Acinetobacter baumannii is a Gram-negative opportunistic pathogen that causes diverse infections, including pneumonia, bacteremia, and wound infections. Due to multiple intrinsic and acquired antimicrobial-resistance mechanisms, A. baumannii isolates are commonly multidrug resistant, and infections are notoriously difficult to treat.
KEYWORDS: Acinetobacter baumannii, hydrogen peroxide, OxyR, RNA sequencing, in vivo imaging, transcriptional regulation
ABSTRACT
Acinetobacter baumannii is a Gram-negative opportunistic pathogen that causes diverse infections, including pneumonia, bacteremia, and wound infections. Due to multiple intrinsic and acquired antimicrobial-resistance mechanisms, A. baumannii isolates are commonly multidrug resistant, and infections are notoriously difficult to treat. The World Health Organization recently highlighted carbapenem-resistant A. baumannii as a “critical priority” for the development of new antimicrobials because of the risk to human health posed by this organism. Therefore, it is important to discover the mechanisms used by A. baumannii to survive stresses encountered during infection in order to identify new drug targets. In this study, by use of in vivo imaging, we identified hydrogen peroxide (H2O2) as a stressor produced in the lung during A. baumannii infection and defined OxyR as a transcriptional regulator of the H2O2 stress response. Upon exposure to H2O2, A. baumannii differentially transcribes several hundred genes. However, the transcriptional upregulation of genes predicted to detoxify hydrogen peroxide is abolished in an A. baumannii strain in which the transcriptional regulator oxyR is genetically inactivated. Moreover, inactivation of oxyR in both antimicrobial-susceptible and multidrug-resistant A. baumannii strains impairs growth in the presence of H2O2. OxyR is a direct regulator of katE and ahpF1, which encode the major H2O2-degrading enzymes in A. baumannii, as confirmed through measurement of promoter binding by recombinant OxyR in electromobility shift assays. Finally, an oxyR mutant is less fit than wild-type A. baumannii during infection of the murine lung. This work reveals a mechanism used by this important human pathogen to survive H2O2 stress encountered during infection.
INTRODUCTION
Acinetobacter baumannii is a Gram-negative coccobacillus and an obligate aerobe. A. baumannii is best known as an agent of ventilator-associated pneumonia and bloodstream infections in critically ill patients but also causes hospital-acquired infections of virtually any body site, including skin and soft tissue infections, wound infections, urinary tract infections, and meningitis (1, 2). Since the original recognition of A. baumannii as a frequent opportunistic pathogen, this organism has emerged as one of the most difficult bacterial infectious agents to treat because of extensive intrinsic and evolved antimicrobial resistance (1, 3–6). Globally, 63% of A. baumannii isolates were found to be multidrug resistant in 2014, an increase from 23% in 2004 (7). In particular, the threat to global human health from increasing rates of carbapenem-resistant A. baumannii infections has led the World Health Organization to name carbapenem-resistant A. baumannii the number one “critical” priority for the development of new antibiotics (8). Therefore, research investigating mechanisms by which A. baumannii survives within hosts and in the environment is urgently needed as a strategy to identify new targets for therapeutic intervention.
A. baumannii does not typically use classical virulence determinants such as effector toxins or immune evasion strategies (3, 9). Instead, A. baumannii utilizes genes that have evolved for survival in the environment to persist during infection (9, 10). Moreover, the ability of A. baumannii to survive within the hospital environment permits this organism to cause outbreaks of hospital-associated infections that are difficult to eradicate (11). We therefore predict that processes involved in sensing and responding to the environment are critical for A. baumannii to be a successful opportunistic pathogen.
During infection, bacteria experience numerous stresses imposed by the host. One stress thought to be encountered during infection is reactive oxygen species produced by effector cells of the innate immune system (12). Neutrophils treated with various proinflammatory stimuli ex vivo generate an oxidative burst initiated by NADPH phagocyte oxidase that results in the production of superoxide, H2O2, hypochlorous acid, and peroxynitrate (12). Mice lacking NADPH phagocyte oxidase are more susceptible to A. baumannii pneumonia (13). Therefore, we hypothesized that H2O2 is formed in the lung during A. baumannii pneumonia and thus is a stress that A. baumannii must overcome in order to survive and infect the lung.
Bacteria have evolved mechanisms to sense damaging molecules and induce an appropriate transcriptional response, such as upregulation of enzymes that detoxify reactive oxygen species. Transcriptional regulators that sense either the reactive molecule itself or the damage caused by reactive molecules control the production of detoxification proteins (14). H2O2 is detoxified by alkyl hydroperoxide reductase (Ahp) and catalase (Kat), which reduce H2O2 to water (15). In A. baumannii, the catalase genes katE and katG and the universal stress protein UspA protect against H2O2 stress (16, 17). However, the regulatory mechanisms that govern the transcriptional response of A. baumannii to H2O2 have not been characterized.
In Gram-negative bacteria, OxyR is the canonical orchestrator of the H2O2 detoxification response (18). OxyR is a highly conserved transcriptional regulator of the LysR family that senses and responds to H2O2 stress (19–21). Oxidation of a conserved cysteine residue causes OxyR to undergo a conformational change, resulting in altered DNA binding (21–25). In Escherichia coli, OxyR serves as a transcriptional activator of genes involved in H2O2 detoxification, including ahp, which encodes alkyl hydroperoxide reductase; kat, which encodes catalase; and dps, which encodes a protein for DNA protection during starvation (19, 26). While OxyR is highly conserved, the function of this protein differs between organisms. For instance, while OxyR is classically described as a transcriptional activator, OxyR in Corynebacterium glutamicum is a transcriptional repressor, and inactivation of oxyR enhances resistance to H2O2 in this organism (27). There are also discrepancies regarding the sensitivity of oxyR mutants to H2O2 killing during log-phase growth. Xanthomonas campestris lacking oxyR is sensitive to H2O2 during log-phase growth, but Neisseria meningitidis and Brucella abortus inactivated for oxyR are resistant to H2O2 killing in exponential growth (28–30). While oxyR homologues in A. baumannii and other Acinetobacter species have been identified and shown to confer resistance to H2O2 on agar plates, the functional role of A. baumannii OxyR in transcriptional regulation and survival in vivo has not been studied (31–33).
We hypothesized that A. baumannii encounters H2O2 stress during infection and that OxyR controls the transcriptional response to this stress. In this study, we demonstrate that H2O2 is formed during A. baumannii infection of the lung, and we characterize the transcriptional response of A. baumannii to H2O2 by RNA sequencing. Furthermore, we define the role of A. baumannii oxyR in defense against H2O2 stress, determine the regulon of OxyR by RNA sequencing, and confirm direct promoter binding by recombinant OxyR. Finally, we study the role of oxyR in A. baumannii infection of the murine lung. By identifying the role and regulon of OxyR in A. baumannii, we have defined a transcriptional regulatory network in this important human pathogen that confers resistance to the stresses it encounters during infection. These findings provide a foundation for the development of novel therapeutics against A. baumannii infections.
RESULTS
A. baumannii lung infection induces H2O2 production.
H2O2 is produced by the neutrophil oxidative burst (12), and neutrophils are recruited in response to A. baumannii infection (34). In order to test our hypothesis that H2O2 functions as an antimicrobial during A. baumannii lung infection, we first wanted to determine if we could directly visualize H2O2 production in vivo. Thus, we employed a recently developed bioluminescence system in which a caged luciferin probe specifically reacts with H2O2 to release d-luciferin, which leads to bioluminescence in mice that constitutively express luciferase (Fig. 1A) (35, 36). Upon intranasal inoculation with A. baumannii ATCC 17978, substantial bioluminescence was detected in the thoracic cavity, whereas minimal bioluminescence was detected following mock infection with phosphate-buffered saline (PBS) (Fig. 1B and C). Based on these data, we conclude that infection with A. baumannii stimulates the production of H2O2 in the lung.
FIG 1.
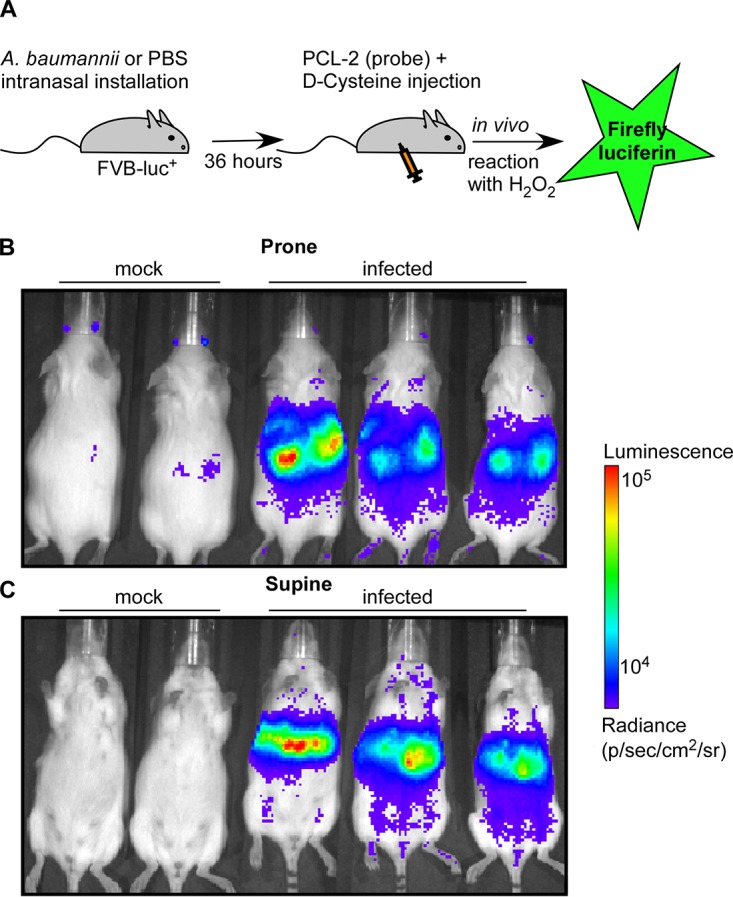
H2O2 is produced during A. baumannii lung infection. (A) Schematic of the experimental design. Mice were either infected with WT A. baumannii or mock infected with PBS. Imaging was performed 36 h postinfection. The production of H2O2 was monitored in vivo by injection of a caged luciferin probe (PCL-2) that specifically reacts with H2O2 to generate bioluminescence (35). (B and C) Mice were placed in the prone position (B) and in the supine position (C). The results are representative of two independent experiments.
H2O2 exposure alters the transcriptome of A. baumannii in an oxyR-dependent manner.
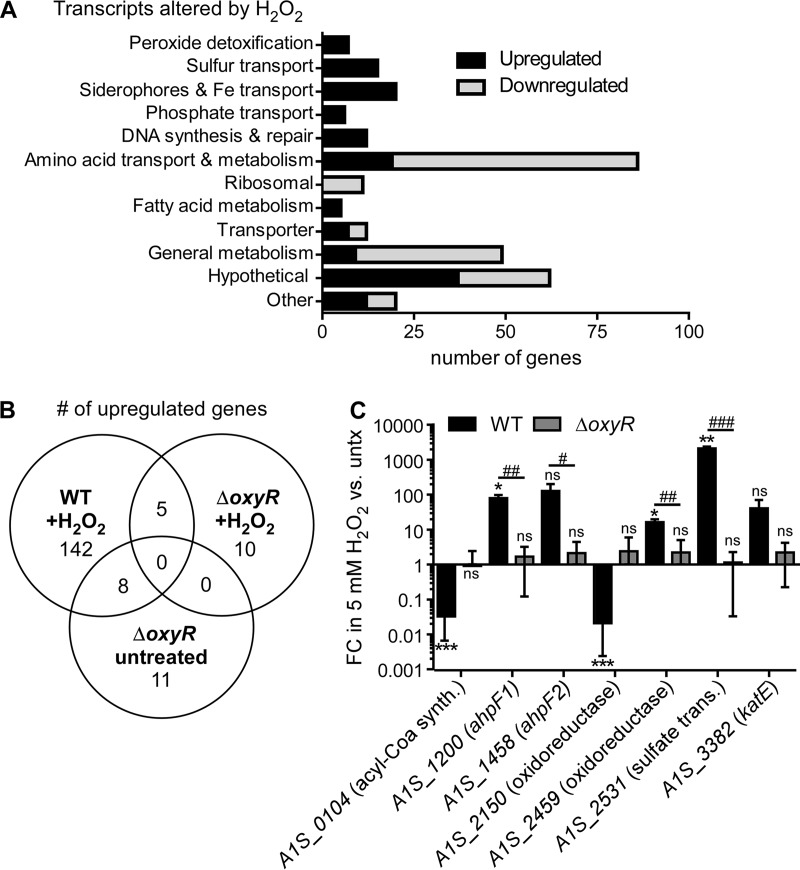
Considering the success of A. baumannii as a pathogen, this organism must survive an onslaught of H2O2 produced in vivo. The mechanisms by which A. baumannii defends against H2O2 are not defined. We characterized the response of A. baumannii to H2O2 exposure by RNA sequencing. Compared to mock-treated cells, cells exposed to H2O2 upregulated the transcription of 155 genes and downregulated the transcription of 151 genes (Fig. 2A and B; Table 1; see also Table S2 in the supplemental material). Genes encoding proteins that directly detoxify H2O2, including ahpF1 (A1S_1200–1201), ahpF2 (A1S_1458–1460), and katE (A1S_3382), were strongly upregulated. Genes involved in sulfur transport and iron homeostasis were also highly upregulated by H2O2 exposure, which we postulate occurs in response to the oxidation of iron-sulfur (Fe-S) clusters by H2O2 (15). Amino acid transport and metabolism pathways were also upregulated by H2O2, perhaps as a repair or replacement mechanism for proteins that sustained oxidative damage. A. baumannii also increases the transcription of phosphate transport and nucleic acid synthesis and repair genes, a response consistent with H2O2-induced DNA damage (15). Treatment with H2O2 predominantly downregulates metabolic and synthetic genes, suggesting a model in which most cellular metabolism is paused while the acute toxicity is managed (Table S2 in the supplemental material).
