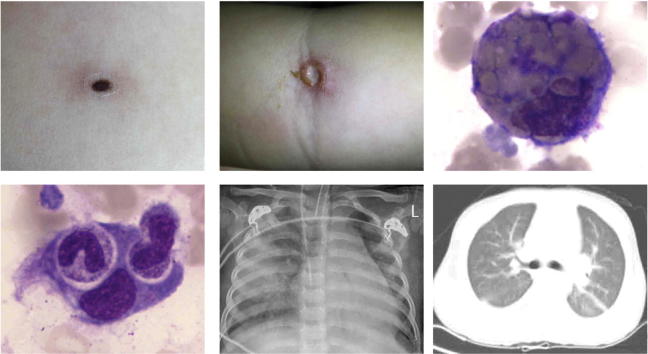
Graphical abstract
Keywords: Rickettsial disease, Hemophagocytic lymphohistiocytosis, Children
Abstract
Hemophagocytic lymphohistiocytosis (HLH) is an uncommon and life-threatening disorder that may rarely complicate the clinical course of Orientia tsutsugamushi disease (scrub typhus). Here, we describe the clinical features, laboratory parameters, management, and outcome of 16 children with scrub typhus-associated HLH. All patients satisfied the HLH-2004 diagnostic criteria. All patients had fever of unknown origin and multisystem damage. Raised hepatic transaminases and abnormalities in routine blood test were observed in all children. Imaging tests showed abnormalities in 10 cases. Six patients were treated with intravenous azithromycin for 5 days, and 10 with intravenous chloramphenicol for 7–10 days because of non-response to 3-day azithromycin treatment. Five patients were treated with intravenous albumin and 3 with intravenous immunoglobulin. Two patients with severe symptoms (shortness of breath, cyanosis) were treated with dexamethasone (0.3 mg/kg/d). Fifteen patients recovered completely after 8–22 days of treatment. One patient died. The occurrence of severe complications draws attention to the need for early diagnosis and effective treatment. Anti-rickettsial antibiotic treatment (azithromycin or chloramphenicol) without the need for chemotherapy may be beneficial in such cases, instead of treatment according to the 2004 HLH protocol.
Introduction
Scrub typhus is an acute febrile illness caused by infection of Orientia tsutsugamushi. It is transmitted by infected chiggers of Trombiculidae family, especially the genus Leptotrombidium [1]. No effective and reliable human vaccine against scrub typhus is currently available [2]. Scrub typhus is characterized by focal or disseminated vasculitis and perivasculitis, involving the lungs, heart, liver, spleen, and central nervous system. It has been an endemic disease in China [3]. Usually, the symptoms of this disease are mild, and its clinical course is uneventful. However, some patients may experience severe or fatal events, such as acute renal failure, respiratory distress and acute liver failure [4]. Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening disorder that can rapidly deteriorate and cause multiple organ failure and even death [5], [6]. HLH can be triggered by rickettsial diseases and other zoonoses [7], [8]. HLH was initially perceived to affect only infants with mutations in these specific genes (PRF1, UNC13D, and STX11), but following studies revealed that this syndrome was also found in adolescents and adults, and the term “secondary HLH (SHLH)” was coined [9], [10]. There are few case reports of scrub typhus-associated HLH in children [9], [10]. This study aimed to investigate the unique presentations of SHLH syndrome and the unusual presentations with unexplained fever and multi-systemic involvement, which may be helpful for the early diagnosis and effective treatment of scrub typhus. Here, we report 16 cases from the Southern part of China: 13 cases from Cang-Nan County, in which scrub typhus is endemic, two cases from Qin-Tian County, and one case from Rui-An County, in which scrub typhus is rare.
Patient and methods
Compliance with ethics guidelines
All procedures followed were in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients included in the study.
Children data
This study was approved by the Ethics Committee of Second Affiliated Hospital. A retrospective medical chart review was performed. Thirty-eight children were diagnosed with scrub typhus, and 16 cases were associated with HLH. All patients were treated in the Yuying Children’s Hospital of Wenzhou Medical University in China and enrolled between January 2010 and July 2016. Residential address, clinical features, laboratory findings, hospital course (hospital stays and hospitalization expenses), complications, and treatments were recorded. OXK titers of 1:160 or more in Weil-Felix test were highly suggestive of rickettsial infection. Treatment response was measured as the time (hours) to becoming afebrile after initiation of specific anti-rickettsial antimicrobial treatment. Children without HLH were excluded from this study.
