Abstract
Development of resistance to chemotherapy is a major obstacle in extending the survival of patients with cancer. Although originally defined as an immune checkpoint molecule, B7-H1 (also named as PD-L1 or CD274) was found to play a role in cancer chemoresistance; however, the underlying mechanism of action of B7-H1 in regulation of chemotherapy sensitivity remains unclear in cancer cells. Here we show that development of chemoresistance depends on an increased activation of ERK in cancer cells overexpressing B7-H1. Conversely, B7-H1 knockout (KO) by CRISPR/Cas9 renders human cancer cells susceptible to chemotherapy in a cell-context dependent manner through a reduced activation of p38 MAPK. B7-H1 was found to associate with the catalytic subunit of DNA-dependent protein kinase (DNA-PKcs) and this association promoted or maintained the activation of ERK or p38 MAPK in cancer cells. Importantly, we found that targeting B7-H1 by anti-B7-H1 monoclonal antibody (H1A) increased the sensitivity of human triple negative breast cancer cells to cisplatin therapy in vivo. Our results suggest that targeting B7-H1 by an antibody capable of disrupting B7-H1 signals may be a new approach to sensitize cancer cells to chemotherapy.
Keywords: Oncology, Cancer research
1. Introduction
Although several molecular mechanisms have been identified to contribute to cancer chemoresistance [1, 2], the role of immune checkpoint molecules in the development of cancer chemoresistance has not been fully recognized. Traditionally, the emergence of chemoresistance and immunoresistance are considered as parallel and unrelated events. Although originally defined as immune checkpoint molecules, B7-homolog 1 (B7-H1) (also named as PD-L1 or CD274) and B7-homolog 3 (B7-H3) are also able to render cancer cells resistant to chemotherapy [3, 4, 5]. Elevated expression of B7-H1 is predictive of an aggressive disease course, including increases in rates of disease progression and cancer-related mortality in a variety of cancers (ovary, breast, liver, pancreas, esophagus, colon lung and mesothelioma) [6, 7, 8]. Even though the poor prognosis of B7-H1 expressing cancers has been attributed to the immune-suppressive function of B7-H1, the emerging role of B7-H1 in cancer chemoresistance may provide a new mechanism responsible for poor clinical outcomes in patients with B7-H1 expressing cancer cells.
It has been previously observed that the overexpression of B7-H1 confers chemoresistance in human multiple myeloma and breast cancers [3, 4], while B7-H1 knockdown in human lymphoma and breast cancer cells renders them sensitive to chemotherapeutic drugs [4, 9]. In this regard, the combination of B7-H1 antibody with chemotherapy has demonstrated promising synergistic therapeutic effects in clinical trials [10, 11, 12]. These studies clearly indicate a therapeutic potential of targeting B7-H1 in cancer chemotherapy, however the mechanism by which B7-H1 governs cancer sensitivity to chemotherapy remains unclear. In this report, we examined the function of B7-H1 in regulation of drug sensitivity of cancer cells using B7-H1 overexpression and knockout models in vitro and in vivo. We report that overexpression of B7-H1 leads to an increased activation of extracellular signal-regulated kinases1/2 (ERK1/2) in cancer cells through an association with DNA-dependent serine/threonine protein kinase catalytic subunit (DNA-PKcs), and B7-H1 knockout by CRISPR/Cas9 renders tumor cells susceptible to chemotherapy in a cell-context dependent manner through reduced activation of p38 MAPK (mitogen-activated protein kinase) pathway. Our results provide new insights into the B7-H1-mediated cancer chemoresistance and suggest that targeting B7-H1 (PD-L1) with an antibody may represent a new approach to overcome chemoresistance in human cancer.
2. Results
2.1. B7-H1 overexpression renders cancer cells resistant to chemotherapy
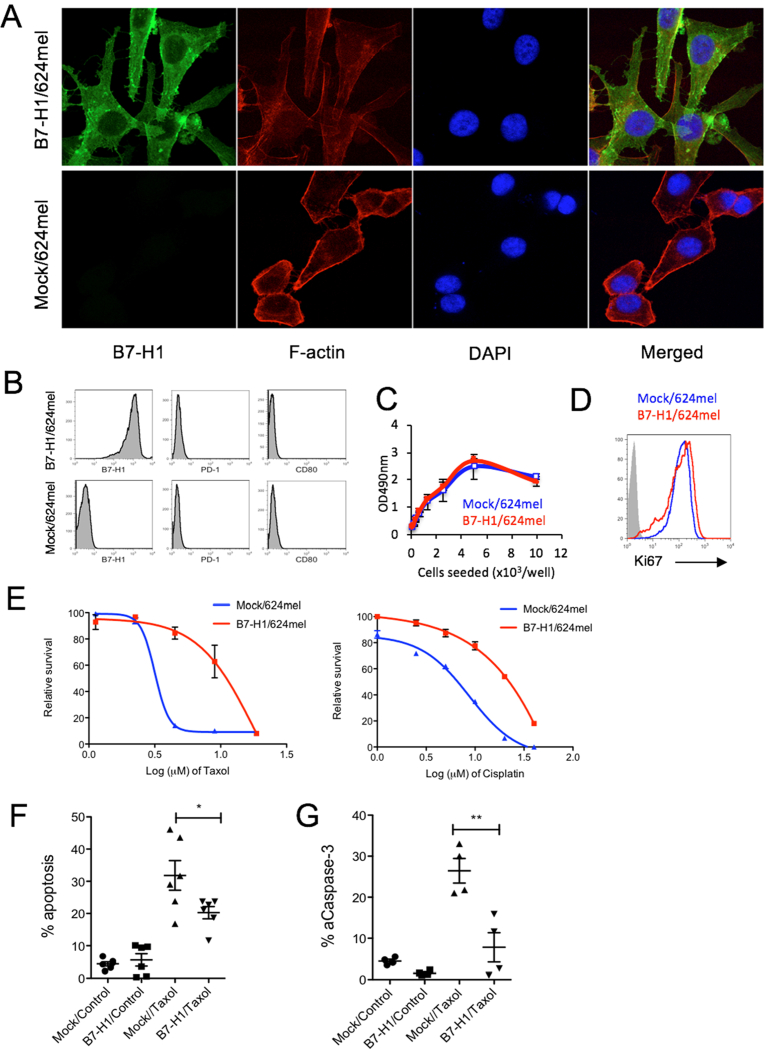
To explore how B7-H1 affects sensitivity to chemotherapy in cancer cells, we transfected a B7-H1 negative human melanoma cell line (624mel) with a full-length human B7-H1 cDNA construct [13]. The B7-H1 cDNA transfected and mock-transfected 624mel cells were confirmed by confocal microscopy (Fig. 1A) and flow cytometry (Fig. 1B) for their B7-H1 expression. B7-H1 protein was found to be located both on the cell membrane and in the cytoplasm of the transfected melanoma cells, whereas both PD-1 and CD80 (two receptors of B7-H1) were not detected in the same melanoma cells (Fig. 1B). The overexpression of B7-H1 did not affect the proliferation of transfected melanoma cells as their cell growth pattern and Ki67 (a nuclear marker for cell proliferation) expression were comparable with mock-transfected cells (Fig. 1C, D).
Fig. 1.
B7-H1 overexpression conferred drug resistance on melanoma cells. (A) B7-H1 expression by B7-H1/624mel cells. (B) No PD-1 and CD80 (B7-1) expression by Mock/624mel and B7-H1/624mel cells. (C–D) Comparable growth rate between B7-H1/624mel and Mock/624mel cells in vitro. The proliferation of both cancer cells was analyzed by MTS assay after 72 hours of culture (C) and flow cytometry assay for the expression of intranuclear protein Ki67 (D). (E) Relative survival was determined by MTS assay. The p-value for area under the curve (dose-response curves) is significant (p < 0.01) in treatment with Taxol (paclitaxel) and cisplatin. One of three experiments is shown. (F–G) Apoptosis of melanoma cells treated with Taxol (2 μg/ml, 48 hours) was analyzed by TMRE and Annexin V staining (F) and intracellular staining for active caspase-3 (G). Numbers of independent experiments (n = 6 in C, n = 4 in D). *P < 0.05, **P < 0.01 compared between Mock/624mel cells and B7-H1/624mel cells.