FIG 2.
H2O2 exposure alters the transcriptome of A. baumannii in an oxyR-dependent manner. (A to C) RNA sequencing was performed on RNA extracted from WT A. baumannii and the ΔoxyR strain, which were grown to mid-exponential phase and treated with 5 mM H2O2 for 10 min. (A) Classification of transcripts significantly up- or downregulated in WT A. baumannii treated with H2O2 relative to expression in untreated cells (>2 log2-fold change; P, <0.05, corrected for the FDR). (B) Venn diagram comparing common upregulated transcripts under the following conditions: the WT strain plus H2O2 (relative to the untreated WT strain), the ΔoxyR strain plus H2O2 (relative to the untreated ΔoxyR strain), and the untreated ΔoxyR strain (relative to the untreated WT strain). (C) Validation of selected RNA sequencing results by qRT-PCR in WT A. baumannii and the ΔoxyR strain treated with H2O2. Results are depicted as the fold change (FC) in gene transcript abundance following a 10-min treatment with 5 mM H2O2 relative to no treatment (untx). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (by t test against a theoretical value of 1.0). #, P < 0.05; ##, P < 0.01; ###, P < 0.001 (by a t test comparing the WT and the ΔoxyR strain). Results are means ± standard deviations for three biological replicates.
TABLE 1.
Genes with increased expression in WT A. baumannii following treatment with H2O2
| Category and locus | Annotation (KEGG)a | Fold change |
|---|---|---|
| Peroxide detoxification | ||
| A1S_1459 | Alkyl hydroperoxide reductase subunit F (ahpF2) | 61.9 |
| A1S_1200 | Alkyl hydroperoxide reductase subunit F (ahpF1) | 49.3 |
| A1S_1458 | Alkyl hydroperoxide reductase subunit F (ahpF2) | 47.2 |
| A1S_1201 | Alkyl hydroperoxide reductase subunit F (ahpF1) | 40.3 |
| A1S_1460 | Alkyl hydroperoxide reductase subunit F (ahpF2) | 35.7 |
| A1S_3382 | Catalase (katE) | 27.7 |
| A1S_2863 | Putative antioxidant protein | 5.9 |
| Sulfur homeostasis | ||
| A1S_2533 | Putative esterase | 459.7 |
| A1S_2532 | Sulfate transport protein | 364.5 |
| A1S_2531 | Sulfate transport system substrate-binding protein | 334.4 |
| A1S_2534 | Sulfate transport system permease protein | 172.9 |
| A1S_0030 | Sulfonate transport system substrate-binding protein | 165.8 |
| A1S_0028 | Alkanesulfonate monooxygenase | 113.7 |
| A1S_2535 | Sulfate transport system permease protein | 106.1 |
| A1S_0029 | Sulfonate transport system substrate-binding protein | 71.2 |
| A1S_0027 | Sulfonate transport system permease protein | 49.6 |
| A1S_3207 | Sulfate transport system substrate-binding protein | 46.0 |
| A1S_1408 | Putative rhodanese-related sulfurtransferase | 25.5 |
| A1S_2536 | Sulfate transport system ATP-binding protein | 18.1 |
| A1S_0977 | Arylsulfatase | 18.0 |
| A1S_3306 | Dimethylsulfone monooxygenase | 17.9 |
| A1S_0026 | Sulfonate transport system ATP-binding protein | 16.2 |
| A1S_2846 | Sulfite reductase (NADPH) hemoprotein beta-component | 5.6 |
| Iron homeostasis | ||
| A1S_2389 | Iron complex transport system permease protein | 26.2 |
| A1S_2381 | 2,3-Dihydroxybenzoate-AMP ligase | 21.8 |
| A1S_1719 | 4Fe-4S ferredoxin iron-sulfur binding | 21.4 |
| A1S_2380 | Bifunctional isochorismate lyase | 18.2 |
| A1S_2379 | Histidine decarboxylase | 16.3 |
| A1S_2382 | BasD | 16.0 |
| A1S_2388 | Iron complex transport system permease protein | 15.8 |
| A1S_2387 | Iron complex transport system ATP-binding protein | 14.9 |
| A1S_2390 | Putative acinetobactin biosynthesis protein | 8.7 |
| A1S_2372 | Isochorismate synthase | 8.2 |
| A1S_2386 | Iron complex transport system substrate-binding protein | 7.4 |
| A1S_2383 | Putative acinetobactin biosynthesis protein | 6.9 |
| A1S_2392 | Putative acinetobactin utilization protein | 6.1 |
| A1S_2077 | Putative outer membrane porin receptor for Fe(III)-coprogen, Fe(III)-ferrioxamine B, and Fe(III)-rhodotorulic acid uptake (fhuE) | 5.7 |
| A1S_3339 | Iron complex outer membrane receptor protein | 5.4 |
| A1S_1607 | Iron complex outer membrane receptor protein | 4.6 |
| A1S_1647 | Putative siderophore biosynthesis protein | 4.4 |
| A1S_1359 | Iron(III) transport system substrate-binding protein | 4.3 |
| A1S_2530 | 4-Amino-4-deoxychorismate lyase | 4.1 |
| A1S_1634 | Rrf2 family transcriptional regulator | 4.1 |
| Phosphate transport | ||
| A1S_2448 | Phosphate transport system substrate-binding protein | 10.5 |
| A1S_2447 | Phosphate transport system permease protein | 6.8 |
| A1S_3374 | Positive Pho regulon response regulator | 6.0 |
| A1S_3376 | Phosphate regulon sensor histidine kinase PhoR | 4.7 |
| A1S_0256 | Phosphate transport system protein | 4.4 |
| Nucleic acid synthesis and repair | ||
| A1S_2273 | RNA 3′-terminal phosphate cyclase (ATP) | 26.4 |
| A1S_2586 | dGTP triphosphohydrolase | 8.6 |
| A1S_0310 | Excinuclease ABC subunit C | 6.4 |
| A1S_1173 | DNA polymerase V | 5.3 |
| A1S_2008 | DNA polymerase V | 4.8 |
| A1S_0636 | DNA polymerase V | 4.8 |
| A1S_1174 | DNA polymerase V | 4.6 |
| A1S_1962 | Recombination protein RecA | 4.4 |
| A1S_3295 | Excinuclease ABC subunit A | 4.2 |
| A1S_3359 | Topoisomerase IV subunit B | 4.1 |
| Amino acid transport and metabolism | ||
| A1S_1443 | Taurine transport system ATP-binding protein | 222.8 |
| A1S_0023 | Putative malic acid transport protein | 135.0 |
| A1S_1397 | Polar amino acid transport system permease protein | 102.2 |
| A1S_1444 | Taurine transport system permease protein | 87.3 |
| A1S_1442 | Taurine transport system substrate-binding protein | 85.9 |
| A1S_1396 | Polar amino acid transport system permease protein | 76.5 |
| A1S_1445 | Taurine dioxygenase | 41.8 |
| A1S_1398 | Polar amino acid transport system ATP-binding protein | 27.1 |
| A1S_0921 | Arginine:ornithine antiporter/lysine permease | 14.7 |
| A1S_1399 | Polar amino acid transport system substrate-binding protein | 11.4 |
| A1S_1485 | d-Methionine transport system substrate-binding protein | 9.9 |
| A1S_2384 | Lysine N6-hydroxylase | 6.6 |
| A1S_1046 | Lysine exporter | 6.3 |
| A1S_1407 | Serine O-acetyltransferase | 6.1 |
| A1S_1400 | Polar amino acid transport system substrate-binding protein | 4.8 |
| Ribosome | ||
| A1S_2271 | tRNA-splicing ligase RtcB | 118.6 |
| A1S_1961 | Ribosome-associated heat shock protein Hsp15 | 5.1 |
| Fatty acid metabolism | ||
| A1S_1436 | Putative acyl-CoA dehydrogenase | 7.8 |
| A1S_1437 | Putative acyl-CoA dehydrogenase | 7.7 |
| A1S_2458 | Linoleoyl-CoA desaturase | 7.5 |
| A1S_0394 | Putative acyl-CoA dehydrogenase | 7.4 |
| Transport | ||
| A1S_1456 | Chromate transporter | 23.1 |
| A1S_1457 | Chromate transporter | 13.1 |
| A1S_2378 | Putative ABC transporter | 8.4 |
| A1S_1720 | NitT/TauT family transport system substrate-binding protein | 8.2 |
| A1S_3272 | MFS transporter, YNFM family, putative membrane transport protein | 5.7 |
| A1S_2377 | Putative ABC transporter | 5.4 |
| A1S_2311 | ABC-2 type transport system ATP-binding protein | 4.4 |
| A1S_1772 | MFS transporter, DHA2 family, multidrug resistance protein | 4.2 |
| Metabolism (other) | ||
| A1S_1488 | Putative acyl-CoA dehydrogenase | 100.9 |
| A1S_1487 | Putative acyl-CoA dehydrogenase | 30.0 |
| A1S_3305 | FMN reductase | 24.6 |
| A1S_1486 | Putative monooxygenase (DszA-like) | 24.5 |
| A1S_0922 | Putative homocysteine S-methyltransferase family protein | 19.9 |
| A1S_0393 | Putative acyl-CoA dehydrogenase | 15.3 |
| A1S_0024 | Adenosylhomocysteine nucleosidase | 10.9 |
| A1S_0463 | Putative alkaline phosphatase | 9.0 |
| A1S_2459 | Putative oxidoreductase | 8.3 |
| A1S_3222 | Homocysteine synthase | 6.9 |
| A1S_2293 | Ferredoxin/flavodoxin–NADP+ reductase | 5.5 |
| A1S_1446 | Allantoin racemase | 4.4 |
| A1S_0131 | Streptomycin 3"-adenylyltransferase | 4.3 |
| Other | ||
| A1S_1717 | GntR family transcriptional regulator | 32.1 |
| A1S_1414 | LrgB-like protein | 27.5 |
| A1S_0466 | Sec-independent protein translocase protein TatA | 25.0 |
| A1S_1665 | Putative membrane protein | 19.9 |
| A1S_3114 | CBS domain-containing membrane protein | 19.0 |
| A1S_1677 | Putative porin precursor | 17.5 |
| A1S_3381 | AnkB protein | 17.4 |
| A1S_0465 | Sec-independent protein translocase protein TatB | 16.1 |
| A1S_1963 | Regulatory protein | 13.2 |
| A1S_0464 | Sec-independent translocation protein TatC | 12.8 |
| A1S_1928 | Putative signal peptide | 12.4 |
| A1S_2473 | Transcriptional regulator, LysR family | 10.4 |
| A1S_1224 | Transposase | 8.9 |
| A1S_0416 | Putative transcriptional regulator (LysR family) | 5.9 |
| A1S_3326 | Putative membrane protein | 5.4 |
| A1S_1438 | Putative coenzyme F420-dependent N5,N10-methylene tetrahydromethanopterin reductase | 5.2 |
| A1S_3375 | Phosphate regulon response regulator PhoB | 5.2 |
| A1S_1539 | ArsR family transcriptional regulator | 4.7 |
| A1S_1767 | Putative acid phosphatase | 4.7 |
| A1S_1503 | Transmembrane pair | 4.5 |
| A1S_3360 | Putative esterase | 4.3 |
| A1S_2319 | Putative membrane protein | 4.2 |
| A1S_0565 | Putative membrane protein | 4.2 |
| A1S_1197 | Putative extracellular nuclease | 4.1 |
| Hypothetical | ||
| A1S_0517 | Hypothetical protein | 139.3 |
| A1S_3813 | Hypothetical protein | 73.1 |
| A1S_3666 | Hypothetical protein | 47.7 |
| A1S_3906 | Hypothetical protein | 43.9 |
| A1S_1718 | Hypothetical protein | 30.1 |
| A1S_2272 | Hypothetical protein | 26.2 |
| A1S_3783 | Hypothetical protein | 21.5 |
| A1S_3116 | Hypothetical protein | 15.4 |
| A1S_3115 | Hypothetical protein | 14.6 |
| A1S_3668 | Hypothetical protein | 13.6 |
| A1S_3804 | Hypothetical protein | 12.5 |
| A1S_0617 | Hypothetical protein | 12.2 |
| A1S_3782 | Hypothetical protein | 9.5 |
| A1S_3913 | Hypothetical protein | 8.1 |
| A1S_0889 | Hypothetical protein | 8.0 |
| A1S_1390 | Hypothetical protein | 7.7 |
| A1S_0976 | Hypothetical protein | 7.0 |
| A1S_2266 | Hypothetical protein | 6.9 |
| A1S_3113 | Hypothetical protein | 5.9 |
| A1S_0224 | Hypothetical protein | 5.6 |
| A1S_1614 | Hypothetical protein | 5.5 |
| A1S_3878 | Hypothetical protein | 5.5 |
| A1S_3479 | Hypothetical protein | 5.2 |
| A1S_3780 | Hypothetical protein | 5.1 |
| A1S_3682 | Hypothetical protein | 5.1 |
| A1S_2267 | Hypothetical protein | 4.9 |
| A1S_3912 | Hypothetical protein | 4.9 |
| A1S_3638 | Hypothetical protein | 4.7 |
| A1S_3849 | Hypothetical protein | 4.1 |
| A1S_0770 | Hypothetical protein | 4.1 |
| A1S_3513 | Hypothetical protein | 4.1 |
CoA, coenzyme A; MFS, major facilitator superfamily; CBS, cystathionine β-synthase; FMN, flavin mononucleotide.