Diagnosis of scrub typhus
The diagnosis of scrub typhus was made once 3 of following criteria were present [11], [12], [13], [14], [15]: (1) history of outdoor activities in epidemic area or grass in summer and autumn; (2) high fever suddenly occurring and lasting for a long period of time with specific ulcer or eschar; (3) presence of lymph node enlargement, splenomegaly, and rash; (4) Weil-Felix test showing OXK titer of ≥1:160 or an increase in OXK titer by 4-fold or greater in the follow-up. The Weil-Felix test is a commonly used serological test in clinical practice, but it has poor sensitivity and specificity [16]. Thus, the behavioral risk factors and clinical characteristics were more important for the early recognition and diagnosis in rickettsial infection.
Diagnosis of HLH
Diagnosis of HLH was based on Diagnostic and Therapeutic guidelines for Hemophagocytic Lymphohistiocytosis (HLH 2004) [17]: (1) persistent fever; (2) splenomegaly; (3) cytopenias, at least two lineages of hematopoiesis (hemoglobin < 9 g/dL, neutrophils < 1.0 × 109/l, platelets < 100 × 109/l); (4) hypofibrinogenemia (<150 mg/dL) and/ or hypertriglyceridemia (>265 mg/dL); (5) hyperferritinemia (>500 ng/mL); (6) hemophagocytosis; (7) low natural killer cell activity; and (8) high concentration of sCD25 (soluble receptor for interleukin 2). Five of eight criteria were sufficient for the diagnosis of HLH. Therefore, patients who were diagnosed with scrub typhus associated with HLH were included in this study.
Statistical analysis
All statistical analyses were performed using Statistical Package for the Social Sciences v. 17.0 (SPSS 17.0). Normality tests were completed for all data, and the normally distributed data were expressed as mean ± standard deviation (SD). Paired-sample t-tests were used for comparison of data before and after treatment in the chloramphenicol group. A value of two-tailed P < 0.05 was considered statistically significant.
Results
Baseline characteristics and clinical manifestations
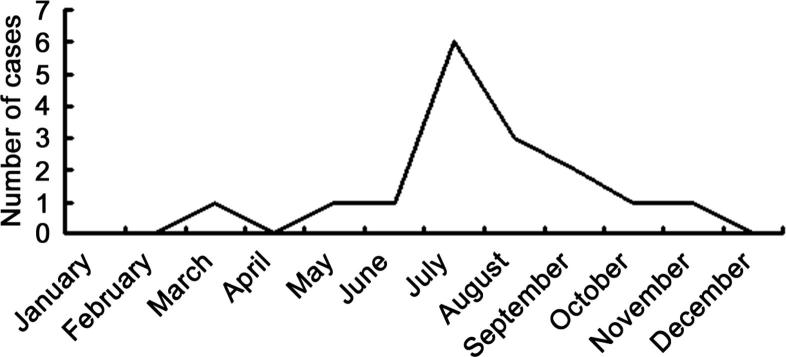
A total of 16 patients with HLH were included in this study. All patients underwent bone marrow examination. Diagnosis of HLH was based on Diagnostic and Therapeutic guidelines for Hemophagocytic Lymphohistiocytosis (HLH 2004). Before admission, 2 patients were diagnosed with sepsis, 3 were treated for pneumonia, and 2 were initiated for the presumptive diagnosis of meningitis. Eighty-one percent (13/16) had a history of outdoor activities in rural areas where scrub typhus was endemic. All children were an average age of 4.69 ± 3.52 years (range: 1–12 years). Among them, 37.5% were boys, and 62.5% were girls. The duration of fever prior to admission ranged from 2 to 14 days (mean: 9.19 ± 3.17 days). The mean hospital stay was 8.56 ± 4.26 days. The scrub typhus was epidemic between June and September, in which 75% of scrub typhus cases were found in the 6 years (Fig. 1). All cases had fever of unknown origin, and the body temperature ranged from 38.3 to 40 °C. The eschar and ulcer were observed in 69% and 31% of patients, respectively (Fig. 2 A and B). Thirteen patients presented with rash on their face and chest. Additionally, cough was found in 10 cases, shortness of breath in 4, and cyanosis in 2. The clinical characteristics of these patients are displayed in Table 1, Table 2.
Fig. 1.
Seasonal variation of scrub typhus with HLH.