To determine whether B7-H1 overexpression specifically regulates the sensitivity toward certain types of chemotherapy, we compared the growth and survival of B7-H1/624mel cells with Mock/624mel cells after treatments with paclitaxel (Taxol, targeting microtubules and blocks mitosis) or cisplatin (crosslinks DNA to stop replication of DNA). After 72 hours of in vitro treatment, the viabilities of melanoma cells were measured using an MTS assay [14]. We found that B7-H1/624mel cells were more resistant to paclitaxel (Taxol) and cisplatin compared to Mock/624mel cells (Fig. 1E). Since a mechanism of action of most cytotoxic drugs is to induce apoptosis [15, 16], we measured and compared the apoptosis of B7-H1/624mel and Mock/624mel cells after treatment with paclitaxel. Apoptosis was measured by the binding of Annexin V (AV) and the levels of tetramethylrhodamine ethyl ester (TMRE, a marker for mitochondria membrane potential) [17]. B7-H1/624mel cells had 1.5-fold less apoptotic cells compared to Mock/624mel cells following the treatment with paclitaxel (Fig. 1F). The level of active caspase-3 (a key executive molecule of apoptosis) was also lower in B7-H1/624mel cells compared with Mock/624mel cells (Fig. 1G). Our results suggest that B7-H1 overexpression renders cancer cells resistant to paclitaxel or cisplatin by reducing their potential for apoptosis.
2.2. B7-H1 knockout renders cancer cells susceptible to chemotherapy
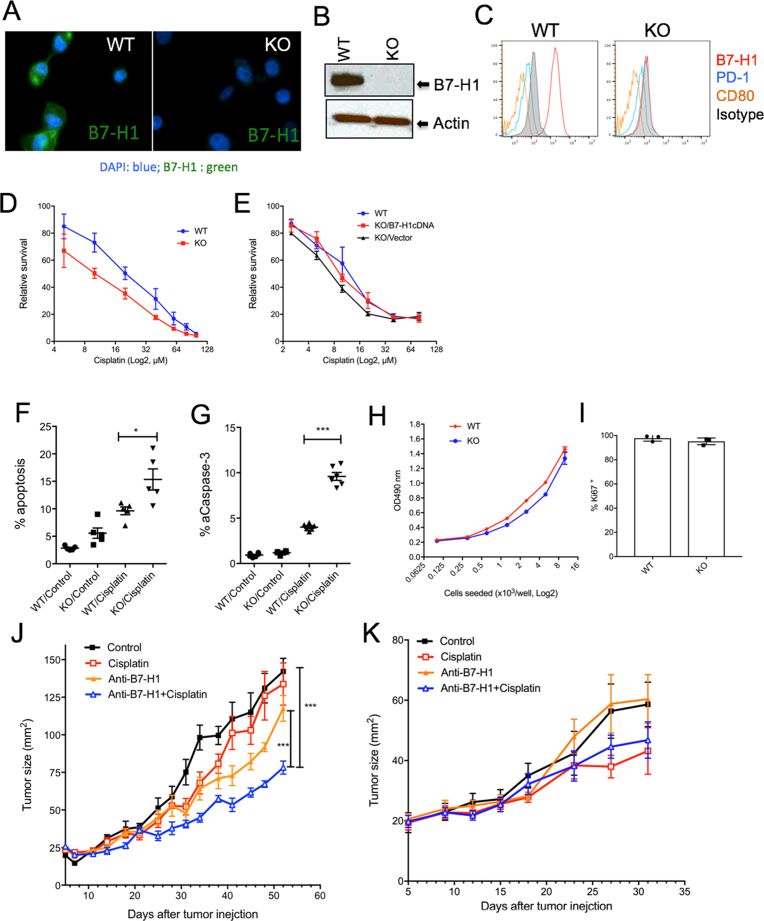
To examine whether knocking out endogenous B7-H1 would affect the sensitivity to chemotherapy in cancer cells, we took the B7-H1 positive human triple negative breast cancer cell line MDA-MB-231 and produced a B7-H1 bi-allelic knockout (KO) subclone by introducing CRISPR/Cas9 constructs carrying a guide RNA (gRNA) sequence specific to human B7-H1 exon 3. The absence of B7-H1 in MDA-MB-231 cells was confirmed by genotyping, confocal microscope, flow cytometry and Western blotting (Fig. 2A–C). We also confirmed that MDA-MB-231 cells did not express B7-H1 receptors PD-1 and CD80 (Fig. 2C). We then compared the sensitivity of B7-H1 WT and KO MDA-MB-231 cells to cisplatin. We found that B7-H1 KO MDA-MB-231 cells were significantly more sensitive to the treatments of cisplatin compared with WT cells (Fig. 2D). To confirm the role of B7-H1 in drug sensitivity, we transfected B7-H1 in B7-H1 KO MDA-MB-231 cells and found the restored expression of B7-H1 reduced drug sensitivity of B7-H1 KO MDA-MB-231 cells (Fig. 2E). Accordingly, we found cisplatin induced more apoptosis and activated caspase-3 in B7-H1 KO MDA-MB-231 cells than in WT cells (Fig. 2F–G). Of note, B7-H1 WT and KO MDA-MB-231 cells had similar growth in vitro (Fig. 2H–I) and in vivo in immune deficient mice (Fig. 2J).
Fig. 2.
Targeting B7-H1 sensitized breast cancer cells to chemotherapy. B7-H1 KO MBA-MD-231 breast cancer cells were generated by CRISPR/Cas9 and confirmed by confocal microscopy (A), Western blotting (B) and flow cytometry (C). The absence of PD-1 and CD80 expression was confirmed in both wild type (WT) and B7-H1 KO cancer cells (C). The whole gel images of (B) were provided as the supplemental materials. (D) B7-H1 KO MBA-MD-231 cells are more sensitive to cisplatin than WT cells as determined by MTS assay. The p-value for area under the curve (dose-response curves) is significant (p < 0.01) in treatment with cisplatin. (E) Restored B7-H1 expression abolished the sensitivity of B7-H1 KO cancer cells to cisplatin. One of three experiments is shown. (F–G) Apoptosis of cancer cells treated with cisplatin (20 μM, 48 hr.) was analyzed by TMRE and Annexin V staining (F) and intracellular staining for active caspase-3 (G). Numbers of independent experiments (n = 5 in F, n = 6 in G). (H) MTS assay of the growth of B7-H1 WT and KO MDA-MB-231 breast cancer cells after 72 hours of culture. (I) Ki67 (a cell proliferation nuclear marker) expression by B7-H1 WT and KO MDA-MB-231 cells analyzed by flow cytometry. (J) WT MDA-MB-231 tumors or (K) B7-H1 KO MDA-MB-231 tumors were treated with B7-H1 antibody (Ab, H1A clone) or cisplatin, or both in vivo. ***P < 0.001 compared between B7-H1 Ab plus cisplatin and B7-H1 Ab along or control group as determined by Two-way ANOVA. One of two independent experiments is shown.
To test whether targeting B7-H1 by antibody would affect their sensitivity to chemotherapy, we treated MDA-MB-231 cells with anti-B7-H1 antibody (H1A clone) alone or in combination with cisplatin in vivo in immunodeficient mice. The treatment with B7-H1 antibody significantly suppressed tumor growth in combination with cisplatin, while B7-H1 antibody alone only modestly suppressed the growth of MDA-MB-231 tumors (Fig. 2J). Of note, the dose of cisplatin when used alone did not suppress the growth of WT MDA-MB-231 tumors in vivo. To exclude any off-target effects of B7-H1 antibody in treatment of tumors, we also treated B7-H1 KO MDA-MB-231 tumors with B7-H1 antibody (H1A) and cisplatin. As expected, B7-H1 antibody (H1A) in combination with cisplatin did not further suppress the growth of B7-H1 KO MDA-MB-231 tumor cells in vivo comapred with cisplatin alone (Fig. 2K), suggesting B7-H1 antibody did not have any off-target effects. We conclude that targeting B7-H1 expression with a B7-H1-specific antibody may increase the sensitivity of breast cancer to cisplatin.
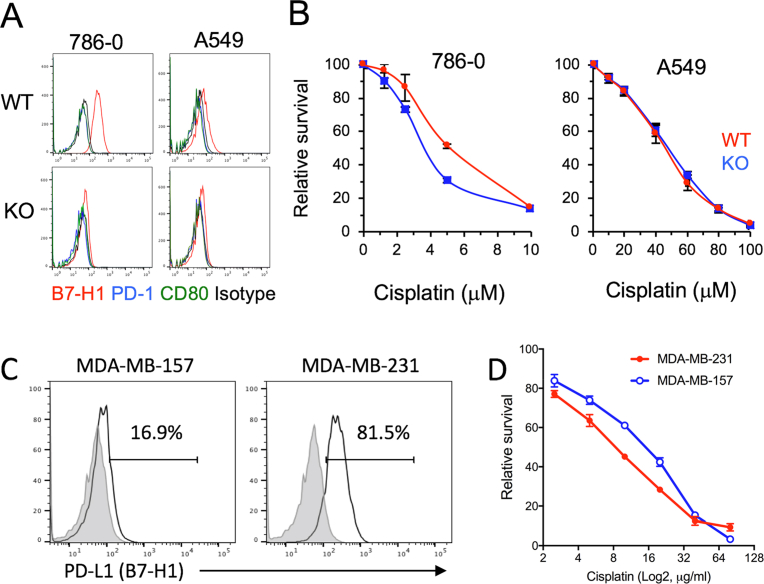
To validate the function of B7-H1 in other cancer cells, we produced B7-H1 KO human kidney 786-0 cancer cells and B7-H1 KO human lung A549 cancer cells (Fig. 3A). While parent 786-0 cancer cells expressed more B7-H1 than the parent A549 cancer cells, B7-H1 KO increased cisplatin susceptibility in B7-H1 KO 786-0 cells but not in B7-H1 KO A549 cells compared with their WT cells (Fig. 3B). On the other hand, although B7-H1 expression was different between wild type MDA-MB-231 and MDA-MB-157 (both are triple negative breast cancer cells), they demonstrated similar sensitivity to the cytotoxicity of cisplatin (Fig. 3C, D). Our results suggest that B7-H1 modulation of chemoresistance may be universal, but the drug sensitivity is dependent on cancer cell-context.
