Abstract
Background
This study aimed to investigate the association between the presence and severity of cardiovascular autonomic neuropathy (CAN) and development of long-term glucose fluctuation in subjects with type 2 diabetes mellitus.
Methods
In this retrospective cohort study, subjects with type 2 diabetes mellitus who received cardiovascular autonomic reflex tests (CARTs) at baseline and at least 4-year of follow-up with ≥6 measures of glycosylated hemoglobin (HbA1c) were included. The severity of CAN was categorized as normal, early, or severe CAN according to the CARTs score. HbA1c variability was measured as the standard deviation (SD), coefficient of variation, and adjusted SD of serial HbA1c measurements.
Results
A total of 681 subjects were analyzed (294 normal, 318 early, and 69 severe CAN). The HbA1c variability index values showed a positive relationship with the severity of CAN. Multivariable logistic regression analysis showed that CAN was significantly associated with the risk of developing higher HbA1c variability (SD) after adjusting for age, sex, body mass index, diabetes duration, mean HbA1c, heart rate, glomerular filtration rate, diabetic retinopathy, coronary artery disease, insulin use, and anti-hypertensive medication (early CAN: odds ratio [OR], 1.65; 95% confidence interval [CI], 1.12 to 2.43) (severe CAN: OR, 2.86; 95% CI, 1.47 to 5.56). This association was more prominent in subjects who had a longer duration of diabetes (>10 years) and lower mean HbA1c (<7%).
Conclusion
CAN is an independent risk factor for future higher HbA1c variability in subjects with type 2 diabetes mellitus. Tailored therapy for stabilizing glucose fluctuation should be emphasized in subjects with CAN.
Keywords: Biological variation, individual; Diabetes mellitus, type 2; Diabetic neuropathies; Glycated hemoglobin A
INTRODUCTION
Cardiovascular autonomic neuropathy (CAN) is common but is one of the most overlooked complications of diabetes. The presence of CAN is associated with various clinical manifestations (e.g., resting tachycardia, orthostatic hypotension), comorbidities (e.g., silent myocardial ischemia, coronary artery disease [CAD], stroke), and overall mortality in patients with diabetes [1,2]. Therefore, the assessment of CAN is usually used for cardiovascular risk stratification in these subjects [3]. In addition to the cardiovascular manifestations, several studies have shown that CAN could be an independent risk factor for severe hypoglycemia in patients with type 1 and 2 diabetes mellitus [4,5,6]. These studies suggested that CAN is a cause of inadequate counter-regulatory responses to hypoglycemia, which results in unexpectedly low blood glucose levels [5,7]. They indicate a harmful effect of CAN on the short-term glucoseb fluctuation in patients with diabetes. However, the effect of CAN on long-term glucose fluctuation has yet to be explored.
Recently, various studies focused on variability of the glycosylated hemoglobin (HbA1c) as an index for long-term glucose fluctuation [8]. Compared to “glucose variability,” which refers to the fluctuation of blood glucose levels during a short-term period such as within-day or day-to-day, “HbA1c variability” refers to visit-to-visit HbA1c fluctuations, which reflect changes in glycemic control over longer periods of time [9,10]. Several studies have reported that HbA1c variability is an independent risk factor for the development of various diabetic complications including microvascular, macrovascular and all-cause mortality in both type 1 and 2 diabetes mellitus [11,12,13,14,15,16]. These relationships were significant even after adjustment of mean HbA1c level, which is a well-known marker for glycemic control [17,18]. Therefore, HbA1c variability should also be considered an additional risk factor that needs to be managed to prevent diabetic complications.
In this study, we aimed to explore the relationship between the presence and severity of CAN and the development of long-term glucose fluctuations, which was assessed by the HbA1c variability indices in subjects with type 2 diabetes mellitus.
METHODS
Study design and subjects
We performed a retrospective cohort study using electronic medical records (EMRs). Eligible subjects were patients with type 2 diabetes mellitus who had undergone cardiovascular autonomic reflex tests (CARTs) in the outpatient clinic of Seoul St. Mary's Hospital between October 2008 and September 2011. Patients were included if they were between 20 and 75 years of age. Patients with prediabetes, gestational diabetes, type 1 diabetes mellitus, latent autoimmune diabetes in adults, history of diabetic ketoacidosis, or a history of admission for poor glycemic status within the 6 months after the CARTs were performed were excluded. Subjects with an additional health condition that could influence their glycemic variability were also excluded, such as the presence of a malignancy except for early stage papillary thyroid cancer; abnormal liver function (aspartate aminotransferase or alanine aminotransferase higher than three times the upper limit of normal) or liver cirrhosis; estimated glomerular filtration rate (eGFR) calculated by the Modification of Diet in Renal Disease (MDRD) formula of under 45 mL/min/1.73 m2; arrhythmia; autoimmune disease; hematologic disease; and use of steroid therapy except for the stable maintenance of physiologic dose (equivalent dose of prednisolone ≤7.5 mg/day). Patients with proliferative diabetic retinopathy and a history of myocardial infarction or stroke within 6 months could not undergo CARTs due to the Valsalva maneuver. To assess long-term glucose variability, those patients with at least 4 years of follow-up after the CARTs and who had at least 6 HbA1c data points were selected among the eligible subjects for inclusion in this study. Finally, a total of 681 subjects were included in the analysis. This study was approved by the Institutional Review Board (IRB) of Seoul St. Mary's Hospital (KC17RESI0131). The written informed consent from the participants was waived by the IRB as only de-identified data were accessed and analyzed.
