Graphical abstract
Keywords: Scopolamine, L-hyoscyamine, Sparteine, Porcine muscle, Egg, Milk, QuEChERS, Residues, LC-MS/MS
Highlights
-
•
A protocol was developed for detecting and quantifying scopolamine, L-hyoscyamine, and sparteine.
-
•
Target analytes were extracted from animal-based food using EN-QuEChERS and analyzed by LC-MS/MS.
-
•
EDTA solution was employed to improve recovery.
-
•
LOQ values of 1–5 µg/kg were obtained for all analytes.
Abstract
We developed a modified Quick, Easy, Cheap, Effective, Rugged, and Safe (CEN QuEChERS) extraction method coupled with liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI+/MS-MS) to identify and quantify residues of three botanical alkaloids, namely, scopolamine, L-hyoscyamine, and sparteine, in animal-derived foods, including porcine muscle, egg, and milk. A combination of ethylenediaminetetraacetic acid disodium buffer and acetonitrile acidified with 0.5% trifluoroacetic acid was used as an extraction solvent, whereas QuEChERS (CEN, 15662) kits and sorbents were applied for cleanup procedures. The proposed method was validated by determining the limits of quantification (LOQs), with values of 1–5 µg/kg achieved for the target analytes in various matrices. Linearity was estimated from matrix-matched calibration curves constructed using six concentration levels ranging from 1- to 6-fold increases in the LOQs of each analyte, and the correlation coefficients (R2) were ≥0.9869. Recoveries (at three concentration levels of 1-, 2-, and 3-fold increases in the LOQ) of 73–104% were achieved with relative standard deviations (RSDs) ≤7.7% (intra-day and inter-day precision). Ten types of each matrix procured from large markets were evaluated, and all tested samples showed negative results. The current protocol is simple and versatile and can be used for routine detection of plant alkaloids in animal food products.
Introduction
In recent decades, concerns regarding plant toxins, such as botanical alkaloids, have increased because their accumulation in animal feed and food may have negative effects on public health. Botanical alkaloids are biosynthesized by numerous plant species, which may result in subchronic toxicity owing to excessive absorption [1]. Two classes of alkaloids have gained attention: tropane alkaloids and quinolizidine alkaloids.
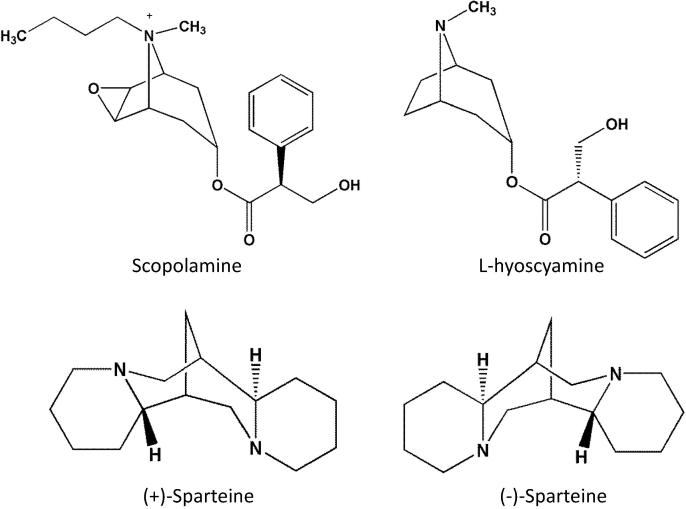
Tropane alkaloids (TAs), which are secondary metabolites, are primarily synthesized by plants in the Solanaceae, Brassicaceae, and Erythroxylaceae families [2]. TAs are found in all parts of the plants and are responsible for the toxic effects of some of these plants. Plant extracts containing TAs have been widely utilized for pharmaceutics in human medicine [3]. Among the 200 TAs reported, atropine and scopolamine (Fig. 1) are representative chemicals in this family [4] and have been used as anticholinergic agents for anaesthesia preparation for many years [5]. However, risk assessment of atropine and scopolamine residues in food and feed by the European Food Safety Authority (EFSA) revealed that TAs may also pose a threat to animal and human health because of their high toxicity [6]. Additionally, atropine is a commercial product containing a racemic mixture of the enantiomers D-hyoscyamine and L-hyoscyamine, but the only effective ingredient showing pharmacological activity is L-hyoscyamine (Fig. 1) [7]. Another class of natural toxins, quinolizidine alkaloids, are derived from Nymophaea or other species in the family Nymphaeaceae [8]. Sparteine (including (+)-sparteine and (−)-sparteine; Fig. 1) has been applied in humans because of its antimuscarinic and oxytocic properties [9] and is widely used as a chiral ligand in the synthesis of some reagents (particularly organolithium reagents); however, the lethal dose of sparteine in 50% of exposed animals (LD50) is 36–67 mg/kg [10], [11], and toxic effects, including cardiac arrhythmia, neurological disorders, and gastrointestinal disorders, were observed following overdose in humans [12].
Fig. 1.
Chemical structures of scopolamine, L-hyoscyamine, (+)-sparteine, and (−)-sparteine.
Plants containing TAs are generally unpalatable and are avoided by most livestock unless other feed are scarce. Therefore, animal exposure to the combination of (−)-hyoscyamine and (−)-scopolamine is primarily from consuming feed contaminated with TA-containing plant material [13]. When wastewater carrying toxins from hospitals flows into rivers, it may be consumed by domestic animals, leading to toxin accumulation in their products (e.g., pork, eggs, and milk) and ultimately, the human body. Therefore, analytical approaches for detecting the contamination levels of these botanical alkaloids are required. Studies have attempted to develop residual detection methods for L-hyoscyamine and scopolamine in a variety of samples, such as grain-based baby food [14], buckwheat grain [15], honey [1], teas and herbal teas [16]. The determination of sparteine levels in human plasma [17], as well as silage, honey, and pig feed [13], has also been reported. However, no studies have examined the residual detection of L-hyoscyamine, scopolamine, and sparteine in animal-derived food products.