Fig. 3.
B7-H1 modulation of chemoresistance is dependent on cancer cell-context. (A) B7-H1, PD-1 and CD80 expression in wild type or B7-H1 KO 786-0 renal cell carcinoma cells and A549 lung cancer cells. (B) B7-H1 KO 786-0 cells, but not B7-H1 KO A549 cells, are more sensitive to cisplatin compared to their WT cells as determined by MTS assay. The p-value for area under the curve (dose-response curves) is significant (p < 0.01) in treatment with cisplatin in B7-H1 KO 786-0 cells. (C) B7-H1 expression by human triple negative breast cancer cells: MDA-MB-231 and MDA-MB-157. (D) MTS assay of the growth of MDA-MB-231 and MDA-MB-157 cancer cells after 72 hours of culture with cisplatin.
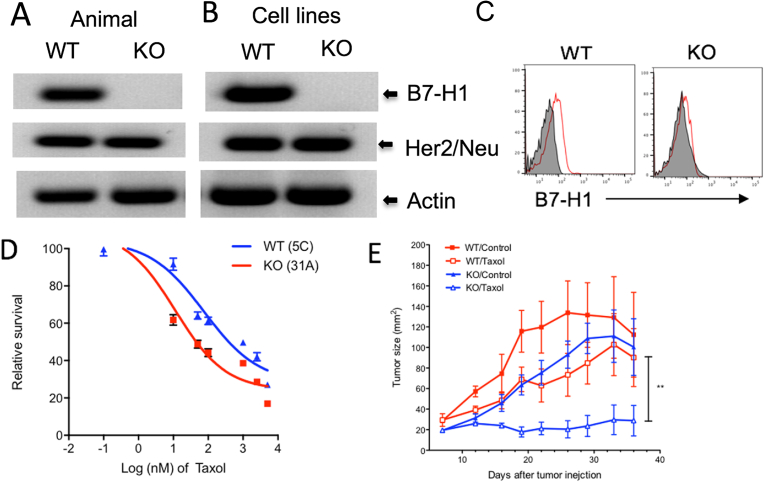
To further determine the cell-specific role of B7-H1 in rendering chemoresistance in vitro and in vivo, we produced B7-H1 KO mouse breast cancer cells lines from B7-H1 KO/NeuT mice that spontaneously generate Her2 expressing breast cancers but do not express B7-H1 (Fig. 4A). From the grown out breast cancers, we established Her2 expressing breast cancer cell lines with (WT/5C) or without (KO/31A) B7-H1 expression (Fig. 4B). The presence or absence of B7-H1 expression was confirmed by flow cytometry (Fig. 4C). Interestingly, we found B7-H1 KO 31A cells are more susceptible to the treatment of paclitaxel, but not to cisplatin, than B7-H1 WT 5C cells in vitro and in vivo (Fig. 4D, E). Collectively, our studies suggest that B7-H1 renders cancer cell resistance to chemotherapy in the context of cell type and drug selection.
Fig. 4.
B7-H1 KO Her2+ breast cancer cells were more sensitive to chemotherapy. B7-H1 WT (5C) and B7-H1 KO (31A) mouse breast cancer cell lines were established from the breast cancer tissues of WT or B7-H1 KO NeuT mice. B7-H1 KO and Her2/Neu expression were confirmed by PCR in genomic DNA of mice (A) or breast cancer cell lines (B), and by flow cytometry (C). The whole gel images of (A) and (B) were provided as the supplemental materials. (D–E) B7-H1 KO cancer cells are more sensitive to Taxol (paclitaxel) than WT cells. (E) B7-H1 WT and KO cancer cells were s.c. injected into Balb/c mice at 5 × 105 cells/mouse. On days of 8 and 14, mice were treated with paclitaxel (Taxol, i.p. 25 mg/kg) or saline (for control groups). Data show the mean tumor size ± SEM, **P < 0.01 compared between WT/Taxol and KO/Taxol (Two-way ANOVA, 5 mice per group).
2.3. B7-H1 is required for DNA-PKcs to promote or maintain the activation of ERK/MAPK pathway in cancer cells
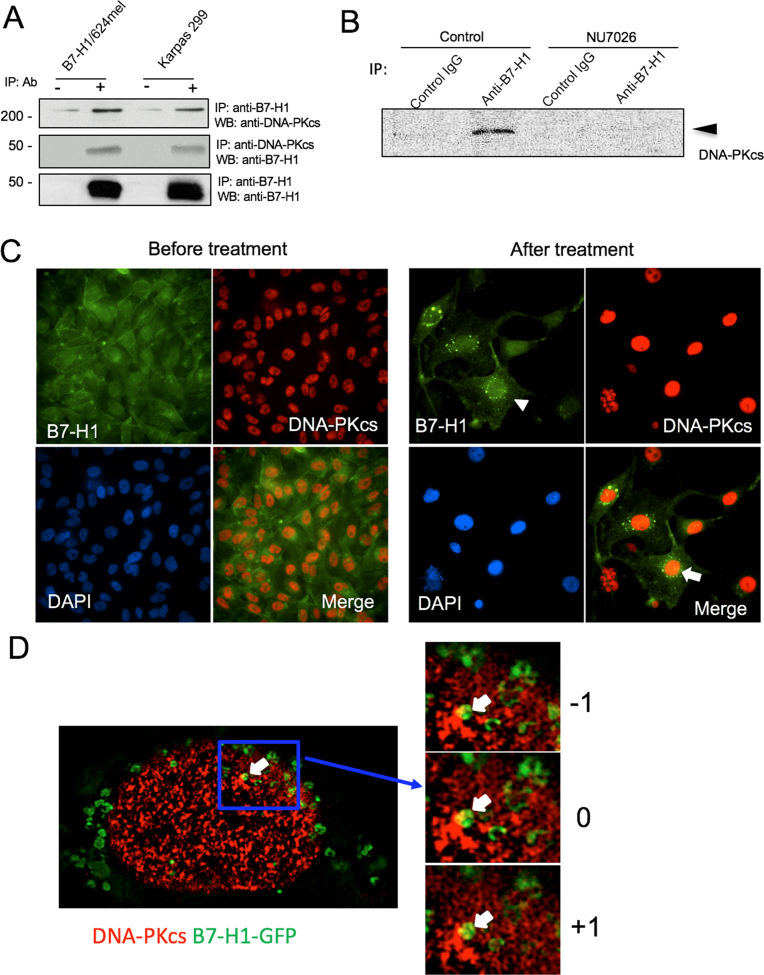
The absence of PD-1 and CD80 (two B7-H1 receptors) expression in human 624mel and MDA-MB-231 cells excluded the possibility of ligand/receptor (B7-H1/PD-1 or B7-H1/CD80) mediated cell signaling in cancer cells (Figs. 1B and 2C). This prompted us to examine whether other intracellular pathways could be used by B7-H1 to regulate the drug sensitivity of these tumor cells. Since we previously found B7-H1 associated with DNA-PKcs in human primary T cells [18], we examined whether B7-H1 would associate with DNA-PKcs in cancer cells. To that end, we performed immunoprecipitation (IP) and Western blotting using anti-B7-H1 antibody (5H1-A3 clone) or anti-DNA-PKcs antibody on lysates from B7-H1 expressing tumor cells B7-H1/624mel and Karpas 299 (human lymphoma expressing B7-H1). When the blot was probed with anti-DNA-PKcs, we identified a DNA-PKcs band in IP samples immunoprecipitated with anti-B7-H1 antibody but not with isotype control antibody (Fig. 5A). Conversely, when the blot probed with anti-B7-H1 antibody, a B7-H1 band was readily identified in IP samples immunoprecipitated with anti-DNA-PKcs antibody (Fig. 5A). The specificity of IP antibody to B7-H1 (clone 5H1-A3) was confirmed by Western blot with another B7-H1 antibody (clone E1L3N) (Fig. 5A). In addition, we identified the endogenous association of B7-H1 and DNA-PKcs in MDA-MB-231 breast cancer cells (Fig. 5B). Interestingly, a DNA-PKcs inhibitor (NU7026) that inhibits the catalytic activity of DNA-PKcs, abolished the association of DNA-PKcs and B7-H1 in MDA-MB-231 cells (Fig. 5B), suggesting the association is dependent on DNA-PKcs activity.
