Abstract
Purpose
This study aimed to investigate the effect of nintedanib on the conversion of human Tenon’s fibroblasts (HTFs) into myofibroblasts and reveal the molecular mechanisms involved.
Methods
Primary cultured HTFs were incubated with transforming growth factor β1 (TGF-β1) alone or combined with nintedanib, and cell proliferation and migration were measured by cell counting kit-8 (CCK8) and the scratch wound assay, respectively. HTF contractility was evaluated with a 3D collagen contraction assay. The mRNA and protein levels of α smooth muscle actin (α-SMA) and Snail and the phosphorylation levels of Smad2/3, p38 mitogen-activated protein kinase (p38MAPK), and extracellular signal-regulated kinase ½ (ERK1/2) were determined by quantitative reverse transcription polymerase chain reaction (RT-PCR), western blot, and immunofluorescence staining.
Results
Nintedanib inhibited the proliferation and migration of HTFs in a dose-dependent manner. Furthermore, nintedanib prevented HTF myofibroblast differentiation via downregulation of mRNA and protein expression of α-SMA and Snail. A three-dimensional (3D) collagen gel contraction assay demonstrated that nintedanib effectively inhibits myofibroblast contraction induced by TGF-β1. Mechanistically, we revealed that nintedanib reduces the TGF-β1-induced phosphorylation of Smad2/3, p38MAPK, and ERK1/2, suggesting that nintedanib acts through both classic and nonclassic signaling pathways of TGF-β1 to prevent HTF activation.
Conclusions
Our study provides new evidence that nintedanib has potent antifibrotic effects in HTFs and suggests that it may be used as a potential therapeutic agent for subconjunctival fibrosis.
Introduction
Glaucoma is a leading cause of irreversible blindness [1]. In 2010, the World Health Organization estimated that 285 million people in the world were visually impaired; in 22.8 million (8%) of these people, the impairment was caused by glaucoma [2]. The vision loss caused by glaucoma is mainly attributed to damage at the optic nerve head (ONH) as a result of elevated intraocular pressure (IOP) [3,4]. At present, glaucoma filtration surgery is the most effective approach to consistently achieving a desirable low IOP, and trabeculectomy is considered the mainstay of treatment [5]. However, a major issue with this surgery is subconjunctival fibrosis, which is robustly associated with surgical failure [5]. To achieve a better operative success rate, several antimitotic agents, such as mitomycin C (MMC) and 5-fluorouracil (5-FU), have been applied postoperatively [6]. However, a significant failure rate persists and is associated with sight-threatening infections [6]. Thus, an alternative antifibrotic agent is urgently needed.
Subconjunctival fibrosis is mediated by the activities of human Tenon’s fibroblasts (HTFs), which initiate myofibroblast differentiation upon stimulation by many fibrogenic factors. Activated HTFs can produce extracellular matrix (ECM) proteins, such as type I, III, V, and VI collagens, all of which are major components of fibrotic tissue [7]. Furthermore, HTFs turn into myofibroblasts with contractile properties leading to ECM shrinkage, which contributes to scar formation. Transforming growth factor β1 (TGF-β) family members, especially TGF-β1, are regarded as the prominent stimuli of HTF activation [8]. Through binding to its heterodimer receptors (TβR I, TβR II), TGF-β1 triggers phosphorylation of the downstream signal proteins Smad2/3, which in turn form a complex with Smad4, thereby translocating to the nucleus and regulating target gene transcription [8]. In addition to the abovementioned classical signaling cascade, recent studies have identified a nonclassical signaling pathway in which TGF-β1 triggers mitogen-activated protein kinase (MAPK) signal cascade activation [9].
Nintedanib is an intracellular multitarget inhibitor of tyrosine kinases, including the platelet-derived growth factor receptors (PDGFRs), vascular endothelial growth factor receptors (VEGFRs), and fibroblast growth factor receptors (FGFRs), and it is approved for the treatment of idiopathic pulmonary fibrosis (IPF) [10,11]. It has shown consistent antifibrotic and anti-inflammatory activities in animal models of lung fibrosis [12] and interference with central processes in fibrosis, such as fibroblast proliferation, migration, and differentiation and ECM protein secretion [11]. These remarkable antifibrotic effects of nintedanib in other tissues have prompted us to hypothesize that it may present similar functions in subconjunctival fibrosis.
In this manuscript, we aimed to test whether nintedanib can prevent the HTF activation induced by TGF-β1. We found that nintedanib offers an antifibrotic effect by counteracting TGF-β1-stimulated HTF myofibroblast transdifferentiation. Furthermore, we revealed that this inhibitory effect of nintedanib acts through suppression of TGF-β/Smad2/3, p38MAPK, and ERK1/2 activation.
Methods
Cell culture
Samples of human Tenon’s capsules were obtained anonymously from tissue explants taken during strabismus or glaucoma filtering surgery at the Zhongshan Ophthalmic Center, Sun Yat-sen University, with the approval of the ethics committee of the Zhongshan Ophthalmic Center and in accordance with the Declaration of Helsinki. The tissues were cut into approximately 2-mm pieces under sterile conditions. Then, the samples were seeded in 60-mm culture dishes and maintained in Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 (DMEM/F12, Invitrogen-Gibco, Karlsruhe, Germany) supplemented with 10% fetal bovine serum (FBS, Invitrogen, Auckland, NZ), 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen) in a 5% CO2 humidified atmosphere at 37 °C. Approximately 8 days after seeding, primary cultures were passaged at 60% confluence with 0.25% trypsin-EDTA (Invitrogen) into 100-mm culture dishes. For subsequent passaging, cells were dissociated using 0.25% trypsin-EDTA (Invitrogen) at 90% confluence and replanted at 1:2 or 1:3 dilutions at a concentration of 1×106 cells per 100-mm plate. HTFs from passages 3 to 6 were used in the experiments. Cells were characterized by immunostaining for fibroblast markers, vimentin (Abcam, Cambridge, UK) and cytokeratin (Abcam).
CCK8 cell viability assay
HTFs were seeded in 96-well plates (1×104 cells/well) and incubated overnight in 100 μl complete culture media. Then, the cells were washed to remove serum and replaced with serum-free culture media overnight for starvation, followed by the addition of 5 ng/ml TGF-β1, a mixture of SB203580 and TGF-β1, or a mixture of nintedanib and TGF-β1 for 24 h. Then, 10 µl CCK-8 reagent was added to each well and incubated at 37 °C for an additional 4 h. The absorbance at 450 nm was measured using a microplate reader (Bio-Rad, Munich, Germany).
