Abstract
Context
The impact of vitamin D deficiency on the success of ovarian stimulation according to underlying infertility diagnosis has not been investigated.
Objective
To evaluate the relationship between vitamin D deficiency and reproductive outcomes after ovarian stimulation in women with either polycystic ovary syndrome (PCOS) or unexplained infertility.
Design
Retrospective cohort study.
Setting
Analysis of randomized controlled trial (RCT) data.
Participants
Participants from the Pregnancy in Polycystic Ovary Syndrome II (PPCOS II) RCT (n = 607); participants from the Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation (AMIGOS) RCT of unexplained infertility (n = 647).
Interventions
Serum 25(OH)D levels measured in banked sera.
Main Outcome Measures
Primary: live birth; secondary: ovulation (PPCOS II), pregnancy, and early pregnancy loss.
Results
In PPCOS II, subjects with vitamin D deficiency [25(OH)D < 20 ng/mL or 50 nmol/L] were less likely to ovulate (adjusted OR, 0.82; 95% CI, 0.68 to 0.99; P = 0.04) and experienced a 40% lower chance of live birth (adjusted OR, 0.63; 95% CI, 0.41 to 0.98; P = 0.04) than those not deficient. In AMIGOS, no significant association between vitamin D deficiency and live birth was noted. In pregnant subjects from both studies, vitamin D deficiency was associated with elevated risk of early pregnancy loss (OR, 1.6; 95% CI, 1.0 to 2.6; P = 0.05).
Conclusions
In this investigation of women pursuing ovarian stimulation, the association between vitamin D deficiency and diminished live birth relied on carrying the diagnosis of PCOS and was not observed in unexplained infertility. Given the generally modest success of ovarian stimulation, addressing vitamin D deficiency may prove an important treatment adjunct for many infertile women.
In women with infertility undergoing ovarian stimulation, pretreatment vitamin D deficiency is associated with diminished live birth rates in those with PCOS but not those with unexplained infertility.
There is a growing acknowledgment of the important role of vitamin D in human reproduction. Vitamin D receptors (site of action) and 1 α-hydroxylase (site of synthesis) are present throughout the female reproductive tract, and vitamin D is known to regulate genes responsible for aspects of ovarian, endometrial, and placental function (1–3). Consistent with the relevance of vitamin D to female reproductive physiology is emerging evidence that women with infertility and vitamin D deficiency have diminished chance of conceiving a pregnancy in response to treatment compared with those who are vitamin D replete. However, the bulk of this evidence derives from studies in women undergoing in vitro fertilization (IVF) (4–7) rather than from non-IVF treatments, which are more commonly performed to treat infertility. The clinical significance of a putative role for vitamin D in reproduction is underscored by the prevalence of vitamin D insufficiency or deficiency in 45% to 90% of reproductive age women (8, 9). The paucity of data evaluating the impact of vitamin D status on the efficacy of non-IVF infertility treatments represents an important gap in reproductive research.
There are numerous etiologies for infertility including polycystic ovary syndrome (PCOS) and unexplained infertility. PCOS, a condition causing anovulatory infertility, is the most common endocrinopathy affecting reproductive-age women (5% to 10% prevalence) (10–12). PCOS and vitamin D deficiency have overlapping metabolic features in their strong associations with obesity and insulin resistance (13, 14). Unexplained infertility affects 15% to 37% of infertile couples and is diagnosed when no clinical barrier to fertility is identified after extensive testing has been performed (11, 15, 16). Current treatment paradigms for each disorder include the use of ovarian stimulation medications to either induce ovulation (in PCOS) or increase the number of follicles recruited and oocytes released (in unexplained infertility) (11, 16, 17). Ovarian stimulation treatment pitfalls include lack of response in up to 25% of patients with PCOS (17, 18) and overall modest per cycle pregnancy rates in all women necessitating numerous repeated treatments (10, 16, 17).
The primary hypothesis of this study was that vitamin D deficiency is an important modifiable contributor to diminished treatment success in women with either PCOS or unexplained infertility undergoing ovarian stimulation (which, going forward, represents either oral ovulation induction or oral/injectable ovarian stimulation). To test this hypothesis, we performed an assessment of vitamin D status in stored sera from completed randomized controlled trials conducted by the Reproductive Medicine Network (RMN): (i) The Pregnancy in Polycystic Ovary Syndrome II (PPCOS II) trial (10) and (ii) The Assessment of Multiple Intrauterine Gestations From Ovarian Stimulation (AMIGOS) trial (19). The RMN is funded by the National Institute of Child Health and Human Development as a cooperative effort of seven clinical sites charged with conducting high-quality clinical studies in reproductive medicine.
Subjects and Methods
Study cohorts
Vitamin D status was determined by measuring serum 25(OH)D, which is the primary circulating form of the vitamin. The study design and methods for each of the RMN trials has been previously described (10, 19). PPCOS II was a double-blind, multicenter trial of 750 women with PCOS who were randomized to receive letrozole or clomiphene citrate to determine rates of ovulation and live birth for up to five treatment cycles (10). The trial enrolled subjects ages 18 to 40 years meeting modified Rotterdam criteria for PCOS and the exclusion of disorders mimicking PCOS. Additional inclusion criteria were ≥1 patent fallopian tube and a normal uterine cavity, a male partner with a sperm concentration of ≥14 million per milliliter, with documented motility according to World Health Organization cutoff points, in at least one ejaculate during the previous year. The primary trial outcomes demonstrated the superiority of letrozole compared with Clomid in achieving ovulation and live birth. Of the original 750 PPCOS II subjects, 607 had banked serum available to assess vitamin D status and were included in this study.
The AMIGOS trial was a randomized multicenter trial that enrolled 900 subjects to assess whether ovulation induction for up to 4 cycles in couples with unexplained infertility treated with the aromatase inhibitor letrozole reduced multiple gestations while maintaining a comparable pregnancy success rate to that achieved by ovulation induction with either gonadotropins or Clomid (19). Women were between 18 and 40 years of age with regular menses, had a normal uterine cavity with ≥1 patent fallopian tube, and had a male partner with a semen specimen of ≥5 million sperm/mL. The trial concluded that in women with unexplained infertility, ovarian stimulation with letrozole resulted in a significantly lower frequency of multiple gestation and a lower frequency of live birth compared with gonadotropin but not as compared with Clomid. Of the original 900 AMIGOS subjects, 647 had banked serum available to assess vitamin D status and were included in this study.