Among the reported analytical methods for target alkaloids evaluated in the present study, liquid chromatography-tandem mass spectrometry (LC-MS/MS) is commonly employed to analyse the sample preparation process using solvents, methanol or acetonitrile, without a cleanup procedure [18], [19], [20], [21]. However, abundant protein and fat, as well as the presence of co-eluting substances of animal-derived matrices, can greatly impact the accuracy and sensitivity of this method. For trace residual analysis of food of animal origin [22], the QuEChERS (Quick, Easy, Cheap, Effective, Rugged and Safe) method [23] was developed to reduce time and labour. Here, a protocol using QuEChERS purification coupled to LC-MS/MS was developed and validated as a feasible analytical method for detecting and quantifying L-hyoscyamine, scopolamine, and sparteine residues in porcine muscle, egg, and milk samples. Maximum residue limits (MRLs) have not been established, and the current findings could assist regulatory authorities [24], [25], [26], [27] in setting the appropriate limits.
Material and methods
Reagents, materials, and solutions
Scopolamine hydrobromide (98% purity), trifluoroacetic acid (99% purity), ethylenediaminetetraacetic acid disodium salt (EDTA) solution (0.5 M in H2O), formic acid (98% purity), and ammonium formate (97% purity) were acquired from Sigma-Aldrich (St. Louis, MO, USA). Hyoscyamine sulfate (83% purity) was purchased from the European Pharmacopoeia Reference Standards (EDQM Council of Europe, Strasbourg, France). (+)-Sparteine (98% purity) and (−)-Sparteine (98% purity) were supplied by the Korean Ministry of Food and Drug Safety (MFDS, Seoul, Republic of Korea). HPLC-grade methanol (99% purity) and acetonitrile (100% purity) were obtained from J.T. Baker Chemicals (Phillipsburg, NJ, USA). GH polypro membranes were provided by Pall Corporation (Port Washington, NY, USA), and syringe filters (0.2-µm) were purchased from MILLEX (Merck Millipore Ltd., Co., Billerica, MA, USA). QuEChERS extraction kits and sorbents were acquired from Agilent Bond Elut (Agilent Technologies, Santa Clara, CA, USA).
Primary stock solutions of the target analytes (1 mg/mL) were prepared by weighing each drug powder, followed by transfer to 10 mL of methanol in brown amber flasks. The amount of each drug powder used was based on the precise purity of the sample. For example, to prepare the L-hyoscyamine stock solution (1 mg/mL), 8.3 mg of hyoscyamine sulfate powder was dissolved in 10 mL of methanol and transferred to a brown amber flask. Intermediate individual standard solutions (1 µg/mL) and working solutions at different concentrations (0.005–0.3 µg/mL for scopolamine; 0.002–0.12 µg/mL for L-hyoscyamine; and 0.001–0.06 µg/mL for (+)-sparteine and (−)-sparteine) were prepared by dilution with methanol. All working solutions were stored in the dark at −20 °C and analysed within one week.
Sample preparation
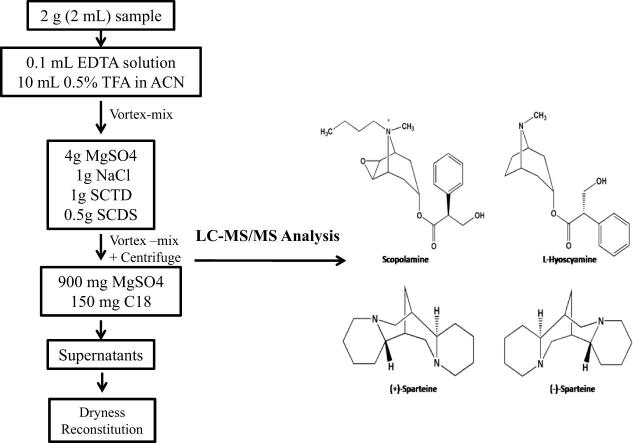
Samples of porcine muscle, egg, and milk were acquired from local markets in Seoul, Republic of Korea. All samples were chopped, homogenized, and weighed. Representative portions (2 g for porcine muscle; 2 mL for milk or egg liquid) were prepared in individual 50-mL centrifuge tubes, fortified with 0.2 mL of working solution, and equilibrated for 10 min [28]. Next, 0.1 mL of EDTA solution was added, followed by the addition of 10 mL of acetonitrile containing 0.5% trifluoroacetic acid. The compounds were thoroughly vortexed by a BenchMixer™ Multi-Tube Vortexer (Benchmark Scientific, NJ, USA) for 5 min prior to adding QuEChERS reagent (4 g of magnesium sulfate, 1 g of sodium chloride, 1 g of sodium citrate tribasic dihydrate, and 0.5 g of sodium citrate dibasic sesquihydrate). Next, the mixture was vortexed for another 5 min and centrifuged at 2600g (Union 32 R Plus, Hanil Science Industrial Co., Ltd., Incheon, Republic of Korea) for 10 min. The supernatants were then transferred to 15-mL QuEChERS d-SPE kits consisting of 150 mg of C18 sorbent and 900 mg of MgSO4, vortexed for 5 min, and centrifuged at 2600g for 10 min. The obtained mixtures were transferred and dried under nitrogen gas at 45 °C until the volume was <0.3 mL. The residues were reconstituted in methanol up to 2 mL, vortexed, centrifuged at 10,840g (MEGA 17R, Hanil Science Industrial Co., Ltd.), and filtered through a 0.2-µm syringe filter prior to LC-MS/MS analysis.
