During infection, the host utilizes a diverse array of processes to combat invaders, including the restriction of availability of essential nutrients such as manganese. Similarly to many other pathogens, Staphylococcus aureus possesses two manganese importers, MntH and MntABC.
KEYWORDS: ABC transporters, calprotectin, infection, manganese, MntABC, MntH, nutritional immunity, Staphylococcus aureus
ABSTRACT
During infection, the host utilizes a diverse array of processes to combat invaders, including the restriction of availability of essential nutrients such as manganese. Similarly to many other pathogens, Staphylococcus aureus possesses two manganese importers, MntH and MntABC. Several infection models have revealed a critical role for MntABC during staphylococcal infection. However, culture-based studies have suggested parity between the two transporters when cells are resisting manganese starvation imposed by the manganese binding immune effector calprotectin. In this investigation, initial elemental analysis revealed that MntABC is the primary transporter responsible for obtaining manganese in culture in the presence of calprotectin. MntABC was also necessary to maintain wild-type levels of manganese-dependent superoxide dismutase activity in the presence of calprotectin. Building on this framework, we investigated if MntABC enabled S. aureus to resist the synergistic actions of nutritional immunity and other host defenses. This analysis revealed that MntABC critically contributes to staphylococcal growth when S. aureus is subjected to manganese limitations and exposed to oxidative stress. This transporter was also important for growth in manganese-limited environments when S. aureus was forced to consume glucose as an energy source, which occurs when it encounters nitric oxide. MntABC also expanded the pH range conducive for S. aureus growth under conditions of manganese scarcity. Collectively, the data presented in this work provide a robust molecular basis for the crucial role of MntABC in staphylococcal virulence. Further, this work highlights the importance of synergy between host defenses and the necessity of evaluating the contribution of virulence factors to pathogenesis in the presence of multiple stressors.
INTRODUCTION
Staphylococcus aureus is a Gram-positive bacterium that asymptomatically colonizes the anterior nares and skin of about one-third of the world’s population and that, upon breach of the epithelial cell barrier, can infect nearly every organ (1–3). The treatment of S. aureus infections is increasingly challenging due to the rise of antibiotic resistance. Both the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) have identified S. aureus as a serious threat to human health, stating that there is a critical need to develop novel therapeutics to treat antibiotic-resistant infections (4, 5). One approach to solving this challenge is targeting the mechanisms that pathogens utilize to subvert or evade host defenses.
During infection, S. aureus and other pathogens must acquire a broad array of essential nutrients such as metal ions from the host. The first-row transition metals iron (Fe), manganese (Mn), and zinc (Zn) are essential for life due to their roles in facilitating the structure and function of proteins (6). Their importance is highlighted by bioinformatic analyses, which suggest that ∼30% of all proteins interact with a metal ion (6, 7). The host leverages the essentiality of first-row transition metals to defend itself against invading pathogens by restricting their availability during infection, a strategy known as nutritional immunity (8–10). The prototypic example of Mn and Zn restriction is the staphylococcal abscess, which is rendered devoid of these two essential metals (11, 12). The innate immune effector calprotectin (CP) is a key component of nutritional immunity and contributes to this host defense by withholding Mn and Zn from invading pathogens (9, 11, 13). CP is highly expressed in neutrophils, where it composes ∼50% of the total protein in the cytoplasm (14, 15). At sites of infection, CP can be found at levels in excess of 1 mg/ml, and CP-deficient mice, which fail to sequester Mn, are more susceptible to a range of both bacterial and fungal pathogens, including Acinetobacter baumannii, Klebsiella pneumoniae, Staphylococcus aureus, and Aspergillus fumigatus (11, 13, 16–20). CP is a heterodimer comprised of S100A8 and S100A9 and features two transition metal-binding sites, which are responsible for sequestration of Mn and Zn (13, 16, 21, 22). The first site (S1) comprises six histidine residues and is able to bind a single Mn or Zn ion with subnanomolar and picomolar affinities, respectively (23). Site 1 also binds Fe(II) and nickel (Ni) (24, 25). However, the physiological relevance and antimicrobial contribution of this activity remain unclear. The second site (S2) consists of an aspartic acid and three histidine residues and binds Zn with subpicomolar affinity (13, 16, 23, 26).
Despite experiencing Mn and Zn starvation during infection, S. aureus and other successful pathogens are still capable of causing infection. A common mechanism employed by pathogens to overcome host-mediated metal ion limitation is expression of dedicated high-affinity acquisition systems. S. aureus expresses two Mn transporters: MntH, a natural resistance-associated macrophage protein (NRAMP) family member, and MntABC, an ATP-binding cassette (ABC) permease (12, 27, 28). MntH is constitutively expressed, with modest modulation of expression occurring in response to Mn availability, while expression of MntABC is repressed when Mn is available and induced by Mn limitation (12, 27). Expression of both mntABC and mntH is controlled by the intracellular Mn sensing regulator MntR. In the presence of Mn, MntR represses expression of mntABC. While MntR is considered to be a canonical repressor, it is necessary for maximal expression of mntH (27). Homologs of both MntABC and MntH are widely distributed throughout prokaryotes and contribute to bacterial virulence (28–39). In the context of S. aureus, data from previous studies showed that simultaneous loss of the two Mn transport systems rendered the bacterium more sensitive to CP treatment in vitro and profoundly impacted pathogenesis in various models of infection (12, 27, 40, 41). Surprisingly, although loss of MntC, the Mn-recruiting protein of the ABC import pathway, resulted in a modest reduction in superoxide dismutase (SOD) activity, a ΔmntC mutant was not more sensitive to CP in the presence or absence of oxidative stress (12). In contrast to those culture-based studies, loss of MntABC resulted in virulence defects in several animal models of infection, implicating this pathway as the dominant Mn import system in the host (12, 42–44). Although in vitro growth defects associated with loss of MntABC have been reported, those previous studies investigated conditions that are presumed to represent metal-replete environments (42, 44). As CP has a greater affinity for Mn than MntC (45), this raises the possibility that MntABC may not be the primary transporter responsible for resisting host-imposed Mn starvation. Therefore, the relative contributions of MntABC and MntH to resisting nutritional immunity remain unclear.
Pathogenic organisms must also contend with other host defenses, such as the oxidative burst of neutrophils (46) and host niches that may impact the function of MntABC and MntH (47–52). Enzymes, such as the superoxide dismutases (SODs), allow bacteria to detoxify damaging reactive oxygen species (53–55). However, this process is more challenging because of the concomitant restriction of essential metal ion availability, which can inactivate metal-dependent proteins such as Mn-dependent SODs (11, 13). Thus, nutritional immunity and the oxidative burst function synergistically during infection, with Mn sequestration sensitizing S. aureus to superoxide generated by immune cells (13). Exposure to host-generated nitric oxide (NO⋅) free radicals forces S. aureus to consume glucose (49). Also, recent work revealed that manganese starvation reduces the ability of S. aureus to utilize glucose as an energy source (50), suggesting that manganese limitation may function synergistically with host defenses beyond oxidative stress. The contribution of Mn to resisting independent host defenses underscores the need to understand how S. aureus obtains this nutrient when Mn availability is limited by the host.
