Novel midwater ocean sampling shows that physiology dictates zooplankton distributions in submesoscale low oxygen features.
Abstract
Oxygen minimum zones (OMZs), large midwater regions of very low oxygen, are expected to expand as a result of climate change. While oxygen is known to be important in structuring midwater ecosystems, a precise and mechanistic understanding of the effects of oxygen on zooplankton is lacking. Zooplankton are important components of midwater food webs and biogeochemical cycles. Here, we show that, in the eastern tropical North Pacific OMZ, previously undescribed submesoscale oxygen variability has a direct effect on the distribution of many major zooplankton groups. Despite extraordinary hypoxia tolerance, many zooplankton live near their physiological limits and respond to slight (≤1%) changes in oxygen. Ocean oxygen loss (deoxygenation) may, thus, elicit major unanticipated changes to midwater ecosystem structure and function.
INTRODUCTION
Oxygen loss in subsurface waters of the world’s oceans is now recognized as a critical environmental and ecological issue associated with ongoing climate change (1–3). Oceanic oxygen minimum zones (OMZs), large midwater regions (100- to 1000-m depth) of very low oxygen, are projected to expand in intensity and extent, based on recent data and models (4–7). Zooplankton are critical components of midwater food webs and biogeochemical cycles, serving as a major trophic link between primary producers (phytoplankton) and larger animals, including marine mammals and commercially important fishes and squids. Their feeding, defecation, respiration, and vertical migration in the water column affect the vertical transport of carbon and particles to depth as part of the biological pump (8, 9). Zooplankton distributions, diel vertical migration, and ecological functions are strongly affected by the vertical oxygen gradients of the OMZ (10–12). For example, there are often subsurface maxima in zooplankton biomass, certain species abundances, and associated trophic activities at the upper and lower OMZ edges (oxyclines). The depth, abundance, and composition of these unique communities in the water column and, thus, their effects on food webs and particle fluxes vary with OMZ thickness (12). Many animals living in the OMZ also have unique physiological adaptations for tolerating extreme hypoxia and maintaining metabolic function at very low oxygen concentrations (13–15). However, species that occur in the most pronounced OMZs appear to be living at oxygen levels lower than previously measured tolerances and must be near their physiological limits (14). Thus, small changes in oxygen concentration may have important consequences for mesopelagic and deep-sea communities.
Here, we describe observations and experiments undertaken during a 2017 research expedition in the eastern tropical North Pacific, one of the world’s major OMZs and an important fisheries and biodiversity region (16). A new approach for investigating submesoscale OMZ features, using integrated and targeted physical and biological sampling, was accomplished using a recently developed towed vertically oscillating hydrographic profiler (Wire Flyer) (17) to identify midwater oxygen features, followed by horizontal tows collecting sequential zooplankton samples through those features with a MOCNESS [Multiple Opening-Closing Net and Environmental Sensing System (18)] (table S1). Wire Flyer transects sampled 325-m-thick midwater depth intervals for distances of ~50 km and revealed small persistent midwater oxygen anomalies a few kilometers wide. Locations of these anomalies were used to position subsequent MOCNESS zooplankton tows conducted at a constant depth. Transects and tows closely overlapped geographically, successfully sampling the same hydrographic features (fig. S1 and table S1). Experiments on live animals quantified the oxygen levels that are critical for aerobic metabolism (hypoxia tolerance) of key zooplankton species collected within, or just outside of, the oxygen features. On the basis of these biological and environmental parameters, we modeled metabolically suitable habitats that closely matched actual distributions and could be used to predict future responses to warming and deoxygenation.
RESULTS
Submesoscale oxygen features
Midwater submesoscale (~5 to 10 km) features, characterized by sharp (but small) shifts in oxygen (~3 to 5 μM) and temperature (<1°C), were evident in the Wire Flyer transects (Fig. 1). Oxygen sections show data with the horizontal MOCNESS track overlaid at the sampled depths of 425, 430, and 800 m, respectively. Horizontal MOCNESS tows crossed the edges of several of these features, collecting consecutive biological samples and continuous hydrographic data (Fig. 2) across a narrow range of low oxygen values. In contrast to surface ocean fronts, there was no obvious density gradient across the oxygen feature edges because salinity changed slightly in concert with temperature, maintaining similar density along depth horizons. Features were persistent during both Wire Flyer and MOCNESS sampling (6- to 8-hour time difference between arrivals) and remained recognizable over several days in repeated Wire Flyer transects.
Fig. 1. Oxygen sections from Wire Flyer transects, showing oxygen concentration (color) with distance.
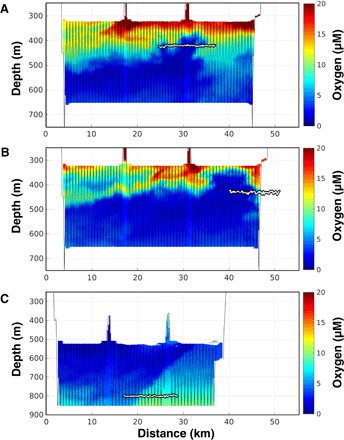
Diagonal black lines are the Wire Flyer path as it oscillated through the indicated depth zone. The light horizontal line is the horizontal MOCNESS tow path that targeted the edges of specific oxygen features seen in the earlier Wire Flyer tow (table S1). (A) Wire Flyer tow #9 and MOCNESS tow #724 (upper oxycline). (B) Wire Flyer tow #10 and MOCNESS tow #726 (upper oxycline). (C) Wire Flyer tow #12 and MOCNESS tow #728 (lower oxycline).
Fig. 2. Hydrographic parameters from the three horizontal MOCNESS tows during zooplankton sample collection at depth.
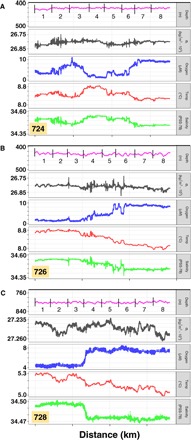
Charts (top to bottom within each set) show MOCNESS depth, density (σt), oxygen, temperature, and salinity. Vertical lines and numbers (depth panel) indicate when each of the eight nets was open. Distance is ~8 km, and sampling time at depth is ~2.5 to 3 hours for each tow. (A) Tow #724 (425-m depth). (B) Tow #726 (430-m depth). (C) Tow #728 (800-m depth).
