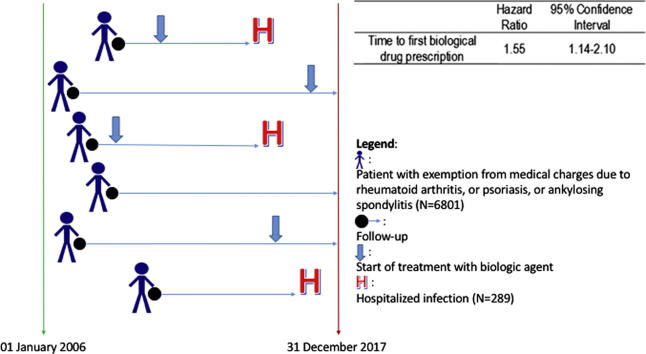
Graphical abstract
Keywords: Rheumatoid, Arthritis, Psoriasis, Biologic drug, Tumor necrosis factor, Infection
Highlights
-
•
In this cohort, adalimumab and etanercept are the most commonly prescribed biologics.
-
•
Risk of hospitalized infections increases under biologic agents.
-
•
Risk is much higher in the elderly and in the presence of comorbidities.
-
•
Upper and lower respiratory tract infections are the most common infections.
-
•
Administrative data are useful for confirming the observation of clinical trials.
Abstract
Risk of hospitalized infections under biologics among patients suffering from chronic inflammatory autoimmune diseases such as rheumatoid arthritis (RA), ankylosing spondylitis (AS), psoriatic arthritis (PSA), or psoriasis was investigated using administrative data. The hospital discharge records database, the medical prescription database, and the database of exemptions from medical charges were linked at the individual patient level. A cohort of patients diagnosed with RA, SA, PSA, and severe psoriasis from 2006 to 2017 was identified and followed-up to either the end of 2017 or hospitalization with the main discharge diagnosis of infection, death, or they moved out of the region. Multiple Cox regression was used to estimate the hazard ratio (HR) of hospitalization associated with bDMARDs and adjusting for age, sex, Charlson’s Comorbidity Index, calendar year, prescription of steroids, and use of csDMARDs. Use of bDMARDs was treated as a time-dependent variable. A total of 5596 patients diagnosed with RA, AS, or PSA/severe psoriasis were included in the cohort. Overall, 289 (4.2%) were hospitalized due to infection. Time to first use of biological drugs was significantly associated with a 55% increased risk of hospitalization for infections. Thus, large cohorts from administrative databases are useful to support observations from registries and clinical trials. Patients with chronic autoimmune inflammatory diseases are at risk of serious infections when starting biologics. This risk is higher in the elderly or those with comorbidities. Upper and lower respiratory tract infections are the most common infections. Our findings support prevention policies such as vaccination.
Introduction
The development of biologic drugs changed the management of several chronic inflammatory autoimmune diseases, including rheumatoid arthritis (RA), ankylosing spondylitis (AS), psoriatic arthritis (PSA), and psoriasis (PSO) [1]. However, while their efficacy has been well established by many clinical trials, it remains uncertain to what extent biologic treatments may be associated with severe safety risks such as serious infections. This relevant topic has been addressed, in particular, using data from national or international observational registries [2], [3], [4], [5], [6], [7].
It is well known that the disease itself or the disease activity is a risk factor for infections. The risk of serious infections with tumor necrosis factor inhibitor (TNFi) agents is particularly increased in the first 6 months of therapy, and this risk is higher compared to the use of conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) [8]. A history of serious infections, glucocorticoid dose, and older age are other important risk factors of serious infections in patients treated with biologics [9]. Individuals with RA had a two-fold increased adjusted risk of hospitalized infection compared to those without RA when adjusted for age, sex, calendar year, comorbidities, and prescription medication use in a retrospective cohort study performed using 1999–2006 claims data from a managed-care database.
Recent results from PSOLAR suggest a higher risk of serious infections with adalimumab and infliximab compared to non-methotrexate and non-biologic therapies in PSO, while no increased risk was observed with ustekinumab or etanercept, suggesting that both the diseases and the biologics may differ regarding the risk of serious or hospitalized infections [10].
Finally, for AS and PSA, in addition the short-term data from clinical trials, specific long-term data are urgently awaited and observational studies are planned [11].
Although not designed for research purposes, administrative health databases have become powerful data sources for studying diseases or the long-term outcomes of procedures or health interventions [12], [13] because of their large sample sizes, comprehensive records, and very long observation periods, providing a further useful and feasible tool to quickly increase the body of knowledge of real-life data on one topic and to develop quality of care improvement programs.
Thus, to locally verify the risk of serious infections under biologics among patients suffering from chronic inflammatory autoimmune diseases such as RA, AS, PSA, and severe psoriasis, 10-year administrative databases of a regional health information system were analyzed as the sources of data in the northeastern region of Friuli Venezia Giulia, Italy, which has approximately 1,200,000 inhabitants.