MntC is highly conserved in staphylococcal strains, is expressed on the surface of S. aureus, and is currently being investigated as a component of a multiantigen vaccine in clinical trials (56). Hence, how MntABC contributes to infection is crucial to understand. In the current study, the individual contributions of MntABC and MntH to S. aureus pathogenicity during Mn limitation were examined. Elemental analyses showed that MntABC is the primary transporter that enables S. aureus to compete with CP for Mn acquisition. Our investigations also revealed that loss of MntABC, but not MntH, makes S. aureus more vulnerable to Mn limitation and concomitant oxidative stress due to a reduced ability to maintain a robust antioxidant defense. The contribution of MntABC to staphylococcal growth when Mn availability is restricted also becomes more important in environments when the cellular demand for this metal is increased, such as when cells are forced to rely on glucose as an energy source. Similarly to studies of other pathogens that utilize both NRAMP and ABC importers to obtain Mn, in the current study we observed an important role for MntABC in the acquisition of Mn under alkaline conditions. Collectively, these results show that MntABC, not MntH, enables S. aureus to compete with the host for Mn during infection and reveal that the crucial role of this transporter is necessitated by the synergistic action of nutritional immunity with other host defenses.
RESULTS
MntABC is the primary transporter responsible for manganese uptake in the presence of calprotectin.
To define the respective contributions of MntABC and MntH to resisting host-imposed Mn limitation, wild-type, ΔmntC, and ΔmntH S. aureus strains were grown in rich medium in the presence and absence of CP, with accumulation of first-row transition metal ions assessed by inductively coupled plasma mass spectrometry (ICP-MS) (Fig. 1). In metal-replete medium, the wild-type and mutant strains accumulated similar levels of Mn (Fig. 1A). Consistent with prior results, CP treatment reduced intracellular Mn levels in all strains (11, 50). However, the ΔmntC mutant accumulated significantly less Mn than either the wild type or the ΔmntH strain, indicating that MntABC is the primary transporter responsible for resisting host-imposed Mn starvation. Similarly to prior observations with wild-type S. aureus, CP did not reduce intracellular Fe or Zn levels in any of the strains examined (Fig. 1B and F). Given the ability of CP to bind other first-row transition metal ions (24, 25), we assessed if loss of MntABC or MntH affected their accumulation. CP did not reduce the accumulation of Co, Ni, or Cu in wild-type bacteria or the ΔmntC and ΔmntH mutants (Fig. 1C to E). Surprisingly, growth in the presence of CP increased staphylococcal accumulation of Co and Ni (Fig. 1C and D), suggesting that even though CP can bind Co and Ni, it does not starve S. aureus for these metals. Collectively, these results indicate that MntABC is the primary system responsible for competing with the host for Mn and that S. aureus can successfully compete with CP for other metals.
FIG 1.
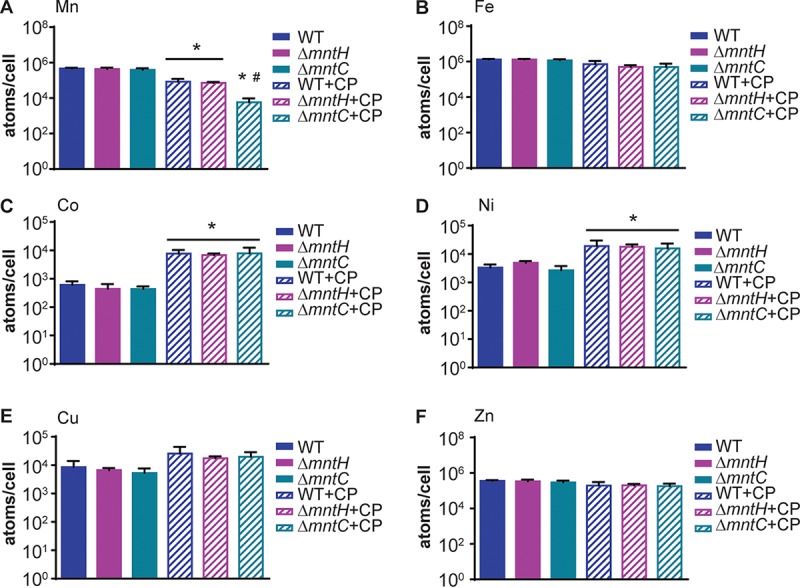
Calprotectin starves S. aureus for Mn. S. aureus wild-type, ΔmntH, and ΔmntC strains were precultured in metal-limited medium (NRPMI) and then grown in rich medium (TSB) in the presence or absence of 240 µg/ml of wild-type (WT) CP, and intracellular Mn (A), Fe (B), Co (C), Ni (D), Cu (E), and Zn (F) levels were determined by ICP-MS. * = P ≤ 0.05 relative to untreated cells and # = P ≤ 0.05 relative to wild-type S. aureus and ΔmntH mutant cells grown in the presence of 240 µg/ml of WT CP by one-way analysis of variance (ANOVA) with Tukey’s posttests of selected means. n ≥ 3. Error bars indicate standard errors of the means (SEM).
MntABC facilitates manganese binding by critical enzymes.
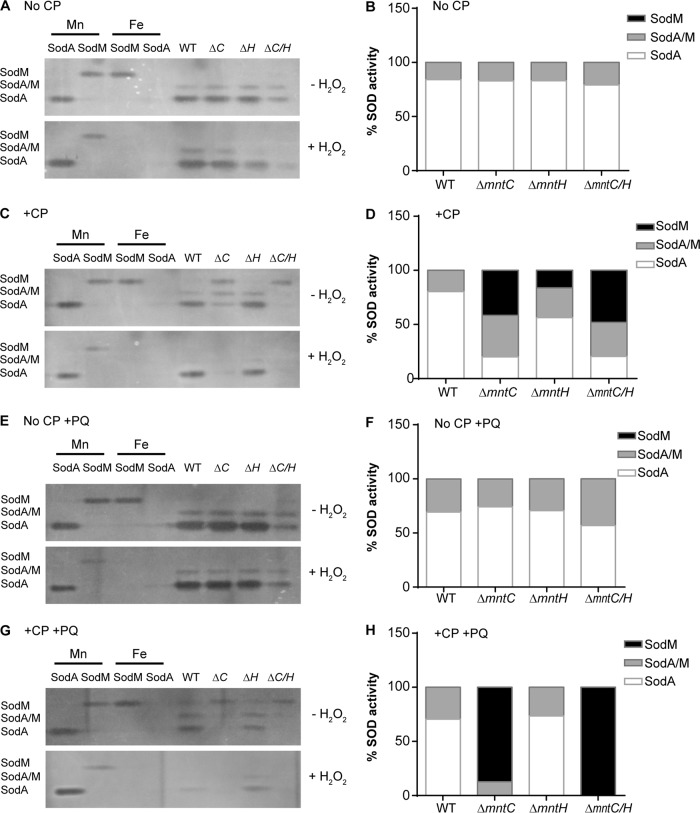
Although MntABC strongly contributes to Mn acquisition in the presence of CP, it may not be essential to prevent inhibition of Mn-dependent enzymes. In S. aureus, the activities of the two SODs, SodA and SodM, strongly correlate with intracellular Mn abundance. In the case of SodA, which is strictly Mn dependent, reduced levels of Mn accumulation result in reduced enzymatic activity (41). In contrast, for SodM, which is cambialistic and can utilize either Mn or Fe as a cofactor, Mn limitation results in increased Fe-dependent activity (41). Hence, we sought to determine if reduced Mn accumulation in the ΔmntC strain in the presence of CP impacted enzyme function and metalation. The individual activities of SodA and SodM in wild-type bacteria and in the ΔmntC and ΔmntH single and ΔmntC ΔmntH double mutants were determined following growth in the presence and absence of CP. In the absence of CP, when Mn was abundant, the predominant SOD activity in all four strains was attributable to SodA (Fig. 2A and B). In the presence of an intermediate concentration of CP (240 µg/ml), SOD activity in both the wild type and the ΔmntH strain was mainly from SodA (Fig. 2C and D). In contrast, for both the ΔmntC and ΔmntC ΔmntH mutants in the presence of CP, the activity associated with SodA decreased whereas that of SodM increased. Hydrogen peroxide treatment, which inactivates Fe-containing but not Mn-containing SODs (41, 57), revealed that the activity associated with SodM came from the Fe-loaded form (Fig. 2C). Taken together, these observations indicate that the reduced Mn accumulation of the ΔmntC mutant in the presence of CP impacts SOD metalation and function.