BrdU cell proliferation assay
Cells were seeded (5000 cells/well) in 96-well culture plates. After serum starvation overnight, the cells were treated with TGF-β1 (5 ng/ml) in the presence or absence of nintedanib (1 μM) for 24 h. Cell proliferation was detected using a 5-Bromo-2-deoxyUridine (BrdU) cell proliferation kit (ab126556, Abcam) according to the manufacturer’s instructions. Briefly, 20 μl of BrdU was added at 37 °C and incubated for 12 h, followed by fixing with fixing solution (3.7% formaldehyde in PBS-1X; Potassium Phosphate monobasic-KH2PO4; 1 mM, Sodium Chloride-NaCl; 155 mM, Sodium Phosphate dibasic-Na2HPO4-7H2O; 3 mM, pH 7.4) at room temperature for 30 min. After washing with PBS, anti-BrdU monoclonal antibody (100 µl/well) was added and incubated for 1 h. Then, the cells were washed with PBS, and peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) antibody (1:2,000) was added and incubated for 30 min. Subsequently, the cells were washed with PBS and incubated with 3,3',5,5'-Tetramethylbenzidine (TMB) peroxidase substrate for 30 min at room temperature in the dark. Finally, 100 µl of stop solution was added, and the absorbance at 450 nm was measured by a microplate reader (Bio-Rad, Munich, Germany).
Cell migration assay
Cells were seeded in six-well plates and allowed to grow to confluence. The cells were starved overnight by removing serum, and a vertical wound was created using a 200 μl pipette tip. The cells were then washed three times with PBS and replaced with fresh DMEM/F12 medium containing the indicated agents. After treatment for 12 h, the migration distance was measured in six random fields under phase-contrast microscopy.
Fibroblast-mediated collagen contraction assay
The effects of nintedanib on HTF contractility were assessed using Cell Biolabs’ (San Diego, CA) collagen contraction assay kit. Briefly, a total of 5×105 HTFs and 500 μl of collagen preparation were added to each well in a 24-well plate. After collagen gel polymerization, nintedanib was added 1 h before application of TGF-β1 (5 ng/ml). After mixing, the gels were detached from the well and photographed at 24 h. The contraction was measured by analyzing gel size using NIH ImageJ software version 1.47.
Western blot assay
The cells were incubated in ice-cold RIPA Lysis Buffer (50 mM Tris-HCl, pH 7.4, 5 mM EDTA, 150 mM NaCl, 1% NP-40, 1% protease inhibitor cocktail, and 1% phosphatase inhibitor cocktail; Beyotime Biotechnology, Nanjing, China) on ice for 10 min, the lysates were centrifuged at 10,000 ×g, and the supernatants were collected and frozen at −80 °C. Protein concentrations were determined with the Micro BCA protein assay kit (Thermo Scientific, Waltham, MA) according to the manufacturer’s instructions. Protein samples were separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) gels and transferred to polyvinylidene fluoride (PVDF) membranes (Thermo Scientific), which were then probed overnight with primary antibodies against human α smooth muscle actin (α-SMA; ab5694, 1:1,000, Abcam, Cambridge, UK), TGF-β1 (sc-130348, 1:1,000, Santa Cruz Biotechnology, Dallas, TX), Smad2 (#5339, 1:1,000, Cell Signaling Technology, Danvers, MA), Smad3 (#9523, 1:1,000, Cell Signaling Technology), phospho-Smad2/3 (#8828, 1:1,000, Cell Signaling Technology), p38MAPK (#8690, 1:1,000, Cell Signaling Technology), phospho-p38MAPK (#4511, 1:1,000, Cell Signaling Technology), ERK1/2 (#4695, 1:1,000, Cell Signaling Technology), phospho-ERK1/2 (#4370, 1:1,000, Cell Signaling Technology), Snail (#3879, 1:1,000, Cell Signaling Technology), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; ab9485, 1:1,000, Abcam), followed by incubating with horseradish peroxidase–conjugated secondary antibodies (sc-2375, 1:2,000, Santa Cruz Biotechnology) for 1 h at room temperature. Labeled proteins were detected by enhanced chemiluminescence.
Quantitative real-time PCR
Total RNA was harvested using RNeasy spin columns (Qiagen, West Sussex, UK) according to the manufacturer’s instructions. cDNA was synthesized by reverse transcriptase from total RNA with PrimeScript RT Master Mix (Takara Biotechnology Co., Ltd., Dalian, China). The expression of specific mRNAs was determined with a LightCycler (Roche) using the SYBR Green PCR Master Mix (Roche). The sequences of the PCR primers used in the experiment are as follows: GAPDH, forward 5′-GGA GCG AGA TCC CTC CAA AAT-3′ and reverse 5′-GGC TGT TGT CAT ACT TCT CAT GG-3′; α-SMA, forward 5′-AAA AGA CAG CTA CGT GGG TG-3′ and reverse 5′-GCC ATG TTC TAT CGG GTA CTT C-3′; TGF-β1, forward 5′-GGC CAG ATC CTG TCC AAG C-3′ and reverse 5′-GTG GGT TTC CAC CAT TAG CAC-3′; Snail, forward 5′-TCG GAA GCC TAA CTA CAG CGA-3′ and reverse 5′-AGA TGA GCA TTG GCA GCG AG-3′.
Immunofluorescence staining
The cells were fixed with 4% paraformaldehyde for 15 min, permeated with 0.1% Triton X-100 for 10 min, and then blocked with 3% bovine serum albumin (BSA; Sigma-Aldrich, Dorset, UK) for 1 h. Following this, the cells were incubated with primary antibodies against α-SMA (ab5694, 1:200, Abcam), vimentin (ab92547, 1:200, Abcam), p-Smad2/3 (ab63399, 1:200, Abcam), or cytokeratin (#5741, 1:200, Cell Signaling Technology) at 4 °C overnight and then exposed to Alexa Fluor® 488-conjugated green fluorescence secondary antibodies (ab150077, 1:500, Abcam) or Alexa Fluor® 555-conjugated red fluorescence secondary antibodies (ab150158, 1:500, Abcam) for 1 h at room temperature. 4’,6-Diamidino-2-phenylindole (DAPI) was used for cell nuclei detection. Images were captured under a Leica fluorescence microscope.
