The biological mediator hydrogen sulfide (H2S) is produced by bacteria and has been shown to be cytoprotective against oxidative stress and to increase the sensitivity of various bacteria to a range of antibiotic drugs. Here we evaluated whether bacterial H2S provides resistance against the immune response, using two bacterial species that are common sources of nosocomial infections, Escherichia coli and Staphylococcus aureus.
KEYWORDS: antibiotic resistance, burn, hydrogen sulfide, opportunistic infections
ABSTRACT
The biological mediator hydrogen sulfide (H2S) is produced by bacteria and has been shown to be cytoprotective against oxidative stress and to increase the sensitivity of various bacteria to a range of antibiotic drugs. Here we evaluated whether bacterial H2S provides resistance against the immune response, using two bacterial species that are common sources of nosocomial infections, Escherichia coli and Staphylococcus aureus. Elevations in H2S levels increased the resistance of both species to immune-mediated killing. Clearances of infections with wild-type and genetically H2S-deficient E. coli and S. aureus were compared in vitro and in mouse models of abdominal sepsis and burn wound infection. Also, inhibitors of H2S-producing enzymes were used to assess bacterial killing by leukocytes. We found that inhibition of bacterial H2S production can increase the susceptibility of both bacterial species to rapid killing by immune cells and can improve bacterial clearance after severe burn, an injury that increases susceptibility to opportunistic infections. These findings support the role of H2S as a bacterial defense mechanism against the host response and implicate bacterial H2S inhibition as a potential therapeutic intervention in the prevention or treatment of infections.
INTRODUCTION
Hydrogen sulfide (H2S) is a gaseous biological mediator that regulates important functions in the nervous, cardiovascular, immune, and gastrointestinal systems. The importance of H2S as an endogenous mediator in mammalian systems is highlighted by discoveries that disruptions in H2S homeostasis are associated with a wide range of disease states, including cardiovascular diseases, diabetes, burn injury, ischemia-reperfusion, and cancer (1). In mammalian cells, H2S is produced by three enzymes: cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST) (1, 2).
Bacteria can also produce H2S via orthologous enzymes (3). While bacterial H2S production was long perceived primarily as a metabolic by-product, recent studies have implicated H2S as an important signaling molecule in bacteria. H2S can protect bacteria from antibiotic-induced damage, at least in part by sequestering free iron to prevent the Fenton reaction that generates toxic hydroxyl radicals (3, 4). Additionally, H2S can regulate intracellular cysteine, which can be toxic at high levels (4). Recently, it was demonstrated that H2S, and downstream reactive sulfur species, can regulate the expression of some bacterial virulence genes through S-sulfhydration of the proteome (5). Given the importance of reactive oxygen species (ROS) in antimicrobial immune responses, especially innate responses (6), we have now evaluated whether bacterial H2S protects bacteria from the host immune response by manipulating H2S levels in bacteria without modulation of host H2S. The findings in this report support the role of H2S as a bacterial defense mechanism and implicate bacterial H2S inhibition as a potential therapeutic intervention for the treatment or prevention of infections.
RESULTS
Because manipulation of host H2S production could have potential effects on the immune response to infection, experiments were designed to specifically manipulate bacterial, but not host, H2S levels. To determine if pharmacological donation of H2S can increase the resistance of bacteria to the immune response, Escherichia coli and Staphylococcus aureus were cultured in the presence or absence of a slow-release H2S donor, GYY4137 (7). GYY4137 is an organic small molecule that slowly decomposes to release low levels of H2S over a prolonged period of time. Its use as a low-level H2S generator in biological systems, both mammalian and nonmammalian, has been well characterized (8, 9). To prevent any direct effects of GYY4137 on host cells, bacteria were preincubated with the H2S donor, which was removed prior to inoculation of the mouse leukocyte cultures. H2S donation to E. coli concentration-dependently reduced leukocyte-mediated clearance of E. coli in vitro (P < 0.05) (Fig. 1). Bacterial clearance was completely prevented at the highest concentrations of GYY4137 tested (0.3 and 1 mM), allowing bacterial growth in the presence of leukocytes, suggesting that H2S can protect E. coli from immune-mediated killing. Similarly, at concentrations of 0.3 and 1 mM, GYY4137 significantly reduced and prevented rapid killing of S. aureus in vitro (P < 0.05) (Fig. 1). This was not due to effects of GYY4137 on bacterial proliferation rates, as bacterial counts were similar in the corresponding control cultures lacking leukocytes (not shown).
FIG 1.
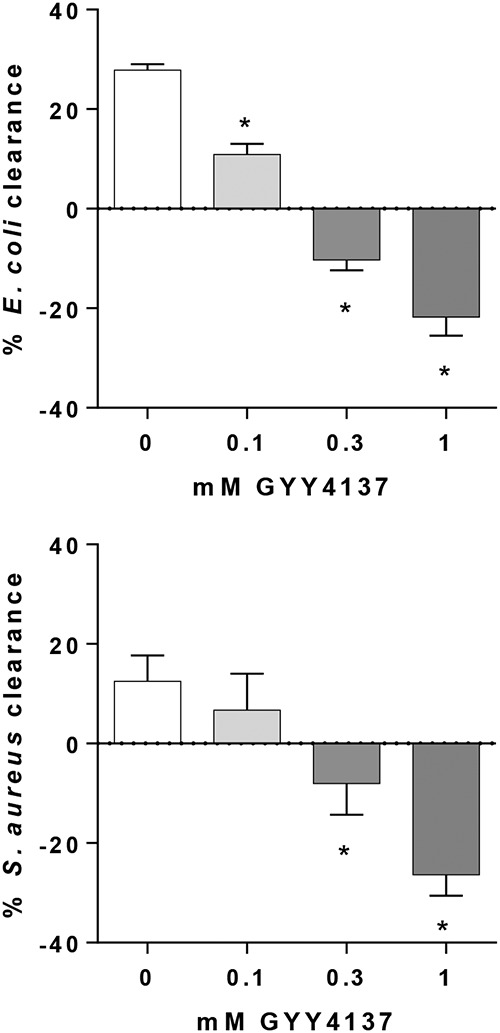
E. coli and S. aureus were cultured with increasing concentrations of up to 1 mM GYY4137 prior to inoculation of mouse leukocyte cultures. Graphs show percentages of inoculum CFU killed after coculture with leukocytes. *, significantly different from 0 mM (n = 4 to 5 replicates per group).