LC-MS/MS analysis
Instrumentation
An Agilent series 1100 HPLC system (Agilent Technologies) equipped with a G1311A Quart pump, a G1313A autosampler, a G1322A degasser, a G1316A column oven, and an API 2000TM liquid chromatography (LC)–triple quadrupole tandem mass spectrometric (MS/MS) detector (Applied Biosystems, Foster City, CA, USA) coupled to an electrospray ionization source (ESI+) was utilized.
LC-MS/MS conditions
Multiple reaction monitoring mode combined with ABI software (version 1.4.2) was implemented for data collection. An ion spray voltage of 5.5 kV, capillary temperature of 350 °C, and pressure of 50 psi were used as optimized conditions for ion source gas 1 (GS1) and ion source gas 2 (GS2). Individual standard solutions (0.1 µg/mL) were injected directly into the MS unit, and the fragments (M + H)+ of the precursor ions were collected; the results are summarized in Table 1.
Table 1.
Multiple reaction monitoring data acquisition parameters for the target alkaloids.
| Analyte | CAS number | Molecular weight | Precursorion (m/z) | Production (m/z) | Collision energy (eV) | Declustering potential (V) |
|---|---|---|---|---|---|---|
| Scopolamine | 51-34-3 | 303 | 304 | 138a | 27 | 51 |
| 103 | 55 | |||||
| 156 | 19 | |||||
| L-hyoscyamine | 101-31-5 | 289 | 290 | 124a | 31 | 51 |
| 93 | 45 | |||||
| 77 | 71 | |||||
| (+)-Sparteine | 90-39-1 | 234 | 235 | 98a | 49 | 86 |
| 70 | 71 | |||||
| 55 | 73 | |||||
| (−)-Sparteine | 90-39-1 | 234 | 235 | 98a | 47 | 61 |
| 70 | 65 | |||||
| 55 | 77 |
Quantification ions.
The ultrahigh-purity water used to prepare the mobile phases was supplied by an aqua MAXTM water purification system (Young Wha, Seoul, Republic of Korea). A binary mobile phase system composed of 0.1% formic acid containing 10 mM ammonium formate in distilled water (solvent A) and methanol (solvent B) was delivered in isocratic gradient mode at a ratio of 10:90 (solvent A:B), with an injection volume of 10 µL and flow rate of 0.2 mL/min.
Method validation
The developed method was validated according to the guidelines described by the Korea Ministry of Food and Drug Safety in 2015 [24] in reference to Codex standards [25]. The method was validated in terms of linearity, accuracy, precision, limits of detection (LODs), and limits of quantitation (LOQs). Six spiking levels equivalent to 1-, 2-, 3-, 4-, 5-, and 6-fold increases in the LOQ values for each compound were prepared to assess the linearity of standards in the solvent and matrices. Accuracy (expressed as recovery) and repeatability (intraday precision) were determined by fortifying blank samples at three spiking levels (n = 5) in a single day. To evaluate the reproducibility (interday precision), the same concentration levels were tested (n = 5) on three consecutive days. The recoveries were determined by comparing the calculated amounts of the analytes spiked in the samples (using matrix-matched calibration curves) with standard solutions. The precision was expressed as the percent relative standard deviation (RSD %). The concentrations that yielded signal-to-noise ratios (S/N) ≥3 and ≥10 were defined as the LOD and LOQ, respectively.
Results and discussion
Optimization of sample preparation
Organic solvents containing acidic or basic additives are commonly used in the extraction of analytes from animal tissues [29]. Thus, methanol and ethyl acetate were assayed as extraction solvents; however, the supernatants were cloudy because of the complexes formed by animal-derived matrices. To improve extraction efficiency, organic solvents are commonly fortified with acids. The effects of adding different acids are dependent upon the properties of the tested analytes [30]. To better understand the effects of different acids on the analyte extraction efficiency, 10 mL of additive-free acetonitrile (for deproteinization) and 10 mL of acetonitrile acidified by (a) 1% acetic acid, (b) 1% formic acid, or (c) 1% trifluoroacetic acid coupled with the CEN QuEChERS purification method were tested at a spiking concentration of 50 µg/kg. When solvent (a), (b), or additive-free acetonitrile was used, a recovery rate of 45–53%, 52–67%, 40–49%, and 37–48% was achieved for scopolamine, L-hyoscyamine, (+)-sparteine, and (−)-sparteine, respectively, in various matrices. Recoveries ≥70% were obtained for all analytes in porcine muscle, egg, and milk when solvent (c) was used. For further comparison, acetonitrile containing various concentrations of trifluoroacetic acid (0.1%, 0.5%, 1%, and 2%; total volume of acetonitrile = 10 mL) was evaluated at a spiking concentration of 50 µg/kg. Based on the obtained recoveries of ≥65%, ≥80%, ≥70%, and ≥68%, respectively, acetonitrile containing 0.5% trifluoroacetic acid showed the highest extraction efficiency and was used throughout the experimental protocol. Notably, EDTA solution was used to improve the accuracy of the developed method, as reported previously [31]. Next, 0.1 mL of EDTA solution (0.5 M) was added, which improved the recoveries by 2.3–4.5%, 3.5–6.1%, 1.9–3.2%, and 2.1–3.5% for scopolamine, L-hyoscyamine, (+)-sparteine, and (−)-sparteine, respectively, in all the matrices (spiking concentration: 50 µg/kg).