Anthropometric data such as height, weight, waist circumference (WC), hip circumference (HC), and blood pressure (BP) were measured by an experienced nurse while wearing light clothing without shoes. Body mass index (BMI) was calculated using the participant's height and weight (kg/m2). The waist-hip ratio (WHR) was calculated as WC divided by HC. BP was measured by the oscillometric method using an appropriate cuff. Diabetic retinopathy was evaluated using fundus photography or ophthalmologic outpatient clinic records. Other clinical data (age, sex, duration of diabetes, insulin therapy, use of lipid-lowering agents and anti-hypertensive agents, smoking history, and laboratory data) were retrieved from the EMR. All of the HbA1c values after the date of the CARTs were collected. HbA1c was measured by high-performance liquid chromatography using DCCT-aligned methods (Tosoh-G8; Tosoh, Tokyo, Japan).
Assessment of cardiovascular autonomic neuropathy
The CARTs were performed at the diabetes complication test clinic by one skilled nurse examiner. The confounding factors that could influence the CARTs were avoided [3]. The patients were advised to fast for 8 hours before the CARTs and to avoid caffeine, tobacco, alcohol, insulin, β-blockers, antihistamines, and antidepressants for 12 hours before the CARTs. The patients were also requested to avoid strenuous physical exercise within 24 hours before the CARTs.
CAN was assessed by five standard CARTs using the Ewing method [19]. The three tests were for parasympathetic function: heart rate responses to deep breathing (maximum–minimum heart rate), postural change from lying to standing (30:15 ratio), and the Valsalva maneuver. These heart rate responses were assessed automatically by the continuous electrocardiogram recoding using the DICAN (Medicore Co. LTD., Seoul, Korea). The two tests for sympathetic function were BP responses to standing up and sustained handgrip. Based on the reference value and scoring system for the classification of CAN described by Bellavere et al. [20] and Boulton et al. [21], each test was scored as normal=0, borderline=1, or abnormal=2 (Supplementary Table 1). Patients' severity of CAN was assessed by the total CARTs score, which was the sum of scores for each of the five CARTs (minimum 0, maximum 10). The scores 0 and 1 were classified as normal autonomic function, scores from 2 to 4 as early CAN, and 5 and over as the severe CAN group. As a sensitivity analysis, we also analyzed the results only using parasympathetic function tests for the diagnosis and staging of CAN [3]. Using this criteria, CAN was defined as the presence of at least one abnormal result in the parasympathetic tests. The CAN staging was defined as follows: (1) one abnormal parasympathetic test result defined as early CAN or (2) at least two abnormal parasympathetic function tests defined as definite CAN.
Assessment of HbA1c variability
HbA1c variability was measured as the standard deviation (SD) of serial HbA1c measurements (HbA1c-SD), the coefficient of variation of HbA1c (HbA1c-CV) to correct for the mean of the serial HbA1c measurements, and the adjusted SD of the serial HbA1c measurements (adj-HbA1c-SD) to adjust for the number of HbA1c assessments [9]. Indices of HbA1c variability were calculated as follows:
x=mean of serially measured HbA1c,
n=number of HbA1c measurements
Since there is no standard cutoff value for HbA1c variability indices, we categorized the subjects into two groups using the median value of each index: a low HbA1c variability group (HbA1c-SD <0.475%, HbA1c-CV <6.913%, adj-HbA1c-SD <0.463%) and a high HbA1c variability group (HbA1c-SD ≥0.475%, HbA1c-CV ≥6.913%, adj-HbA1c-SD ≥0.463%).
Statistical analysis
Continuous variables are presented as the mean±SD, and categorical variables are presented as number (%). Differences between groups of continuous variables were evaluated with a t-test or the Mann-Whitney U test. Differences between three groups were evaluated with a one-way analysis of variance and post hoc analysis, and P for trend was evaluated with a Jonckheere-Terpstra test. Categorical variables were analyzed with a chi-square test or Fisher's exact test. Multivariable logistic regression analysis was performed to test the association between the CAN and HbA1c variability indices and to compute the odds ratio (OR) (95% confidence interval [CI]) after adjusting for potential confounders. Model 1 was adjusted for age, sex, and BMI. Model 2 was further adjusted for duration of diabetes and mean of serial HbA1c. Model 3 was further adjusted for heart rate, eGFR, pre-existing CAD (imaging evidence with multi-detector coronary computed tomography or coronary angiography: ≥50% stenosis of at least one of the coronary artery, or history of myocardial infarction), presence of diabetic retinopathy, insulin treatment, and use of hypertension medication. The analyses were repeated entering HbA1c-CV or adj-HbA1c-SD instead of HbA1c-SD as a measure of HbA1c variability. Subgroup analysis was performed after categorizing the subjects according to the mean serial HbA1c of 7% and the duration of diabetes of 10 years. The statistical analysis was performed using SPSS version 21.0 (IBM Co., Armonk, NY, USA). All P values were two-sided, and a P value <0.05 was considered significant.
RESULTS
Baseline characteristics of study subjects
The median (interquartile range [IQR]) follow-up period of the study subjects was 5.5 years (IQR, 4.9 to 6.0 years). Of the 681 total subjects, 294 (43.2%) showed normal CARTs, 318 (46.7%) early CAN, and 69 (10.1%) showed severe CAN at baseline. Table 1 shows the baseline characteristics of the subjects according to CAN severity. As the severity of CAN increased from normal to severe, the subjects were older, more likely to be female, to have a longer duration of diabetes, a higher systolic BP, a lower eGFR, a higher high density lipoprotein cholesterol level, a higher prevalence of diabetic retinopathy, and they used insulin and antiplatelet agents more frequently. There were no differences in the follow-up period and the number of HbA1c measurements among the three groups. The mean of the serial HbA1c values was higher in proportion to the severity of the baseline CAN.
