Abstract
Background
Dry eye disease (DED) is characterized by a loss of homeostasis of the tear film. It goes along with ocular symptoms, in which ocular surface inflammation and damage play etiological roles. High-mobility group box 1 protein (HMGB1) is a pro-inflammatory protein found in the tear fluid during conjunctivitis, blepharitis and DED. Glycyrrhizin binds to HMGB1, inhibiting cytokine activities, thus potentially improving DED.
Aim
To assess the efficacy and tolerance of glycyrrhizin in moderate DED.
Methods
Multicenter, open-label, prospective, nonrandomized clinical pilot study of glycyrrhizin 2.5% eye drops twice daily over 28 days in adult patients with moderate DED using standard evaluation parameters.
Results
The overall mean age of the 37 patients included was 59.6±19.0 years, 70.3% of the patients were female and 77.0% of the patients had an Oxford score of II. After 28 days, 60.8% of the patients had an Oxford score of 0 or I; a significant mean improvement in the score of 0.97±0.86 (P<0.001) from 2.20±0.44 at day 1 to 1.23±0.88 at day 28 was observed. Tear break-up time and Schirmer scores had significantly improved while the number of patient-reported symptoms had significantly decreased (all P≤0.010). A large majority of patients still had a few spots on their naso-bulbar conjunctiva (86.1%), temporal-bulbar conjunctiva (81.4%) and cornea (84.7%). The investigators considered that DED had improved in 71.6% of the patients. Patients appreciated the eye drops for their efficacy and good tolerance profile, leading to a decreased use of artificial tears. No changes in intraocular pressure and visual acuity were observed; glycyrrhizin 2.5% eye drops were safe, with only one patient reporting a moderate, transient treatment-related contact allergy leading to the withdrawal of the patient. Overall, two patients reported three adverse events, two (moderate contact allergy in both eyes) were related to the eye drops and experienced by the same patient; treatment was stopped; the third event was not treatment-related.
Conclusion
In this pilot study, glycyrrhizin 2.5% eye drops were well tolerated and provide a good clinical benefit to patients with moderate DED after 28 days of continued daily use.
Keywords: dry eye disease, hyaluronic sodium, glycyrrhizin, inflammation, ocular lesions
Introduction
Dry eye disease (DED) or keratoconjunctivitis sicca is a multifactorial disease of the ocular surface. It is characterized by a loss of homeostasis of the tear film and goes along with ocular symptoms in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, as well as neurosensory abnormalities play etiological roles.1
Meibomian gland dysfunction (MGD) is the most frequent cause of DED. The pathogenesis of both MGD and DED can be described in terms of a ‘vicious circle’: the underlying pathophysiological mechanisms of DED and MGD interact, resulting in a double vicious circle. Meibomian gland blockage, dropout and inflammation directly link these two vicious circles. MGD-associated tear film instability provides an entry point into the vicious circle of DED and leads to hyperosmolarity and inflammation, which are both a cause and consequence of DED.2 Other factors such as topical preservative toxicity and xerophthalmia, which is associated with a loss of conjunctival goblet cells or altered mucin expression may also trigger DED.4
Regardless of how it is initiated and its etiology, the clinical consequences of DED are the same on the ocular surface. These may include punctate epitheliopathy, filamentary keratitis, superior limbic keratitis, goblet cell loss, modification of the epithelial glycocalyx, lid parallel conjunctival folds, changes to Marx’s line and MGD.4
There are two predominant and non-mutually exclusive categories of DED: 1) aqueous deficient dry eye (ADDE) and 2) evaporative dry eye (EDE). ADDE describes conditions affecting lacrimal gland function, while EDE is understood to occur with conditions affecting the eyelid (eg, MGD and blink abnormalities) or the ocular surface (eg, related to mucin deficiency or contact lens wear).1
DED is common; its prevalence around the world varies from 5% to 34%.5 Large epidemiological studies confirm that female sex and older age increase the risk for DED.6,7 Other risk factors include MGD, hematopoietic stem cell transplantation, computer use, Asian race, contact lens wear, Sjögren’s syndrome, environmental hazardous conditions such as pollution, low humidity, systemic connective diseases, certain classes of medication including antihistamines, antidepressants, anxiolytics and isotretinoin, and possibly androgen deficiency.3,8,9