Fig. 5.
B7-H1 was associated with DNA-PKcs in cancer cells. (A) Immunoprecipitation (IP) and Western blot (WB) identified an association of B7-H1 with DNA-PKcs in B7-H1/624mel cells and B7-H1 positive Karpas 299 cells. The specificity of IP antibody to B7-H1 (clone 5H1-A3) was confirmed by WB with another antibody to B7-H1 (clone E1L3N). (B) The association of B7-H1 with DNA-PKcs was identified in MDA-MB-231 cells and this association was abolished by NU7026 (1 μM), an inhibitor of DNA-PKcs. The whole gel images of (A) and (B) were provided as the supplemental materials. (C) Co-localization of B7-H1 and DNA-PKcs in B7-H1-GFP transfected MDA-MB-231 cells before and after treatment with cisplatin (40 μM) for 72 hours. Co-localizations of B7-H1 and DNA-PKcs were identified in the nuclei. Arrowhead and arrow indicated B7-H1 at nuclear areas and co-localization with DNA-PKcs. (D) Z-section images (0.11 μm of depth per section at a range of -1, 0, +1) showed the association of DNA-PKcs (red) and B7-H1-GFP (green) within a nucleus upon treatment with cisplatin as in (C).
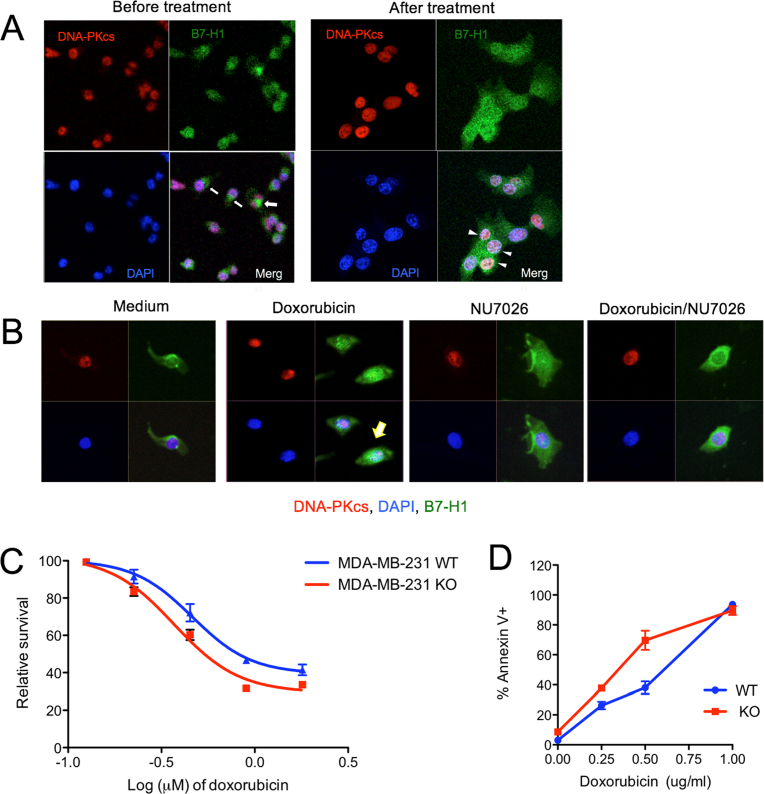
Since chemotherapy may change the location of B7-H1 in cancer cells [4], we examined the subcellular localization of B7-H1 and DNA-PKcs before and after treatment with chemotherapy in MDA-MB-231 cells. Before chemotherapy we found DNA-PKcs mainly localized to the nuclei while B7-H1 (endogenous or transfected) was mainly localized in the cytoplasm but was also seen on cell membranes (Figs. 5C, D and 6A). After cisplatin treatment, B7-H1 accumulated at the perinuclear and intranuclear areas where B7-H1 partially co-localized with DNA-PKcs (Fig. 5C). The co-localization of B7-H1 and DNA-PKcs was confirmed with Z-section imaging at perinuclear areas (Fig. 5D). Similar to a previous report [4], we observed that doxorubicin induced B7-H1 translocation in MDA-MB-231 cells, and the nuclear co-localization of B7-H1 and DNA-PKcs was reduced upon treatment with the DNA-PKcs inhibitor NU7026 (Fig. 6B). However, MDA-MB-231 KO cells were modestly sensitive to the cytotoxic effects of doxorubicin compared with wild type cells (Fig. 6C, D). Although B7-H1 was originally defined as a transmembrane protein mainly on cell surface of cancer cells [19], our new data suggest that chemotherapy may cause B7-H1 translocation or redistribution into nuclei where B7-H1 will associate with DNA-PKcs.
Fig. 6.
Chemotherapy-induced association of B7-H1 and DNA-PKcs in cancer cells. (A) Co-localization of B7-H1 and DNA-PKcs. MDA-MB-231 cells were treated with or without doxorubicin (2 μg/ml) for 2 hours. Co-localizations of B7-H1 and DNA-PKcs were identified in the nuclei. Arrows or arrowheads indicate B7-H1 in plasma or in nuclei, respectively. (B) DNA-PKcs inhibitor NU7026 disrupted the co-localization of B7-H1 and DNA-PKcs were identified in the nuclei of tumor cells. Arrow indicates B7-H1 in nuclei with DNA-PKcs. B7-H1 KO MBA-MD-231 cells are modestly sensitive to doxorubicin than WT cells as determined by MTS assay (C) and by apoptosis assay (D).
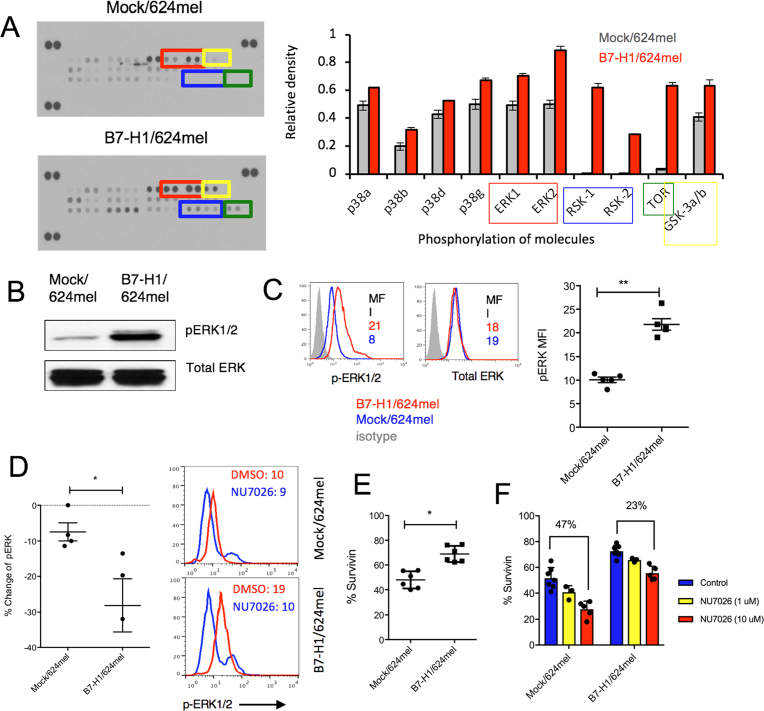
Although DNA-PKcs is mainly involved in DNA damage repair, it also activates MAPK/ERK signaling pathways to promote cell survival [20, 21]. In this regard, we examined whether B7-H1 would affect the activation of MAPK/ERK pathway through DNA-PKcs. First, we screened and compared the phosphorylated proteins (phospho-proteins) involved in the MAPK/ERK pathway between B7-H1/624mel cells and Mock/624mel cells using antibody arrays (R&D Systems). Among 26 phospho-proteins, we found phospho-ERK1/2, phospho-RSK1/2 (downstream targets of ERK pathway), phospho-p38 MAPK and phospho-mTOR significantly increased in B7-H1/624mel cells compared with Mock/624mel cells (Fig. 7A). The increase of phosphorylated ERK1/2 in B7-H1/624mel cells was confirmed by Western blotting and intracellular flow cytometry. Our data showed a >2-fold increase of phospho-ERK1/2 in B7-H1/624mel compared with Mock/624mel cells, while the total ERK levels remained comparable between these two melanoma cell lines (Fig. 7B, C). In line with other reports about the role of B7-H1 in activation of PI3K/mTOR pathway [22], our studies extended the essential role of B7-H1 to the activation of ERK1/2 and p38 MAPK in cancer cells.
Fig. 7.
