There is established evidence that cytotoxic CD8+ T cells are important mediators of immunity against the bovine intracellular protozoan parasite Theileria parva. However, the mechanism by which the specific CD8+ T cells kill parasitized cells is not understood.
KEYWORDS: cattle, granzyme B, T cells, Theileria parva, cytotoxicity, substrate specificity
ABSTRACT
There is established evidence that cytotoxic CD8+ T cells are important mediators of immunity against the bovine intracellular protozoan parasite Theileria parva. However, the mechanism by which the specific CD8+ T cells kill parasitized cells is not understood. Although the predominant pathway used by human and murine CD8+ T cells to kill pathogen-infected cells is granule exocytosis, involving the release of perforin and granzyme B, there is to date a lack of published information on the biological activities of bovine granzyme B. The present study set out to define the functional activities of bovine granzyme B and determine its role in mediating the killing of T. parva-parasitized cells. DNA constructs encoding functional and nonfunctional forms of bovine granzyme B were produced, and the proteins expressed in Cos-7 cells were used to establish an enzymatic assay to detect and quantify the expression of functional granzyme B protein. Using this assay, the levels of killing of different T. parva-specific CD8+ T cell clones were found to be significantly correlated with the levels of granzyme B protein but not the levels of mRNA transcript expression. Experiments using inhibitors specific for perforin and granzyme B confirmed that CD8+ T cell killing of parasitized cells is dependent on granule exocytosis and, specifically, granzyme B. Further studies showed that the granzyme B-mediated death of parasitized cells is independent of caspases and that granzyme B activates the proapoptotic molecule Bid.
INTRODUCTION
Antigen-specific CD8+ T cell responses have been shown to play a key role in immunity to a number of viral, bacterial, and parasitic infections. One such parasite is the tick-borne protozoan Theileria parva. T. parva infects and transforms bovine lymphocytes, resulting in an acute, often fatal lymphoproliferative disease, which is a major constraint to cattle production in a large part of eastern and southern Africa (1). Following invasion of host lymphocytes, the parasite enters the cytosol, where it develops to the schizont stage, which triggers a number of signaling pathways that promote host cell proliferation and inhibit apoptosis. By associating with the mitotic spindle of the activated lymphocyte, the parasite is able to divide at the same time as the host cell, ensuring that infection is retained in both daughter cells. Hence, the parasite remains in an intracellular location during this stage of development. Cattle that recover from infection with T. parva are solidly immune to subsequent challenge with the same parasite strain but show variable susceptibility to other parasite strains (2). Development of immunity is associated with a potent parasite-specific CD8+ T cell response directed against the parasitized lymphoblasts (3, 4), and transfer of purified CD8+ T cells from immune to naive twin calves has been shown to confer immunity to parasite challenge (5). The mechanism by which CD8+ T cells mediate protection against T. parva is poorly understood. They exhibit strong major histocompatibility complex (MHC)-restricted cytotoxic activity and secrete gamma interferon and tumor necrosis factor alpha; however, unlike other intracellular protozoa (6, 7), these cytokines do not appear to have a direct effector role against the parasite (8). Hence, cytotoxicity is considered likely to have an important role in immunity, although direct evidence for this is lacking, and at present, there is no information on the molecular mediators of cell killing.
As an initial step toward investigating the development of subunit vaccines, T. parva-specific CD8+ T cell lines have successfully been used to identify a number of target antigens by employing high-throughput screens of expressed parasite cDNAs. Although prime-boost immunization of cattle with recombinant poxviruses expressing some of these antigens was found to generate specific CD8+ T cell responses, the immunized animals exhibited only partial protection against parasite challenge. A striking feature of the CD8+ T cells induced by this immunization protocol is that they showed poor cytotoxic activity compared to CD8+ T cells generated by immunization with live parasites, suggesting poor functional differentiation of the T cell response (9). As with similar results derived from other vaccine trials, these findings highlight a paucity of knowledge of the molecular mechanisms that determine the effector function of vaccine-induced CD8+ T cells. Understanding the mechanisms of killing of T. parva-infected cells by bovine CD8 T cells is required to identify relevant molecular markers that can be used to monitor vaccine-induced immune responses and accelerate vaccine development.
Killing of target cells by CD8+ T cells is achieved by release of the contents of secretory lysosomes, known as lytic granules, at the immunological synapse formed upon recognition of class I MHC-bound antigenic peptides by the T cell receptor. Cell killing is initiated by perforin, which creates transient pores in the membrane of the target cell, facilitating uptake into the cytosol of a family of serine proteases known as granzymes. Granzymes exhibit different primary substrate specificities and are able to act on various cellular protein substrates to trigger programmed cell death (10). Five granzymes (A, B, K, H, and M) have been identified in humans; mice express four of these granzymes (A, B, K, and M) and six additional granzymes (C, E, D, F, G, and N) (11). We have recently shown that cattle express the same five granzymes described in humans plus a novel granzyme (designated granzyme O) (12). Granzymes have been classified into three distinct evolutionary groups, based on their primary substrate specificities, namely, trypsin-like (granzymes A and K), chymotrypsin-like (granzymes B, H, C, E, M, D, F, G, and N), and metase-like (granzyme M) granzymes (13). The most extensively studied of these proteases, granzyme B, cleaves aspartic acid residues. In vitro studies have demonstrated that granzyme B induces target cell death by two main pathways, one involving direct proteolytic activation of caspases (leading to DNA damage) and the other by triggering outer mitochondrial membrane permeabilization via cleavage of the proapoptotic protein BH3-interaction domain death agonist (Bid) (14). The relative physiological roles of these activities in vivo remain unclear, particularly in view of the potential functional redundancy among the granzymes. Nevertheless, gene knockout mice deficient in granzyme B have been shown to have reduced levels of CD8+ T cell-mediated cytotoxicity and have increased susceptibility to some viral infections. Despite the residual ability of CD8+ T cells from granzyme B−/− mice to kill target cells, they were unable to induce DNA fragmentation (15). Extrapolation of findings in mice to other mammalian species is also complicated by the finding of differences in protein substrate specificity between murine and human granzyme B; in contrast to human granzyme B, mouse granzyme B is inefficient at cleaving Bid and is therefore believed to rely largely on the direct activation of caspases (16).
In view of the potential importance of CD8+ T cell-mediated cytotoxicity as an effector mechanism against T. parva, the current study set out to examine the biological activity of bovine granzyme B and to investigate its role in CD8+ T cell-mediated killing of T. parva-infected cells. The results demonstrate that granzyme B plays a key role in the killing of parasitized cells, that it is able to cleave Bid, and that killing occurs predominantly by a caspase-independent pathway.
RESULTS
Establishing an in vitro assay of granzyme B activity.
In order to assess the role of granzyme B in the killing of T. parva-infected cells, it was necessary to develop methods for measuring its biological activity. Bovine granzyme B expressed in Cos-7 cells using the pFLAG eukaryotic expression vector (Fig. 1A) was tested for enzymatic activity using a substrate assay employing acetyl (Ac)–IEPD–p-nitroaniline (pNA), which contains a tetrapeptide recognized specifically by human and murine granzyme B. As shown in Fig. 1B, the active form of granzyme B (labeled Function in Fig. 1B, in which the prodipeptide is removed) displayed strong activity against the substrate, whereas the native form (labeled wild type [WT]) and a version containing a mutation in the active tripeptide site (labeled Mutant) were inactive. As a substrate-specific control, the chymotrypsin substrate Suc-GGF-pNA was used in the assay, and no signal was detected with any of the cattle granzyme B constructs.
FIG 1.