Since pharmacological donation of H2S increased the resistance of both E. coli and S. aureus to killing by leukocytes, we attempted to pharmacologically inhibit H2S production in these two bacterial species. E. coli lacks homologues for the mammalian H2S-producing enzymes CBS and CSE but expresses the 3-MST homologue that is encoded by the sseA gene (3). Because there are currently no inhibitors available that are specific for bacterial 3MST, an inhibitor of mouse 3-MST (10, 11) was preincubated with E. coli, and removed, prior to inoculation of leukocytes. E. coli production of H2S, detected by reaction with lead acetate, was decreased but not completely inhibited in the presence of increasing concentrations of the 3-MST inhibitor. The ability of this inhibitor to inhibit bacterial 3MST was confirmed, as the activity of purified bacterial 3MST was decreased but not completely inhibited in the presence of increasing concentrations of the 3-MST inhibitor (Fig. 2A). In vitro clearance of E. coli was significantly increased in the presence of the 3-MST inhibitor, in a dose-dependent manner (P < 0.05) (Fig. 2A).
FIG 2.
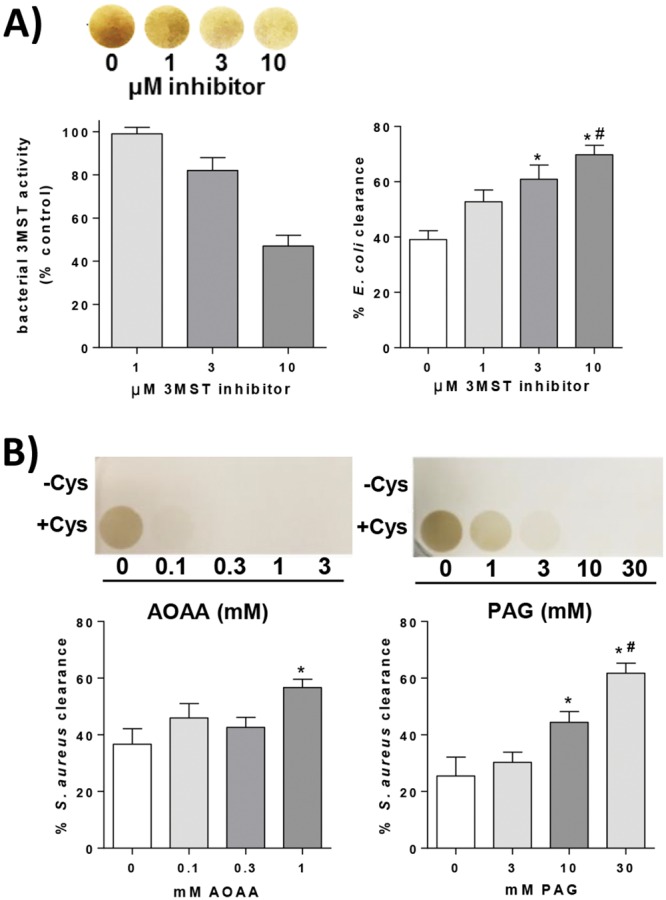
(A) Image showing brown lead sulfide staining produced by reaction of lead acetate with H2S produced by E. coli cultured with or without the 3-MST inhibitor. The graph on the left shows the activity of bacterial 3MST in the presence of increasing concentrations of the 3-MST inhibitor, expressed as a percentage of 3MST activity in the control (no inhibitor) (n = 3 replicates/group). The graph on the right shows in vitro elimination of E. coli by leukocytes after 45 min. WT E. coli bacteria were cultured in the presence of the 3-MST inhibitor prior to inoculation of leukocytes. *, significantly different from 0 μM; #, significantly different from 1 μM (n = 5/group). (B) Brown lead sulfide stain produced by reaction of lead acetate with H2S from S. aureus cultured with or without increasing concentrations of AOAA or PAG, in the presence (+Cys) or absence (−Cys) of 200 µM cysteine supplementation. Graphs show in vitro elimination of S. aureus by leukocytes after 60 min. WT S. aureus bacteria were cultured with inhibitors prior to inoculation of leukocytes. *, significantly different from 0 mM; #, significantly different from 3 mM (n = 5/group).
S. aureus lacks 3MST but expresses CBS and CSE homologues (3). It was observed that the level of H2S production by S. aureus was markedly lower than that of E. coli and nearly undetectable by reaction with lead. However, supplementation of cultures with 200 µM cysteine, a substrate for both CBS and CSE, increased H2S production to detectable levels (Fig. 2B). Because bacterium-specific CBS and CSE inhibitors are not available, commonly used inhibitors of the mammalian homologues were used in an attempt to decrease H2S production by S. aureus. Amino-oxyacetic acid (AOAA) is an inhibitor of human CBS that also has some inhibitory activity against human CSE. Propargylglycine (PAG) inhibits human CSE but not CBS (12). These inhibitors were previously demonstrated to reduce H2S production by S. aureus (3). S. aureus bacteria were preincubated with either AOAA or PAG, which was subsequently removed prior to inoculation of leukocytes. As shown in Fig. 2B, AOAA effectively decreased H2S production in S. aureus cultures. AOAA (at a concentration of 1 mM) increased the clearance of S. aureus by leukocytes (P < 0.05) (Fig. 2B). Similarly, H2S production by S. aureus was inhibited by PAG in a dose-dependent manner, as was in vitro clearance of S. aureus by leukocytes (P < 0.05) (Fig. 2B).
Because the 3-MST, CBS, and CSE inhibitors used here would inhibit the activity of the respective enzymes in host (mouse) cells, which could potentially affect the host response to infection, we were unable to include the inhibitors during the in vitro bacterial killing assays; therefore, rapid bacterial clearance was assessed immediately after the removal of the inhibitors from bacteria and within a short time frame, during which bacterial proliferation was negligible. Additionally, we were unable to treat infected mice to determine the therapeutic potential of bacterial 3MST, CBS, or CSE inhibition for clearance of E. coli or S. aureus infections in vivo, as continued treatment with inhibitors would be required while bacteria replicate and disseminate in vivo, making it difficult to distinguish effects caused by bacterial versus host H2S inhibition. Therefore, bacteria that are genetically deficient in specific H2S-synthesizing enzymes were utilized for further studies.
