminated cancer cells and resident stromal cells plays a critical role in lung metastasis of breast cancer. Microenvironment components are involved, such as TAMs, CAFs, TGF-β.
Therapeutic strategies
Patients' overall survival has improved dramatically secondary to early diagnosis and improved treatments in breast cancer. However, the 5-y overall survival rate of metastatic breast cancer is less than 30%.161 Despite available therapies for metastatic breast cancer, such as cytotoxic chemotherapies, endocrine therapies, and targeted therapies, survival rate is still low. This may be secondary to the lower response rate to systemic chemotherapy and stronger therapeutic resistance of metastatic breast cancer.162
Furthermore, we need more targeted treatments which build on the mechanism of metastatic breast cancer in addition to standard therapies. To date, cytotoxic chemotherapy is the only standard of care systemic treatment for TNBC. Given BCSCs are enriched in TNBC, targeting CSC-associated pathways may be an effective therapeutic approach. Inhibitors against Wnt and Hh signaling are under preclinical and clinical tests for TNBC treatment.163-165
Some genomics-based studies continue to shed light on the molecular understanding of TNBC tumorigenesis and heterogeneity and may provide implications for developing TNBC-targeted therapies.166-169 Of note, Bartholomeusz et al. found that the MEK inhibitor selumetinib inhibits and prevents lung metastasis of TNBC in xenograft models,170 suggesting that MAPK pathway could be a potential therapeutic target for preventing TNBC lung metastasis. Similarly, Cao et al. used succinobucol (SCB), a selective vascular cell adhesion molecule-1 (VCAM-1) inhibitor, to suppress lung metastasis in breast cancer.4 In addition, Citterio et al. reported that Rho GEFs could be a potential target for breast cancer lung metastasis therapy.171
Recently, immunotherapy has become a hotly pursued therapeutic option for breast cancer, especially metastatic breast cancer. Combining cancer vaccines with standard cancer treatments could increase therapeutic efficiency.172-174 To date, the most efficient immunotherapy relies on the infusion of antibodies that directly mediates anti-tumor effector activity, without directly impacting the patients' own immune response.175 A study using a breast cancer mouse model found that combining phosphatidylserine-targeting antibody with anti-PD-1 therapy could significantly enhance anti-tumor activity.176
Although the results from these animal in vivo studies could provide some basic information to clinical therapy, we still face daunting challenges in treating metastatic breast cancer. Can we use markers to screen for a higher likelihood of developing metastases? Can we screen for drug resistance? There are still many problems to be solved, and having a full understanding of the genetic, environmental, and immune pathways may lead to improved care.
Conclusions
In our review, we summarize the studies related to the biology of lung metastasis in breast cancer. Critical regulators for breast cancer dissemination to the lung include CSCs and related signaling pathways, chemokines, and microenvironmental cues. Our knowledge of breast cancer progression has grown exponentially in recent y. However, it is not well understood whether these regulators connect and cooperate with each other to control breast cancer metastasis or whether some play a more dominant role. In addition, there remains a daunting challenge to develop biomarkers to predict and prognosticate lung metastasis at initial diagnosis in patients with early stage disease. Some markers and mechanisms identified in cell and mouse models need to be validated in clinical studies. This may require matched primary breast cancer and lung metastasis samples, a key barrier in establishing the clinical relevance of research results from preclinical models. Undoubtedly, further understanding of the underlying mechanism for breast cancer migration to and colonization of distant sites will create the foundation to develop more effective therapies for metastatic breast cancer.
Disclosure of potential conflict of interests
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the National Institutes of Health (2R01CA151610) and the Avon Foundation for Women (02-2014-063) to Xiaojiang Cui, and the Fashion Footwear Charitable Foundation of New York, Inc., the Entertainment Industry Foundation, the Margie and Robert E. Petersen Foundation, and the Linda and Jim Lippman Research Fund to Armando Giuliano.
References
- 1.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64(1):52–62. doi: 10.3322/caac.21203. PMID:24114568. [DOI] [PubMed] [Google Scholar]
- 2.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–92. doi: 10.1016/j.cell.2011.09.024. PMID:22000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckhardt BL, Francis PA, Parker BS, Anderson RL. Strategies for the discovery and development of therapies for metastatic breast cancer. Nat Rev Drug Discov. 2012;11(6):479–97. doi: 10.1038/nrd2372. PMID:22653217. [DOI] [PubMed] [Google Scholar]
- 4.Cao H, Zhang Z, Zhao S, He X, Yu H, Yin Q, Zhang Z, Gu W, Chen L, Li Y. Hydrophobic interaction mediating self-assembled nanoparticles of succinobucol suppress lung metastasis of breast cancer by inhibition of VCAM-1 expression. J Control Release. 2015;205:162–71. doi: 10.1016/j.jconrel.2015.01.015. PMID:25598420. [DOI] [PubMed] [Google Scholar]
- 5.Pei S, Yang X, Wang H, Zhang H, Zhou B, Zhang D, Lin D. Plantamajoside, a potential anti-tumor herbal medicine inhibits breast cancer growth and pulmonary metastasis by decreasing the activity of matrix metalloproteinase-9 and -2. BMC Cancer. 2015;15:965. doi: 10.1186/s12885-015-1960-z. PMID:26674531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaffan J, Dacre J, Jones A. Educating undergraduate medical students about oncology: a literature review. J Clin Oncol. 2006;24(12):1932–9. doi: 10.1200/JCO.2005.02.6617. PMID:16622269. [DOI] [PubMed] [Google Scholar]