FIG 2.
Loss of MntC reduces the ability of S. aureus to populate metal-dependent enzymes with manganese. S. aureus wild-type, ΔmntC (ΔC), ΔmntH (ΔH), and ΔmntC ΔmntH (ΔC/H) strains were prestarved for metals and then grown in rich medium in the absence (A, B, E, and F) or presence (C, D, G, and H) of 240 µg/ml of CP as well as in the absence (A to D) or presence (E to H) of 0.1 mM PQ. The fractional contribution of SodA and SodM to total SOD activity in cell lysates (5.17 µg of total protein) was determined. The lower gel was treated with hydrogen peroxide prior to assessing SOD activity to inactivate Fe-containing SODs. Purified recombinant SodA and SodM (0.3 µg), loaded with either Mn or Fe, were included as controls. Graphs represent averages of results from three independent experiments. Representative gels are shown. “SodA/M” refers to the heterodimer formed between SodA and SodM.
Oxidative stress may potentially alter the relative importance of MntABC and MntH pathways in acquiring Mn. To evaluate this possibility, wild-type S. aureus and the three transporter mutants were grown in the presence of the superoxide-generating compound paraquat (PQ). In the absence of CP, exposure to PQ did not alter the pattern of SOD activity for any of the strains, with the majority of activity coming from SodA (Fig. 2E and F). In the presence of CP and PQ, SodA was the dominant SOD in the wild type and the ΔmntH strain, while the ΔmntC and ΔmntC ΔmntH mutants showed reduced SodA activity and increased Fe-dependent SodM activity (Fig. 2G and H). If anything, the treatment with PQ resulted in a CP-driven transition to SodM as the primary SOD in the ΔmntC and ΔmntC ΔmntH mutants that was more pronounced. These observations highlight the critical role of MntABC as the primary Mn transporter and its contributions to maintaining the activity of Mn-dependent enzymes when Mn starved by the host.
Oxidative stress increases the importance of MntABC.
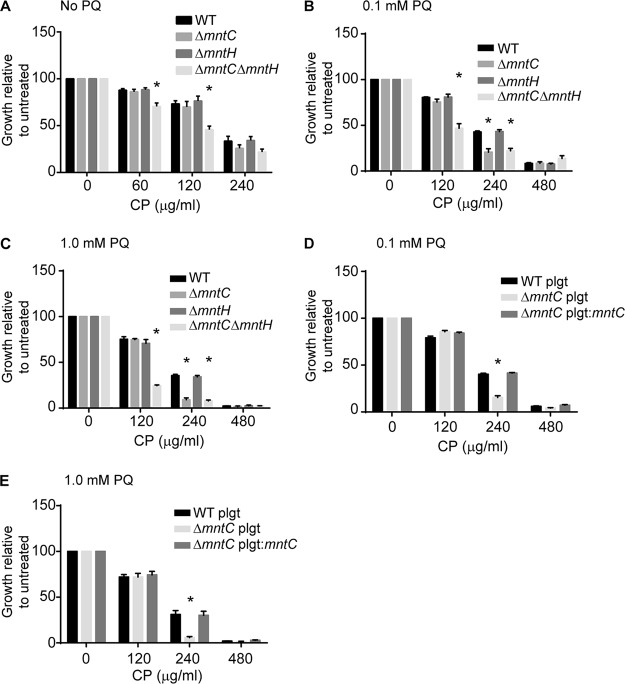
The data reported above indicate that MntABC is the primary Mn transporter and serves to facilitate Mn-SOD activity in the presence of CP. Despite this, no growth defect has previously been observed for the ΔmntC strain in the presence of CP (12). One plausible explanation for the lack of a phenotypic impact could be that the previous study used a rich growth medium (tryptic soy broth [TSB]) that permits sufficient Mn accumulation. To address this inference, we precultured the wild-type and ΔmntC, ΔmntH, and ΔmntC ΔmntH strains in Mn-restricted medium (Chelex-treated RPMI medium plus 1% Casamino Acids [NRPMI]) and performed CP growth assays. Differing from those used in prior studies, these culture conditions better recapitulate the observed contributions of SodA and SodM to maintaining a defense against oxidative stress during infection (41). When precultured in a Mn-restricted medium, the ΔmntC ΔmntH mutant showed increased sensitivity to CP, while the ΔmntC and ΔmntH strains were unaffected (Fig. 3A). In the presence of CP and PQ, both the ΔmntC and ΔmntC ΔmntH strains were more sensitive to CP treatment than the wild-type bacteria (Fig. 3B). By contrast, the ΔmntH mutant showed no increase in sensitivity. Increasing the PQ concentration further diminished the growth of the ΔmntC strain, but not of the ΔmntH strain, compared to the wild-type results (Fig. 3C). Ectopic expression of MntC reversed the phenotype (Fig. 3D and E), confirming that the growth defect was attributable to impaired Mn import by MntABC. These results show that MntABC is crucial for resisting Mn starvation when bacteria are exposed to the synergistic insults of Mn limitation and oxidative stress.
FIG 3.
Oxidative stress increases the importance of MntABC. (A to C) S. aureus wild-type, ΔmntC, ΔmntH, and ΔmntC ΔmntH cells were prestarved for metals, and growth assays were performed in rich medium in the presence of increasing concentrations of CP and in the absence (A) and the presence of 0.1 mM PQ (B) and 1.0 mM PQ (C). (D and E) Growth assays were performed in the presence of increasing concentrations of CP for wild-type and ΔmntC strains containing either pOS1 plgt (plgt) or pOS1 plgt:mntC (plgt:mntC) in the presence of 0.1 mM PQ (D) or 1.0 mM PQ (E). * = P ≤ 0.05 (two-way ANOVA with Tukey’s posttest corrected for repeated measurements). n ≥ 3. Error bars indicate SEM.
Metabolic demands during infection increase the importance of MntABC.
Building on the observation that oxidative stress increases the importance of MntABC, we examined the impact of other stresses associated with host resistance to bacterial infection. Previously, we observed that CP treatment shifts S. aureus metabolism from sugar to amino acid catabolism due to the increased cellular requirement for Mn imposed by using glucose as a carbon source (50, 58, 59). This shift appears to occur in response to glycolysis being disrupted by Mn limitation, a phenotype that has also been observed in Streptococcus pneumoniae and Bradyrhizobium japonicum (58, 59). At the same time, it is also well established that S. aureus resistance to NO⋅ requires glucose consumption (48, 49, 60). Given these divergent pressures, we investigated whether factors beyond oxidative stress, such as the need to consume glucose, contribute to the importance of MntABC during infection. Using a defined medium supplemented with glucose alone, Casamino Acids alone, or glucose and Casamino Acids as carbon sources, the sensitivity of wild-type and mutant variant strains to CP was evaluated. When glucose was the sole carbon source, the wild-type and ΔmntH strains grew similarly in the presence of CP. By contrast, the ΔmntC and ΔmntC ΔmntH mutants showed increased sensitivity to metal starvation (Fig. 4A). The diminished ability of the ΔmntC and ΔmntC ΔmntH mutants to resist Mn starvation relative to wild-type bacteria was largely abolished when Casamino Acids were provided as the sole carbon source, with the only exception occurring in the double mutant at 120 µg/ml of CP (Fig. 4B). Surprisingly, in the presence of both glucose and Casamino Acids, the ΔmntC and ΔmntC ΔmntH mutants were still more sensitive to CP than either the wild type or the ΔmntH mutant (Fig. 4C). Collectively, these results show that loss of MntABC impairs S. aureus growth in Mn-limited environments when glucose is present. Consequently, our findings demonstrate that MntABC provides a crucial mechanism that enhances the capacity of S. aureus to retain glycolytic function in Mn-limited environments.