Statistical analysis
All experiments were repeated at least three times. Values are expressed as mean ± standard deviation (SD). GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA) was used for statistical analysis. Comparisons among multiple groups involved one-way analysis of variance (ANOVA) with post hoc intergroup comparison with the Tukey test. Statistical significance was set at p<0.05.
Results
Culture and identification of HTFs
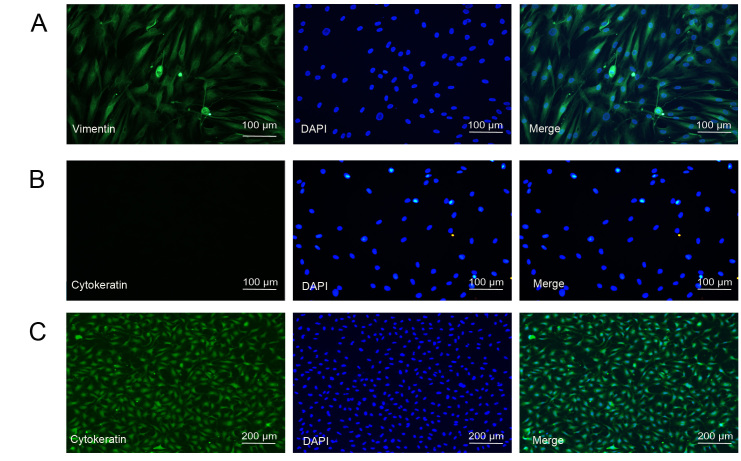
Before performing the subsequent experiments, we first characterized primary cultured HTFs isolated from tissue samples from strabismus or glaucoma filtering surgery. The cells were grown in monolayers and exhibited a spindly, generally flat, elongated shape under the microscope (Figure 1). Furthermore, we performed immunostaining for vimentin, a specific fibroblast marker, and cytokeratin, a marker for epithelial cells, in HTFs. As a positive control, we also performed immunostaining for cytokeratin in lens epithelial cells. As shown in Figure 1C, the cultured lens epithelial cells were positive for cytokeratin, indicating that the antibody for cytokeratin was effective. The cultured HTFs were positive for vimentin and negative for cytokeratin (Figure 1A,B). These results suggested that the isolated HTFs are of high purity and suitable for subsequent study.
Figure 1.
Characterization of human Tenon’s fibroblasts (HTFs). A: HTFs were identified by immunostaining with fibroblast markers, vimentin (green), and nuclei (blue) were labeled with 4’,6-diamidino-2-phenylindole (DAPI). Scale bar = 100 μm. B: HTFs were immunostained with the epithelial cell marker, cytokeratin, and nuclei (blue) were labeled with DAPI. Scale bar = 100 μm. C: Lens epithelial cells were immunostained with the epithelial cell marker, cytokeratin, and nuclei (blue) were labeled with DAPI. Scale bar = 100 μm.
Nintedanib suppresses TGF-β1-induced HTF proliferation and migration
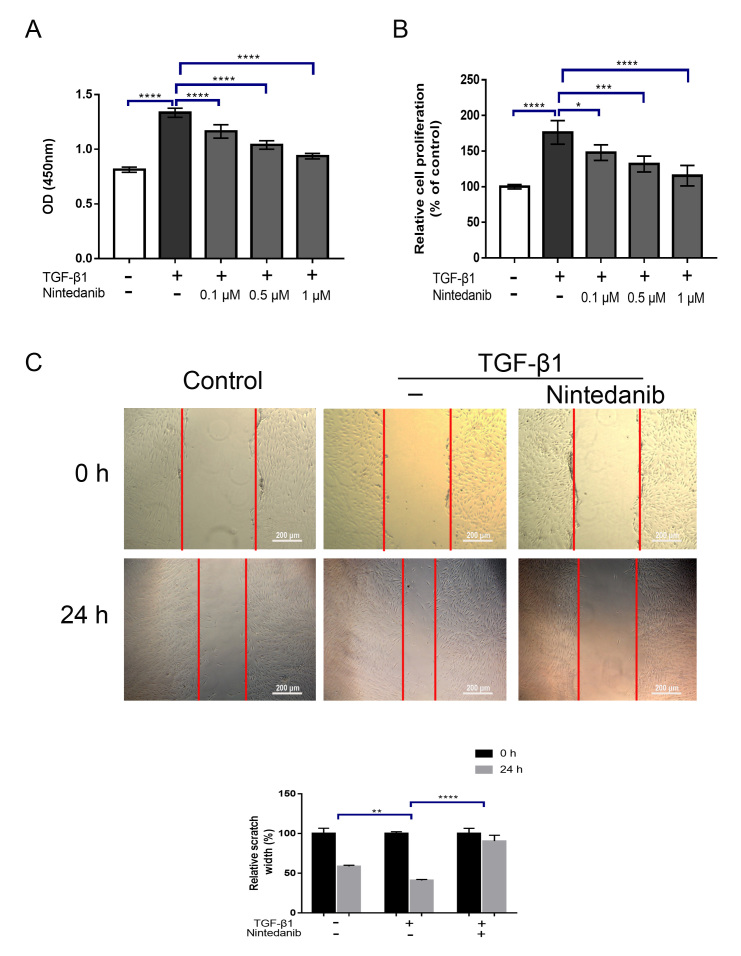
To determine whether nintedanib can suppress HTF proliferation induced by TGF-β1, we treated HTFs with TGF-β1 (5 ng/ml) in the presence or absence of different concentrations of nintedanib (0.1, 0.5, and 1 μM) for 24 h. Cell proliferation was evaluated via the CCK-8 assay. Previous studies demonstrated that the concentration of TGF-β1 used to stimulate fibroblasts ranged between 1 and 5 ng/ml [13–15], and our previous data indicate that treatment with 5 ng/ml of TGF-β presented the maximal activation of HTFs (data not shown). Thus, we chose to use 5 ng/ml of TGF-β in our experiment. The results showed that, while TGF-β1 significantly increased the proliferation of HTFs after 24 h (p<0.0001; Figure 2A), pretreatment with nintedanib largely attenuated this effect in a dose-dependent manner (p<0.05; Figure 2A). To further confirm the effect of nintedanib on proliferation, we also performed a BrdU cell proliferation assay, which was consistent with the CCK-8 assay (Figure 2B). Next, we performed wound-healing assays to determine the effect of nintedanib on HTF migration. As shown in Figure 2C, wound closure was significantly accelerated by TGF-β1 treatment compared with the vehicle control (migration distance 40.97% ± 1.24% versus 58.72% ± 1.38% vehicle control, p<0.05; Figure 2C). This effect was abrogated by pretreatment with nintedanib (migration distance 90.44% ± 7.36% versus 58.72% ± 1.38% TGF-1β, p<0.05; Figure 2C). These data indicated that nintedanib could potently suppress HTF proliferation and migration induced by TGF-β1.