- 7.Gennari A, Conte P, Rosso R, Orlandini C, Bruzzi P. Survival of metastatic breast carcinoma patients over a 20-y period: a retrospective analysis based on individual patient data from six consecutive studies. Cancer. 2005;104(8):1742–50. doi: 10.1002/cncr.21359. PMID:16149088. [DOI] [PubMed] [Google Scholar]
- 8.Gligorov J, Lotz JP. Optimal treatment strategies in postmenopausal women with hormone-receptor-positive and HER2-negative metastatic breast cancer. Breast Cancer Res Treat. 2008;112 Suppl 1:53–66. doi: 10.1007/s10549-008-0232-x. PMID:19101794. [DOI] [PubMed] [Google Scholar]
- 9.Smid M, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, Foekens JA, Martens JW. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68(9):3108–14. doi: 10.1158/0008-5472.CAN-07-5644. PMID:18451135. [DOI] [PubMed] [Google Scholar]
- 10.Dan Z, Cao H, He X, Zhang Z, Zou L, Zeng L, Xu Y, Yin Q, Xu M, Zhong D, et al.. A pH-responsive host-guest nanosystem loading succinobucol suppresses lung metastasis of breast cancer. Theranostics. 2016;6(3):435–45. doi: 10.7150/thno.13896. PMID:26909117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang Y, Xu X, Wang T, Li Y, You W, Fu J, Liu Y, Jin S, Ji Q, Zhao W, et al.. The EGFR/miR-338-3p/EYA2 axis controls breast tumor growth and lung metastasis. Cell Death Dis. 2017;8(7):e2928. doi: 10.1038/cddis.2017.325. PMID:28703807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen TW, Ray T, Wang J, Li X, Naritoku WY, Han B, Bellafiore F, Bagaria SP, Qu A, Cui X, et al.. Diagnosis of Basal-Like Breast Cancer Using a FOXC1-Based Assay. J Natl Cancer Inst. 2015;107(8). doi: 10.1093/jnci/djv148.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ray PS, Wang J, Qu Y, Sim MS, Shamonki J, Bagaria SP, Ye X, Liu B, Elashoff D, Hoon DS, et al.. FOXC1 is a potential prognostic biomarker with functional significance in basal-like breast cancer. Cancer Res. 2010;70(10):3870–6. doi: 10.1158/0008-5472.CAN-09-4120. PMID:20406990. [DOI] [PubMed] [Google Scholar]
- 14.Cardoso F, Costa A, Norton L, Cameron D, Cufer T, Fallowfield L, Francis P, Gligorov J, Kyriakides S, Lin N, et al.. 1st International consensus guidelines for advanced breast cancer (ABC 1). Breast. 2012;21(3):242–52. doi: 10.1016/j.breast.2012.03.003. PMID:22425534. [DOI] [PubMed] [Google Scholar]
- 15.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–95. doi: 10.1016/j.cell.2006.11.001. PMID:17110329. [DOI] [PubMed] [Google Scholar]
- 16.Early Breast Cancer Trialists' Collaborative G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-y survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–717. doi: 10.1016/S0140-6736(05)66544-0. PMID:15894097. [DOI] [PubMed] [Google Scholar]
- 17.Soni A, Ren Z, Hameed O, Chanda D, Morgan CJ, Siegal GP, Wei S. Breast cancer subtypes predispose the site of distant metastases. Am J Clin Pathol. 2015;143(4):471–8. doi: 10.1309/AJCPYO5FSV3UPEXS. PMID:25779997. [DOI] [PubMed] [Google Scholar]
- 18.Largillier R, Ferrero JM, Doyen J, Barriere J, Namer M, Mari V, Courdi A, Hannoun-Levi JM, Ettore F, Birtwisle-Peyrottes I, et al.. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. 2008;19(12):2012–9. doi: 10.1093/annonc/mdn424. PMID:18641006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang J, Clark GM, Allred DC, Mohsin S, Chamness G, Elledge RM. Survival of patients with metastatic breast carcinoma: importance of prognostic markers of the primary tumor. Cancer. 2003;97(3):545–53. doi: 10.1002/cncr.11083. PMID:12548595. [DOI] [PubMed] [Google Scholar]
- 20.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28(20):3271–7. doi: 10.1200/JCO.2009.25.9820. PMID:20498394. [DOI] [PubMed] [Google Scholar]
- 21.Bartmann C, Wischnewsky M, Stüber T, Stein R, Krockenberger M, Häusler S, Janni W, Kreienberg R, Blettner M, Schwentner L, et al.. Pattern of metastatic spread and subcategories of breast cancer. Arch Gynecol Obstet. 2017;295(1):211–23. doi: 10.1007/s00404-016-4225-4. PMID:27832352. [DOI] [PubMed] [Google Scholar]
- 22.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–48. doi: 10.1056/NEJMra1001389. PMID:21067385. [DOI] [PubMed] [Google Scholar]
- 23.Yhim HY, Han SW, Oh DY, Han W, Im SA, Kim TY, Kim YT, Noh DY, Chie EK, Ha SW, et al.. Prognostic factors for recurrent breast cancer patients with an isolated, limited number of lung metastases and implications for pulmonary metastasectomy. Cancer. 2010;116(12):2890–901. doi: 10.1002/cncr.25054. PMID:20564396. [DOI] [PubMed] [Google Scholar]
- 24.Gerratana L, Fanotto V, Bonotto M, Bolzonello S, Minisini AM, Fasola G, Puglisi F. Pattern of metastasis and outcome in patients with breast cancer. Clin Exp Metastasis. 2015;32(2):125–33. doi: 10.1007/s10585-015-9697-2. PMID:25630269. [DOI] [PubMed] [Google Scholar]
- 25.Wu Q, Li J, Zhu S, Wu J, Chen C, Liu Q, Wei W, Zhang Y, Sun S. Breast cancer subtypes predict the preferential site of distant metastases: a SEER based study. Oncotarget. 2017. 8(17):27990–6. doi: 10.18632/oncotarget.15856. PMID:28427196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–8. doi: 10.1073/pnas.0530291100. PMID:12629218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R, et al.. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15(3):504–14. doi: 10.1038/sj.cdd.4402283. PMID:18049477. [DOI] [PubMed] [Google Scholar]
- 28.Czerwinska P, Kaminska B. Regulation of breast cancer stem cell features. Contemp Oncol (Pozn). 2015;19(1A):A7–A15. PMID:25691826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morel AP, Lièvre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3(8):e2888. doi: 10.1371/journal.pone.0002888. PMID:18682804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotiyal S, Bhattacharya S. Breast cancer stem cells, EMT and therapeutic targets. Biochem Biophys Res Commun. 2014;453(1):112–6. doi: 10.1016/j.bbrc.2014.09.069. PMID:25261721. [DOI] [PubMed] [Google Scholar]