Oxygen and zooplankton distributions
For statistical comparisons, we identified nets from each horizontal MOCNESS tow that sampled entirely at “low” (≤5 μM, 0.11 ml/liter) or “high” (≥8 μM, 0.18 ml/liter) oxygen (within or outside a feature). Many zooplankton taxa, including species of copepods, euphausiids, and fishes (fig. S2), showed significant abundance differences between these sample categories (Fig. 3 and table S2). In most cases, abundances and biomass were significantly higher at high oxygen. Note that the high and low oxygen categories differed in oxygen concentration limits by only 3 μM, and all values were below 10 μM, indicating that these taxa responded to very small oxygen differences at very low oxygen concentrations.
Fig. 3. Zooplankton abundances, biomass, and oxygen from MOCNESS horizontal tows.
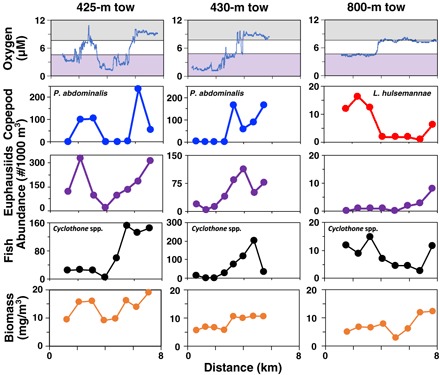
Each column shows data from one tow. For oxygen (top row), purple shading indicates low oxygen (≤5 μM, 0.11 ml/liter), and gray shading indicates high oxygen (≥8 μM, 0.18 ml/liter). For copepods (second row), Pleuromamma abdominalis is shown for shallower tows and L. hulsemannae is shown for the deep tow. The next rows show total euphausiids, the fish Cyclothone spp., and zooplankton biomass. All graphed taxa show significant abundance differences between samples in high versus low oxygen categories (see table S2 for data in each category), except 800-m euphausiids and 800-m total biomass (which are sparse at depth) (see fig. S2 for photographs of these taxa).
Several taxa, however, had a reverse abundance response to oxygen. At 800 m, both the copepod Lucicutia hulsemannae and the midwater fish Cyclothone spp. were significantly more abundant in low oxygen samples versus higher oxygen ones (Fig. 3). This tow was within the lower oxycline of the OMZ, where oxygen concentration was increasing with depth, and thus, lower oxygen conditions occurred shallower in warmer water toward the core of the OMZ. In vertical profiles, both taxa were most abundant in nets incorporating the oxygen inflection points just above and below the narrow OMZ core (depth of lowest oxygen) (Fig. 4) and were considered indicator species of this habitat (12). Neither of these species showed evidence of diel vertical migration. L. hulsemannae remained at 500 to 800 m (peak abundance at 500 to 600 m, 6.8° to 7.8°C) day and night, experiencing constant low oxygen levels (1.2 to 6.8 μM, 0.08 to 0.48 kPa). (In net tow collections, it is unknown where exactly within a single sampling interval the animals occurred and consequently the precise environmental conditions within the sampled range that they experienced.)
Fig. 4. Day and night vertical distributions of zooplankton abundance for the same taxa shown in Fig. 3 and for total zooplankton biomass.
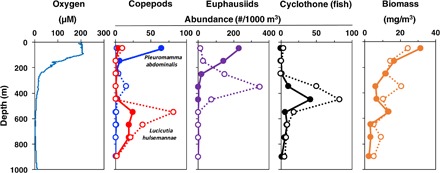
Data come from vertically stratified MOCNESS tow #716 and #725 (table S1). Oxygen is shown in the left graph (tow #725). Abundances are plotted at the mid-depth of each net (table S2). Open circles, day; closed circles, night. For copepods, blue lines represent P. abdominalis and red lines represent L. hulsemannae.
Many taxa migrated during the day into the very low oxygen OMZ core or upper oxycline and returned to shallower more oxygenated habitats at night (Fig. 4). For example, the copepod Pleuromamma abdominalis experienced a daytime oxygen concentration of 11.2 to 19.3 μM (peak abundance at 300 to 400 m, 8.5° to 9.9°C), while at night, it was in warmer well-oxygenated near-surface water (0 to 100 m, 19.8° to 22.7°C, 170 to 210 μM oxygen) (table S2). The euphausiid Nematobrachion flexipes migrated between depth intervals of 400 to 500 m (day) and 100 to 200 m (night), encountering a similar wide oxygen range. At their low oxygen daytime depth (horizontal tows), these species preferred slightly higher oxygen (Figs. 3 and 5).
Fig. 5. Hypoxia tolerance influences the abundance of dominant mesopelagic crustaceans in the OMZ.
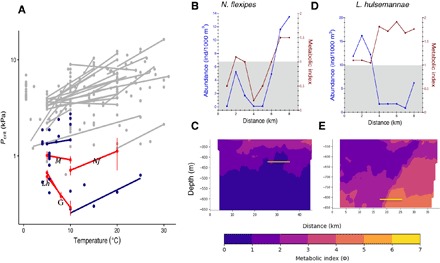
(A) Critical PO2 (Pcrit, kPa) in diverse marine crustaceans (gray; table S4), including species from the current study (L. hulsemannae, Lh; N. flexipes, Nf; Gennadas spp., G; and Megacalanus spp., M; red, means ± SE; table S3) and from the eastern Pacific OMZ (blue). Lines connect intraspecific measurements at different temperatures. Note that the y axis is on a log scale. (B to E) MI calculated for N. flexipes (B and C) at 400 m and L. hulsemannae (D and E) at 800 m from metabolic and environmental data across the horizontal MOCNESS [red lines in (B) and (D)] and Wire Flyer transects (C and E). The yellow line on MI plots illustrates the corresponding MOCNESS tows. The corresponding abundance of each species is also plotted [blue lines in (B) and (D)]. Gray shading (B and D) indicates metabolically unavailable habitat (MI < 1).
Zooplankton respiration and hypoxia tolerance
The critical oxygen partial pressure (Pcrit, the oxygen pressure below which aerobic metabolism can no longer be sustained) was determined for several euphausiids, copepods, and other taxa (Fig. 5, figs. S3 and S4, and table S3). Compared to the published measurements for similar species in more oxygenated regions, Pcrits measured here were much lower. The lower oxycline species, including the copepod L. hulsemannae (mean Pcrit = 0.38 ± 0.04 kPa; n = 34, 8°C) and the penaeid shrimp Gennadas spp. (Pcrit = 0.29 ± 0.05 kPa, n = 12, 10°C), had among the lowest mean Pcrits (greatest hypoxia tolerance) of any animals previously reported (Fig. 5A and table S4), comparable only to the pelagic red crab, Pleuroncodes planipes (Pcrit = 0.27 ± 0.2 kPa), which also inhabits OMZs of the eastern tropical Pacific (19). Because some individuals, perhaps due to stress or injury, reached an apparent oxygen limitation well above the PO2 (oxygen partial pressure) at which they were captured, the mean Pcrit is likely an underestimate of their in situ hypoxia tolerance. Some individuals maintained (regulated) their oxygen consumption rate to the limits of detectable oxygen (~0.05 kPa). Metabolic measurements could not be done for the fish Cyclothone spp. because individuals did not remain alive after capture.