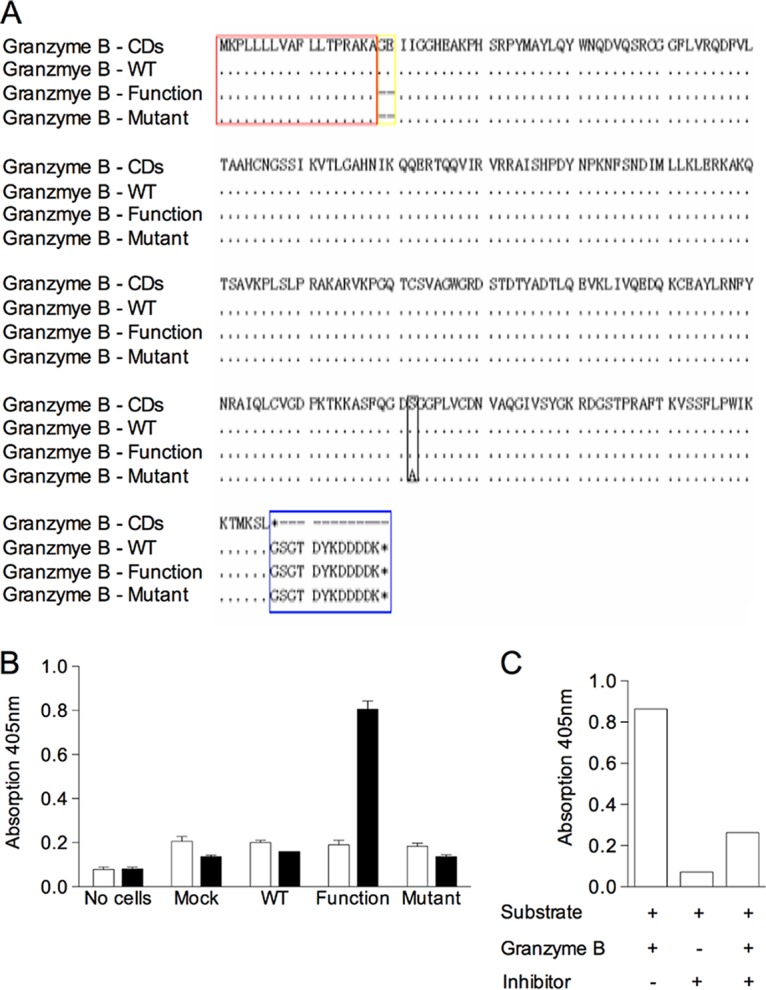
(A) Amino acid sequences from the nucleotide sequences of three recombinant forms of bovine granzyme B cDNA aligned with the reference sequence from the genome database. Granzyme B-CDs, the full-length cDNA from the bovine genome assembly, UMD3.1 (accession number corrected_ENSBTAG00000010057); Granzyme B-WT, the pFLAG-CMV-5a vector containing wild-type granzyme B; Granzyme B-Function, the pFLAG-CMV-5a vector containing functional granzyme B; Granzyme B-Mutant, the pFLAG-CMV-5a vector containing functional granzyme B with a Ser195-to-Ala195 mutation; dots, identical residues; dashes, gaps; red box, leader peptide; yellow box, dipeptide/GE; black box, Ser195Ala; blue box, FLAG epitope tag sequence of the pFLAG-CMV-5a vector. (B) Enzymatic activity of different recombinant forms of bovine granzyme B tested on a granzyme B-specific substrate, Ac-IEPD-pNA (filled bars), and a control substrate, Suc-GGF-pNA (empty bars). Cos-7 cells were transiently transfected with unmodified granzyme B cDNA (WT), cDNA with the GE dipeptide deleted (Function), or cDNA containing a deletion of the dipeptide and an alanine substitution at position 195 (Mutant). The transfection efficiencies of Cos-7 cells with the three granzyme B constructs were 35%, 33%, and 33%, respectively. Lysates of the transfected cells collected after 48 h were incubated with the substrates for 4 h. Controls consisted of lysates of cells transfected with pFLAG without an insert (Mock) and buffer (No cells) added to the substrate. The color reaction generated after 4 h by cleavage of the pNA substrate was measured at a wavelength of 405 nm using a Synergy HT multimode microplate reader (BioTek). (C) Inhibition of the functional recombinant cattle granzyme B by preincubating with 10 μM granzyme B-specific inhibitor Ac-IEPD-CHO for 0.5 h.
To confirm the specificity of the expressed granzyme B, the enzymatic activity was measured in the presence or absence of the granzyme B inhibitor Ac-IEPD-aldehyde (CHO). The specific inhibitor dramatically reduced the activity of the cattle granzyme B preparation by about 4-fold, which was a level close to the background level (Fig. 1C), indicating the effective inhibitory capacity of Ac-IEPD-CHO for cattle granzyme B.
Relationship of cytotoxic activity and granzyme B transcript profiles.
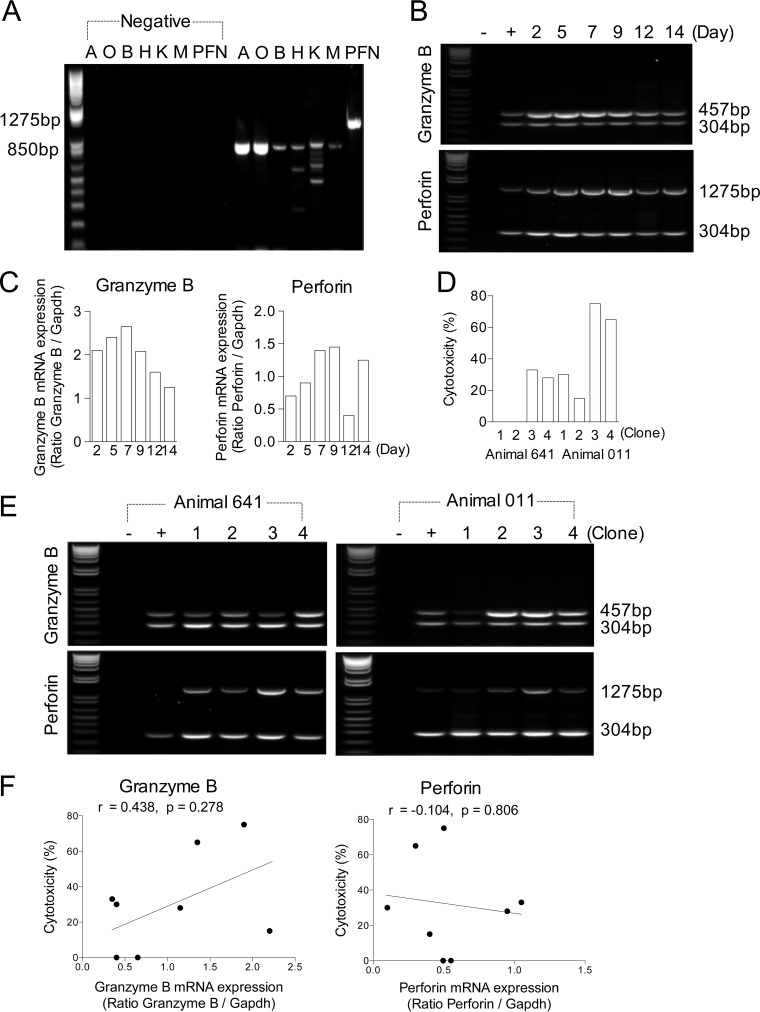
Analysis of cDNA from T. parva-specific CD8+ T cell lines by PCR employing primers that amplify transcripts for six defined bovine granzymes demonstrated expression of the genes for all six, including granzyme B (Fig. 2A). The kinetics of granzyme B mRNA expression were examined using a semiquantitative PCR to determine whether expression was strongly influenced by the time interval after antigenic stimulation. Examination of cDNA prepared from CD8+ T cells at 2- to 3-day intervals at between 2 and 14 days after stimulation with gamma-irradiated T. parva-infected cells demonstrated that near maximal levels of gene expression were achieved between 5 and 7 days after antigenic stimulation, with a subsequent decline in expression (Fig. 2B and C). Cells harvested 6 to 7 days after antigenic stimulation were used for subsequent experiments. To determine whether the levels of killing by CD8+ T cells were related to granzyme B and perforin mRNA expression, CD8+ T cell clones exhibiting different levels of killing were analyzed using a semiquantitative PCR. Two sets of cloned CD8+ T cell lines derived from different animals (animals 641 and 011) were examined; each set of lines expressed identical T cell receptor β chains and recognized the same epitope but exhibited different levels of cytotoxic activity (range, 0% to 75%) on autologous parasitized cells (Fig. 2D). Transcripts for granzyme B and perforin were detected in all 8 T cell clones (Fig. 2E). Overall, there was no consistent pattern of either granzyme B (r = 0.438, P = 0.278) or perforin (r = −0.104, P = 0.806) mRNA transcript expression that correlated with killing activity (Fig. 2F).
FIG 2.