- 31.Langerod A, Zhao H, Ø Borgan, Nesland JM, Bukholm IR, Ikdahl T, Kåresen R, Børresen-Dale AL, Jeffrey SS. TP53 mutation status and gene expression profiles are powerful prognostic markers of breast cancer. Breast Cancer Res. 2007;9(3):R30. doi: 10.1186/bcr1675. PMID:17504517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitt F, Ricardo S, Vieira AF, Dionísio MR, Paredes J. Cancer stem cell markers in breast neoplasias: their relevance and distribution in distinct molecular subtypes. Virchows Arch. 2012;460(6):545–53. doi: 10.1007/s00428-012-1237-8. PMID:22562130. [DOI] [PubMed] [Google Scholar]
- 33.Chekhun SV, Zadvorny TV, Tymovska YO, Anikusko MF, Novak OE, Polishchuk LZ. capital ES, CyrillicD44+/CD24- markers of cancer stem cells in patients with breast cancer of different molecular subtypes. Exp Oncol. 2015;37(1):58–63. PMID:25804234. [PubMed] [Google Scholar]
- 34.Honeth G, Bendahl PO, Ringnér M, Saal LH, Gruvberger-Saal SK, Lövgren K, Grabau D, Fernö M, Borg A, Hegardt C. The CD44+/CD24- phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008. 10(3):R53. doi: 10.1186/bcr2108. PMID:18559090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu H, Patel MR, Prescher JA, Patsialou A, Qian D, Lin J, Wen S, Chang YF, Bachmann MH, Shimono Y, et al.. Cancer stem cells from human breast tumors are involved in spontaneous metastases in orthotopic mouse models. Proc Natl Acad Sci U S A. 2010;107(42):18115–20. doi: 10.1073/pnas.1006732107. PMID:20921380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Croker AK, Goodale D, Chu J, Postenka C, Hedley BD, Hess DA, Allan AL. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J Cell Mol Med. 2009;13(8B):2236–52. doi: 10.1111/j.1582-4934.2008.00455.x. PMID:18681906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yae T, Tsuchihashi K, Ishimoto T, Motohara T, Yoshikawa M, Yoshida GJ, Wada T, Masuko T, Mogushi K, Tanaka H, et al.. Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. Nat Commun. 2012;3:883. doi: 10.1038/ncomms1892. PMID:22673910. [DOI] [PubMed] [Google Scholar]
- 38.Hu J, Li G, Zhang P, Zhuang X, Hu G. A CD44v+ subpopulation of breast cancer stem-like cells with enhanced lung metastasis capacity. Cell Death Dis. 2017;8(3):e2679. doi: 10.1038/cddis.2017.72. PMID:28300837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17(5):548–58. doi: 10.1016/j.ceb.2005.08.001. PMID:16098727. [DOI] [PubMed] [Google Scholar]
- 40.Nigam A. Breast cancer stem cells, pathways and therapeutic perspectives 2011. Indian J Surg. 2013;75(3):170–80. doi: 10.1007/s12262-012-0616-3. PMID:24426422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barolo S, Posakony JW. Three habits of highly effective signaling pathways: principles of transcriptional control by developmental cell signaling. Genes Dev. 2002;16(10):1167–81. doi: 10.1101/gad.976502. PMID:12023297. [DOI] [PubMed] [Google Scholar]
- 42.Garcia A, Kandel JJ. Notch: a key regulator of tumor angiogenesis and metastasis. Histol Histopathol. 2012;27(2):151–6. PMID:22207549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrison H, Farnie G, Howell SJ, Rock RE, Stylianou S, Brennan KR, Bundred NJ, Clarke RB. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 2010;70(2):709–18. doi: 10.1158/0008-5472.CAN-09-1681. PMID:20068161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6(6):R605–15. doi: 10.1186/bcr920. PMID:15535842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sansone P, Storci G, Giovannini C, Pandolfi S, Pianetti S, Taffurelli M, Santini D, Ceccarelli C, Chieco P, Bonafé M. p66Shc/Notch-3 interplay controls self-renewal and hypoxia survival in human stem/progenitor cells of the mammary gland expanded in vitro as mammospheres. Stem Cells. 2007;25(3):807–15. doi: 10.1634/stemcells.2006-0442. PMID:17158237. [DOI] [PubMed] [Google Scholar]
- 46.Pal D, Kolluru V, Chandrasekaran B, Baby BV, Aman M, Suman S, Sirimulla S, Sanders MA, Alatassi H, Ankem MK, et al. Targeting aberrant expression of Notch-1 in ALDH+ cancer stem cells in breast cancer. Mol Carcinog. 2017;56(3):1127–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suman S, Das TP, Damodaran C. Silencing NOTCH signaling causes growth arrest in both breast cancer stem cells and breast cancer cells. Br J Cancer. 2013;109(10):2587–96. doi: 10.1038/bjc.2013.642. PMID:24129237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGovern M, Voutev R, Maciejowski J, Corsi AK, Hubbard EJ. A “latent niche” mechanism for tumor initiation. Proc Natl Acad Sci U S A. 2009;106(28):11617–22. doi: 10.1073/pnas.0903768106. PMID:19564624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen W, Cao G, Yuan X, Zhang X, Zhang Q, Zhu Y, Dong Z, Zhang S. Notch-1 knockdown suppresses proliferation, migration and metastasis of salivary adenoid cystic carcinoma cells. J Transl Med. 2015;13:167. doi: 10.1186/s12967-015-0520-2. PMID:25990317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17(1):45–51. doi: 10.1016/j.gde.2006.12.007. PMID:17208432. [DOI] [PubMed] [Google Scholar]
- 51.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. PMID:19619488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khramtsov AI, Khramtsova GF, Tretiakova M, Huo D, Olopade OI, Goss KH. Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am J Pathol. 2010;176(6):2911–20. doi: 10.2353/ajpath.2010.091125. PMID:20395444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lopez-Knowles E, Zardawi SJ, McNeil CM, Millar EK, Crea P, Musgrove EA, Sutherland RL, O'Toole SA, et al.. Cytoplasmic localization of beta-catenin is a marker of poor outcome in breast cancer patients. Cancer Epidemiol Biomarkers Prev. 2010;19(1):301–9. doi: 10.1158/1055-9965.EPI-09-0741. PMID:20056651. [DOI] [PubMed] [Google Scholar]
- 54.Geyer FC, et al.. beta-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Mod Pathol. 2011;24(2):209–31. doi: 10.1038/modpathol.2010.205. PMID:21076461. [DOI] [PubMed] [Google Scholar]