FIG 4.
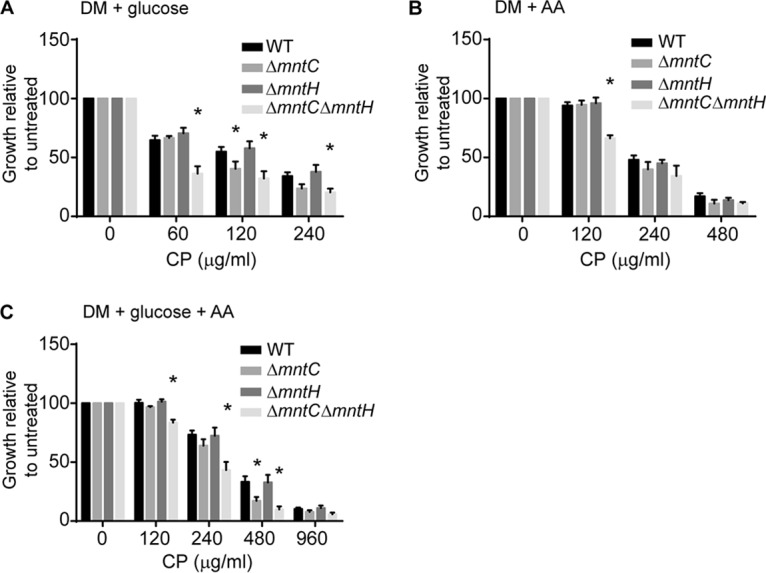
MntABC facilitates glucose consumption when manganese starved. Growth assays were performed with S. aureus wild-type, ΔmntC, ΔmntH, and ΔmntC ΔmntH strains in defined medium (DM) containing glucose only (A), Casamino Acids (AA) only (B), or glucose and Casamino Acids (AA) (C) as a carbon source in the presence of increasing concentrations of CP. * = P ≤ 0.05 (two-way ANOVA with Tukey’s posttest corrected for repeated measurements). n ≥ 3. Error bars indicate SEM.
MntABC facilitates growth in alkaline metal-restricted environments.
During human infection, bacteria are exposed to pH stress, such as the acidic conditions of the phagosome and the slightly alkaline nature of the blood (51, 61). Notably, alkalization precedes infection by S. aureus in burn wounds (52). In Salmonella enterica serovar Typhimurium, and in other pathogenic bacteria that contain homologs of both MntH and MntABC transporters, MntH has been shown to be a proton-driven transporter that functions more effectively under acidic conditions, whereas MntABC is more active under alkaline conditions (62, 63). To determine if the same were true for S. aureus MntH and MntABC, the sensitivity of the wild-type strain and the ΔmntC, ΔmntH, and ΔmntC ΔmntH mutants to CP was evaluated in defined medium supplemented with glucose and buffered to pH 6.4 (acidic), pH 7.4 (neutral), and pH 8.4 (alkaline). In metal-replete medium, all three of the mutants grew similarly to wild-type S. aureus. In the presence of CP, however, the ΔmntC ΔmntH strain had a pronounced growth defect in all three media. In alkaline and neutral media, the ΔmntC strain showed a phenotype similar to that seen with the ΔmntC ΔmntH strain. In contrast, the ΔmntH mutant grew the same as the wild type (Fig. 5A and B). In acidic medium, however, both the ΔmntC and ΔmntH mutant strains grew similarly to wild-type S. aureus (Fig. 5C). Taken together, these observations indicate that extracellular pH influences the contribution of MntABC and MntH to competing with CP for Mn. Thus, the environment to which the pathogen is exposed during infection may also impact the relative contributions of the two staphylococcal Mn transporters to resisting host-imposed metal starvation.
FIG 5.
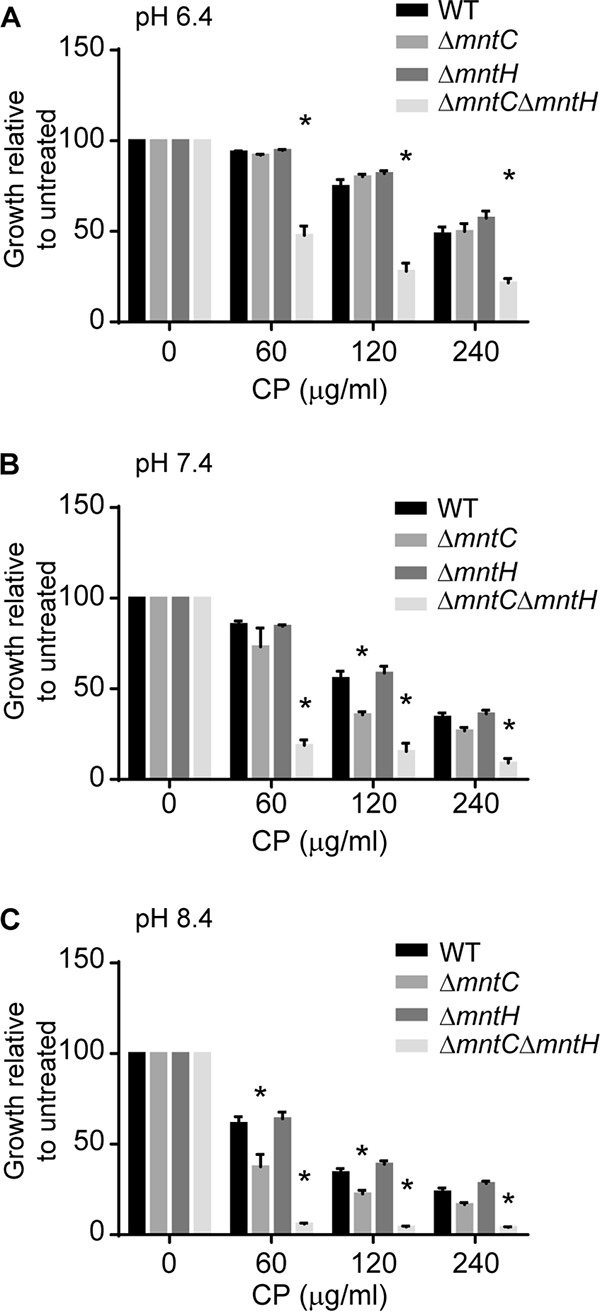
Alkaline environments increase the importance of MntABC to staphylococcal growth. Growth assays were performed in the presence of increasing concentrations of CP with S. aureus wild-type, ΔmntC, ΔmntH, and ΔmntC ΔmntH strains in defined medium containing glucose as a carbon source and adjusted to pH 6.4 (A), pH 7.4 (B), and pH 8.4 (C). * = P ≤ 0.05 (two-way ANOVA with Tukey’s posttest corrected for repeated measurements). n ≥ 3. Error bars indicate SEM.