Overexpression of B7-H1 enhanced the activation of MAPK/ERK pathway in melanoma cells. (A) The relative phosphorylation levels of proteins in the MAPK/ERK pathway were measured by antibody arrays in B7-H1/624mel cells and Mock/624mel cells. (B–C) The levels of phospho-ERK1/2 and total ERK were analyzed by Western blotting (B) and flow cytometry (C). Numbers are mean fluorescence intensity (MFI). **P < 0.01 compared between Mock and B7-H1/624mel cells. The whole gel images of (B) were provided as the supplemental materials. (D) Dot plot graph shows the changes of phosphor-ERK1/2 induced by NU7026 (10 μM, 24 hours). Numbers of independent experiments (n = 4 in Mock/624mel; n = 3 in B7-H1/624mel). *P < 0.05 compared between % changes of phospho-ERK1/2 in Mock/624mel and B7-H1/624mel cells. Right panel shows the representative expression of phospho-ERK1/2 measured by flow cytometry. (E) Survivin expression increased in B7-H1/624mel cells in comparison with Mock/624mel (*P < 0.05). (F) NU7026 (10 μM) inhibited the expression of Survivin in B7-H1/624mel cells and Mock/624mel cells after 24 hours of culture. Numbers are percentages of change from control groups.
To examine whether the activation of ERK1/2 is dependent on the association of DNA-PKcs and B7-H1, we treated melanoma cells with a DNA-PKcs specific inhibitor (NU7026) that can abolish the association of DNA-PKcs and B7-H1 as shown in Figs. 5B and 6B. The results of Fig. 7D show that NU7026 dramatically decreased (∼2-fold) the activation of ERK1/2 in B7-H1/624mel cells but not in Mock/624mel cells. To examine the consequences of increased activation of ERK1/2, we measured the expression of survivin, an anti-apoptotic molecule and a downstream target of ERK1/2 [23]. We found that the expression of survivin was significantly increased in B7-H1/624mel compare with Mock/624mel cells (Fig. 7E). NU7026 dramatically decreased the expression of survivin in both B7-H1/624mel cells and Mock/624mel cells in a dose dependent way, however the decrease of survivin expression was more significant in Mock/624mel cells than in B7-H1/624mel cells (47% vs. 23%, Fig. 7F). Our data suggest that overexpression of B7-H1 may work through DNA-PKcs to promote ERK activation that results in an elevated survivin expression in cancer cells.
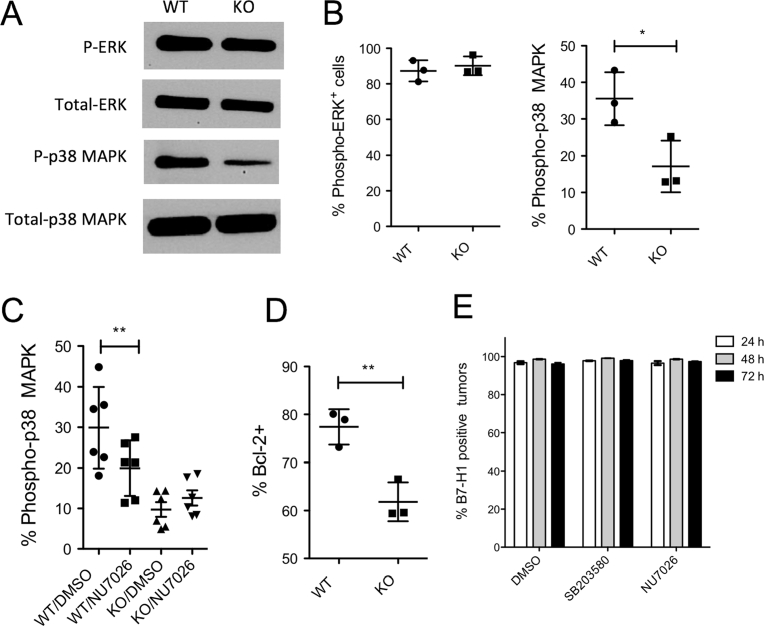
Besides the B7-H1 overexpression model, we examined the activation of ERK/MAPK pathway in B7-H1 KO cancer cells. In contrast to 624mel melanoma cells with B7-H1 overexpression, B7-H1 KO MDA-MB-231 breast cancer cells expressed comparable levels of phospho-ERK1/2 as B7-H1 WT cells, but expressed lower levels of phospho-p38 MAPK as shown by Western blot (Fig. 8A). The quantitative levels of phospho-ERK and phospho-p38 MAPK were confirmed with flow cytometry assays that demonstrated less activation of p38 MAPK in B7-H1 KO cancer cells compared with WT cells (Fig. 8B). To examine whether the activation of p38 MAPK is dependent on the association of B7-H1 and DNA-PKcs, we measured the phospho-p38 MAPK in the presence of NU7026. Interestingly, DNA-PKcs inhibitor NU7026 significantly reduced the activation of p38 MAPK in B7-H1 WT MDA-MB-231 cells (WT/NU7026), but not in B7-H1 KO MDA-MB-231 cells (KO/NU7-26) (Fig. 8C). Our results suggest that B7-H1 may be required for DNA-PKcs to promote or maintain the activation of p38 MAPK in breast cancer cells. As a consequence of decreased activation of p38 MAPK, we found reduced expression of Bcl-2 (a pro-survival molecule regulated by p38 MAPK) [24] in B7-H1 KO MDA-MB-231 cells compared to wild type cells (Fig. 8D). The decreased levels of Bcl-2 may explain why B7-H1 KO MDA-MB-231 cells were more susceptible to cisplatin-induced apoptosis compared with WT MDA-MB-231 cells. Since DNA-PKcs or p38 MAPK inhibitors did not change the expression of B7-H1 in these cancer cells (Fig. 8E), it seems that both of DNA-PKcs and p38 MAPK are downstream targets of the signaling pathway of B7-H1 in cancer cells.
Fig. 8.
B7-H1 was required in the activation of p38 MAPK through DNA-PKcs in breast cancer cells. (A–B) The levels of phospho-ERK or phospho-p38 MAPK and total ERK or total p38 MAPK in WT or B7-H1 KO MDA-MB-231 cells were analyzed by Western blotting (A) and flow cytometry (B). *P < 0.01 compared between B7-H1 WT and KO MDA-MB-231 cells. The whole gel images of (A) were provided as supplemental material. (C) The expression of phospho-p38 MAPK after incubation with NU7026 (10 μM, 24 hours). **P < 0.01 compared between NU7026 treated or not treated WT MDA-MB-231 cells. (D) Bcl-2 expression decreased in B7-H1 KO MDA-MB-231 cells compared to WT cells (**P < 0.01). Numbers of independent experiments (n = 3 in B, n = 6 in C, n = 3 in D). (E) B7-H1 wild type MDA-MB-231 cancer cells were incubated with DNA-PKcs inhibitor (NU7026 at 10 μM) or p38 MAPK inhibitor (SB203580 at 10 μM). Bar graph showed the expression of B7-H1 by MDA-MB-231 cells after 24–72 hours of culture with inhibitors or carrier control DMSO.
3. Discussion
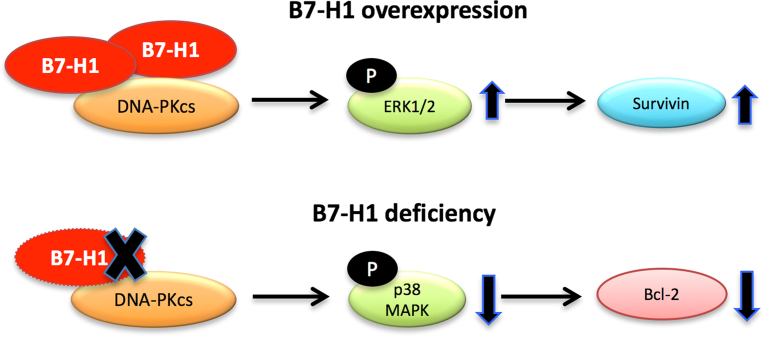
In this report we found that B7-H1 promotes cancer survival by cell-context dependent activation of ERK or MAPK pathway through association with DNA-PKcs (Fig. 9). The cancer cell-intrinsic pro-survival function of B7-H1 may contribute to cancer chemoresistance. Not only did CRISPR/cas9-mediated B7-H1 knock out render cancer cells to be more sensitive to chemotherapy, but targeting B7-H1 with a monoclonal antibody also sensitized cancer cells to chemotherapy. Thus, our results provided new insights to the action of B7-H1 in cancer cell survival and chemoresistance, and supports a new approach to improve the efficacy of cancer chemotherapy.
Fig. 9.
Diagram of potential functions of B7-H1 in cancer cell survival and chemoresistance. Overexpression of B7-H1 increases ERK activation through association with DNA-PKcs and leads to increased expression of survivin. B7-H1 deficiency decreased the activation of p38 MAPK that is dependent on the association of DNA-PKcs and B7-H1 followed by a downregulation of Bcl-2. Collectively, B7-H1 has a tumor-cell intrinsic pro-survival function and targeting B7-H1 signal pathways may sensitize cancer cells to chemotherapy.