Patient and methods
The Regional Health Information System of Friuli Venezia Giulia was used as the source of information for this retrospective cohort study. The system covers the entire regional population and includes various electronic health administrative databases that can be linked with one another on an individual basis through a unique encrypted identifier. The database of the regional potential health care beneficiaries (including demographic information and the residential history of all of the subjects living in the region), the hospital discharge database, the pharmaceutical prescription database, and the database of exemptions from medical charges were used for this study.
The hospital discharge database includes records from all of the regional hospitals (either public or private accredited to the public health system) and those regarding admissions of regional residents to extra-regional hospitals. The pharmaceutical prescription database contains information on all of the medications prescribed by the physicians working in the public health system except those paid out-of-pocket. The database of exemptions from medical charges includes records on all of the potential health care beneficiaries who are entitled, because of low income, age, or chronic diseases, to receive free medications and outpatient specialist care. The Italian Ministry of Health assigns codes to all of the diseases that entitle patients to exemptions. Currently, they include approximately 100 chronic and disabling diseases including RA, AS, and PSA/PSO (pustular or erythrodermic), (exemption codes 006, 054, and 045, respectively) [14] and groups of rare diseases [15].
The cohort included all of the subjects living in Friuli Venezia Giulia who received an exemption from medical charges because of a diagnosis of either RA, AS, or PSA/PSO according to the corresponding exemption code from 2006 to 2017. The subjects were observed from the date of first release of the exemption and followed until they moved outside the region, died, the outcome of interest occurred, or December 31, 2017, whichever came first.
The outcome of interest was severe infection defined as a hospitalization event with main discharge diagnosis ICD-9-CM code in the following list: 001-139 (infectious and parasitic diseases, except 009.1 (colitis, enteritis, and gastroenteritis of presumed infectious origin), 078.3 (cat-scratch disease), 078.11 (condyloma acuminatum), 084.0 (Falciparum malaria [malignant tertian]), 088.81 (Lyme disease), 099.3 (Reiter's disease), 135 (sarcoidosis), 136.1 (Behçet's syndrome), 320 (bacterial meningitis), 321 (meningitis due to other organisms), 382 (suppurative and unspecified otitis media), 421 (acute and subacute endocarditis), 460 (acute nasopharyngitis), 461 (acute sinusitis), 462 (acute pharyngitis), 463 (acute tonsillitis), 464 (acute laryngitis and tracheitis), 465 (acute upper respiratory infections of multiple or unspecified sites), 466 (acute bronchitis and bronchiolitis), 480 (viral pneumonia), 481 (pneumococcal pneumonia), 482 (other bacterial pneumonia), 483 (pneumonia due to other specified organisms), 484 (pneumonia in infectious diseases classified elsewhere), 485 (bronchopneumonia, organism unspecified), 486 (pneumonia, organism unspecified), 528.3 (oral cellulitis and abscess), 528.5 (diseases of the lips), 566 (abscess of the anal and rectal regions), 567 (peritonitis and retroperitoneal infections), 590 (infections of the kidney), 595 (cystitis, except 595.1 [chronic interstitial cystitis] and 595.2 [other chronic cystitis]), 597.0 (urethral abscess), 680 (carbuncles and furuncles, except 680.2 [trunk]), 686 (other local infections of the skin and subcutaneous tissue), and 711 (septic arthritis). If a patient had multiple events, only the first was considered.
Information on all of the medications prescribed from the exemption date to 2017 was abstracted for each patient. In particular, the prescriptions of traditional DMARDs were identified according to their Anatomical Therapeutic Chemical (ATC) classification codes (ATC L01BA01 or L04AX03 for methotrexate, L04AA13 for leflunomide, A07EC01 for sulfasalazine, P01BA02 for hydroxychloroquine, and P01BA01 for chloroquine) and biological agents (ATC L04AB02 for infliximab, L04AB04 for adalimumab, L04AB01 for etanercept, L04AB05 for certolizumab, L04AB06 for golimumab, L04AC03 for anakinra, L01XC02 for rituximab, L04AA24 for abatacept, and L04AC07 for tocilizumab). The total duration of therapy and number of traditional DMARD prescriptions were calculated. The date of the first biological DMARD prescription was also recorded, if any.
Information on the patient’s age at the start of follow-up, prescriptions for the steroids methylprednisolone (ATC H02AB04) and prednisone (H02AB07) were abstracted as well as the discharge diagnoses of possible hospitalizations that had occurred in the 12 months prior to the release of the rheumatic disease exemption, which were used to calculate Charlson’s Comorbidity Index [16] for each patient at cohort entry.
Statistical analysis
The frequency distribution of the baseline cohort characteristics and events of interest was calculated. The statistical significance of differences in the variable distribution between patients who experienced the event of interest and the others was assessed using the chi-squared test for categorical variables, the t-test for continuous variables with normal distribution, and Wilcoxon’s rank-sum test for continuous variables with non-normal distribution. Normality was assessed using the Kolmogorov-Smirnov test.
Kaplan-Meier curves were calculated to describe the event-free survival of patients, both overall and by treatment groups. The log-rank test and Wilcoxon’s test were used to assess the significance of differences in survival. P < 0.05 was considered statistically significant.