Permission was received from the RMN Resource and Data Access committee to access serum and clinical data on study participants from PPCOS II and AMIGOS. The study protocol was submitted to the University of Pennsylvania institutional review board and considered eligible for institutional review board review exemption authorized by 45 CFR 46.101, category 4. Both PPCOS II (NCT00719186) and AMIGOS (NCT01044862) were registered with ClinicalTrials.gov.
Data collection
For each RMN study, a baseline study visit before randomization and treatment initiation occurred; at this visit, blood was collected from subjects providing consent to have serum stored in the RMN biorepository (10, 19). In properly banked serum, 25(OH)D is stable for at least 6 years and after up to four freeze–thaw cycles (20, 21). Quantification of total 25(OH)D was performed using liquid-liquid extraction and liquid chromatography-tandem mass spectrometry in the Department of Laboratory Medicine at the University of Washington (22). This assay has been shown to meet the target criteria for precision and bias established by the Vitamin D Standardization Program (23). Subject-level data regarding demographics and relevant clinical variables including outcomes of interest from PPCOS II and AMIGOS were abstracted and deidentified.
Clinical measures and outcomes
The primary study outcome was live birth. Secondary outcomes included ovulation, pregnancy, and pregnancy loss. In PPCOS II subjects, ovulation was defined by a progesterone level ≥3 ng/mL (10 nmol/L) measured at the monthly midluteal visit 3 weeks after initiation of study medication ± 4 days. Pregnancy was defined as a serum level of human chorionic gonadotropin >10 mIU/mL (10). Vitamin D status was characterized according to Endocrine Society cutoffs (24). Vitamin D deficiency is characterized as a 25(OH)D level <20 ng/mL (50 nmol/L); a 25(OH)D level of 20 to 29 ng/mL (52 to 72 nmol/L) is consistent with vitamin D insufficiency, and a level ≥30 ng/mL (75 pmol/L) indicates sufficient vitamin D status (13, 24, 25). In this analysis, vitamin D status was dichotomized as 25(OH)D < 20 ng/mL (D deficiency) or 25(OH)D ≥ 20 ng/mL groups. These categories reflected the most robust cutpoint when performing univariate tests of the associations between 25(OH)D levels and live birth and based on locally weighted regression of live birth on 25(OH)D levels depicted graphically using a locally weighted scatterplot smoothing curve (Supplemental Fig 1) .
Statistical analysis and power
Analyses were conducted separately for the PPCOS II and AMIGOS subjects. Comparisons of continuous data were analyzed using Student t test or Wilcoxon rank-sum tests, whereas categorical data were analyzed using the χ2 test. Graphical and statistical tests of normality were used to examine distributional assumptions of continuous variables. Logistic regression was used to calculate ORs to perform initial tests of association between D deficiency and outcomes of interest. Multivariable logistic regression was used to adjust OR estimates for relevant covariates. For the primary study outcome of live birth, prepregnancy body mass index (BMI), and study treatment arm were selected a priori to be included in multivariable models. Otherwise, model covariates were selected if the marginal association between the variable and the outcome was present at the P ≤ 0.1 level of significance. Variables were removed in a backward stepwise fashion if they did not maintain statistical significance at P < 0.05 unless there was strong biological plausibility for retention in the model. For the PPCOS II ovulation and live birth multivariable models, total testosterone was treated as a categorical variable and examined as quartiles to limit the effect of extreme values. A final logistic regression model for live birth associated with D deficiency in PPCOS II subjects was adjusted for age, BMI, race, quartiles of total testosterone, and severity of insulin resistance. Collinearity diagnostics were evaluated for the final multivariable model to ensure the stability of regression coefficients and tests of statistical significance. Likelihood ratio tests were performed to determine whether the associations between D deficiency and chance of live birth were modified across strata of race and treatment.
Kaplan-Meier curves were generated to graphically depict time from randomization to positive pregnancy in subjects who ultimately achieved a live birth according to vitamin D status. A log-rank test was used to determine the significance of this association. A detailed power calculation is described in the Supplemental Methods. SAS, version 9.2, was used for statistical analysis.
Results
Vitamin D status was evaluated in serum from a total of 607 PPCOS II subjects (81% of 750 original participants) and 647 AMIGOS subjects (71.9% of 900 original participants). Baseline demographics and clinical features of the study cohorts are summarized in Table 1. In PPCOS II and AMIGOS, 41% and 25% of subjects met criteria for vitamin D deficiency, respectively. Poor vitamin D status was significantly more prevalent in PPCOS participants than AMIGOS participants (P < 0.0001).
Table 1.
Baseline Demographic and Clinical Factors by Study
| Demographics/Clinical Factors | PPCOS II n = 607 | AMIGOS n = 647 |
|---|---|---|
| Age, mean ± SD | 28.8 ± 4.21 | 32.3 ± 4.19 |
| Race | ||
| White | 81 (486) | 84 (517) |
| Black | 13 (80) | 7 (45) |
| Other | 5.4 (32) | 8 (51) |
| Ethnicity | ||
| Non-Hispanic | 80 (479) | 88 (539) |
| Hispanic | 20 (119) | 12 (74) |
| BMI, mean ± SD, kg/m2 | 35.5 ± 9.21 | 27.1 ± 6.64 |
| Pretreatment vitamin D status | ||
| 25(OH)D, mean ± SD, ng/dL | 22.3 ± 7.6 | 25.4 ± 8.7 |
| <20 (deficient), ng/mL | 41 (246) | 25 (155) |
| 20–29.9 (insufficient), ng/mL | 45 (270) | 49 (303) |
| ≥30 (sufficient), ng/mL | 14 (82) | 25 (155) |
| Gravidity (Y/N) | 34 (202) | 41 (249) |
| Smoking (Y/N) | 15 (90) | 8 (47) |
| SHBG, mean ± SD, nmol/L | 33.2 ±,22.8 | 59.6 ± 28.4 |
| Free androgen index, mean ± SDa | 64.5 ± 47.7 | 18.1 ± 54.1 |
| Total testosterone, mean ± SD, ng/dL | 54.6 ± 28.0 | 25.1 ± 16.0 |
| HOMA IR, mean ± SDb | 4.43 ± 9.79 | 2.11 ± 3.76 |
| HOMA IR categories of insulin resistance, % (N) | ||
| Normal | 50 (297) | 84 (515) |
| Moderate | 22 (130) | 9 (56) |
| Severe | 29 (171) | 7 (42) |
| AMH, mean ± SD, ng/mL | 8.08 ± 7.19 | 2.61 ± 2.09 |
To convert to SI units for 25(OH)D, multiply by 2.5; for total testosterone, multiply by 0.0347; for AMH, multiply by 7.14.