Both L. hulsemannae and Gennadas spp. had a reverse response to temperature, with a lower Pcrit at higher temperature despite a higher metabolic rate (Fig. 5A and table S3). This highly unusual metabolic response facilitates life within the lower oxycline depth range, where oxygen is inversely related to temperature in vertical profiles (more oxygen in deeper colder water below the OMZ core). N. flexipes, which inhabits the upper oxycline and OMZ during the day but moves shallower at night to warmer more oxygenated water, had a mean Pcrit of 0.68 kPa at 10°C that increased with increasing temperature (Fig. 5A). This Pcrit is substantially higher than for the lower oxycline copepod and shrimp species, but within the range found for other OMZ euphausiids (15, 20).
Metabolic limits and distributions
A Metabolic Index (MI), defined as the ratio of oxygen supply to demand for a given species, is effectively a measure of potential sustained aerobic scope for that species in a given spatiotemporal location (21). The MI typically declines with increasing temperature or decreasing oxygen toward a species-specific critical value that may delineate latitudinal and depth distributions of many marine species (21). We calculated the MI across the horizontal transects for the euphausiid N. flexipes and the copepod L. hulsemannae. These and other OMZ zooplankton with similar hypoxia tolerance live very close to their physiological limits with an MI of <2 (Fig. 5, B and D), indicating that ambient PO2 is less than two times that required at rest at ambient temperature. The horizontal tows showed that N. flexipes spent daytime at depths with an MI of ~1 to 1.5, and its abundance declined strongly when oxygen decreased by even a few micromolar (Figs. 3 and 5B). N. flexipes, like many migrating zooplankton, has greater scope for energetic activities, such as locomotion, growth, and reproduction, in warmer more oxygenated waters at night where the MI is much higher. Some euphausiids and many other migrating zooplankton and nekton suppress metabolism when in subcritical oxygen (MI < 1) during the daytime (14, 15, 19). The decrease in abundance of euphausiids at their daytime depth in the lowest oxygen (Figs. 3 and 4) suggests that they depend strongly on the little oxygen available there to support even their suppressed rate of metabolism. This may also explain the decreased fish abundance (22) and global shoaling of the scattering layer (23) in low oxygen regions.
The strong reverse temperature effect on Pcrit for species living in the lower oxycline partially offsets the increase in oxygen demand at higher temperature. This reduced the variability in MI across the 800-m transect, but only slightly, as the temperature variability was quite small (<0.05°C). For L. hulsemannae, the lowest measured Pcrit at 5°C (0.16 kPa) resulted in an MI ranging from 1.8 to 3.3 across the transect. Using the mean Pcrit for the species, a reduced MI range results (~1 to 2; Fig. 5D). Whereas many species would be precluded from these waters, L. hulsemannae was not strictly limited by oxygen provision at this depth. Its preference for the lowest oxygen in its range likely provides refuge from predators or possibly a competitive edge to access food unavailable to less tolerant taxa.
DISCUSSION
This study revealed an unexpected level of submesoscale variability in oxygen concentration at midwater OMZ depths. Changes in abundance of OMZ zooplankton and fish were associated with environmental changes of only a few micromolar oxygen at very low oxygen concentrations (<10 μM). This unexpected sensitivity matched the results from shipboard metabolic experiments on live animals. These OMZ zooplankton not only live at levels of oxygen often assumed to be biological “dead zones” for animals (and well below the ~60 μM traditional definition of “hypoxia”) (2, 3) but also have the ability to respond to extremely small oxygen gradients. Our findings suggest that zooplankton in the eastern tropical Pacific OMZ have very little in situ scope for energetic activity and virtually no capacity to tolerate further ocean oxygen loss.
Although some species may be able to shift depth (to waters with higher oxygen) in some situations of decreasing oxygen, this is probably impossible for many others because of associated changes in temperature, pressure, food, light, and predation, among other environmental factors. There may also be differences in deoxygenation responses, depending on life history stage or age. For example, although all identifiable (intact) specimens of Cyclothone spp. in these tows appeared to be Cyclothone acclinidens, individuals at 425 to 430 m were usually smaller in size and had the opposite distributional response to oxygen than the larger individuals at 800 m, suggesting a change in oxygen tolerance or preference with age (Fig. 3). In the Arabian Sea, different life history stages of the lower oxycline copepod Lucicutia grandis, a sibling species of L. hulsemannae, had slightly different depth distributions and oxygen preferences as development progressed (24). For lower oxycline taxa, an inverse response of abundance to oxygen would help maintain the animals within that habitat, perhaps as a refuge from the large bathypelagic predators below in more oxygenated water.
Through their feeding activities, zooplankton fragment sinking particles, thus facilitating microbial remineralization, an important component of the vertical flux of particulate organic carbon (9). Zooplankton abundance and distribution responses to decreasing oxygen in OMZs may therefore affect carbon cycles by altering remineralization and carbon sequestration to depth (25). OMZ zooplankton may also use submesoscale oxygen variability and gradients as a key part of their life processes (reproduction and feeding activities) in ocean regions where animals and ecosystems have adapted over evolutionary time to very low oxygen, and these functions could be disrupted by further oxygen loss (24).
These factors suggest the possibility of unanticipated effects of deoxygenation on OMZ ecosystems. In addition, submesoscale oxygen variability in Wire Flyer transects raises questions about defining longer-term deoxygenation trends based on sparse midwater sampling with standard CTDs (Conductivity-Temperature-Depth instruments) and net tows at particular locations and times. It may also alias interpretations of the drivers of animal responses. If the estimated 10 to 15 μM per decade decrease in ocean oxygen (2) continues, the associated changes in zooplankton distributions and function could have major ecosystem-wide and potential economic (fisheries) ramifications. Ocean oxygen loss may, thus, elicit major changes to midwater ecosystem structure and function.