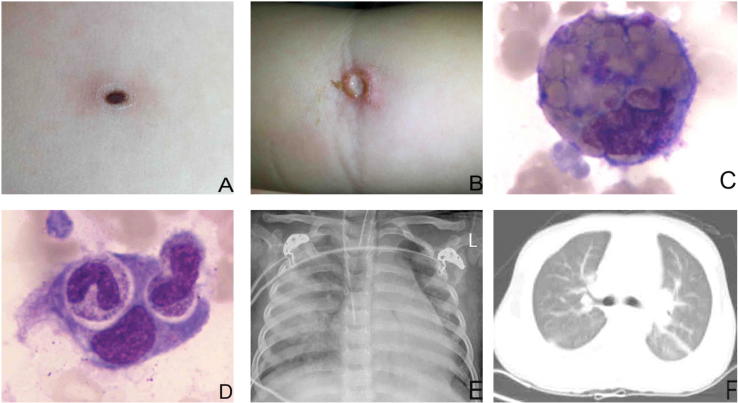
Fig. 2.
A: A 17-month-old boy with a 0.3 × 0.3 cm eschar in the left chest. B: A 3-year-old girl with a 0.5 × 0.5 cm ulcer in the right fossa cubitalia. C, D: Hemophagocytes in the bone marrow after bone marrow aspiration. E: Chest X-ray showed discrete, scattered, patchy shadows on both lungs in a 3-year-old girl. F: CT showed bilateral punctuated change and mild pleural effusion in a 2-year-old girl.
Table 1.
Clinical findings of eschar or ulcer in patients HLH associated scrub typhus.
| No | Age (yrs) | Gender | Position (eschar or ulcer) | Size (cm) (eschar or ulcer) | Weil-Felix test | Hospital stay (days) |
|---|---|---|---|---|---|---|
| 1 | 3 | F | Right fossa cubitalia | 0.5 × 0.5 | + | 22 |
| 2 | 2 | F | Right lower quadrant | 0.2 × 0.2 | + | 8 |
| 3 | 4 | F | Right auricle | 0.1 × 0.1 | − | 12 |
| 4 | 10 | M | Light forearm | 0.3 × 0.3 | + | 8 |
| 5 | 2 | F | Left neck | 0.5 × 0.5 | + | 7 |
| 6 | 2 | F | Right popliteal fossa | 0.3 × 0.3 | + | 10 |
| 7 | 4 | M | Upper chest | 0.5 × 0.5 | − | 7 |
| 8 | 2 | F | Lower abdomen | 0.5 × 0.5 | + | 8 |
| 9 | 1 | F | Left chest | 0.3 × 0.3 | − | 7 |
| 10 | 5 | M | Left groin | 0.2 × 0.2 | − | 7 |
| 11 | 10 | M | Left forearm | 0.4 × 0.4 | − | 5 |
| 12 | 6 | F | Right neck | 0.3 × 0.3 | + | 5 |
| 13 | 3 | F | Left armpit | 0.5 × 0.5 | − | 7 |
| 14 | 1 | M | Right groin | 0.2 × 0.2 | + | 6 |
| 15 | 8 | M | Left armpit | 0.4 × 0.4 | + | 5 |
| 16 | 12 | F | Left neck | 0.5 × 0.5 | − | 13 |
Table 2.
HLH-04 criteria met by the scrub typhus patients.
| No | Days of fever | Splenomegaly | Cytopenias ANC(109/L,)/HB(g/L)/PLT(109/L) | FR (mg/dL)/ TG (mmol/L) | Hyperferriti nemia (ng/mL) | Hemophagocytosis | NK%(8.1–25.6%) | sCD25 |
|---|---|---|---|---|---|---|---|---|
| 1 | 13 | Y | 2.1/88/61 | 237/3.54 | 897. | + | 4.67 | NT |
| 2 | 16 | Y | 5.04/86/55 | 65/3.17 | 995.0 | + | 5.75 | NT |
| 3 | 13 | Y | 3.78/79/22 | 124/4.53 | >1500 | + | 6.6 | NT |
| 4 | 17 | Y | 6.735/69/36 | 94/4.53 | >1500 | + | 5.04 | NT |
| 5 | 10 | Y | 2.929/81/57 | 177/3.01 | 708.5 | + | 5.76 | NT |
| 6 | 8 | N | 0.895/133/76 | 297/3.05 | 657.6 | + | 7.23 | NT |
| 7 | 11 | Y | 6.735/69/11 | 45/4.4 | 820.6 | + | 9.16 | NT |
| 8 | 12 | Y | 3.466/89/25 | 134/3.5 | >1500 | + | 7.16 | NT |
| 9 | 9 | Y | 0.705/89/65 | 346/3.32 | 573.6 | + | 5.08 | NT |
| 10 | 11 | Y | 7.60/88/48 | 233/3.26 | 810.1 | + | 6.40 | NT |
| 11 | 11 | Y | 10.843/84/11 | 246/3.05 | 657.5 | + | 8.25 | NT |
| 12 | 14 | Y | 0.725/90/22 | 116/3.53 | >1500 | + | 6.40 | NT |
| 13 | 11 | Y | 0.854/90/52 | 246/4.05 | 890.6 | + | 6.58 | NT |
| 14 | 15 | Y | 4.519/83/26 | 143/2.87 | >1500 | + | 5.90 | NT |
| 15 | 14 | Y | 1.08/89/65 | 166/3.25 | 995.0 | + | 5.15 | NT |
| 16 | 9 | Y | 3.062/84/55 | 305/2.05 | 580.0 | + | 9.23 | NT |
Notes: ANC: absolute neutrophil count; PLT: platelet; HB: hemoglobin; TG: serum triglyceride; FR: fibrinogenemia; NK: natural killer cell; sCD25: soluble interleukin-2 receptor. NT: not tested;Y:yes;N:No.