- 55.Lindvall C, Zylstra CR, Evans N, West RA, Dykema K, Furge KA, Williams BO. The Wnt co-receptor Lrp6 is required for normal mouse mammary gland development. PLoS One. 2009;4(6):e5813. doi: 10.1371/journal.pone.0005813. PMID:19503830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu CC, Prior J, Piwnica-Worms D, Bu G. LRP6 overexpression defines a class of breast cancer subtype and is a target for therapy. Proc Natl Acad Sci U S A. 2010;107(11):5136–41. doi: 10.1073/pnas.0911220107. PMID:20194742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18(5):523–7. doi: 10.1038/cr.2008.47. PMID:18392048. [DOI] [PubMed] [Google Scholar]
- 58.Pires BR, Amorim ÍS DE, Souza LD, Rodrigues JA, Mencalha AL. Targeting cellular signaling pathways in breast cancer stem cells and its implication for cancer treatment. Anticancer Res. 2016. 36(11):5681–91. doi: 10.21873/anticanres.11151. PMID:27793889. [DOI] [PubMed] [Google Scholar]
- 59.Dey N, Barwick BG, Moreno CS, Ordanic-Kodani M, Chen Z, Oprea-Ilies G, Tang W, Catzavelos C, Kerstann KF, Sledge GW Jr, et al.. Wnt signaling in triple negative breast cancer is associated with metastasis. BMC Cancer. 2013;13:537. doi: 10.1186/1471-2407-13-537. PMID:24209998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DiMeo TA, Anderson K, Phadke P, Fan C, Perou CM, Naber S, Kuperwasser C. A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancer. Cancer Res. 2009;69(13):5364–73. doi: 10.1158/0008-5472.CAN-08-4135. PMID:19549913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klauzinska M, Baljinnyam B, Raafat A, Rodriguez-Canales J, Strizzi L, Greer YE, Rubin JS, Callahan R. Rspo2/Int7 regulates invasiveness and tumorigenic properties of mammary epithelial cells. J Cell Physiol. 2012;227(5):1960–71. doi: 10.1002/jcp.22924. PMID:21732367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou XL, Qin XR, Zhang XD, Ye LH. Downregulation of Dickkopf-1 is responsible for high proliferation of breast cancer cells via losing control of Wnt/beta-catenin signaling. Acta Pharmacol Sin. 2010;31(2):202–10. doi: 10.1038/aps.2009.200. PMID:20139903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brennan KR, Brown AM. Wnt proteins in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2004;9(2):119–31. doi: 10.1023/B:JOMG.0000037157.94207.33. PMID:15300008. [DOI] [PubMed] [Google Scholar]
- 64.Zhang K, Zhang J, Han L, Pu P, Kang C. Wnt/beta-catenin signaling in glioma. J Neuroimmune Pharmacol. 2012;7(4):740–9. doi: 10.1007/s11481-012-9359-y. PMID:22454041. [DOI] [PubMed] [Google Scholar]
- 65.Zhuang X, Zhang H, Li X, Li X, Cong M, Peng F, Yu J, Zhang X, Yang Q, Hu G. Differential effects on lung and bone metastasis of breast cancer by Wnt signalling inhibitor DKK1. Nat Cell Biol. 2017;19(10):1274–85. doi: 10.1038/ncb3613. PMID:28892080. [DOI] [PubMed] [Google Scholar]
- 66.Benhaj K, Akcali KC, Ozturk M. Redundant expression of canonical Wnt ligands in human breast cancer cell lines. Oncol Rep. 2006;15(3):701–7. PMID:16465433. [PubMed] [Google Scholar]
- 67.Leris AC, Roberts TR, Jiang WG, Newbold RF, Mokbel K. WNT5A expression in human breast cancer. Anticancer Res. 2005;25(2A):731–4. PMID:15868903. [PubMed] [Google Scholar]
- 68.Jiang W, Crossman DK, Mitchell EH, Sohn P, Crowley MR, Serra R. WNT5A inhibits metastasis and alters splicing of Cd44 in breast cancer cells. PLoS One. 2013;8(3):e58329. doi: 10.1371/journal.pone.0058329. PMID:23484019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trifa F, Karray-Chouayekh S, Jmal E, Jmaa ZB, Khabir A, Sellami-Boudawara T, Frikha M, Daoud J, Mokdad-Gargouri R. Loss of WIF-1 and Wnt5a expression is related to aggressiveness of sporadic breast cancer in Tunisian patients. Tumour Biol. 2013;34(3):1625–33. doi: 10.1007/s13277-013-0694-2. PMID:23417837. [DOI] [PubMed] [Google Scholar]
- 70.Zhong Z, Shan M, Wang J, Liu T, Shi Q, Pang D. Decreased Wnt5a expression is a poor prognostic factor in triple-negative breast cancer. Med Sci Monit. 2016;22:1–7. doi: 10.12659/MSM.894821. PMID:26721633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Borcherding N, Kusner D, Kolb R, Xie Q, Li W, Yuan F, Velez G, Askeland R, Weigel RJ, Zhang W. Paracrine WNT5A signaling inhibits expansion of tumor-initiating cells. Cancer Res. 2015;75(10):1972–82. doi: 10.1158/0008-5472.CAN-14-2761. PMID:25769722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jonsson M, Andersson T. Repression of Wnt-5a impairs DDR1 phosphorylation and modifies adhesion and migration of mammary cells. J Cell Sci. 2001;114(Pt 11):2043–53. PMID:11493640. [DOI] [PubMed] [Google Scholar]
- 73.Medrek C, Landberg G, Andersson T, Leandersson K. Wnt-5a-CKI{alpha} signaling promotes {beta}-catenin/E-cadherin complex formation and intercellular adhesion in human breast epithelial cells. J Biol Chem. 2009;284(16):10968–79. doi: 10.1074/jbc.M804923200. PMID:19244247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jonsson M, Dejmek J, Bendahl PO, Andersson T. Loss of Wnt-5a protein is associated with early relapse in invasive ductal breast carcinomas. Cancer Res. 2002;62(2):409–16. PMID:11809689. [PubMed] [Google Scholar]
- 75.Shi J, Wang Y, Zeng L, Wu Y, Deng J, Zhang Q, Lin Y, Li J, Kang T, Tao M, et al.. Disrupting the interaction of BRD4 with diacetylated Twist suppresses tumorigenesis in basal-like breast cancer. Cancer Cell. 2014;25(2):210–25. doi: 10.1016/j.ccr.2014.01.028. PMID:24525235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu Y, Shen T, Liu J, Zheng J, Zhang Y, Xu R, Sun C, Du J, Chen Y, Gu L. Rab35 is required for Wnt5a/Dvl2-induced Rac1 activation and cell migration in MCF-7 breast cancer cells. Cell Signal. 2013;25(5):1075–85. doi: 10.1016/j.cellsig.2013.01.015. PMID:23353182. [DOI] [PubMed] [Google Scholar]