Multiple Cox regressions were used to estimate the risk of hospitalization for patients starting biological treatment compared to the others, adjusting for the potential confounding effect of the following variables: the patient’s age, sex, Charlson’s Comorbidity Index, the calendar year of first exemption from medical charges (<2011 vs ≥2011), the overall DDDs of the steroids prescribed to the patient up to the end of the follow-up, and the average annual number of prescriptions for csDMARDs up to the end of follow-up. Biological medications were included in the models as time-varying variables, that is, for each time, it was assessed whether or not the patient had started biological treatment. The results were expressed using hazard ratios (HRs) and 95% confidence intervals (95% CI).
Cox models stratified by underlying rheumatic disease were also conducted.
All of the analyses were assessed using SAS v9.4 (SAS Institute Inc., Cary, NC, USA.).
Compliance with ethical standards
The authors assert that all of the procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and the Helsinki Declaration of 1975 as revised in 2008. This article does not contain any studies of human or animal subjects performed by any of the authors. Since this analysis was based on anonymous administrative data, patient informed consent and Ethical Committee approval were not required in Italy.
Results
From 2006 to 2017, 6801 people living in Friuli Venezia Giulia received new exemptions from medical charges because of a diagnosis of RA, AS, or PSA/PSO and were included in the cohort. Of these, 289 (4.2%) experienced a hospitalization with the main discharge diagnosis among those of interest during the follow-up period. The median follow-up time was 1910 days. The characteristics of the cohort patients are shown in Table 1, Table 2.
Table 1.
Characteristics of the cohort of 6801 Italian patients with rheumatoid arthritis, psoriatic arthritis/severe psoriasis, or ankylosing spondylitis (categorical variables).
| No hospitalization for infection (N = 6512) | Hospitalization for infection (N = 289) | Total (N = 6801) | P of Chi-squared test | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Sex | 0.1032 | |||
| Female | 4225 (95.5) | 201 (4.5) | 4426 (1 0 0) | |
| Male | 2287 (96.3) | 88 (3.7) | 2375 (1 0 0) | |
| Age category | <0.0001 | |||
| <40 | 1197 (97.8) | 27 (2.2) | 1224 (1 0 0) | |
| 40–64 | 3952 (97.1) | 119 (2.9) | 4071 (1 0 0) | |
| ≥65 | 1363 (90.5) | 143 (9.5) | 1506 (1 0 0) | |
| Rheumatic disease | <0.0001 | |||
| Rheumatoid arthritis | 3656 (94.8) | 200 (5.2) | 3856 (1 0 0) | |
| Psoriatic arthritis/severe psoriasis | 2074 (97.0) | 65 (3.0) | 2139 (1 0 0) | |
| Ankylosing spondylitis | 782 (97.0) | 24 (3.0) | 806 (1 0 0) | |
| First exemption before 2011 | <0.0001 | |||
| No | 3961 (97.4) | 105 (2.6) | 4067 (1 0 0) | |
| Yes | 2551 (93.3) | 183 (6.7) | 2734 (1 0 0) | |
| Cumulative steroid use >180 days | <0.0001 | |||
| No | 5779 (96.3) | 223 (3.7) | 6002 (1 0 0) | |
| Yes | 733 (91.7) | 66 (8.3) | 799 (1 0 0) | |
| Any biological drug prescription | 0.9336 | |||
| No | 5263 (95.8) | 233 (4.2) | 5496 (1 0 0) | |
| Yes | 1249 (95.7) | 56 (4.3) | 1305 (1 0 0) |
Table 2.
Characteristics of a cohort of 6801 Italian patients with rheumatoid arthritis, psoriatic arthritis/severe psoriasis, or ankylosing spondylitis (continuous variables).
| No hospitalization for infection (N = 6512) | Hospitalization for infection (N = 289) | P of Wilcoxon’s rank-sum test | |
|---|---|---|---|
| Charlson’s Comorbidity Index | 0.10 ± 0.50 (0) | 0.290 ± 0.89 (0) | <0.0001 |
| Cumulative steroid use, days | 83 ± 228 (3) | 169 ± 378 (12) | <0.0001 |
| Conventional DMARDs, prescriptions/year | 3.3 ± 4.1 (2.5) | 3.9 ± 3.9 (3.0) | 0.0229 |
Results are expressed as mean ± standard deviation (median).
The most commonly prescribed biological medications in this cohort were adalimumab and etanercept, together representing approximately 80% of prescriptions, followed by golimumab (Table 3).
Table 3.
Active principles of biological medications prescribed in the cohort of 5596 Italian patients with rheumatoid arthritis, psoriatic arthritis/severe psoriasis, or ankylosing spondylitis from 2006 to 2017.