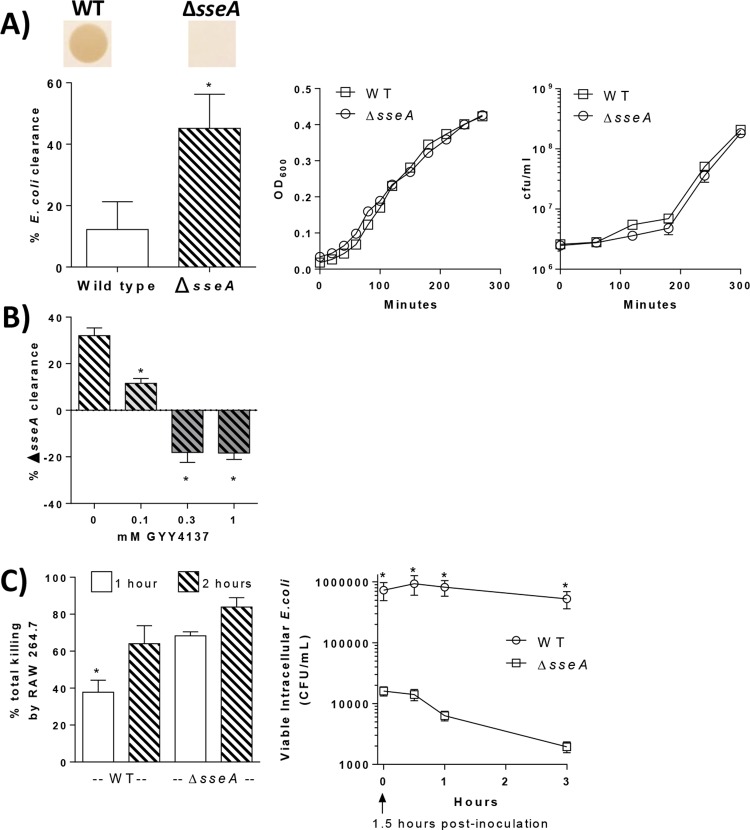
To establish a role of bacterial 3MST in E. coli defense against the immune response, wild-type (WT) E. coli and sseA-deficient E. coli strains were utilized. As shown in Fig. 3A, H2S production by 3MST-deficient E. coli (ΔsseA) is negligible compared to that by WT E. coli. Additionally, the level of rapid clearance of E. coli by leukocytes in vitro was significantly higher in cultures inoculated with the ΔsseA strain (45% bacterial clearance) than in those inoculated with the WT (12% clearance; P < 0.05) (Fig. 3A). Levels of bacterial proliferation were negligible during the bacterial clearance assay and were similar between WT E. coli and the ΔsseA strain. The susceptibility of sseA-deficient E. coli bacteria was reversed when they were preincubated in the presence of the H2S donor (P < 0.05) (Fig. 3B), and bacterial clearance was completely prevented at higher GYY4137 concentrations (0.3 and 1 mM).
FIG 3.
(A) Images showing brown lead sulfide staining produced by reaction of lead acetate with H2S produced by bacterial cultures. WT, wild-type E. coli; ΔsseA, sseA-deficient E. coli. (Left) Bacterial elimination by leukocytes after 45 min in vitro. *, significantly different from the WT (n = 6/group). (Right) Densities of E. coli WT and ΔsseA bacteria in liquid cultures over 5 h, measured by the OD600, and viability in cell culture medium, measured as CFU per milliliter (n = 3/group). (B) E. coli ΔsseA bacteria were cultured in the presence of GYY4137 prior to inoculation of mouse leukocytes. The graph shows bacterial elimination after 45 min of coculture. *, significantly different from 0 mM (n = 4 per group). (C, left) Total killing of E. coli bacteria by RAW 264.7 macrophages after 1 and 2 h in vitro. *, significantly different from all other groups (n = 7/group). (Right) Levels (CFU per milliliter) of viable intracellular E. coli bacteria recovered from RAW 264.7 cells. *, significantly different from the ΔsseA group at the corresponding time point (n = 6/group and time point).
Significant differences in the clearance of opsonized bacteria by total leukocytes were detected within an hour, suggesting H2S-mediated protection from early innate immune responses. To further define a role of 3MST in E. coli defense against phagocytic killing, the macrophage-like RAW 264.7 cell line was used. The 3MST-deficient E. coli bacteria were killed more rapidly than WT E. coli bacteria. Specifically, in cultures inoculated with WT E. coli, 38% of the bacteria were killed by 1 h, and 64% were killed by 2 h (Fig. 3C), whereas 68% of ΔsseA strain bacteria were killed by 1 h, and 84% were killed by 2 h. To compare resistances of the two strains to intracellular killing, intracellular viability was measured by a gentamicin protection assay. At the start of the assay (time zero), which was 1.5 h following inoculation of macrophage cultures with E. coli, there were significantly more viable intracellular wild-type E. coli bacteria than ΔsseA strain bacteria, and the numbers of viable intracellular WT E. coli bacteria remained steady throughout the assay, whereas numbers of viable intracellular E. coli ΔsseA bacteria consistently decreased and were significantly lower at all time points (P < 0.05) (Fig. 3C).