(A) PCR products obtained for each of the bovine granule enzymes from an uncloned T. parva-specific CD8+ T cell line (from animal 641). The sizes of the PCR products obtained were as follows: granzyme A (lane A), 838 bp; granzyme O (lane O), 849 bp; granzyme B (lane B), 818 bp; granzyme H (lane H), 820 bp; granzyme K (lane K), 889 bp; granzyme M (lane M), 833 bp; perforin (lane PFN), 1,275 bp. Negative controls (primers with no added cDNA template) were included in the lane to the left. (B) Agarose gels showing the PCR products for granzyme B (457 bp), perforin (1,275 bp), and the GAPDH control (304 bp). The numbers of days after antigenic stimulation are shown. (C) Changes in the quantity of the PCR product (vertical axis) at different times following antigenic stimulation, normalized in relation to that of the GAPDH product obtained from the same sample. (D) Cytotoxic activity of 8 T. parva-specific CD8+ T cell clones from two different animals (animals 641 and 011) assayed on autologous T. parva-infected targets. (E) Agarose gels showing the PCR products for granzyme B (457 bp), perforin (1,275 bp), and the GAPDH control (304 bp) from 8 T. parva-specific CD8+ T cell clones (D). (F) Correlation of killing of Theileria-infected target cells by CD8+ T cell clones with the levels of mRNA expression of granzyme B (r = 0.438, P = 0.278) and perforin (r = −0.104, P = 0.806). Changes in the quantity of the PCR product (vertical axis) in different T cell clones normalized in relation to that of the GAPDH product obtained from the same sample. (B, E) A negative control (lanes −) without added template and a positive control (lanes +) consisting of primers with a cDNA template of an uncloned T. parva-specific CD8+ T cell line (from animal 641) obtained on day 7 after the 3rd stimulation are included. The density of all PCR amplicon bands was measured by Kodak 1D software (version 3.6). The correlation between variables was analyzed by Pearson’s correlation test. P values of <0.05 were considered significant.
Relationship of cytotoxic activity and level of granzyme B protein expression.
A series of CD8+ T cell clones specific for the same epitope in the Tp1 T. parva antigen from residues 214 to 224 (Tp1214–224) was used to examine the relationship between killing activity and granzyme B protein expression. These CD8+ clones exhibited maximal levels of killing of infected target cells ranging from 1% to 47% at effector-to-target cell ratios of 1:1 or greater (see Fig. S1 in the supplemental material). Assays of granzyme B were conducted at a standard effector-to-target cell ratio of 2:1 to ensure maximal killing activity (Fig. 3A). Granzyme B activity in the culture supernatants and in the cell lysates of these clones following incubation with infected cells was measured using the in vitro substrate-specific assay established in the present study and described above. As shown in Fig. 3A, the T cell clones showed variable levels of granzyme B release following exposure to antigen-expressing cells (which prior assays had confirmed do not express granzyme B protein; data not shown). The levels of granzyme activity in cell supernatants showed a highly significant correlation with the levels of granzyme protein in lysates of the respective clones (r = 0.953, P < 0.0001; Fig. 3B), indicating that the levels of enzyme release reflect the cell content rather than inherent differences in the rates of release during degranulation. The levels of granzyme B of the clones also showed a statistically significant correlation (r = 0.732, P = 0.007) with the levels of cytotoxicity of the T cell clones (Fig. 3C).
FIG 3.
(A) Cytotoxic activity and the levels of granzyme B content and release of 12 T. parva-specific CD8+ T cell clones isolated from two animals (animals 641 and 633) were assayed with autologous T. parva-infected target cells. A standard effector-to-target cell ratio of 2:1 was used. (B, C) Correlation of granzyme B cellular activity with the levels of granzyme B released following antigenic stimulation (r = 0.953, P < 0.0001) (B) and the levels of killing of Theileria-infected target cells by CD8+ T cell clones (r = 0.732, P = 0.007) (C). The correlation between variables was analyzed by Pearson’s correlation test. P values of <0.05 were considered significant.
The cytotoxic activity of T cells is dependent on perforin and granzyme B.
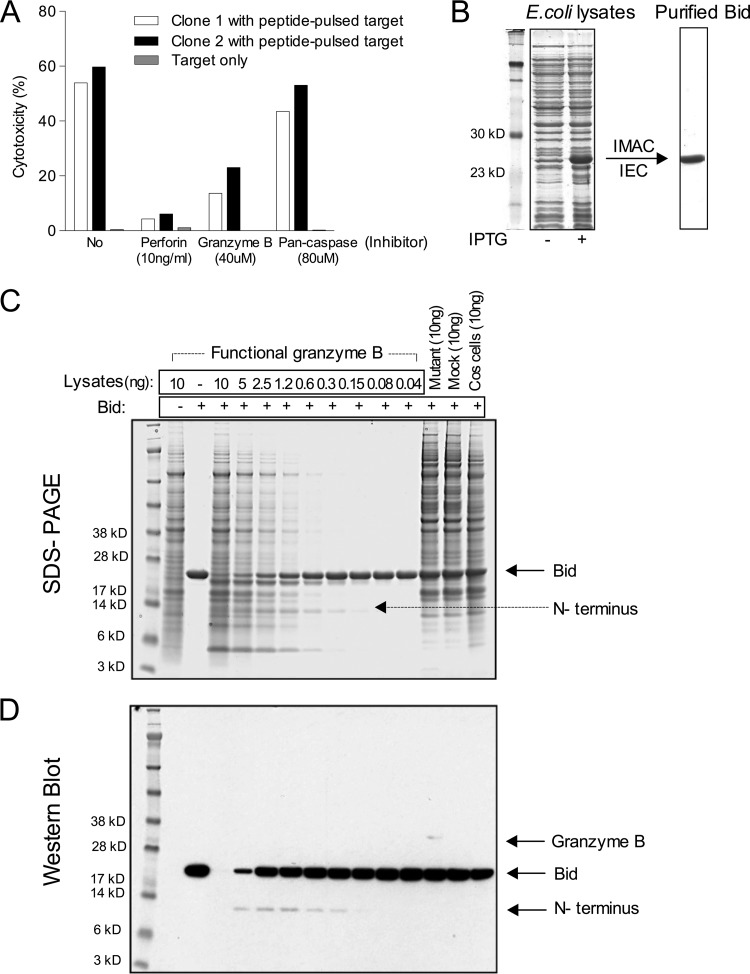
The involvement of lytic granule exocytosis and, specifically, the role of granzyme B in cell killing by bovine CD8+ T cells were investigated by testing the effects of specific inhibitors of perforin and granzyme B. Cytotoxicity assays were first conducted in the presence of a range of concentrations of concanamycin A (CMA), an inhibitor of vacuolar-type H+-ATPase (17), which raises the pH of the lytic granule and, thus, induces the degradation of perforin (18). The effect of CMA on cytotoxic activity was examined using an uncloned CD8+ T cell line assayed on either T. parva-infected or peptide-pulsed target cells and three cloned CD8+ T cell lines assayed on infected target cells. CMA concentrations of 10 ng/ml or greater were found to completely ablate the killing of all T cell lines but did not affect the viability of the target cells (Fig. 4A and B) or the CD8+ T cells (data not shown). The results indicate that lysis of T. parva-infected cells by CD8+ T cells is dependent on perforin, implying that killing is mediated by the release of granule enzymes.
FIG 4.

Inhibition of the cytotoxic activity of an uncloned (bulk) CD8+ T cell line from animal 011 (A) and three CD8+ T cell lines from animal 592 (B) by incubation with the perforin inhibitor concanamycin A (CMA) and three CD8+ T cell lines from animal 641 by incubation with the granzyme B inhibitor Z-IETD-FMK (C). (A, B) Effectors (1 × 104) were preincubated with various concentrations of CMA for 2 h and tested in a 4-h cytotoxicity assay with 111In-labeled autologous TpM target cells and MHC-matched target cells pulsed with a peptide consisting of T. parva antigen Tp2 from residues 49 to 59 (Tp249–59) (1,000 ng/ml). (C) Three cloned CD8+ T cell lines (1 × 104) were preincubated for 1 h with 40 μM Z-IETD-FMK and a negative control, Z-VAD-FMK. Labeled target cells alone were also incubated with the inhibitors in the assay. A standard effector-to-target cell ratio of 2:1 was used.
To examine the role of granzyme B in cell killing, several specific inhibitors used in studies of murine and human CD8+ T cells were first tested for their ability to inhibit granzyme B activity in bovine CD8+ T cell lysates tested using the in vitro substrate-specific assay. Although Ac-IEPD-CHO was the most potent inhibitor, reducing granzyme B activity by approximately 80% (Fig. S2), its lack of membrane permeation prohibits its use in cellular assays. The membrane-permeant agent carbobenzoxy-IETD-fluoromethylketone (Z-IETD-FMK), which inhibits killing by human CD8+ T cells (19, 20), inhibited bovine granzyme B activity by approximately 50% in the substrate assay (Fig. S2) and so was used in subsequent experiments. Preincubation of T. parva-specific CD8+ T cells with Z-IETD-FMK for 1 h prior to use in a cytotoxicity assay resulted in the complete inhibition of the cytotoxic activity of all three cloned T cell lines tested (Fig. 4C). A control compound, carbobenzoxy-valyl-alanyl-aspartyl-(O-methyl)-fluoromethylketone (Z-VAD-FMK; a caspase inhibitor that does not affect the granzyme B activity of effector cells), did not affect cell killing.