Both discomfort and visual disturbance symptoms remain fundamental to DED. It causes irritation, pain and affects ocular and general health and well-being, the perception of visual function, and visual performance. Pain associated with DED can have psychological and physical impacts, while blurred vision may impose restrictions in daily life activities such as reading, driving, watching television, and operating smartphones.8 Some DED patients may exhibit signs of ocular surface disease, but do not report any symptoms of discomfort due to reduced corneal sensitivity.1
High-mobility group box 1 protein (HMGB1) is a pro-inflammatory protein found in tear fluid during conjunctivitis, blepharitis and DED, including primary Sjögren’s syndrome.10,11 It belongs to the “alarmin” family, inflammatory mediators with intracellular localization under resting conditions but released extracellularly to activate the innate immune response. Released HMGB1 brings about a pro-inflammatory vicious circle, sustaining the immune response and participating in tissue damage.12,13
In vitro assays showed that glycyrrhizin, a triterpene glycoside isolated from licorice root, binds to HMGB1, inhibiting its cytokine activities.14 Thus, it has anti-inflammatory properties with a different mode of action from the corticosteroid dexamethasone at 0.1%.15–18 It was hypothesized that targeting HMGB1-dependent immune activation may represent a valid strategy to reduce inflammation in structures of the external eye.10 In 2013, Mencucci et al demonstrated that an ophthalmic solution containing glycyrrhizin 5% and copolymer PEG/PPG significantly decreased inflammation by reducing symptoms in patients suffering from blepharitis when associated with appropriate eyelid hygiene by 64%.19
Current management of DED is largely addressed by the suppression of triggering factors and the prescription of a wide range of artificial tears (eye drops, fluid solutions or ophthalmic gels, which reduce the severity of DED) although these do not, however, provide a cure.20 One of the mainstays in the first step of DED management is treatment with topical lubricants and artificial tears, as confirmed by the Management and Therapy Subcommittee of the International Dry Eye Workshop.20–22 According to these guidelines, non-glucocorticoid immunomodulatory treatments such as cyclosporine or other molecules may be added in a second step to reduce inflammation.20
The aim of this pilot study was to assess the clinical benefit and tolerance of eye drops containing the non-glucocorticoid anti-inflammatory molecule dipotassium glycyrrhizin at 2.5%, sodium hyaluronate, PEG/PPG copolymer, EDTA disodium salt, and stabilized oxychloro complex (SOC), isotonic buffered solution at pH 7.2 in the symptomatic treatment of moderate DED.
SOC, a new preservative system keeps the solution sterile and breaks down into oxygen, water and sodium chloride, as soon as it comes into contact with the eye. These substances, naturally present in the tears, do not irritate the eye mucosa. Thus, the tested eye drops are preservative free in the eyes.
Methods
This was a multicenter, open-label, prospective, nonrandomized clinical pilot study. The study was conducted between March 2016 and December 2017 at three investigational sites in France under the following dossier number: 2015-A01860-49. The study was not registered internationally. The study complied with all local legal requirements for the conduct of a clinical study and was conducted in accordance with good clinical practices and the principles of the Declaration of Helsinki. The study received approval from the French ethics committee (approval no 2015–055 CPP Sud Est II) prior to the inclusion of any patient and all patients provided signed informed consent prior to study start.
Suitable patients were to be at least 18 years of age, with a clinically confirmed diagnosis of moderate DED, corresponding to a dry eye syndrome caused by hyposecretion, related or not to Sjörgen’s syndrome or a DED caused by excessive evaporation. All patients had to present with ocular surface damages (score of at least II) at selection and at day 1 according to the Oxford scoring system.23 Moreover, patients could have used artificial tears continuously and for a prolonged period without reporting any improvement in the symptoms related to their chronic DED. Patients wearing contact lenses, presenting with signs or symptoms of ocular inflammation or infection not caused by DED or patients with a glaucoma or herpetic keratitis or with an intraocular pressure (IOP) of more than 22 mmHg were not allowed to participate in this study. Moreover, patients receiving orally an anticholinergic or antibiotic treatment, such as cyclins, during the 14 days prior to inclusion or patients receiving a topical ocular treatment during the 7 days preceding their participation were not suitable for inclusion.