Laboratory findings
Serological examinations showed that all patients were negative for EBV (Epstein-Barr virus), TORCH (toxoplasmosis, other, rubella, cytomegalovirus, and herpes), HIV (human immunodeficiency virus), parvovirus B19, hepatitis B and C, and mycoplasma pneumonia. Bacterial culture of the blood and sputum were negative. Raised hepatic transaminases and abnormal blood values were observed in all the children. Bone marrow examinations of all cases disclosed hemophagocytosis (Fig. 2 C and D), but malignancy was not observed. A Weil-Felix agglutination test (for the diagnosis of rickettsial infections) revealed a high anti-OXK titer of ≥1:160 in 56% (9/16) of patients. Abnormalities on X-ray or CT were noted in 10 cases. Chest radiography showed discrete, scattered, and patchy shadows in both lungs of a patient (Fig. 2E). CT showed bilateral punctuated changes and mild pleural effusion in 5 cases (Fig. 2F). The remaining 5 cases showed the thickness or turbulence of texture in bilateral lungs. The laboratory findings of these patients are shown in Table 3. Five cases had headache and lethargy, 4 had abnormal electroencephalogram, but the routine test and biochemistry of the cerebrospinal fluid and magnetic resonance imaging (MRI) examinations were normal.
Table 3.
Findings from routine laboratory examinations at admission and discharge by retrospective analysis.
| Findings | At admission (n = 16) | At discharge (n = 16) | t | P |
|---|---|---|---|---|
| WBC count (109/L) | 7.344 ± 4.897 | 8.950 ± 3.543 | −1.063 | 0.296 |
| PLT count (109/L) | 42.938 ± 20.917 | 158.563 ± 91.030 | −4.952 | 0.000 |
| HGB (g/L) | 86.938 ± 13.978 | 105.063 ± 15.792 | −3.438 | 0.002 |
| CRP (mg/L) | 76.938 ± 31.480 | 13.875 ± 10.012 | 7.636 | 0.000 |
| Albumin (g/L) | 27.312 ± 5.391 | 33.356 ± 3.667 | −3.708 | 0.001 |
| GPT | 166.8123 ± 117.287 | 45.188 ± 14.593 | 4.116 | 0.000 |
Notes: WBC: white blood cells; PLT: platelet; HGB: hemoglobin; CRP: C-reactive protein; GPT: glutamic-pyruvic transaminase
Treatment
The patients with HLH-associated scrub typhus received appropriate treatment with antibiotics against scrub typhus. Six patients were treated with intravenous azithromycin (10 mg/kg/d) for 5 consecutive days, and 10 with intravenous chloramphenicol for 7–10 days because of non-response to azithromycin treatment for 3 days (continuous fever > 38.5 °C). The dosage was reduced from 50 mg/kg/d (divided every 6 h) to 25 mg/kg/d (divided every 6 h) as soon as body temperature normalized. Five patients were treated with intravenous albumin 1 g/kg/d and 3 with intravenous immunoglobulin (IVIG) 500 mg/kg/d for 2 days. Two patients with severe symptoms (shortness of breath, cyanosis) were treated with dexamethasone (0.3 mg/kg/d). Fifteen patients recovered completely after 8–22 days of treatment. One patient with HLH died of multiple organ failure.