Many Gram-negative organisms defend against H2O2 by inducing the OxyR regulon, which includes genes encoding the H2O2-reducing enzymes catalase and alkyl hydroperoxide reductase (19, 26, 37). Since A. baumannii is known to encode an OxyR homologue (33), we hypothesized that OxyR is important for the transcriptional changes induced by H2O2. To test this hypothesis, an A. baumannii mutant (ΔoxyR) that contained an in-frame replacement of oxyR (A1S_0992) with the kanamycin resistance cassette aph1 was generated. The transcriptional profile of the ΔoxyR strain in the presence and absence of H2O2 was characterized by RNA sequencing (Fig. 2B; Table 2; see also Table S3 in the supplemental material). In accord with our hypothesis, only 15 genes were upregulated by H2O2 in the ΔoxyR mutant, demonstrating that oxyR is important for activating the transcriptional response to H2O2. To validate the RNA-sequencing results, reverse transcription-quantitative PCR (qRT-PCR) was performed to measure the transcript abundance of selected genes that exhibited the most significant changes in expression in the transcriptome sequencing (RNA-seq) data (Fig. 2C). The abundances of the A1S_1200 (ahpF1), A1S_1458 (ahpF2), A1S_2459 (oxidoreductase), and A1S_2531 (sulfate transporter) transcripts were significantly increased following H2O2 treatment in an oxyR-dependent manner, while the abundances of the A1S_0104 (acyl coenzyme A [acyl-CoA] synthesis) and A1S_2150 (oxidoreductase) transcripts were significantly decreased in an oxyR-dependent manner. Together, these results demonstrate that in A. baumannii, OxyR is an important global regulator of the transcriptional response to H2O2.
TABLE 2.
Genes with increased expression in the ΔoxyR strain following treatment with H2O2
| Category and locus | Annotation | Fold change |
|---|---|---|
| Nucleic acid synthesis and repair | ||
| A1S_2008 | DNA polymerase V | 4.9 |
| A1S_1389 | DNA polymerase V | 4.2 |
| Transport | ||
| A1S_3272 | MFS transporter, YNFM family, putative membrane transport protein | 7.8 |
| Metabolism (other) | ||
| A1S_0567 | NAD(P) transhydrogenase subunit alpha | 12.4 |
| A1S_0566 | NAD(P) transhydrogenase subunit alpha | 11.3 |
| A1S_0568 | NAD(P) transhydrogenase subunit beta | 9.8 |
| Hypothetical | ||
| A1S_3510 | Hypothetical protein | 9.8 |
| A1S_0617 | Hypothetical protein | 9.4 |
| A1S_3704 | Hypothetical protein | 7.8 |
| A1S_1151 | Hypothetical protein | 7.0 |
| A1S_3705 | Hypothetical protein | 5.0 |
| A1S_3116 | Hypothetical protein | 4.5 |
| A1S_3115 | Hypothetical protein | 4.5 |
| A1S_1150 | Hypothetical protein | 4.4 |
Inactivation of oxyR impairs A. baumannii growth in the presence of H2O2.
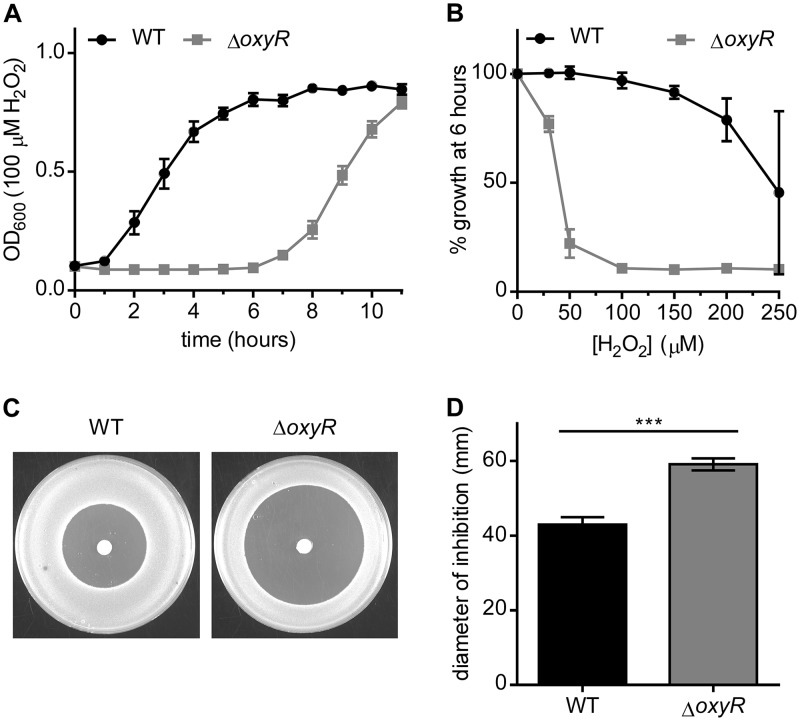
Based on the inability of the ΔoxyR strain to upregulate detoxification genes upon exposure to H2O2, we hypothesized that the ΔoxyR strain would have a growth deficiency in a medium containing H2O2. The A. baumannii ΔoxyR strain did not have a significant growth defect in lysogeny broth (LB) and, in fact, grew to a higher cell density than the wild type (WT) in stationary phase (see Fig. S1A in the supplemental material). However, when the strains were grown in the presence of 100 μM H2O2, the lag time for the ΔoxyR strain was 6 h longer than that for the WT (Fig. 3A). The growth lag of the ΔoxyR strain in H2O2 was complemented by expressing oxyR in trans under the control of a constitutive promoter (Fig. S1B in the supplemental material). The growth of the ΔoxyR strain was impaired by concentrations of H2O2 as low as 50 μM (Fig. 3B). Similarly, in a H2O2 disc diffusion assay, the ΔoxyR strain exhibited a substantially greater zone of inhibition by H2O2 than the WT (Fig. 3C and D).
FIG 3.
Inactivation of oxyR impairs the growth of A. baumannii in the presence of H2O2. (A to D) The growth of WT A. baumannii and the ΔoxyR strain was monitored under various conditions. (A) The growth of A. baumannii in LB containing 100 μM H2O2 was monitored by the OD600. (B) Inhibition of growth by H2O2 at the concentrations indicated on the x axis at the 6-h time point. Growth as a percentage of the growth of the corresponding untreated strain. For panels A and B, growth curves are from a single experiment performed in biological triplicate and are representative of the results of three independent experiments. (C) Zone of inhibition by 10 μl of 9.8 M H2O2 spotted onto discs placed on solid medium. Representative plates are shown after overnight growth. (D) Quantification of the diameter of inhibition by H2O2. Data are combined from three independent experiments with three biological replicates in each experiment. Triple asterisks (***) indicate a P value of <0.001 by Student’s t test.
A gene encoding OxyR is present in every annotated A. baumannii genome, suggesting that its regulatory role is conserved within the species (see Table S4 in the supplemental material). We evaluated the role of oxyR in the growth of the A. baumannii strain AB5075, a multidrug-resistant clinical isolate. AB5075 mutant strains with transposons (Tn) within the oxyR allele (ABUW_2905) were obtained from the University of Washington sequenced-transposon library (38). Four independent ABUW_2905::Tn mutants were evaluated for growth in LB in the absence or presence of H2O2. In contrast to the 17978 oxyR mutant, AB5075 ABUW_2905::Tn mutants exhibited impaired growth in LB (Fig. S1C in the supplemental material). Decreased growth in the absence of exogenous H2O2 has been observed previously for oxyR mutants in other organisms (39). In the presence of H2O2, ABUW_2905::Tn mutants show striking growth impairment (Fig. S1D and E in the supplemental material). Taken together, these results demonstrate that oxyR is important for the growth of multiple A. baumannii strains in the presence of H2O2.
A conserved cysteine residue is important for OxyR function.
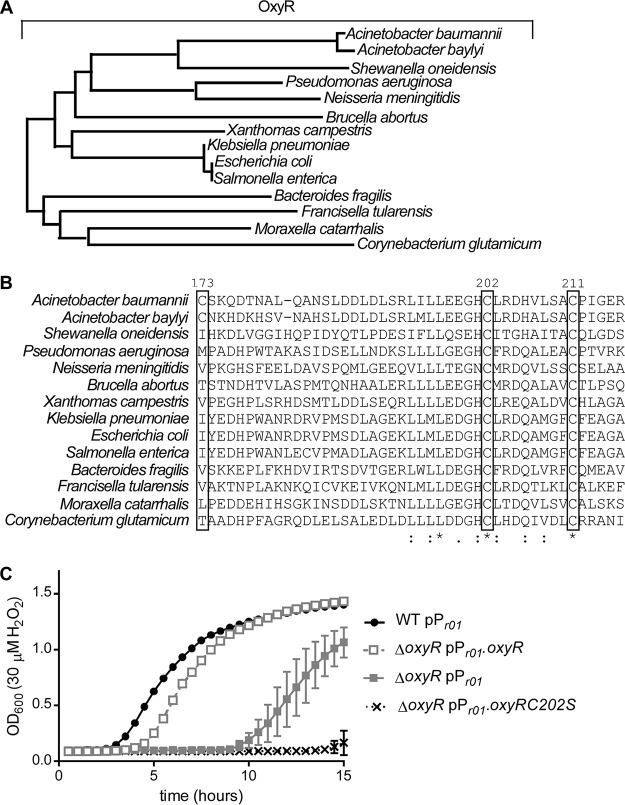
A phylogenetic analysis comparing A. baumannii OxyR to other characterized OxyR proteins revealed that A. baumannii OxyR is in the same clade as OxyR from other Proteobacteria (Fig. 4A). In E. coli, OxyR senses H2O2 by a hyperreactive thiol in a conserved cysteine residue (C199) that is rapidly oxidized by H2O2 (37). Mutational analysis of E. coli demonstrated that mutation of this cysteine residue to serine locks OxyR in the reduced state (40). Alignment of the primary amino acid sequence revealed that C202 in A. baumannii OxyR aligns with C199 in E. coli (Fig. 4B). Therefore, we hypothesized that C202 is important for the function of A. baumannii OxyR. Expression of oxyR with cysteine 202 mutated to serine in trans did not complement the growth defect of the ΔoxyR strain in H2O2 (Fig. 4C). These data suggest that this conserved cysteine residue is critical for the function of OxyR in A. baumannii.
FIG 4.
A. baumannii OxyR contains a conserved cysteine residue that is important for function. (A) Phylogenetic tree based on protein sequence alignment of A. baumannii and Acinetobacter baylyi OxyR with experimentally characterized OxyR proteins in other Gram-negative organisms. Branch lengths are representative of sequence divergence. (B) Amino acid sequence alignment for a portion of the substrate-binding domain of OxyR that contains conserved cysteine residues. An asterisk indicates complete conservation; one dot indicates similar residues at a particular position; and two dots indicate highly similar residues at a particular position. Boxed amino acid positions contain a cysteine in the A. baumannii OxyR sequence. (C) Growth of WT A. baumannii with an empty vector, the ΔoxyR strain with an empty vector, the ΔoxyR strain expressing WT oxyR in trans, or the ΔoxyR strain expressing oxyR C202S in trans in 30 µM H2O2. The growth curves are from a single experiment performed in biological triplicate and are representative of the results of three independent experiments.