Included patients instilled one drop of the glycyrrhizin 2.5% eye drops in their eyes twice daily for a period of 28 days. Additionally, patients were provided with artificial tears (Aqualarm®, Bausch & Lomb Incorporated, Bridgewater, NJ, USA) to be instilled three times per day, at least 30 minutes before or after instillation of the glycyrrhizin 2.5% eye drops.
Efficacy was assessed at days 1 and 28. The primary efficacy parameter was the severity of ocular surface damage using the Oxford scoring system. Secondary parameters included the assessment of ocular surface damage (OSD) using the van Bijsterveld scale,24 ocular dryness using the tear break-up-time (TBUT) and Schirmer test, IOP, visual acuity (VA) on a scale from 0 to 10, evaluation of signs using the global improvement scale (from 0=severe worsening to 4=important improvement) and patient reported symptoms using a visual analog scale ranging from 0 (absent) to 100 (maximum intensity). Moreover, at day 28, patients completed a treatment satisfaction questionnaire.
Adverse events were collected throughout the study.
As this was a pilot study, no proper sample size calculation was performed.
For summary statistics, categorical variables were summarized by frequency and percentage for each response category. Continuous variables were summarized using mean and standard deviation (mean ± SD). All local tolerability and efficacy endpoints were summarized per visit.
SAS version 9.3 or above was used for all statistical analyses (SAS Institute Inc., Cary, NC, USA).
The authors do not plan to share any individual de-identified data generated through this presently reported study.
Results
Overall, 39 patients were selected and 37 included; one patient withdrew from the study due to an adverse event. A total of 37 patients were suitable for the intent-to-treat analysis and 33 for the per protocol (PP) analysis.
At day 1, a large majority of patients (70.3%) were women. The mean ± SD age was 59.6±19.0 years; slightly more patients (54.1%) were older than 64 years and 78.4% of patients suffered from DED caused by excessive evaporation. The mean duration of the disease was 6.60±5.42 years. An Oxford score of II was reported for 77.0% of the patients; the mean Oxford score was 2.2±0.44; one patient (1.4%) had a score of I and 21.6% had a score of III (16 patients). The mean TBUT was 5.84±0.86 seconds, and that of the Schirmer test was 8.3±1.4 mm/5 minutes; the mean IOP was 13.6±3.4 mmHg. At baseline, a majority of patients had a small number of separated spots on the naso-bulbar conjunctiva (65.7%) or temporal-bulbar conjunctiva (68.1%) and 55.7% of the patients had a small number of separated spots on the cornea; all were secondary to DED. The mean VA was 8.70±2.04.
Detailed demographic data and clinical assessments at day 1 are provided in Table 1.
Table 1.
Demographic data and clinical assessments at day 1
| Intent-to-treat population N=37 | |
|---|---|
| Age, years | |
| N | 37 |
| Mean ± SD | 59.6±19.0 |
| Median | 66.0 |
| (Min, max) | (18, 82) |
| Age, years | |
| N | 37 |
| 18–64 | 17 (45.9%) |
| 65–84 | 20 (54.1%) |
| Gender | |
| N | 37 |
| Male | 11 (29.7%) |
| Female | 26 (70.3%) |
| Diagnosis | |
| N | 37 |
| DED caused by hypersecretion related or not to a Sjögren’s syndrome | 8 (21.6%) |
| DED caused by excessive evaporation | 29 (78.4%) |
| Duration, years | |
| N | 36 |
| Mean ± SD | 6.60±5.42 |
| Median | 5.10 |
| (Min, max) | (0.0, 21.9) |
| Oxford grade | |
| N | 74 |
| I | 1 (1.4%) |
| II | 57 (77.0%) |
| III | 16 (21.6%) |
| Mean ± SD | 2.20±0.44 |
| Median | 2.00 |
| (Min, max) | (1, 3) |
| Tear break-up time, seconds | |
| N | 74 |
| Mean ± SD | 5.841±0.861 |
| Median | 5.70 |
| (Min, max) | (4.3, 8.3) |
| Schirmer, mm/5 minutes | |
| N | 74 |
| Mean ± SD | 8.30±1.41 |
| Median | 8.00 |
| (Min, max) | (5, 12) |
| Intraocular pressure, mmHg | |
| N | 74 |
| Mean ± SD | 13.59±3.36 |
| Median | 14.00 |
| (Min, max) | (6, 20) |
| Naso-bulbar conjunctival lesion, score | |
| N | 70 |
| Mean ± SD | 1.36±0.50 |
| Median | 1.00 |
| (Min, max) | (1, 3) |
| Temporal-bulbar conjunctival lesion, score | |
| N | 74 |
| Mean ± SD | 1.35±0.53 |
| Median | 1.00 |
| (Min, max) | (1, 3) |
| Cornea, score | |
| N | 74 |
| Mean ± SD | 1.44±0.49 |
| Median | 1.00 |
| (Min, max) | (1, 2) |
| Visual acuity | |
| N | 74 |
| Mean ± SD | 8.7076±2.0437 |
| Median | 10.00 |
| (Min, max) | (0.06, 10.00) |
Abbreviation: DED, dry eye disease.