Abbreviations: HOMA IR, Homeostatic Model Assessment of Insulin Resistance; N, no; SHBG, sex hormone binding globulin; Y, yes.
Free androgen index: total testosterone (nmol/L)/SHBG × 100.
HOMA IR: fasting insulin × tasting glucose/405; HOMA-IR cutpoints for insulin resistance: normal, <3; moderate, 3–5; severe, >5.
Demographic variables and hormonal and metabolic markers as functions of vitamin D status: PPCOS II
Consistent with established risk factors for poor vitamin D status, black race (P < 0.0001) and obesity (P < 0.0001) were associated with higher prevalence of D deficiency in PPCOS II subjects (Table 2). Subjects with D deficiency were significantly more likely than those without D deficiency to have higher degrees of hyperandrogenemia and insulin resistance. Although mean anti-Müllerian hormone (AMH) was comparable across vitamin D groups, a statistically significant positive linear association between AMH and 25(OH)D was observed (Pearson r, 0.2; P < 0.0001).
Table 2.
Clinical and Demographic Factors Compared According to Vitamin D Status in PPCOS II
| 25(OH)D < 20 ng/dL n = 247 | 25(OH)D ≥ 20 ng/dL n = 360 | P | |
|---|---|---|---|
| Age, mean ± SD | 28.7 ± 4.45 | 29.0 ± 4.04 | 0.5 |
| Race, % (N) | <0.0001 | ||
| White | 70 (172) | 89 (314) | |
| Black | 23 (56) | 7 24) | |
| Other | 7 (18) | 4 (14) | |
| Ethnicity, % (N) | 0.002 | ||
| Non-Hispanic | 75 (184) | 84 (295) | |
| Hispanic | 25 (62) | 16 (57) | |
| BMI, % (N) | <0.0001 | ||
| <30 | 17.8 (44) | 41.4 (149) | |
| ≥30 | 82.2 (202) | 59 (211) | |
| Ever pregnant, % (N) | 35.1 (72) | 68.8 (21.9) | 0.046 |
| AMH, mean ± SD, ng/mL | 7.7 ± 7.11 | 8.3 ± 7.24 | 0.3 |
| LH, mean ± SD | 9.7 | 11.6 | 0.002 |
| Treatment | 0.2 | ||
| Clomid | 46 (113) | 51 (184) | |
| Letrozole | 54 (134) | 49 (176) | |
| Testosterone, mean ± SD, ng/dL | 58.4 ± 31.2 | 51.9 ± 25.3 | 0.005 |
| Free Androgen Index, mean ± SDa | 77.3 ± 55.7 | 55.6 ± 38.9 | <0.0001 |
| SHBG, nmol/L | 28.3 | 36.8 | <0.0001 |
| HOMA IR categories of insulin resistance, % (N)b | <0.0001 | ||
| Normal | 36 (88) | 59 (209) | |
| Moderate | 25 (62) | 19 (68) | |
| Severe | 39 (96) | 21 (75) |
To convert to SI units for 25(OH)D, multiply by 2.5; for total testosterone, multiply by 0.0347.
Free androgen index: total testosterone (nmol/L)/SHBG × 100.
HOMA IR: fasting insulin × tasting glucose/405; HOMA-IR cutpoints for insulin resistance: normal, <3; moderate, 3–5; severe, >5.
Ovulation as a function of vitamin D status: PPCOS II
A total of 2262 ovulation induction cycles were carried out among the 595 PPCOS II subjects evaluated. The cumulative ovulation rate of 42.4% (959 ovulatory cycles of a total of 2262 treatment cycles) in subjects with D deficiency was significantly lower than the cumulative ovulation rate of 57.6% (1303 ovulatory cycles of 2262 total treatment cycles) in subjects without D deficiency (P = 0.008). Moreover, 23% of the PPCOS II subjects demonstrated resistance to ovarian stimulation by completing the trial without any evidence of ovulation in response to either Clomid or letrozole. Subjects with D deficiency were 21% less likely to ovulate at all in response to ovarian stimulation than those without D deficiency (P = 0.03, Table 3). After adjusting for the effects of race, obesity, treatment received during PPCOSII (Clomid vs letrozole), baseline AMH, LH, testosterone, and degree of insulin resistance, D deficiency remained a significant predictor of resistance to ovulation induction [adjusted OR (AOR), 0.82, 95% CI, 0.68 to 0.99; P = 0.04; Table 3].
Table 3.