A. baumannii ΔoxyR is preadapted to H2O2 stress.
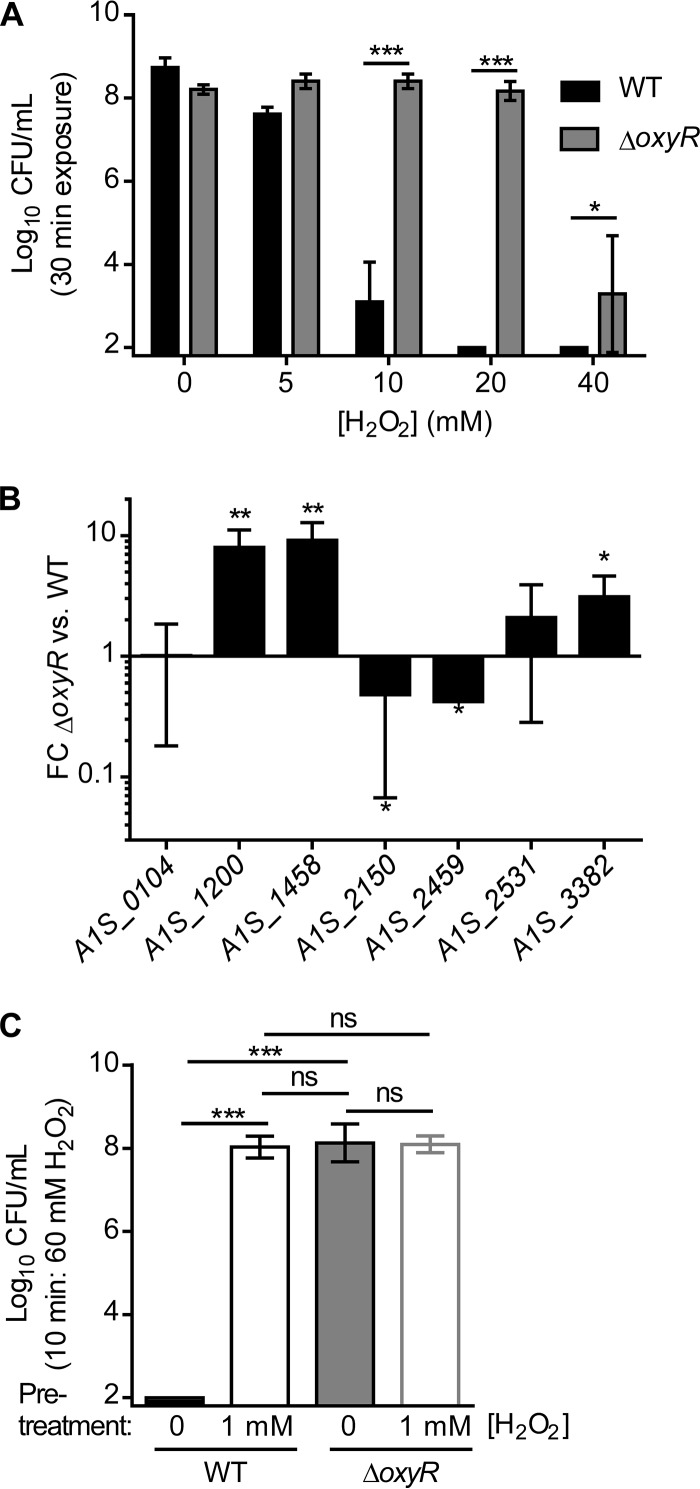
We next investigated whether OxyR altered the survival of A. baumannii cultures in a H2O2 killing assay. As expected, increasing concentrations of H2O2 resulted in decreased survival of WT A. baumannii (Fig. 5A). However, the ΔoxyR strain was resistant to killing by H2O2 under these conditions, with no decrease in survival until 40 mM H2O2 was applied (Fig. 5A). Based on these results, we hypothesized that the ΔoxyR strain is protected from H2O2 killing because this strain has altered expression of the peroxide detoxification machinery in LB. To test this hypothesis, the RNA-seq data were analyzed to compare the transcriptional profiles of WT A. baumannii and the ΔoxyR strain in LB (Table 3; see also Table S5 in the supplemental material). In results consistent with our model, the ΔoxyR strain expressed higher levels of ahpF1 (A1S_1200–1201), ahpF2 (A1S_1458–1460), and katE (A1S_3382) in LB, and these findings were confirmed by qRT-PCR (Fig. 5B). Because these genes are also induced by H2O2 exposure, we posited that low-dose H2O2 exposure adapts A. baumannii to H2O2 stress, protecting against killing by higher concentrations of H2O2. Indeed, pretreatment with 1 mM H2O2 enhanced the survival of WT A. baumannii following high-dose H2O2 stress (Fig. 5C). However, pretreatment of the ΔoxyR strain with 1 mM H2O2 did not alter the survival of this strain, suggesting that the ΔoxyR strain is preadapted to H2O2 stress (Fig. 5C). Together, these results reveal that A. baumannii adapts to H2O2 stress and that the ΔoxyR strain is phenotypically preadapted to H2O2 stress and resistant to H2O2 killing. This result is consistent with the finding that ahpF1 (A1S_1200–1201), ahpF2 (A1S_1458–1460), and katE (A1S_3382) expression does not increase with H2O2 treatment in the ΔoxyR strain (Fig. 2C) but that expression is high in the absence of H2O2 (Fig. 5B). Mechanistically, these data suggest that OxyR functions as a repressor of the ahpF1 (A1S_1200–1201), ahpF2 (A1S_1458–1460), and katE (A1S_3382) genes, since the ΔoxyR strain overexpresses these genes.
FIG 5.
A. baumannii with oxyR inactivated is preadapted to H2O2 stress during exponential growth. (A) Mid-exponential-phase cultures of WT A. baumannii and the ΔoxyR strain were treated with bolus doses of H2O2 at the indicated concentrations for 30 min. CFU enumerated following bolus exposure are shown on a log10 scale. The limit of detection was 2 log10 CFU. Asterisks indicate significant differences (*, P < 0.05; ***, P < 0.001) by t test. Bars indicate means ± standard deviations. (B) qRT-PCR quantification of the relative abundances of H2O2-responsive transcripts in WT A. baumannii and the ΔoxyR strain in LB alone. FC, fold change. Asterisks indicate significant differences (*, P < 0.05; **, P < 0.01) by t test against a theoretical value of 1.0. Six biological replicates were run. Results are means ± standard deviations. For panels A and B, data are from a single experiment performed in biological triplicate and are representative of five independent experiments. (C) Mid-exponential-phase cultures of the A. baumannii WT and ΔoxyR strains were pretreated with 1 mM H2O2 or a vehicle for 30 min. Following pretreatment, cultures were exposed to a bolus of 60 mM H2O2 for 10 min. CFU enumerated following bolus exposure are shown on a log10 scale with the limit of detection at 2 log10 CFU. Asterisks indicate significant differences (*, P < 0.05; ***, P < 0.001) by one-way analysis of variance with Tukey’s multiple-comparison test. ns, no significant difference. Bars indicate means ± standard deviations.
TABLE 3.
Genes with increased expression in the ΔoxyR strain in LB
| Category and locus | Annotation (KEGG) | Fold change |
|---|---|---|
| Peroxide detoxification | ||
| A1S_1458 | Alkyl hydroperoxide reductase subunit F (ahpF2) | 8.4 |
| A1S_1200 | Alkyl hydroperoxide reductase subunit F (ahpF1) | 8.3 |
| A1S_1201 | Alkyl hydroperoxide reductase subunit F (ahpF1) | 7.9 |
| A1S_1459 | Alkyl hydroperoxide reductase subunit F (ahpF2) | 7.4 |
| A1S_1460 | Alkyl hydroperoxide reductase subunit F (ahpF2) | 7.3 |
| A1S_1458 | Alkyl hydroperoxide reductase subunit F (ahpF2) | 8.4 |
| Sulfur homeostasis | ||
| A1S_2531 | Sulfate transport system substrate-binding protein | 4.6 |
| A1S_2533 | Putative esterase | 4.5 |
| Iron homeostasis | ||
| A1S_1647 | Putative siderophore biosynthesis protein | 4.4 |
| Amino acid transport and metabolism | ||
| A1S_1528 | Hypothetical | 24.0 |
| A1S_3046 | Oligopeptidase A | 5.2 |
| A1S_3047 | Oligopeptidase A | 5.1 |
| Chaperones | ||
| A1S_2959 | Molecular chaperone GrpE | 5.1 |
| A1S_2960 | Molecular chaperone DnaK | 4.6 |
| A1S_2665 | K04078 chaperonin GroES | 4.4 |
| Metabolism (other) | ||
| A1S_0804 | Trehalose 6-phosphate phosphatase | 4.6 |
| Other | ||
| A1S_0646 | Intracellular multiplication protein IcmB | 4.5 |
| A1S_0647 | Intracellular multiplication protein IcmO | 4.5 |
| Hypothetical | ||
| A1S_3873 | Hypothetical protein | 19.0 |
| A1S_3546 | Hypothetical protein | 4.9 |
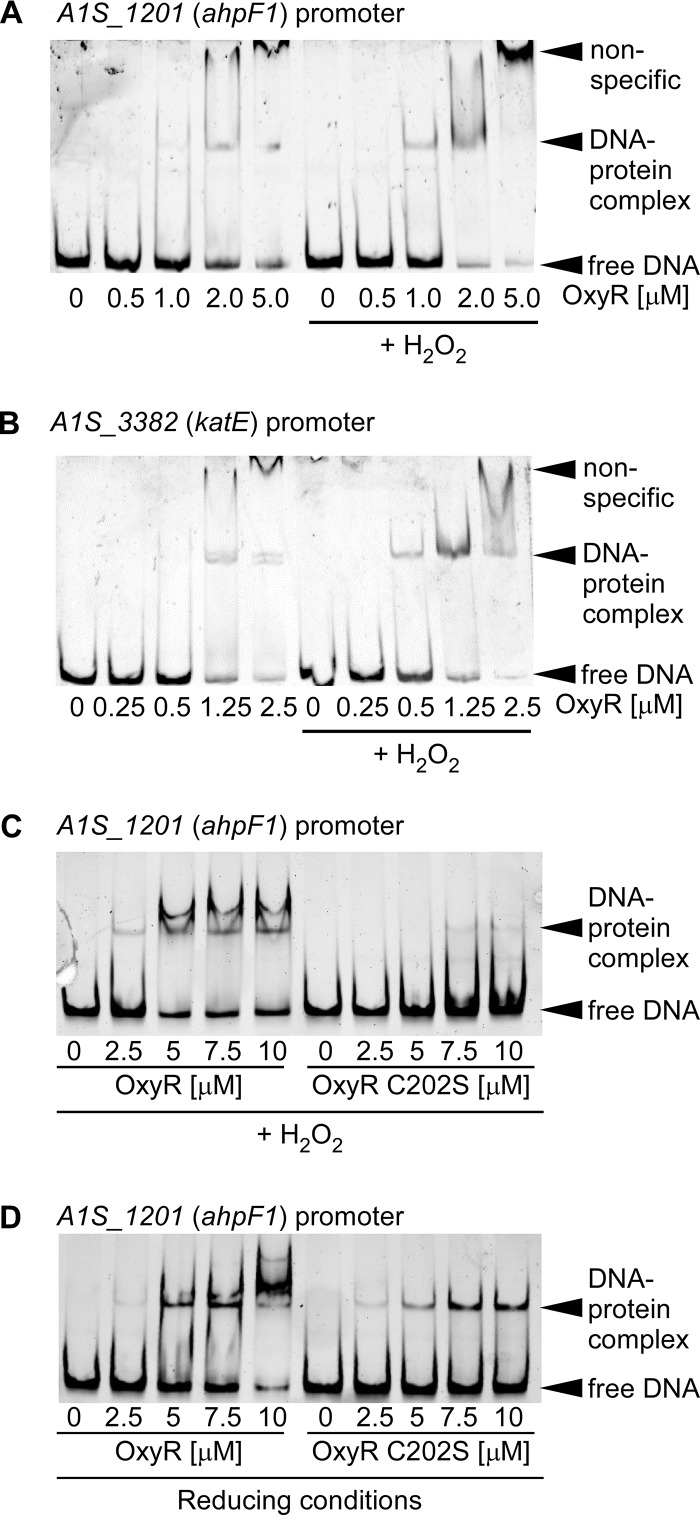
OxyR binds to the promoters of ahp and kat.
We hypothesized that OxyR directly binds to the promoter regions of genes encoding H2O2 detoxification machinery. To determine if OxyR directly binds the promoter regions of putative target genes in a H2O2-dependent manner, electromobility shift assays (EMSAs) were carried out with OxyR bearing an N-terminal hexahistidine tag (see Fig. S2A in the supplemental material). OxyR was purified under reducing conditions, and promoter binding was tested in the presence or absence of excess H2O2. OxyR binds the promoter regions of A1S_1201 (ahpF1) and A1S_3382 (katE), and binding occurs with lower concentrations of OxyR following incubation with H2O2 (Fig. 6A and B). To test the hypothesis that the cysteine at position 202 is important for the regulation of DNA binding activity, the binding of recombinant OxyR C202S to the promoter region of A1S_1201 (ahpF1) was assessed. In the presence of H2O2, a DNA mobility shift was present at lower concentrations of protein for WT OxyR than for OxyR C202S, suggesting that under these conditions, C202 is important for DNA binding activity (Fig. 6C). Interestingly, under reducing conditions, OxyR C202S had DNA binding activity similar to that of WT OxyR, suggesting that C202 is not important for DNA binding activity in the absence of H2O2 (Fig. 6D). In addition, OxyR C202S was capable of binding the promoter region of A1S_3382 (katE) similarly to WT OxyR under reducing conditions but demonstrated decreased binding in the presence of H2O2 (Fig. S2B in the supplemental material). WT OxyR and OxyR C202S did not cause DNA shifts of the negative-control mumT promoter (Fig. S2C in the supplemental material). Together, these data demonstrate that A. baumannii OxyR is a DNA-binding protein that exhibits differential DNA binding ability for target genes following exposure to H2O2. Furthermore, C202 modulates binding to target DNA promoters in the presence of H2O2 but not in the absence of H2O2. The fact that OxyR binds to the promoter regions of ahpF1 and katE in the absence of H2O2 is consistent with the model that A. baumannii OxyR is a repressor of these genes in the absence of H2O2.