After one month of daily treatment, the mean Oxford score had significantly decreased by 0.97±0.86 (P<0.001), reaching 1.23±0.88 (Figure 1); 23.0% of the patients had a score of 0, 37.8% had a score of I, 32.4% a score of II and 6.8% a score of III. Results were confirmed in the PP population.
Figure 1.
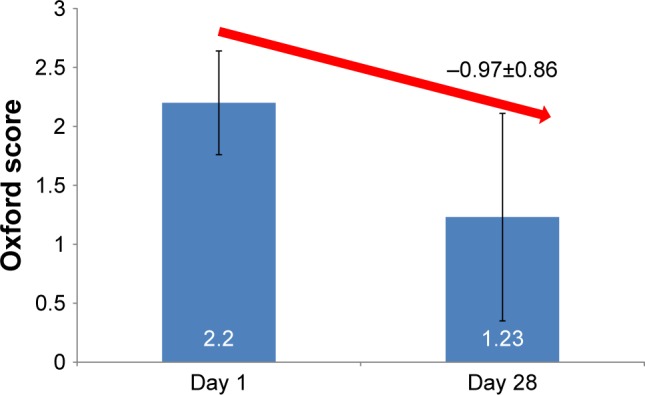
Oxford score at days 1 and 28.
Note: The decrease was statistically significant between days 1 and 28 (P<0.001).
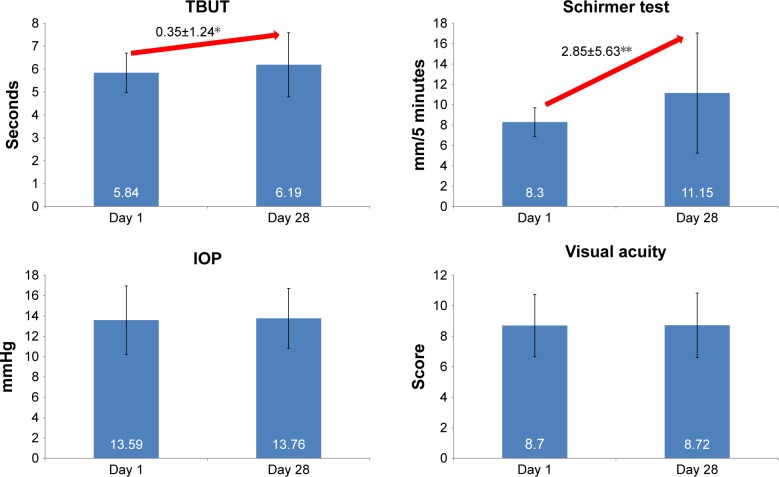
TBUT and Schirmer score had significantly increased by 0.35±1.24 to 6.19±1.40 (P=0.10) and by 2.85±5.63 to 11.15±5.90 (P<0.001), respectively, while IOP and VA remained stable over time (Figure 2).
Figure 2.
Tear break-up time, Schirmer score, intraocular pressure and visual acuity at days 1 and 28.
Notes: Visual acuity was assessed on a scale from 0 to 10. Improvements were statistically significant between days 1 and 28 for TBUT (*P=0.010) and Schirmer score (**P<0.001).