- 77.Han B, et al.. FOXC1-induced non-canonical WNT5A-MMP7 signaling regulates invasiveness in triple-negative breast cancer. Oncogene. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Flemban A, Qualtrough D. The potential role of hedgehog signaling in the Luminal/Basal Phenotype of breast epithelia and in breast cancer invasion and metastasis. Cancers (Basel). 2015;7(3):1863–84. doi: 10.3390/cancers7030866. PMID:26389956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao C, Chen A, Jamieson CH, Fereshteh M, Abrahamsson A, Blum J, Kwon HY, Kim J, Chute JP, Rizzieri D, et al.. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458(7239):776–9. doi: 10.1038/nature07737. PMID:19169242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Santini R, Vinci MC, Pandolfi S, Penachioni JY, Montagnani V, Olivito B, Gattai R, Pimpinelli N, Gerlini G, Borgognoni L, et al.. Hedgehog-GLI signaling drives self-renewal and tumorigenicity of human melanoma-initiating cells. Stem Cells. 2012;30(9):1808–18. doi: 10.1002/stem.1160. PMID:22730244. [DOI] [PubMed] [Google Scholar]
- 81.Varnat F, Duquet A, Malerba M, Zbinden M, Mas C, Gervaz P, Ruiz i Altaba A. Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Mol Med. 2009;1(6–7):338–51. doi: 10.1002/emmm.200900039. PMID:20049737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hui M, Cazet A, Nair R, Watkins DN, O'Toole SA, Swarbrick A. The Hedgehog signalling pathway in breast development, carcinogenesis and cancer therapy. Breast Cancer Res. 2013;15(2):203. doi: 10.1186/bcr3401. PMID:23547970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mukherjee S, Frolova N, Sadlonova A, Novak Z, Steg A, Page GP, Welch DR, Lobo-Ruppert SM, Ruppert JM, Johnson MR, et al.. Hedgehog signaling and response to cyclopamine differ in epithelial and stromal cells in benign breast and breast cancer. Cancer Biol Ther. 2006;5(6):674–83. doi: 10.4161/cbt.5.6.2906. PMID:16855373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thomas ZI, Gibson W, Sexton JZ, Aird KM, Ingram SM, Aldrich A, Lyerly HK, Devi GR, Williams KP. Targeting GLI1 expression in human inflammatory breast cancer cells enhances apoptosis and attenuates migration. Br J Cancer. 2011;104(10):1575–86. doi: 10.1038/bjc.2011.133. PMID:21505458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O'Toole SA, Machalek DA, Shearer RF, Millar EK, Nair R, Schofield P, McLeod D, Cooper CL, McNeil CM, McFarland A, et al.. Hedgehog overexpression is associated with stromal interactions and predicts for poor outcome in breast cancer. Cancer Res. 2011;71(11):4002–14. doi: 10.1158/0008-5472.CAN-10-3738. PMID:21632555. [DOI] [PubMed] [Google Scholar]
- 86.Inaguma S, Riku M, Ito H, Tsunoda T, Ikeda H, Kasai K. GLI1 orchestrates CXCR4/CXCR7 signaling to enhance migration and metastasis of breast cancer cells. Oncotarget. 2015;6(32):33648–57. doi: 10.18632/oncotarget.5203. PMID:26413813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hayashi H, Kume T. Forkhead transcription factors regulate expression of the chemokine receptor CXCR4 in endothelial cells and CXCL12-induced cell migration. Biochem Biophys Res Commun. 2008;367(3):584–9. doi: 10.1016/j.bbrc.2007.12.183. PMID:18187037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Han B, Qu Y, Jin Y, Yu Y, Deng N, Wawrowsky K, Zhang X, Li N, Bose S, Wang Q, et al.. FOXC1 activates smoothened-independent hedgehog signaling in basal-like breast cancer. Cell Rep. 2015;13(5):1046–58. doi: 10.1016/j.celrep.2015.09.063. PMID:26565916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zuo HD, Wu Yao W. The role and the potential regulatory pathways of high expression of forkhead box C1 in promoting tumor growth and metastasis of basal-like breast cancer. J BUON. 2016;21(4):818–25. PMID:27685901. [PubMed] [Google Scholar]
- 90.Liu J, Chen S, Wang W, Ning BF, Chen F, Shen W, Ding J, Chen W, Xie WF, Zhang X. Cancer-associated fibroblasts promote hepatocellular carcinoma metastasis through chemokine-activated hedgehog and TGF-beta pathways. Cancer Lett. 2016;379(1):49–59. doi: 10.1016/j.canlet.2016.05.022. PMID:27216982. [DOI] [PubMed] [Google Scholar]
- 91.Zardawi SJ, O'Toole SA, Sutherland RL, Musgrove EA. Dysregulation of Hedgehog, Wnt and Notch signalling pathways in breast cancer. Histol Histopathol. 2009;24(3):385–98. PMID:19130408. [DOI] [PubMed] [Google Scholar]
- 92.Salem ML, El-Badawy AS, Li Z. Immunobiology and signaling pathways of cancer stem cells: implication for cancer therapy. Cytotechnology. 2015;67(5):749–59. doi: 10.1007/s10616-014-9830-0. PMID:25516358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arnold KM, Pohlig RT, Sims-Mourtada J. Co-activation of Hedgehog and Wnt signaling pathways is associated with poor outcomes in triple negative breast cancer. Oncol Lett. 2017;14(5):5285–92. PMID:29142600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Okuhashi Y, Itoh M, Tohda S. Hedgehog stimulation suppresses clonogenicity and activates NOTCH signalling in T-lymphoblastic Leukaemia Jurkat cells. Anticancer Res. 2017;37(9):5005–9. PMID:28870926. [DOI] [PubMed] [Google Scholar]
- 95.Maeda O, Kondo M, Fujita T, Usami N, Fukui T, Shimokata K, Ando T, Goto H, Sekido Y. Enhancement of GLI1-transcriptional activity by beta-catenin in human cancer cells. Oncol Rep. 2006;16(1):91–6. PMID:16786128. [PubMed] [Google Scholar]
- 96.Kikuchi I, Takahashi-Kanemitsu A, Sakiyama N, Tang C, Tang PJ, Noda S, Nakao K, Kassai H, Sato T, Aiba A, et al.. Dephosphorylated parafibromin is a transcriptional coactivator of the Wnt/Hedgehog/Notch pathways. Nat Commun. 2016;7:12887. doi: 10.1038/ncomms12887. PMID:27650679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. doi: 10.1146/annurev.immunol.18.1.217. PMID:10837058. [DOI] [PubMed] [Google Scholar]
- 98.Murphy PM, Baggiolini M, Charo IF, Hébert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52(1):145–76. PMID:10699158. [PubMed] [Google Scholar]
- 99.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12(2):121–7. doi: 10.1016/S1074-7613(00)80165-X. PMID:10714678. [DOI] [PubMed] [Google Scholar]
- 100.Locati M, Otero K, Schioppa T, Signorelli P, Perrier P, Baviera S, Sozzani S, Mantovani A. The chemokine system: tuning and shaping by regulation of receptor expression and coupling in polarized responses. Allergy. 2002. 57(11):972–82. doi: 10.1034/j.1398-9995.2002.02166.x. PMID:12358993. [DOI] [PubMed] [Google Scholar]