In conclusion, these findings reveal that Z-IETD-FMK specifically and effectively blocks the activity of cattle granzyme B and inhibits the killing of target cells by bovine CD8+ T cells, indicating that granzyme B is an important mediator for killing of T. parva-infected cells.
The cytotoxic activity of T cells is not dependent on caspases but is associated with activation of Bid.
To examine the role of caspases in cell killing, experiments were undertaken to test the ability of the pan-caspase inhibitor Z-VAD-FMK and its control, Z-FA-FMK, to block killing by two T. parva-specific CD8+ T cell clones. In contrast to previous experiments in which this inhibitor was preincubated with effector cells (as a negative control), these experiments involved preincubation with the target cells. The cytotoxic activity of the CD8+ T cell clones was blocked by inclusion of inhibitors of perforin and granzyme B (CMA and Z-IETD-FMK, respectively) but was unaffected by preincubation with Z-VAD-FMK (Fig. 5A), demonstrating that the granzyme B-dependent killing by these clones was independent of caspase activity. In contrast, Z-VAD-FMK specifically blocked the lysis of Theileria-infected cells induced by the proapoptotic agent cisplatin (Fig. S3), which is known to mediate cytotoxicity through caspase induction. These results therefore indicate that granzyme B-mediated killing of Theileria-infected cells by specific CD8+ T cells is not dependent on caspases.
FIG 5.
(A) 111In-labeled peptide-pulsed target cells (5 × 103 MHC-matched target cells plus Tp1214–224 at 100 ng/ml) were preincubated with the pan-caspase inhibitor Z-VAD-FMK (80 μM) for 1 h and tested in a 4-h cytotoxicity assay with two Tp1-specific cloned CD8+ T cell lines from animal 641. As controls, effector cells (1 × 104) preincubated with the perforin inhibitor CMA (10 ng/ml) for 2 h or the granzyme B inhibitor Z-IETD-FMK (40 μM) for 1 h were tested in the same experiment. Labeled target cells alone were also incubated with these inhibitors in the assay. A standard effector-to-target cell ratio of 2:1 was used. (B) Expression vector pET-15b carrying an N-terminal His tag sequence followed by the full-length coding sequence of bovine Bid was expressed in E. coli BL21(DE3) in the presence (lane +) or absence (lane −) of IPTG, and the expressed products were purified using automated immobilized metal affinity chromatography (IMAC) and automated ion-exchange chromatography (IEC). The products were separated by SDS-PAGE and visualized by Coomassie blue staining. The predicted size of bovine recombinant Bid is 23.7 kDa. (C, D) Purified recombinant bovine Bid proteins (3 μg) were incubated with the indicated concentrations of active bovine granzyme B for 2 h at 37°C. (C) The reaction products were separated by SDS-PAGE and visualized by Coomassie blue staining. (D) Full-length recombinant Bid and truncated Bid (N terminus) were detected by anti-His tag antibody, and recombinant granzyme B was detected by anti-FLAG M2 antibody in a Western blot. Inactive bovine granzyme B mutant cells (with an alanine substitution at position 195), mock-transfected cells (pFLAG without an insert), and Cos-7 cells alone were included as negative controls for granzyme B proteolysis specificity.
The other known mechanism by which granzyme B induces cell death is through cleavage and, therefore, activation of the proapoptotic molecule Bid. To investigate this, we sought to examine the ability of bovine granzyme B to cleave bovine Bid. Wild-type bovine Bid was expressed in Escherichia coli BL21 with cDNA incorporated into the pET-15b expression vector, which carries an N-terminal His tag sequence. Purified recombinant bovine Bid protein (Fig. 5B) was incubated for 2 h with serially titrated concentrations of the active form of bovine granzyme B (confirmed using the substrate-specific assay), and the reaction products were separated by SDS-PAGE (Fig. 5C). Bovine recombinant Bid was cleaved by active bovine granzyme B, as revealed by the detection of an N-terminal 11-kDa fragment of bovine recombinant Bid of the expected size (based on the predicted cleavage site) by an anti-His tag antibody in a Western blot (Fig. 5D). A reduction in the concentration of bovine granzyme B was associated with a declining ability to cleave bovine recombinant Bid. Additional smaller bands of approximately 5 kDa and 8 kDa, present in the Coomassie blue-stained gels but not detected in the Western blot, may represent additional smaller fragments of Bid. As controls, an inactive form of bovine granzyme B with a serine-to-alanine substitution at position 195, mock-transfected cells (cells transfected with pFLAG without an insert), and Cos-7 cells alone were analyzed; none yielded truncated Bid products, indicating an inability to cleave bovine recombinant Bid. In conclusion, these results demonstrate that bovine granzyme B cleaves Bid, indicating that cytotoxicity may be mediated by activation of Bid.
DISCUSSION
In this study, we aimed to examine the role of bovine granzyme B in the cytotoxic function of T. parva-specific CD8+ T cell responses. To achieve this, we established an in vitro substrate-specific assay to detect and quantify the expression of bovine granzyme B protein, employing recombinant bovine granzyme B expressed in Cos-7 cells. Using this assay, we showed that the levels of killing of different T. parva-specific CD8+ T cell clones are significantly correlated with the levels of granzyme B protein and that the killing of infected cells by bovine CD8+ T cells is mediated by the granule exocytosis pathway and critically requires granzyme B for induction of cell death. Furthermore, we provide evidence that the granzyme B-mediated death of parasitized cells is independent of caspases, suggesting that instead cell death may be induced via activation of Bid, which we show is cleaved by bovine granzyme B.
Granzyme B was selected for analysis in this study as it has been shown to be the most potent effector molecule utilized by CD8+ T cells to kill infected cells in both humans and mice. Due to the lack of prior information on bovine granzyme B, studies of its biological activity were required to investigate its role in the killing of T. parva-infected cells. Results obtained with recombinant bovine granzyme B expressed in Cos-7 cells demonstrated many similarities to its human and murine orthologues. This included evidence that processing of the translated polypeptide is similar to that described for humans and mice, with deletion of the dipeptide/GE being a prerequisite for activation of cattle as well as human and murine granzyme B (21, 22). Similarly, mutation of Ser195, one of the functional triad of residues at the conserved catalytic site (His, Asp, and Ser), was demonstrated to ablate the enzymatic activity of the active form of bovine granzyme B, confirming that, as with the murine and human proteins, this residue is a critical component of the enzyme’s active site (23). These similarities extended to the substrate specificities of the human, murine, and bovine forms of granzyme B, with recombinant mature bovine granzyme B showing the capacity to cleave Ac-IEPD-pNA. This activity forms the basis of a sensitive and reliable in vitro method to measure murine and human granzyme B activity (24).
By exploiting this cross-species similarity, we were able to generate an equivalent assay for cattle and so investigate the levels of biologically active bovine granzyme B and its relation to the cytotoxic activity of bovine CD8+ T cells specific for T. parva-infected cells, overcoming an obstacle posed by the lack of specific antibodies for bovine granzyme B. The demonstration of strong activity against this substrate confirms that cattle granzyme B displays aspase activity, which is a characteristic feature of granzyme B, with no other known serine protease in mammals having a preference for cleaving aspartic acid-containing substrates (25). We also demonstrated that the non-cell-permeant and cell-permeant compounds Ac-IEPD-CHO and Z-IETD-FMK, respectively, which are known inhibitors of human and rodent granzyme B (19, 26), efficiently inhibit bovine granzyme B, further highlighting the cross-species functional similarities. However, the inability of another two inhibitors of human and murine granzyme B (Z-AAD-chloromethylketone and Ac-AAVALLPAVLLALLAPIETD-CHO) to block bovine granzyme B (data not shown) emphasizes that extrapolating functional parameters based on orthology cannot be assumed for granzymes and must be empirically validated.
This also applies to the pathways utilized by granzyme B to mediate killing, which are known to be species dependent. Mouse granzyme B predominantly functions through the direct activation of caspases to promote apoptosis, whereas human granzyme B acts mainly via a Bid-dependent pathway (16, 27). The work described in this study demonstrates that bovine granzyme B, like its human orthologue, is capable of cleaving the Bid protein in vitro, thus providing evidence indicating that Bid activation can potentially be utilized by bovine granzyme B for cell death induction. Although activation of caspases was initially thought to be important in granzyme B-mediated cell death, studies by many groups revealed that the requirement for caspase activation, even in mice, is not absolute. For example, an in vitro study of mouse CD8+ T cells showed that the apoptotic nuclear damage induced by granule exocytosis was abrogated by the caspase inhibitor Z-VAD-FMK, whereas lysis of the cells was unaffected. In contrast, target cell lysis induced by the proapoptotic drug cisplatin was specifically blocked by this inhibitor (28). Similar results have been obtained in studies with purified human granzyme B, with caspase inhibition preventing granzyme-induced DNA damage but not cell lysis (29). These observations are consistent with the results obtained in this study, which showed that Z-VAD-FMK inhibited the cisplatin-induced apoptosis of Theileria-infected cells but did not inhibit the granzyme B-mediated cytolytic activity of cattle CD8+ T cells.