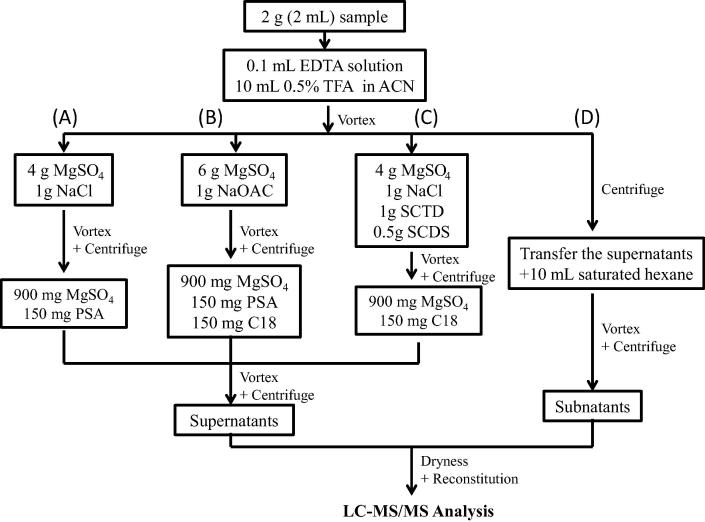
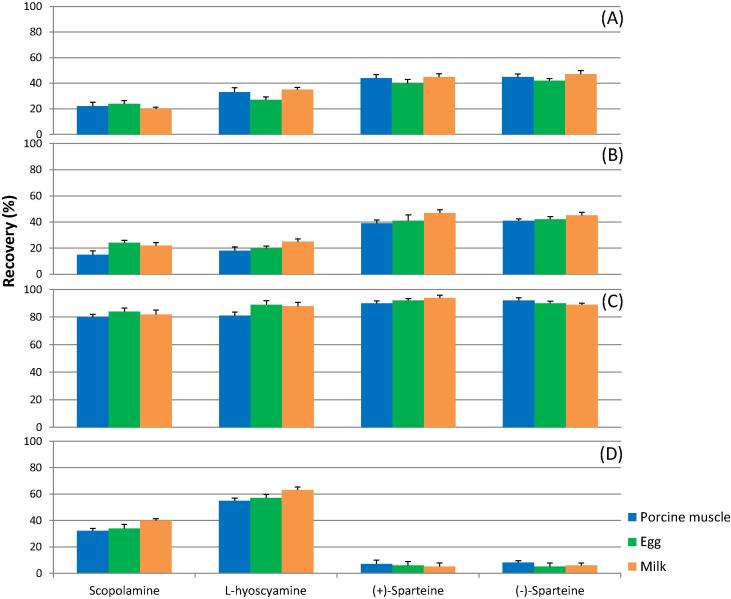
Furthermore, for animal-derived products, the purification process is vital because these samples are rich in proteins, fats, and endogenous substances [29]. Therefore, four protocols based on (A) the original QuEChERS methodology, (B) the AOAC QuEChERS methodology, (C) the CEN QuEChERS methodology (CEN, 15662) [23], [32], [33], and (D) conventional liquid-liquid extraction methodology were compared (at a spiking concentration of 50 µg/kg), as shown in Scheme 1. The duration of vortexing was 5 min, and the speed of centrifugation was 2600g throughout the optimization process. As shown in Fig. 2, recoveries ranging from 20–47%, 15–48%, 80–94%, and 5–63% were obtained when protocols (A), (B), (C), and (D) were utilized, respectively, for the tested analytes in various matrices. Because the d-SPE C18 sorbent in the CEN QuEChERS method can adsorb fatty acids [30], the cleanup step in (C) is an appropriate methodology for animal matrices. Additionally, the components of the modified CEN QuEChERS, magnesium sulfate and sodium chloride, were separately used to eliminate excess water and transfer the analytes from the aqueous phase to the organic phase [30]. The extraction solvent of EDTA solution and acetonitrile containing 0.5% trifluoroacetic acid coupled with CEN QuEChERS purification was utilized in all experiments.
Scheme 1.
Different protocols used for purification of the tested analytes in various matrices.
Fig. 2.
Effects of various cleanup procedures (according to Scheme 1) on the extraction efficiency of scopolamine, L-hyoscyamine, (+)-sparteine, and (−)-sparteine in porcine muscle, egg, and milk (spiking level: 50 µg/kg).
Optimization of chromatographic conditions
Optimized signals were found for all targets when using methanol as a solvent in ESI turbo-positive ion mode. The (a) CAPCELL PAK C18 column, (b) Zorbax Elipse XDB-C18 column, (c) Phenomenex Kinetex EVO C18 column, and (d) Waters Xbridge C18 column were assayed to detect the best separation; the Phenomenex Kinetex EVO C18 column presented the best results.
Acetonitrile or methanol coupled to distilled water is commonly used as the LC mobile phase [34]; therefore, (a) 1 mM ammonium formate, (b) 0.1% formic acid, (c) 0.1% acetic acid, (d) 0.1% formic acid containing 1 mM ammonium formate, and (e) 0.1% formic acid containing 10 mM ammonium formate in distilled water were separately combined with methanol or acetonitrile to test the LC conditions. Ultimately, the mixture of 0.1% formic acid and 10 mM ammonium formate in distilled water (solvent A) and methanol (solvent B) showed the best signal response. Moreover, a membrane filter was utilized to purify the extracts and protect the instrument and column prior to LC-MS/MS analysis [35].