Vitamin D Deficiency and Clinical Factors Associated With Ovulation in PPCOS II
| OR | 95% CI | AOR a | 95% CI | P | |
|---|---|---|---|---|---|
| Vitamin D status | 0.03 | ||||
| 25(OH)D ≥ 20 | 1.00 | — | 1.00 | — | |
| 25(OH)D < 20 | 0.79 | 0.68–0.94 | 0.81 | 0.67–0.98 | |
| Race | <0.0001 | ||||
| White | 1.00 | — | 1.00 | — | |
| Black | 2.0 | 1.52–2.52 | 2.36 | 1.794–3.104 | |
| Other | 1.5 | 1.06–2.14 | 1.326 | 0.916–1.920 | |
| BMI | <0.0001 | ||||
| <30 | 1.00 | — | 1.00 | — | |
| ≥30 | 0.54 | 0.45–0.64 | 0.546 | 0.434–0.687 | |
| Treatment | <0.0001 | ||||
| Letrozole | 1.00 | — | 1.00 | — | |
| Clomiphene | 0.58 | 0.49–0.68 | 0.567 | 0.477–0.675 | |
| AMH | 0.97 | 0.95–0.98 | 0.947 | 0.932–0.961 | <0.0001 |
| LH | 1.02 | 1.01–1.04 | 1.01 | 1.0–1.024 | 0.04 |
| Quartiles of total testosterone, ng/dL | 0.04 | ||||
| Q1 (4–33.1) | 1.00 | — | 1.00 | — | |
| Q2 (33.2–49.3) | 0.90 | 0.71–1.14 | 1.04 | 0.81–1.33 | |
| Q3 (49.5–66.6) | 0.78 | 0.61–1.00 | 1.01 | 0.77–1.31 | |
| Q4 (66.7–226) | 0.53 | 0.42–0.68 | 0.74 | 0.56–0.98 | |
| HOMA IR categories of insulin resistance | 0.0003 | ||||
| Normal | 1.0 | — | 1.00 | — | |
| Moderate | 0.68 | 0.55–0.84 | 0.89 | 0.7–1.13 | |
| Severe | 0.5 | 0.42–0.61 | 0.63 | 0.5–0.79 |
AOR for race, obesity, treatment received during PPCOS II (Clomid vs letrozole), AMH, LH, testosterone, and degree of insulin resistance.
Pregnancy as a function of vitamin D status: PPCOS II
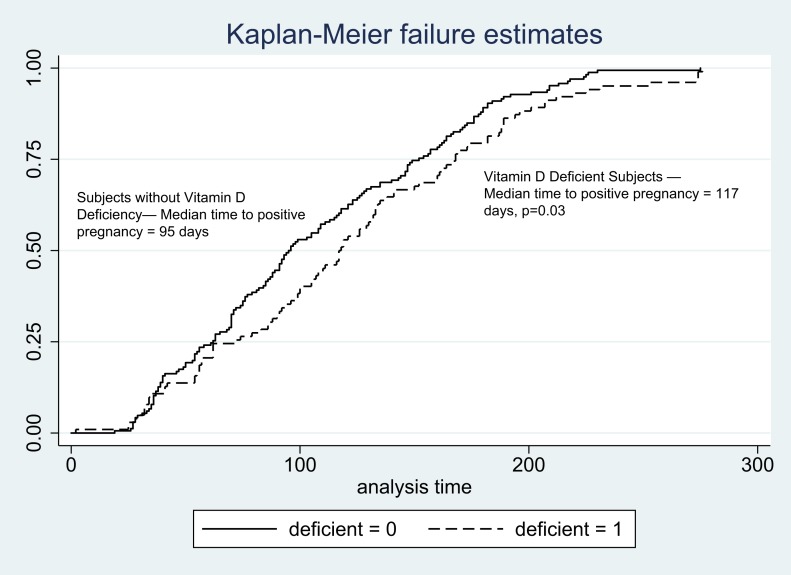
A total of 205 PPCOS II subjects (33.8% of the PPCOS II subjects evaluated) achieved a pregnancy with ovarian stimulation. PPCOS II subjects with D deficiency had a 28% positive pregnancy rate, whereas those who were not vitamin D deficient had a 37.8% positive pregnancy rate (P = 0.01, Table 4). Subjects who were vitamin D deficient were significantly less likely to achieve a pregnancy than those who were not deficient after controlling for the effects of age, study treatment (Clomid vs letrozole), and obesity (AOR, 0.69; 95% CI, 0.48 to 0.99; P = 0.049). Of the subjects ultimately achieving a live birth, median time from randomization to initial positive pregnancy test was 22 days longer in those with D deficiency than in those who were not deficient (P = 0.03). This difference is depicted graphically in Fig. 1.
Table 4.
PPCOS II Early Pregnancy Outcomes as a Function of Vitamin D Status
| Vitamin D Status | Pregnancy (n) | Pregnancy OR (95% CI) | P | Pregnancy AOR (95% CI) a | P | Pregnancy Loss OR (95% CI) | P |
|---|---|---|---|---|---|---|---|
| 25(OH)D ≥ 20 | 136 | 1.0 | 0.01 | 1.0 | 0.049 | 1.0 | 0.3 |
| 25(OH)D < 20 | 69 | 0.64 (0.45–0.9) | 0.69 (0.48–0.99) | 1.4 (0.7–2.8) |
AOR for age, treatment received during PPCOS II (Clomid vs letrozole), and obesity.
Figure 1.
Time from PPCOS II randomization to pregnancy (d) according to vitamin D status.
Early pregnancy loss as a function of vitamin D status: PPCOS II
Of the PPCOS II subjects evaluated who achieved a pregnancy, 45 (22%) experienced a subsequent pregnancy loss by gestation week 12. Although pregnancy loss was more likely to occur in subjects with D deficiency (26%) than in those who were non-D deficient (18%), this association did not achieve statistical significance (Table 4,P = 0.3).
Live birth as a function of vitamin D status: PPCOS II
Of the PPCOS II subjects evaluated, 25.7% (n = 156) achieved a live birth. The live birth rate in subjects who were D-deficient was 19% compared with 30% in in those without vitamin D deficiency (P = 0.02). Independent of the effects of age, race, obesity, study treatment, degree of insulin resistance, and quartiles of total testosterone, women with D deficiency were 37% less likely to achieve a live birth than those were not vitamin D deficient (AOR, 0.63; 95% CI, 0.41 to 0.98; P = 0.04; Table 5). Season of the blood draw that generated subject samples for 25(OH)D assessment was not included in the final live birth model because adjusting for season resulted in minimal change to the point estimate for vitamin D deficiency (AOR, 0.61; 95% CI, 0.39 to 0.95; P = 0.03) and the season variables did not have a substantial association with live birth in the model. The correlation between the coefficients for BMI and vitamin D deficiency in the live birth model was assess to the degree of collinearity between these variables as a potential challenge to the validity of our findings. The weak correlation (−0.145) between the vitamin D deficiency and BMI coefficients indicates that these variables were not collinear in our live birth model and supports the stability of our estimates.