T. parva has been shown to enhance the resistance of infected cells to apoptosis by utilizing NF-κB activation to induce the expression of antiapoptotic proteins, such as FLIP (which functions as a catalytically inactive form of caspase-8) and X-chromosome-linked inhibitor of apoptosis protein (XIAP) and cellular inhibitor of apoptosis (c-IAP) (which block caspase-9 and also downstream executioner caspases 3 and 7) (30). Studies by Guergnon and colleagues in 2003 showed that drug-induced parasite death in Theileria-infected cells results in apoptosis involving activation of caspases 9 and 3 and is inhibited by Z-VAD-FMK (31). These findings confirm that bovine caspases in non-granzyme B-mediated killing are capable of inducing cell death and that Z-VAD-FMK is an effective inhibitor of bovine caspases. The inhibition of killing by T. parva-specific CD8+ T cell clones by Z-IETD-FMK but not Z-VAD-FMK in the current study demonstrates that T cell-mediated killing of T. parva-infected cells is dependent on granzyme B but independent of caspases. Although this may be universally applicable to bovine granzyme B-mediated cytotoxicity, it is important to note that as a consequence of the negative regulation of caspases by intracellular inhibitors induced by the NF-κB pathway in T. parva-infected cells, the apparent redundancy of caspases might be a feature of this specific biological context.
The prime rationale for conducting this study was to better understand the molecular mechanisms that underlie the functional capacity of T. parva-specific CD8+ T cells. The critical role that these cells play in mediating immunological protection against T. parva (29) has led to considerable efforts to identify CD8 T cell target antigens for use in generating novel subunit vaccines (32, 33). A number of T. parva antigens recognized by CD8 T cells from immune cattle have been identified, and although they have proved to be immunogenic when used in prime-boost immunization protocols, the CD8+ T cells elicited generally exhibited poor cytotoxicity and were poorly protective upon in vivo parasite challenge (9). Understanding the discrepancy between immunogenicity and protective efficacy will be critical to defining correlates of protection that can guide subsequent vaccine development. Ongoing work is applying transcriptomics to address this issue. However, such approaches used in isolation have limitations and need to be supplemented by analyses of the functional activities of the specific T cell responses, including the cytotoxic activity of CD8+ T cells. By confirming the central role of granzyme B in the cytotoxic function, this study provides the knowledge and tools that can be used to refine and enhance the immunological evaluation of T cell responses induced in future vaccine trials.
Our data from assays of expressed biologically active granzyme B revealed a statistically significant correlation between the levels of granzyme B enzymatic activity in cell lysates (and supernatants) of cloned CD8+ T cell lines and the levels of killing of T. parva-infected cells. Direct evidence that granzyme B is a dominant effector molecule in CD8+ T cell-mediated killing of these parasitized cells was provided by a subsequent analysis showing that the membrane-permeant inhibitor of granzyme B, Z-IETD-FMK, reduced T. parva-infected cell lysis by these CD8+ T cells by 70% to 100%. The highly significant association of the levels of granzyme B with the cytotoxic activity of a series of cloned CD8+ T cell lines indicates that a relatively high granzyme B cell content is usually required to achieve maximal cell killing. Nevertheless, one clone (clone 2, which was inhibited by the granzyme B inhibitor; Fig. 4C) consistently showed low levels of granzyme B content and release but displayed relatively strong killing (Fig. 3A). The strong killing shown by this single clone, despite the modest granzyme B content, likely reflects variations in other factors that influence cytotoxicity. This does not detract from the overall conclusion from the study that, at the polyclonal T. parva-specific CD8+ T cell response, granzyme B is a critical mediator of cytotoxic function. There is evidence from in vitro studies in humans and mice that other granzymes, in addition to directly mediating cell death in some situations, can synergistically increase the activity of granzyme B. Examples from the literature include (i) cotransfection of rat basophilic leukemia (RBL) cells with granzyme A and granzyme B in the presence of perforin, resulting in enhanced killing of tumor targets in a synergistic manner (34); (ii) human granzyme H augmentation of granzyme B-mediated killing of adenovirus-infected cells (35–37) by neutralizing the viral inhibitor of granzyme B (L4-100K assembly protein) (36, 37); and (iii) the ability of human granzyme M, in addition to inducing the death of tumor cells directly (38–40), to hydrolyze PI-9, thereby inactivating its inhibitory effect on granzyme B (41). Thus, although our finding of strong inhibition of killing by a granzyme B inhibitor indicates that, in general, other granzymes do not play a prominent role in the killing of T. parva-infected cells, there are clear mechanisms by which for individual T cells complementary granzyme activities may contribute to CD8+ T cell killing of T. parva-infected cells. Unfortunately, further investigation of these interactions in cattle is hampered by the current lack of specific antibodies and biological assays to measure other bovine granzyme proteins.
In conclusion, the work described in this paper developed molecular and biochemical methods for measuring the functional activity of bovine granzyme B, in order to determine its role in the killing of T. parva-infected cells by CD8+ T cells. The results provide evidence that the killing of parasitized cells occurs by granule-mediated lysis and is substantially dependent on granzyme B. However, cell killing was shown not to be caspase dependent, and the finding that Bid is cleaved by granzyme B suggests that Bid activation through cleavage is a feasible alternative/parallel killing mechanism. This study represents the first dissection of the effector mechanisms employed in the killing of target cells by bovine CD8+ T cells and specifically provides the first evidence that granzyme B plays a key role in the killing of T. parva-infected cells by specific CD8+ T cells.
MATERIALS AND METHODS
Animals and T cell lines.
Four Holstein-Friesian animals (animals 011, 592, 641, and 633) homozygous for the A10 or A18 MHC class I (MHC-I) haplotype were used for the study. Their MHC types were determined by a combination of serological typing (42) and an MHC-I allele-specific PCR (43). The animals were aged 18 to 36 months at the outset of the study and were maintained indoors on rations of hay and concentrate. Cattle were immunized against the Muguga stock of T. parva (TpM) by infection with cryopreserved sporozoites and the simultaneous administration of a long-acting formulation of oxytetracycline as described previously (2). The animals were challenged with a lethal dose of sporozoites on two occasions at ∼18-month intervals following immunization. All animal experiments were completed in accordance with the Animal (Scientific Procedures) Act 1986. T. parva-specific CD8+ T cell lines and clones were generated from the immune cattle and maintained as described previously (44).
Standard and semiquantitative PCR assays.
Total RNA was extracted from T. parva-specific CD8+ T cell lines from immunized cattle using the TRI Reagent (Sigma), and cDNA was synthesized using a reverse transcription system (Promega) with priming by an oligo(dT)15 primer. Both of these procedures were done according to the manufacturers’ instructions. The primers for granzymes and perforin and the protocols for standard PCRs were as previously described (12). For semiquantitative PCR, the sequences of the primers were as follows: for granzyme B, 5′-ACT GGA ATC AGG ATG TCC AGA G-3′ (forward) and 5′-TTT GGG TCC CCC ACA CAC AG-3′ (reverse), and for GAPDH (glyceraldehyde-3-phosphate dehydrogenase), 5′-ACC CCT TCA TTG ACC TTC AC-3′ (forward) and 5′-TTC ACG CCC ATC ACA AAC ATG-3′ (reverse). The PCR mixtures were composed of 20 pmol of granzyme B/perforin primers and 10 pmol of GAPDH primers, 2.5 units BIOTAQ DNA polymerase (5 units/μl; Bioline), 2.5 μl SM-0005 buffer (ABgene), 0.05 μg of cDNA template, and nuclease-free water to give a final volume of 25 μl. The primers for perforin and the protocol for the PCR program were as described above. The semiquantified PCR products were analyzed by 1.5% agarose gel electrophoresis, and the density of the specific bands was measured with computer software (Kodak 1D software, version 3.6).
Cloning of bovine granzyme B cDNA constructs.