DISCUSSION
Mn and other transition metals are essential for the survival of pathogens during infection. In the case of Mn, it is an important cofactor for bacterial enzymes, including enzymes involved in carbon metabolism, the stringent response, and detoxification of reactive oxygen species (62). Additionally, Mn can prevent oxidative damage of mononuclear enzymes by replacing Fe in the active site of metalloenzymes (64). Therefore, it is unsurprising that the ability of pathogens to successfully infect a host depends on their ability to efficiently acquire Mn and that the host uses the restriction of Mn as a defense (9, 10, 65). However, successful pathogens, including S. aureus, have evolved mechanisms that allow them to circumvent this host defense, with the most common mechanism being the use of dedicated high-affinity acquisition systems (12, 27–35). S. aureus possesses two Mn transporters, MntABC and MntH (12, 27, 28), and loss of MntABC has been shown to reduce staphylococcal virulence during infection (27, 42, 43). However, knowledge of the molecular basis for this defect has remained elusive, and recent work has suggested that MntABC may not be the primary transporter responsible for competing with the host for Mn (45). The current investigation revealed that MntABC is the primary transporter responsible for resisting host-imposed Mn limitation and that additive insults of nutritional immunity and other host defenses drive the importance of this transporter during infection.
Mn import by MntH- or MntABC-like Mn importers is required for the full virulence of numerous pathogens, including Salmonella enterica serovar Typhimurium, Brucella abortus, Shigella flexneri, multiple Yersinia and Streptococcus species, and others (28, 29, 31–33, 35, 66–68). Mn acquisition has crucial importance in the pathogenesis of numerous bacterial species, and yet there remains significant heterogeneity in the types of Mn transporters employed. The most common Mn importers in pathogenic bacteria are NRAMP systems, such as MntH, and the ABC permeases, such as MntABC. Many species encode one of these transporters, while others, including S. aureus, express both (28–35). In vitro studies have suggested near-parity between MntABC and MntH with respect to their contributions to growth in Mn-limited environments. However, binding studies have shown that CP can outcompete MntC for Mn (45), suggesting that MntABC might not be the primary staphylococcal Mn transporter during infection. Despite this, multiple animal models have shown that MntABC has a critical role in staphylococcal pathogenesis (27, 42, 43). Our current work unequivocally shows that while expression of mntABC does not enable the bacteria to accumulate levels of Mn that are equivalent to those accumulated by bacteria grown in the absence of CP, loss of MntC, but not of MntH, diminished the ability of S. aureus to compete with CP. Further, this function was critical for population of S. aureus superoxide dismutases with Mn. Notably, loss of MntABC did not reduce the ability of S. aureus to obtain any of the other assayed metals. Collectively, these results indicate that, despite CP having a greater affinity for Mn in vitro (45), MntABC is necessary, as it enables S. aureus to obtain sufficient (but not optimal) quantities of Mn during infection. Further, our work also suggested that pathogens that express MntABC homologs compete more effectively with the host for Mn than those that express only homologs of MntH. Examples include Salmonella enterica serovar Typhimurium (69, 70) and avian Escherichia coli (34), where the MntABC-like Mn transporter is more important than the MntH-like transporter.
During infection, S. aureus and other pathogens must simultaneously overcome nutritional immunity and a plethora of other host defenses. In the current study, loss of MntC alone was sufficient to sensitize S. aureus to oxidative stresses in the presence of CP. This observation differs from those reported from previous studies, where loss of both MntABC and MntH was necessary to sensitize S. aureus to oxidative stress in the presence of CP (12). The current study differed from the prior work in that we used a medium that limits intracellular Mn levels to a greater extent than the media used in previous investigations. Glucose is the preferred carbon source for S. aureus and many other bacteria (71–74). However, the consumption of glucose in S. aureus increases the cellular demand for Mn (50). In response to Mn limitation, both S. aureus and S. pneumoniae reroute metabolism toward the consumption of amino acids (50, 59). Similarly, in response to Zn limitation, A. baumannii increases the consumption of histidine (75). In the case of S. aureus, these observations are in tension with the observation that people with diabetes, especially those with hyperglycemia, are more susceptible to S. aureus infection than healthy individuals (76–80). Those prior observations suggest that S. aureus prefers to consume glucose during infection. Additionally, glycolysis enables S. aureus to grow when challenged by NO⋅ produced by activated phagocytes (47, 49). In the presence of CP, loss of MntC impaired the ability to grow when glucose was provided as the sole carbon source. Highlighting the preference of S. aureus for consuming glucose, inactivation of MntC also impaired growth in defined medium when both glucose and amino acids were present. These observations suggest that an increased ability to compete with the host for Mn enabled by MntC allows S. aureus to consume its preferred carbon source and thereby resist NO⋅ stress during infection. This inference is supported by results from a screen that showed that MntABC contributes to NO⋅ resistance (48). These observations highlight the necessity of maintaining cellular Mn levels during infection and hence the importance of MntABC in staphylococcal infection.
A rodent model of S. pneumoniae infection revealed that shortly after infection the pH in the blood increased from neutral to slightly alkaline (51). Burn wound infections have an elevated pH prior to the manifestation of clinical symptoms, and colonization of S. aureus or Staphylococcus epidermidis was shown previously to be favored by alkaline conditions (52). Therefore, it is highly likely that invading pathogens encounter both alkaline and acidic conditions during infection. Previous studies have investigated the impact that pH has on the relative importance of MntABC and MntH. In Salmonella enterica serovar Typhimurium, which encodes both MntH and MntABC homologs, studies have shown that MntABC operates best under slightly alkaline conditions whereas MntH is more active under acidic conditions (62, 63). Similarly to prior work, our findings show that pH impacts the efficacy of the staphylococcal transporters, with MntH functioning optimally in acidic environments. Differing from other pathogens (62, 63), the staphylococcal MntABC appears capable of fully compensating for loss of MntH under the conditions tested. It is possible that MntH may have increased importance in environments that are more acidic than those examined in the current study. While the results of the previous studies have generally been considered from the perspective of transporter function, our current work suggested that altered cellular demand could also contribute to the relative importance of the Mn transporters in acidic and alkaline environments.
If MntABC is important for resisting nutritional immunity and is sufficient under acidic and neutral as well as alkaline conditions, an issue arises as to the necessity of MntH. Our studies failed to reveal physiological conditions that favor MntH, although we did observe that the presence of MntH was sufficient in Mn-replete environments and Mn-depleted acidic environments in the absence of additional stressors. It should be noted that Mn import by the proton-driven MntH is energetically cheaper than that by the ATP-driven MntABC system. Similarly to Mn importers, S. aureus expresses multiple inorganic phosphate importers, including a proton-driven importer, PitA, and an ATP-driven ABC transporter, PstSCAB. PitA is constitutively expressed, while PstSCAB is expressed in response to nutrient limitation. Forcing S. aureus to rely on PstSCAB in phosphate-replete medium results in a reduced growth rate (81). Hence, it is not unreasonable to speculate that, similarly to PitA, MntH is retained because of its greater energy efficiency for the pathogen.
Since being characterized as a Mn- and Zn-binding protein, CP has been reported to bind nearly all of the first-row transition metals, including Fe and Ni, in vitro (24, 25). This has led to suggestions that the antimicrobial activity of CP is driven by its ability to bind a broad array of metal ions. Nevertheless, subsequent studies showed that for S. aureus, A. baumannii, and Candida albicans, the antimicrobial activity of CP in culture is not driven by Fe sequestration (20, 50, 82). Consistent with prior studies (20, 50, 83), our elemental analysis showed that S. aureus successfully competes with CP for Fe. Further diminishing the likelihood of a potential role of CP in Fe sequestration is the observation that extracellular Fe exists as Fe(III) during infection and cannot be bound by CP but is bound avidly by transferrin and lactoferrin (16, 84). Hence, the evidence to date does not support the idea of a role for CP as a general metal sequestration protein during infection. The ability of CP to bind Cu and Zn does enable it to limit the ability of Candida albicans to obtain both of these metals (82). The current analysis revealed that CP does not reduce the cellular levels of Ni, Co, or Cu in S. aureus. Notably, cellular levels of both Ni and Co increased in the presence of CP. This is in contrast to a prior study, which showed reduced cellular Ni concentrations in the presence of CP (25). Differing from the current investigations, the prior study assessed metal accumulation using a metal-depleted defined medium supplemented with excess Ni (25). In the prior study, the observed accumulation of Ni was dependent on the staphylopine-Cnt Zn importer (85). In addition to inducing the expression of Mn transporters, growth of S. aureus in the presence of CP also leads to the induction of zinc uptake systems, including the CntABCDF-staphylopine system (83). While this system functions physiologically as a zinc importer, it is also capable of importing other metals, most notably Co and Ni (85). Induction of this transporter would provide a plausible explanation for the increased accumulation of Co and Ni in the presence of CP. The basis for the difference between the current results and prior results regarding Ni accumulation in the presence of CP is most likely attributable to media formulation. The prior studies used a superphysiological concentration of Ni in the growth medium, facilitating increased accumulation of this metal (85). Thus, despite the ability of CP to bind an array of metals, the antimicrobial activity of this immune effector toward S. aureus appears to be driven by Mn limitation, with some contribution from Zn limitation at higher concentrations (16, 50, 83).