| First biological medicine prescribed for each patient | Total number of prescriptions | |
|---|---|---|
| Active principle | N (%) | N (%) |
| Abatacept | 53 (4.1) | 1220 (4.1) |
| Adalimumab | 565 (43.3) | 11,746 (39.3) |
| Anakinra | 17 (1.3) | 255 (0.8) |
| Certolizumab pegol | 66 (5.1) | 1288 (4.3) |
| Etanercept | 460 (35.2) | 11,008 (36.9) |
| Golimumab | 68 (5.2) | 2964 (9.9) |
| Infliximab | 31 (2.4) | 317 (1.1) |
| Rituximab | 7 (0.5) | 24 (0.1) |
| Tocilizumab | 38 (2.9) | 1043 (3.5) |
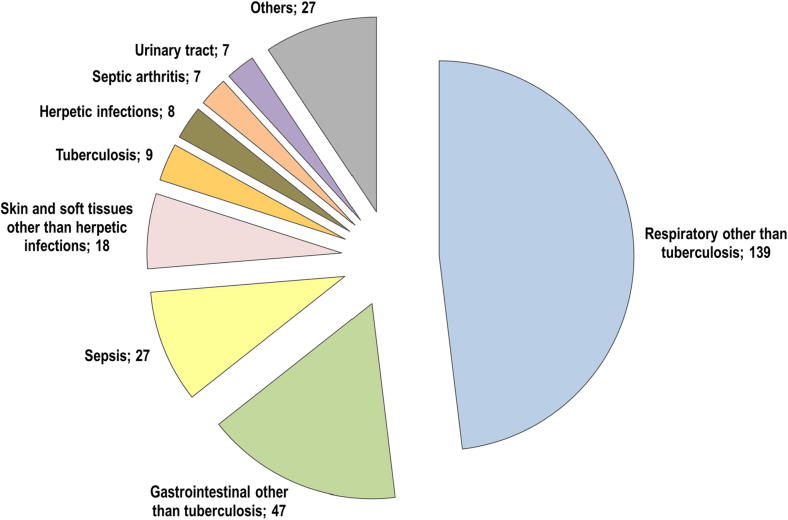
Of the patients hospitalized for infections, 200 had RA, 65 had PSA/PSO, and 24 had AS. Infections affected a variety of organs and systems. Overall, the upper and lower respiratory airways were the most common sites of infection (N = 139, 45.3%), followed by the gastrointestinal region (N = 47, 16.3%). Interestingly, sepsis (N = 27, 9.3%) was more frequent than skin and/or soft tissue infections (N = 18, 6.2%) (Fig. 1).
Fig. 1.
Hospitalized infections in the cohort of 6801 Italian patients with rheumatoid arthritis, psoriatic arthritis/severe psoriasis, or ankylosing spondylitis from 2006 to 2017.
Among the patients with RA, the most common discharge diagnosis was acute respiratory infections: N = 107, 53.5%. Among patients with PSA/PSO and AS, respiratory infections were less common (N = 23, 35.4%, N = 9, 37.5%), whereas infections of the gastrointestinal tract, including anal rectal abscess and peritonitis, were more common than in RA (N = 13, 20.0%, and N = 6, 25.0%) (Table 4).
Table 4.
Hospitalized infections in the cohort of 6801 Italian patients with rheumatoid arthritis, psoriatic arthritis/severe psoriasis, or ankylosing spondylitis from 2006 to 2017, by underlying disease.
| Total | Rheumatoid arthritis (N = 3856) |
Psoriatic arthritis or severe psoriasis (N = 2139) |
Ankylosing spondylitis (N = 806) |
|
|---|---|---|---|---|
| Hospitalized infections | 289 | 200 | 65 | 24 |
| Respiratory other than tuberculosis | 139 (45.3%) | 107 (53.5%) | 23 (35.4%) | 9 (37.5%) |
| Gastrointestinal* other than tuberculosis | 47 (16.3%) | 28 (14.0%) | 13 (20.0%) | 6 (25.0%) |
| Sepsis | 27 (9.3%) | 18 (9.0%) | 7 (10.8%) | 2 (8.3%) |
| Skin and soft tissues other than herpetic infections | 18 (6.2%) | 13 (6.5%) | 5 (7.7%) | – |
| Tuberculosis | 9 (3.1%) | 4 (2.0%) | 2 (3.1%) | 3 (12.5%) |
| Herpetic infections | 8 (2.8%) | 6 (3.0%) | 2 (3.1%) | – |
| Septic arthritis | 7 (2.4%) | 2 (1.0%) | 4 (6.1%) | 1 (4.2%) |
| Urinary tract | 7 (2.4%) | 4 (2.0%) | 2 (3.1) | 1 (4.2%) |
| Others | 27 (9.3%) | 18 (9.0%) | 7 (10.8%) | 2 (8.3%) |
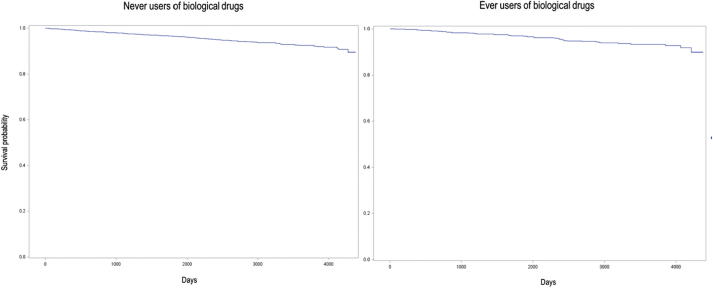
Event-free survival was high both in the patients who used biological drugs and in those who did not use them: after 12 years of follow-up, event-free survival was 89.9% among ever users of biological agents and 89.6% among never users, without statistical differences (Fig. 2; P of the log-rank test was 0.4898, P of Wilcoxon’s test was 0.3619). However, after adjusting for the potential aforementioned confounders, the time to the first use of biological drugs was significantly associated with a 55% increased risk of hospitalization for infections (Table 5). Other factors associated with the risk of hospitalization for infections were age (the elderly patients had a four-fold increased risk compared to those younger than 40), Charlson’s Comorbidity Index (the risk increased with increasing score), the use of steroids (use for more than 180 cumulative days increased the risk by 31%, with borderline statistical significance), and the annual average number of csDMARD prescriptions (8% increase in risk for each increase of one prescription per year).