Table 5.
Vitamin D Deficiency and Clinical Factors Associated With Live Birth in PPCOSII
| OR | 95% CI | AOR a | 95% CI | P | |
|---|---|---|---|---|---|
| Vitamin D status | 0.04 | ||||
| 25(OH)D ≥ 20 | 1.00 | — | 1.00 | — | |
| 25(OH)D < 20 | 0.54 | 0.37–0.8 | 0.63 | 0.41–0.98 | |
| Age | 0.95 | 0.91–0.99 | 0.94 | 0.9–0.99 | 0.01 |
| Race | 0.6 | ||||
| White | 1.00 | — | 1.00 | — | |
| Black | 0.92 | 0.53–1.58 | 1.1 | 0.61–2.0 | |
| Other | 0.65 | 0.26–1.61 | 0.64 | 0.25–1.7 | |
| BMI | 0.0003 | ||||
| <30 | 1.00 | — | 1.00 | — | |
| 30+ | 0.4 | 0.27–0.58 | 0.33 | 0.23–0.58 | |
| Treatment | 0.005 | ||||
| Letrozole | 1.00 | — | 1.00 | — | |
| Clomiphene | 0.63 | 0.43–0.91 | 0.57 | 0.39–0.84 | |
| HOMA IR categories of insulin resistance | 0.5 | ||||
| Normal | 1.00 | — | 1.00 | — | |
| Moderate | 0.71 | 0.44–1.14 | 1.4 | 0.79–2.4 | |
| Severe | 0.57 | 0.36–0.89 | 1.1 | 0.62–1.9 | |
| Quartiles of total testosterone, ng/dL | 0.04 | ||||
| Q1 (4–33.1) | 1.00 | — | 1.00 | — | |
| Q2 (33.2–49.3) | 0.67 | 0.41–1.1 | 0.64 | 0.38–1.1 | |
| Q3 (49.5–66.) | 0.69 | 0.41–1.16 | 0.73 | 0.42–1.3 | |
| Q4 (66.7–226) | 0.43 | 0.25–0.74 | 0.43 | 0.24–0.77 |
AOR for age, BMI, race, degree of insulin resistance, total testosterone, and treatment received during PPCOS II (Clomid vs letrozole). Other AORs are controlled for vitamin D status and all other covariates in the table.
There was no important interaction between D deficiency and treatment arm or between D deficiency and race on the odds of live birth.
Reproductive outcomes as a function of vitamin D status: AMIGOS
A total of 228 AMIGOS subjects (34.7%) achieved a positive pregnancy with study treatment. Pregnancy rates were comparable in subjects with and without D deficiency (35.9% and 34.2%, respectively; OR 1.07 95% CI 0.73-1.58, P = 0.7). Of the pregnancies achieved by AMIGOS participants (n = 228), nearly 21% were complicated by a subsequent early pregnancy loss (n = 47). Subjects with D deficiency experienced an early pregnancy loss rate of 28.3% which was 80% higher (OR 1.82, 95% CI 0.92-3.61, P = 0.09) than in those who were not deficient (18%). The cumulative live birth rate in AMIGOS subjects evaluated was 30% (n = 194). In subjects with D deficiency the live birth rate was 32% compared with 29% in those who were not vitamin D deficient (OR 1.1, 95% CI 0.7-1.7, P = 0.5).
Early pregnancy loss as a function of vitamin D status—AMIGOS and PPCOS II combined
When combining subjects from both RMN studies to evaluate the association between preconception vitamin D status and subsequent early pregnancy loss, a total of 92 early losses were observed (21.3%). Subjects with D deficiency were 60% more likely to experience a pregnancy loss than were subjects without vitamin D deficiency (pregnancy loss rate 27.1% and 18.8%, respectively; OR, 1.6; 95% CI, 1.0 to 2.6; P = 0.05).
Discussion
This investigation demonstrates that the association between vitamin D deficiency and ovarian stimulation treatment outcomes differs according to infertility diagnosis. The data showed that vitamin D deficiency in women with PCOS who underwent ovarian stimulation for the treatment of infertility was associated with significantly diminished rates of ovulation, of pregnancy, and ultimately a reduced chance of live birth. In contrast, the live birth rate in women with unexplained infertility treated with ovarian stimulation did not diminish in association with vitamin D deficiency. Our findings support the concept that vitamin D deficiency negatively affects pathways specific and integral to the phenotype of women with PCOS. Furthermore, the association between vitamin D deficiency and increased risk of miscarriage observed when pregnant subjects from the AMIGOS and PPCOS II cohorts were evaluated together suggests that vitamin D deficient status may negatively affect pathways involved in the maintenance of early pregnancy, regardless of the underlying infertility diagnosis (6).
Vitamin D deficiency was associated with lack of ovulation independent of baseline reproductive/ metabolic features of PCOS severity and demographic factors, suggesting that additional pathways regulated by vitamin D that could not be accounted for in this investigation. One potential pathway involves advanced glycation end-products (AGEs), which are proinflammatory molecules that have been identified in the ovarian granulosa and theca layers of women with PCOS (26, 27). Current evidence suggests that correcting vitamin D deficiency in women with PCOS leads to a substantial increase in levels of sRAGE, the soluble AGE receptor that binds to AGEs, reducing their ability to activate receptors in the ovary that could disrupt follicular development (28, 29). Ongoing studies should clarify the effect of vitamin D deficiency and its treatment on pathways of reproductive function.
The association between high AMH and diminished ovulatory response to ovarian stimulation has been demonstrated in a recent secondary analysis of the PPCOS II cohort and by additional investigators (30, 31). In vitro and epidemiologic studies have suggested a role for vitamin D in regulating AMH and AMHR-II expression such that vitamin D deficiency could result in AMH elevation or altered signaling through the AMH receptor (28, 32, 33). Although we did not observe an important association between vitamin D deficiency and mean AMH, a substantial positive correlation between total 25(OH)D and AMH was noted. This result is consistent with evidence in the epidemiologic literature (34), but contrasts with the data supporting vitamin D as a negative regulator of AMH action. The relationship among vitamin D status, AMH, and ovulation in women with PCOS is undeniably complex, requiring additional study.