The current study identified a diverse collection of host stresses that increase the importance of the MntABC system to infection and provided a molecular rationale for the critical contribution of this system to staphylococcal infection. While diverse, they are unified by increasing the cellular demand for Mn. Thus, nutritional immunity can be viewed as augmenting the activity of well-established host defenses or vice versa. Regardless of perspective, these results highlight the consideration that should be given to potentiation of host defenses in evaluating the contribution of potential virulence factors to the development of disease.
MATERIALS AND METHODS
Bacterial strains.
For routine overnight cultures, bacteria were grown in 5 ml of tryptic soy broth (TSB) or Chelex-treated RPMI medium plus 1% Casamino Acids (NRPMI) supplemented with 1 mM MgCl2, 100 µM CaCl2, and 1 µM FeCl2 (12) in 15-ml conical tubes at 37°C on a roller drum. As needed, 10 µg/ml of chloramphenicol was added for plasmid maintenance. S. aureus strain Newman and its derivatives were used for all of the experiments. S. aureus strains Newman ΔmntC, Newman ΔmntH, and Newman ΔmntC ΔmntH and complementation constructs were generated in a previous study (12).
CP growth assays.
CP growth assays were performed as described previously (13, 16), with slight modifications. Briefly, for assays using defined medium, bacteria were grown in TSB overnight. The overnight cultures were then back-diluted 1:50 into fresh TSB and grown for 1 h at 37°C. The cultures were diluted 1:100 into 96-well round-bottom plates containing 100 µl of growth medium in the presence of various concentrations of CP. The growth medium for assays using defined medium consisted of 38% medium and 62% CP buffer (20 mM Tris [pH 7.5], 100 mM NaCl, 1 mM CaCl2, 10 mM β-mercaptoethanol) (50). The defined medium consisted of 1.3 g/liter NaCl, 2.6 g/liter NH4Cl, 5.2 g/liter KH2PO4, 18.2 g/liter Na2HPO4, 0.593 µg/liter biotin, 0.593 mg/liter nicotinic acid, 0.593 mg/liter pyridoxine-HCl, 0.593 mg/liter thiamine-HCl, 0.296 mg/liter riboflavin, 1.778 g/liter calcium pantothenate, 0.104 g/liter phenylalanine, 0.078 g/l isoleucine, 0.13 g/l tyrosine, 0.053 g/liter cysteine, 0.26 g/liter glutamic acid 0.026 g/liter lysine, 0.182 g/liter methionine, 0.078 g/liter histidine, 0.026 g/liter tryptophan, 0.234 g/liter leucine, 0.234 g/liter aspartic acid, 0.182 g/liter arginine, 0.078 g/liter serine, 0.15 g/liter alanine, 0.078 g/liter threonine, 0.130 g/liter glycine, 0.208 g/liter valine and 0.026 g/liter proline. The defined medium was supplemented with 6 mM MgSO4, 1 µM FeCl2, 1 µM MnCl2, and 1 µM ZnSO4. Casamino Acids (6.5%) and glucose (1.3%) were provided as carbon sources as indicated. Defined medium supplemented with glucose and adjusted to pH 6.4, pH 7.4, or pH 8.4 was similar to the medium described above and was prepared as described previously (81), with MOPS (morpholinepropanesulfonic acid), HEPES, and Tris buffers being used to adjust the pH. When a metal starvation step was included, the bacteria were grown overnight in NRPMI supplemented with 1 mM MgCl2, 100 µM CaCl2, and 1 µM FeCl2, diluted 1:10 into fresh NRPMI, and then inoculated 1:100 into the assay medium. The assay medium consisted of 38% TSB and 62% CP buffer (20 mM Tris [pH 7.5], 100 mM NaCl, 3 mM CaCl2, 10 mM β-mercaptoethanol). For all assays, the bacteria were incubated with shaking at 37°C and growth was measured by assessing optical density at 600 nm (OD600). In the figures, growth is shown as “growth relative to untreated”; i.e., growth was normalized for each strain to growth in the absence of CP. Calprotectin was purified as previously described (13, 16). Where indicated, 0.1 mM or 1.0 mM paraquat (PQ) was added to the assay medium.
SOD activity.
Individual superoxide dismutase activity (SOD) was assessed using a gel-based nitroblue tetrazolium assay, as previously described (41, 86). Bacteria were grown overnight in NRPMI supplemented with 1 mM MgCl2, 100 µM CaCl2 and 1 µM FeCl2, diluted 1:10 into fresh NRPMI, and then inoculated 1:100 into the assay medium (38% TSB and 62% CP buffer supplemented with 1 µM MnCl2 and 1 µM ZnSO4) and grown in the presence and absence of 240 µg/ml of CP and in the presence and absence of 0.1 mM PQ. The bacteria were incubated with shaking at 37°C, and growth was measured by assessing optical density (OD600). The bacteria were harvested in exponential phase (OD600 = ∼0.1), and cells were collected and resuspended in 0.5 mM KPO4 at pH 7.8 with 0.1 mM EDTA (41). The bacteria were lysed via mechanical disruption, insoluble material was removed by centrifugation, and protein concentrations were determined using a bicinchoninic acid (BCA) assay kit. In order to evaluate individual SOD activity levels, cell lysates with normalized protein concentrations were resolved on 10% native polyacrylamide gels. The gels were then incubated in buffer containing 0.05 M KPO4 at pH 7.8 with 1 mM EDTA, 0.25 mM nitro blue tetrazolium chloride, and 0.05 mM riboflavin and exposed to light, as previously described (86). In order to evaluate if SOD was iron loaded, gels were incubated with 20 mM H2O2 or water for 20 min prior to gel staining. Gels were imaged using a Bio-Rad Universal Hood II imager, and the fractional distribution of SOD activity was determined using Bio-Rad Quantity One software.
Elemental analyses.
For whole-cell metal accumulation, S. aureus strains were grown overnight in NRPMI supplemented with 1 mM MgCl2, 100 µM CaCl2 and 1 µM FeCl2, diluted 1:10 into fresh NRPMI, and then inoculated 1:100 into the assay medium. The assay medium consisted of 38% TSB and 62% CP buffer supplemented with 1 µM MnCl2 and 1 µM ZnSO4. Bacteria were harvested during the exponential phase (OD600 = 0.1), washed twice with 100 mM EDTA, and washed twice with water. Bacterial pellets were resuspended in 1 ml of water, and a small aliquot was taken to determine CFU. The bacteria were then pelleted, the supernatant was removed, and pellets were desiccated at 96°C overnight. The dry cell weight was determined, the pellets were resuspended in 35% HNO3, and samples were boiled at 95°C for 1 h prior to removal of debris by centrifugation. Samples were diluted to a final concentration of 3.5% HNO3 and analyzed by inductively coupled plasma mass spectrometry (ICP-MS) on an Agilent 8900 ICP mass spectrometer (Adelaide Microscopy, University of Adelaide), as described previously (87, 88).