Discussion
Rickettsial diseases have been reported in various parts of India and are showing a trend of re-emergence [18], [19], [20], [21]. In our study, most patients (81%) were from rural areas, and this finding was similar to those in other studies, which showed that the rural population was more susceptible to rickettsial infection [22], [23]. Rickettsial diseases in 75% of patients in our study occurred between June and September in the past 6 years. The occurrence of rickettsial infection depends on the climate, temperature, and degree of rainfall in a specific region [24]. These factors in turn are also related to the reproduction of trombiculid mites. The bite of this mite leaves a characteristic black eschar that is useful for its diagnosis. In the present study, the average duration of fever was 9.19 ± 3.17 days (range: 2–14 days) before admission. The patients with continuous high fever of unknown origin should be carefully examined to find eschar or ulcers, which may be located in any (hidden) site. The reported percentage of eschar formation varies significantly across studies. Previous studies from some new endemic areas in northern China showed that the percentages of scrub typhus cases with eschar were 15% and 100%, respectively. In our study, 68.75% of children had eschars and 31.25% had ulcers. This was relatively higher as compared to other reports, which could be explained as follows: (1) The skin color may be a possible explanation for the absence of an eschar in scrub typhus; (2) physicians in our study were very familiar with eschar. Therefore, a careful examination of the entire body should be performed to identify eschar in patients from an endemic area [25]. In our study thorough body examination was carried out in each patient with suspected scrub typhus, which may contribute to the higher detection rate of eschar. Eschar and hepatosplenomegaly with elevated C-reactive protein (CRP) is also helpful for the diagnosis of scrub typhus [26]. In addition, it needs to be identified with other rickettsial organisms, such as Ehrlichia-induced Hemophagocytic Lymphohistiocytosis. Ehrlichia infected patients typically present with fever, headache, malaise, myalgia, arthralgia, nausea, and anorexia [27]. A non-specific maculopapular rash may be present in a percentage of patients [27]. But the most important distinction between Scrub typhus and other Rickettsia is the eschar or ulcer of the skin.
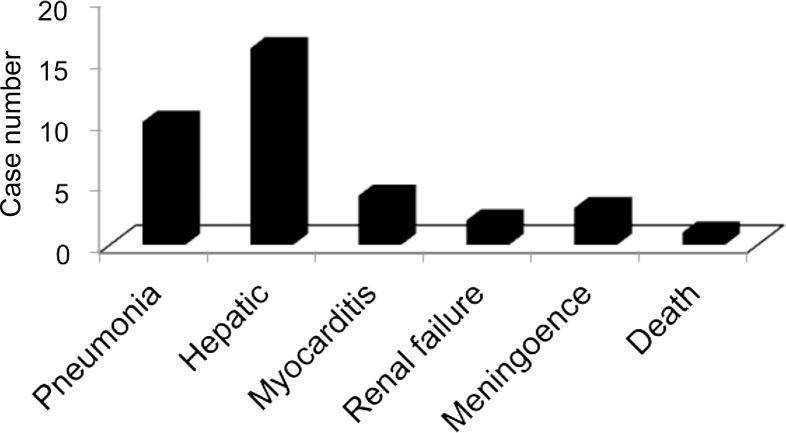
In this study, 75% of patients with a definitive diagnosis of scrub typhus had albumin levels ≤3.0 g/dL, and the serum albumin levels showed significant difference at admission compared with discharge (P < 0.05). Although the pathogenic mechanisms for hypoalbuminemia in patients with scrub typhus have not yet been elucidated, it is postulated that vasculitis, the main pathology of scrub typhus, increases vascular permeability, and thus plasma protein leaks from the blood vessels, subsequently leading to hypoalbuminemia. Hypoalbuminemia has a significant correlation with the severity and mortality rate of this disease [28]. Elevated CRP and liver enzymes were observed in all children, but they returned to normal before the children were discharged from the hospital. Our study showed that the CRP increased significantly at admission against discharge (P < 0.05). Findings from blood examinations at admission and discharge by retrospective analysis showed significant differences in platelets and hemoglobin (P < 0.05). However, the mean of white blood cells remained normal at admission and discharge (P > 0.05) (Table 3). In addition, scrub typhus must be excluded in the differential diagnosis of febrile patients with elevated liver enzymes. Scrub typhus should be considered in the differential diagnosis of acute febrile illness with acute kidney injury (AKI). AKI in scrub typhus is usually mild and non-oliguric, and the kidney function is recovered in most patients. Rhabdomyolysis may be related to AKI. Thrombocytopenia and intensive care requirement are significant predictors of AKI in scrub typhus patients [29]. In our cases, kidney damage was found in two patients but the kidney function returned to normal within 3 days. Neyaz et al. [30] reported brain MRI findings in a patient with scrub typhus. Central nervous system (CNS) clinical or imaging is often seen in HLH of patients [31]. But in our study, results of MRI examination were normal in 4 patients with headache and lethargy. Our results differ from those reported formerly. The reasons for the difference in the proportion of CNS abnormalities may be related to the small sample size and the different causes of HLH. Abnormalities on X-ray or CT were noted in 10 cases. If the patient is diagnosed with HLH who complicated with pulmonary infection, it is necessary to rule out scrub typhus disease. Complications of patients with HLH-associated scrub typhus are shown in Fig. 3. In addition, it should be noted that identification of hemophagocytosis in bone marrow aspirate represents only one of 5/8 criteria needed for the diagnosis of HLH and that a bone marrow aspirate lacking hemophagocytosis does not rule out the diagnosis of HLH [32].