Full-length bovine wild-type (WT) granzyme B was amplified from cDNA by a high-fidelity PCR, using primers flanking the coding sequence, as previously described (12). The PCR mixture used for the high-fidelity protocol was composed of 10 pmol of primers, 1.2 unit Pfu DNA polymerase (3 units/μl; Promega), 10× buffer with MgSO4 (Promega), 10 mM deoxynucleoside triphosphates, 0.5 μg of cDNA template, and nuclease-free water to give a final volume of 50 μl. The program used was as follows: 95°C for 2 min, 30 cycles of 95°C for 1 min followed by 55°C for 0.5 min and 72°C for 2.5 min, and a final extension period of 72°C for 5 min. To generate cDNA encoding active granzyme B, 6 nucleotides encoding a dipeptide segment in the wild-type granzyme B cDNA (which inhibits granzyme B function and is present in pro-granzyme B but absent in fully mature granzyme B) were deleted by PCR splice overlap extension (PCR-SOE), based on procedures described for human granzyme B (21). Briefly, two PCR assays were initially performed to generate two overlapping fragments that carry the 6-nucleotide deletion in the overlapping segment. These reactions utilized the external flanking primers described above with the following internal primers: 5′-CAAAGGCAATCATCGGGGGCCATG-3′ (forward) and 5′-CCCGATGATTGCCTTTGCCCTGGG-3′ (reverse). The resulting two fragments were mixed, denatured, and annealed to produce deletion mutant DNA templates, and amplification of the extended DNAs was performed with flanking primers in a further PCR. Replacement of Ser with Ala at the active site of the dipeptide-knockout cDNA was performed by megaprimer PCR mutagenesis (45, 46) using an internal mutagenic forward primer incorporating the mutation, as follows: 5′-AGAAAGCTTCCTTTCAGGGGGACGCGG-3′. Briefly, an initial 5 cycles of a PCR with a PCR mixture containing 50 pmol of an internal mutagenic forward primer and 2.5 pmol of a flanking reverse primer (as described above) were followed by a prolonged extension step to generate mutant megafragments. Fifty picomoles of the other flanking primer (as described above) was added to the mutant templates, and the PCR was subjected to a further 25 cycles to generate a full-length product containing the mutation. All three bovine granzyme B cDNAs were subcloned into the pFLAG-CMV-5a expression vector (Sigma), and nucleotide sequencing was performed by DBS Genomic (Durham University).
Expression of granzyme B in Cos-7 cells.
Cos-7 cells were maintained in Dulbecco’s minimal essential medium (DMEM; Invitrogen) supplemented with 10% fetal calf serum (FCS), 5 × 10−5 M 2-mercaptoethanol, 4 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C with 5% CO2. Cos-7 cells were transfected in 75-cm2 flasks with the pFLAG-CMV-5a vector containing each of the three cattle granzyme B recombinant cDNAs (the wild type [60 μg], the dipeptide knockout [60 μg], and the knockout with a Ser195Ala substitution [40 μg]) or the vector only (60 μg). The transient transfection was performed by using the Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s protocol. Transfected cells were harvested after 48 h, washed, suspended in cold phosphate-buffered saline (PBS), and analyzed in further experiments. To test for expression of transfected DNA products, cytospin smears of cells were examined microscopically with anti-FLAG M2 antibody (1:500 dilution; IgG1; Sigma). The transfection efficiency of the pFLAG vectors containing three bovine granzyme B recombinant cDNAs (the WT, the dipeptide knockout, and the knockout with an additional Ser195Ala substitution) was 35%, 33%, and 33%, respectively.
Granzyme B protease activity in transfected Cos-7 cells.
Cell lysis and assay of protease activity were performed as previously described for equine granzyme B (47). Briefly, aliquots of 1 ml of PBS-washed Cos-7 cells adjusted to 2 × 106 cells/ml in PBS were pelleted and lysed by addition of 0.2 ml lysis buffer (1% Triton X-100, 50 mM Tris, pH 8.0, 2 μl of 25 U/ml Benzonase nuclease; purity, >99%; Merck). Following incubation on ice for 20 min, the lysed cells were centrifuged at 21,000 × g for 10 min at 0°C to pellet the cell nuclei and other cell debris. Supernatants were harvested and assayed in duplicate for protease activity; aliquots of 25 μl of lysis supernatant, the granzyme B substrate acetyl (Ac)–IEPD–p-nitroaniline (pNA) (Calbiochem) at a final concentration of 300 μM, and reaction buffer (0.1 M HEPES, pH 7.0, 0.3 M NaCl, 1 mM EDTA) in a total volume of 250 μl/well were added into the wells of Falcon 96-well flat-bottomed microplates (BD). Chymotrypsin substrate I, Suc-GGF-pNA (Calbiochem), was used as a negative control for substrate specificity. The reaction mixture was composed of 25 μl of lysis supernatant, Suc-GGF-pNA at a final concentration of 1 mM, and the reaction buffer (50 mM Tris, 100 mM NaCl, pH 8.0) in a total volume of 125 μl. The reaction mixtures were incubated at 37°C for 4 h, and the color reaction generated by cleavage of the pNA substrate was measured at a wavelength of 405 nm by using a Synergy HT multimode microplate reader (BioTek). For inhibition of active bovine granzyme B protease activity in lysates, aliquots of 25 μl of lysis supernatant containing active bovine granzyme B were preincubated with 10 uM Ac-IEPD-CHO (the granzyme B inhibitor; Calbiochem) at 37°C for 0.5 h.
Granzyme B activity in CD8+ T cell lines.
The methods used for measurement of granzyme B in T cell lysates and supernatants were based on procedures previously described for human and equine granzyme B (24, 47). CD8+ T cells washed in PBS were adjusted to 1 × 106 cells/ml in PBS, pelleted, and lysed by addition of 50 μl of a lysis buffer per ml as described above. To examine granzyme B release, aliquots of 1 × 106 CD8+ T cells were distributed into the wells of 96-well V-bottomed plates together with 5 × 105 target cells in a total volume of 200 μl phenol red-free complete medium (RPMI 1640 with 5% FCS; Invitrogen). Control wells containing effector cells and medium were also included. After incubation in an atmosphere of 5% CO2 at 37°C for 4 h, the plates were centrifuged for 10 min at 400 × g and the supernatants were collected. Granzyme B activity was measured by adding aliquots of 10 μl of cell lysates or 40 μl of culture supernatants in duplicate to wells of Falcon 96-well flat-bottomed microplates (BD) together with 200 μM granzyme B substrate, Ac-IEPD-pNA (Calbiochem), and reaction buffer (0.1 M HEPES, pH 7.0, 0.3 M NaCl, 1 mM EDTA) in a total volume of 100 μl/well. Wells containing the reaction buffer and substrate control were also included as controls. The reaction mixtures were incubated at 37°C for 4 h, and the color reaction generated by cleavage of the pNA substrate was measured at a wavelength of 405 nm using a Synergy HT multimode microplate reader (BioTek). To test for the specificity of the reaction, CD8+ T cells were preincubated with the cell-permeant granzyme B inhibitor Z-IETD-FMK (40 μM) for 1 h prior to preparation and testing of cell lysates as describe above. Z-VAD-FMK (40 μM), a pan-caspase inhibitor, was used as a negative control, whereas a non-cell-permeant granzyme B inhibitor, Ac-IEPD-CHO (10 μM), was used to inhibit granzyme B activity in lysates as a positive control.
Cytotoxicity assays.
Standard 4-h 111In-release cytotoxicity assays were used to measure the cytotoxicity of CD8+ T cell clones, using as target cells either autologous T. parva-infected cells or autologous T. annulata-transformed cells incubated with peptide for 0.5 h prior to the assay (44). Peptides were supplied by Pepscan Systems (Lelystad, The Netherlands). All assays were conducted in duplicate, and controls included T. annulata-infected target cells without added peptide and, where appropriate, MHC-mismatched T. parva-infected target cells. Cytotoxicity assays were established, and specific lysis was measured as described previously (44). For inhibition of perforin activity, effector cells were preincubated with 10-fold dilutions of concanamycin A (CMA) at final concentrations ranging from 0.1 μg/ml to 1,000 μg/ml for 2 h at 37°C. For inhibition of granzyme B activity, effector cells were preincubated for 1 h at 37°C with 40 μM Z-IETD-FMK and the negative control, the pan-caspase inhibitor Z-VAD-FMK (40 μM). For inhibition of caspase activity, 111In-labeled target cells were preincubated with 80 μM Z-VAD-FMK and the negative control, cathepsin B inhibitor Z-FA-FMK (80 μM), for 1 h at 37°C.
Generation of recombinant bovine Bid.