Fig. 2.
Kaplan-Meier curves of event-free survival in a cohort of 6801 Italian patients with rheumatoid arthritis, psoriatic arthritis/severe psoriasis, or ankylosing spondylitis by their use of biological drugs from 2006 to 2017.
Table 5.
Hazard ratios of hospitalization for infections in a cohort of 6801 Italian patients with rheumatoid arthritis, psoriasis, or ankylosing spondylitis.
| Hazard ratioa | 95% confidence interval | P | |
|---|---|---|---|
| Sex | |||
| Female | 1.06 | 0.82–1.37 | 0.6589 |
| Male | 1.0 | – | |
| Age category | |||
| <40 | 1.0 | – | |
| 40–64 | 1.24 | 0.81–1.90 | 0.3154 |
| ≥65 | 4.21 | 2.74–6.46 | <0.0001 |
| Rheumatic disease | |||
| Rheumatoid arthritis | 1.0 | – | |
| Psoriatic arthritis/severe psoriasis | 1.01 | 0.75–1.37 | 0.9206 |
| Ankylosing spondylitis | 1.04 | 0.67–1.63 | 0.8499 |
| First exemption before 2011 | |||
| No | 1.0 | – | |
| Yes | 0.85 | 0.64–1.12 | 0.2500 |
| Cumulative steroid use > 180 days | |||
| No | 1.0 | – | |
| Yes | 1.31 | 0.99–1.75 | 0.0617 |
| Charlson’s Comorbidity Index (continuous) | 1.35 | 1.19–1.52 | <0.0001 |
| Annual number of traditional DMARD prescriptions | 1.08 | 1.05–1.12 | <0.0001 |
| Time to first biological drug prescription | 1.55 | 1.14–2.10 | 0.0047 |
Adjusted for all of the variables listed in the Table.
The underlying rheumatic disease did not significantly modify the effect of biologic drug use: in the Cox regression models stratified by underlying disease, the HRs associated with time to first biological drug prescription were 1.49 (95% CI 1.01–2.21, P = 0.00446) for RA, 1.11 (95% CI 0.56–2.21, P = 0.7575) for PSA/PSO, and 2.91 (95% CI 1.28–6.62, P = 0.0111) for AS (Table 6).
Table 6.
Hazard ratios of hospitalization for infections in a cohort of 6801 Italian patients with rheumatoid arthritis, psoriasis, or ankylosing spondylitis, by underlying disease.
| Rheumatoid arthritis (N = 3856) |
Psoriatic arthritis or severe psoriasis (N = 2139) |
Ankylosing spondylitis (N = 806) |
||||
|---|---|---|---|---|---|---|
| Hazard ratio (95% confidence interval)a |
P | Hazard ratio (95% confidence interval)a |
P | Hazard ratio (95% confidence interval)a |
P | |
| Sex | ||||||
| Female | 0.91 (0.67–1.25) | 0.5781 | 1.45 (0.88–2.40) | 0.1444 | 1.14 (0.48–2.72) | 0.7713 |
| Male | 1.00 (–) | 1.00 (–) | 1.00 (–) | |||
| Age category | ||||||
| <40 | 1.00 (–) | 1.00 (–) | 1.00 (–) | |||
| 40–64 | 1.28 (0.69–2.39) | 0.4371 | 1.09 (0.53–2.27) | 0.9132 | 1.19 (0.44–3.18) | 0.7291 |
| ≥65 | 5.07 (2.78–9.25) | <0.0001 | 1.92 (0.81–4.56) | 0.1397 | 3.09 (0.84–11.41) | 0.0904 |
| First exemption before 2011 | ||||||
| No | 1.00 (–) | 1.00 (–) | 1.00 (–) | |||
| Yes | 0.94 (0.66–1.32) | 0.7116 | 0.82 (0.46–1.47) | 0.5048 | 0.47 (0.191.17) | 0.1048 |
| Cumulative steroid use > 180 days | ||||||
| No | 1.00 (–) | 1.00 (–) | 1.00 (–) | |||
| Yes | 1.31 (0.95–1.80) | 0.0964 | 1.39 (0.63–2.95) | 0.3980 | 2.01 (0.58–7.00) | 0.2742 |
| Charlson’s Comorbidity Index (continuous) | 1.31 (1.15–1.49) | <0.0001 | 2.07 (1.44–2.98) | <0.0001 | n/a | |
| Annual number of traditional DMARD prescriptions | 1.07 (1.03–1.12) | 0.0008 | 1.11 (1.02–1.20) | 0.0129 | 1.14 (1.08–1.27) | 0.0115 |
| Time to first biological drug prescription | 1.49 (1.01–2.21) | 0.0446 | 1.11 (0.56–2.21) | 0.7575 | 2.91 (1.28–6.62) | 0.0111 |
Adjusted for all of the variables listed in the table.