Table 1. Baseline characteristics according to CAN severity.
| Characteristic | Normal (n=294) | Early CAN (n=318) | Severe CAN (n=69) | P for trend |
|---|---|---|---|---|
| Age, yr | 56.4±9.3 | 57.7±9.6 | 61.4±7.7 | <0.001 |
| Male sex | 188 (63.9) | 181 (56.9) | 37 (53.6) | 0.043 |
| Duration of diabetes, yr | 8.5±7.1 | 10.0±8.2 | 12.1±8.0 | 0.001 |
| Current smoker | 50 (17.0) | 58 (18.2) | 14 (20.3) | 0.509 |
| BMI, kg/m2 | 24.8±3.0 | 25.0±3.2 | 24.6±3.5 | 0.876 |
| WC, cm | 86.6±7.8 | 86.2±8.9 | 86.5±9.9 | 0.587 |
| WHR | 0.93±0.06 | 0.92±0.06 | 0.92±0.07 | 0.152 |
| Systolic BP, mm Hg | 129.3±16.3 | 131.1±16.1 | 130.9±15.4 | 0.044 |
| Diastolic BP, mm Hg | 82.5±8.5 | 82.1±8.9 | 82.3±7.9 | 0.811 |
| Heart rate, beats/min | 71.5±12.0 | 70.8±12.5 | 72.3±13.2 | 0.661 |
| Fasting glucose, mg/dL | 131.8±30.9 | 134.1±38.3 | 133.7±46.1 | 0.923 |
| Baseline HbA1c, % | 6.97±0.93 | 7.05±1.11 | 7.37±1.35 | 0.085 |
| Serum creatinine, mg/dL | 0.85±0.19 | 0.84±0.19 | 0.88±0.20 | 0.473 |
| eGFR (MDRD), mL/min/1.73 m2 | 88.5±17.7 | 87.5±19.2 | 79.3±20.9 | 0.002 |
| Total cholesterol, mg/dL | 164.2±33.4 | 163.9±32.2 | 165.1±33.4 | 0.870 |
| Triglyceride, mg/dL | 134.2±106.3 | 120.9±73.6 | 139.5±92.6 | 0.775 |
| HDL-C, mg/dL | 47.1±10.9 | 48.7±11.4 | 49.5±13.5 | 0.038 |
| LDL-C, mg/dL | 91.0±26.3 | 91.2±27.9 | 90.8±29.4 | 0.902 |
| Diabetic retinopathy | 65 (22.1) | 90 (28.3) | 29 (42.0) | 0.001 |
| Previous CAD | 20 (6.8) | 29 (9.1) | 6 (8.7) | 0.368 |
| Previous CVA | 12 (4.1) | 18 (5.7) | 4 (5.8) | 0.383 |
| Diabetes treatment | ||||
| Life style modification only | 22 (7.5) | 18 (5.7) | 2 (2.9) | 0.134 |
| OHA only | 234 (79.6) | 243 (76.4) | 51 (73.9) | 0.228 |
| Insulin±OHA | 38 (12.9) | 57 (17.9) | 16 (23.2) | 0.019 |
| Use of hypertension medication | 163 (55.4) | 179 (56.3) | 39 (56.5) | 0.824 |
| Use of statin | 182 (61.9) | 189 (59.4) | 45 (65.2) | 0.957 |
| Use of antiplatelet | 156 (53.1) | 201 (63.2) | 44 (63.8) | 0.014 |
| During follow-up | ||||
| Follow-up period, yr | 5.4 (5.0–5.9) | 5.5 (4.9–6.0) | 5.6 (4.7–6.2) | 0.192 |
| No. of HbA1c measurements | 18.0±4.8 | 18.0±4.9 | 18.6±4.9 | 0.706 |
| Mean of serial HbA1c, % | 6.97±0.67 | 7.07±0.73 | 7.27±0.88 | 0.011 |
Values are presented as mean±standard deviation, number (%), or median (interquartile range).
CAN, cardiovascular autonomic neuropathy; BMI, body mass index; WC, waist circumference; WHR, waist-hip ratio; BP, blood pressure; HbA1c, glycosylated hemoglobin; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; CAD, coronary artery disease; CVA, cerebrovascular accident; OHA, oral hypoglycemic agent.
The comparison of baseline characteristics between lower and higher HbA1c-SD groups is shown in Supplementary Table 2. The subjects found to have higher HbA1c variability (SD) during the follow-up period were younger; had higher BMI, WC, WHR, systolic and diastolic BP; had faster heart rates; had higher fasting glucose values, baseline HbA1c, and triglyceride levels; had a higher prevalence of diabetic retinopathy, CAN and CAD; and more frequently used insulin at baseline compared with those subjects with lower HbA1c-SD. Although there was no difference in the follow-up period between the two groups, the higher HbA1c-SD group had more HbA1c measurements during the follow-up. In addition, subjects with higher HbA1c-SD had higher mean values of serial HbA1c, HbA1c-CV, and adj-HbA1c-SD compared with those subjects with lower HbA1c-SD. These findings were similar when the subjects were categorized according to HbA1c-CV or adj-HbA1c-SD (data not shown).