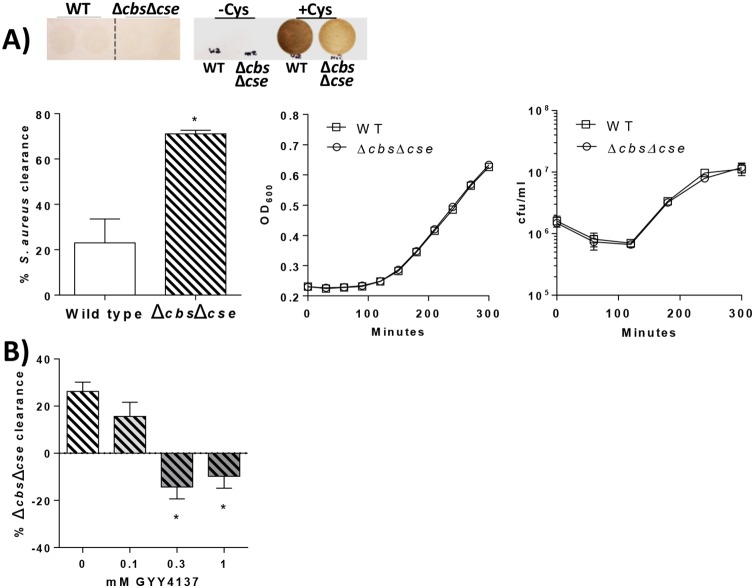
To determine if deficiency in H2S-producing enzymes similarly increases susceptibility of S. aureus to immune responses, clearance of wild-type and cbs- and cse-deficient S. aureus in vitro was measured when levels of proliferation were negligible and similar between the two strains (Fig. 4A). As observed previously, the level of S. aureus production of H2S was low in the absence of cysteine supplementation. Figure 4A shows a very faint precipitate with WT S. aureus that was not observed with the Δcbs Δcse strain. Supplementation of cultures with cysteine increased H2S production to detectable levels, with substantially lower levels being produced by cbs- and cse-deficient S. aureus. The rate of bacterial clearance was significantly higher in cultures inoculated with the Δcbs Δcse strain (71%) than in cultures inoculated with WT S. aureus (23% elimination; P < 0.05) (Fig. 4A). The susceptibility of Δcbs Δcse strain bacteria to leukocytes was reversed in the presence of increasing concentrations of the H2S donor GYY4137 (P < 0.05) (Fig. 4B).
FIG 4.
(A) Images showing brown lead sulfide staining produced by reaction of lead acetate with H2S produced by bacteria grown with or without cysteine (Cys) (200 μM) supplementation. WT, wild-type S. aureus; Δcbs Δcse, S. aureus lacking the cbs and cse genes. The graph on the left shows bacterial clearance by leukocytes after 60 min in vitro. *, significantly different from the WT (n = 6/group). The graphs on the right show densities of S. aureus WT and Δcbs Δcse bacteria in liquid cultures over 5 h, measured by the OD600, and viability in cell culture medium, measured as CFU per milliliter (n = 3/group). (B) S. aureus Δcbs Δcse bacteria were cultured in the presence of GYY4137 prior to inoculation of mouse leukocytes. The graph shows the percentage of the inoculum cleared after coculture with leukocytes. *, significantly different from 0 and 0.1 mM (n = 5 per group).
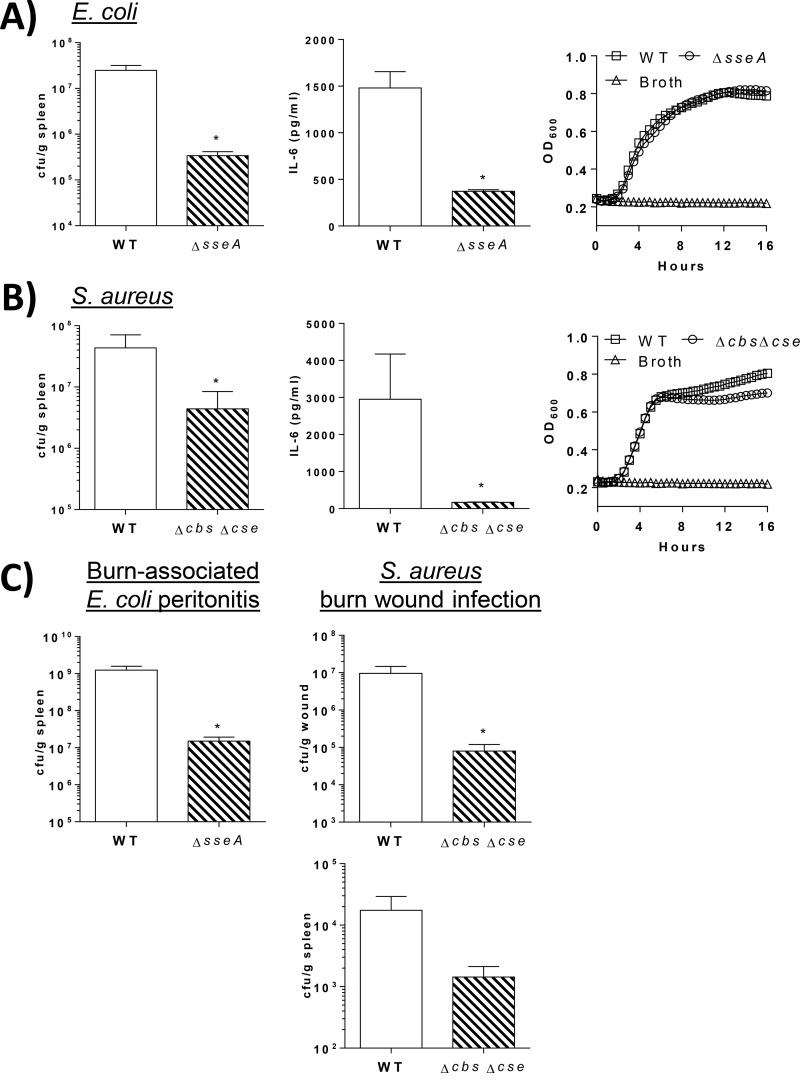
To determine if H2S deficiency affects bacterial clearance in vivo, mice were infected intraperitoneally (i.p.) with WT or H2S-deficient bacteria. Bacterial burden and systemic levels of interleukin-6 (IL-6), a marker of systemic inflammation and an indicator of poor outcomes during sepsis in mice and humans (13, 14), were measured 16 h later. When mice were inoculated i.p. with equal numbers of WT or 3MST-deficient E. coli bacteria, all mice infected with WT E. coli developed bacteremia, with a mean bacterial burden of 1.5 × 103 CFU/ml in the blood, whereas only 1 of 5 mice inoculated with the ΔsseA strain had a positive blood culture, which was negligible (200 CFU/ml). The bacterial burden in the spleen was similarly and significantly lower in ΔsseA strain-infected mice (3.4 × 105 CFU/g in the ΔsseA strain group versus 2.5 × 107 CFU/g in the WT E. coli group; P < 0.05) (Fig. 5A). Additionally, systemic levels of IL-6 were lower in ΔsseA strain-infected mice (372 ± 17.9 pg/ml) than in those infected with WT E. coli (1,480 ± 175 pg/ml; P < 0.05). This was not caused by differences in basic growth rates between the two strains, as levels of proliferation over 16 h (time frame of the in vivo infection) were similar between E. coli WT and ΔsseA bacteria (Fig. 5A). Similarly, when mice were given an i.p. inoculation with equal numbers of S. aureus WT and Δcbs Δcse bacteria, mean bacterial counts in the spleen 16 h later were significantly lower in Δcbs Δcse strain-infected mice (4.4 × 106 CFU/g) than in mice infected with WT S. aureus (4.3 × 107 CFU/g; P < 0.05) (Fig. 5B). Additionally, there was a significant reduction in the level of systemic inflammation marker IL-6 in Δcbs Δcse strain-infected mice compared to WT-infected mice (2,950 ± 1,222 pg/ml for the wild type; 170.0 ± 6.401 pg/ml for the Δcbs Δcse strain; P < 0.05) (Fig. 5B). Bacterial growth rates were nearly identical between the two strains within the first 8 h, after which time growth of the Δcbs Δcse strain was slightly slower than that of the WT strain (Fig. 5B).