Discussion
The use of biologics is associated with high rates of improvement in disease symptoms and signs in many chronic inflammatory conditions, and they have become an integral and important part of the treatment strategy when traditional immunosuppressors fail [17], [18].
Biologics are categorized based on their targets. Biologics used for the treatment of RA, AS, PSA, or PSO variably include TNFi, such as etanercept, infliximab, golimumab, certolizumab pegol, adalimumab, and, specifically for RA, non-TNF biologics, including interleukin-1 (anakinra), interleukin-6 receptor (tocilizumab), CD80/86 (abatacept), and B lymphocytes (rituximab). Even if these drugs allowed us to improve the symptoms, signs, and quality of life of moderate to severe forms of chronic arthritides and psoriasis, the harms of biologics must be balanced against of their use benefits when conducting a risk-benefit assessment of their use in patients with systemic autoimmune conditions. Patients and physicians worry about risks including not only common side effects such as injection site reactions but also infections and particularly serious infections that are less common.
In this study, taken together, the patients suffering from RA, AS, PSA, or PSO demonstrated a statistically significant approximately two-fold risk of hospitalized infection from the moment they started biologic treatment. Although the large majority of this cohort suffered from RA, the increase in risk of serious infections was similar for all of the specific diseases. This result is consistent with the level of risk estimated in previous studies of both chronic arthritides and PSO and indirectly supports the integration of administrative databases as an alternative source of data for better understanding long-term outcomes and improving the health system.
Clinical trials on biologics usually enroll patients 18–80 years old; however, extreme ages (both young and elderly) are usually underrepresented, thus both the observed clinical efficacy and safety are not directly attributable to all classes of age. Risk of infections in elderly patients taking biologics has been not well studied and contrasting results have been published up to the present, with few ad hoc studies addressing this issue [8], [19], [20], [21]. In this work, the elderly patients (≥65 years) had a four-fold increased risk of serious infections compared to those <40 years. Similarly, the correction for some other clinical confounders revealed that this risk of infection was associated with comorbidities as measured by Charlson’s Comorbidity Index, chronic exposure to glucocorticoids, or concomitant exposure to traditional immunosuppressors.
The most frequent infections as expected from many data from clinical trials and registries were upper and lower respiratory tract infections. Thus, clinicians who prescribe and patients who undergo biologic treatments must be aware that all of the comorbidities affecting the respiratory tract further increase the risk of serious infections and can worsen infection outcomes. Physicians may postpone prescribing biological and less frequently administer TNFi biological drugs to patients with multimorbidity. Comorbidity may also have a negative effect on the treatment response [22]. In this context, the balance between the risks and benefits of biologic treatment must be carefully evaluated and all health interventions for improving infection control must be followed, such as vaccination, stopping smoking, glucocorticoid tapering, and suspension. Also, since traditional immunosuppressors and biologics can decrease the vaccines’ immunogenicity and efficacy, vaccinations should be proposed to patients at the time of diagnosis, before starting treatment, if clinically appropriate [23].
Finally, the diagnosis before or after 2011 (introduced as a possible confounder) when the concept of “treat to target” was globally proposed in the management of RA [24] was not significantly associated with the risk of infection. On the one hand, it can be speculated that a much more aggressive and intense management of RA did not increase the risk of serious infections; however, on the other hand, the improvement in the diagnosis and cure for RA in recent years may lower the risk of infections in subsequent years by decreasing the patients’ exposure to glucocorticoids or NSAIDs and the number of iatrogenic comorbidities [25], [26].
Because the type of biologic prescriptions herein largely involved TNF inhibitors, in particular adalimumab and etanercept, the results can be mainly applied to the anti-TNF category of biologics. This is a limitation of the study, but it clearly reflects real-world experience. Furthermore, it was not possible to distinguish PSO (erythrodermic or pustular) from PSA as separate categories, since the Italian code for exception (045) comprises both clinical conditions. However, none of the three disease categories (RA, AS, and PSA/PSO) affected the risk of serious infections. Indeed, patients with rheumatic conditions such as RA and AS are often thought to have PSO, when the estimated outcomes are more linked to the treatment employed than to the disease, and this is the case for the risk of infections [7], [27], [28]. In addition, severe patterns of psoriasis other than plaque are rare [29], accounting for less than 10% of psoriatic patients, while PSA is much more prevalent (35%) [29]. Finally, the licensed indications for biologic drugs for psoriasis are limited to chronic moderate to severe plaque psoriasis. Thus, the category of PSA/PSO patients is likely more representative of PSA patients than PSO patients.
Furthermore, patients with PSA or PSO are collectively defined as affected by psoriatic disease; in fact, the cardiovascular risk in this setting is usually studied as psoriatic disease as a whole entity [30]. The efficacy of traditional immunosuppressors such as methotrexate, biologics such as TNF inhibitors, and more recently IL-17 inhibitors [31] for both PSA and PSO supports this notion.