Abbreviations: IOP, intraocular pressure; TBUT, tear break-up time.
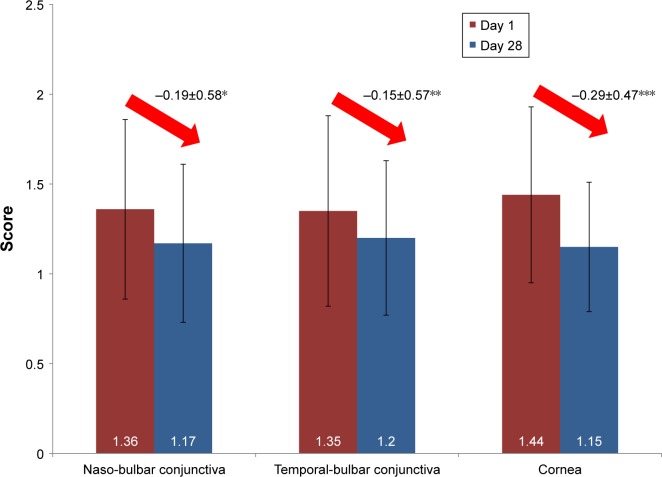
Mean scores for OSD after one month had significantly improved (naso-bulbar conjunctiva: −0.19±0.58, temporal-bulbar conjunctiva: −0.15±0.57 and cornea −0.29±0.47, all P≤0.027) (Figure 3). While at day 1, 34.3%, 32.0% and 44.3% of the patients had many separated or confluent spots on the naso-bulbar conjunctiva, temporal-bulbar conjunctiva and cornea respectively; 28 days after initiation of the treatment, their prevalence had decreased to 13.9%, 18.5% and 15.3%, respectively.
Figure 3.
Ocular surface damage (mean scores) at days 1 and 28.
Note: Improvement of surface damage was significant at day 28 from day 1 (*P=0.003, **P=0.027, ***P<0.001).
Globally, the investigators considered that glycyrrhizin 2.5% eye drops had improved DED in 71.6% of the patients, while no changes were observed in 27.0% of the patients and 1.4%, corresponding to one patient, experienced a worsening of the condition. This patient discontinued the study due to treatment-related contact allergy.
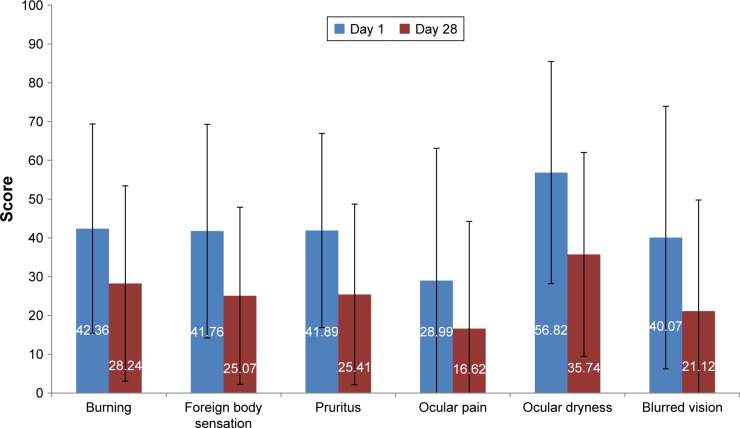
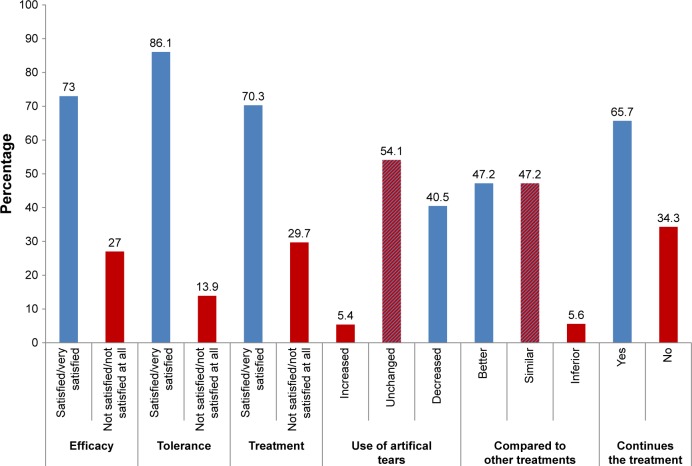
Patients stated that DED symptoms had significantly improved (all P<0.001) with the tested eye drops (Figure 4) and a majority of the patients appreciated their glycyrrhizin 2.5% eye drops (Figure 5). A total of 40.5% of the patients reported that they had decreased the daily use of artificial tears.