FIG 5.
(A, left) Bar graphs showing bacterial counts in spleen and IL-6 levels in plasma 16 h after i.p. injection of mice with 1 × 108 CFU E. coli. *, significantly different from the WT (n = 5 mice/group). (Right) Densities of E. coli WT and ΔsseA bacteria in liquid cultures, measured by the OD600 over 16 h. (B, left) Bar graphs showing bacterial counts in spleens and plasma IL-6 levels in mice 16 h after i.p. injection of 5 × 108 CFU S. aureus. *, significantly different from the WT (n = 5/group). (Right) Densities of S. aureus WT and Δcbs Δcse bacteria in liquid cultures, measured by the OD600 over 16 h. (C, left) Graph showing bacterial counts in spleens of burned mice 16 h after i.p. injection of mice with 1 × 108 CFU E. coli. *, significantly different from the WT (n = 5 mice/group). (Right) Graphs showing bacterial counts in burn wounds and spleens of mice 24 h after inoculation of wounds with 1 × 106 CFU S. aureus. *, significantly different from the WT (n = 3 to 4/group).
To determine if bacterial H2S deficiency has therapeutic potential under conditions in which immune responses to infection are deficient, bacterial clearance was measured in mouse models of burn injury-associated infections. Burn patients are susceptible to life-threatening infections, due largely to burn-induced alterations in immune function that decrease patient defense against opportunistic microorganisms, and similar immunological perturbations occur in mice after burns. First, burn-injured mice were infected i.p. with E. coli as a model of burn-associated bacterial peritonitis. Peritonitis frequently develops into abdominal sepsis, and E. coli is a common contributor (15). As shown in Fig. 5C, the bacterial burden was significantly lower in burned mice infected with the ΔsseA strain (1.5 × 107 CFU/g) than in mice infected with WT E. coli (1.2 × 109 CFU/g) (P < 0.05). Next, the effects of bacterial H2S deficiency on clearance of S. aureus burn wound infection were examined. Wound infections are a common contributor to sepsis in burn patients, and methicillin-resistant S. aureus (MRSA) is a common early colonizer of burn wounds (16, 17). Burn wounds were inoculated with S. aureus WT or Δcbs Δcse bacteria, and bacterial growth within the wound and dissemination were measured. At 24 h postinoculation, there were significantly fewer bacteria in the wounds of mice inoculated with the Δcbs Δcse strain (7.9 × 104 CFU/g) than in mice inoculated with WT S. aureus (9.6 × 106 CFU/g) (P < 0.05) (Fig. 5C). Similarly, the bacterial burden in the spleen was lower in Δcbs Δcse strain-infected mice than in those infected with WT S. aureus (1.4 × 103 CFU/g with the Δcbs Δcse strain; 1.7 × 104 CFU/g with the WT).
DISCUSSION
The observations that elevations in H2S levels make both E. coli and S. aureus resistant to leukocyte-mediated killing and that decreases in bacterial H2S levels increase the susceptibility of these bacteria to killing by the host both in vivo and in vitro demonstrate that H2S can provide some protection of bacteria against early immune responses. This is further supported by the fact that E. coli and S. aureus utilize different enzymatic pathways for the synthesis of H2S, and a loss of the respective enzyme activities in each bacterial species induces susceptibility to the host immune response. Our data show that the effects of bacterial H2S are not likely due to effects on bacterial proliferation, as levels of growth of wild-type and H2S-deficient E. coli and S. aureus were similar during the lag and exponential phases (Fig. 3 to 5). While specific mechanisms of protection are not known, previous reports provide some insight into possible bacterial defenses that could be regulated by H2S. H2S can prevent oxidative damage to bacteria through stimulation of superoxide dismutase and catalase activities, sequestration of free iron to prevent hydroxyl radical production via the Fenton reaction, and control of intracellular cysteine, which can stimulate hydroxyl radical production and inhibit electron transport at high levels (3, 4, 18). Additionally, hydrogen sulfide can inhibit the activity of myeloperoxidase (19). The increased susceptibility of opsonized H2S-deficient bacteria to rapid killing by total leukocytes in vitro suggests that bacterial H2S protects against rapid innate responses, which could include extracellular reactive oxygen species (ROS) and reactive nitrogen species (RNS) and intracellular ROS/RNS in phagosomes. This is supported by the susceptibility of H2S-deficient E. coli to intracellular killing by macrophages, which is largely mediated by the generation of toxic radicals. However, the results from this study show significantly improved clearance of H2S-deficient bacteria by total splenic leukocytes, of which the percentage of phagocytic cells is relatively low in the mouse. Therefore, there are likely other immune responses that may be resisted by bacterial H2S, and further studies are needed to determine which specific immunological mechanisms can be resisted or avoided in the presence of bacterial H2S. Therefore, although this study found that bacterial H2S can protect bacteria from the host immune response, it is limited by a lack of identified mechanisms.