- 101.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. PMID:15032599. [DOI] [PubMed] [Google Scholar]
- 102.Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. PMID:17291188. [DOI] [PubMed] [Google Scholar]
- 103.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0. PMID:11229684. [DOI] [PubMed] [Google Scholar]
- 104.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540–50. doi: 10.1038/nrc1388. PMID:15229479. [DOI] [PubMed] [Google Scholar]
- 105.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29(6):313–26. doi: 10.1089/jir.2008.0027. PMID:19441883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maru Y. The lung metastatic niche. J Mol Med (Berl). 2015;93(11):1185–92. doi: 10.1007/s00109-015-1355-2. PMID:26489606. [DOI] [PubMed] [Google Scholar]
- 107.Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-Todorova K, Leversha M, Hogg N, Seshan VE, et al.. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150(1):165–78. doi: 10.1016/j.cell.2012.04.042. PMID:22770218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al.. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–6. doi: 10.1038/35065016. PMID:11242036. [DOI] [PubMed] [Google Scholar]
- 109.Mukherjee D, Zhao J. The Role of chemokine receptor CXCR4 in breast cancer metastasis. Am J Cancer Res. 2013;3(1):46–57. PMID:23359227. [PMC free article] [PubMed] [Google Scholar]
- 110.Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23(7):879–94. doi: 10.1634/stemcells.2004-0342. PMID:15888687. [DOI] [PubMed] [Google Scholar]
- 111.Atretkhany KN, et al.. Chemokines, cytokines and exosomes help tumors to shape inflammatory microenvironment. Pharmacol Ther. 2016;168:98–112. doi: 10.1016/j.pharmthera.2016.09.011. PMID:27613100. [DOI] [PubMed] [Google Scholar]
- 112.Gong HY, Hu WG, Hu QY, Li XP, Song QB. Radiation-induced pulmonary injury accelerated pulmonary metastasis in a mouse model of breast cancer. Oncol Lett. 2015. 10(6):3613–8. doi: 10.3892/ol.2015.3810. PMID:26788178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen HW, Du CW, Wei XL, Khoo US, Zhang GJ. Cytoplasmic CXCR4 high-expression exhibits distinct poor clinicopathological characteristics and predicts poor prognosis in triple-negative breast cancer. Curr Mol Med. 2013;13(3):410–6. PMID:23331013. [PubMed] [Google Scholar]
- 114.Boimel PJ, Smirnova T, Zhou ZN, Wyckoff J, Park H, Coniglio SJ, Qian BZ, Stanley ER, Cox D, Pollard JW, et al.. Contribution of CXCL12 secretion to invasion of breast cancer cells. Breast Cancer Res. 2012;14(1):R23. doi: 10.1186/bcr3108. PMID:22314082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bachelder RE, Wendt MA, Mercurio AM. Vascular endothelial growth factor promotes breast carcinoma invasion in an autocrine manner by regulating the chemokine receptor CXCR4. Cancer Res. 2002;62(24):7203–6. PMID:12499259. [PubMed] [Google Scholar]
- 116.Hall JM, Korach KS. Stromal cell-derived factor 1, a novel target of estrogen receptor action, mediates the mitogenic effects of estradiol in ovarian and breast cancer cells. Mol Endocrinol. 2003;17(5):792–803. doi: 10.1210/me.2002-0438. PMID:12586845. [DOI] [PubMed] [Google Scholar]
- 117.Helbig G, Christopherson KW 2nd, Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE, Nakshatri H. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem. 2003;278(24):21631–8. doi: 10.1074/jbc.M300609200. PMID:12690099. [DOI] [PubMed] [Google Scholar]
- 118.Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol. 2006;6(12):907–18. doi: 10.1038/nri1964. PMID:17124512. [DOI] [PubMed] [Google Scholar]
- 119.Wang J, Ou ZL, Hou YF, Luo JM, Chen Y, Zhou J, Shen ZZ, Ding J, Shao ZM. Duffy antigen receptor for chemokines attenuates breast cancer growth and metastasis: an experiment with nude mice. Zhonghua Yi Xue Za Zhi. 2005;85(29):2033–7. PMID:16313795. [PubMed] [Google Scholar]
- 120.Galzi JL, Hachet-Haas M, Bonnet D, Daubeuf F, Lecat S, Hibert M, Haiech J, Frossard N. Neutralizing endogenous chemokines with small molecules. Principles and potential therapeutic applications. Pharmacol Ther. 2010;126(1):39–55. doi: 10.1016/j.pharmthera.2009.12.003. PMID:20117133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu FY, Ou ZL, Feng LY, Luo JM, Wang LP, Shen ZZ, Shao ZM. Chemokine decoy receptor d6 plays a negative role in human breast cancer. Mol Cancer Res. 2008;6(8):1276–88. doi: 10.1158/1541-7786.MCR-07-2108. PMID:18708360. [DOI] [PubMed] [Google Scholar]
- 122.Mantovani A, Savino B, Locati M, Zammataro L, Allavena P, Bonecchi R. The chemokine system in cancer biology and therapy. Cytokine Growth Factor Rev. 2010;21(1):27–39. doi: 10.1016/j.cytogfr.2009.11.007. PMID:20004131. [DOI] [PubMed] [Google Scholar]
- 123.Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell. 2010;18(6):884–901. doi: 10.1016/j.devcel.2010.05.012. PMID:20627072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Polyak K, Kalluri R. The role of the microenvironment in mammary gland development and cancer. Cold Spring Harb Perspect Biol. 2010;2(11):a003244. doi: 10.1101/cshperspect.a003244. PMID:20591988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ursini-Siegel J, Siegel PM. The influence of the pre-metastatic niche on breast cancer metastasis. Cancer Lett. 2016;380(1):281–8. [DOI] [PubMed] [Google Scholar]