Figure 2.
Nintedanib inhibits human Tenon’s fibroblast (HTF) proliferation and motility induced by transforming growth factor β1 (TGF-β1). A: HTFs were treated with TGF-β1 (5 ng/ml) in the presence or absence of different concentrations of nintedanib (0.1 μM, 0.5 μM, 1 μM) for 24 h. Cell proliferation was determined by cell counting kit-8 (CCK-8) assay. B: HTFs were treated with TGF-β1 (5 ng/ml) in the presence or absence of different concentrations of nintedanib (0.1 μM, 0.5 μM, 1 μM) for 24 h. Cell proliferation was determined by a 5-Bromo-2-deoxyUridine (BrdU) incorporation assay. C: HTFs were stimulated with TGF-β1 (5 ng/ml) for 24 h in the presence or absence of nintedanib (1 μM). Cell migration was evaluated by wound healing assay. Data are presented as the means ± standard deviations (SDs; n = 3) of independent repeated experiments (* p<0.01; ** p<0.01; *** p<0.001; **** p<0.0001 compared with control or TGF-β1 group).
Nintedanib prevents the HTF transdifferentiation into myofibroblasts by TGF-β1
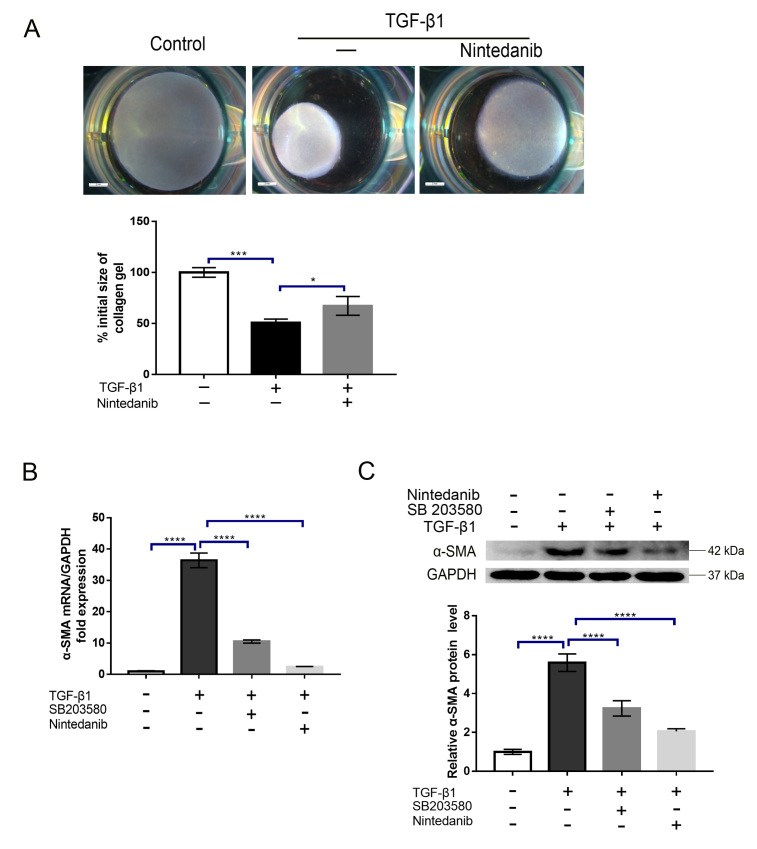
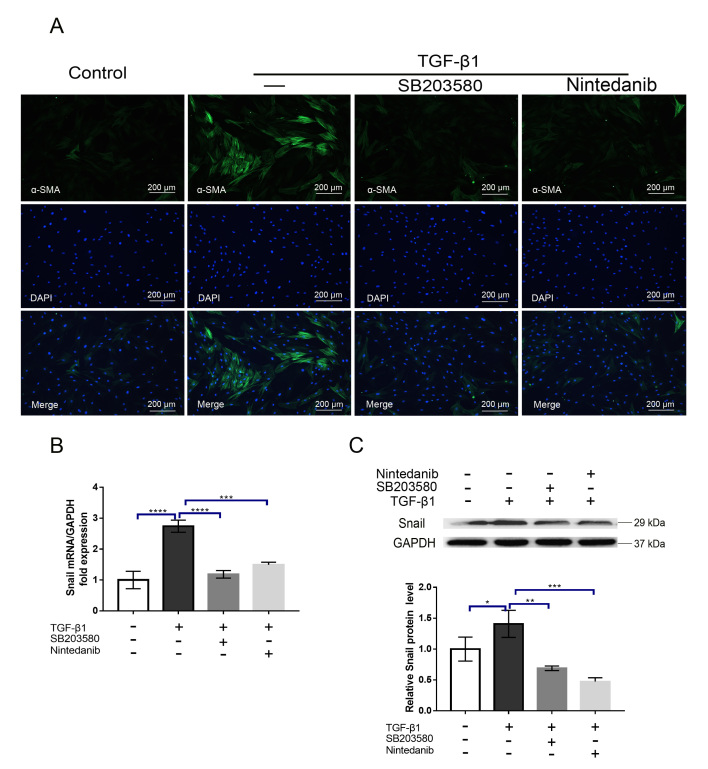
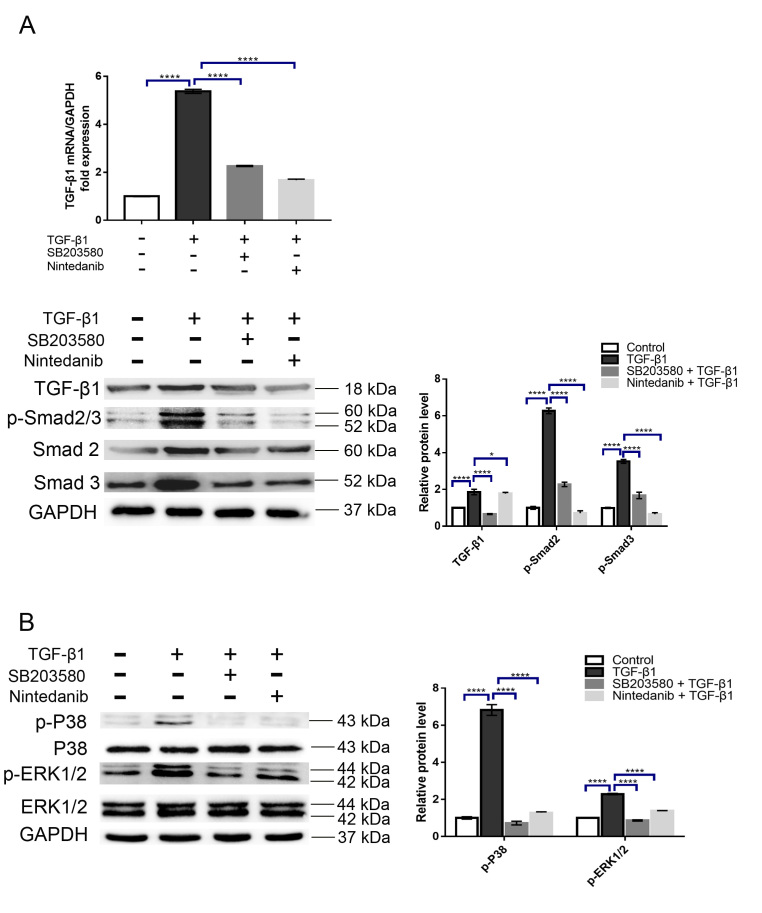
As mentioned above, the contraction of the ECM mediated by myofibroblasts greatly contributes to scar formation. To determine whether nintedanib can inhibit myofibroblast contraction induced by TGF-β1, we treated HTFs with TGF-β1 (5 ng/ml) in the presence or absence of nintedanib (1 μM). The collagen contraction response was evaluated by a three-dimensional (3D) collagen contraction assay. The results demonstrated that TGF-β1 significantly enhanced collagen matrix contraction compared with the vehicle control (p<0.001, Figure 3A), and this effect was significantly inhibited by preincubation with nintedanib (p<0.05, Figure 3A). The conversion of fibroblasts into myofibroblasts is a hallmark of fibroblast activation. At the molecular level, this process is characterized by α-SMA upregulation. To address whether this process can be impeded by nintedanib, we treated HTFs with TGF-β1 (5 ng/ml) in the presence or absence of nintedanib (1 μM); we then performed RT-qPCR and western blot experiments to detect the mRNA and protein levels of α-SMA, respectively. As shown in Figure 3B,C, while treatment with TGF-β1 upregulated α-SMA mRNA and protein expression, as expected, preincubation with nintedanib substantially alleviated the effect of TGF-β1 (p<0.0001). Because a previous study reported that p38MAPK plays a crucial role in HTF transdifferentiation [15], we treated cells with a p38MAPK specific inhibitor, SB203580, and tested its efficacy in our model. Our results showed that inhibition of p38MAPK significantly attenuated HTF activation, which is in line with the previous study, and this effect was comparable to the nintedanib treatment effects (Figure 3B,C). To confirm the effect of nintedanib on α-SMA expression, we performed