While nearly all bacterial species express orthologues of at least one mammalian H2S-synthesizing enzyme (3), the relative importance of H2S as a bacterial defense mechanism across a wide range of bacteria may vary and remains to be determined. Baseline levels of H2S production vary between bacterial species, as demonstrated here (S. aureus < E. coli), and may further vary when bacteria are stressed by the host immune response. Additionally, the presence of different pathogenic and virulence factors that can affect the host response and bacterial infectivity may influence the relative importance of H2S in bacterial self-defense. Nonetheless, as the two bacterial species used here differ not only in their pathogenicity and elicitation of specific host responses but also in their utilization of H2S-producing enzymes, the results presented here implicate H2S as a potentially global bacterial defense mechanism against the host immune response that may be targeted in the development of novel antimicrobial agents. Inhibition of bacterial H2S may be a particularly beneficial approach to antimicrobial therapy in patients with inadequate immune functions, such as severe-burn patients. Burn injury induces impairments in both innate and acquired immune functions that can decrease the ability of the patient to respond effectively to an infection (20–22). The finding that both E. coli peritonitis and S. aureus burn wound infections are better controlled in burned mice when the respective bacteria are H2S deficient suggests that bacterial H2S inhibition may have potential as a prophylactic measure to prevent infections in high-risk patients.
While the magnitude of the effects of H2S deficiency on bacterial clearance suggests that H2S inhibition alone would not be sufficient to treat an ongoing infection, it may be useful as an adjunct to increase the efficacy of antibiotics. H2S can also provide resistance for many bacterial species against a broad range of antibiotics (3). Therefore, bacterial H2S is implicated as a potential therapeutic target to enhance bacterial killing by both immune cells and antibiotics. However, the differential dependence of various bacterial species on the different H2S-producing enzymes during the response to infection or antibiotic-induced stress mandates that the H2S-inhibitory strategy be matched to the particulars of the H2S-producing system in the respective bacterial strain(s). For example, the greater effect of PAG (than of AOAA) on the clearance of S. aureus (Fig. 2) suggests that cse activity may be more important for defense of S. aureus against the early immune response. Alternatively, combined treatment with multiple inhibitors that target all 3 primary bacterial H2S-synthesizing enzymes may be more appropriate as a global treatment to be used prophylactically in high-risk patients or in conjunction with antibiotics. Unfortunately, the ability to further advance the current studies is restricted by a lack of inhibitors with specificity for the bacterial enzymes. While the mammalian enzyme inhibitors used here (Fig. 2) show some effects on bacterial H2S production and susceptibility to leukocyte-mediated killing in vitro, the potency of each inhibitor against the mammalian homologues is higher (10). In mammals, H2S is important for a wide range of important biological functions, including cellular bioenergetics and cardiovascular and neuronal functions (1). Additionally, H2S has been reported to have both pro- and anti-inflammatory effects (23, 24). Therefore, these inhibitors are not suitable for the treatment of bacterial infections due to their inhibitory effects on host enzymes. Given the apparent role of bacterial H2S in defense against both the immune response and antibiotics, there is a need for the development of inhibitors that are specific for bacterial H2S-synthesizing enzymes to be considered novel antimicrobial agents.
MATERIALS AND METHODS
Bacterial strains.
Two different wild-type species (and their H2S mutant counterparts) that differ in their utilization of H2S-producing enzymes were used. E. coli (MG1655) is an avirulent Gram-negative rod that primarily utilizes 3-MST, encoded by sseA, for H2S production. To generate 3MST-deficient E. coli (ΔsseA), the sseA gene was excised from E. coli (3) by λInt/Xis site-specific recombination, using the pMWts-λInt/Xis-helper plasmid as described previously (25) and sseA-specific primer sequences (3). The S. aureus strain (USA300) is Gram positive and methicillin resistant and lacks 3MST but carries the cbs-cse operon (3). S. aureus wild-type and Δcbs Δcse strains were obtained from the Nebraska Transposon Mutants Library. Bacteria were grown in Luria-Bertani (LB) broth with shaking (200 rpm) at 37°C, and CFU were determined by plating diluted aliquots on LB agar plates. Growth rate curves were established by measuring the optical density at 600 nm (OD600) over time. Culture medium for the Δcbs Δcse strain was supplemented with 10 µg/ml erythromycin. Bacterial growth and viability in cell culture medium (RPMI 1640 with 10% fetal bovine serum [FBS]) were measured by plating serial dilutions of cultures over time for determination of CFU per milliliter.
Animals.
Male BALB/c mice (10 to 12 weeks of age; Envigo) were housed in a biosafety level 2 (BSL-2) animal facility under the supervision of the University of Texas Medical Branch (UTMB) Animal Resource Center and veterinarians, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Animal care and all procedures were compliant with NIH guidelines for the care and use of experimental animals and were approved by the University of Texas Medical Branch Institutional Animal Care and Use Committee. Some mice received a full-thickness scald burn to approximately 35% of the body surface area, as described previously (13), under deep anesthesia (2% isoflurane) with preemptive analgesia (0.1 mg/kg of body weight buprenorphine). Fluid resuscitation (2 ml lactated Ringer’s solution, i.p.) was administered after injury.
Bacterial clearance assays.
(i) In vitro. For in vitro bacterial elimination assays, total leukocytes were isolated from the spleens of male BALB/c mice as described previously (26). Bacteria were opsonized by incubation with 5% mouse serum (Sigma-Aldrich) at room temperature for 15 min and then incubated at 37°C with leukocytes in RPMI 1640 supplemented with 10% FBS at a multiplicity of infection (MOI) of ∼2 for 45 to 60 min. Back-plating of inocula was performed to confirm that all groups within an experiment received the same starting number of bacteria. To measure bacterial killing by macrophages, RAW 264.7 cells (American Type Culture Collection) were used and maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin (Gibco) at 37°C in a humidified incubator with 5% CO2. Briefly, 1 × 105 RAW cells were seeded onto 13-mm coverslips in 24-well plates and allowed to adhere overnight. Nonadherent cells were removed with Hanks’ balanced salt solution (HBSS), and bacteria were added at an MOI of ∼5. Plates were centrifuged at 500 × g and incubated at 37°C for 1 h. To enumerate total viable bacteria, 0.03% Triton X-100 was added, and serial dilutions were plated. Percent elimination was calculated as [(CFUtime zero − CFUfinal)/CFUtime zero] × 100. To enumerate viable intracellular bacteria, cells were washed twice with HBSS after 1 h of incubation with bacteria, treated with 100 µg/ml gentamicin (Sigma-Aldrich) for 30 min to kill extracellular bacteria, rinsed twice with HBSS, and incubated with 50 µg/ml gentamicin (time zero). After 0, 0.5, 1, and 3 h of incubation, cells were washed twice with HBSS and lysed with 0.3% Triton X-100, and serial dilutions were plated for determination of CFU. In some experiments, bacteria were cultured (8 to 12 h) in the presence of the following reagents (all reviewed in reference 10) prior to inoculation of leukocytes to manipulate bacterial levels of H2S: the slow-release H2S donor GYY4137 [morpholin-4-ium 4 methoxyphenyl(morpholino) phosphinodithioate] (0 to 1 mM; Sigma-Aldrich), the 3-MST inhibitor 2-[(4-hydroxy-6-methylpyrimidin-2-yl) sulfanyl]-1-(naphthalen-1-yl)ethan-1-one (0 to 10 μM; Molport), the CSE inhibitor d-l-propargylglycine (PAG) (0 to 30 mM; Sigma), or the CBS inhibitor amino-oxyacetic acid (AOAA) (0 to 1 mM; Sigma).