Method performance
Specificity and linearity
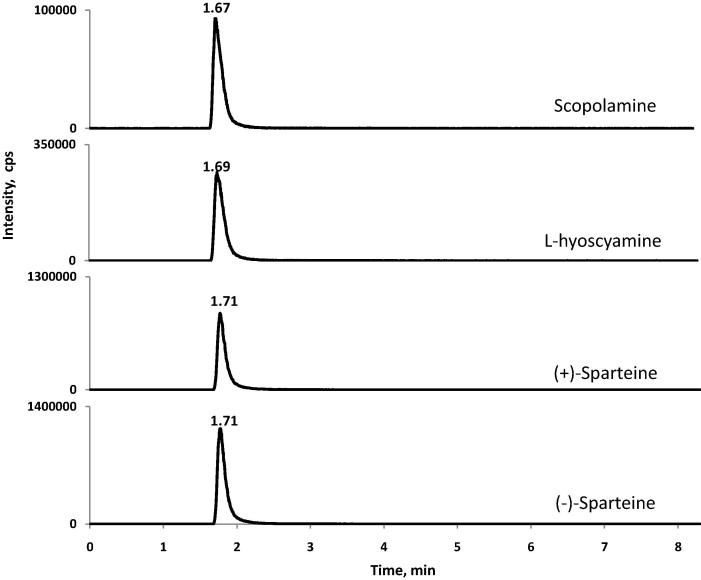
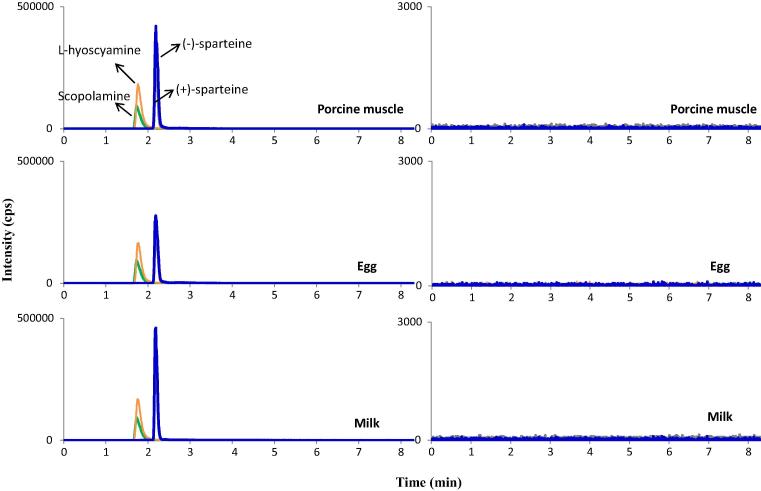
Specificity was evaluated by analysing the working standard and blank porcine muscle, egg, and milk samples (n = 5), which are shown in Fig. 3, Fig. 4. High specificity was observed, with no interfering peaks around the retention times of scopolamine, L-hyoscyamine, (+)-sparteine, and (−)-sparteine.
Fig. 3.
LC-MS/MS chromatograms of standard solutions of scopolamine, L-hyoscyamine, (+)-sparteine, and (−)-sparteine (spiking level: 50 µg/kg).
Fig. 4.
LC-MS/MS chromatograms of standard solutions of scopolamine, L-hyoscyamine, (+)-sparteine, and (−)-sparteine in spiked blank samples (left) and market samples (right) of porcine muscle, egg, and milk (spiking level: 50 µg/kg).
Standard and matrix-fortified determinate calibrations should be performed at six spiking levels according to the Korea MFDS guidelines [24]. Therefore, concentrations of 5, 10, 15, 20, 25, and 30 µg/kg for scopolamine, 2, 4, 6, 8, 10, and 12 µg/kg for L-hyoscyamine, and 1, 2, 3, 4, 5, and 6 µg/kg for sparteine ((+)-sparteine and (−)-sparteine), which are equivalent to 1-, 2-, 3-, 4-, -5, and 6-fold increases in the LOQs for each analyte (n = 5), were evaluated. Calibration curves were acquired by plotting the response for the peak area of the standard at different concentrations. Obtained coefficients of determination (R2) ≥ 0.9869 confirmed the satisfactory linearities of the developed approach (shown in Table 2).
Table 2.
Method performance for the analytes in samples of porcine muscle, egg, and milk.
| Compound | Spiking level (µg/kg) | Intraday (n = 5) Recovery (RSD) (%) |
Interday (n = 15) Recovery (RSD) (%) |
Linear range (µg/kg) | R2 | LOD (µg/kg) | LOQ (µg/kg) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Porcine muscle | Egg | Milk | Porcine muscle | Egg | Milk | ||||||
| Scopolamine | 5 | 74 (2.6) | 87 (2.7) | 85 (4.1) | 74 (3.7) | 85 (3.0) | 87 (2.3) | 5–30 | 0.9869 | 1 | 5 |
| 10 | 84 (5.1) | 98 (3.5) | 80 (3.0) | 86 (2.4) | 99 (2.6) | 81 (1.6) | |||||
| 15 | 85 (5.2) | 92 (4.1) | 82 (1.5) | 89 (1.2) | 93 (5.4) | 85 (4.8) | |||||
| L-hyoscyamine | 2 | 92 (3.3) | 95 (4.1) | 92 (5.5) | 91 (3.1) | 91 (7.7) | 89 (1.9) | 2–12 | 0.9904 | 0.8 | 2 |
| 4 | 89 (1.3) | 83 (5.4) | 97 (2.5) | 91 (3.1) | 83 (4.4) | 94 (3.1) | |||||
| 6 | 86 (3.0) | 85 (2.1) | 99 (5.1) | 86 (1.5) | 85 (2.4) | 97 (4.4) | |||||
| (+)-Sparteine | 1 | 75 (3.6) | 90 (3.2) | 76 (4.1) | 74 (2.0) | 86 (7.0) | 79 (6.1) | 1–6 | 0.9882 | 0.4 | 1 |
| 2 | 90 (1.9) | 82 (2.9) | 82 (2.6) | 91 (1.8) | 77 (4.5) | 82 (2.3) | |||||
| 3 | 94 (4.5) | 84 (5.3) | 86 (1.4) | 92 (4.7) | 82 (3.7) | 82 (4.0) | |||||
| (−)-Sparteine | 1 | 77 (3.3) | 84 (2.3) | 73 (5.2) | 76 (3.2) | 83 (3.9) | 74 (3.4) | 1–6 | 0.994 | 0.4 | 1 |
| 2 | 82 (4.1) | 79 (4.0) | 75 (2.7) | 81 (2.7) | 80 (2.1) | 73 (1.8) | |||||
| 3 | 85 (5.8) | 104 (3.3) | 89 (2.6) | 86 (4.0) | 102 (5.5) | 88 (1.8) | |||||
Accuracy and precision
Accuracy is expressed as recovery, while precision (intraday and interday) is expressed as relative standard deviation (RSD) [30]. The results for accuracy and precision were determined by fortifying blank samples at three concentration levels (1-, 2-, and 3-fold increases the LOQ): 5, 10, and 15 µg/kg for scopolamine; 2, 4, and 6 µg/kg for L-hyoscyamine; and 1, 2, and 3 µg/kg for (+)-sparteine and (−)-sparteine. Five replicates (for each matrix at each concentration level) were prepared to evaluate intraday reproducibility and repeatability (n = 5), and samples were measured on three consecutive days to determine interday values (n = 15). The recoveries and RSDs obtained were evaluated based on the standards described by the Codex Alimentarius Commission [36], which states that when the spiking concentrations range from 1 to 10 ppb, the recoveries and within-laboratory repeatability (RSDs) should be in the range of 60–120% and not above 30%, respectively; in addition, when the spiking concentrations range from 10 to 100 ppb, the recoveries should be in the range of 70–110% with RSDs not above 20%. Herein, the obtained recovery rates were 73–104% with RSDs ≤ 7.7% (intraday and interday) for all analytes in porcine muscle, egg, and milk, indicating that the proposed method is accurate and precise.