immunofluorescence staining of α-SMA after treatment with nintedanib or SB203580 and obtained a similar result (Figure 4A). Snail is a transcription factor that plays a critical role in the epithelial–mesenchymal transition (EMT) and the switching of fibroblasts into myofibroblasts. To further verify the inhibitory effect of nintedanib on HTFs transdifferentiation, we evaluated the effect of nintedanib or SB203580 on Snail expression. The results showed that HTF cells treated with TGF-β1 (5 ng/ml) for 24 h exhibited markedly increased expression of Snail (Figure 4E,F) and nintedanib or SB203580 preincubation largely reduced the mRNA and protein expression of Snail induced by TGF-β1(Figure 4E,F). It is noteworthy that the inhibitory effect did not show a significant difference between the nintedanib and SB203580 groups. These results strongly indicated that nintedanib could potently inhibit the conversion of HTFs into myofibroblasts.
Figure 3.
Nintedanib inhibits three-dimensional (3D) collagen gel contraction and α smooth muscle actin (α-SMA) expression induced by transforming growth factor β1 (TGF-β1). A: Human Tenon’s fibroblasts (HTFs) were treated with TGF-β1 (5 ng/ml) in the presence or absence of nintedanib (1 μM) for 24 h. The gel contraction was detected by a three-dimensional (3D) collagen lattice contraction experiment. B,C: HTFs were treated with TGF-β1 (5 ng/ml) in the presence or absence of SB203580 or nintedanib (1 μM) for 24 h. The mRNA (B) and protein (C) expression of α-SMA were analyzed by quantitative reverse transcription polymerase chain reaction (RT-PCR) and western blot, respectively, and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression. Data are presented as the means ± standard deviations (SDs; n = 3) of independent repeated experiments (* p<0.01; *** p<0.001; **** p<0.0001 compared with the control or TGF-β1 group).
Figure 4.
Nintedanib inhibits the expression of α smooth muscle actin (α-SMA) and Snail induced by transforming growth factor β1 (TGF-β1). A: The cells were treated with TGF-β1 (5 ng/ml) in the presence or absence of SB203580 or nintedanib (1 μM) for 24 h and then stained with anti-α-SMA linked to fluorescein isothiocyanate (FITC) and 4’,6-diamidino-2-phenylindole (DAPI; nuclei). Immunofluorescence was detected by confocal microscopy. Scale bar = 200 μm. B,C: Human Tenon’s fibroblasts (HTFs) were treated with TGF-β1 (5 ng/ml) in the presence or absence of SB203580 or nintedanib (1 μM) for 24 h. The mRNA (B) and protein (C) expressions of Snail were analyzed by quantitative reverse transcription polymerase chain reaction (RT-PCR) and western blot, respectively, and normalized to GAPDH expression. Data are presented as the means ± standard deviations (SDs; n = 3) of independent repeated experiments (* p<0.01;** p<0.01;*** p<0.001; **** p<0.0001 compared with the control or TGF-β1 group).