Fig. 3.
Complications of patients with scrub typhus-associated HLH.
Chloramphenicol is a broad-spectrum antibiotic and used as a bacteriostatic agent. Chloramphenicol was isolated in 1947 from Streptomyces Venezuela. The main side effects of chloramphenicol treatment were aplastic anemia and bone marrow suppression. The former was very rare [33], and the latter was fully reversible once the drug was stopped. Doxycycline is banned for use in children under 8 years old in China (due to negative impact on bone growth and discoloration of permanent teeth). Intravenous azithromycin was preferred in all cases because of the side effects of chloramphenicol (bone marrow suppression). However, 10 patients received intravenous chloramphenicol for 7–10 days because of non-response to azithromycin treatment for 3 days (continuous fever > 38.5 °C). The dosage was reduced from 50 mg/kg/d (divided every 6 h) to 25 mg/kg/d (divided every 6 h) as soon as body temperature normalized. In our study, the patients were treated with chloramphenicol, and their fever subsided immediately within 24 h. These patients had blood counts checked twice weekly during treatment. There was no significant decline of the WBC after treatment (P > 0.05). In addition, the HGB and PLT increased significantly after therapy (P < 0.05), indicating that chloramphenicol treatment may not inhibit the blood cells, which might be associated with small sample size in this study. They also had blood counts checked once monthly until 6 months after discharge. No aplastic anemia or bone marrow suppression was observed, indicating that chloramphenicol was relatively safe if the patients were closely monitored.
The HLH 2004 guidelines are suitable for all patients with HLH, with or without evidence of genetic or familial disease, regardless of documented or suspected viral infection [17]. Dexamethasone, etoposide, and cyclosporin A are recommended as the initial therapy in this protocol. Kleynberg and Schiller emphasized the importance of etoposide for the treatment of EBV infection associated with HLH [34]. In our study, two patients with severe symptoms (shortness of breath, cyanosis) were treated with dexamethasone (0.3 mg/kg/d). Fifteen cases without chemotherapy (e.g., etoposide) resulted in complete resolution. Therefore, the use of chemotherapy in the treatment of scrub typhus-associated HLH in children may be unnecessary.
The clinical presentations of patients were unusual: (1) in this study, all patients had high fever of unknown origin, and the response to appropriate treatment can be described as early defervescence; (2) abnormalities in routine blood test, and elevated CRP and liver enzymes; (3) examination of the entire body, which may be helpful for the identification of eschar or ulcer is important for patients with suspected scrub typhus; (4) infected children developed various systemic symptoms, although the prognosis of systemic infection is good in most cases, and rickettsia may be severe and potentially lethal.
Conclusions
The current study highlights that scrub typhus associated with HLH in children is a life-threatening complication. Scrub typhus-associated HLH should be taken into consideration among patients with acute systemic febrile illness, pancytopenia, significantly elevated levels of CRP, hypoalbuminemia, thrombocytopenia, splenomegaly, pneumonitis with pleural effusion, and especially those with suspected exposure history. Early recognition of disease and prompt treatment are critical for the improvement of survival. The Weil-Felix assay may be used as an initial screening test but is not a unique laboratory examination due to its poor sensitivity. Eschar and ulcer on the skin are important manifestations of scrub typhus. Azithromycin was the preferred drug, but it was not effective in some scrub typhus patients with HLH. Chloramphenicol without the need for chemotherapy was effective in such patients. Anti-rickettsial antibiotic treatment combined with an immunomodulatory treatment (steroids and IVIG) may be beneficial in such cases, instead of treatment according to the 2004-HLH protocol.
Conflict of interest
The authors have declared no conflict of interest.