MATERIALS AND METHODS
Experimental design
A month-long research expedition from Manzanillo, Mexico, to San Diego, CA, on the R/V Sikuliaq, cruise number SKQ201701S, occurred from 19 January to 15 February 2017 and was centered at 21.6°N 117.8°W, an area with a strong OMZ. Interactive targeted physical and biological sampling was accomplished as follows (table S1 and fig. S1). First, the Wire Flyer, a recently developed towed deepwater oscillating profiler (17), was deployed on ~50-km-long transects between depths of 325 to 650 m or 525 to 850 m with 1-km repeats to obtain high-resolution oxygen and environmental data for elucidating spatial and temporal variability of OMZ oxygen gradients and features. In contrast to other oscillating profilers that are generally limited to the upper few hundred meters of the water column, the Wire Flyer system incorporates a heavy clump weight (955 kg) that allows it to oscillate at programmable depth intervals to 1000 m at a ship speed of 4 knots. The Wire Flyer includes a 16-Hz Sea-Bird 49 FastCAT CTD and an Aanderaa 4831F oxygen sensor, plus other sensors. Hydrographic sections of environmental parameters were generated in near real time for the transects (Fig. 1). The location of specific midwater features showing abrupt oxygen gradients within a section was targeted for zooplankton sampling. The ship reversed direction and was positioned at the appropriate location to allow sufficient time for net deployment to depth before reaching the feature. Then, horizontally sequenced zooplankton samples along with hydrographic data were collected through the feature with a 1-m2 MOCNESS net system (222-μm mesh nets) (18), towed at a constant depth along the same track as the Wire Flyer (at 425-, 430-, or 800-m depth) (Fig. 2 and fig. S1). The coalignment of the two sampling transects was based on the ship position and estimates of the corresponding instrument locations at depth; we encountered the oxygen features at approximately the same geographic location with both instruments but cannot be certain that the exact track at depth was repeated. Eight sequential samples, along with environmental data from MOCNESS sensors, were collected in each of the three horizontal tows; all horizontal tows were done during the day (tables S1 and S2). MOCNESS depth was maintained within ~5 m above or below the target depths (Fig. 2). Each net during a tow filtered ~1000 m3 of water and was open for ~20 min while being towed at a speed of ~1.5 to 2 knots, thus covering a distance of ~1 km; the entire sampling portion of a tow was ~8 km long and took ~2.5 to 3 hours. Sampling this large volume of water was essential because zooplankton are sparse in OMZs. Day and night vertically stratified MOCNESS tows along with environmental data, from 1000 m to the surface (nine nets per tow), provided broader context to interpret the horizontal distributions (tables S1 and S2 and Fig. 4).
A modified MOCNESS, which incorporated a Sea-Bird SBE 911plusCTD and updated software in place of the original sensors, was used (Scripps Institution of Oceanography modifications). The MOCNESS oxygen sensor was a Sea-Bird SBE43 dissolved oxygen sensor. The Wire Flyer and MOCNESS oxygen sensors were cross calibrated during the cruise and by postcruise analyses. Depth, temperature, salinity, fluorescence, light transmission, and volume filtered were also measured by MOCNESS sensors. MOCNESS environmental data collection during the tow while the zooplankton were being captured was done using the in situ sensors on the MOCNESS relayed through the electronic tow cable to the ship and displayed on a shipboard computer; MOCNESS software onboard the ship was used by the tow director to trigger each net based on MOCNESS sensor data, tow duration, and other factors. On deck, nets were rinsed with filtered seawater, and whole samples were photographed and preserved in 4% sodium borate–buffered formaldehyde. In the laboratory, wet weight biomass of large (>2 mm) and small (<2 mm) size fractions was determined. Species and taxa were identified from whole samples or quantitative splits (see fig. S2 for animal photos).
Statistical analysis (abundances)
Abundances from the horizontal tows were separated into two groups for Mann-Whitney U statistical tests (P < 0.05 for significance): those from nets taken entirely at high oxygen and those from nets taken entirely at low oxygen (defined below) based on the continuous data from the MOCNESS oxygen sensor during the time of collection (table S2). High oxygen was defined as having at least some part of a net sample taken where oxygen was ≥8 μM (~0.54 kPa, 0.18 ml/liter) (i.e., there were at least some high values), while low oxygen was defined as having the maximum oxygen ≤5 μM (~0.34 kPa, 0.11 ml/liter) (i.e., all of a sample was taken at low oxygen). Because a single net spanned some time and distance (Fig. 2), it is unknown where exactly within its oxygen range animals may have occurred. Nets that bridged this range were not included for statistical purposes. The samples from the 425- and 430-m tows (tow #724 and #726) were combined for statistics, while the 800-m samples (tow #728) were separately tested because species composition changed substantially between these two depth zones. It should be noted that both of these oxygen limits, separated by only 3 μM (0.07 ml/liter), are well below most definitions of hypoxic water and OMZ oxygen limits (2, 3).
Respiration experiments
Zooplankton for live experiments were collected by Tucker trawls done at similar locations and depths as the MOCNESS tows (table S1). The Tucker trawl used standard MOCNESS control software and sensors and had a large insulated cod end (26) so that many species remained alive when brought to the surface in water at their ambient temperature (13). Shipboard respiration measurements of key species (copepods, euphausiids, krill, shrimps, lophogastrids, crabs, squids, and octopods) determined their physiological tolerances [critical oxygen partial pressure (Pcrit)] and metabolic rates at 5°, 8°, 10°, or 20°C that could be related to species-specific distributions and habitat preferences. Following 6- to 12-hour acclimation at experimental temperature and air-saturated water, animals were placed in darkened sealed chambers filled with 0.2-μm filtered seawater treated with antibiotics (25 mg/liter each of streptomycin and actinomycin) to minimize bacterial respiration. Chamber size ranged from 80 μl to 750 ml, resulting in a ratio of chamber volume to animal mass of ~10 to 100. Seawater PO2 (oxygen partial pressure) was measured optically with a Loligo Systems Witrox 4 meter, PyroScience FireStingO2 meter, or Loligo Systems Sensor Dish Reader. Upon placement in the chamber, animals were allowed to breathe down the ambient oxygen until their oxygen consumption rate could no longer be sustained. Individual trial durations ranged from 6 to 48 hours. Temperature was maintained with Lauda E100 and Thermo Fisher Scientific NESLAB RTE-7 water baths. Oxygen meters were calibrated with air-saturated seawater and concentrated NaSO3 solution. Chambers were stirred with magnetic stirrers (Cole-Parmer Immersible Stirrer EW-04636-50) or via a shaker table. After the experiments were completed, animals were frozen at −80°C before being weighed and measured.
Statistical analysis (metabolic data)
Metabolic data were analyzed using the R package “respirometry” (27). Oxygen consumption rates (MO2) were calculated from the linear rate of PO2 decrease in the respirometer over time (figs. S3 and S4). The first section of each trial, where MO2 was obviously higher because of handling stress, was removed. Each trial was divided into discrete time bins to calculate multiple MO2 values over each trial. Bins of 1/10th the trial duration were used at the highest PO2 values (where good precision was a priority) and 1/100th the trial duration at the lowest PO2 values (where good PO2 resolution was a priority). The critical partial pressure of oxygen, or Pcrit, was defined as the PO2 below which MO2 can no longer be maintained independent of PO2. For each trial, a linear breakpoint relationship was fit to the MO2-PO2 relationship using the R package “segmented” (28).