CAN severity and future HbA1c variability
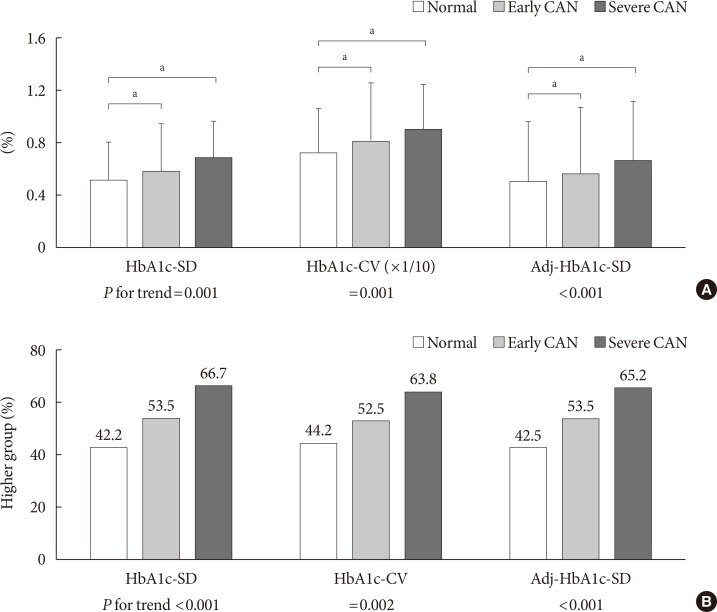
Fig. 1 shows three HbA1c variability indices during the follow-up period according to baseline CAN severity. All the HbA1c variability indices showed positive correlations with the severity of baseline CAN (Fig. 1A); (1) HbA1c-SD: normal, 0.514%±0.287%; early CAN, 0.582%±0.350%; severe CAN, 0.687%±0.467%; P for trend=0.001; (2) HbA1c-CV: normal, 7.228%±3.642%; early CAN, 8.077%±4.533%; severe CAN, 9.053%±5.044%; P for trend=0.001; and (3) adj-HbA1c-SD: normal, 0.499%±0.280%; early CAN, 0.565%±0.339%; severe CAN 0.668%±0.456%; P for trend <0.001. Furthermore, the percentage of the higher HbA1c variability group increased according to the baseline CAN severity in a dose-dependent manner (Fig. 1B).
Fig. 1. Future glycosylated hemoglobin (HbA1c) variability indices according to baseline cardiovascular autonomic neuropathy (CAN) severity. (A) The value of each HbA1c variability index according to the severity of CAN at baseline. (B) The percentage of the higher HbA1c variability group according to the severity of CAN at baseline. Data are expressed as the mean±standard deviation (SD). CV, coefficient of variation; Adj, adjusted. aP<0.05.
Risk factors associated with higher HbA1c variability during follow-up
To identify risk factors associated with future HbA1c variability, multivariable logistic regression analysis was performed. Compared to subjects in the normal group, the subjects with early and severe CAN were significantly associated with developing higher HbA1c variability (SD) during the follow-up period (Table 2). The ORs (95% CI) of early CAN (OR, 1.65; 95% CI, 1.12 to 2.43) and severe CAN (OR, 2.86; 95% CI, 1.47 to 5.56) were significantly increased, even after adjusting for multiple covariates. In addition to baseline CAN severity, younger age, higher BMI, shorter duration of diabetes, higher mean value of serial HbA1c, faster heart rate, and the presence of diabetic retinopathy and pre-existing CAD were significantly associated with higher HbA1c variability. These results were largely consistent when HbA1c-CV or adj-HbA1c-SD were used instead of HbA1c-SD (Supplementary Table 3). However, in the analysis using HbA1c-CV, BMI, duration of diabetes, and early CAN lost statistical significance in models 2 and 3.
Table 2. Risk factors associated with higher HbA1c-SD during follow-up.
| Variable | OR (95% CI) | ||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Early CAN (vs. normal group) | 1.65 (1.19–2.30)b | 1.64 (1.13–2.39)a | 1.65 (1.12–2.43)a |
| Severe CAN (vs. normal group) | 3.41 (1.92–6.05)b | 2.83 (1.49–5.36)b | 2.86 (1.47–5.56)b |
| Age (per 1 yr increment) | 0.96 (0.95–0.98)b | 0.98 (0.95–1.00)a | 0.96 (0.94–0.99)b |
| Sex (male vs. female) | 1.16 (0.84–1.61) | 0.99 (0.69–1.44) | 1.06 (0.72–1.55) |
| BMI (per 1 kg/m2 increment) | 1.09 (1.03–1.14)b | 1.08 (1.02–1.14)a | 1.07 (1.01–1.13)a |
| Duration of diabetes (per 1 yr increment) | 0.98 (0.95–1.01) | 0.96 (0.93–0.99)a | |
| Mean of serial HbA1c (per 1% increment) | 8.45 (5.55–12.87)b | 8.32 (5.38–12.88)b | |
| Heart rate (per 1 beats/min increment) | 1.02 (1.00–1.03)a | ||
| eGFR (per 1 mL/min/1.73 m2 increment) | 1.00 (0.99–1.01) | ||
| Diabetic retinopathy (yes vs. no) | 2.05 (1.29–3.28)b | ||
| Coronary artery disease (yes vs. no) | 2.00 (1.00–3.97)a | ||
| Diabetes treatment (insulin use vs. no) | 0.99 (0.54–1.81) | ||
| Hypertension medication (yes vs. no) | 1.37 (0.92–2.02) | ||
Model 1: adjusted for age, sex, and BMI; Model 2: adjusted for model 1+diabetes duration and mean serial HbA1c; Model 3: adjusted for model 2+heart rate, eGFR, diabetic retinopathy, coronary artery disease, diabetes treatment (insulin use), and use of hypertension medication.
HbA1c, glycosylated hemoglobin; SD, standard deviation; OR, odds ratio; CI, confidence interval; CAN, cardiovascular autonomic neuropathy; BMI, body mass index; eGFR, estimated glomerular filtration rate.
aP<0.05, bP<0.01.