LODs, LOQs, and matrix effects
The LODs and the LOQs were calculated when the signal/noise intensity ratio was 3 and 10, respectively. LOD values of 1, 0.8, 0.4, and 0.4 µg/kg and LOQ values of 5, 2, 1, and 1 µg/kg were achieved for scopolamine, L-hyoscyamine, (+)-sparteine, and (−)-sparteine, respectively (n = 10). Remarkably, no MRLs have been established by any regulatory agency [24], [25], [26], [27], and no studies have reported the LODs and LOQs of the target analytes in animal foods.
The high selectivity of tandem mass spectrometry does not greatly reduce the interference from endogenous impurities [37]. Additionally, electrospray ionization (ESI), a soft ionization technique, is more prone to non-volatile components that are competitively co-eluted with the analytes during bioanalysis, thus producing a suppression or enhancement effect, a phenomenon commonly referred to as matrix effects (MEs) [37]. Endogenous substances, including salts, carbohydrates, amines, urea, lipids, peptides, and metabolites [38], and exogenous substances, such as mobile phase additives (as trifluoroacetic acid) and buffer salts [39], could contribute to MEs. Such effects could diminish the reproducibility, linearity, and accuracy of the method and lead to erroneous quantitation [37]. Therefore, such effects should be estimated to ensure the accurate quantification of the tested analytes. The ME (%) was calculated according to the following equation:
ME values of −40 to −25%, −36 to −23%, −27 to −19%, and −25 to −20% were obtained for scopolamine (spiking level: 15 µg/kg), L-hyoscyamine (spiking level: 6 µg/kg), (+)-sparteine (spiking level: 3 µg/kg), and (−)-sparteine (spiking level: 3 µg/kg), respectively, in the samples of porcine muscle, eggs, and milk. Only ion suppression (expressed as negative ME values) was observed for the target analytes in porcine muscle, eggs, and milk samples in the current study. As all matrices contain different percentages of fat, the suppression effect is likely related to particular phospholipids [37] and might also be analyte specific. Overall, matrix-matched calibrations were used throughout the experimental work to quantify the tested analytes in various animal-based food matrices.
Method application
Market samples of porcine muscle, chicken eggs, and milk (including whole milk and low-fat milk) were obtained from different sources in the Republic of Korea. Ten types of each matrix were collected and handled based on the procedures described above, followed by evaluation using the developed LC-MS/MS analytical method. None of the market samples were quantified positive for the tested analytes, as shown in Fig. 4. As swine and poultry are raised in a farmhouse, the transfer of botanical alkaloids to porcine muscle and chicken eggs is therefore limited. Milk might be contaminated if cattle are grazed on botanical plants containing TAs and/or quinolizidine alkaloids.
Conclusions
In this study, a process using an extraction solvent of 0.1 mL of EDTA solution and 10 mL of acetonitrile acidified with 0.5% trifluoroacetic acid combined with the CEN QuEChERS method was developed to detect and quantify three botanical alkaloids, scopolamine, L-hyoscyamine, and sparteine ((+)-sparteine and (−)-sparteine), in samples of porcine muscle, egg, and milk. The LC-MS/MS technique using a Phenomenex Kinetex EVO C18 reversed-phase analytical column coupled to the mobile phase combination of 0.1% formic acid containing 10 mM ammonium formate in distilled water (A) and methanol (B) showed the best separation. Recoveries of 73–104% were acquired, and LOQs of 5, 2, 1, and 1 µg/kg were obtained for scopolamine, L-hyoscyamine, (+)-sparteine, and (−)-sparteine, respectively, in all matrices. Therefore, the proposed protocol is a versatile approach for the simultaneous detection of scopolamine, L-hyoscyamine, and sparteine in animal-derived food products. We suggest further research to monitor other plant alkaloids in food and feed.
Acknowledgments
Acknowledgements
This work was supported by a grant (16162MFDS582) from the Ministry of Food and Drug Safety Administration, Republic of Korea, in 2016.
Conflict of Interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Footnotes
Peer review under responsibility of Cairo University.
Contributor Information
A.M. Abd El-Aty, Email: abdelaty44@hotmail.com.
Ho-Chul Shin, Email: hshin@konkuk.ac.kr.