Although B7-H1 was originally defined as a ligand of PD-1 expressed by T cells [25], recent studies suggest that B7-H1 can regulate cancer cell metabolism and growth through PD-1 [26, 27]. Ligation of B7-H1 by antibody causes B7-H1 internalization [26] and disrupts the role of B7-H1 in maintenance of Akt/mTOR signaling [27]. Clark et al showed B7-H1 sensitizes mouse melanoma cells to pharmacologic autophagy inhibitors in vivo [22]. In contrast to these studies using PD-1 positive cancer (most of them are melanoma) cells, we explored the downstream signaling pathway of B7-H1 in melanoma and breast cancer cells that do not express PD-1 or CD80 (two receptors of B7-H1). In our models, we unraveled a new signaling pathway of B7-H1 within cancer cells, i.e. the DNA-PKcs/ERK/MAPK pathway that may promote cancer cell survival and chemoresistance. In association with DNA-PKcs, B7-H1 increased activation of ERK in melanoma cells and maintained the activation of p38 MAPK in triple negative breast cancer cells.
DNA-PKcs localizes to the nucleus where it regulates DNA damage repair in response to cytotoxic drugs [28]. In addition, the role of DNA-PKcs in cell survival has been identified in activation of ERK and AKT in the nucleus or cytoplasm [20, 21]. We found that upon treatment with chemotherapy, B7-H1 accumulated in the nuclei where B7-H1 formed an association with DNA-PKcs. The accumulation of B7-H1 in the nuclei of cancer cells suggest that B7-H1 may transmit signals to nucleus for cell stress management through DNA-PKcs that is involved in both DNA damage repair and signal transduction. In this regard, the activation of ERK and p38 MAPK seems function as downstream signal molecules of DNA-PKcs.
B7-H1–mediated cancer chemoresistance seems to be cell-context dependent according to drug selection. We found that knocking out B7-H1 sensitized cancer cells to chemotherapy in some cancer cells (MDA-MB-231 and 786-0) but not in other cancer cells (A549). It is possible that higher expression of B7-H1 by MDA-MB-231 and 786-0 cells may increase the threshold of apoptosis by the activation of p38 MAPK pathway along with a high level of Bcl-2 [24]. Accordingly, disruption of B7-H1 expression in these cancer cells would reduce the threshold of apoptosis and render cancer cells more susceptible to chemotherapy. In line with this hypothesis, we showed that B7-H1 KO human breast cancer cells are more sensitive to chemotherapy and undergo a high rate of apoptosis. Thus, high expression of B7-H1 by certain cancer cells seems to be a sign of their “apoptosis-primed” status in which B7-H1 is used to counteract intrinsic or external pro-apoptosis signals. This may explain why in cancer cells with low or absent B7-H1 expression, the knocking out of B7-H1 did not significantly affect their sensitivity to chemotherapy as these cancer cells may not be “apoptosis-primed”.
As ERK activation plays key roles in cell survival and drug resistance [29, 30], the increased ERK activation in B7-H1 overexpressing melanoma cells may explain why these cancer cells are more resistant to chemotherapy. However, ERK activation is not always dependent on the expression of B7-H1. We found B7-H1 KO was not able to reduce the activation of ERK in MDA-MB-231 breast cancer cells. The reason could be that MDA-MB-231 cells harbor mutant ras that leads to a high activation of ERK [31] although MDA-MB-231 cells constitutively express high levels of B7-H1. Accordingly, although MDA-MB-231 cells express higher levels of B7-H1 than MDA-MB-157 cells (a human triple negative breast cancer cell line) [32], both of them demonstrated similar sensitivity to cisplatin in vitro. Besides their different B7-H1 expression, these two cell lines have multiple different gene mutations in p53 and RB pathways that also regulate sensitivity to chemotherapy [33, 34]. In this regard, B7-H1 expression alone may not be able to predict chemoresistance as multiple factors are responsive for drug resistance in cancer cells. However, our results support the therapeutic potential of targeting B7-H1 to promote the efficacy of chemotherapy in cancer cells that express B7-H1. In fact, we found that B7-H1 antibody (H1A) sensitized human breast cancer cells to cisplatin in vivo, suggesting B7-H1 antibody may disrupt B7-H1 signals in cancer cells along with blocking B7-H1 and PD-1 interaction that suppress antitumor immunity.
In summary, our studies identify a pro-survival function of B7-H1 in cancer cells. B7-H1 may promote cancer cell survival by activation of ERK and p38 MAPK pathways through the association with DNA-PKcs. The pro-survival function of B7-H1 could be used by “apoptosis-primed” cancer cells to counteract the cytotoxicity of chemotherapy. To that end, we propose that targeting B7-H1 by monoclonal antibody to B7-H1 capable of disrupting B7-H1 signals may be a new approach to promote the efficacy of cancer chemotherapy. Recent clinical trials that have demonstrated the superiority of adding B7-H1 or PD-1 inhibitors to chemotherapy compared to chemotherapy alone further support our findings [10, 11, 12, 35].
4. Methods and materials
4.1. Cell lines and reagents
Human cancer cell lines (MDA-MB-231, MDA-MB-157, 786-0, A549) were purchased from ATCC (Manassas, VA). Tumor cells were cultured and maintained in medium indicated by ATCC. B7-H1 or OVA (mock) transfected 624mel cells were cultured in RPMI 1640 medium (Cellgro) and supplemented as described previously [13]. Cells were cultured in a 37 °C humidified chamber at 5% CO2. Chemotherapy drugs were purchased form Mayo Pharmacy or Sigma.
4.2. B7-H1 transfection and knockout
Human B7-H1 was knocked-out by CRISPR/Cas9 technology. The guide sequence (5′-ATTTACTGTCACGGTTCCCA-3′) specific to human B7-H1 exon 3 (second coding exon), designed using CRISPR DESIGN tool (http://crispr.mit.edu) and cloned into px458 plasmid coexpressing GFP (Addgene, #52961). Thirty-six hours after transfection, cells were sorted for GFP and sub-cloned using flow cytometry. Two weeks later, single cell subclones were genotyped by PCR and validated Western blotting for B7-H1 protein depletion. B7-H1 expression level was determined by flow cytometry and Western blotting.
4.3. Immunofluorescence staining
Following growth on PBS and medium pre-rinsed coverslips, cells were fixed with 4% formalin or paraformaldehyde for 15 min., washed 4x with PBS, and permeabilized for 10 min. with 0.2% Triton X-100 or 0.5% IGEPAL ca-360. After washing with PBS, cells were blocked with 3% milk/PBS, then incubated at 4 °C overnight with primary antibodies (1:100 anti-DNA-PKcs and 1:300 anti-B7-H1 antibody 5H1) diluted in blocking solution. Six 3% milk/PBS washes were performed prior to 1-hour incubation with secondary antibody (Life Technologies Fluorescein-conjugated goat anti-mouse and Alexa 594-conjugated goat anti-rabbit IgG) diluted 1:100 in blocking solution. Following five PBS washes, re-fixation for 10 min. with 4% paraformaldehyde, and two dH2O washes, coverslips were mounted with SlowFade Gold antifade reagent with DAPI (Invitrogen) and cured for 24 hrs in dark at RT. Nail-polish sealed coverslips were visualized using a Zeiss LSM 510 confocal microscope. The two-dimensional Z-section images were acquired and performed using a Zeiss ELYRA super-resolution structured illumination microscopy.
4.4. MTS cytotoxicity assay
1 × 104 cells were seeded into 96-well plates and chemo-drug was applied. Following 72-hour incubation, 20 μl/well CellTiter 96 Aqueous One Solution Reagent (Promega) was added. After 2 hours of incubation, absorbance at 490 nm was recorded using an ELISA plate reader. Control and all concentrations of drug were assayed in triplicate, and the absorbance at each drug concentration was normalized relative to that of untreated controls.
4.5. Flow cytometry analysis
Fluorochrome-conjugated Abs against human B7-H1 (MIH1), PD-1 (EH12.2H7) and CD80 (L307.4) were purchased from BD Biosciences (Mountain View, CA), BioLegend (San Diego, CA), or eBioscience (San Diego, CA). To detect intracellular Survivin (clone 32.1, Novus), and Bcl-2 (Clone 50E3, Cell Signaling Inc.), tumor cells were incubated in Fixation Buffer (BioLegend) for 20 min at room temperature, followed by permeabilization using Permeabilization Wash Buffer (BioLegend). After staining with antibody, cells were washed three times with washing buffer before analysis. Apoptosis of tumor cells was analyzed by staining with Annexin V (BD Biosciences) and tetramethylrhodamine (TMRE; ethyl ester) (Invitrogen-Molecular Probes) for 10 min at room temperature. At least 100,000 viable cells were live gated on FACSCailbur (BD Biosciences) instrumentation. Flow cytometry analysis was performed using FlowJo software (Tree Star, Ashland, OR).