FIG 6.
OxyR binds the promoters of ahp and kat. (A to D) PCR-amplified promoter regions for A1S_1201 (ahpF1) (A, C, and D) or A1S_3382 (katE) (B) were incubated with purified recombinant His6-OxyR alone (A and B) or with WT His6-OxyR or His6-OxyR C202S (C and D). The protein was preincubated for 10 min with or without 50 mM H2O2. DNA was visualized using SYBR green. Each gel is representative of experiments performed on at least two separate days.
oxyR promotes the fitness of A. baumannii in a murine model of pneumonia.
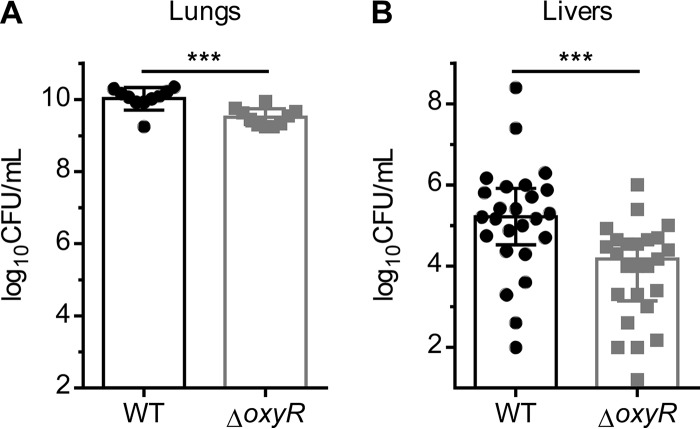
Since H2O2 is produced in the lung during A. baumannii pneumonia and OxyR orchestrates the transcriptional response to H2O2 in vitro, we hypothesized that OxyR promotes the fitness of A. baumannii during infection of the murine lung. Mice were coinfected intranasally with WT A. baumannii and the ΔoxyR strain, and bacterial burdens were enumerated 36 h postinfection. The ΔoxyR strain was recovered at significantly lower numbers than WT A. baumannii in the lung (Fig. 7A), indicating that the WT is more fit than the ΔoxyR strain. The ΔoxyR strain was also less able to disseminate to the liver (Fig. 7B). Therefore, oxyR promotes the fitness of A. baumannii during infection.
FIG 7.
oxyR promotes the fitness of A. baumannii in a murine model of pneumonia. (A and B) Bacterial burdens were enumerated from the lungs (A) and livers (B) of mice 36 h following intranasal inoculation of a 1:1 mixture of WT A. baumannii or the ΔoxyR strain. (A) Lung CFU. Data are from one experiment (n = 10). (B) Liver CFU. Data are combined from two independent experiments (n = 25). Triple asterisks (***) indicate a P value of <0.001 by the Mann-Whitney test.
DISCUSSION
We report here that A. baumannii encounters H2O2 during infection of the murine lung and employs a sophisticated strategy to survive and grow in the presence of H2O2. Transcriptional profiling of A. baumannii exposed to H2O2 revealed that the canonical oxyR regulon is induced by H2O2 in an oxyR-dependent manner. A. baumannii lacking oxyR has impaired growth in the presence of H2O2, but the ΔoxyR strain overexpresses ahp and katE during aerobic growth and is protected from H2O2-mediated killing. We demonstrate that A. baumannii OxyR contains a functionally important conserved cysteine residue and exhibits H2O2-dependent promoter binding activity. Finally, oxyR confers a fitness advantage in a competitive murine model of pneumonia.
People who lack an effective neutrophil oxidative burst due to mutations in NADPH oxidase develop frequent and severe infections due to catalase-producing organisms, emphasizing the importance of the neutrophil oxidative burst in protection against infection (41). While the production of H2O2 by neutrophils can be monitored easily in cell culture assays (42), visualization of the neutrophil oxidative burst that occurs during infection presents a technical challenge, because reactive oxygen and nitrogen species are, by definition, highly reactive. Here we used a recently described chemical probe to detect in vivo H2O2 production in real time in a live animal. We found that infection of the lung with A. baumannii instigated H2O2 production specifically within the thoracic cavity, illustrating the harsh environment that A. baumannii must endure in order to successfully colonize the lung and cause disease.
RNA sequencing of A. baumannii exposed to H2O2 revealed a robust transcriptional response that shared some transcriptional changes with the findings of previously published RNA-sequencing and microarray experiments in other bacteria (27, 43–53). Interestingly, RNA sequencing revealed that H2O2 exposure leads to the downregulation of many metabolic genes, including the paa operon, which has been shown to enhance the virulence of A. baumannii in a murine sepsis model and a zebrafish infection model (54, 55). Analysis of the RNA-sequencing data provides insight into genes that may be activated by OxyR in the presence of H2O2. Compared to the well-studied OxyR regulon in E. coli (21), A. baumannii shares upregulation of ahpF1, ahpF2, and catalase, but not other genes known to be regulated by E. coli OxyR, including fur, hemH, trxB, trxC, gorA, or grxA. Thus, while OxyR is highly conserved among Gram-negative bacteria, the OxyR regulon is not conserved across the Gammaproteobacteria, highlighting the functional diversity of this regulator.
A. baumannii ΔoxyR shares characteristics with oxyR mutants in multiple organisms. Inactivation of oxyR in A. baumannii increases the zone of inhibition by H2O2 in a disc diffusion assay, a finding similar to those for B. abortus, Pseudomonas aeruginosa, Moraxella catarrhalis, and E. coli (28, 48, 53, 56). Elucidating the contribution of oxyR to H2O2 stress resistance adds to existing knowledge of genes that protect against H2O2 toxicity in A. baumannii, including uspA, katE, and katG (16, 17).
In addition to its role in coordinating the response to exogenous H2O2, OxyR may also be important in the context of endogenous H2O2. Indeed, in A. baumannii strain AB5075, inactivation of oxyR leads to a decreased growth rate in the absence of exogenously added H2O2. In contrast, inactivation of oxyR in A. baumannii strain ATCC 17978 leads to higher optical density (OD) of stationary-phase cultures. One potential explanation for the finding that oxyR mutants have growth phenotypes in the absence of exogenous H2O2 is that A. baumannii OxyR may be important for protecting against endogenously produced H2O2, as has been shown in E. coli, where OxyR induces H2O2-scavenging enzymes in response to phenylethylamine oxidase-generated H2O2 (57). However, another possible explanation of these data is that OxyR plays a role in fundamental cellular metabolism. Loss of oxyR reduces the efficacy of oxygen respiration in Shewanella oneidensis and increases the transcription of cytochrome bd (39); while no significant differences in cyd operon expression were noted in the RNA sequencing of the A. baumannii ΔoxyR strain in LB, it is possible that other aspects of central metabolism are altered in this strain. Much remains to be learned about the function of OxyR in pathogenic organisms, including its potential role in central metabolism. For instance, all Mycobacterium tuberculosis isolates carry inactivated oxyR alleles (58), suggesting that oxyR may be detrimental in some infectious niches and that the biological role of this protein remains incompletely understood. Exploring central metabolism using oxyR-null strains may provide insight into this biology.
Like B. abortus and N. meningitidis (28, 30), the A. baumannii ΔoxyR strain is resistant to H2O2 in exponential phase. This resistance correlates with the increased transcription of katE, ahpF1, and ahpF2 in the ΔoxyR strain in the absence of H2O2. There are at least two possible models to explain the upregulation of ahpF1, ahpF2, and katE in the oxyR mutant. The first model is that loss of oxyR induces the upregulation of oxyR-independent pathways that lead to increased transcription of hydrogen peroxide detoxification genes. The second model is that OxyR has a dual function as both a repressor and an activator of ahpF1, ahpF2, and katE depending on the oxidation state of OxyR, which has been proposed previously for OxyR in other organisms, including N. meningitidis and S. oneidensis (30, 59). In this dual repressor-activator model, reduced OxyR binding to the promoter represses transcription and oxidized OxyR binding to the promoter activates transcription. Based on this hypothesis, loss of oxyR would lead to derepression of ahpF1, ahpF2, and katE in the absence of hydrogen peroxide and thus to an increase in the basal level of transcription. We propose that the A. baumannii ΔoxyR strain is resistant to H2O2 killing because of the higher transcription of katE, ahpF1, and ahpF2 in the absence of H2O2, and increased transcription of the detoxification machinery provides resistance to bolus dosing of H2O2. It would be interesting to test the hypothesis that the increased resistance to H2O2 in the oxyR deletion strain is due to upregulation of ahpF1, ahpF2, and katE through the generation of double, triple, or quadruple mutant strains, although this undertaking would be technically challenging, and analysis of the combination mutants would not be straightforward, since each mutant is likely to have a phenotype independently. Taken together, these results highlight the importance of OxyR as a transcriptional regulator in both reduced and oxidative environments.
This work provides evidence that A. baumannii OxyR functions as both a transcriptional repressor and a transcriptional activator. H2O2 induces expression of OxyR gene targets, including ahpF1 and katE, in an OxyR-dependent manner. This result suggests that OxyR is an activator. However, ahpF1 and katE have higher expression in the ΔoxyR strain in the absence of exogenous H2O2, in accord with repression by OxyR at these promoters. OxyR binds the ahpF1 and katE promoter regions with greater affinity following incubation with H2O2, in accord with a mechanism of OxyR activation by oxidation of C202, but binding is not abolished in the absence of H2O2. The finding that OxyR C202S is capable of binding the promoters of ahpF1 and katE under reducing conditions, but binds less well under oxidizing conditions, is consistent with the model that this residue is important for regulating DNA binding in the presence of H2O2. Therefore, we propose that OxyR binds to these promoters in the absence of a signal and represses transcription. Following activation by H2O2, OxyR undergoes a conformational change and activates transcription of the target genes. This dual repressor/activator function has been described previously for regulation of the ahpC promoter by OxyR in X. campestris (60). However, another possible explanation for increased expression of ahpF1, ahpF2, and katE in the absence of oxyR is that another transcriptional regulator can be activated by endogenous peroxide when OxyR is absent.
Previous studies with E. coli have demonstrated that sensing of H2O2 requires a conserved cysteine residue at position 199. Oxidation of C199 results in a significant structural change to the regulatory domain of E. coli OxyR (22). In this work, we demonstrate that C202 is important for the function of A. baumannii OxyR. Interestingly, the oxyR deletion strain complemented with oxyR C202S grows more poorly in the presence of H2O2 than that oxyR deletion strain alone. We expect that the OxyR C202S strain is “locked” in the reduced form, as was demonstrated previously for E. coli OxyR C199S (22, 61). Therefore, the presence of reduced OxyR appears to have a detrimental impact on A. baumannii growth in the presence of H2O2. Since OxyR C202S is capable of DNA binding, we hypothesize that this mutant causes dysregulated expression of the OxyR regulon in the presence of H2O2, leading to impaired growth.
Finally, we report that oxyR increases the fitness of A. baumannii in a murine pneumonia model. Importantly, this result corroborates the imaging data, demonstrating that H2O2 is produced in vivo during A. baumannii infection. The finding that oxyR contributes to A. baumannii pathogenesis builds on work with P. aeruginosa, E. coli, Francisella tularensis, Klebsiella pneumoniae, and Bacteroides fragilis demonstrating that oxyR mutants are less fit than the WT in vivo (62–66). These results define oxyR as a factor in A. baumannii that is important for fitness during infection, and they contribute to understanding the pathogenesis of this organism, a critical multidrug-resistant threat to human health.
MATERIALS AND METHODS
Bacterial strains and reagents.
The strains used in this study are described in Table 4. Unless otherwise noted, all strains are derivatives of the human clinical isolate A. baumannii ATCC 17978. Transposon mutants in the A. baumannii AB5075 background were purchased from the University of Washington A. baumannii mutant library (38). Transposon insertion sites were confirmed by PCR amplification between transposon-specific primer Pgro-172 and primers external to the oxyR allele (ERG19 for 5′ and ERG24 for 3′) (see Table S1 in the supplemental material). Cloning was performed in E. coli DH5α. Bacteria were routinely grown in lysogeny broth (LB) at 37°C unless otherwise noted. Solid medium contained 1.5% agar. Antibiotics were added at the following concentrations: 75 μg ml−1 carbenicillin and 40 μg ml−1 kanamycin. All antibiotics were purchased from Sigma (St. Louis, MO).
TABLE 4.