References
- 1.Martinello M., Borin A., Stella R., Bovo D., Biancotto G., Gallina A. Development and validation of a QuEChERS method coupled to liquid chromatography and high resolution mass spectrometry to determine pyrrolizidine and tropane alkaloids in honey. Food Chem. 2017;234:295–302. doi: 10.1016/j.foodchem.2017.04.186. [DOI] [PubMed] [Google Scholar]
- 2.Adamse P., Van Egmond H., Noordam M., Mulder P., De Nijs M. Tropane alkaloids in food: poisoning incidents. Qual Assur Saf Crops Foods. 2014;6(1):15–24. [Google Scholar]
- 3.Grynkiewicz G., Gadzikowska M. Tropane alkaloids as medicinally useful natural products and their synthetic derivatives as new drugs. Pharmacol Rep. 2008;60(4):439. [PubMed] [Google Scholar]
- 4.Chen H., Marín-Sáez J., Romero-González R., Frenich A.G. Simultaneous determination of atropine and scopolamine in buckwheat and related products using modified QuEChERS and liquid chromatography tandem mass spectrometry. Food Chem. 2017;218:173–180. doi: 10.1016/j.foodchem.2016.09.075. [DOI] [PubMed] [Google Scholar]
- 5.Xu A., Havel J., Linderholm K., Hulse J. Development and validation of an LC/MS/MS method for the determination of L-hyoscyamine in human plasma. J Pharm Biomed Anal. 1995;14(1–2):33–42. doi: 10.1016/0731-7085(95)01630-9. [DOI] [PubMed] [Google Scholar]
- 6.Beuerle T., Benford D., Brimer L., Cottrill B., Doerge D., Dusemund B. Scientific opinion on pyrrolizidine alkaloids in food and feed: EFSA Panel on Contaminants in the Food Chain (CONTAM) J Efsa. 2011;9:(11). [Google Scholar]
- 7.Heine S., Ebert K., Blaschke G. Determination of L-hyoscyamine in atropine and d-hyoscyamine in L-hyoscyamine by chiral capillary electrophoresis as an alternative to polarimetry. Electrophoresis. 2003;24(15):2687–2692. doi: 10.1002/elps.200305492. [DOI] [PubMed] [Google Scholar]
- 8.Vescan A., Vari C.-E., Vlase L. Alkaloid content of some potential isoflavonoids sources (native Genista species). Long-term safety implications. Farmacia. 2014;62(6):1109–1117. [Google Scholar]
- 9.Daly J.W. Nicotinic agonists, antagonists, and modulators from natural sources. Cell Mol Neurobiol. 2005;25(3–4):513–552. doi: 10.1007/s10571-005-3968-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yovo K. Les alcaloïdes quinolizidiniques des graines des lupins: contribution à une étude pharmacologique et toxicologique comparée de la spartéine et de la lupanine, 1982.
- 11.Wink M. Advances in lupin Research. ISA Press; Lisbon: 1994. Biological activities and potential application of lupin alkaloids; pp. 161–178. [Google Scholar]
- 12.Flores-Soto M., Banuelos-Pineda J., Orozco-Suárez S., Schliebs R., Beas-Zarate C. Neuronal damage and changes in the expression of muscarinic acetylcholine receptor subtypes in the neonatal rat cerebral cortical upon exposure to sparteine, a quinolizidine alkaloid. Int J Dev Neurosci. 2006;24(6):401–410. doi: 10.1016/j.ijdevneu.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Mol H., VanDam R., Zomer P., Mulder P.P. Screening of plant toxins in food, feed and botanicals using full-scan high-resolution (Orbitrap) mass spectrometry. Food Addit Contam: Part A. 2011;28(10):1405–1423. doi: 10.1080/19440049.2011.603704. [DOI] [PubMed] [Google Scholar]
- 14.Mulder P.P., Pereboom-de Fauw D.P., Hoogenboom R.L., de Stoppelaar J., Nijs M. Tropane and ergot alkaloids in grain-based products for infants and young children in the Netherlands in 2011–2014. Food Addit Contam: Part B. 2015;8(4):284–290. doi: 10.1080/19393210.2015.1089947. [DOI] [PubMed] [Google Scholar]
- 15.Perharič L., Juvan K.A., Stanovnik L. Acute effects of a low-dose atropine/scopolamine mixture as a food contaminant in human volunteers. J Appl Toxicol. 2013;33(9):980–990. doi: 10.1002/jat.2797. [DOI] [PubMed] [Google Scholar]
- 16.Romera-Torres A., Romero-González R., Martínez Vidal J.L., Garrido Frenich A. Simultaneous analysis of tropane alkaloids in teas and herbal teas by liquid chromatography coupled to high-resolution mass spectrometry (Orbitrap) J Sep Sci. 2018;41(9):1897–2104. doi: 10.1002/jssc.201701485. [DOI] [PubMed] [Google Scholar]
- 17.Yin O.Q., Lam S.S., Lo C.M., Chow M.S. Rapid determination of five probe drugs and their metabolites in human plasma and urine by liquid chromatography/tandem mass spectrometry: application to cytochrome P450 phenotyping studies. Rapid Commun Mass Spectrom. 2004;18(23):2921–2933. doi: 10.1002/rcm.1704. [DOI] [PubMed] [Google Scholar]
- 18.Adams M., Wiedenmann M., Tittel G., Bauer R. HPLC-MS trace analysis of atropine in Lycium barbarum berries. Phytochemical Anal. 2006;17(5):279–283. doi: 10.1002/pca.915. [DOI] [PubMed] [Google Scholar]
- 19.Jakabová S., Vincze L., Farkas Á., Kilár F., Boros B., Felinger A. Determination of tropane alkaloids atropine and scopolamine by liquid chromatography–mass spectrometry in plant organs of Datura species. J Chromatogr A. 2012;1232:295–301. doi: 10.1016/j.chroma.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 20.Ng S.W., Ching C.K., Chan A.Y.W., Mak T.W.L. Simultaneous detection of 22 toxic plant alkaloids (aconitum alkaloids, solanaceous tropane alkaloids, sophora alkaloids, strychnos alkaloids and colchicine) in human urine and herbal samples using liquid chromatography–tandem mass spectrometry. J Chromatogr B. 2013;942:63–69. doi: 10.1016/j.jchromb.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Shimshoni J.A., Duebecke A., Mulder P.P., Cuneah O., Barel S. Pyrrolizidine and tropane alkaloids in teas and the herbal teas peppermint, rooibos and chamomile in the Israeli market. Food Addit Contam: Part A. 2015;32(12):2058–2067. doi: 10.1080/19440049.2015.1087651. [DOI] [PubMed] [Google Scholar]
- 22.Wilkowska A., Biziuk M. Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chem. 2011;125(3):803–812. [Google Scholar]
- 23.Anastassiades M., Lehotay S.J., Štajnbaher D., Schenck F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int. 2003;86(2):412–431. [PubMed] [Google Scholar]
- 24.Ministry of Food and Drug Safety (MFDS). Maximum residue limits (MRLs) of veterinary medicine, Republic of Korea, 2015, http://fse.foodnara.go.kr/residue/RS/jsp/menu 02 01 03.jsp?idx=828 [accessed 26.03.15.].