Pcrits along with environmental oxygen and temperature were used to calculate the MI along these transects. The MI is the temperature-dependent ratio of environmental oxygen supply to oxygen demand (21). It was calculated as the environmental PO2/Pcrit normalized to temperature using the measured temperature sensitivity of Pcrit. MI maps effectively show the factorial increase in oxygen above that just sufficient to sustain aerobic metabolism for particular taxa. This parameter has been used to predict global spatial responses of different taxa to future and past deoxygenation (21). Modeled MI predictions along Wire Flyer transects were compared with the actual animal abundances and zooplankton biomass from MOCNESS net tows (Fig. 5).
Supplementary Material
Acknowledgments
We thank the captain and crew of the R/V Sikuliaq (University of Alaska) and Scripps Institution of Oceanography for additional technical services. Thanks also to D. Ullman and D. Casagrande for Wire Flyer assistance; C. Matson and J. Calderwood for MOCNESS upgrades; S. Gordon (professional photographer, Open Boat Films LLC) for the photographs and movies; and A. Dymowska, J. Ivory, Y. Jin, J. McGreal, and N. Redmond for help at sea. Funding: Funding was provided by the NSF grants OCE1459243 (to K.F.W., C.R., and B.A.S.), OCE1458967 (to C.D.), DGE1244657 (to M.A.B.), and OCE1460819 (URI REU SURFO program to S.R.) plus funding from our respective institutions. Author contributions: K.F.W., B.A.S., C.R., and C.D. conceived the project. K.F.W. led the writing effort, with substantial contributions from all the authors. K.F.W. directed the MOCNESS component including zooplankton abundance and biomass quantification. B.A.S. directed the metabolic experiments and Tucker trawls. C.R. directed the Wire Flyer work. B.A.S., C.D., K.A.S.M., and M.A.B. developed the MI models. D.O., C.T.S., D.M., and S.R. processed and analyzed the zooplankton data. T.J.A. processed the MOCNESS hydrographic data. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Extensive files of continuous hydrographic data from transects are available from C.R. (Wire Flyer) and K.F.W. (MOCNESS). Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/12/eaau5180/DC1
Fig. S1. Map showing the spatial overlap of Wire Flyer transects and MOCNESS tows.
Fig. S2. Photographs of animals discussed in the paper.
Fig. S3. Lucicutia hulsemannae and Gennadas spp. oxygen consumption rates versus body mass and critical oxygen partial pressures.
Fig. S4. Representative respirometry trials showing the effect of PO2 on oxygen consumption rates.
Table S1. Cruise sampling data for all gear.
Table S2. MOCNESS sampling data and abundances for each net.
Table S3. Metabolic rates and critical oxygen partial pressures.
Table S4. Compilation of critical oxygen partial pressures from the literature.
REFERENCES AND NOTES
- 1.Stramma L., Schmidtko S., Levin L. A., Johnson G. C., Ocean oxygen minima expansions and their biological impacts. Deep Sea Res. Pt. 1. 57, 587–595 (2010). [Google Scholar]
- 2.Breitburg D., Levin L. A., Oschlies A., Grégoire M., Chavez F. P., Conley D. J., Garçon V., Gilbert D., Gutiérrez D., Isensee K., Jacinto G. S., Limburg K. E., Montes I., Naqvi S. W. A., Pitcher G. C., Rabalais N. N., Roman M. R., Rose K. A., Seibel B. A., Telszewski M., Yasuhara M., Zhang J., Declining oxygen in the global ocean and coastal waters. Science 359, eaam7240 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Levin L. A., Manifestation, drivers, and emergence of open ocean deoxygenation. Ann. Rev. Mar. Sci. 10, 229–260 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Stramma L., Johnson G. C., Sprintall J., Mohrholz V., Expanding oxygen-minimum zones in the tropical oceans. Science 320, 655–658 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Oschlies A., Schulz K. G., Riebesell U., Schmittner A., Simulated 21st century’s increase in oceanic suboxia by CO2-enhanced biotic carbon export. Glob. Biogeochem. Cycl. 22, GB4008 (2008). [Google Scholar]
- 6.Karstensen J., Stramma L., Visbeck M., Oxygen minimum zones in the eastern tropical Atlantic and Pacific oceans. Prog. Oceanogr. 77, 331–350 (2008). [Google Scholar]
- 7.Keeling R. F., Körtzinger A., Gruber N., Ocean deoxygenation in a warming world. Ann. Rev. Mar. Sci. 2, 199–229 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Robinson C., Steinberg D. K., Anderson T. R., Arístegui J., Carlson C. A., Frost J. R., Ghiglione J.-F., Hernández-León S., Jackson G. A., Koppelmann R., Quéguiner B., Ragueneau O., Rassoulzadegan F., Robison B. H., Tamburini C., Tanaka T., Wishner K. F., Zhang J., Mesopelagic zone ecology and biogeochemistry – a synthesis. Deep Sea Res. Pt. 2 57, 1504–1518 (2010). [Google Scholar]
- 9.Steinberg D. K., Landry M. R., Zooplankton and the ocean carbon cycle. Ann. Rev. Mar. Sci. 9, 413–444 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Wishner K. F., Gelfman C., Gowing M. M., Outram D. M., Rapien M., Williams R. L., Vertical zonation and distributions of calanoid copepods through the lower oxycline of the Arabian Sea oxygen minimum zone. Prog. Oceanogr. 78, 163–191 (2008). [Google Scholar]
- 11.Wishner K. F., Ashjian C. J., Gelfman C., Gowing M. M., Kann L., Levin L. A., Mullineaux L. S., Saltzman J., Pelagic and benthic ecology of the lower interface of the Eastern Tropical Pacific oxygen minimum zone. Deep Sea Res. Pt. 1 42, 93–115 (1995). [Google Scholar]
- 12.Wishner K. F., Outram D. M., Seibel B. A., Daly K. L., Williams R. L., Zooplankton in the eastern tropical north Pacific: Boundary effects of oxygen minimum zone expansion. Deep Sea Res. Pt. 1 79, 122–140 (2013). [Google Scholar]