Sensitivity analysis
We also performed a sensitivity analysis using only three parasympathetic CARTs for the diagnosis and staging of CAN. When CAN was defined as the presence of at least one abnormal result of the parasympathetic tests (Supplementary Table 4), 378 subjects (55.5%) had CAN. The OR (95% CI) for developing higher HbA1c-SD was significantly increased, even after adjusting for multiple covariates (OR, 1.52; 95% CI, 1.05 to 2.18). When the CAN was staged according to the numbers of abnormal parasympathetic tests (Supplementary Table 5), 303 (44.5%) showed normal CARTs, 240 (35.2%) early CAN, and 138 (20.3%) showed definite CAN at baseline. The subjects with definite CAN showed a significantly increased OR (95% CI) for developing a higher HbA1c-SD, even after adjusting for multiple covariates (OR, 1.76; 95% CI, 1.07 to 2.88). The results were similar when using adj-HbA1c-SD, although significance was lost with analysis using HbA1c-CV. The other risk factors that were significantly associated with higher HbA1c variability were similar with the original analysis.
Subgroup analysis
Table 3 shows the subgroup analysis according to the mean value of serial HbA1c during the observation period (<7% vs. ≥7%) and the duration of diabetes (≤10 years vs. >10 years) at baseline. In the analysis of subjects with mean HbA1c <7%, a stepwise increase in the OR (95% CI) for higher HbA1c-SD was noted according to the severity of CAN (model 3: early CAN [OR, 1.72; 95% CI, 1.02 to 2.90]; severe CAN [OR, 3.34; 95% CI, 1.41 to 7.91]). However, this association was insignificant in the subject of mean HbA1c ≥7%. In the analysis of subjects with diabetes duration >10 years, severe CAN showed a significant association with higher HbA1c-SD (OR, 4.75; 95% CI, 1.71 to 13.20). In subjects with diabetes duration ≤10 years, only early CAN was significantly associated with higher HbA1c-SD. These results were similar when using HbA1c-CV or adj-HbA1c-SD instead of HbA1c-SD (Supplementary Table 6), except that the association between early CAN and higher HbA1c-CV was insignificant in the subgroups of mean HbA1c <7% and diabetes duration ≤10 years.
Table 3. The risk of developing higher HbA1c-SD in subgroups according to the mean HbA1c and diabetes duration.
| OR (95% CI) | |||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Mean of serial HbA1c | |||
| <7% (n=360) | |||
| Normal | 1 (reference) | 1 (reference) | 1 (reference) |
| Early CAN | 1.67 (1.02–2.71)a | 1.73 (1.04–2.86)a | 1.72 (1.02–2.90)a |
| Severe CAN | 3.28 (1.47–7.30)b | 3.19 (1.41–7.26)b | 3.34 (1.41–7.91)b |
| ≥7% (n=321) | |||
| Normal | 1 (reference) | 1 (reference) | 1 (reference) |
| Early CAN | 1.70 (1.01–2.89)a | 1.44 (0.81–2.58) | 1.47 (0.81–2.68) |
| Severe CAN | 3.59 (1.47–9.24)b | 2.17 (0.767–6.16) | 2.12 (0.71–6.31) |
| Duration of diabetes | |||
| ≤10 yr (n=434) | |||
| Normal | 1 (reference) | 1 (reference) | 1 (reference) |
| Early CAN | 1.73 (1.14–2.63)a | 1.77 (1.09–2.87)a | 1.74 (1.05–2.87)a |
| Severe CAN | 1.94 (0.87–4.35) | 2.18 (0.88–5.41) | 2.03 (0.79–5.23) |
| >10 yr (n=247) | |||
| Normal | 1 (reference) | 1 (reference) | 1 (reference) |
| Early CAN | 1.44 (0.81–2.54) | 1.44 (0.77–2.68) | 1.52 (0.80–2.89) |
| Severe CAN | 5.23 (2.09–13.05)b | 3.81 (1.43–10.13)b | 4.75 (1.71–13.20)b |
bModel 1: adjusted for age, sex, and BMI; Model 2: adjusted for model 1+diabetes duration and mean serial HbA1c; Model 3: adjusted for model 2+heart rate, eGFR, diabetic retinopathy, coronary artery disease, diabetes treatment (insulin use), and use of hypertension medication.
HbA1c, glycosylated hemoglobin; SD, standard deviation; OR, odds ratio; CI, confidence interval; CAN, cardiovascular autonomic neuropathy.
aP<0.05, bP<0.01.
DISCUSSION
Glucose variability is a product of the complex interplay between various pathophysiological, behavioral, and treatment factors. In this study, we demonstrated that CAN is also an independent risk factor for developing higher HbA1c variability, which reflects glucose fluctuation over longer periods of time, in subjects with type 2 diabetes mellitus. This association was more prominent in subjects with lower mean HbA1c levels during the follow-up period and a longer duration of diabetes at baseline.
CAN is caused by damaged autonomic nerve fibers that innervate the heart and blood vessels, resulting in the impairment of autonomic control in the cardiovascular system [1,22]. The autonomic nervous system also innervates many other internal organs and plays an important role in maintaining the body's internal environmental homeostasis including glucose control. For balancing blood glucose levels within the suitable range, the autonomic nervous system and endocrine system function intricately together during homeostasis [23]. Previous studies have shown that subjects with CAN have delayed sympathetic and parasympathetic activation in response to the fluctuation of blood glucose levels [4,5,7], causing disruption of the glucose homeostasis and ultimately leading to higher glucose variability. Our study showed a stepwise increase in the risk of developing higher HbA1c variability according to the severity of CAN, suggesting a negative influence of CAN in a dose-dependent manner.