Wild-type bovine Bid cDNA was amplified using primers flanking the full-length coding region of bovine Bid, as follows: 5′-TAGCATATGGATTTGAAGGTTA-3′ (forward) and 5′-TGCTGGATCCGAGTGGTCACTCAGTCCAT-3′ (reverse). The amplified PCR products were purified and subcloned into the NdeI and BamHI sites of the pET-15b vector (Novagen), and nucleotide sequencing was performed by DBS Genomic (Durham University). The protocols for expression and purification of recombinant bovine Bid proteins were performed as previously described for human Bid (48). Briefly, pET-15b expression vectors containing wild-type bovine Bid cDNA were transformed in E. coli BL21(DE3)/pLYsS (Novagen) and expressed in the presence of IPTG (isopropyl-β-d-thiogalactopyranoside). The expressed products, which carry an N-terminal His tag sequence, were purified by automated immobilized metal affinity chromatography (IMAC) using a nickel affinity column (Qiagen) and further purified by automated ion-exchange chromatography (IEC) using a Mono Q column (Pharmacia).
Proteolysis of recombinant bovine Bid by bovine granzyme B.
Twofold dilutions of lysates containing active bovine granzyme B at final concentrations ranging from 10 ng to 0.04 ng in 10-μl reaction volumes were incubated with 3 μg of recombinant bovine Bid for 2 h at 37°C. Cells transfected with inactive mutated bovine granzyme B (with an alanine substitution at position 195), mock-transfected cells (transfected with pFLAG without an insert), and Cos-7 cells alone were used as negative controls for granzyme B proteolysis specificity. The reaction products were separated by SDS-PAGE (NuPAGE 4% to 12% bis-Tris gel; Thermo Fisher) and visualized by Coomassie blue staining. The reaction products were transferred using an iBlot system (Thermo Fisher) for Western blotting, according to the manufacturer’s instructions. The blots were probed with anti-His tag antibody (1:2,500 dilution; Thermo Fisher) and anti-FLAG M2 antibody (1:1,000 dilution; Sigma) and detected by chemiluminescence using horseradish peroxidase-labeled rabbit anti-mouse IgG (H + L) secondary antibody (1:5,000 dilution; Thermo Fisher).
Statistical analysis.
Statistical analyses were performed using Minitab software (Minitab, version 15.1.20.0; Minitab Inc.). The correlation between variables was analyzed by Pearson’s correlation test. P values of <0.05 were considered significant.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by a grant awarded by the Bill and Melinda Gates Foundation jointly with the UK Department for International development (DfID) (no. OPP1078791) and a Biotechnology and Biological Sciences Research Council (BBSRC) Institute ISP grant (grant number BB/J004227/1). Jie Yang was supported by a China Scholarship Council/University of Edinburgh scholarship.
We also thank Kathryn Degnan and Robyn Cartwright for expert technical assistance, Andy Gill for technical assistance with protein purification and analysis, and Darren Shaw for statistical help and advice.
Footnotes
[This article was published on 19 December 2018 with a CC BY 4.0 copyright line (“© 2018 Yang et al. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International license.”). The authors elected to remove open access for the article after publication, necessitating replacement of the original copyright line, and this change was made on 24 April 2019.]
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00386-18..
REFERENCES
- 1.Morrison WI, Connelley T, Hemmink JD, MacHugh ND. 2015. Understanding the basis of parasite strain-restricted immunity to Theileria parva. Annu Rev Anim Biosci 3:397–418. doi: 10.1146/annurev-animal-022513-114152. [DOI] [PubMed] [Google Scholar]
- 2.Radley DE, Brown CGD, Burridge MJ, Cunningham MP, Kirimi IM, Purnell RE, Young AS. 1975. East coast fever. 1. Chemoprophylactic immunization of cattle against Theileria parva (Muguga) and five Theileria strains. Vet Parasitol 1:35–41. doi: 10.1016/0304-4017(75)90005-9. [DOI] [Google Scholar]
- 3.Morrison WI, Goddeeris BM, Teale AJ, Groocock CJ, Kemp S, Stagg DA. 1987. Cytotoxic T-cells elicited in cattle challenged with Theileria parva (Muguga): evidence for restriction by class I MHC determinants and parasite strain specificity. Parasite Immunol 9:563–578. doi: 10.1111/j.1365-3024.1987.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 4.Goddeeris BM, Morrison WI, Toye PG, Bishop R. 1990. Strain specificity of bovine Theileria parva-specific cytotoxic T cells is determined by the phenotype of the restricting class I MHC. Immunology 69:38–44. [PMC free article] [PubMed] [Google Scholar]
- 5.McKeever DJ, Taracha EL, Innes E, MacHugh ND, Awino E, Goddeeris BM, Morrison WI. 1994. Adoptive transfer of immunity to Theileria parva in the CD8+ fraction of responding efferent lymph. Proc Natl Acad Sci U S A 91:1959–1963. doi: 10.1073/pnas.91.5.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vassalli P. 1992. The pathophysiology of tumor necrosis factors. Annu Rev Immunol 10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 7.Boehm U, Klamp T, Groot M, Howard JC. 1997. Cellular responses to interferon-gamma. Annu Rev Immunol 15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 8.DeMartini JC, Baldwin CL. 1991. Effects of gamma interferon, tumor necrosis factor alpha, and interleukin-2 on infection and proliferation of Theileria parva-infected bovine lymphoblasts and production of interferon by parasitized cells. Infect Immun 59:4540–4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham SP, Pelle R, Honda Y, Mwangi DM, Tonukari NJ, Yamage M, Glew EJ, de Villiers EP, Shah T, Bishop R, Abuya E, Awino E, Gachanja J, Luyai AE, Mbwika F, Muthiani AM, Ndegwa DM, Njahira M, Nyanjui JK, Onono FO, Osaso J, Saya RM, Wildmann C, Fraser CM, Maudlin I, Gardner MJ, Morzaria SP, Loosmore S, Gilbert SC, Audonnet JC, van der Bruggen P, Nene V, Taracha EL. 2006. Theileria parva candidate vaccine antigens recognized by immune bovine cytotoxic T lymphocytes. Proc Natl Acad Sci U S A 103:3286–3291. doi: 10.1073/pnas.0511273103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jenne DE, Tschopp J. 1988. Granzymes, a family of serine proteases released from granules of cytolytic T lymphocytes upon T cell receptor stimulation. Immunol Rev 103:53–71. doi: 10.1111/j.1600-065X.1988.tb00749.x. [DOI] [PubMed] [Google Scholar]
- 11.Grossman WJ, Revell PA, Lu ZA, Johnson H, Bredemeyer AJ, Ley TJ. 2003. The orphan granzymes of humans and mice. Curr Opin Immunol 15:544–552. doi: 10.1016/S0952-7915(03)00099-2. [DOI] [PubMed] [Google Scholar]
- 12.Yang J, Vrettou C, Connelley T, Morrison WI. 2018. Identification and annotation of bovine granzyme genes reveals a novel granzyme encoded within the trypsin-like locus. Immunogenetics 70:585–597. doi: 10.1007/s00251-018-1062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smyth MJ, O’Connor MA, Trapani JA. 1996. Granzymes: a variety of serine protease specificities encoded by genetically distinct subfamilies. J Leukoc Biol 60:555–562. doi: 10.1002/jlb.60.5.555. [DOI] [PubMed] [Google Scholar]
- 14.Lieberman J. 2003. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol 3:361–370. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- 15.Anthony D, Andrews ADM, Watt SV, Trapani JA, Smyth MJ. 2010. Functional dissection of the granzyme family: cell death and inflammation. Immunol Rev 235:73–92. doi: 10.1111/j.0105-2896.2010.00907.x. [DOI] [PubMed] [Google Scholar]
- 16.Cullen SP, Adrain C, Luthi AU, Duriez PJ, Martin SJ. 2007. Human and murine granzyme B exhibit divergent substrate preferences. J Cell Physiol 176:435–444. doi: 10.1083/jcb.200612025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kataoka T, Shinohara N, Takayama H, Takaku K, Kondo S, Yonehara S, Nagai K. 1996. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J Immunol 156:3678–3686. [PubMed] [Google Scholar]