The unique association between vitamin D deficiency and features of PCOS may explain the potential salutary effects of normalizing vitamin D on reproductive (28, 32) and metabolic (28, 35) pathways related to ovarian stimulation treatment outcomes. The underpinnings of unexplained infertility for any given couple could encompass subtle derangements in a wide array and combination of female and male factors, many of which may not be prone to the effects of vitamin D deficiency. In a separate secondary analysis of the AMIGOS trial, Hansen et al. (15) described increased duration of infertility and annual income <$50,000 as substantial independent predictors of diminished rate of live birth in women with unexplained infertility, whereas obesity and insulin resistance were not associated with live birth. This suggests that barriers to accessing care impede the likelihood of treatment success in women with unexplained infertility undergoing ovarian stimulation in ways that metabolic and anthropometric indicators do not.
Correcting vitamin D deficiency in women with PCOS has been shown to significantly improve ovarian follicular growth, dominant follicle formation, and regularity of menstrual cycles (36, 37). These findings are consistent with recent evidence that vitamin D may have a direct trophic impact upon folliculogenesis and oocyte maturation that is dose and follicle stage dependent (38). We propose that the reproductive effect of vitamin D status may be influential in the context of aberrant folliculogenesis present in women with PCOS.
Among the strengths of this study is using large, well-characterized study populations allowing for statistical power to test associations and the ability to thoroughly address confounding. The association between vitamin D deficiency and live birth in PPCOS II was independent of the effects of race, age, treatment, degree of hyperandrogenemia, degree of insulin resistance, and obesity. Our results support the idea that vitamin D deficiency is detrimental to reproduction in women with modified Rotterdam criteria for PCOS, which was used to enroll subjects in PPCOS II. Prior work demonstrating diminished live birth in sujbects who were vitamin D deficient in the PPCOS I study focused on women with PCOS based on National Institutes of Health diagnostic criteria (39). The reproducibility of the finding that vitamin D deficiency has negative reproductive consequences for women with PCOS regardless of which diagnostic criteria are applied speaks to the robustness of this association.
As with prior studies in reproduction (4, 7, 39), 25(OH)D was measured at a single pretreatment time point, making it impossible to characterize variation resulting from season or vitamin supplementation. However, any potential bias introduced by vitamin D variation was likely nondifferential with respect to the outcomes under study. It is extremely unlikely that such variation would entirely explain the null finding between vitamin D deficiency and treatment outcomes in the AMIGOS study. In this investigation, we did not have access to serum from the male partners and were unable to ascertain whether partner vitamin D deficiency contributes to treatment outcomes in either AMIGOS or PPCOS II. The receptor for vitamin D is present on sperm and in the male reproductive tract, making this an important line of future investigation in fertility (40).
The presence of a 60% increased risk of early pregnancy loss among all pregnant patients with preconception vitamin D deficiency (AMIGOS and PPCOS II combined) approached statistical significance (P = 0.05). Although this finding is in line with existing epidemiological evidence of detrimental decidual-placental effects of vitamin D deficiency (6), it does not preclude the possibility of embryonic effects of vitamin D deficiency contributing to pregnancy loss. Additional studies exploring larger numbers of early pregnancies are needed to clarify this association.
Our finding that women with PCOS who are vitamin D deficient have impaired chances of ovulation, pregnancy, and live birth with ovarian stimulation builds upon the evidence supporting a role for vitamin D in human reproduction. Considering the prevalence of PCOS and vitamin D deficiency alongside the vast number of unsuccessful ovarian stimulation cycles conducted in this country every year, these results are pertinent to the reproductive outcomes of a substantial number of infertile women. As the results of future studies of vitamin D and reproduction accumulate, potentially building toward well-designed intervention studies, a reexamination of current periconceptual screening, and treatment guidelines may be warranted.
Supplementary Material
Acknowledgments
We thank the members and principal investigators of the Reproductive Medicine Network for their contributions to the original trials upon which this research is based. In addition to the authors, other members of the National Institute of Child Health and Human Development Reproductive Medicine Network were: University of Pennsylvania: C. Coutifaris; University of Florida: G. Christman; University of Texas Health Science Center at San Antonio: R. Robinson, R. Brzyski; University of Colorado: W. Schlaff; University of Vermont: P. Casson; SUNY Upstate Medical University: J. C. Trussell; and Eunice Kennedy Shriver National Institute of Child Health and Human Development: E. Eisenberg, C. Lamar, L. DePaolo. We also thank Charikleia Kalliora for assistance with editing the manuscript. Presented as an oral presentation at the Annual Meeting of the American Society for Reproductive Medicine Society 27 October–1 November 2017, San Antonio, Texas.
Financial Support: This study was supported by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant U10 HD27049 (to C. Coutifaris, University of Pennsylvania), Grant U10 HD38992 (to R.S.L.); Grant U10 HD055925 (to H.Z.); Grant U10 HD39005 (to M.P. D.); Grant U10 HD38998 (to W. Schlaff and R. Alvero, University of Colorado); Grant U10 HD055936 (to G. Christman, University of Florida); Grant U10 HD055942 (to R. Brzyski and R. Robinson, University of Texas Health Science Center at San Antonio); Grant U10 HD055944 (to P. Casson, University of Vermont); Grant U54 HD068157 (to S.F.B); and U54-HD29834 to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core of the Specialized Cooperative Centers Program in Reproduction and Infertility Research. S.F.B. is supported by the Penn Presbyterian Harrison Fund. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health. Both the Pregnancy in Polycystic Ovary Syndrome II (NCT00719186) and Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation trials (NCT01044862) from which data were derived for this study were registered with ClinicalTrials.gov.
Clinical Trial Information: ClinicalTrials.gov nos. NCT00719186 (registered 15 May 2009) and NCT01044862 (registered 8 January 2010).