The association between CAN and glucose variability may also be partly attributable to the abnormal gastrointestinal (GI) motility of subjects with CAN. It is well-known that CAN is associated with other diabetic autonomic neuropathies, including GI autonomic neuropathy [24,25]. Ohlsson et al. [26] showed that the subjects with proven esophagogastric dysmotility by gastric emptying scintigraphy or esophageal manometry had delayed and decreased glucose uptake from the upper GI tract using 72 hours continuous glucose monitoring. Other studies also suggested the bidirectional relationship between glycemic disturbances and GI dysmotility, which could create a mismatch between insulin delivery and glucose absorption [27,28]. In addition, delayed gastric emptying can cause difficulty in maintaining glycemic control because of the unpredictable emptying of food from the stomach [29,30].
Although the effect was small, younger age, higher BMI, and shorter duration of diabetes were also associated with future higher HbA1c variability in our study. The Renal Insufficiency And Cardiovascular Events (RIACE) cohort, which consisted of 15,933 patients of 19 hospital-based diabetes clinics in Italy, showed similar results, including that higher HbA1c variability was associated with younger age, shorter duration of diabetes, higher HbA1c, and higher BMI in subjects with type 2 diabetes mellitus [13,31]. Of note, increasing mean HbA1c was associated with longer diabetes duration, whereas higher HbA1c variability was associated with shorter diabetes duration. In addition, the presence of diabetic retinopathy and CAD were also associated with higher HbA1c variability in our study. Diabetic retinopathy and CAD are representative of micro- and macrovascular complications of diabetes, respectively. The association with these diabetic complications could partially be explained by vasculopathy as a common pathophysiology. As in the case with neuropathy, subjects with vasculopathy could be predisposed to a delayed response of hormone and glucose uptake by peripheral tissues, leading to higher glucose fluctuations [32,33].
Among the clinical manifestations of CAN, orthostatic hypotension occurs usually late in diabetes and subsequent to abnormalities in the heart rate tests [3]. Moreover, orthostatic hypotension test could be more affected by a number of external factors than heart rate tests [3]. In the sensitivity analysis, we excluded two sympathetic CARTs and used only three parasympathetic CARTs for the diagnosis and staging of CAN like prior other studies [3,6]. With this different definition of CAN, the main results were similar to our original analysis.
In this study, we also performed subgroup analyses according to the diabetes duration of 10 years and mean HbA1c level of 7%. The β-cell dysfunction progresses and insulin secretory capacity worsens over time after diagnosis of diabetes [34]. The complementary interaction of the hormones and peptides involved in glucose homeostasis is usually more disrupted as the diabetes progresses, and subjects are prone to showing a high swing in glucose levels from the external stimuli. Thus, the effect of CAN on glucose fluctuations could be more marked in the subjects with long-standing diabetes. The association between CAN and HbA1c variability was more prominent in the subjects with mean HbA1c under 7% during the observation period. This finding suggests that the presence of CAN could be a more significant barrier to reaching stable glycemic control in subjects with relatively well-controlled glycemia. In the RIACE study [13], all the HbA1c variability indices progressively increased throughout mean HbA1c quartiles. Therefore, the high impact of mean HbA1c on HbA1c variability might mask the impact of CAN on HbA1c variability in the higher mean HbA1c subgroup (HbA1c ≥7%).
In addition to being an independent risk factor of various diabetic complications [8], HbA1c variability also showed a significant association with an increased risk of advanced CAN in subjects with type 2 diabetes mellitus [35]. Additionally, we demonstrated a significant effect of CAN on the development of higher HbA1c variability in the present study. Therefore, we could deduce that the presence of CAN and higher HbA1c variability have a bidirectional influence, therefore creating a vicious cycle.
There are several limitations in the present study. First, this was a retrospective study performed in a single center. Therefore, the causal relationship could not be confirmed. Second, diet, exercise, compliance and alterations in diabetic medications that could affect the glucose variability could not be assessed. Third, there were various numbers and intervals in HbA1c measurements for each subject during the observation period, although the general strategy was to measure every 3 to 6 months. To minimize this limitation, we also used the SD of serially measured HbA1c levels adjusted by the number of measurements (adj-HbA1c-SD) and HbA1c-CV to account for the different mean HbA1c levels. Fourth, we categorized the subjects into two groups using the median value of each HbA1c variability index since there is no standard cutoff value. As a result, the HbA1c-SD and adj-HbA1c-SD were similar to that of the RIACE study (median value: 0.40% and 0.46% respectively) [13,31]. Further studies suggesting the gold standard cut-off values of each index are needed.
This study has a strength that included relatively large numbers of type 2 diabetes mellitus subjects with CAN evaluations and long-term follow-up. We only included subjects with at least 4 years of follow-up data, and the median follow-up period was approximately 5.5 years. To the best of our knowledge, this is the first study that investigated the association between the CAN and long-term visit-to-visit HbA1c variability in subjects with type 2 diabetes mellitus. CARTs are non-invasive, easy to perform, and clinically relevant tests that enable high quality evaluation of the autonomic function with a high strength of evidence [36,37]. In addition to the cardiovascular risk stratification, detection of CAN could help lead to more tailored therapeutic strategies for patients with diabetes, such as selecting medications that are more favorable for reducing glucose variability.
In conclusion, CAN is associated with a future high HbA1c variability in subjects with type 2 diabetes mellitus. The presence and severity of CAN should be considered not only for cardiovascular risk stratification but also for optimal glycemic control.