Bacterial strains used in this study
| Strain | Relevant characteristics | Reference or source |
|---|---|---|
| Acinetobacter baumannii 17978 | Wild-type Acinetobacter baumannii | ATCC |
| Acinetobacter baumannii 17978 ΔoxyR | In-frame ΔoxyR::aphA | This study |
| Acinetobacter baumannii 17978 pWH1266 | Empty-vector control with plasmid pPr01WH1266 | 78 |
| Acinetobacter baumannii 17978 ΔoxyR pWH1266 | Empty-vector control with plasmid pPr01WH1266 | This study |
| Acinetobacter baumannii 17978 ΔoxyR poxyRWH1266 | Complementation strain containing plasmid p.Pr01.oxyR.WH1266 | This study |
| Acinetobacter baumannii 17978 ΔoxyR poxyR C202S WH1266 | Complementation strain containing plasmid p.Pr01.oxyR.WH1266 with C202S mutation | This study |
| Acinetobacter baumannii 17978 pAT02 | Wild type harboring recAb machinery for recombineering | 33 |
| Escherichia coli DH5α p.oxyR | Cloning strain containing plasmid p.Pr01.oxyR.WH1266 | This study |
| Escherichia coli DH5α p.oxyR C202S | Cloning strain containing plasmid p.Pr01.oxyR.WH1266 with C202S mutation | This study |
| Escherichia coli DH5α p.oxyR pCRBlunt | Cloning strain containing oxyR in pCR-Blunt | This study |
| Escherichia coli DH5α p.oxyR C202S pCRBlunt | Cloning strain containing oxyR with C202S mutation in pCR-Blunt | This study |
| Escherichia coli BL21(DE3) p.oxyR.ET15B | Protein expressing strain for oxyR cloned into pET15b | This study |
| Escherichia coli DH5α p.oxyR.ET15B | Cloning strain for oxyR cloned into pET15b | This study |
| Escherichia coli BL21(DE3) p.oxyRC202S.ET15B | Protein-expressing strain for OxyR C202S cloned into pET15b | This study |
| Escherichia coli DH5α p.oxyRC202S.ET15B | Cloning strain for oxyR C202S cloned into pET15b | This study |
| Acinetobacter baumannii AB5075 | Wild-type parental strain for transposon library | 38 |
| Acinetobacter baumannii AB07598 | AB5075 transposon mutant ABUW_2905-184::T26 | 38 |
| Acinetobacter baumannii AB07599 | AB5075 transposon mutant ABUW_2905-143::T26 | 38 |
| Acinetobacter baumannii AB07600 | AB5075 transposon mutant ABUW_2905-146::T26 | 38 |
| Acinetobacter baumannii AB07601 | AB5075 transposon mutant ABUW_2905-156::T26 | 38 |
Strain generation.
The primers used in this study are listed in Table S1 in the supplemental material. The in-frame deletion ΔoxyR strain was generated via one-step recombineering as described previously (33). Briefly, the aph1 kanamycin resistance cassette from pUC18K1 was amplified using primer pairs LEJ_209 and LEJ_220, which contained 120-bp regions of homology flanking the oxyR open reading frame (ORF). The PCR product was purified using column PCR purification (Qiagen, Hilden, Germany), concentrated to >1 μg/μl, and electroporated into WT A. baumannii containing pAT02 (33). RecAb expression was induced with 2 mM isopropyl β-d-1-thiogalactopyranoxide (IPTG), cultures were grown at 37°C for 4 h, and half of the transformed cells were plated onto LB agar containing kanamycin. Kanamycin-resistant colonies were restreaked onto LB agar with kanamycin and were screened by PCR for replacement of the oxyR allele with aph1 by use of primer pairs LEJ_213 and LEJ_214. Clones with the correct insertion were restreaked to solid medium containing kanamycin or carbenicillin to screen for the loss of the pAT02 plasmid. Kanr CarbS clones were saved at –80°C. The oxyR complementation vectors were constructed in pWH1266 under the control of the 16S rRNA promoter (r01) by PCR amplification of the ORF, double digestion of the vector and PCR product with BamHI-HF and SalI-HF (New England Biolabs, Ipswich, MA), and ligation with T4 DNA ligase (Promega, Madison WI). Site-directed mutagenesis was performed by subcloning the oxyR ORF into pCR-Blunt (Invitrogen, Carlsbad, CA) and amplifying the vector with Pfu Turbo polymerase (Thermo Fisher, Waltham, MA) using primer pairs LEJ_236 and LEJ_237. The 50-μl PCR product was treated with 1.5 μl DpnI (New England Biolabs, Ipswich, MA) for 2 h and was transformed into DH5α, and transformants were plated onto LB agar containing kanamycin. Clones that contained the desired mutations were confirmed by Sanger sequencing, plasmids were miniprepped and digested, and inserts were subcloned into pPr01WH1266 to generate complementation vectors with the desired point mutations.
H2O2 growth assays.
LB was used as the growth medium for all assays and contained antibiotics as necessary for the maintenance of plasmids. Bacterial strains were freshly streaked onto solid agar and grown to stationary phase in 3-ml overnight cultures. Overnight cultures were subcultured 1:50 in LB for 1 h prior to 1:100 inoculation of medium containing H2O2 (30%; EMD Millipore, Darmstadt, Germany) at the concentrations indicated in the figures. All growth assays were carried out in 96-well plates in a 100-µl volume, and growth was measured by optical density at 600 nm (OD600).
H2O2 disc diffusion assays.
Disc diffusion assays were modified from published protocols (63, 67). Freshly streaked strains were grown to stationary phase in 3-ml overnight cultures and were diluted 1:10 in LB, and 100 μl of diluted culture was added to 4 ml of melted soft agar (0.75% agar) and immediately poured onto LB agar plates, which were allowed to dry for 5 min. Sterile 6-mm paper discs were placed on the center of the plate and were loaded with 10 μl of 9.8 M H2O2 (30% H2O2; EMD Millipore, Darmstadt, Germany). After incubation at 37°C for 20 h, the zone of growth inhibition was determined as the mean of three separate diameter measurements.
H2O2 killing assays.
Biological-triplicate overnight cultures of WT A. baumannii and the ΔoxyR strain were diluted 1:1,000 into 10 ml LB in 50-ml conical tubes and were grown to mid-exponential phase. Cultures were treated with various concentrations of H2O2 (EMD Millipore, Darmstadt, Germany) ranging from 1 to 40 mM for 10 or 30 min. At each time point, 10 μl of culture was collected, serially diluted in PBS containing catalase (2,000 U/ml, catalase from bovine liver; Sigma, St. Louis, MO), and spot-plated onto LB agar for CFU enumeration.
OxyR protein alignment.
The primary amino acid sequences of selected OxyR orthologues were downloaded from ncbi.gov. Multiple sequence alignment was performed using ClustalOmega on the EBI Web server, which can be found at https://www.ebi.ac.uk/Tools/msa/clustalo/ (68).
OxyR phylogenetic tree.
Phylogenetic tree analysis was performed on the Phylogeny.fr platform (69). Sequences were aligned with MUSCLE (v3.8.31) using default settings (70). After alignment, ambiguous regions were removed with Gblocks (v0.91b) (71) with the following settings: a minimum block length of 10, no gap positions allowed, a maximum contiguous segment of nonconserved positions of 8, and a minimum number of sequences for a flank position of 85%. The phylogenetic tree was reconstructed using the maximum likelihood method implemented in the PhyML program (v3.1/3.0, approximate likelihood-ratio test [aLRT]) (72, 73) using the following settings: WAG model, aLRT statistical test, 4 categories, estimated gamma, and estimated invariable sites. Graphical representation of the phylogenetic tree was performed with TreeDyn (v198.3) (74) using the following settings: rectangular conformation, legend displayed, and bootstrap branch annotation.
Purification of recombinant protein.
The oxyR ORF was cloned into pET15b (Novagen, EMD Millipore, Darmstadt, Germany) to generate N-terminal hexahistidine-tagged constructs. The WT oxyR ORF was amplified by primer pair LEJ_227 and LEJ_228, double digested with NdeI and BamHI-HF (New England Biolabs, Ipswich, MA), ligated into pET15b with T4 DNA ligase (Promega, Madison, WI), and transformed into DH5α. The OxyR C202S ORF was cloned by PCR amplification of the ORF from miniprepped plasmid p.oxyR C202S using primer pairs LEJ_227 and LEJ_228 and ligation into pCR-Blunt. Digestion and ligation were performed as described for WT oxyR. E. coli BL21(DE3) was transformed with the resultant vector and was grown at 37°C to an OD600 of 0.4 to 0.6 before induction with 1 mM IPTG (Sigma, St. Louis, MO). Following induction, bacteria were maintained at 30°C for an additional 8 to 10 h. Cells were harvested by centrifugation at 6,000 × g for 10 min, washed once with LB, and stored at –70°C. Cells were thawed, resuspended in lysis buffer (50 mM Tris [pH 8], 300 mM NaCl, 20 mM imidazole, 1 mg/ml lysozyme, protease inhibitor cocktail [Sigma, St. Louis, MO], and DNase [Sigma, St. Louis, MO]), Dounce homogenized, and lysed at 20,000 lb/in2 for 5 min in an EmulsiFlex homogenizer (Aventin, Inc., Ottawa, ON, Canada). Following disruption, lysates were centrifuged at 15,000 rpm for 1 h at 4°C to remove the insoluble fraction. The supernatants were passed through a 45-μm filter and were applied to a Ni-nitrilotriacetic acid (NTA) column (Qiagen, Hilden, Germany). The column was washed with 20 bed volumes of wash buffer (50 mM Tris [pH 8], 300 mM NaCl, 25 mM imidazole) followed by sequential washes with increasing concentrations of imidazole (100 mM, 150 mM, 200 mM, 250 mM, 300 mM, 500 mM). OxyR eluted free of contaminating proteins at 200 mM imidazole. Buffer exchange was performed using PD-10 desalting columns (GE Healthcare Life Sciences, Little Chalfont, UK) to the final buffer (20 mM Tris [pH 8], 500 mM NaCl, 5% glycerol, 10 mM dithiothreitol). The OxyR C202S protein was purified using the same protocol as that for WT OxyR.
Electromobility shift assays.
Promoter DNA was amplified by PCR using the primer pairs outlined in Table S1 in the supplemental material and was purified by a Qiagen PCR cleanup kit (Qiagen, Hilden, Germany). Forty nanograms of promoter was incubated for 30 min at room temperature with freshly purified OxyR or OxyR C202S at the concentrations indicated in the figures in EMSA binding buffer (20 mM Tris HCl [pH 7.5], 2.5 mM MgCl2, 0.45 mM EDTA, 0.05% Nonidet P-40, 10% glycerol, and 5 mM dithiothreitol) that was modified with H2O2 as noted in Fig. 6. The molarity of promoters in final solution was 13.5 nM for A1S_1201 (ahpF1), 16.4 nM for A1S_3382 (katE), and 10 nM for mumT. Samples were separated by electrophoresis in 6% polyacrylamide-0.5× Tris-buffered EDTA (TBE) gels (5× TBE contained 89 mM Tris base, 89 mM boric acid, and 1 mM EDTA) that were prerun at 100 V for 20 min. Following electrophoresis, gels were stained with SYBR green (Invitrogen, Carlsbad, CA) diluted 1:10,000 in 0.5× TBE for 20 min in the dark, washed twice with H2O, and visualized with a gel imager (Bio-Rad, Hercules, CA).
Mouse infections.
A murine competitive infection model of pneumonia was utilized. Wild-type A. baumannii and the isogenic, kanamycin-marked ΔoxyR strain were freshly streaked from frozen stocks onto LB agar or LB agar containing 40 μg ml−1 kanamycin, respectively, 2 days prior to infection. Overnight cultures were grown in LB without antibiotic selection. On the day of the infection, overnight cultures were subcultured 1:1,000 in 10 ml of LB and were grown to mid-exponential phase. Cells were then harvested by centrifugation, washed twice in PBS, and resuspended in PBS to a final concentration of 1 × 1010 CFU ml−1. Suspensions of wild-type A. baumannii and the ΔoxyR strain were then combined in a 1:1 ratio, mixed thoroughly, and immediately utilized for infection. Mice were anesthetized by intraperitoneal injection of 2,2,2-tribromoethanol diluted in PBS. Anesthetized mice were inoculated intranasally with 5 × 108 CFU in a 45-μl volume. Infection proceeded for 36 h. Mice were then euthanized with CO2, and lungs and livers were removed and placed on ice. Organs were homogenized in 1 ml PBS, serially diluted in PBS with catalase (2,000 U/ml; catalase from bovine liver; Sigma, St. Louis, MO), and dilutions were spot-plated onto LB agar and LB agar containing 40 μg ml−1 kanamycin. Isogenic mutant burdens were enumerated by counting colonies recovered on kanamycin-containing plates. Infections were performed at the Vanderbilt University Medical Center under the principles and guidelines described in the Guide for the Care and Use of Laboratory Animals (75) using Institutional Animal Care and Use Committee (IACUC)-approved protocol M1600123-00. The Vanderbilt University Medical Center is an American Association for Laboratory Animal Science (AALAS)-accredited facility and is registered with the Office of Laboratory Animal Welfare (OLAW), assurance number A-3227-01.
Imaging of H2O2 production in vivo.
Imaging was carried out similarly to published methods (35). FVB-luc+ [FVB-Tg(CAG-luc-GFP)L2G85Chco/J] mice were infected using the protocol described above with WT A. baumannii. FVB-luc+ mice were purchased from The Jackson Laboratory (stock no. 008450) and were bred in-house at Vanderbilt University. After 36 h of infection, mice were injected intraperitoneally with 0.05 μmol of Peroxy Caged Luciferin-2 (PCL-2) and 0.05 μmol of d-cysteine in 50 μl dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO). Chemical probes were provided by Chris Chang (UC Berkeley). Mice were imaged 30 min postinjection using a Xenogen IVIS 200 system (Caliper Life Sciences, Hopkinton, MA). Mice were anesthetized prior to injection and during imaging via inhalation of isoflurane (Piramal, Bethlehem, PA). Infections were performed at the Vanderbilt University Medical Center under the principles and guidelines described in the Guide for the Care and Use of Laboratory Animals (75) using IACUC-approved protocol M/14/243.