Statistical analysis.
All statistical analyses were performed using GraphPad Prism version 6. For details of the specific statistical tests used, see figure legends.
ACKNOWLEDGMENTS
We thank the members of the Kehl-Fie laboratory for critical reading of the manuscript.
This work was supported by grants from the National Institutes of Health (K22 AI104805 and RO1 AI18880), by a March of Dimes Basil O’Conner award and a Vallee Scholars award to T.E.K.-F., and by grants from the National Health and Medical Research Council (project grants 1122582 and 1140554) and the Australian Research Council (Discovery Project DP170102102 and Future Fellowship FT170100006) to C.A.M. This work does not represent the views of the March of Dimes or the National Institutes of Health.
REFERENCES
- 1.Wertheim HF, Vos MC, Ott A, van Belkum A, Voss A, Kluytmans JA, van Keulen PH, Vandenbroucke-Grauls CM, Meester MH, Verbrugh HA. 2004. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364:703–705. doi: 10.1016/S0140-6736(04)16897-9. [DOI] [PubMed] [Google Scholar]
- 2.Kluytmans J, van Belkum A, Verbrugh H. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 10:505–520. doi: 10.1128/CMR.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 4.CDC. 2013. Antibiotic resistance threats in the United States, 2013. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf.
- 5.WHO. 2014. Antimicrobial resistance global report on surveillance. http://apps.who.int/iris/bitstream/handle/10665/112642/9789241564748_eng.pdf;jsessionid=CED8E9C818604145CEE877A03FA6E5E0?sequence=1.
- 6.Andreini C, Bertini I, Cavallaro G, Holliday GL, Thornton JM. 2008. Metal ions in biological catalysis: from enzyme databases to general principles. J Biol Inorg Chem 13:1205–1218. doi: 10.1007/s00775-008-0404-5. [DOI] [PubMed] [Google Scholar]
- 7.Waldron KJ, Rutherford JC, Ford D, Robinson NJ. 2009. Metalloproteins and metal sensing. Nature 460:823–830. doi: 10.1038/nature08300. [DOI] [PubMed] [Google Scholar]
- 8.Weinberg ED. 2009. Iron availability and infection. Biochim Biophys Acta 1790:600–605. doi: 10.1016/j.bbagen.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Kehl-Fie TE, Skaar EP. 2010. Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol 14:218–224. doi: 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hood MI, Skaar EP. 2012. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, Caprioli RM, Nacken W, Chazin WJ, Skaar EP. 2008. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319:962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- 12.Kehl-Fie TE, Zhang Y, Moore JL, Farrand AJ, Hood MI, Rathi S, Chazin WJ, Caprioli RM, Skaar EP. 2013. MntABC and MntH contribute to systemic Staphylococcus aureus infection by competing with calprotectin for nutrient manganese. Infect Immun 81:3395–3405. doi: 10.1128/IAI.00420-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kehl-Fie TE, Chitayat S, Hood MI, Damo S, Restrepo N, Garcia C, Munro KA, Chazin WJ, Skaar EP. 2011. Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe 10:158–164. doi: 10.1016/j.chom.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clohessy PA, Golden BE. 1995. Calprotectin-mediated zinc chelation as a biostatic mechanism in host defence. Scand J Immunol 42:551–556. doi: 10.1111/j.1365-3083.1995.tb03695.x. [DOI] [PubMed] [Google Scholar]
- 15.Gebhardt C, Nemeth J, Angel P, Hess J. 2006. S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol 72:1622–1631. doi: 10.1016/j.bcp.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Damo SM, Kehl-Fie TE, Sugitani N, Holt ME, Rathi S, Murphy WJ, Zhang Y, Betz C, Hench L, Fritz G, Skaar EP, Chazin WJ. 2013. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc Natl Acad Sci U S A 110:3841–3846. doi: 10.1073/pnas.1220341110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. 2009. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog 5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bianchi M, Niemiec MJ, Siler U, Urban CF, Reichenbach J. 2011. Restoration of anti-Aspergillus defense by neutrophil extracellular traps in human chronic granulomatous disease after gene therapy is calprotectin-dependent. J Allergy Clin Immunol 127:1243–1252e7. doi: 10.1016/j.jaci.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Achouiti A, Vogl T, Urban CF, Rohm M, Hommes TJ, van Zoelen MA, Florquin S, Roth J, van 't Veer C, de Vos AF, van der Poll T. 2012. Myeloid-related protein-14 contributes to protective immunity in gram-negative pneumonia derived sepsis. PLoS Pathog 8:e1002987. doi: 10.1371/journal.ppat.1002987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hood MI, Mortensen BL, Moore JL, Zhang Y, Kehl-Fie TE, Sugitani N, Chazin WJ, Caprioli RM, Skaar EP. 2012. Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLoS Pathog 8:e1003068. doi: 10.1371/journal.ppat.1003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayden JA, Brophy MB, Cunden LS, Nolan EM. 2013. High-affinity manganese coordination by human calprotectin is calcium-dependent and requires the histidine-rich site formed at the dimer interface. J Am Chem Soc 135:775–787. doi: 10.1021/ja3096416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brophy MB, Hayden JA, Nolan EM. 2012. Calcium ion gradients modulate the zinc affinity and antibacterial activity of human calprotectin. J Am Chem Soc 134:18089–18100. doi: 10.1021/ja307974e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brophy MB, Nakashige TG, Gaillard A, Nolan EM. 2013. Contributions of the S100A9 C-terminal tail to high-affinity Mn(II) chelation by the host-defense protein human calprotectin. J Am Chem Soc 135:17804–17817. doi: 10.1021/ja407147d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakashige TG, Zhang B, Krebs C, Nolan EM. 2015. Human calprotectin is an iron-sequestering host-defense protein. Nat Chem Biol doi: 10.1038/nchembio.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakashige TG, Zygiel EM, Drennan CL, Nolan EM. 2017. Nickel sequestration by the host-defense protein human calprotectin. J Am Chem Soc 139:8828–8836. doi: 10.1021/jacs.7b01212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korndorfer IP, Brueckner F, Skerra A. 2007. The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting alpha-helices can determine specific association of two EF-hand proteins. J Mol Biol 370:887–898. doi: 10.1016/j.jmb.2007.04.065. [DOI] [PubMed] [Google Scholar]
- 27.Horsburgh MJ, Wharton SJ, Cox AG, Ingham E, Peacock S, Foster SJ. 2002. MntR modulates expression of the PerR regulon and superoxide resistance in Staphylococcus aureus through control of manganese uptake. Mol Microbiol 44:1269–1286. doi: 10.1046/j.1365-2958.2002.02944.x. [DOI] [PubMed] [Google Scholar]
- 28.Anderson ES, Paulley JT, Gaines JM, Valderas MW, Martin DW, Menscher E, Brown TD, Burns CS, Roop RM II.. 2009. The manganese transporter MntH is a critical virulence determinant for Brucella abortus 2308 in experimentally infected mice. Infect Immun 77:3466–3474. doi: 10.1128/IAI.00444-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Champion OL, Karlyshev A, Cooper IA, Ford DC, Wren BW, Duffield M, Oyston PC, Titball RW. 2011. Yersinia pseudotuberculosis mntH functions in intracellular manganese accumulation, which is essential for virulence and survival in cells expressing functional Nramp1. Microbiology 157:1115–1122. doi: 10.1099/mic.0.045807-0. [DOI] [PubMed] [Google Scholar]