Acknowledgments
This work was supported by the Department of Pediatric Emergency, The Second Affiliated Hospital and Yuying Children’s Hospital, Wenzhou Medical University.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Lyu Y., Tian L., Zhang L., Dou X., Wang X., Li W. A case-control study of risk factors associated with scrub typhus infection in Beijing, China. PLoS ONE. 2013;8(5):e63668. doi: 10.1371/journal.pone.0063668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valbuena G., Walker D.H. Approaches to vaccines against Orientia tsutsugamushi. Front Cell Infect Microbiol. 2012;2:170. doi: 10.3389/fcimb.2012.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lijuan Z., Si H., Yuming J., Liang L., Xuemei L., Lianying L. A rapid, sensitive and reliable diagnostic test for scrub typhus in China. Indian J Med Microbiol. 2011;29(4):368–371. doi: 10.4103/0255-0857.90166. [DOI] [PubMed] [Google Scholar]
- 4.Lai C.H., Huang C.K., Weng H.C., Chung H.C., Liang S.H., Lin J.N. The difference in clinical characteristics between acute Q fever and scrub typhus in southern Taiwan. Int J Infect Dis. 2009;13(3):387–393. doi: 10.1016/j.ijid.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Karapinar B., Yilmaz D., Balkan C., Akin M., Ay Y., Kvakli K. An unusual cause of multiple organ dysfunction syndrome in the pediatric intensive care unit: hemophagocytic lymphohistiocytosis. Pediatr Crit Care Med. 2009;10(3):285–290. doi: 10.1097/PCC.0b013e318198868b. [DOI] [PubMed] [Google Scholar]
- 6.Narendra A.M., Varun Kumar G., Krishna Prasad A., Shetty M., Uppin M.S., Srinivasan V.R. Hemophagocytic lymphohistiocytosis. Indian J Hematol Blood Transfus. 2014;30(3):204–207. doi: 10.1007/s12288-012-0210-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cascio A., Giordano S., Dones P., Venezia S., Iaria C., Ziino O. Haemophagocytic syndrome and rickettsial diseases. J Med Microbiol. 2011;60(Pt 4):537–542. doi: 10.1099/jmm.0.025833-0. [DOI] [PubMed] [Google Scholar]
- 8.Cascio A., Pernice L.M., Barberi G., Delfino D., Biondo C., Beninati C. Secondary hemophagocytic lymphohistiocytosis in zoonoses. A systematic review. Eur Rev Med Pharmacol Sci. 2012;16(10):1324–1337. [PubMed] [Google Scholar]
- 9.Jayakrishnan M.P., Veny J., Feroze M. Rickettsial infection with hemophagocytosis. Trop Doct. 2011;41(2):111–112. doi: 10.1258/td.2010.100303. [DOI] [PubMed] [Google Scholar]
- 10.Kwon H.J., Yoo I.H., Lee J.W., Chung N.G., Cho B., Kim H.K. Life-threatening scrub typhus with hemophagocytosis and acute respiratory distress syndrome in an infant. J Trop Pediatr. 2013;59(1):67–69. doi: 10.1093/tropej/fms030. [DOI] [PubMed] [Google Scholar]
- 11.Iqbal N., Viswanathan S., Remalayam B., Muthu V., George T. Pancreatitis and MODS due to scrub typhus and dengue co-infection. Trop Med Health. 2012;40(1):19–21. doi: 10.2149/tmh.2012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar S., Kumar P.S., Kaur G., Bhalla A., Sharma N., Varma S. Rare concurrent infection with scrub typhus, dengue and malaria in a young female. J Vector Borne Dis. 2014;51(1):71–72. [PubMed] [Google Scholar]
- 13.Lee C.H., Liu J.W. Coinfection with leptospirosis and scrub typhus in Taiwanese patients. Am J Trop Med Hyg. 2007;77(3):525–527. [PubMed] [Google Scholar]
- 14.Mahajan S.K., Kaushik M., Raina R., Thakur P. Scrub typhus and malaria co-infection causing severe sepsis. Trop Doct. 2014;44(1):43–45. doi: 10.1177/0049475513512640. [DOI] [PubMed] [Google Scholar]
- 15.Watt G., Jongsakul K., Suttinont C. Possible scrub typhus coinfections in Thai agricultural workers hospitalized with leptospirosis. Am J Trop Med Hyg. 2003;68(1):89–91. [PubMed] [Google Scholar]
- 16.Kim D.M., Lee Y.M., Back J.H., Yang T.Y., Lee J.H., Song H.J. A serosurvey of Orientia tsutsugamushi from patients with scrub typhus. Clin Microbiol Infect. 2010;16(5):447–451. doi: 10.1111/j.1469-0691.2009.02865.x. [DOI] [PubMed] [Google Scholar]