Growth for RNA-seq and RNA isolation.
Biological-triplicate overnight cultures of WT A. baumannii and the ΔoxyR strain were diluted 1:1,000 into 10 ml LB in 50-ml conical tubes and were grown to mid-exponential phase. Cultures were treated with 5.65 μl of 30% H2O2 (EMD Millipore, Darmstadt, Germany), for a final concentration of 5 mM, or were left untreated, at 37°C with shaking at 180 rpm for 10 min. Following treatment, 10 μl of bacterial culture was saved for CFU plating, while the remaining bacterial culture was immediately mixed with 10 ml of a 1:1 mixture of ice-cold acetone (JT Baker, Center Valley, PA) and ethanol (Sigma, St. Louis, MO), and the mixture was frozen at –70°C. CFU were enumerated by serially diluting cultures in PBS containing catalase (2,000 U/ml; catalase from bovine liver; Sigma, St. Louis, MO) and spot-plating to LB agar. For RNA purification, thawed cell suspensions were centrifuged at 7,000 rpm for 10 min, supernatants were decanted, and pellets were dried on paper towels. Pellets were resuspended in LETS buffer (0.1 M LiCl, 10 mM EDTA, 10 mM Tris HCl [pH 7.4], 1% SDS), homogenized in a bead beater (Fastprep-24; MP Biomedicals, Santa Ana, CA) with Lysing Matrix B beads (MP Biomedicals, Santa Ana, CA) at a speed of 6 m/s for 45 s, heated at 55°C for 5 min, and centrifuged for 10 min at 15,000 rpm. The upper phase was collected, mixed with 1 ml TRI reagent (Sigma, St. Louis, MO), and incubated for 5 min at room temperature. Chloroform (0.2 ml; Acros Organics, Waltham, MA) was added, samples were vigorously shaken for 15 s, and samples were incubated at room temperature for 2 min. Following centrifugation at 4°C for 15 min, 600 μl of the upper aqueous phase was collected, and RNA was precipitated with 1 ml isopropanol (Sigma, St. Louis, MO). RNA was washed with 70% ethanol (Sigma, St. Louis, MO) and resuspended in 100 μl DNase-free, RNase-free water (Thermo Fisher, Waltham, MA). DNA contamination was removed by treatment with 8 μl RQ1 enzyme (Promega, Madison, WI), 12 μl 10× RQ1 buffer, and 2 μl RiboLock RNase inhibitor (Thermo Fisher, Waltham, MA) for 2 h at 37°C. DNase was removed and samples further purified by the RNeasy kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions, and RNA was stored long-term at –80°C. Each biological replicate was submitted for sequencing without technical replicates.
RNA-seq library preparation and sequencing.
RNA-seq library construction and sequencing were performed by HudsonAlpha (Huntsville, AL). The concentration and integrity of the total RNA were estimated with a Qubit 2.0 fluorometer (Invitrogen, Carlsbad, CA) and an Agilent 2100 bioanalyzer (Applied Biosystems, Carlsbad, CA), respectively. Five hundred nanograms of total RNA was required for proceeding to downstream RNA-seq applications. First, rRNA was removed using a Ribo-Zero Gold (Epidemiology) kit (Illumina, San Diego, CA) according to the manufacturer's recommended protocol. Immediately after the rRNA removal, the RNA was fragmented and primed for the first-strand synthesis using the NEBNext First Strand synthesis module (New England Biolabs, Inc., Ipswich, MA). Directional second-strand synthesis was performed using a NEBNext Ultra Directional second-strand synthesis kit. Following this, the samples underwent a standard library preparation protocol using the NEBNext DNA Library Prep Master Mix Set for Illumina with slight modifications. Briefly, end-repair was done followed by poly(A) addition and custom adapter ligation. Postligated materials were individually barcoded with unique in-house Genomic Services Lab (GSL) primers and were amplified through 12 cycles of PCR. The library quantity was assessed with a Qubit 2.0 fluorometer, and the library quality was estimated by utilizing a DNA High Sense chip on a Caliper Gx system (Perkin-Elmer). Accurate quantification of the final libraries for sequencing applications was determined using the qPCR-based Kapa Biosystems Library Quantification kit (Kapa Biosystems, Inc., Woburn, MA). Each library was diluted to a final concentration of 12.5 nM and pooled in equimolar amounts prior to clustering. Paired-end (PE) sequencing was performed on an Illumina HiSeq 2500 sequencer (Illumina, Inc.).
Processing of RNA-seq reads.
RNA-seq analysis was performed by HudsonAlpha (Huntsville, AL). Approximately 25 million 100-bp PE reads were generated from each sample. Further downstream analysis of the sequenced reads from each sample was performed as per the HudsonAlpha unique in-house pipeline. Briefly, quality control checks on raw sequence data from each sample were performed using FastQC (Babraham Bioinformatics, London, UK). Raw reads were imported onto the commercial data analysis platform Avadis NGS (Strand Scientifics, CA, USA) and were mapped to the A. baumannii ATCC 17978 reference genome. After quality inspection, the aligned reads were filtered on the basis of read quality metrics, where reads with a base quality score less than 30, an alignment score less than 95, and a mapping quality less than 40 were removed. The remaining reads were then filtered on the basis of their read statistics, where missing mates and translocated, unaligned, and flipped reads were removed. The read list was then filtered to remove duplicates. Samples were grouped, and transcript abundance was quantified on this final read list using Trimmed Means of M-values (TMM) (76) as the normalization method. Differential expression of genes was calculated on the basis of the fold change (using a default cutoff greater than or equal to ±2.0) observed between defined conditions, and the P value of the differentially expressed gene list was estimated by Z-score calculations using by a Benjamini-Hochberg false-discovery-rate (FDR) correction of 0.05 (77).
Quantitative RT-PCR.
Two micrograms of RNA was reverse transcribed by Moloney murine leukemia virus (M-MLV) reverse transcriptase (Fisher Scientific, Waltham, MA) in the presence of random hexamers (Promega, Madison, WI) and RiboLock RNase inhibitor (Thermo Fisher, Waltham, MA). Reaction mixtures containing no reverse transcriptase were used to control for DNA contamination. The resultant cDNA was diluted 1:100 and was subjected to qRT-PCR using iQ SYBR green supermix (Bio-Rad, Hercules, CA) with the primer pairs listed in Table S1 in the supplemental material. Amplification was performed on a CFX96 qPCR cycler (Bio-Rad, Hercules, CA) using a 3-step melt curve program. Threshold cycle (CT) values for each transcript were normalized by 16S rRNA, and fold changes were calculated using the ΔΔCT method.
Statistical analyses.
All raw numerical data were saved in Excel files and imported into GraphPad Prism for statistical analysis. The specific statistical tests employed in each experiment are outlined in the figure legends.
Availability of data and materials.
Any materials and data will be made available to members of the scientific community upon communication with the corresponding author.
Accession number(s).
Raw RNA sequencing data and processed data are deposited in the NCBI GEO under accession number GSE114130.
Supplementary Material
ACKNOWLEDGMENTS
L.J.J. conceived of the project, performed experiments, and wrote the manuscript. E.P.S. supervised all studies and helped to write the manuscript. E.R.G. carried out EMSAs. Z.R.L. performed the bioluminescent experiment. C.J.C. and M.C.H. provided the bioluminescent probe.
We thank members of the Skaar laboratory for reviewing the manuscript and Joe Zackular for uploading the RNA-Seq data to GEO.
Work in E.P.S.’s laboratory was supported by Public Health Service grants AI101171, AI069233, and AI073843 and the Defense Advanced Research Projects Agency (DARPA). L.J.J. was supported by American Heart Association grant 15PRE25060007 and Public Health Service award T32 GM07347 from the National Institute of General Medical Studies for the Vanderbilt Medical-Scientist Training Program. L.J.J. is a P.E.O. Scholar. E.R.G. was supported by training grant T32HL094296. Z.R.L. was supported by T32 ES007028 and F31 AI136255. We thank the NIH for grant support of C.J.C. (GM79465). C.J.C. is an Investigator with the Howard Hughes Medical Institute. M.C.H. thanks the University of California President’s Postdoctoral Program for postdoctoral fellowship support.
We declare that no conflicts of interest exist.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00413-18.
REFERENCES
- 1.Dijkshoorn L, Nemec A, Seifert H. 2007. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol 5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 2.Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. 2017. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev 30:409–447. doi: 10.1128/CMR.00058-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antunes LC, Visca P, Towner KJ. 2014. Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis 71:292–301. doi: 10.1111/2049-632X.12125. [DOI] [PubMed] [Google Scholar]
- 4.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. 2007. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 51:3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Potron A, Poirel L, Nordmann P. 2015. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: mechanisms and epidemiology. Int J Antimicrob Agents 45:568–585. doi: 10.1016/j.ijantimicag.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Giammanco A, Cala C, Fasciana T, Dowzicky MJ. 2017. Global assessment of the activity of tigecycline against multidrug-resistant Gram-negative pathogens between 2004 and 2014 as part of the tigecycline evaluation and surveillance trial. mSphere 2:e00310-16. doi: 10.1128/mSphere.00310-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. http://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf.
- 9.Harding CM, Hennon SW, Feldman MF. 2018. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat Rev Microbiol 16:91–102. doi: 10.1038/nrmicro.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McConnell MJ, Actis L, Pachon J. 2013. Acinetobacter baumannii: human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol Rev 37:130–155. doi: 10.1111/j.1574-6976.2012.00344.x. [DOI] [PubMed] [Google Scholar]
- 11.Roca I, Espinal P, Vila-Farrés X, Vila J. 2012. The Acinetobacter baumannii oxymoron: commensal hospital dweller turned pan-drug-resistant menace. Front Microbiol 3:148. doi: 10.3389/fmicb.2012.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hampton MB, Kettle AJ, Winterbourn CC. 1998. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92:3007–3017. [PubMed] [Google Scholar]
- 13.Qiu H, Kuolee R, Harris G, Chen W. 2009. Role of NADPH phagocyte oxidase in host defense against acute respiratory Acinetobacter baumannii infection in mice. Infect Immun 77:1015–1021. doi: 10.1128/IAI.01029-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demple B. 1991. Regulation of bacterial oxidative stress genes. Annu Rev Genet 25:315–337. doi: 10.1146/annurev.ge.25.120191.001531. [DOI] [PubMed] [Google Scholar]
- 15.Imlay JA. 2013. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol 11:443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elhosseiny NM, Amin MA, Yassin AS, Attia AS. 2015. Acinetobacter baumannii universal stress protein A plays a pivotal role in stress response and is essential for pneumonia and sepsis pathogenesis. Int J Med Microbiol 305:114–123. doi: 10.1016/j.ijmm.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Sun D, Crowell SA, Harding CM, De Silva PM, Harrison A, Fernando DM, Mason KM, Santana E, Loewen PC, Kumar A, Liu Y. 2016. KatG and KatE confer Acinetobacter resistance to hydrogen peroxide but sensitize bacteria to killing by phagocytic respiratory burst. Life Sci 148:31–40. doi: 10.1016/j.lfs.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christman MF, Morgan RW, Jacobson FS, Ames BN. 1985. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell 41:753–762. doi: 10.1016/S0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- 19.Tao K, Makino K, Yonei S, Nakata A, Shinagawa H. 1991. Purification and characterization of the Escherichia coli OxyR protein, the positive regulator for a hydrogen peroxide-inducible regulon. J Biochem 109:262–266. [PubMed] [Google Scholar]
- 20.Christman MF, Storz G, Ames BN. 1989. OxyR, a positive regulator of hydrogen peroxide-inducible genes in Escherichia coli and Salmonella typhimurium, is homologous to a family of bacterial regulatory proteins. Proc Natl Acad Sci U S A 86:3484–3488. doi: 10.1073/pnas.86.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiang SM, Schellhorn HE. 2012. Regulators of oxidative stress response genes in Escherichia coli and their functional conservation in bacteria. Arch Biochem Biophys 525:161–169. doi: 10.1016/j.abb.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Choi H, Kim S, Mukhopadhyay P, Cho S, Woo J, Storz G, Ryu SE. 2001. Structural basis of the redox switch in the OxyR transcription factor. Cell 105:103–113. doi: 10.1016/S0092-8674(01)00300-2. [DOI] [PubMed] [Google Scholar]
- 23.Jo I, Chung IY, Bae HW, Kim JS, Song S, Cho YH, Ha NC. 2015. Structural details of the OxyR peroxide-sensing mechanism. Proc Natl Acad Sci U S A 112:6443–6448. doi: 10.1073/pnas.1424495112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Storz G, Tartaglia LA, Ames BN. 1990. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science 248:189–194. doi: 10.1126/science.2183352. [DOI] [PubMed] [Google Scholar]