ACKNOWLEDGMENTS
The authors thank Dr. Hae Kyung Yang for helpful discussions. This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science & ICT (NRF-2018M-3A9E8021503).
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
SUPPLEMENTARY MATERIALS
Reference values of the CARTs and scoring system used in this study
Baseline characteristics according to HbA1c-SD
Risk factors associated with higher HbA1c-CV and adjusted-HbA1c-SD during follow-up
Risk factors associated with higher HbA1c variability during follow-up (CAN was diagnosed using three parasympathetic CARTs)
Risk factors associated with higher HbA1c variability during follow-up (CAN was staged using three parasympathetic CARTs)
The risk of developing higher HbA1c-CV or adjusted-HbA1c-SD in subgroups according to the mean HbA1c and diabetes duration
References
- 1.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115:387–397. doi: 10.1161/CIRCULATIONAHA.106.634949. [DOI] [PubMed] [Google Scholar]
- 2.Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care. 2003;26:1895–1901. doi: 10.2337/diacare.26.6.1895. [DOI] [PubMed] [Google Scholar]
- 3.Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, Stevens M, Kempler P, Hilsted J, Tesfaye S, Low P, Valensi P Toronto Consensus Panel on Diabetic Neuropathy. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011;27:639–653. doi: 10.1002/dmrr.1239. [DOI] [PubMed] [Google Scholar]
- 4.Stephenson JM, Kempler P, Perin PC, Fuller JH. Is autonomic neuropathy a risk factor for severe hypoglycaemia? The EURODIAB IDDM Complications Study. Diabetologia. 1996;39:1372–1376. doi: 10.1007/s001250050585. [DOI] [PubMed] [Google Scholar]
- 5.Meyer C, Grossmann R, Mitrakou A, Mahler R, Veneman T, Gerich J, Bretzel RG. Effects of autonomic neuropathy on counterregulation and awareness of hypoglycemia in type 1 diabetic patients. Diabetes Care. 1998;21:1960–1966. doi: 10.2337/diacare.21.11.1960. [DOI] [PubMed] [Google Scholar]
- 6.Yun JS, Kim JH, Song KH, Ahn YB, Yoon KH, Yoo KD, Park YM, Ko SH. Cardiovascular autonomic dysfunction predicts severe hypoglycemia in patients with type 2 diabetes: a 10-year follow-up study. Diabetes Care. 2014;37:235–241. doi: 10.2337/dc13-1164. [DOI] [PubMed] [Google Scholar]
- 7.Bottini P, Boschetti E, Pampanelli S, Ciofetta M, Del Sindaco P, Scionti L, Brunetti P, Bolli GB. Contribution of autonomic neuropathy to reduced plasma adrenaline responses to hypoglycemia in IDDM: evidence for a nonselective defect. Diabetes. 1997;46:814–823. doi: 10.2337/diab.46.5.814. [DOI] [PubMed] [Google Scholar]
- 8.Gorst C, Kwok CS, Aslam S, Buchan I, Kontopantelis E, Myint PK, Heatlie G, Loke Y, Rutter MK, Mamas MA. Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care. 2015;38:2354–2369. doi: 10.2337/dc15-1188. [DOI] [PubMed] [Google Scholar]
- 9.Kilpatrick ES, Rigby AS, Atkin SL. A1C variability and the risk of microvascular complications in type 1 diabetes: data from the Diabetes Control and Complications Trial. Diabetes Care. 2008;31:2198–2202. doi: 10.2337/dc08-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suh S, Kim JH. Glycemic variability: how do we measure it and why is it important? Diabetes Metab J. 2015;39:273–282. doi: 10.4093/dmj.2015.39.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waden J, Forsblom C, Thorn LM, Gordin D, Saraheimo M, Groop PH Finnish Diabetic Nephropathy Study Group. A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes. 2009;58:2649–2655. doi: 10.2337/db09-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu CC, Chang HY, Huang MC, Hwang SJ, Yang YC, Lee YS, Shin SJ, Tai TY. HbA1c variability is associated with microal buminuria development in type 2 diabetes: a 7-year prospective cohort study. Diabetologia. 2012;55:3163–3172. doi: 10.1007/s00125-012-2700-4. [DOI] [PubMed] [Google Scholar]
- 13.Penno G, Solini A, Bonora E, Fondelli C, Orsi E, Zerbini G, Morano S, Cavalot F, Lamacchia O, Laviola L, Nicolucci A, Pugliese G Renal Insufficiency And Cardiovascular Events Study Group. HbA1c variability as an independent correlate of nephropathy, but not retinopathy, in patients with type 2 diabetes: the Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicenter study. Diabetes Care. 2013;36:2301–2310. doi: 10.2337/dc12-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prince CT, Becker DJ, Costacou T, Miller RG, Orchard TJ. Changes in glycaemic control and risk of coronary artery disease in type 1 diabetes: findings from the Pittsburgh Epidemiology of Diabetes Complications Study (EDC) Diabetologia. 2007;50:2280–2288. doi: 10.1007/s00125-007-0797-7. [DOI] [PubMed] [Google Scholar]
- 15.Hirakawa Y, Arima H, Zoungas S, Ninomiya T, Cooper M, Hamet P, Mancia G, Poulter N, Harrap S, Woodward M, Chalmers J. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: the ADVANCE trial. Diabetes Care. 2014;37:2359–2365. doi: 10.2337/dc14-0199. [DOI] [PubMed] [Google Scholar]
- 16.Lee EJ, Kim YJ, Kim TN, Kim TI, Lee WK, Kim MK, Park JH, Rhee BD. A1c variability can predict coronary artery disease in patients with type 2 diabetes with mean a1c levels greater than 7. Endocrinol Metab (Seoul) 2013;28:125–132. doi: 10.3803/EnM.2013.28.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Currie CJ, Peters JR, Tynan A, Evans M, Heine RJ, Bracco OL, Zagar T, Poole CD. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375:481–489. doi: 10.1016/S0140-6736(09)61969-3. [DOI] [PubMed] [Google Scholar]