- 17.Henter J.I., Horne A., Arico M., Egeler R.M., Filipovich A.H., Imashuku S. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 18.Kumar M., Krishnamurthy S., Delhikumar C.G., Narayanan P., Biswal N., Srinivasan S. Scrub typhus in children at a tertiary hospital in southern India: clinical profile and complications. J Infect Public Health. 2012;5(1):82–88. doi: 10.1016/j.jiph.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Mittal V., Gupta N., Bhattacharya D., Kumar K., Ichhpujani R.L., Singh S. Serological evidence of rickettsial infections in Delhi. Indian J Med Res. 2012;135(4):538–541. [PMC free article] [PubMed] [Google Scholar]
- 20.Palanivel S., Nedunchelian K., Poovazhagi V., Raghunadan R., Ramachandran P. Clinical profile of scrub typhus in children. Indian J Pediatr. 2012;79(11):1459–1462. doi: 10.1007/s12098-012-0721-0. [DOI] [PubMed] [Google Scholar]
- 21.Rathi N., Rathi A. Rickettsial infections: Indian perspective. Indian Pediatr. 2010;47(2):157–164. doi: 10.1007/s13312-010-0024-3. [DOI] [PubMed] [Google Scholar]
- 22.Liu J.R., Xu B.P., Li S.G., Liu J., Tian B.L., Zhao S.Y. Clinical features of four atypical pediatric cases of endemic typhus with pneumonia. Zhonghua Er Ke Za Zhi. 2013;51(10):775–778. [PubMed] [Google Scholar]
- 23.Wang Y.C., Chen P.C., Lee K.F., Wu Y.C., Chiu C.H. Scrub typhus cases in a teaching hospital in Penghu, Taiwan, 2006–2010. Vector Borne Zoonotic Dis. 2013;13(3):154–159. doi: 10.1089/vbz.2012.1059. [DOI] [PubMed] [Google Scholar]
- 24.Li T., Yang Z., Dong Z., Wang M. Meteorological factors and risk of scrub typhus in Guangzhou, southern China, 2006–2012. BMC Infect Dis. 2014;14:139. doi: 10.1186/1471-2334-14-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shikino K., Ohira Y., Ikusaka M. Scrub typhus (Tsutsugamushi Disease) presenting as fever with an eschar. J Gen Intern Med. 2016;31(5):582. doi: 10.1007/s11606-015-3371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishna M.R., Vasuki B., Nagaraju K. Scrub typhus: audit of an outbreak. Indian J Pediatr. 2015;82(6):537–540. doi: 10.1007/s12098-014-1664-4. [DOI] [PubMed] [Google Scholar]
- 27.Hanson D., Walter A.W., Powell J. Ehrlichia-induced hemophagocytic lymphohistiocytosis in two children. Pediatr Blood Cancer. 2011;56(4):661–663. doi: 10.1002/pbc.22814. [DOI] [PubMed] [Google Scholar]
- 28.Kim D.M., Kim S.W., Choi S.H., Yun N.R. Clinical and laboratory findings associated with severe scrub typhus. BMC Infect Dis. 2010;10:108. doi: 10.1186/1471-2334-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Attur R.P., Kuppasamy S., Bairy M., Nagaraju S.P., Pammidi N.R., Kamath V. Acute kidney injury in scrub typhus. Clin Exp Nephrol. 2013;17(5):725–729. doi: 10.1007/s10157-012-0753-9. [DOI] [PubMed] [Google Scholar]
- 30.Neyaz Z., Bhattacharya V., Muzaffar N., Gurjar M. Brain MRI findings in a patient with scrub typhus infection. Neurol India. 2016;64(4):788–792. doi: 10.4103/0028-3886.185397. [DOI] [PubMed] [Google Scholar]
- 31.Song Y., Pei R.J., Wang Y.N., Zhang J., Wang Z. Central nervous system involvement in hemophagocytic lymphohistiocytosis in adults: a retrospective analysis of 96 patients in a single center. Chin Med J (Engl) 2018;131(7):776–783. doi: 10.4103/0366-6999.228234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta A., Tyrrell P., Valani R., Benseler S., Weitzman S., Abdelhaleem M. The role of the initial bone marrow aspirate in the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2008;51(3):402–404. doi: 10.1002/pbc.21564. [DOI] [PubMed] [Google Scholar]
- 33.Wallerstein R.O., Condit P.K., Kasper C.K., Brown J.W., Morrison F.R. Statewide study of chloramphenicol therapy and fatal aplastic anemia. JAMA. 1969;208(11):2045–2050. [PubMed] [Google Scholar]
- 34.Kleynberg R.L., Schiller G.J. Secondary hemophagocytic lymphohistiocytosis in adults: an update on diagnosis and therapy. Clin Adv Hematol Oncol. 2012;10(11):726–732. [PubMed] [Google Scholar]