The results of this study should be interpreted considering some limitations depending on the administrative nature of the data sources. First, the diagnoses were based on the disease exemptions. However, most sensitivity and specificity estimates for administrative data-based case definitions were >90% in several systemic rheumatic diseases [32]. Second, there may have been subjects with RA, AS, PSA, or PSO who had no exemption recorded with the codes corresponding to these diseases. This may happen, for instance, among patients with other reasons for exemption from medical charges, such as low income, which is considered more powerful than exemptions due to diseases. This cohort did not include such subjects. In addition, there may be some information bias regarding the outcomes, since the infections were identified from hospital discharge records and the validity of the estimates depends on the quality of the discharge diagnosis coding. Finally, as in all studies using data on medicine prescription, there is some degree of uncertainty regarding the actual drug intake. Despite these limitations, the use of administrative data allowed the assessment of many patients, with full coverage of the regional population, over a substantial timespan and with no recall bias.
Conclusions
Administrative data are novel and promising for the local support of observations coming from clinical trials and registries. The analysis of the administrative data of patients with inflammatory chronic arthritis or psoriasis confirmed an increased risk of hospitalized infections under biologic agents. This risk is much higher in the elderly and those with comorbidities. Upper and lower respiratory tract infections are the most common infections, supporting prevention policies by vaccination, particularly in senior citizens undergoing long-term biologic treatments. Future follow-up studies of this patient cohort and the inclusion of newly diagnosed cases will enable the more precise assessment of such diseases.
Conflict of interest
The authors have declared no conflict of interest.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Bulpitt K.J. Biologic therapies in rheumatoid arthritis. Curr Rheumatol Rep. 1999;1(2):157–163. doi: 10.1007/s11926-999-0013-5. [DOI] [PubMed] [Google Scholar]
- 2.Listing J., Gerhold K., Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology (Oxford) 2013;52(1):53–61. doi: 10.1093/rheumatology/kes305. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe F., Caplan L., Michaud K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: associations with prednisone, disease-modifying antirheumatic drugs, and antitumor necrosis factor therapy. Arthritis Rheum. 2006;54(2):628–634. doi: 10.1002/art.21568. [DOI] [PubMed] [Google Scholar]
- 4.Doran M.F., Crowson C.S., Pond G.R., O’Fallon W.M., Gabriel S.E. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46(9):2287–2293. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 5.Smitten A.L., Choi H.K., Hochberg M.C., Suissa S., Simon T.A., Testa M.A. The risk of hospitalized infection in patients with rheumatoid arthritis. J Rheumatol. 2008;35(3):387–393. [PubMed] [Google Scholar]
- 6.Au K., Reed G., Curtis J.R., Kremer J.M., Greenberg J.D., Strand V. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(5):785–791. doi: 10.1136/ard.2010.128637. [DOI] [PubMed] [Google Scholar]
- 7.Grijalva C.G., Chen L., Delzell E., Baddley J.W., Beukelman T., Winthrop K.L. Initiation of tumor necrosis factor-alpha antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA. 2011;306(21):2331–2339. doi: 10.1001/jama.2011.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galloway J.B., Hyrich K.L., Mercer L.K., Dixon W.G., Fu B., Ustianowski A.P. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology (Oxford) 2011;50(1):124–131. doi: 10.1093/rheumatology/keq242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh J.A., Cameron C., Noorbaloochi S., Cullis T., Tucker M., Christensen R. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet. 2015;386(9990):258–265. doi: 10.1016/S0140-6736(14)61704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalb R.E., Fiorentino D.F., Lebwohl M.G., Toole J., Poulin Y., Cohen A.D. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR) JAMA Dermatol. 2015;151(9):961–969. doi: 10.1001/jamadermatol.2015.0718. [DOI] [PubMed] [Google Scholar]
- 11.Macfarlane G.J., Barnish M.S., Jones E.A., Kay L., Keat A., Meldrum K.T. The British Society for Rheumatology Biologics Registers in Ankylosing Spondylitis (BSRBR-AS) study: protocol for a prospective cohort study of the long-term safety and quality of life outcomes of biologic treatment. BMC Musculoskelet Disord. 2015;16:347. doi: 10.1186/s12891-015-0805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen S., Gilutz H., Marelli A.J., Iserin L., Benis A., Bonnet D. Administrative health databases for addressing emerging issues in adults with CHD: a systematic review. Cardiol Young. 2018;29:1–11. doi: 10.1017/S1047951118000446. [DOI] [PubMed] [Google Scholar]
- 13.Valent F., Busolin A., Boscutti G. Inception and utility of a renal replacement registry using administrative health data in North-East Italy. J Ren Care. 2017;43(2):121–127. doi: 10.1111/jorc.12192. [DOI] [PubMed] [Google Scholar]
- 14.Ministero della Salute. Elenco malattie e condizioni croniche e invalidanti. Allegato 8 DPCM 12 gennaio 2017. <http://www.trovanorme.salute.gov.it/norme/renderPdf.spring?seriegu=SG&datagu=18/03/2017&redaz=17A02015&artp=13&art=1&subart=1&subart1=10&vers=1&prog=001> [accessed 24 April 2018].