Disclosure Summary: D.B.S. is co-inventor of using anti-Müllerian hormone as a means of accessing ovarian reserve and receives royalties from a license agreement between Rutgers Medical School/Massachusetts General Hospital and Beckman-Coulter. M.P.D. is a board member and advanced reproductive care consultant for Halt Medical, Genzyme, Auxogyn, Actamax, and ZSX Medical; an investigator for Abbvie, Novartis, Boeringher Ingelheim, Ferring, EMD Serono, and Biosante. R.S.L. is a speaker for Ferring Pharmaceuticals. A.N.H. receives grant funding from Waters, a mass spectrometry company, and is a consultant for Roche. S.A.K. receives support from the Merck 2016 Grant for Fertility Innovation and EIC Systems Biology in Reproductive Medicine. The remaining authors have nothing to disclose.
Glossary
- Abbreviations
AGE, advanced glycation end-product
- AMH
anti-Müllerian hormone
- AMIGOS
Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation
- BMI
body mass index
- IVF
in vitro fertilization
- PCOS
polycystic ovary syndrome
- PPCOS II
Pregnancy in Polycystic Ovary Syndrome II
- RCT
randomized controlled trial
- RMN
Reproductive Medicine Network
References
- 1. Levine MJ, Teegarden D. 1alpha,25-dihydroxycholecalciferol increases the expression of vascular endothelial growth factor in C3H10T1/2 mouse embryo fibroblasts. J Nutr. 2004;134(9):2244–2250. [DOI] [PubMed] [Google Scholar]
- 2. Barrera D, Avila E, Hernández G, Méndez I, González L, Halhali A, Larrea F, Morales A, Díaz L. Calcitriol affects hCG gene transcription in cultured human syncytiotrophoblasts. Reprod Biol Endocrinol. 2008;6(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Evans KN, Bulmer JN, Kilby MD, Hewison M. Vitamin D and placental-decidual function. J Soc Gynecol Investig. 2004;11(5):263–271. [DOI] [PubMed] [Google Scholar]
- 4. Ozkan S, Jindal S, Greenseid K, Shu J, Zeitlian G, Hickmon C, Pal L. Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil Steril. 2010;94(4):1314–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Irani M, Merhi Z.. Role of vitamin D in ovarian physiology and its implication in reproduction: a systematic review. Fertil Steril. 2014;102(2):460–468. [DOI] [PubMed] [Google Scholar]
- 6. Rudick BJ, Ingles SA, Chung K, Stanczyk FZ, Paulson RJ, Bendikson KA. Influence of vitamin D levels on in vitro fertilization outcomes in donor-recipient cycles. Fertil Steril. 2014;101(2):447–452. [DOI] [PubMed] [Google Scholar]
- 7. Polyzos NP, Anckaert E, Guzman L, Schiettecatte J, Van Landuyt L, Camus M, Smitz J, Tournaye H. Vitamin D deficiency and pregnancy rates in women undergoing single embryo, blastocyst stage, transfer (SET) for IVF/ICSI. Hum Reprod. 2014;29(9):2032–2040. [DOI] [PubMed] [Google Scholar]
- 8. Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr. 2007;137(2):447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nesby-O’Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr. 2002;76(1):187–192. [DOI] [PubMed] [Google Scholar]
- 10. Legro RS, Brzyski RG, Diamond MP, Coutifaris C, Schlaff WD, Casson P, Christman GM, Huang H, Yan Q, Alvero R, Haisenleder DJ, Barnhart KT, Bates GW, Usadi R, Lucidi S, Baker V, Trussell JC, Krawetz SA, Snyder P, Ohl D, Santoro N, Eisenberg E, Zhang H; NICHD Reproductive Medicine Network . Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371(2):119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fritz MA, Speroff L. Clinical gynecologic endocrinology and infertility. 8th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2011.
- 12. Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352(12):1223–1236. [DOI] [PubMed] [Google Scholar]
- 13. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. [DOI] [PubMed] [Google Scholar]
- 14. Kelly A, Brooks LJ, Dougherty S, Carlow DC, Zemel BS. A cross-sectional study of vitamin D and insulin resistance in children. Arch Dis Child. 2011;96(5):447–452. [DOI] [PubMed] [Google Scholar]
- 15. Hansen KR, He AL, Styer AK, Wild RA, Butts S, Engmann L, Diamond MP, Legro RS, Coutifaris C, Alvero R, Robinson RD, Casson P, Christman GM, Huang H, Santoro N, Eisenberg E, Zhang H, Eunice Kennedy Shriver National Institute of Child Health and Human Development Reproductive Medicine Network. Predictors of pregnancy and live-birth in couples with unexplained infertility after ovarian stimulation-intrauterine insemination. Fertil Steril. 2016;105(6):1575–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Practice Committee of the American Society for Reproductive Medicine Effectiveness and treatment for unexplained infertility. Fertil Steril. 2006;86(Suppl 1):S111–S114. [DOI] [PubMed] [Google Scholar]
- 17. Practice Committee of the American Society for Reproductive Medicine Use of clomiphene citrate in infertile women: a committee opinion. Fertil Steril. 2013;100(2):341–348. [DOI] [PubMed] [Google Scholar]
- 18. Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, Steinkampf MP, Coutifaris C, McGovern PG, Cataldo NA, Gosman GG, Nestler JE, Giudice LC, Leppert PC, Myers ER; Cooperative Multicenter Reproductive Medicine Network . Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356(6):551–566. [DOI] [PubMed] [Google Scholar]
- 19. Diamond MP, Legro RS, Coutifaris C, Alvero R, Robinson RD, Casson P, Christman GM, Ager J, Huang H, Hansen KR, Baker V, Usadi R, Seungdamrong A, Bates GW, Rosen RM, Haisenleder D, Krawetz SA, Barnhart K, Trussell JC, Ohl D, Jin Y, Santoro N, Eisenberg E, Zhang H; NICHD Reproductive Medicine Network . Letrozole, gonadotropin, or clomiphene for unexplained infertility. N Engl J Med. 2015;373(13):1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Antoniucci DM, Black DM, Sellmeyer DE. Serum 25-hydroxyvitamin D is unaffected by multiple freeze-thaw cycles. Clin Chem. 2005;51(1):258–261. [DOI] [PubMed] [Google Scholar]