- 25.Codex Alimentarius Commission, July 2014. Updated as at the 37th Session http://www.codexalimentarius.org/standards/veterinary-drugs-mrls/en.
- 26.The Japanese Positive List System for Agricultural Chemical Residues in Foods Ministry of Health, Labour and Welfare, Tokyo, Japan, 2014. http://www.ffcr.or.jp/zaidan/FFCRHOME.nsf/pages/MRLs-p [Accessed 10 March 2015].
- 27.U.S. Food and Drug Administration CFR – Code of Federal Regulations Title 21 Part 556. Tolerances for Residues of New Animal Drugs in Food, 2014 http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?CFRPart=556&showFR=1.
- 28.Zhang D., Park J.A., Kim S.K., Cho S.H., Jeong D., Cho S.M. Simultaneous detection of flumethasone, dl-methylephedrine, and 2-hydroxy-4,6-dimethylpyrimidine in porcine muscle and pasteurized cow milk using liquid chromatography coupled with triple-quadrupole mass spectrometry. J Chromatogr B. 2016;1012–1013:8–16. doi: 10.1016/j.jchromb.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Zheng W., Park J.-A., Abd El-Aty A.M., Kim S.-K., Cho S.-H., Choi J.-M. Bithionol residue analysis in animal-derived food products by an effective and rugged extraction method coupled with liquid chromatography–tandem mass spectrometry. J Chromatogr B. 2017;1064:100–108. doi: 10.1016/j.jchromb.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 30.Zheng W., Park J.-A., Zhang D., Abd El-Aty A.M., Kim S.-K., Cho S.-H. Determination of fenobucarb residues in animal and aquatic food products using liquid chromatography-tandem mass spectrometry coupled with a QuEChERS extraction method. J Chromatogr B. 2017;1058:1–7. doi: 10.1016/j.jchromb.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Choi J.-H., Mamun M., Abd El-Aty A.M., Kim K.T., Koh H.-B., Shin H.-C. Inert matrix and Na4EDTA improve the supercritical fluid extraction efficiency of fluoroquinolones for HPLC determination in pig tissues. Talanta. 2009;78(2):348–357. doi: 10.1016/j.talanta.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 32.Lehotay S.J., Tully J., Garca A.V., Contreras M., Mol H., Heinke V. Determination of pesticide residues in foods by acetonitrile extraction and partitioning with magnesium sulfate: collaborative study. J AOAC Int. 2007;90(2):485–520. [PubMed] [Google Scholar]
- 33.Lehotay S.J., Son K.A., Kwon H., Koesukwiwat U., Fu W., Mastovska K. Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J Chromatogr A. 2010;1217(16):2548–2560. doi: 10.1016/j.chroma.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 34.Gan J., Lv L., Peng J., Li J., Xiong Z., Chen D. Multi-residue method for the determination of organofluorine pesticides in fish tissue by liquid chromatography triple quadrupole tandem mass spectrometry. Food Chem. 2016;207:195–204. doi: 10.1016/j.foodchem.2016.02.098. [DOI] [PubMed] [Google Scholar]
- 35.Thompson R.D., Carlson M. Liquid chromatographic determination of dehydroepiandrosterone (DHEA) in dietary supplement products. J AOAC Int. 2000;83(4):847–857. [PubMed] [Google Scholar]
- 36.Codex Alimentarius Commission Codex Guidelines for the Establishment of a Regulatory Programme for Control of Veterinary Drug Residues in Foods. Part III Attributes of Analytical Methods for Residue of Veterinary Drugs in Foods, 1993. CA C/GL 16: 41.
- 37.Park J.A., Abd El-Aty A.M., Zheng W., Kim S.K., Cho S.H., Choi J.M. Simultaneous determination of clanobutin, dichlorvos, and naftazone in pork, beef, chicken, milk, and egg using liquid chromatography-tandem mass spectrometry. Food Chem. 2018;252:40–48. doi: 10.1016/j.foodchem.2018.01.085. [DOI] [PubMed] [Google Scholar]
- 38.Ismaiel O.A., Zhang T., Jenkins R.G., Karnes H.T. Investigation of endogenous blood plasma phospholipids, cholesterol and glycerides that contribute to matrix effects in bioanalysis by liquid chromatography/mass spectrometry. J Chromatogr B. 2010;878(31):3303–3316. doi: 10.1016/j.jchromb.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 39.Garcia M. The effect of the mobile phase additives on sensitivity in the analysis of peptides and proteins by high-performance liquid chromatography–electrospray mass spectrometry. J Chromatogr B. 2005;825(2):111–123. doi: 10.1016/j.jchromb.2005.03.041. [DOI] [PubMed] [Google Scholar]