- 25.Zheng M, Aslund F, Storz G. 1998. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279:1718–1721. doi: 10.1126/science.279.5357.1718. [DOI] [PubMed] [Google Scholar]
- 26.Tartaglia LA, Storz G, Ames BN. 1989. Identification and molecular analysis of oxyR-regulated promoters important for the bacterial adaptation to oxidative stress. J Mol Biol 210:709–719. doi: 10.1016/0022-2836(89)90104-6. [DOI] [PubMed] [Google Scholar]
- 27.Milse J, Petri K, Ruckert C, Kalinowski J. 2014. Transcriptional response of Corynebacterium glutamicum ATCC 13032 to hydrogen peroxide stress and characterization of the OxyR regulon. J Biotechnol 190:40–54. doi: 10.1016/j.jbiotec.2014.07.452. [DOI] [PubMed] [Google Scholar]
- 28.Kim JA, Mayfield J. 2000. Identification of Brucella abortus OxyR and its role in control of catalase expression. J Bacteriol 182:5631–5633. doi: 10.1128/JB.182.19.5631-5633.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mongkolsuk S, Sukchawalit R, Loprasert S, Praituan W, Upaichit A. 1998. Construction and physiological analysis of a Xanthomonas mutant to examine the role of the oxyR gene in oxidant-induced protection against peroxide killing. J Bacteriol 180:3988–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ieva R, Roncarati D, Metruccio MM, Seib KL, Scarlato V, Delany I. 2008. OxyR tightly regulates catalase expression in Neisseria meningitidis through both repression and activation mechanisms. Mol Microbiol 70:1152–1165. doi: 10.1111/j.1365-2958.2008.06468.x. [DOI] [PubMed] [Google Scholar]
- 31.Longkumer T, Parthasarathy S, Vemuri SG, Siddavattam D. 2014. OxyR-dependent expression of a novel glutathione S-transferase (Abgst01) gene in Acinetobacter baumannii DS002 and its role in biotransformation of organophosphate insecticides. Microbiology 160:102–112. doi: 10.1099/mic.0.070664-0. [DOI] [PubMed] [Google Scholar]
- 32.Geissdorfer W, Kok RG, Ratajczak A, Hellingwerf KJ, Hillen W. 1999. The genes rubA and rubB for alkane degradation in Acinetobacter sp. strain ADP1 are in an operon with estB, encoding an esterase, and oxyR. J Bacteriol 181:4292–4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tucker AT, Nowicki EM, Boll JM, Knauf GA, Burdis NC, Trent MS, Davies BW. 2014. Defining gene-phenotype relationships in Acinetobacter baumannii through one-step chromosomal gene inactivation. mBio 5:e01313-14. doi: 10.1128/mBio.01313-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Faassen H, KuoLee R, Harris G, Zhao X, Conlan JW, Chen W. 2007. Neutrophils play an important role in host resistance to respiratory infection with Acinetobacter baumannii in mice. Infect Immun 75:5597–5608. doi: 10.1128/IAI.00762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van de Bittner GC, Bertozzi CR, Chang CJ. 2013. Strategy for dual-analyte luciferin imaging: in vivo bioluminescence detection of hydrogen peroxide and caspase activity in a murine model of acute inflammation. J Am Chem Soc 135:1783–1795. doi: 10.1021/ja309078t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van de Bittner GC, Dubikovskaya EA, Bertozzi CR, Chang CJ. 2010. In vivo imaging of hydrogen peroxide production in a murine tumor model with a chemoselective bioluminescent reporter. Proc Natl Acad Sci U S A 107:21316–21321. doi: 10.1073/pnas.1012864107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imlay JA. 2015. Transcription factors that defend bacteria against reactive oxygen species. Annu Rev Microbiol 69:93–108. doi: 10.1146/annurev-micro-091014-104322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallagher LA, Ramage E, Weiss EJ, Radey M, Hayden HS, Held KG, Huse HK, Zurawski DV, Brittnacher MJ, Manoil C. 2015. Resources for genetic and genomic analysis of emerging pathogen Acinetobacter baumannii. J Bacteriol 197:2027–2035. doi: 10.1128/JB.00131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wan F, Shi M, Gao H. 2017. Loss of OxyR reduces efficacy of oxygen respiration in Shewanella oneidensis. Sci Rep 7:42609. doi: 10.1038/srep42609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kullik I, Toledano MB, Tartaglia LA, Storz G. 1995. Mutational analysis of the redox-sensitive transcriptional regulator OxyR: regions important for oxidation and transcriptional activation. J Bacteriol 177:1275–1284. doi: 10.1128/jb.177.5.1275-1284.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arnold DE, Heimall JR. 2017. A review of chronic granulomatous disease. Adv Ther 34:2543–2557. doi: 10.1007/s12325-017-0636-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wymann MP, von Tscharner V, Deranleau DA, Baggiolini M. 1987. Chemiluminescence detection of H2O2 produced by human neutrophils during the respiratory burst. Anal Biochem 165:371–378. doi: 10.1016/0003-2697(87)90284-3. [DOI] [PubMed] [Google Scholar]
- 43.Nobre LS, Saraiva LM. 2013. Effect of combined oxidative and nitrosative stresses on Staphylococcus aureus transcriptome. Appl Microbiol Biotechnol 97:2563–2573. doi: 10.1007/s00253-013-4730-3. [DOI] [PubMed] [Google Scholar]
- 44.Hajaj B, Yesilkaya H, Shafeeq S, Zhi X, Benisty R, Tchalah S, Kuipers OP, Porat N. 2017. CodY regulates thiol peroxidase expression as part of the pneumococcal defense mechanism against H2O2 stress. Front Cell Infect Microbiol 7:210. doi: 10.3389/fcimb.2017.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masloboeva N, Reutimann L, Stiefel P, Follador R, Leimer N, Hennecke H, Mesa S, Fischer HM. 2012. Reactive oxygen species-inducible ECF sigma factors of Bradyrhizobium japonicum. PLoS One 7:e43421. doi: 10.1371/journal.pone.0043421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jang HJ, Nde C, Toghrol F, Bentley WE. 2009. Microarray analysis of Mycobacterium bovis BCG revealed induction of iron acquisition related genes in response to hydrogen peroxide. Environ Sci Technol 43:9465–9472. doi: 10.1021/es902255q. [DOI] [PubMed] [Google Scholar]
- 47.Voskuil MI, Bartek IL, Visconti K, Schoolnik GK. 2011. The response of Mycobacterium tuberculosis to reactive oxygen and nitrogen species. Front Microbiol 2:105. doi: 10.3389/fmicb.2011.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoopman TC, Liu W, Joslin SN, Pybus C, Brautigam CA, Hansen EJ. 2011. Identification of gene products involved in the oxidative stress response of Moraxella catarrhalis. Infect Immun 79:745–755. doi: 10.1128/IAI.01060-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harrison A, Ray WC, Baker BD, Armbruster DW, Bakaletz LO, Munson RS Jr. 2007. The OxyR regulon in nontypeable Haemophilus influenzae. J Bacteriol 189:1004–1012. doi: 10.1128/JB.01040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang W, Small DA, Toghrol F, Bentley WE. 2005. Microarray analysis of Pseudomonas aeruginosa reveals induction of pyocin genes in response to hydrogen peroxide. BMC Genomics 6:115. doi: 10.1186/1471-2164-6-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schroeter R, Voigt B, Jurgen B, Methling K, Pother DC, Schafer H, Albrecht D, Mostertz J, Mader U, Evers S, Maurer KH, Lalk M, Mascher T, Hecker M, Schweder T. 2011. The peroxide stress response of Bacillus licheniformis. Proteomics 11:2851–2866. doi: 10.1002/pmic.201000461. [DOI] [PubMed] [Google Scholar]
- 52.Salunkhe P, Topfer T, Buer J, Tummler B. 2005. Genome-wide transcriptional profiling of the steady-state response of Pseudomonas aeruginosa to hydrogen peroxide. J Bacteriol 187:2565–2572. doi: 10.1128/JB.187.8.2565-2572.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng M, Wang X, Templeton LJ, Smulski DR, LaRossa RA, Storz G. 2001. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J Bacteriol 183:4562–4570. doi: 10.1128/JB.183.15.4562-4570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhuiyan MS, Ellett F, Murray GL, Kostoulias X, Cerqueira GM, Schulze KE, Mahamad Maifiah MH, Li J, Creek DJ, Lieschke GJ, Peleg AY. 2016. Acinetobacter baumannii phenylacetic acid metabolism influences infection outcome through a direct effect on neutrophil chemotaxis. Proc Natl Acad Sci U S A 113:9599–9604. doi: 10.1073/pnas.1523116113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cerqueira GM, Kostoulias X, Khoo C, Aibinu I, Qu Y, Traven A, Peleg AY. 2014. A global virulence regulator in Acinetobacter baumannii and its control of the phenylacetic acid catabolic pathway. J Infect Dis 210:46–55. doi: 10.1093/infdis/jiu024. [DOI] [PubMed] [Google Scholar]
- 56.Ochsner UA, Vasil ML, Alsabbagh E, Parvatiyar K, Hassett DJ. 2000. Role of the Pseudomonas aeruginosa oxyR-recG operon in oxidative stress defense and DNA repair: OxyR-dependent regulation of katB-ankB, ahpB, and ahpC-ahpF. J Bacteriol 182:4533–4544. doi: 10.1128/JB.182.16.4533-4544.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ravindra Kumar S, Imlay JA. 2013. How Escherichia coli tolerates profuse hydrogen peroxide formation by a catabolic pathway. J Bacteriol 195:4569–4579. doi: 10.1128/JB.00737-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deretic V, Philipp W, Dhandayuthapani S, Mudd MH, Curcic R, Garbe T, Heym B, Via LE, Cole ST. 1995. Mycobacterium tuberculosis is a natural mutant with an inactivated oxidative-stress regulatory gene: implications for sensitivity to isoniazid. Mol Microbiol 17:889–900. doi: 10.1111/j.1365-2958.1995.mmi_17050889.x. [DOI] [PubMed] [Google Scholar]
- 59.Wan F, Kong L, Gao H. 2018. Defining the binding determinants of Shewanella oneidensis OxyR: implications for the link between the contracted OxyR regulon and adaptation. J Biol Chem 293:4085–4096. doi: 10.1074/jbc.RA117.001530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loprasert S, Fuangthong M, Whangsuk W, Atichartpongkul S, Mongkolsuk S. 2000. Molecular and physiological analysis of an OxyR-regulated ahpC promoter in Xanthomonas campestris pv. phaseoli. Mol Microbiol 37:1504–1514. doi: 10.1046/j.1365-2958.2000.02107.x. [DOI] [PubMed] [Google Scholar]
- 61.Toledano MB, Kullik I, Trinh F, Baird PT, Schneider TD, Storz G. 1994. Redox-dependent shift of OxyR-DNA contacts along an extended DNA-binding site: a mechanism for differential promoter selection. Cell 78:897–909. doi: 10.1016/S0092-8674(94)90702-1. [DOI] [PubMed] [Google Scholar]
- 62.Hennequin C, Forestier C. 2009. oxyR, a LysR-type regulator involved in Klebsiella pneumoniae mucosal and abiotic colonization. Infect Immun 77:5449–5457. doi: 10.1128/IAI.00837-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sund CJ, Rocha ER, Tzinabos AO, Wells WG, Gee JM, Reott MA, O'Rourke DP, Smith CJ. 2007. The Bacteroides fragilis transcriptome response to oxygen and H2O2: the role of OxyR and its effect on survival and virulence. Mol Microbiol 67:129–142. doi: 10.1111/j.1365-2958.2007.06031.x. [DOI] [PubMed] [Google Scholar]
- 64.Ma Z, Russo VC, Rabadi SM, Jen Y, Catlett SV, Bakshi CS, Malik M. 2016. Elucidation of a mechanism of oxidative stress regulation in Francisella tularensis live vaccine strain. Mol Microbiol 101:856–878. doi: 10.1111/mmi.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnson JR, Clabots C, Rosen H. 2006. Effect of inactivation of the global oxidative stress regulator oxyR on the colonization ability of Escherichia coli O1:K1:H7 in a mouse model of ascending urinary tract infection. Infect Immun 74:461–468. doi: 10.1128/IAI.74.1.461-468.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lau GW, Britigan BE, Hassett DJ. 2005. Pseudomonas aeruginosa OxyR is required for full virulence in rodent and insect models of infection and for resistance to human neutrophils. Infect Immun 73:2550–2553. doi: 10.1128/IAI.73.4.2550-2553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.LeBlanc JJ, Brassinga AK, Ewann F, Davidson RJ, Hoffman PS. 2008. An ortholog of OxyR in Legionella pneumophila is expressed postexponentially and negatively regulates the alkyl hydroperoxide reductase (ahpC2D) operon. J Bacteriol 190:3444–3455. doi: 10.1128/JB.00141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, Thompson JD, Higgins DG. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O. 2008. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36:W465–W469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Castresana J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17:540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- 72.Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 73.Anisimova M, Gascuel O. 2006. Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative. Syst Biol 55:539–552. doi: 10.1080/10635150600755453. [DOI] [PubMed] [Google Scholar]
- 74.Chevenet F, Brun C, Banuls AL, Jacq B, Christen R. 2006. TreeDyn: towards dynamic graphics and annotations for analyses of trees. BMC Bioinformatics 7:439. doi: 10.1186/1471-2105-7-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 76.Robinson MD, Oshlack A. 2010. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol 11:R25. doi: 10.1186/gb-2010-11-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Statist Soc Ser B Methodol 57:289–300. [Google Scholar]
- 78.Hood MI, Mortensen BL, Moore JL, Zhang Y, Kehl-Fie TE, Sugitani N, Chazin WJ, Caprioli RM, Skaar EP. 2012. Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLoS Pathog 8:e1003068. doi: 10.1371/journal.ppat.1003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any materials and data will be made available to members of the scientific community upon communication with the corresponding author.