Nintedanib alleviates the TGF-β1-stimulated phosphorylation of Smad2/3, p38MAPK, and ERK1/2 in HTFs
TGF-β1 has been reported to exert biological functions via activation of the Smad2/3 and MAPK signaling pathways. To reveal the molecular mechanisms underlying the inhibitory effects of nintedanib in our experiments, we sought to determine whether nintedanib can inhibit the activation of these signaling cascades by TGF-β1. The results showed that, while treatment with TGF-β1 (5 ng/ml) alone resulted in a significant increase in Smad2/3, p38MAPK, and ERK1/2 phosphorylation, as expected, these effects were significantly alleviated by preincubation with nintedanib or SB203580 (Figure 5A,B). Importantly, we observed that the reduced phosphorylation of Smad2/3 was accompanied by reduced translocation of Smad2/3 into the nucleus (Appendix 1), further supporting the functional blockade of TGF-β1 signaling by nintedanib. As we measured the molecular activation of this signaling at a later time point (24 h), an indirect activation of these signaling molecules by TGF-β1 cannot be ruled out, such as via transactivation of PDGFR or FGFR, which has been reported in other studies [16,17]. To address this, we further determined whether TGF-β1 can rapidly activate these signaling molecules. As shown in Appendix 2, treatment with TGF-β for 10 min can stimulate Smad, p38 and ERK1/2 phosphorylation, which are all blocked by nintedanib. These results supported the notion that these signaling molecules are directly activated by TGF-β and can be inhibited by nintedanib at an early timepoint. Intriguingly, TGF-β1 also enhanced TGF-β1 mRNA and protein expression, suggesting that TGF-β1 induced a positive feedback circuit in HTFs. Accordingly, this effect can be largely attenuated by nintedanib or SB203580 (Figure 5A,B). Taken together, these data suggested that nintedanib prevented HTF activation through suppression of the Smad2/3, p38MAPK, and ERK1/2 signaling pathways.
Figure 5.
Nintedanib suppresses Smad2/3, p38MAPK, and ERK1/2 activation induced by transforming growth factor β1 (TGF-β1) at later timepoints. Human Tenon’s fibroblasts (HTFs) were treated with TGF-β1 (5 ng/ml) in the presence or absence of SB203580 or nintedanib (1 μM) for 24 h. TGF-β1 and p-Smad2/3 (A), p-p38MAPK and p-ERK1/2 (B) mRNA and protein expression were analyzed by quantitative reverse transcription polymerase chain reaction (RT-PCR) and western blot, respectively, and normalized to total Smad2/3, ERK1/2, or GAPDH. Data are presented as the means ± standard deviations (SDs; n = 3) of independent repeated experiments (* p<0.01;**** p<0.0001 compared with the control or TGF-β1 group).
Discussion
Subconjunctival fibrosis is the major reason for the primary failure of glaucoma filtering surgery. Despite the application of anti-mitotic agents, such as MMC and 5-FU, which have postoperatively largely prolonged the longevity of the bleb [18], these therapeutic strategies are closely associated with serious postoperative complications, including bleb leakage, hypotony maculopathy, and endophthalmitis [19]. We are the first to show that nintedanib, an intracellular inhibitor of tyrosine kinase receptors, may serve as a potential therapeutic agent for subconjunctival fibrosis.
We employed primary cultured HTFs isolated from tissue explants taken during strabismus or glaucoma filtering surgery as our in vitro cell model. The present study provides evidence that nintedanib exhibits a potent antifibrotic effect in HTFs through inhibition of cell proliferation and migration, myofibroblast differentiation, and 3D collagen gel contraction, all of which are fundamental events during fibrosis and scar formation. We discovered that the underlying mechanisms of these effects are that nintedanib targets not only the classic Smad2/3 signaling cascade but also the newly identified nonclassic p38MAPK and ERK1/2 pathways of TGF-β1 in HTFs.
Nintedanib is a well-known broad-spectrum tyrosine kinase inhibitor that targets multiple receptor kinases, such as PDGFR, VEGFR, FGFR, and Src family kinases, in a variety of tissue cells [20-22]. At present, nintedanib is widely used for the treatment of IPF [23]. Information from two repeated Phase III clinical trials on IPF demonstrated that patients receiving nintedanib treatment showed successfully reduced disease progression and annual rates of decline in forced vital capacity relative to their placebo counterparts [10]. In addition to IPF, recent studies have suggested that nintedanib can be used to target tumor growth, metastasis and angiogenesis in non–small cell lung cancer (NSCLC) and colorectal cancer [24]. The wide application of nintedanib in disease treatments is not surprising because its targets are involved in numerous critical cellular functions. Considering that the targets of nintedanib, such as PDGFR and FGFR, are also involved in the pathogenesis of intraocular fibrosis [25], these findings in the abovementioned diseases may have direct implications for subconjunctival fibrosis. Indeed, the present study provides new evidence that nintedanib has the potential to exhibit important antifibrotic effects in HTFs, suggesting that it may be exploited as an alternative agent for the treatment of intraocular fibrosis. Our results offer an experimental basis for further clinical trials with nintedanib in subconjunctival fibrosis. Because of the broad spectrum of nintedanib targets, there are concerns about the safety and tolerability of this drug in ocular applications. However, recent evidence from clinical trials of IPF have suggested that oral administration of 150 mg of nintedanib twice daily is well tolerated, with the most frequent adverse side effect being diarrhea, which resulted in less than 5% of patients terminating the use of the study medication [10]. Nevertheless, further investigations are warranted for its ocular application.