Figure 4.
Ocular symptoms (mean scores) at days 1 and 28.
Notes: Symptoms were rated on a scale from 0 (absent) to 100 (maximum intensity). Improvement of all symptoms at day 28 from day 1 was significant (all P<0.001).
Figure 5.
Patient satisfaction of treatment after 28 days.
Overall, two patients reported three adverse events, two (transient, moderate contact allergy in both eyes) were related to the eye drops and experienced by the same patient; treatment was stopped; the third event was not treatment-related.
Discussion
Results from this open-label, prospective pilot study conducted in 37 patients demonstrated that eye drops containing glycyrrhizin at 2.5% improved moderate DED after 28 days of daily use.
A significant mean decrease in the Oxford score of 0.97±0.86 (P<0.001) from 2.20±0.44 at day 1 −1.23±0.88 at day 28 was observed; a majority of patients (60.8%) had an Oxford score of 0 or I reported after 28 days. Moreover, the study demonstrated that TBUT and Schirmer scores had significantly (P=0.010 and <0.001, respectively) improved while patient-reported symptoms including burning, foreign body sensation, pruritus, ocular pain and dryness and blurred vision had significantly (all P<0.001) reduced. The daily use of the glycyrrhizin 2.5% eye drops over 28 days significantly reduced ocular lesions on the naso-bulbar conjunctiva: −0.19±0.58, temporal-bulbar conjunctiva: −0.15±0.57 and cornea −0.29±0.47, all P≤0.027. Overall, the investigator considered that the glycyrrhizin 2.5% eye drops had improved DED in 71.6% of the patients. Patients appreciated their eye drops for their efficacy and good tolerance profile leading to a decreased use of artificial tears in more than 40% of all patients. No changes in IOP or VA were observed and glycyrrhizin 2.5% eye drops were well tolerated with only one patient reporting a moderate and transient treatment-related contact allergy.
Demographic data confirmed that DED is mainly observed in the older population and in women with a mean age of the studied population of 59.6±19.0 years and 70.3% of the patients being female.6,7
The open-label and non-comparative study design of this pilot study has certain inherent limitations and biases. One main concern is that it did not allow comparison of the tested product with other DED treatments or with a placebo. Nevertheless, with the perceived clinical benefit in patients with moderate DED, results obtained confirmed the primary study objective demonstrating that the eye drops containing glycyrrhizin 2.5% were efficacious and well tolerated while the use of artificial tears had decreased. Artificial tears are indicated in mild DED. However, if DED becomes more severe, artificial tears may be insufficient and a pharmacological-active treatment may be initiated.5 Thus, the present results about the patient-reported reduced frequency of use of artificial tears, due to improved symptoms may indirectly confirm that a regular use of the tested eye drops improves moderate DED.
Vehicle or active controlled clinical trials of 12 weeks or longer will be necessary to further investigate the clinical benefit of the eye drops in patients with moderate DED.
Conclusion
This pilot study demonstrated that in patients with moderate DED eye drops containing glycyrrhizin 2.5%, in addition to the standard DED care recommending artificial tears, are well tolerated and provide a good clinical benefit after 28 days of continued daily use.
Acknowledgments
The authors acknowledge the support in conducting the study of Catherine Bosc, Tailorpharma, France, and the writing assistance of Karl Patrick Göritz, Scientific and Medical Writing Services, France.
Funding for the study was made by Laboratoire Densmore, Monaco.
Footnotes
Disclosure
The authors received honoraria from Laboratoire Densmore, Monaco to conduct this study. Moreover, Carole Burillon received honoraria from Chiesi. The authors report no other conflicts of interest in this work.