(ii) In vivo. To measure bacterial clearance in vivo, mice were injected i.p. with ∼1 × 108 CFU E. coli or 5 × 108 CFU S. aureus, and tissues were harvested 12 to 16 h later. To measure bacterial growth and spread within burn wounds, 1 × 106 CFU S. aureus were injected under the upper half of the burn wound, and the lower half of the wound was harvested 24 h later. Inocula were back-plated to confirm doses. Tissues were homogenized in sterile saline, and serial dilutions were plated on agar. Data for all in vivo inoculation experiments shown are representative of results from 2 to 4 independently performed experiments. To assess systemic inflammation in response to infection, blood was collected when tissues were harvested for cultures, and IL-6 was measured by an enzyme-linked immunosorbent assay (ELISA) (Invitrogen, ThermoFisher).
Bacterial H2S production.
Lead acetate was used to detect and compare H2S production by bacteria under different conditions, as described previously (3), with some modifications. Briefly, equal numbers of bacteria were cultured in 12-well plates at 37°C at 140 rpm overnight. The lid covering the culture plate had filter paper (Bio-Rad), saturated with 2% lead acetate (Sigma-Aldrich), affixed to the inside, above, but not in contact with, the bacterial cultures. H2S reaction with lead acetate produces a brown lead sulfide stain that is visible on the filter paper (3).
Bacterial 3MST activity.
Bacterial 3MST gene (sseA) (NCBI accession number NC_000913.3) was cloned and expressed in an E. coli vector system (pET43.1a) and purified (GenScript Inc., Piscataway, NJ, USA). The effect of the 3-MST inhibitor on the activity of the bacterial enzyme was determined as previously described (27), with modifications. Briefly, the inhibitor was added to assay buffer to yield final concentrations of 1 to 10 μM in a total assay mixture volume of 200 μl. The assay solution contained Tris HCl (50 mM; pH 8.0), bacterial full-length 3MST (sseA) (50 ng/well), the 3-MST substrate 3-mercaptopyruvate (100 µM final concentration), glutathione (2 μM final concentration), and the H2S-specific fluorescent probe 7-azido-4-methylcoumarin (AzMc) (10 μM final concentration). The 96-well plates were incubated at 37°C for 2 h, and the increase in the AzMc fluorescence in each well was read at 450 nm (excitation wavelength [λex] of 365 nm).
Statistical analysis.
Data are presented as means ± standard errors of the means (SEM) and were analyzed using GraphPad Prism 7.0 for Windows. Multiple groups were compared by one-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparison test, and two groups were compared by unpaired Student’s t test. When unequal variances were detected by an F-test, data were log transformed for analyses. A P value of <0.05 was considered statistically significant.
ACKNOWLEDGMENTS
This work was supported by NIH grant 5R21AI124009-02 (to T.T.-K. and C.S.), Shriners Hospitals for Children grants 85400 and 369553 (to T.T.-K. and C.S.), the Blavatnik Family Foundation, DoD grant W81XWH1810625 (to K.S. and E.N.), and the Howard Hughes Medical Institute (E.N.).
W.C., G.T., S.-J.L., and K.S. performed experimental work. T.T.-K., C.S., and E.N. directed and designed experimentation and analyzed data. T.T.-K. and C.S. wrote the manuscript, and E.N. edited the manuscript.
We thank Aaron Cherry for his assistance with animal experiments.
We declare that we have no conflict of interest.