- 18.Kataoka T, Takaku K, Magae J, Shinohara N, Takayama H, Kondo S, Nagai K. 1994. Acidification is essential for maintaining the structure and function of lytic granules of CTL. Effect of concanamycin A, an inhibitor of vacuolar type H(+)-ATPase, on CTL-mediated cytotoxicity. J Immunol 153:3938–3947. [PubMed] [Google Scholar]
- 19.Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, Houtzager VM, Nordstrom PA, Roy S, Vaillancourt JP, Chapman KT, Nicholson DW. 1997. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem 272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 20.Watchmaker PB, Urban JA, Berk E, Nakamura Y, Mailliard RB, Watkins SC, van Ham SM, Kalinski P. 2008. Memory CD8+ T cells protect dendritic cells from CTL killing. J Immunol 180:3857–3865. doi: 10.4049/jimmunol.180.6.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smyth MJ, McGuire MJ, Thia KY. 1995. Expression of recombinant human granzyme B. A processing and activation role for dipeptidyl peptidase I. J Immunol 154:6299–6305. [PubMed] [Google Scholar]
- 22.Caputo A, Garner RS, Winkler U, Hudig D, Bleackley RC. 1993. Activation of recombinant murine cytotoxic cell proteinase-1 requires deletion of an amino-terminal dipeptide. J Biol Chem 268:17672–17675. [PubMed] [Google Scholar]
- 23.Caputo A, James MN, Powers JC, Hudig D, Bleackley RC. 1994. Conversion of the substrate specificity of mouse proteinase granzyme B. Nat Struct Biol 1:364–367. doi: 10.1038/nsb0694-364. [DOI] [PubMed] [Google Scholar]
- 24.Ewen C, Kane KP, Shostak I, Griebel PG, Bertram EM, Watts TH, Bleackley RC, McElhaney JE. 2003. A novel cytotoxicity assay to evaluate antigen-specific CTL responses using a colorimetric substrate for granzyme B. J Immunol Methods 276:89–101. doi: 10.1016/S0022-1759(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 25.Poe M, Blake JT, Boulton DA, Gammon M, Sigal NH, Wu JK, Zweerink HJ. 1991. Human cytotoxic lymphocyte granzyme B. Its purification from granules and the characterization of substrate and inhibitor specificity. J Biol Chem 266:98–103. [PubMed] [Google Scholar]
- 26.Harris JL, Peterson EP, Hudig D, Thornberry NA, Craik CS. 1998. Definition and redesign of the extended substrate specificity of granzyme B. J Biol Chem 273:27364–27373. doi: 10.1074/jbc.273.42.27364. [DOI] [PubMed] [Google Scholar]
- 27.Kaiserman D, Bird CH, Sun J, Matthews A, Ung K, Whisstock JC, Thompson PE, Trapani JA, Bird PI. 2006. The major human and mouse granzymes are structurally and functionally divergent. J Cell Physiol 175:619–630. doi: 10.1083/jcb.200606073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarin A, Williams MS, Alexander-Miller MA, Berzofsky JA, Zacharchuk CM, Henkart PA. 1997. Target cell lysis by CTL granule exocytosis is independent of ICE/Ced-3 family proteases. Immunity 6:209–215. doi: 10.1016/S1074-7613(00)80427-6. [DOI] [PubMed] [Google Scholar]
- 29.Trapani JA, Jans DA, Jans PJ, Smyth MJ, Browne KA, Sutton VR. 1998. Efficient nuclear targeting of granzyme B and the nuclear consequences of apoptosis induced by granzyme B and perforin are caspase-dependent, but cell death is caspase-independent. J Biol Chem 273:27934–27938. doi: 10.1074/jbc.273.43.27934. [DOI] [PubMed] [Google Scholar]
- 30.Kuenzi P, Schneider P, Dobbelaere DA. 2003. Theileria parva-transformed T cells show enhanced resistance to Fas/Fas ligand-induced apoptosis. J Immunol 171:1224–1231. doi: 10.4049/jimmunol.171.3.1224. [DOI] [PubMed] [Google Scholar]
- 31.Guergnon J, Dessauge F, Langsley G, Garcia A. 2003. Apoptosis of Theileria-infected lymphocytes induced upon parasite death involves activation of caspases 9 and 3. Biochimie 85:771–776. doi: 10.1016/j.biochi.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Nene V, Morrison WI. 2016. Approaches to vaccination against Theileria parva and Theileria annulata. Parasite Immunol 38:724–734. doi: 10.1111/pim.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graham SP, Pelle R, Yamage M, Mwangi DM, Honda Y, Mwakubambanya RS, de Villiers EP, Abuya E, Awino E, Gachanja J, Mbwika F, Muthiani AM, Muriuki AM, Nyanjui JK, Onono JO, Osaso J, Riitho V, Saya RM, Ellis SA, McKeever DJ, MacHugh ND, Gilbert SC, Audonnet JC, Morrison WI, van der Bruggen P, Taracha EL. 2008. Characterization of the fine specificity of bovine CD8 T-cell responses to defined antigens from the protozoan parasite Theileria parva. Infect Immun 76:685–694. doi: 10.1128/IAI.01244-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakajima H, Park HL, Henkart PA. 1995. Synergistic roles of granzymes A and B in mediating target cell death by rat basophilic leukemia mast cell tumors also expressing cytolysin/perforin. J Exp Med 181:1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waterhouse NJ, Trapani JA. 2007. H is for helper: granzyme H helps granzyme B kill adenovirus-infected cells. Trends Immunol 28:373–375. doi: 10.1016/j.it.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Andrade F, Fellows E, Jenne DE, Rosen A, Young CS. 2007. Granzyme H destroys the function of critical adenoviral proteins required for viral DNA replication and granzyme B inhibition. EMBO J 26:2148–2157. doi: 10.1038/sj.emboj.7601650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrade F, Bull HG, Thornberry NA, Ketner GW, Casciola-Rosen LA, Rosen A. 2001. Adenovirus L4-100K assembly protein is a granzyme B substrate that potently inhibits granzyme B-mediated cell death. Immunity 14:751–761. doi: 10.1016/S1074-7613(01)00149-2. [DOI] [PubMed] [Google Scholar]
- 38.Bovenschen N, de Koning PJ, Quadir R, Broekhuizen R, Damen JM, Froelich CJ, Slijper M, Kummer JA. 2008. NK cell protease granzyme M targets alpha-tubulin and disorganizes the microtubule network. J Immunol 180:8184–8191. doi: 10.4049/jimmunol.180.12.8184. [DOI] [PubMed] [Google Scholar]
- 39.Kelly JM, Waterhouse NJ, Cretney E, Browne KA, Ellis S, Trapani JA, Smyth MJ. 2004. Granzyme M mediates a novel form of perforin-dependent cell death. J Biol Chem 279:22236–22242. doi: 10.1074/jbc.M401670200. [DOI] [PubMed] [Google Scholar]
- 40.Cullen SP, Afonina IS, Donadini R, Luthi AU, Medema JP, Bird PI, Martin SJ. 2009. Nucleophosmin is cleaved and inactivated by the cytotoxic granule protease granzyme M during natural killer cell-mediated killing. J Biol Chem 284:5137–5147. doi: 10.1074/jbc.M807913200. [DOI] [PubMed] [Google Scholar]
- 41.Mahrus S, Kisiel W, Craik CS. 2004. Granzyme M is a regulatory protease that inactivates proteinase inhibitor 9, an endogenous inhibitor of granzyme B. J Biol Chem 279:54275–54282. doi: 10.1074/jbc.M411482200. [DOI] [PubMed] [Google Scholar]
- 42.Ellis SA, Morrison WI, MacHugh ND, Birch J, Burrells A, Stear MJ. 2005. Serological and molecular diversity in the cattle MHC class I region. Immunogenetics 57:601–606. doi: 10.1007/s00251-005-0027-8. [DOI] [PubMed] [Google Scholar]
- 43.Ellis SA, Staines KA, Stear MJ, Hensen EJ, Morrison WI. 1998. DNA typing for BoLA class I using sequence-specific primers (PCR-SSP). Eur J Immunogenet 25:365–370. [DOI] [PubMed] [Google Scholar]
- 44.Goddeeris BM, Morrison WI. 1988. Techniques for the generation, cloning, and characterization of bovine cytotoxic T cells specific for the protozoan Theileria parva. J Tissue Culture Methods 11:101–110. doi: 10.1007/BF01404140. [DOI] [Google Scholar]
- 45.Tyagi R, Lai R, Duggleby RG. 2004. A new approach to ‘megaprimer’ polymerase chain reaction mutagenesis without an intermediate gel purification step. BMC Biotechnol 4:2. doi: 10.1186/1472-6750-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Picard V, Ersdal-Badju E, Lu A, Bock SC. 1994. A rapid and efficient one-tube PCR-based mutagenesis technique using Pfu DNA polymerase. Nucleic Acids Res 22:2587–2591. doi: 10.1093/nar/22.13.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piuko K, Bravo IG, Muller M. 2007. Identification and characterization of equine granzyme B. Vet Immunol Immunopathol 118:239–251. doi: 10.1016/j.vetimm.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481–490. doi: 10.1016/S0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.