References
- 1.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Baudouin C, Messmer EM, Aragona P, et al. Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. Br J Ophthalmol. 2016;100(3):300–306. doi: 10.1136/bjophthalmol-2015-307415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2012;130(1):90–100. doi: 10.1001/archophthalmol.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438–510. doi: 10.1016/j.jtos.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(5):71–81. doi: 10.3238/arztebl.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chia EM, Mitchell P, Rochtchina E, Lee AJ, Maroun R, Wang JJ. Prevalence and associations of dry eye syndrome in an older population: the Blue Mountains Eye Study. Clin Exp Ophthalmol. 2003;31(3):229–232. doi: 10.1046/j.1442-9071.2003.00634.x. [DOI] [PubMed] [Google Scholar]
- 7.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136(2):318–326. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 8.Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334–365. doi: 10.1016/j.jtos.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol. 2013;32(1):19–41. doi: 10.3109/08830185.2012.748052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavone L, Muzzi M, Mencucci R, et al. 18β-glycyrrhetic acid inhibits immune activation triggered by HMGB1, a pro-inflammatory protein found in the tear fluid during conjunctivitis and blepharitis. Ocul Immunol Inflamm. 2011;19(3):180–185. doi: 10.3109/09273948.2010.538121. [DOI] [PubMed] [Google Scholar]
- 11.Dupire G, Nicaise C, Gangji V, Soyfoo MS. Increased serum levels of high-mobility group box 1 (HMGB1) in primary Sjögren’s syndrome. Scand J Rheumatol. 2012;41(2):120–123. doi: 10.3109/03009742.2011.633099. [DOI] [PubMed] [Google Scholar]
- 12.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17(4):359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 14.Mollica L, De Marchis F, Spitaleri A, et al. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol. 2007;14(4):431–441. doi: 10.1016/j.chembiol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Fu Y, Zhou E, Wei Z, et al. Glycyrrhizin inhibits the inflammatory response in mouse mammary epithelial cells and a mouse mastitis model. FEBS J. 2014;281(11):2543–2557. doi: 10.1111/febs.12801. [DOI] [PubMed] [Google Scholar]
- 16.Feng X, Ding L, Qiu F. Potential drug interactions associated with glycyrrhizin and glycyrrhetinic acid. Drug Metab Rev. 2015;47(2):229–238. doi: 10.3109/03602532.2015.1029634. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka H, Hasegawa T, Matsushita M, Miichi H, Hayashi S. Quantitative evaluation of ocular anti-inflammatory drugs based on measurements of corneal temperature in rabbits: dexamethasone and glycyrrhizin. Ophthalmic Res. 1987;19(4):213–220. doi: 10.1159/000265496. [DOI] [PubMed] [Google Scholar]
- 18.Takei H, Baba Y, Hisatsune A, et al. Glycyrrhizin inhibits interleukin-8 production and nuclear factor-kappaB activity in lung epithelial cells, but not through glucocorticoid receptors. J Pharmacol Sci. 2008;106(3):460–468. doi: 10.1254/jphs.FP0072378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mencucci R, Favuzza E, Menchini U. Assessment of the tolerability profile of an ophthalmic solution of 5% glycyrrhizin and copolymer PEG/PPG on healthy volunteers and evaluation of its efficacy in the treatment of moderate to severe blepharitis. Clin Ophthalmol. 2013;7:1403–1410. doi: 10.2147/OPTH.S47657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575–628. doi: 10.1016/j.jtos.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Wozniak PA, Schmidl D, Bata AM, et al. Effect of different lubricant eye gels on tear film thickness as measured with ultrahigh-resolution optical coherence tomography. Acta Ophthalmol. 2017;95(4):e307–e313. doi: 10.1111/aos.13342. [DOI] [PubMed] [Google Scholar]
- 22.Schmidl D, Schmetterer L, Witkowska KJ, et al. Tear film thickness after treatment with artificial tears in patients with moderate dry eye disease. Cornea. 2015;34(4):421–426. doi: 10.1097/ICO.0000000000000358. [DOI] [PubMed] [Google Scholar]
- 23.Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22(7):640–650. doi: 10.1097/00003226-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 24.van Bijsterveld OP. Diagnostic tests in the Sicca syndrome. Arch Ophthalmol. 1969;82(1):10–14. doi: 10.1001/archopht.1969.00990020012003. [DOI] [PubMed] [Google Scholar]