REFERENCES
- 1.Szabo C. 2018. A timeline of hydrogen sulfide (H2S) research: from environmental toxin to biological mediator. Biochem Pharmacol 149:5–19. doi: 10.1016/j.bcp.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kabil O, Banerjee R. 2014. Enzymology of H2S biogenesis, decay and signaling. Antioxid Redox Signal 20:770–782. doi: 10.1089/ars.2013.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shatalin K, Shatalina E, Mironov A, Nudler E. 2011. H2S: a universal defense against antibiotics in bacteria. Science 334:986–990. doi: 10.1126/science.1209855. [DOI] [PubMed] [Google Scholar]
- 4.Mironov A, Seregina T, Nagornykh M, Luhachack LG, Korolkova N, Lopes LE, Kotova V, Zavilgelsky G, Shakulov R, Shatalin K, Nudler E. 2017. Mechanism of H2S-mediated protection against oxidative stress in Escherichia coli. Proc Natl Acad Sci U S A 114:6022–6027. doi: 10.1073/pnas.1703576114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng H, Zhang Y, Palmer LD, Kehl-Fie TE, Skaar EP, Trinidad JC, Giedroc DP. 2017. Hydrogen sulfide and reactive sulfur species impact proteome S-sulfhydration and global virulence regulation in Staphylococcus aureus. ACS Infect Dis 3:744–755. doi: 10.1021/acsinfecdis.7b00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dupré-Crochet S, Erard M, Nüβe O. 2013. ROS production in phagocytes: why, when, and where? J Leukoc Biol 94:657–670. doi: 10.1189/jlb.1012544. [DOI] [PubMed] [Google Scholar]
- 7.Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, Moore PK. 2008. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation 117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 8.Qabazard B, Li L, Gruber J, Peh MT, Ng LF, Kumar SD, Rose P, Tan CH, Dymock BW, Wei F, Swain SC, Halliwell B, Sturzenbaum SR, Moore PK. 2014. Hydrogen sulfide is an endogenous regulator of aging in Caenorhabditis elegans. Antioxid Redox Signal 20:2621–2630. doi: 10.1089/ars.2013.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rose P, Dymock BW, Moore PK. 2015. GYY4137, a novel water-soluble, H2S-releasing molecule. Methods Enzymol 554:143–167. doi: 10.1016/bs.mie.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Szabo C, Papapetropoulos A. 2017. International Union of Basic and Clinical Pharmacology. CII. Pharmacological modulation of H2S levels: H2S donors and H2S biosynthesis inhibitors. Pharmacol Rev 69:497–564. doi: 10.1124/pr.117.014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanaoka K, Sasakura K, Suwanai Y, Toma-Fukai S, Shimamoto K, Takano Y, Shibuya N, Terai T, Komatsu T, Ueno T, Ogasawara Y, Tsuchiya Y, Watanabe Y, Kimura H, Wang C, Uchiyama M, Kojima H, Okabe T, Urano Y, Shimizu T, Nagano T. 2017. Discovery and mechanistic characterization of selective inhibitors of H2S-producing enzyme: 3-mercaptopyruvate sulfurtransferase (3MST) targeting active-site cysteine persulfide. Sci Rep 7:40227. doi: 10.1038/srep40227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asimakopoulou A, Panopoulos P, Chasapis CT, Coletta C, Zhou Z, Cirino G, Giannis A, Szabo C, Spyroulias GA, Papapetropoulos A. 2013. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE). Br J Pharmacol 169:922–932. doi: 10.1111/bph.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bohannon J, Cui W, Cox R, Przkora R, Sherwood E, Toliver-Kinsky T. 2008. Prophylactic treatment with fms-like tyrosine kinase-3 ligand after burn injury enhances global immune responses to infection. J Immunol 180:3038–3048. doi: 10.4049/jimmunol.180.5.3038. [DOI] [PubMed] [Google Scholar]
- 14.Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. 2002. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock 17:463–467. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Goswami M, Sharma D, Khan NM, Checker R, Sandur SK, Jawali N. 2014. Antioxidant supplementation enhances bacterial peritonitis in mice by inhibiting phagocytosis. J Med Microbiol 63(Part 3):355–366. doi: 10.1099/jmm.0.067173-0. [DOI] [PubMed] [Google Scholar]
- 16.Branski LK, Al-Mousawi A, Rivero H, Jeschke MG, Sanford AP, Herndon DN. 2009. Emerging infections in burns. Surg Infect (Larchmt) 10:389–397. doi: 10.1089/sur.2009.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rezaei E, Safari H, Naderinasab M, Aliakbarian H. 2011. Common pathogens in burn wound and changes in their drug sensitivity. Burns 37:805–807. doi: 10.1016/j.burns.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Pittman MS, Corker H, Wu G, Binet MB, Moir AJ, Poole RK. 2002. Cysteine is exported from the Escherichia coli cytoplasm by CydDC, an ATP-binding cassette-type transporter required for cytochrome assembly. J Biol Chem 277:49841–49849. doi: 10.1074/jbc.M205615200. [DOI] [PubMed] [Google Scholar]
- 19.Pálinkás Z, Furtmüller PG, Nagy A, Jakopitsch C, Pirker KF, Magierowski M, Jasnos K, Wallace JL, Obinger C, Nagy P. 2015. Interactions of hydrogen sulfide with myeloperoxidase. Br J Pharmacol 172:1516–1532. doi: 10.1111/bph.12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Church D, Elsayed S, Reid O, Winston B, Lindsay R. 2006. Burn wound infections. Clin Microbiol Rev 19:403–434. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hazeldine J, Hampson P, Lord JM. 2014. The impact of trauma on neutrophil function. Injury 45:1824–1833. doi: 10.1016/j.injury.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 22.Schneider DF, Glenn CH, Faunce DE. 2007. Innate lymphocyte subsets and their immunoregulatory roles in burn injury and sepsis. J Burn Care Res 28:365–379. doi: 10.1097/BCR.0B013E318053D40B. [DOI] [PubMed] [Google Scholar]
- 23.Badiei A, Rivers-Auty J, Ang AD, Bhatia M. 2013. Inhibition of hydrogen sulfide production by gene silencing attenuates inflammatory activity of LPS-activated RAW264.7 cells. Appl Microbiol Biotechnol 97:7845–7852. doi: 10.1007/s00253-013-5080-x. [DOI] [PubMed] [Google Scholar]
- 24.Yuan S, Shen X, Kevil CG. 2017. Beyond a gasotransmitter: hydrogen sulfide and polysulfide in cardiovascular health and immune response. Antioxid Redox Signal 27:634–653. doi: 10.1089/ars.2017.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minaeva NI, Gak ER, Zimenkov DV, Skorokhodova A, Biryukova IV, Mashko SV. 2008. Dual-in/out strategy for genes integration into bacterial chromosome: a novel approach to step-by-step construction of plasmid-less marker-less recombinant E. coli strains with predesigned genome structure. BMC Biotechnol 8:63. doi: 10.1186/1472-6750-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toliver-Kinsky TE, Lin CY, Herndon DN, Sherwood ER. 2003. Stimulation of hematopoiesis by the Fms-like tyrosine kinase 3 ligand restores bacterial induction of Th1 cytokines in thermally injured mice. Infect Immun 71:3058–3067. doi: 10.1128/IAI.71.6.3058-3067.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Druzhyna N, Szczesny B, Olah G, Módis K, Asimakopoulou A, Pavlidou A, Szoleczky P, Gerö D, Yanagi K, Törö G, López-García I, Myrianthopoulos V, Mikros E, Zatarain JR, Chao C, Papapetropoulos A, Hellmich MR, Szabo C. 2016. Screening of a composite library of clinically used drugs and well-characterized pharmacological compounds for cystathionine β-synthase inhibition identifies benserazide as a drug potentially suitable for repurposing for the experimental therapy of colon cancer. Pharmacol Res 113(Part A):18–37. doi: 10.1016/j.phrs.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]