- 19.Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care. 1985;8:491–498. doi: 10.2337/diacare.8.5.491. [DOI] [PubMed] [Google Scholar]
- 20.Bellavere F, Bosello G, Fedele D, Cardone C, Ferri M. Diagnosis and management of diabetic autonomic neuropathy. Br Med J (Clin Res Ed) 1983;287:61. doi: 10.1136/bmj.287.6384.61-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D American Diabetes Association. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 22.Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niijima A. Nervous regulation of metabolism. Prog Neurobiol. 1989;33:135–147. doi: 10.1016/0301-0082(89)90037-3. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann R, Borovicka J, Kunz P, Crelier G, Boesiger P, Fried M, Schwizer W, Spinas GA. Evaluation of delayed gastric emptying in diabetic patients with autonomic neuropathy by a new magnetic resonance imaging technique and radio-opaque markers. Diabetes Care. 1996;19:1075–1082. doi: 10.2337/diacare.19.10.1075. [DOI] [PubMed] [Google Scholar]
- 25.Darwiche G, Almer LO, Bjorgell O, Cederholm C, Nilsson P. Delayed gastric emptying rate in type 1 diabetics with cardiac autonomic neuropathy. J Diabetes Complications. 2001;15:128–134. doi: 10.1016/s1056-8727(00)00143-4. [DOI] [PubMed] [Google Scholar]
- 26.Ohlsson B, Melander O, Thorsson O, Olsson R, Ekberg O, Sundkvist G. Oesophageal dysmotility, delayed gastric emptying and autonomic neuropathy correlate to disturbed glucose homeostasis. Diabetologia. 2006;49:2010–2014. doi: 10.1007/s00125-006-0354-9. [DOI] [PubMed] [Google Scholar]
- 27.Parthasarathy G, Kudva YC, Low PA, Camilleri M, Basu A, Bharucha AE. Relationship between gastric emptying and diurnal glycemic control in type 1 diabetes mellitus: a randomized trial. J Clin Endocrinol Metab. 2017;102:398–406. doi: 10.1210/jc.2016-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones KL, Horowitz M, Carney BI, Wishart JM, Guha S, Green L. Gastric emptying in early noninsulin-dependent diabetes mellitus. J Nucl Med. 1996;37:1643–1648. [PubMed] [Google Scholar]
- 29.Hongo M, Okuno Y. Diabetic gastropathy in patients with autonomic neuropathy. Diabet Med. 1993;10(Suppl 2):79S–81S. doi: 10.1111/j.1464-5491.1993.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 30.Lyrenas EB, Olsson EH, Arvidsson UC, Orn TJ, Spjuth JH. Prevalence and determinants of solid and liquid gastric emptying in unstable type I diabetes. Relationship to postprandial blood glucose concentrations. Diabetes Care. 1997;20:413–418. doi: 10.2337/diacare.20.3.413. [DOI] [PubMed] [Google Scholar]
- 31.Penno G, Solini A, Zoppini G, Orsi E, Fondelli C, Zerbini G, Morano S, Cavalot F, Lamacchia O, Trevisan R, Vedovato M, Pugliese G Renal Insufficiency and Cardiovascular Events (RIACE) Study Group. Hemoglobin A1c variability as an independent correlate of cardiovascular disease in patients with type 2 diabetes: a cross-sectional analysis of the Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicenter study. Cardiovasc Diabetol. 2013;12:98. doi: 10.1186/1475-2840-12-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark MG, Wallis MG, Barrett EJ, Vincent MA, Richards SM, Clerk LH, Rattigan S. Blood flow and muscle metabolism: a focus on insulin action. Am J Physiol Endocrinol Metab. 2003;284:E241–E258. doi: 10.1152/ajpendo.00408.2002. [DOI] [PubMed] [Google Scholar]
- 33.Clark MG. Impaired microvascular perfusion: a consequence of vascular dysfunction and a potential cause of insulin resistance in muscle. Am J Physiol Endocrinol Metab. 2008;295:E732–E750. doi: 10.1152/ajpendo.90477.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jun JE, Jin SM, Baek J, Oh S, Hur KY, Lee MS, Lee MK, Kim JH. The association between glycemic variability and diabetic cardiovascular autonomic neuropathy in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:70. doi: 10.1186/s12933-015-0233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spallone V, Bellavere F, Scionti L, Maule S, Quadri R, Bax G, Melga P, Viviani GL, Esposito K, Morganti R, Cortelli P Diabetic Neuropathy Study Group of the Italian Society of Diabetology. Recommendations for the use of cardiovascular tests in diagnosing diabetic autonomic neuropathy. Nutr Metab Cardiovasc Dis. 2011;21:69–78. doi: 10.1016/j.numecd.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Assessment: clinical autonomic testing report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 1996;46:873–880. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Reference values of the CARTs and scoring system used in this study
Baseline characteristics according to HbA1c-SD
Risk factors associated with higher HbA1c-CV and adjusted-HbA1c-SD during follow-up
Risk factors associated with higher HbA1c variability during follow-up (CAN was diagnosed using three parasympathetic CARTs)
Risk factors associated with higher HbA1c variability during follow-up (CAN was staged using three parasympathetic CARTs)
The risk of developing higher HbA1c-CV or adjusted-HbA1c-SD in subgroups according to the mean HbA1c and diabetes duration