- 126.Soikkeli J, Podlasz P, Yin M, Nummela P, Jahkola T, Virolainen S, Krogerus L, Heikkilä P, von Smitten K, Saksela O, et al.. Metastatic outgrowth encompasses COL-I, FN1, and POSTN up-regulation and assembly to fibrillar networks regulating cell adhesion, migration, and growth. Am J Pathol. 2010;177(1):387–403. doi: 10.2353/ajpath.2010.090748. PMID:20489157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Oskarsson T, Acharyya S, Zhang XH, Vanharanta S, Tavazoie SF, Morris PG, Downey RJ, Manova-Todorova K, Brogi E, Massagué J. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med. 2011;17(7):867–74. doi: 10.1038/nm.2379. PMID:21706029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.O'Connell JT, Sugimoto H, Cooke VG, MacDonald BA, Mehta AI, LeBleu VS, Dewar R, Rocha RM, Brentani RR, Resnick MB, et al.. VEGF-A and Tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc Natl Acad Sci U S A. 2011;108(38):16002–7. doi: 10.1073/pnas.1109493108. PMID:21911392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Malanchi I, Santamaria-Martine A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481(7379):85–9. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 130.Gao D, Joshi N, Choi H, Ryu S, Hahn M, Catena R, Sadik H, Argani P, Wagner P, Vahdat LT, et al.. Myeloid progenitor cells in the premetastatic lung promote metastases by inducing mesenchymal to epithelial transition. Cancer Res. 2012;72(6):1384–94. doi: 10.1158/0008-5472.CAN-11-2905. PMID:22282653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sharma R, Sharma R, Khaket TP, Dutta C, Chakraborty B, Mukherjee TK. Breast cancer metastasis: Putative therapeutic role of vascular cell adhesion molecule-1. Cell Oncol (Dordr). 2017;40(3):199–208. doi: 10.1007/s13402-017-0324-x. PMID:28534212. [DOI] [PubMed] [Google Scholar]
- 132.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massagué J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436(7050):518–24. doi: 10.1038/nature03799. PMID:16049480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, Massagué J. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133(1):66–77. doi: 10.1016/j.cell.2008.01.046. PMID:18394990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hembruff SL, Jokar I, Yang L, Cheng N. Loss of transforming growth factor-beta signaling in mammary fibroblasts enhances CCL2 secretion to promote mammary tumor progression through macrophage-dependent and -independent mechanisms. Neoplasia. 2010;12(5):425–33. doi: 10.1593/neo.10200. PMID:20454514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Drabsch Y, ten Dijke P. TGF-beta signaling in breast cancer cell invasion and bone metastasis. J Mammary Gland Biol Neoplasia. 2011;16(2):97–108. doi: 10.1007/s10911-011-9217-1. PMID:21494783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fazilaty H, Gardaneh M, Bahrami T, Salmaninejad A, Behnam B. Crosstalk between breast cancer stem cells and metastatic niche: emerging molecular metastasis pathway? Tumour Biol. 2013;34(4):2019–30. doi: 10.1007/s13277-013-0831-y. PMID:23686802. [DOI] [PubMed] [Google Scholar]
- 137.Ye Y, Liu S, Wu C, Sun Z. TGFbeta modulates inflammatory cytokines and growth factors to create premetastatic microenvironment and stimulate lung metastasis. J Mol Histol. 2015;46(4–5):365–75. doi: 10.1007/s10735-015-9633-4. PMID:26208571. [DOI] [PubMed] [Google Scholar]
- 138.Park CY, Min KN, Son JY, Park SY, Nam JS, Kim DK, Sheen YY. An novel inhibitor of TGF-beta type I receptor, IN-1130, blocks breast cancer lung metastasis through inhibition of epithelial-mesenchymal transition. Cancer Lett. 2014;351(1):72–80. doi: 10.1016/j.canlet.2014.05.006. PMID:24887560. [DOI] [PubMed] [Google Scholar]
- 139.Son JY, Park SY, Kim SJ, Lee SJ, Park SA, Kim MJ, Kim SW, Kim DK, Nam JS, Sheen YY. EW-7197, a novel ALK-5 kinase inhibitor, potently inhibits breast to lung metastasis. Mol Cancer Ther. 2014;13(7):1704–16. doi: 10.1158/1535-7163.MCT-13-0903. PMID:24817629. [DOI] [PubMed] [Google Scholar]
- 140.Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A, Geng Y, Gray JW, Moses HL, Yang L. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70(15):6139–49. doi: 10.1158/0008-5472.CAN-10-0706. PMID:20631080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pekarek LA, Starr BA, Toledano AY, Schreiber H. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995;181(1):435–40. doi: 10.1084/jem.181.1.435. PMID:7807024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, Verstegen NJM, Ciampricotti M, Hawinkels LJAC, Jonkers J, et al.. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522(7556):345–8. doi: 10.1038/nature14282. PMID:25822788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8(12):1369–75. doi: 10.1038/ncb1507. PMID:17128264. [DOI] [PubMed] [Google Scholar]
- 144.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15(1):35–44. doi: 10.1016/j.ccr.2008.11.012. PMID:19111879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al.. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–7. doi: 10.1038/nature04186. PMID:16341007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yu PF, Huang Y, Han YY, Lin LY, Sun WH, Rabson AB, Wang Y, Shi YF. TNFalpha-activated mesenchymal stromal cells promote breast cancer metastasis by recruiting CXCR2+ neutrophils. Oncogene. 2016;36(4):482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Acharyya S, Massague J. Arresting supporters: targeting neutrophils in metastasis. Cell Res. 2016;26(3):273–4. doi: 10.1038/cr.2016.17. PMID:26823207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. 2015;528(7582):413–7. doi: 10.1038/nature16140. PMID:26649828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–8. doi: 10.1038/nrc1256. PMID:14708027. [DOI] [PubMed] [Google Scholar]
- 150.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–5. doi: 10.1038/nature10138. PMID:21654748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen J, Yao Y, Gong C, Yu F, Su S, Chen J, Liu B, Deng H, Wang F, Lin L, et al.. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell. 2011;19(4):541–55. doi: 10.1016/j.ccr.2011.02.006. PMID:21481794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lin X, Chen L, Yao Y, Zhao R, Cui X, Chen J, Hou K, Zhang M, Su F, Chen J, et al.. CCL18-mediated down-regulation of miR98 and miR27b promotes breast cancer metastasis. Oncotarget. 2015;6(24):20485–99. doi: 10.18632/oncotarget.4107. PMID:26244871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen Q, Zhang XH, Massague J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell. 2011;20(4):538–49. doi: 10.1016/j.ccr.2011.08.025. PMID:22014578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li XJ, Gangadaran P, Kalimuthu S, Oh JM, Zhu L, Jeong SY, Lee SW, Lee J, Ahn BC. Role of pulmonary macrophages in initiation of lung metastasis in anaplastic thyroid cancer. Int J Cancer. 2016;139(11):2583–92. doi: 10.1002/ijc.30387. PMID:27537102. [DOI] [PubMed] [Google Scholar]