4.6. Measurement of phosphorylation of ERK and p38 MAPK
Human Phospho-MAPK Array Kit (R&D Systems) was used to screen phosphorylated proteins between B7-H1 and Mock transfected 624mel cells. After incubation, cells are fixed in 4% formaldehyde for 10 min at 37 °C followed with permeabilization in ice-cold methanol for 30 min on ice. After washing, cells were blocked with Fc receptor blockers and incubated with rabbit mAb to phospho-ERK1/2 (T202/Y204) (clone 197G2) or p38 MAPK (Thr180/Tyr182) (Clone D3F9, Cell Signaling #4511) or Isotype control IgG for 1 hour at room temperature, followed with incubation with secondary anti-rabbit IgG (H + L), F (ab')2 Fragment (Alexa Fluor® 488 Conjugate) (Cell Signaling #4412) for 30 min at room temperature. To inhibit the activity of DNA-PKcs, NU7026 (Selleckchem #S2893) was added at 1 or 10 μM in cell culture an hour before analysis. To inhibit the kinase activity of p38 MAPK, SB203580 (Cell Signaling #5633) was added at 10 μM in cell culture. The intracellular levels of ERK or p38 MAPK activation was analyzed by flow cytometry.
4.7. Immunoprecipitation (IP), Western blotting and Mass spectrometry
Immunoprecipitation of endogenous proteins that may be associated with B7-H1 was performed using B7-H1/624mel and B7-H1 positive Karpas-299 cells (a human T cell lymphoma cell line) purchased from American Type Culture Collection and propagated in complete media (RPMI, 10% FBS, 20 mmol/L HEPES, and penicillin/streptomycin). Cells were harvested in 1%NP40 lysis buffer containing 50 mM Tris-HCL pH 8.0, 150 mM NaCl, 5 mM EGTA, 5 mM EDTA, 30 mM MgCl2, 1.3% Beta-glycerophosphate, 1 mM DTT, 0.1 mM Na-Vanadate, 0.1 mM HaF, 0.4% p-nitrophenyl phosphate and protease inhibitors. Extracts were incubated overnight with protein G beads pre-coated with anti-B7-H1 mAb (5H1-A3), control IgG or polyclonal anti-DNA-PKcs antibody (H163, Santa Cruz Biotechnology, Santa Cruz, CA). The proteins eluted from the protein G beads were resolved 5–7.5% SDS gel and detected by Coomassie Blue and Western blotting (WB). Rabbit anti-DNA-PKcs antibody at 1:1000 dilution (H-163, Santa Cruz Biotechnology) and mouse anti-B7-H1 antibody at 1:500 dilution (H1A or B11, established at Dong lab) were used as the primary antibodies for Western blotting. To confirm the specificity of IP antibody 5H1, rabbit anti-human B7-H1/PD-L1 antibody (Cell signalling, clone E1L3N) was used in Western blot. Mass spectrometry analysis of protein bands were performed at Mayo Clinic Proteomics Research Core Facility.
4.8. Animal studies
Both B7-H1 WT and KO MDA-MB-231 cells were subcutaneously injected into NOD SCID mice (Jackson lab) at 2 × 106 cells per mouse. Cisplatin was administrated intraperitoneally (i.p.) at 5 mg/kg for 2 doses on days 7 and 13. Anti-human B7-H1 monoclonal antibody (clone H1A, produced at Dong lab) [18] or isotype control (mouse IgG) was i.p. injected at 200 μg/mouse on days 6, 9, 12, 15 and 18. B7-H1 wild type or knockout tumor cells were isolated from wild type breast cancer from the NeuT mice (provided by Dr. L. Pease, Mayo Clinic, Rochester, MN) or from B7-H1 knockout NeuT mice (Dong lab). Mouse breast tumor cells (5 × 105 cells) were injected subcutaneously at the right flank of Balb/c purchased from Taconic Farms (Germantown, NY) and used at age of 12 weeks. On day 8 and 14, paclitaxel (Taxol) was injected i.p. at 25 mg/kg, and control groups were treated with saline. Tumor growth was evaluated every 2–3 days until days 35–55 when all mice were euthanized that were not already sacrificed for rapid tumor growth or ulceration. In compliance with animal care guidelines, mice were euthanatized when either primary or secondary tumors reached ethical endpoints or developed ulcerations. Mayo Clinic's Institutional Animal Care and Use Committee approved this study.
4.9. Statistical analysis
Mann-Whitney test or unpaired two-side t test was used to compare phenotype between independent groups (treatments) or cell populations. The drug cytotoxicity assay was analyzed by the area under curve between the control cell lines and the tested drugs was calculated between cancer cell lines. The therapeutic effects of chemotherapy were analyzed by two-way ANOVA because two independent factors (B7-H1 phenotype or drug treatment) were analyzed. All statistical analyses were performed using GraphPad Prism software 5.0 (GraphPad Software, Inc., San Diego, CA). A P value < 0.05 was considered statistically significant.
Declarations
Author contribution statement
Xiaosheng Wu: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Yanli Li: Performed the experiments; Analyzed and interpreted the data.
Xin Liu, Chunhua Chen, Susan Harrington, Siyu Cao, Tiancheng Xie, Tu Pham: Performed the experiments.
Aaron Mansfield, Yiyi Yan: Analyzed and interpreted the data; Wrote the paper.
Eugene Kwon, Liewei Wang, Kun Ling, Haidong Dong: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This study was supported by Richard M. Schulze Family Foundation (EK and HD), Mayo Clinic Breast Cancer SPORE (HD and LW), Center of Biomedical Discovery fund (KL and HD), NIH K12 CA090628 (ASM), NIH K12 CA090628 (YY) and Fraternal Order of Eagles Cancer Research Fund awards (YY).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Brozovic A., Osmak M. Activation of mitogen-activated protein kinases by cisplatin and their role in cisplatin-resistance. Cancer Lett. 2007;251:1–16. doi: 10.1016/j.canlet.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 2.McCubrey J.A., Steelman L.S., Chappell W.H., Abrams S.L., Wong E.W., Chang F., Lehmann B., Terrian D.M., Milella M., Tafuri A., Stivala F., Libra M., Basecke J., Evangelisti C., Martelli A.M., Franklin R.A. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamura H., Ishibashi M., Yamashita T., Tanosaki S., Okuyama N., Kondo A., Hyodo H., Shinya E., Takahashi H., Dong H., Tamada K., Chen L., Dan K., Ogata K. Marrow stromal cells induce B7-H1 expression on myeloma cells, generating aggressive characteristics in multiple myeloma. Leukemia. 2013;27:464–472. doi: 10.1038/leu.2012.213. [DOI] [PubMed] [Google Scholar]
- 4.Ghebeh H., Lehe C., Barhoush E., Al-Romaih K., Tulbah A., Al-Alwan M., Hendrayani S.F., Manogaran P., Alaiya A., Al-Tweigeri T., Aboussekhra A., Dermime S. Doxorubicin downregulates cell surface B7-H1 expression and upregulates its nuclear expression in breast cancer cells: role of B7-H1 as an anti-apoptotic molecule. Breast Cancer Res. 2010;12:R48. doi: 10.1186/bcr2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu H., Tekle C., Chen Y.W., Kristian A., Zhao Y., Zhou M., Liu Z., Ding Y., Wang B., Maelandsmo G.M., Nesland J.M., Fodstad O., Tan M. B7-H3 silencing increases paclitaxel sensitivity by abrogating Jak2/Stat3 phosphorylation. Mol. Canc. Therapeut. 2011;10:960–971. doi: 10.1158/1535-7163.MCT-11-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson R.H., Gillett M.D., Cheville J.C., Lohse C.M., Dong H., Webster W.S., Krejci K.G., Lobo J.R., Sengupta S., Chen L., Zincke H., Blute M.L., Strome S.E., Leibovich B.C., Kwon E.D. Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zang X., Allison J.P. The B7 family and cancer therapy: costimulation and coinhibition. Clin. Canc. Res. 2007;13:5271–5279. doi: 10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
- 8.Mansfield A.S., Roden A.C., Peikert T., Sheinin Y.M., Harrington S.M., Krco C.J., Dong H., Kwon E.D. B7-H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis. J. Thorac. Oncol. 2014;9:1036–1040. doi: 10.1097/JTO.0000000000000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y., Wang J., Li C., Ke X.Y. Contribution of PD-L1 to oncogenesis of lymphoma and its RNAi-based targeting therapy. Leuk. Lymphoma. 2012;53:2015–2023. doi: 10.3109/10428194.2012.673228. [DOI] [PubMed] [Google Scholar]