The traditional signaling pathway of TGF-β1 is through Smad2/3 regulation of target gene transcription [26]. In addition to the canonical pathway, there is increasing evidence suggesting that TGF-β can also stimulate the activation of MAPK signaling cascades, such as p38MAPK, ERK1/2, and c-Jun-N-terminal kinase (JNK) [9,27]. Recent studies have demonstrated that both canonical and noncanonical signaling kinases are involved in multiple cellular processes induced by TGF-β, such as cell proliferation, migration, and transdifferentiation in fibroblasts and other cell types [15,28–30]. It is worth noting that there are complex interactions between the two signaling pathways. They may function cooperatively or counteract each other, depending on the specific cellular context. For example, ERK1/2 and stress activate protein kinase (SAPK)/JNK activation can inhibit or enhance Smad2 phosphorylation, nuclear translocation, and transcriptional activity in different cellular contexts [31,32]. Furthermore, TGF-β-induced activation of ERK1/2 signaling can induce TGF-β expression, thereby amplifying the TGF-β response [33]. Thus, it is suggested that the balance between direct activation of the two pathways often defines cellular responses to TGF-β [9]. In an effort to uncover the molecular mechanisms that underlie the inhibitory effects of nintedanib in our study, we examined the activation status of classic and nonclassic signaling pathways of TFG-β1 after treatment with nintedanib. We observed that TGF-β1 triggers Smad2/3, p38MAPK, and ERK1/2 phosphorylation, and inhibition of p38MAPK with SB203580 leads to HTF inactivation, in line with the previous study. Moreover, treatment with nintedanib prevented Smad2/3, p38MAPK, and ERK1/2 phosphorylation in HTFs, suggesting that nintedanib acts through both classic and nonclassic signaling pathways of TFG-β1 to inhibit HTF activation. Notably, the effect of nintedanib is comparable with that of SB203580, indicating that suppression of all three signaling pathways has no additive effect. Furthermore, preincubation with SB203580 significantly attenuated TGF-β-induced Smad2/3 and ERK1/2 activation. We hypothesize that p38MAPK may function as the upstream signal kinase of Smad2/3 and ERK1/2 in HTFs. Thus, inhibition of p38MAPK is sufficient for producing an effect similar to that of all other signals.
In summary, we demonstrated that nintedanib effectively prevents HTF myofibroblast differentiation by TGF-β1. Our study suggests that nintedanib may be used as a potential intervention agent for fibrosis at the filtering bleb of trabeculectomy.
Acknowledgments
This work is funded by Guangdong Natural Science Foundation (2015A030313171), National Natural Science Foundation of China (81200685), and Program for Outstanding Young Teacher of Sun Yat-sen University (17ykpy76), The Guangdong Province Science & Technology Plan (2014B020228002). Author contributions: JW and XL conceived and performed the experiments. JW did the statistical analysis. XL and MY wrote the manuscript with input from WG, BQ, and RL. All authors declare that they have no competing interests. Prof. Minbin Yu (yuminbin@mail.sysu.edu.cn) and Dr. Xiancai Lin (xianchai.lin@qq.com) are co-corresponding authors on this paper.
Appendix 1.
Nintedanib prevents p-Smad2/3 translocation to the nucleus induced by TGF-β1. HTFs were treated with TGF-β1 (5 ng/ml) in the presence or absence of nintedanib (1 μM) for 30 min, and immunofluorescence was performed to detect the cellular location of p-Smad2/3 (green). The nuclei were stained with DAPI (blue). Data are presented as the means ± SD (n=3) of independently repeated experiments. (**** p<0.0001 compared to control or TGF-β1 group). Scale bar=100 μm. To access the data, click or select the words “Appendix 1.”
Appendix 2.
Nintedanib suppresses Smad2/3, P38MAPK and ERK1/2 activation induced by TGF-β1 at earlier time points. HTFs were treated with TGF-β1 (5 ng/ml) in the presence or absence of nintedanib (1 μM) for 15 min. p-Smad2/3 (A), p-P38MAPK and p-ERK1/2 (B) protein expression were analyzed by western blot and normalized to total Smad2/3, ERK1/2 or GAPDH, respectively. Data are presented as the means ± SD (n=3) of independent repeated experiments (* p<0.01; ** p<0.01;*** p<0.001; **** p<0.0001 compared to control or TGF-β1 group). To access the data, click or select the words “Appendix 2.”
References
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311:1901–11. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hnin H, Tan HY, Agrawal K, Agrawal R. Pursuit Of An Ideal Model For Community Eye Health - Non-Governmental Organisations (NGOs). Nepal J Ophthalmol. 2017;9:108–11. doi: 10.3126/nepjoph.v9i2.19251. [DOI] [PubMed] [Google Scholar]
- 3.Sigal IA, Yang H, Roberts MD, Burgoyne CF, Downs JC. IOP-induced lamina cribrosa displacement and scleral canal expansion: an analysis of factor interactions using parameterized eye-specific models. Invest Ophthalmol Vis Sci. 2011;52:1896–907. doi: 10.1167/iovs.10-5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sigal IA, Flanagan JG, Ethier CR. Factors influencing optic nerve head biomechanics. Invest Ophthalmol Vis Sci. 2005;46:4189–99. doi: 10.1167/iovs.05-0541. [DOI] [PubMed] [Google Scholar]
- 5.Fan Gaskin JC, Nguyen DQ, Soon Ang G, O’Connor J, Crowston JG. Wound Healing Modulation in Glaucoma Filtration Surgery—Conventional Practices and New Perspectives: The Role of Antifibrotic Agents (Part I). Journal of Current Glaucoma Practice. 2014;8:37–45. doi: 10.5005/jp-journals-10008-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitazawa Y, Kawase K, Matsushita H, Minobe M. Trabeculectomy With Mitomycin.A Comparative Study With Fluorouracil. Arch Ophthalmol. 1991;109:1693–8. doi: 10.1001/archopht.1991.01080120077030. [DOI] [PubMed] [Google Scholar]