- 13.Childress J. J., Seibel B. A., Life at stable low oxygen levels: Adaptations of animals to oceanic oxygen minimum layers. J. Exp. Biol. 201, 1223–1232 (1998). [DOI] [PubMed] [Google Scholar]
- 14.Seibel B. A., Critical oxygen levels and metabolic suppression in oceanic oxygen minimum zones. J. Exp. Biol. 214, 326–336 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Seibel B. A., Schneider J. L., Kaartvedt S., Wishner K. F., Daly K. L., Hypoxia tolerance and metabolic suppression in oxygen minimum zone euphausiids: Implications for ocean deoxygenation and biogeochemical cycles. Integr. Comp. Biol. 56, 510–523 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Paulmier A., Ruiz-Pino D., Oxygen minimum zones (OMZs) in the modern ocean. Prog. Oceanogr. 80, 113–128 (2009). [Google Scholar]
- 17.Roman C., Ullman D. S., Hebert D., Licht S., The Wire Flyer towed profiling system. J. Atmos. Oceanic Tech. 10.1175/JTECH-D-17-0180.1 (2018). [Google Scholar]
- 18.Wiebe P. H., Morton A. W., Bradley A. M., Backus R. H., Craddock J. E., Barber V., Cowles T. J., Flierl G. R., New development in the MOCNESS, an apparatus for sampling zooplankton and micronekton. Mar. Biol. 87, 313–323 (1985). [Google Scholar]
- 19.Seibel B. A., Luu B. E., Tessier S. N., Towanda T., Storey K. B., Metabolic suppression in the pelagic crab, Pleuroncodes planipes, in oxygen minimum zones. Comp. Biochem. Physiol. Pt. B Biochem. Mol. Biol. 224, 88–97 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Kiko R., Hauss H., Buchholz F., Melzner F., Ammonium excretion and oxygen respiration of tropical copepods and euphausiids exposed to oxygen minimum zone conditions. Biogeosciences 13, 2241–2255 (2016). [Google Scholar]
- 21.Deutsch C., Ferrel A., Seibel B., Pörtner H.-O., Huey R. B., Climate change tightens a metabolic constraint on marine habitats. Science 348, 1132–1135 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Koslow J. A., Goericke R., Lara-Lopez A., Watson W., Impact of declining intermediate-water oxygen on deepwater fishes in the California Current. Mar. Ecol. Prog. Ser. 436, 207–218 (2011). [Google Scholar]
- 23.Bianchi D., Galbraith E. D., Carozza D. A., Mislan K. A. S., Stock C. A., Intensification of open-ocean oxygen depletion by vertically migrating animals. Nat. Geosci. 6, 545–548 (2013). [Google Scholar]
- 24.Wishner K. F., Gowing M. M., Gelfman C., Living in suboxia: Ecology of an Arabian Sea oxygen minimum zone copepod. Limnol. Oceanogr. 45, 1576–1593 (2000). [Google Scholar]
- 25.Cavan E. L., Trimmer M., Shelley F., Sanders R., Remineralization of particulate organic carbon in an ocean oxygen minimum zone. Nat. Commun. 8, 14847 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Childress J. J., Barnes A. T., Quetin L. B., Robison B. H., Thermally protecting cod ends for recovery of living deep-sea animals. Deep Sea Res. 25, 419–422 (1978). [Google Scholar]
- 27.M. A. Birk, respirometry: Tools for conducting respirometry experiments. R package version 0.6.0 (2018); https://CRAN-R-project.org/package=respirometry.
- 28.Muggeo V. M. R., segmented: An R package to fit regression models with broken-line relationships. R News 8, 20–25 (2008). [Google Scholar]
- 29.Agnew D. J., Jones M. B., Metabolic adaptations of Gammarus duebeni liljeborg (Crustacea, Amphipoda) to hypoxia in a sewage treatment plant. Comp. Biochem. Physiol. A 84, 475–478 (1986). [Google Scholar]
- 30.Agnew D. J., Taylor A. C., The effect of oxygen tension on the physiology and distribution of Echniogammarus pirloti (Sexton and Spooner) and E. obtusatus (Dahl) (Crustacea: Amphipoda). J. Exp. Mar. Biol. Ecol. 87, 169–190 (1985). [Google Scholar]
- 31.Allan G. L., Maguire G. B., Lethal levels of low dissolved oxygen and effects of short-term oxygen stress on subsequent growth of juvenile Penaeus monodon. Aquaculture 94, 27–37 (1991). [Google Scholar]
- 32.Alter K., Paschke K., Gebauer P., Cumillaf J.-P., Pörtner H.-O., Differential physiological responses to oxygen availability in early life stages of decapods developing in distinct environments. Mar. Biol. 162, 1111–1124 (2015). [Google Scholar]
- 33.Anderson S. J., Atkinson R. J. A., Taylor A. C., Behavioral and respiratory adaptations of the mud-burrowing shrimp Calocaris macandreae bell (Thalassinidea: crustacean) to the burrow environment. Ophelia 34, 143–156 (2012). [Google Scholar]
- 34.Bradford S. M., Taylor A. C., The respiration of Cancer pagurus under normoxia and hypoxic conditions. J. Exp. Biol. 97, 273–288 (1982). [Google Scholar]
- 35.Bridges C. R., Brand A. R., Oxygen consumption and oxygen-independence in marine crustaceans. Mar. Ecol. Prog. Ser. 2, 133–141 (1980). [Google Scholar]
- 36.Brill R. W., Bushell P. G., Elton T. A., Small H. J., The ability of blue crab (Callinectes sapidus, Rathbun 1886) to sustain aerobic metabolism during hypoxia. J. Exp. Mar. Biol. Ecol. 471, 126–136 (2015). [Google Scholar]
- 37.Burd B. J., Respiration of a low oxygen tolerant galatheid crab, Munida quadrispina (Benedict, 1902). Can. J. Zool. 63, 2538–2542 (1985). [Google Scholar]
- 38.Butler P. J., Taylor E. W., McMahon B. R., Respiratory and circulatory changes in the lobster (Homarus vulgaris) during long term exposure to moderate hypoxia. J. Exp. Biol. 73, 131–146 (1978). [Google Scholar]
- 39.Childress J. J., The respiratory rates of midwater crustaceans as a function of depth of occurrence and relation to the oxygen minimum layer off southern California. Comp. Biochem. Physiol. 50, 787–799 (1975). [DOI] [PubMed] [Google Scholar]
- 40.Childress J. J., Effects of pressure, temperature and oxygen on the oxygen consumption rate of the midwater copepod Gaussia princeps. Mar. Biol. 39, 19–24 (1976). [Google Scholar]