- 155.Cao H, Dan Z, He X, Zhang Z, Yu H, Yin Q, Li Y. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano. 2016;10(8):7738–48. doi: 10.1021/acsnano.6b03148. PMID:27454827. [DOI] [PubMed] [Google Scholar]
- 156.Vadrevu SK, Sharma S, Chintala N, Patel J, Karbowniczek M, Markiewski M. Studying the role of alveolar macrophages in breast cancer metastasis. J Vis Exp. 2016;(112). doi: 10.3791/54306. PMID:27403530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xu K, Tian X, Oh SY, Movassaghi M, Naber SP, Kuperwasser C, Buchsbaum RJ. The fibroblast Tiam1-osteopontin pathway modulates breast cancer invasion and metastasis. Breast Cancer Res. 2016;18(1):14. doi: 10.1186/s13058-016-0674-8. PMID:26821678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim HM, Jung WH, Koo JS. Expression of cancer-associated fibroblast related proteins in metastatic breast cancer: an immunohistochemical analysis. J Transl Med. 2015;13:222. doi: 10.1186/s12967-015-0587-9. PMID:26163388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hasebe T. Tumor-stromal interactions in breast tumor progression–significance of histological heterogeneity of tumor-stromal fibroblasts. Expert Opin Ther Targets. 2013;17(4):449–60. doi: 10.1517/14728222.2013.757305. PMID:23297753. [DOI] [PubMed] [Google Scholar]
- 160.Takai K, Le A, Weaver VM, Werb Z. Targeting the cancer-associated fibroblasts as a treatment in triple-negative breast cancer. Oncotarget. 2016;7(50):82889–901. doi: 10.18632/oncotarget.12658. PMID:27756881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ci Y, Qiao J, Han M. Molecular mechanisms and metabolomics of natural polyphenols interfering with breast cancer metastasis. Molecules. 2016;21(12):E1634. doi: 10.3390/molecules21121634. PMID:27999314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. PMID:17993229. [DOI] [PubMed] [Google Scholar]
- 163.Jang GB, Hong IS, Kim RJ, Lee SY, Park SJ, Lee ES, Park JH, Yun CH, Chung JU, Lee KJ, et al.. Wnt/beta-Catenin small-molecule inhibitor CWP232228 preferentially inhibits the growth of breast cancer stem-like cells. Cancer Res. 2015;75(8):1691–702. doi: 10.1158/0008-5472.CAN-14-2041. PMID:25660951. [DOI] [PubMed] [Google Scholar]
- 164.Kurebayashi J, Koike Y, Ohta Y, Saitoh W, Yamashita T, Kanomata N, Moriya T. Anti-cancer stem cell activity of a hedgehog inhibitor GANT61 in estrogen receptor-positive breast cancer cells. Cancer Sci. 2017;108(5):918–30. doi: 10.1111/cas.13205. PMID:28211214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Koike Y, Ohta Y, Saitoh W, Yamashita T, Kanomata N, Moriya T, Kurebayashi J. Anti-cell growth and anti-cancer stem cell activities of the non-canonical hedgehog inhibitor GANT61 in triple-negative breast cancer cells. Breast Cancer. 2017;24(5):683–93. doi: 10.1007/s12282-017-0757-0. PMID:28144905. [DOI] [PubMed] [Google Scholar]
- 166.Huebschman ML, Lane NL, Liu H, Sarode VR, Devlin JL, Frenkel EP. Molecular heterogeneity in adjacent cells in triple-negative breast cancer. Breast Cancer (Dove Med Press). 2015;7:231–7. PMID:26316815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kreike B, van Kouwenhove M, Horlings H, Weigelt B, Peterse H, Bartelink H, van de Vijver MJ. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res. 2007;9(5):R65. doi: 10.1186/bcr1771. PMID:17910759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–67. doi: 10.1172/JCI45014. PMID:21633166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Turner N, Lambros MB, Horlings HM, Pearson A, Sharpe R, Natrajan R, Geyer FC, van Kouwenhove M, Kreike B, Mackay A, et al.. Integrative molecular profiling of triple negative breast cancers identifies amplicon drivers and potential therapeutic targets. Oncogene. 2010;29(14):2013–23. doi: 10.1038/onc.2009.489. PMID:20101236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bartholomeusz C, Xie X, Pitner MK, Kondo K, Dadbin A, Lee J, Saso H, Smith PD, Dalby KN, Ueno NT. MEK inhibitor Selumetinib (AZD6244; ARRY-142886) Prevents lung metastasis in a triple-negative breast cancer xenograft model. Mol Cancer Ther. 2015. 14(12):2773–81. doi: 10.1158/1535-7163.MCT-15-0243. PMID:26384399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Citterio C, Menacho-Márquez M, García-Escudero R, Larive RM, Barreiro O, Sánchez-Madrid F, Paramio JM, Bustelo XR. The rho exchange factors vav2 and vav3 control a lung metastasis-specific transcriptional program in breast cancer cells. Sci Signal. 2012;5(244):ra71. doi: 10.1126/scisignal.2002962. PMID:23033540. [DOI] [PubMed] [Google Scholar]
- 172.Emens LA. Chemotherapy and tumor immunity: an unexpected collaboration. Front Biosci. 2008;13:249–57. doi: 10.2741/2675. PMID:17981543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Emens LA, Jaffee EM. Leveraging the activity of tumor vaccines with cytotoxic chemotherapy. Cancer Res. 2005. 65(18):8059–64. doi: 10.1158/0008-5472.CAN-05-1797. PMID:16166275. [DOI] [PubMed] [Google Scholar]
- 174.Finn OJ. Cancer immunology. N Engl J Med. 2008;358(25):2704–15. doi: 10.1056/NEJMra072739. PMID:18565863. [DOI] [PubMed] [Google Scholar]
- 175.Varn FS, Mullins DW, Arias-Pulido H, Fiering S, Cheng C. Adaptive immunity programmes in breast cancer. Immunology. 2016;150(1):25–34. PMID:27564847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gray MJ, Gong J, Hatch MM, Nguyen V, Hughes CC, Hutchins JT, Freimark BD. Phosphatidylserine-targeting antibodies augment the anti-tumorigenic activity of anti-PD-1 therapy by enhancing immune activation and downregulating pro-oncogenic factors induced by T-cell checkpoint inhibition in murine triple-negative breast cancers. Breast Cancer Res. 2016;18(1):50. doi: 10.1186/s13058-016-0708-2. PMID:27169467. [DOI] [PMC free article] [PubMed] [Google Scholar]