- 15.Ministero della Salute. Elenco malattie rare esentate dalla partecipazione al costo. Allegato 7 DPCM 12 gennaio; 2017. <http://www.trovanorme.salute.gov.it/norme/renderPdf.spring?seriegu=SG&datagu=18/03/2017&redaz=17A02015&artp=12&art=1&subart=1&subart1=10&vers=1&prog=001> [accessed 24 April 2018].
- 16.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Singh J.A., Hossain A., Mudano A.S., Tanjong Ghogomu E., Suarez-Almazor M.E., Buchbinder R. Biologics or tofacitinib for people with rheumatoid arthritis naive to methotrexate: a systematic review and network meta-analysis. Cochrane Database Syst Rev. 2017;5 doi: 10.1002/14651858.CD012657. CD012657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terenzi R., Monti S., Tesei G., Carli L. One year in review 2017: spondyloarthritis. Clin Exp Rheumatol. 2018;36(1):1–14. [PubMed] [Google Scholar]
- 19.Kawashima H., Kagami S.I., Kashiwakuma D., Takahashi K., Yokota M., Furuta S. Long-term use of biologic agents does not increase the risk of serious infections in elderly patients with rheumatoid arthritis. Rheumatol Int. 2017;37(3):369–376. doi: 10.1007/s00296-016-3631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugihara T., Harigai M. Targeting low disease activity in elderly-onset rheumatoid arthritis: current and future roles of biological disease-modifying antirheumatic drugs. Drugs Aging. 2016;33(2):97–107. doi: 10.1007/s40266-015-0341-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lahaye C., Soubrier M., Mulliez A., Bardin T., Cantagrel A., Combe B. Society of Rheumatology. Effectiveness and safety of abatacept in elderly patients with rheumatoid arthritis enrolled in the French Society of Rheumatology's ORA registry. Rheumatology (Oxford) 2016;55(5):874–878. doi: 10.1093/rheumatology/kev437. [DOI] [PubMed] [Google Scholar]
- 22.Armagan B., Sari A., Erden A., Kilic L., Erdat E.C., Kilickap S. Starting of biological disease modifying antirheumatic drugs may be postponed in rheumatoid arthritis patients with multimorbidity: single center real life results. Medicine (Baltimore) 2018;97(13):e9930. doi: 10.1097/MD.0000000000009930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman M.A., Winthrop K.L. Vaccines and disease-modifying antirheumatic drugs: practical implications for the rheumatologist. Rheum Dis Clin North Am. 2017;43(1):1–13. doi: 10.1016/j.rdc.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Smolen J.S., Landewé R., Bijlsma J., Burmester G., Chatzidionysiou K., Dougados M. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–977. doi: 10.1136/annrheumdis-2016-210715. [DOI] [PubMed] [Google Scholar]
- 25.Dixon W.G., Suissa S., Hudson M. The association between systemic glucocorticoid therapy and the risk of infection in patients with rheumatoid arthritis: systematic review and meta-analyses. Arthritis Res Ther. 2011;13(4):R139. doi: 10.1186/ar3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hua C., Daien C.I., Combe B., Landewe R. Diagnosis, prognosis and classification of early arthritis: results of a systematic review informing the 2016 update of the EULAR recommendations for the management of early arthritis. RMD Open. 2017;3(1):e000406. doi: 10.1136/rmdopen-2016-000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Accortt N.A., Bonafede M.M., Collier D.H., Iles J., Curtis J.R. Risk of subsequent infection among patients receiving tumor necrosis factor inhibitors and other disease-modifying antirheumatic drugs. Arthritis Rheumatol. 2016;68(1):67–76. doi: 10.1002/art.39416. [DOI] [PubMed] [Google Scholar]
- 28.Saunte D.M., Mrowietz U., Puig L., Zachariae C. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177(1):47–62. doi: 10.1111/bjd.15015. [DOI] [PubMed] [Google Scholar]
- 29.Kimball A.B., Leonardi C., Stahle M., Gulliver W., Chevrier M., Fakharzadeh S. Steering Committee. Demography, baseline disease characteristics and treatment history of patients with psoriasis enrolled in a multicentre, prospective, disease-based registry (PSOLAR) Br J Dermatol. 2014;171:137–147. doi: 10.1111/bjd.13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sobchak C., Eder L. Cardiometabolic disorders in psoriatic disease. Curr Rheumatol Rep. 2017;26;19(10):63. doi: 10.1007/s11926-017-0692-2. [DOI] [PubMed] [Google Scholar]
- 31.Abrouk M., Gandy J., Nakamura M., Lee K., Brodsky M., Singh R. Secukinumab in the treatment of psoriasis and psoriatic arthritis: a review of the literature. Skin Therapy Lett. 2017;22(4):1–6. [PubMed] [Google Scholar]
- 32.Bernatsky S., Linehan T., Hanly J.G. The accuracy of administrative data diagnoses of systemic autoimmune rheumatic diseases. J Rheumatol. 2011;38:1612–1616. doi: 10.3899/jrheum.101149. [DOI] [PubMed] [Google Scholar]