- 41.Chu J. W. F., Gale K. S. P., Ecophysiological limits to aerobic metabolism in hypoxia determine epibenthic distributions and energy sequestration in the northeast Pacific ocean. Limnol. Oceanogr. 62, 59–74 (2017). [Google Scholar]
- 42.Cochran R. E., Burnett L. E., Respiratory responses of the salt marsh animals, Fundulus heteroclitus, Leiostomus xanthurus, and Palaemonetes pugio to environmental hypoxia and hypercapnia and to the organiphosphate pesticide, azinphosmethyl. J. Exp. Mar. Biol. Ecol. 195, 125–144 (1996). [Google Scholar]
- 43.Cowles D. L., Childress J. J., Wells M. E., Metabolic rates of midwater crustaceans as a function of depth of occurrence off the Hawaiian Islands: Food availability as a selective factor? Mar. Biol. 110, 75–83 (1991). [Google Scholar]
- 44.Crear B. J., Forteath G. N. R., The effect of extrinsic and intrinsic factors on oxygen consumption by the southern rock lobster, Jasus edwardsii. J. Exp. Mar .Biol. Ecol. 252, 129–147 (2000). [DOI] [PubMed] [Google Scholar]
- 45.Dall W., Estimation of routine metabolic rate in a penaeid prawn, Penaeus esculentus Haswell. J. Exp. Mar. Biol. Ecol. 96, 57–74 (1986). [Google Scholar]
- 46.Das T., Stickle W. B., Sensitivity of crabs Callinectes sapidus and C. similis and the gastropod Stramonita haemastoma to hypoxia and anoxia. Mar. Ecol. Prog. Ser. 98, 263–274 (1993). [Google Scholar]
- 47.Diamond D. W., Scott L. K., Forward R. B. J. Jr., Kirby-Smith W., Respiration and osmoregulation of the estuarine crab, Rhithropanopeus harrisii (Gould): effects of the herbicide, alachlor. Comp. Biochem. Physiol. A 93, 313–318 (1989). [DOI] [PubMed] [Google Scholar]
- 48.Donnelly J., Torres J. J., Oxygen consumption of midwater fishes and crustaceans from the eastern Gulf of Mexico. Mar. Biol. 97, 483–494 (1988). [Google Scholar]
- 49.Dupont-Prinet A., Pillet M., Chabot D., Hansen T., Tremblay R., Audet C., Northern shrimp (Pandalus borealis) oxygen consumption and metabolic enzyme activities are severely constrained by hypoxia in the Estuary and Gulf of St. Lawrence. J. Exp. Mar. Biol. Ecol. 448, 298–307 (2013). [Google Scholar]
- 50.Felder D. L., Respiratory adaptations of the estuarine mud shrimp, Callianassa jamaicense (Schmitt, 1935) (Crustacea, Decapoda, Thalassinidea). Biol. Bull. 157, 125–137 (1979). [Google Scholar]
- 51.Forgue J., Massabuau J.-C., Truchot J.-P., What are resting water-breathers lacking O2? Arterial PO2 at the anaerobic threshold in crab. Respir. Physiol. 88, 247–256 (1992). [DOI] [PubMed] [Google Scholar]
- 52.Hagerman L., Weber R. E., Respiratory rate, haemolymph oxygen tension and haemocyanin level in the shrimp Palaemon adspersus: Rathke. J. Exp. Mar. Ecol. 54, 13–20 (1981). [Google Scholar]
- 53.Jawed M., Effects of environmental factors and body size on rates of oxygen consumption in Archaemysis grebnitzkii and Neomysis awatschensis (Crustacea: Mysidae). Mar. Biol. 21, 173–179 (1973). [Google Scholar]
- 54.Kiko R., Hauss H., Dengler M., Sommer S., Melzner F., The squat lobster Pleuroncodes monodon tolerates anoxic “dead zone” conditions off Peru. Mar. Biol. 162, 1913–1921 (2015). [Google Scholar]
- 55.Leffler C. W., Metabolic rate in relation to body size and environmental oxygen concentration in two species of xanthid crabs. Comp. Biochem. Physiol. A. 44, 1047–1052 (1973). [DOI] [PubMed] [Google Scholar]
- 56.Marshall S. M., Nicholls A. G., Orr A. P., On the biology of Calanus finmarchicus. Part VI. Oxygen consumption in relation to environmental conditions. J. Mar. Biol. Assoc. U.K. 20, 1–27 (1935). [Google Scholar]
- 57.McAllen R., Taylor A. C., Davenport J., The effects of temperature and oxygen partial pressure on the rate of oxygen consumption of the high-shore rock pool copepod Tigriopus brevicornis. Comp. Biochem. Physiol. A 123, 195–202 (1999). [Google Scholar]
- 58.McLeese D. W., Watson J., Oxygen consumption of the spider crab (Chionoecetes opilio) and the American Lobster (Homarus americanus) at a low temperature. J. Fish. Res. Board Can. 25, 1729–1732 (2011). [Google Scholar]
- 59.McMahon B. R., McDonald D. G., Wood C. M., Ventilation, oxygen uptake and haemolymph oxygen transport, following enforced exhausting activity in the Dungeness crab Cancer magister. J. Exp. Biol. 80, 271–285 (1979). [Google Scholar]
- 60.Mickel T. J., Childress J. J., Effects of temperature, pressure and oxygen concentration on the oxygen consumption rate of the hydrothermal vent crab Bythograea thermydron (Brachyura). Physiol. Biochem. Zool. 55, 199–207 (1982). [Google Scholar]
- 61.Morris S., Taylor A. C., The respiratory response of the intertidal prawn Palaemon elegans (Rathke) to hypoxia and hyperoxia. Comp. Biochem. Physiol. A 81, 633–639 (1985). [Google Scholar]
- 62.Mukai H., Koike I., Behavior and respiration of the burrowing shrimps Upogebia major (De Haan) and Calianassa japonica (De Haan). J. Crustacean Biol. 4, 191–200 (1984). [Google Scholar]
- 63.Nielsen A., Hagerman L., Effects of short-term hypoxia on metabolism and haemocyanin oxygen transport in the prawns Palaemon adspersus and Palaemonetes varians. Mar. Ecol. Prog. Ser. 167, 177–183 (1998). [Google Scholar]
- 64.Ocampo L., Patiño D., Ramírez C., Effect of temperature on hemolymph lactate and glucose concentrations in the spiny lobster Panulirus interuptus during progressive hypoxia. J. Exp. Mar. Biol. Ecol. 296, 71–77 (2003). [Google Scholar]
- 65.Paschke K., Cumillaf J. P., Loyola S., Gebauer P., Urbina M., Chimal M. E., Pascual C., Rosas C., Effect of dissolved oxygen level on respiratory metabolism, nutritional physiology, and immune condition of southern king crab Lithodes santolla (Molina, 1782) (Decapoda, Lithodidae). Mar. Biol. 157, 7–18 (2010). [Google Scholar]