- 10.Schmid P., Adams S., Rugo H.S., Schneeweiss A., Barrios C.H., Iwata H., Dieras V., Hegg R., Im S.A., Shaw Wright G., Henschel V., Molinero L., Chui S.Y., Funke R., Husain A., Winer E.P., Loi S., Emens L.A., Investigators I.M.T. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 11.Liu S.V., Camidge D.R., Gettinger S.N., Giaccone G., Heist R.S., Hodi F.S., Ready N.E., Zhang W., Wallin J., Funke R., Waterkamp D., Foster P., Iizuka K., Powderly J. Long-term survival follow-up of atezolizumab in combination with platinum-based doublet chemotherapy in patients with advanced non-small-cell lung cancer. Eur. J. Cancer. 2018;101:114–122. doi: 10.1016/j.ejca.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 12.Horn L., Mansfield A.S., Szczesna A., Havel L., Krzakowski M., Hochmair M.J., Huemer F., Losonczy G., Johnson M.L., Nishio M., Reck M., Mok T., Lam S., Shames D.S., Liu J., Ding B., Lopez-Chavez A., Kabbinavar F., Lin W., Sandler A., Liu S.V., Group I.M.S. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 13.Dong H., Strome S.E., Salomao D.R., Tamura H., Hirano F., Flies D.B., Roche P.C., Lu J., Zhu G., Tamada K., Lennon V.A., Celis E., Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 14.Pei H., Li L., Fridley B.L., Jenkins G.D., Kalari K.R., Lingle W., Petersen G., Lou Z., Wang L. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. 2009;16:259–266. doi: 10.1016/j.ccr.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sedletska Y., Giraud-Panis M.J., Malinge J.M. Cisplatin is a DNA-damaging antitumour compound triggering multifactorial biochemical responses in cancer cells: importance of apoptotic pathways. Curr. Med. Chem. Anti Cancer Agents. 2005;5:251–265. doi: 10.2174/1568011053765967. [DOI] [PubMed] [Google Scholar]
- 16.Wang S., Konorev E.A., Kotamraju S., Joseph J., Kalivendi S., Kalyanaraman B. Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms. intermediacy of H(2)O(2)- and p53-dependent pathways. J. Biol. Chem. 2004;279:25535–25543. doi: 10.1074/jbc.M400944200. [DOI] [PubMed] [Google Scholar]
- 17.Jayaraman S. Flow cytometric determination of mitochondrial membrane potential changes during apoptosis of T lymphocytic and pancreatic beta cell lines: comparison of tetramethylrhodamineethylester (TMRE), chloromethyl-X-rosamine (H2-CMX-Ros) and MitoTracker Red 580 (MTR580) J. Immunol. Methods. 2005;306:68–79. doi: 10.1016/j.jim.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Liu X., Wu X., Cao S., Harrington S.M., Yin P., Mansfield A.S., Dong H. B7-H1 antibodies lose antitumor activity due to activation of p38 MAPK that leads to apoptosis of tumor-reactive CD8+ T cells. Sci. Rep. 2016;6:36722. doi: 10.1038/srep36722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong H., Zhu G., Tamada K., Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 20.Dragoi A.M., Fu X., Ivanov S., Zhang P., Sheng L., Wu D., Li G.C., Chu W.M. DNA-PKcs, but not TLR9, is required for activation of Akt by CpG-DNA. EMBO J. 2005;24:779–789. doi: 10.1038/sj.emboj.7600539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yotsumoto S., Saegusa K., Aramaki Y. Endosomal translocation of CpG-oligodeoxynucleotides inhibits DNA-PKcs-dependent IL-10 production in macrophages. J. Immunol. 2008;180:809–816. doi: 10.4049/jimmunol.180.2.809. [DOI] [PubMed] [Google Scholar]
- 22.Clark C.A., Gupta H.B., Sareddy G., Pandeswara S., Lao S., Yuan B., Drerup J.M., Padron A., Conejo-Garcia J., Murthy K., Liu Y., Turk M.J., Thedieck K., Hurez V., Li R., Vadlamudi R., Curiel T.J. Tumor-intrinsic PD-L1 signals regulate cell growth, pathogenesis, and autophagy in ovarian cancer and melanoma. Cancer Res. 2016;76:6964–6974. doi: 10.1158/0008-5472.CAN-16-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye Q., Cai W., Zheng Y., Evers B.M., She Q.B. ERK and AKT signaling cooperate to translationally regulate survivin expression for metastatic progression of colorectal cancer. Oncogene. 2014;33:1828–1839. doi: 10.1038/onc.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phong M.S., Van Horn R.D., Li S., Tucker-Kellogg G., Surana U., Ye X.S. p38 mitogen-activated protein kinase promotes cell survival in response to DNA damage but is not required for the G(2) DNA damage checkpoint in human cancer cells. Mol. Cell Biol. 2010;30:3816–3826. doi: 10.1128/MCB.00949-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freeman G.J., Long A.J., Iwai Y., Bourque K., Chernova T., Nishimura H., Fitz L.J., Malenkovich N., Okazaki T., Byrne M.C., Horton H.F., Fouser L., Carter L., Ling V., Bowman M.R., Carreno B.M., Collins M., Wood C.R., Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang C.H., Qiu J., O'Sullivan D., Buck M.D., Noguchi T., Curtis J.D., Chen Q., Gindin M., Gubin M.M., van der Windt G.J., Tonc E., Schreiber R.D., Pearce E.J., Pearce E.L. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleffel S., Posch C., Barthel S.R., Mueller H., Schlapbach C., Guenova E., Elco C.P., Lee N., Juneja V.R., Zhan Q., Lian C.G., Thomi R., Hoetzenecker W., Cozzio A., Dummer R., Mihm M.C., Jr., Flaherty K.T., Frank M.H., Murphy G.F., Sharpe A.H., Kupper T.S., Schatton T. Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth. Cell. 2015;162:1242–1256. doi: 10.1016/j.cell.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedmann B.J., Caplin M., Savic B., Shah T., Lord C.J., Ashworth A., Hartley J.A., Hochhauser D. Interaction of the epidermal growth factor receptor and the DNA-dependent protein kinase pathway following gefitinib treatment. Mol. Canc. Therapeut. 2006;5:209–218. doi: 10.1158/1535-7163.MCT-05-0239. [DOI] [PubMed] [Google Scholar]
- 29.Schluter C., Duchrow M., Wohlenberg C., Becker M.H., Key G., Flad H.D., Gerdes J. The cell proliferation-associated antigen of antibody Ki-67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J. Cell Biol. 1993;123:513–522. doi: 10.1083/jcb.123.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mebratu Y., Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: is subcellular localization the answer? Cell Cycle. 2009;8:1168–1175. doi: 10.4161/cc.8.8.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eckert L.B., Repasky G.A., Ulku A.S., McFall A., Zhou H., Sartor C.I., Der C.J. Involvement of Ras activation in human breast cancer cell signaling, invasion, and anoikis. Cancer Res. 2004;64:4585–4592. doi: 10.1158/0008-5472.CAN-04-0396. [DOI] [PubMed] [Google Scholar]
- 32.Mittendorf E.A., Philips A.V., Meric-Bernstam F., Qiao N., Wu Y., Harrington S., Su X., Wang Y., Gonzalez-Angulo A.M., Akcakanat A., Chawla A., Curran M., Hwu P., Sharma P., Litton J.K., Molldrem J.J., Alatrash G. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollestelle A., Nagel J.H., Smid M., Lam S., Elstrodt F., Wasielewski M., Ng S.S., French P.J., Peeters J.K., Rozendaal M.J., Riaz M., Koopman D.G., Ten Hagen T.L., de Leeuw B.H., Zwarthoff E.C., Teunisse A., van der Spek P.J., Klijn J.G., Dinjens W.N., Ethier S.P., Clevers H., Jochemsen A.G., den Bakker M.A., Foekens J.A., Martens J.W., Schutte M. Distinct gene mutation profiles among luminal-type and basal-type breast cancer cell lines. Breast Canc. Res. Treat. 2010;121:53–64. doi: 10.1007/s10549-009-0460-8. [DOI] [PubMed] [Google Scholar]
- 34.Lai D., Visser-Grieve S., Yang X. Tumour suppressor genes in chemotherapeutic drug response. Biosci. Rep. 2012;32:361–374. doi: 10.1042/BSR20110125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gandhi L., Rodriguez-Abreu D., Gadgeel S., Esteban E., Felip E., De Angelis F., Domine M., Clingan P., Hochmair M.J., Powell S.F., Cheng S.Y., Bischoff H.G., Peled N., Grossi F., Jennens R.R., Reck M., Hui R., Garon E.B., Boyer M., Rubio-Viqueira B., Novello S., Kurata T., Gray J.E., Vida J., Wei Z., Yang J., Raftopoulos H., Pietanza M.C., Garassino M.C., Investigators K.-. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.