- 21. Agborsangaya C, Toriola AT, Grankvist K, Surcel HM, Holl K, Parkkila S, Tuohimaa P, Lukanova A, Lehtinen M. The effects of storage time and sampling season on the stability of serum 25-hydroxy vitamin D and androstenedione. Nutr Cancer. 2010;62(1):51–57. [DOI] [PubMed] [Google Scholar]
- 22. Sachs MC, Brunzell JD, Cleary PA, Hoofnagle AN, Lachin JM, Molitch ME, Steffes MW, Zinman B, de Boer IH; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group . Circulating vitamin D metabolites and subclinical atherosclerosis in type 1 diabetes. Diabetes Care. 2013;36(8):2423–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wise SA, Phinney KW, Tai SS, Camara JE, Myers GL, Durazo-Arvizu R, Tian L, Hoofnagle AN, Bachmann LM, Young IS, Pettit J, Caldwell G, Liu A, Brooks SPJ, Sarafin K, Thamm M, Mensink GBM, Busch M, Rabenberg M, Cashman KD, Kiely M, Kinsella M, Galvin K, Zhang JY, Oh K, Lee SW, Jung CL, Cox L, Goldberg G, Guberg K, Prentice A, Carter GD, Jones J, Brannon PM, Lucas RM, Crump PM, Cavalier E, Merkel J, Betz JM, Sempos CT. Baseline assessment of 25-hydroxyvitamin D assay performance: a Vitamin D Standardization Program (VDSP) Interlaboratory Comparison Study. J AOAC Int. 2017;100(5):1244–1252. [DOI] [PubMed] [Google Scholar]
- 24. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM; Endocrine Society . Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. [DOI] [PubMed] [Google Scholar]
- 25. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab. 2012;97(4):1153–1158. [DOI] [PubMed] [Google Scholar]
- 26. Diamanti-Kandarakis E, Katsikis I, Piperi C, Kandaraki E, Piouka A, Papavassiliou AG, Panidis D. Increased serum advanced glycation end-products is a distinct finding in lean women with polycystic ovary syndrome (PCOS). Clin Endocrinol (Oxf). 2008;69(4):634–641. [DOI] [PubMed] [Google Scholar]
- 27. Diamanti-Kandarakis E, Piperi C, Patsouris E, Korkolopoulou P, Panidis D, Pawelczyk L, Papavassiliou AG, Duleba AJ. Immunohistochemical localization of advanced glycation end-products (AGEs) and their receptor (RAGE) in polycystic and normal ovaries. Histochem Cell Biol. 2007;127(6):581–589. [DOI] [PubMed] [Google Scholar]
- 28. Irani M, Minkoff H, Seifer DB, Merhi Z. Vitamin D increases serum levels of the soluble receptor for advanced glycation end products in women with PCOS. J Clin Endocrinol Metab. 2014;99(5):E886–E890. [DOI] [PubMed] [Google Scholar]
- 29. Merhi Z. Advanced glycation end products and their relevance in female reproduction. Hum Reprod. 2014;29(1):135–145. [DOI] [PubMed] [Google Scholar]
- 30. Mumford SL, Legro RS, Diamond MP, Coutifaris C, Steiner AZ, Schlaff WD, Alvero R, Christman GM, Casson PR, Huang H, Santoro N, Eisenberg E, Zhang H, Cedars MI. Baseline AMH level associated with ovulation following ovulation induction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2016;101(9):3288–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mahran A, Abdelmeged A, El-Adawy AR, Eissa MK, Shaw RW, Amer SA. The predictive value of circulating anti-Müllerian hormone in women with polycystic ovarian syndrome receiving clomiphene citrate: a prospective observational study. J Clin Endocrinol Metab. 2013;98(10):4170–4175. [DOI] [PubMed] [Google Scholar]
- 32. Merhi Z, Doswell A, Krebs K, Cipolla M. Vitamin D alters genes involved in follicular development and steroidogenesis in human cumulus granulosa cells. J Clin Endocrinol Metab. 2014;99(6):E1137–E1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wojtusik J, Johnson PA. Vitamin D regulates anti-Mullerian hormone expression in granulosa cells of the hen. Biol Reprod. 2012;86(3):91. [DOI] [PubMed] [Google Scholar]
- 34. Merhi ZO, Seifer DB, Weedon J, Adeyemi O, Holman S, Anastos K, Golub ET, Young M, Karim R, Greenblatt R, Minkoff H. Circulating vitamin D correlates with serum antimüllerian hormone levels in late-reproductive-aged women: Women’s Interagency HIV Study. Fertil Steril. 2012;98(1):228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pal L, Berry A, Coraluzzi L, Kustan E, Danton C, Shaw J, Taylor H. Therapeutic implications of vitamin D and calcium in overweight women with polycystic ovary syndrome. Gynecol Endocrinol. 2012;28(12):965–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fang F, Ni K, Cai Y, Shang J, Zhang X, Xiong C. Effect of vitamin D supplementation on polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Complement Ther Clin Pract. 2017;26:53–60. [DOI] [PubMed] [Google Scholar]
- 37. Thys-Jacobs S, Donovan D, Papadopoulos A, Sarrel P, Bilezikian JP. Vitamin D and calcium dysregulation in the polycystic ovarian syndrome. Steroids. 1999;64(6):430–435. [DOI] [PubMed] [Google Scholar]
- 38. Xu J, Hennebold JD, Seifer DB. Direct vitamin D3 actions on rhesus macaque follicles in three-dimensional culture: assessment of follicle survival, growth, steroid, and antimullerian hormone production. Fertil Steril. 2016;106(7):1815–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pal L, Zhang H, Williams J, Santoro NF, Diamond MP, Schlaff WD, Coutifaris C, Carson SA, Steinkampf MP, Carr BR, McGovern PG, Cataldo NA, Gosman GG, Nestler JE, Myers E, Legro RS; Reproductive Medicine Network . Vitamin D status relates to reproductive outcome in women with polycystic ovary syndrome: secondary analysis of a multicenter randomized controlled trial. J Clin Endocrinol Metab. 2016;101(8):3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Luk J, Torrealday S, Neal Perry G, Pal L. Relevance of vitamin D in reproduction. Hum Reprod. 2012;27(10):3015–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.