- 66.Paterson B. D., Thorne M. J., Measurements of oxygen uptake, heart and gill bailer rates of the callianassid burrowing shrimp Trpaea australiensis Dana and its responses to low oxygen tensions. J. Exp. Mar. Biol. Ecol. 194, 39–52 (1995). [Google Scholar]
- 67.Quetin L. B., Childress J. J., Respiratory Adaptations of Pleuroncodes planipes to its environment off Baja California. Mar. Biol. 38, 327–334 (1976). [Google Scholar]
- 68.Rantin F. T., Kalinin A. L., de Freitas J. C., Cardio-respiratory function of swimming blue crab Callinectes danae Smith, during normoxia and graded hypoxia. J. Exp. Mar. Biol. Ecol. 198, 1–10 (1996). [Google Scholar]
- 69.Robertson R. F., Meagor J., Taylor E. W., Specific dynamic action in the shore crab, Carcinus maenas (L.), in relation to acclimation temperature and to the onset of the emersion response. Physiol. Biochem. Zool. 75, 350–359 (2002). [DOI] [PubMed] [Google Scholar]
- 70.Schmitt A. S. C., Uglow R. F., Metabolic responses of Nephrops norvegicus to progressive hypoxia. Aqua. Living Res. 11, 87–92 (1998). [Google Scholar]
- 71.Spoek G. L., The relationship between blood haemocyanin level, oxygen uptake and the heart-beat and scaphonathite-beat frequencies in the lobster Homarus gammarus. Netherlands J. Sea Res. 8, 1–26 (1974). [Google Scholar]
- 72.Stickle W. B., Kapper M. A., Liu L.-L., Gnaiger E., Wang S. Y., Metabolic adaptations of several species of crustaceans and molluscs to hypoxia: Tolerances and microcalorimetric studies. Biol. Bull. 177, 303–312 (1989). [Google Scholar]
- 73.Subrahmanyam C. B., Oxygen consumption in relation to body weight and oxygen tension in the prawn Penaeus indicus (Milne Edwards). Proc. Indian Acad. Sci. 55, 152–161 (1962). [Google Scholar]
- 74.Tankersley R. A., Wieber M. G., Physiological responses of postlarval and juvenile blue crabs Callinectes sapidus to hypoxia and anoxia. Mar. Ecol. Prog. Ser. 194, 179–191 (2000). [Google Scholar]
- 75.Taylor A. C., The respiratory responses of Carcinus maenas to declining oxygen tension. J. Exp. Biol. 65, 309–322 (1976). [DOI] [PubMed] [Google Scholar]
- 76.Thompson R. K., Pritchard A. W., Respiratory adaptations of two burrowing crustaceans, Callianassa californiensis and Upogebia pugettensis (Decapoda, Thalassinidae). Biol. Bull. 136, 274–287 (1969). [Google Scholar]
- 77.Torres J. J., Childress J. J., Respiration and chemical composition of the bathypelagic euphausiid Bentheuphausia amblyops. Mar. Biol. 87, 267–272 (1985). [Google Scholar]
- 78.Torres J. J., Aarset A. V., Donnelly J., Hopkins T. L., Lancraft T. M., Ainley D. G., Metabolism of Antarctic micronektonic crustacean as a function of depth of occurrence and season. Mar. Ecol. Prog. Ser. 113, 207–219 (1994). [Google Scholar]
- 79.Cerezo Valverde J., Aguado-Giménez F., Hernández M. D., García B. G., Oxygen consumption response to gradual hypoxia in spider crab, Maja brachydactyla: Critical and lethal oxygen saturations and recovery ability. J. World Aquacult. Soc. 43, 433–441 (2012). [Google Scholar]
- 80.Vetter R.-A. H., Franke H.-D., Buchholz F., Habitat-related differences in the responses to oxygen deficiencies in Idotea baltica and Idotea emarginata (Isopoda, Crustacea). J. Exp. Mar. Biol. Ecol. 239, 259–272 (1999). [Google Scholar]
- 81.Villarreal H., Ocampo L., Effects of size and temperature on the oxygen consumption of the brown shrimp Penaeus californiensis (Holmes, 1900). Comp. Biochem. Physiol. A 106, 97–101 (1993). [Google Scholar]
- 82.F. M. Waldron, Respiratory and acid-base physiology of the New Zealand rock lobster, Jasus edwardsii (Hutton), 1991, thesis, University of Cantebury, Christchurch, New Zealand. [Google Scholar]
- 83.Weymouth F. W., Crimson J. M., Hall V. E., Belding H. S., Field J. II, Total and tissue respiration in relation to body weight. A comparison of the kelp crab with other crustaceans and with mammals. Physiol. Zool. 17, 50–71 (1944). [Google Scholar]
- 84.J. N. C. Whyte, B. L. Carswell, Determinants for live holding the spot prawn Pandalus platyceros, Brandt. Fisheries and Aquatic Sciences, Canadian Technical Report No. 1129 (1982), 29 pp.
- 85.Winget R. R., Oxygen consumption and respiratory energetics in the spiny lobster, Panulirus interruptus (Randall). Biol. Bull. 136, 301–312 (1969). [DOI] [PubMed] [Google Scholar]
- 86.Wu R. S. S., Or Y. Y., Bioenergetics, growth and reproduction of amphipods are affected by moderately low oxygen regimes. Mar. Ecol. Prog. Ser. 297, 215–223 (2005). [Google Scholar]
- 87.Wu R. S. S., Lam P. K. S., Wan K. L., Tolerance to, and avoidance of, hypoxia by the penaeid shrimp (Metapenaeus ensis). Environ. Poll. 118, 351–355 (2002). [DOI] [PubMed] [Google Scholar]
- 88.Zou E., Steuben B., Acute exposure to naphthalene reduces oxyregulating capacity of the brown shrimp, Penaeus aztecus, subjected to progressive hypoxia. Mar. Biol. 149, 1411–1415 (2006). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/12/eaau5180/DC1
Fig. S1. Map showing the spatial overlap of Wire Flyer transects and MOCNESS tows.
Fig. S2. Photographs of animals discussed in the paper.
Fig. S3. Lucicutia hulsemannae and Gennadas spp. oxygen consumption rates versus body mass and critical oxygen partial pressures.
Fig. S4. Representative respirometry trials showing the effect of PO2 on oxygen consumption rates.
Table S1. Cruise sampling data for all gear.
Table S2. MOCNESS sampling data and abundances for each net.
Table S3. Metabolic rates and critical oxygen partial pressures.
Table S4. Compilation of critical oxygen partial pressures from the literature.