- 7.Pardali E, Sanchez-Duffhues G, Gomez-Puerto MC, Ten Dijke P. TGF-beta-Induced Endothelial-Mesenchymal Transition in Fibrotic Diseases. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18102157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li DQ, Lee SB, Tseng SC. Differential expression and regulation of TGF-beta1, TGF-beta2, TGF-beta3, TGF-betaRI, TGF-betaRII and TGF-betaRIII in cultured human corneal, limbal, and conjunctival fibroblasts. Curr Eye Res. 1999;19:154–61. doi: 10.1076/ceyr.19.2.154.5321. [DOI] [PubMed] [Google Scholar]
- 9.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 10.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, Noble PW, Selman M, Taniguchi H, Brun M, Le Maulf F, Girard M, Stowasser S, Schlenker-Herceg R, Disse B, Collard HR, INPULSIS Trial Investigators Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–82. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 11.Wollin L, Wex E, Pautsch A, Schnapp G, Hostettler KE, Stowasser S, Kolb M. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J. 2015;45:1434–45. doi: 10.1183/09031936.00174914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J Pharmacol Exp Ther. 2014;349:209–20. doi: 10.1124/jpet.113.208223. [DOI] [PubMed] [Google Scholar]
- 13.Hocevar BA, Brown TL, Howe PH. TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 1999;18:1345–56. doi: 10.1093/emboj/18.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Liu PP, Liu L, Zheng XS, Zheng H, Yang CC, Luobu CR, Liu Y. Triptolide inhibits TGF-β-induced matrix contraction and fibronectin production mediated by human Tenon fibroblasts. Int J Ophthalmol. 2018;11:1108–13. doi: 10.18240/ijo.2018.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer-Ter-Vehn T, Gebhardt S, Sebald W, Buttmann M, Grehn F, Schlunck G, Knaus P. p38 inhibitors prevent TGF-beta-induced myofibroblast transdifferentiation in human tenon fibroblasts. Invest Ophthalmol Vis Sci. 2006;47:1500–9. doi: 10.1167/iovs.05-0361. [DOI] [PubMed] [Google Scholar]
- 16.Heinzelmann K, Noskovičová N, Merl-Pham J, Preissler G, Winter H, Lindner M, Hatz R, Hauck SM, Behr J, Eickelberg O. Surface proteome analysis identifies platelet derived growth factor receptor-alpha as a critical mediator of transforming growth factor-beta-induced collagen secretion. Int J Biochem Cell Biol. 2016;74:44–59. doi: 10.1016/j.biocel.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Strutz F, Zeisberg M, Renziehausen A, Raschke B, Becker V, van Kooten C, Müller G. TGF-beta 1 induces proliferation in human renal fibroblasts via induction of basic fibroblast growth factor (FGF-2). Kidney Int. 2001;59:579–92. doi: 10.1046/j.1523-1755.2001.059002579.x. [DOI] [PubMed] [Google Scholar]
- 18.Fan Gaskin JC, Nguyen DQ, Soon Ang G, O’Connor J, Crowston JG. Wound Healing Modulation in Glaucoma Filtration Surgery- Conventional Practices and New Perspectives: Antivascular Endothelial Growth Factor and Novel Agents (Part II). J Curr Glaucoma Pract. 2014;8:46–53. doi: 10.5005/jp-journals-10008-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuchshofer R, Kottler UB, Ohlmann AV, Schlötzer-Schrehardt U, Jünemann A, Kruse FE, Ohlmann A. SPARC is expressed in scars of the Tenon’s capsule and mediates scarring properties of human Tenon’s fibroblasts in vitro. Mol Vis. 2011;17:177–85. [PMC free article] [PubMed] [Google Scholar]
- 20.Hilberg F, Roth GJ, Krssak M, Kautschitsch S, Sommergruber W, Tontsch-Grunt U, Garin-Chesa P, Bader G, Zoephel A, Quant J, Heckel A, Rettig WJ. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008;68:4774–82. doi: 10.1158/0008-5472.CAN-07-6307. [DOI] [PubMed] [Google Scholar]
- 21.Roth GJ, Binder R, Colbatzky F, Dallinger C, Schlenker-Herceg R, Hilberg F, Wollin SL, Kaiser R. Nintedanib: from discovery to the clinic. J Med Chem. 2015;58:1053–63. doi: 10.1021/jm501562a. [DOI] [PubMed] [Google Scholar]
- 22.McCormack PL. Nintedanib: first global approval. Drugs. 2015;75:129–39. doi: 10.1007/s40265-014-0335-0. [DOI] [PubMed] [Google Scholar]
- 23.Tepede A, Yogaratnam D. Nintedanib for Idiopathic Pulmonary Fibrosis. J Pharm Pract. 2017;•••:897190017735242. doi: 10.1177/0897190017735242. [DOI] [PubMed] [Google Scholar]
- 24.Rossi A, Latiano TP, Parente P, Chiarazzo C, Limosani F, Di Maggio G, Maiello E. The potential role of nintedanib in treating colorectal cancer. Expert Opin Pharmacother. 2017;18:1153–62. doi: 10.1080/14656566.2017.1346086. [DOI] [PubMed] [Google Scholar]
- 25.Thomopoulos S, Das R, Sakiyama-Elbert S, Silva MJ, Charlton N, Gelberman RH. bFGF and PDGF-BB for tendon repair: controlled release and biologic activity by tendon fibroblasts in vitro. Ann Biomed Eng. 2010;38:225–34. doi: 10.1007/s10439-009-9844-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pardali E, Sanchez-Duffhues G, Gomez-Puerto MC, Ten Dijke P. TGF-β-Induced Endothelial-Mesenchymal Transition in Fibrotic Diseases. Int J Mol Sci. 2017;18:2157. doi: 10.3390/ijms18102157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo X, Wang XF. Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res. 2009;19:71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu L, Hébert MC, Zhang YE. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J. 2002;21:3749–59. doi: 10.1093/emboj/cdf366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci. 2002;115:3193–206. doi: 10.1242/jcs.115.15.3193. [DOI] [PubMed] [Google Scholar]
- 30.Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005;118:3573–84. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 31.Scherer A, Graff JM. Calmodulin differentially modulates Smad1 and Smad2 signaling. J Biol Chem. 2000;275:41430–8. doi: 10.1074/jbc.M005727200. [DOI] [PubMed] [Google Scholar]
- 32.Brown JD, DiChiara MR, Anderson KR, Gimbrone MA, Jr, Topper JN. MEKK-1, a component of the stress (stress-activated protein kinase/c-Jun N-terminal kinase) pathway, can selectively activate Smad2-mediated transcriptional activation in endothelial cells. J Biol Chem. 1999;274:8797–805. doi: 10.1074/jbc.274.13.8797. [DOI] [PubMed] [Google Scholar]
- 33.Yue J, Mulder KM. Activation of the mitogen-activated protein kinase pathway by transforming growth factor-beta. Methods Mol Biol. 2000;142:125–31. doi: 10.1385/1-59259-053-5:125. [DOI] [PubMed] [Google Scholar]