- 30.Janulczyk R, Ricci S, Bjorck L. 2003. MtsABC is important for manganese and iron transport, oxidative stress resistance, and virulence of Streptococcus pyogenes. Infect Immun 71:2656–2664. doi: 10.1128/IAI.71.5.2656-2664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paik S, Brown A, Munro CL, Cornelissen CN, Kitten T. 2003. The sloABCR operon of Streptococcus mutans encodes an Mn and Fe transport system required for endocarditis virulence and its Mn-dependent repressor. J Bacteriol 185:5967–5975. doi: 10.1128/JB.185.20.5967-5975.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perry RD, Craig SK, Abney J, Bobrov AG, Kirillina O, Mier I Jr, Truszczynska H, Fetherston JD. 2012. Manganese transporters Yfe and MntH are Fur-regulated and important for the virulence of Yersinia pestis. Microbiology 158:804–815. doi: 10.1099/mic.0.053710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Runyen-Janecky L, Dazenski E, Hawkins S, Warner L. 2006. Role and regulation of the Shigella flexneri sit and MntH systems. Infect Immun 74:4666–4672. doi: 10.1128/IAI.00562-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabri M, Caza M, Proulx J, Lymberopoulos MH, Bree A, Moulin-Schouleur M, Curtiss R III, Dozois CM. 2008. Contribution of the SitABCD, MntH, and FeoB metal transporters to the virulence of avian pathogenic Escherichia coli O78 strain chi7122. Infect Immun 76:601–611. doi: 10.1128/IAI.00789-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wichgers Schreur PJ, Rebel JM, Smits MA, van Putten JP, Smith HE. 2011. TroA of Streptococcus suis is required for manganese acquisition and full virulence. J Bacteriol 193:5073–5080. doi: 10.1128/JB.05305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartsevich VV, Pakrasi HB. 1995. Molecular identification of an ABC transporter complex for manganese: analysis of a cyanobacterial mutant strain impaired in the photosynthetic oxygen evolution process. EMBO J 14:1845–1853. doi: 10.1002/j.1460-2075.1995.tb07176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Que Q, Helmann JD. 2000. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol Microbiol 35:1454–1468. [DOI] [PubMed] [Google Scholar]
- 38.Makui H, Roig E, Cole ST, Helmann JD, Gros P, Cellier MF. 2000. Identification of the Escherichia coli K-12 Nramp orthologue (MntH) as a selective divalent metal ion transporter. Mol Microbiol 35:1065–1078. doi: 10.1046/j.1365-2958.2000.01774.x. [DOI] [PubMed] [Google Scholar]
- 39.Kehres DG, Zaharik ML, Finlay BB, Maguire ME. 2000. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol Microbiol 36:1085–1100. doi: 10.1046/j.1365-2958.2000.01922.x. [DOI] [PubMed] [Google Scholar]
- 40.Diep BA, Phung Q, Date S, Arnott D, Bakalarski C, Xu M, Nakamura G, Swem DL, Alexander MK, Le HN, Mai TT, Tan MW, Brown EJ, Nishiyama M. 2014. Identifying potential therapeutic targets of methicillin-resistant Staphylococcus aureus through in vivo proteomic analysis. J Infect Dis 209:1533–1541. doi: 10.1093/infdis/jit662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia YM, Barwinska-Sendra A, Tarrant E, Skaar EP, Waldron KJ, Kehl-Fie TE. 2017. A superoxide dismutase capable of functioning with iron or manganese promotes the resistance of Staphylococcus aureus to calprotectin and nutritional immunity. PLoS Pathog 13:e1006125. doi: 10.1371/journal.ppat.1006125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coady A, Xu M, Phung Q, Cheung TK, Bakalarski C, Alexander MK, Lehar SM, Kim J, Park S, Tan MW, Nishiyama M. 2015. The Staphylococcus aureus ABC-type manganese transporter MntABC is critical for reinitiation of bacterial replication following exposure to phagocytic oxidative burst. PLoS One 10:e0138350. doi: 10.1371/journal.pone.0138350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Handke LD, Gribenko AV, Timofeyeva Y, Scully IL, Anderson AS. 2018. MntC-dependent manganese transport is essential for Staphylococcus aureus oxidative stress resistance and virulence. mSphere 3:e00336-18. doi: 10.1128/mSphere.00336-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Handke LD, Hawkins JC, Miller AA, Jansen KU, Anderson AS. 2013. Regulation of Staphylococcus aureus MntC expression and its role in response to oxidative stress. PLoS One 8:e77874. doi: 10.1371/journal.pone.0077874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hadley RC, Gagnon DM, Brophy MB, Gu Y, Nakashige TG, Britt RD, Nolan EM. 2018. Biochemical and spectroscopic observation of Mn(II) sequestration from bacterial Mn(II) transport machinery by calprotectin. J Am Chem Soc 140:110–113. doi: 10.1021/jacs.7b11207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rigby KM, DeLeo FR. 2012. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin Immunopathol 34:237–259. doi: 10.1007/s00281-011-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fang FC. 2004. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol 2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 48.Grosser MR, Paluscio E, Thurlow LR, Dillon MM, Cooper VS, Kawula TH, Richardson AR. 2018. Genetic requirements for Staphylococcus aureus nitric oxide resistance and virulence. PLoS Pathog 14:e1006907. doi: 10.1371/journal.ppat.1006907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vitko NP, Spahich NA, Richardson AR. 2015. Glycolytic dependency of high-level nitric oxide resistance and virulence in Staphylococcus aureus. mBio 6:e00045-15. doi: 10.1128/mBio.00045-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Radin JN, Kelliher JL, Parraga Solorzano PK, Kehl-Fie TE. 2016. The two-component system ArlRS and alterations in metabolism enable Staphylococcus aureus to resist calprotectin-induced manganese starvation. PLoS Pathog 12:e1006040. doi: 10.1371/journal.ppat.1006040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elwell MR, Sammons ML, Liu CT, Beisel WR. 1975. Changes in blood pH in rats after infection with Streptococcus pneumoniae. Infect Immun 11:724–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ono S, Imai R, Ida Y, Shibata D, Komiya T, Matsumura H. 2015. Increased wound pH as an indicator of local wound infection in second degree burns. Burns 41:820–824. doi: 10.1016/j.burns.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 53.Beyer W, Imlay J, Fridovich I. 1991. Superoxide dismutases. Prog Nucleic Acids Res Mol Biol 40:221–253. doi: 10.1016/S0079-6603(08)60843-0. [DOI] [PubMed] [Google Scholar]
- 54.Imlay JA. 2003. Pathways of oxidative damage. Annu Rev Microbiol 57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 55.Storz G, Imlay JA. 1999. Oxidative stress. Curr Opin Microbiol 2:188–194. doi: 10.1016/S1369-5274(99)80033-2. [DOI] [PubMed] [Google Scholar]
- 56.Anderson AS, Scully IL, Timofeyeva Y, Murphy E, McNeil LK, Mininni T, Nunez L, Carriere M, Singer C, Dilts DA, Jansen KU. 2012. Staphylococcus aureus manganese transport protein C is a highly conserved cell surface protein that elicits protective immunity against S. aureus and Staphylococcus epidermidis. J Infect Dis 205:1688–1696. doi: 10.1093/infdis/jis272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bray RC, Cockle SA, Fielden EM, Roberts PB, Rotilio G, Calabrese L. 1974. Reduction and inactivation of superoxide dismutase by hydrogen peroxide. Biochem J 139:43–48. doi: 10.1042/bj1390043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hohle TH, O'Brian MR. 2012. Manganese is required for oxidative metabolism in unstressed Bradyrhizobium japonicum cells. Mol Microbiol 84:766–777. doi: 10.1111/j.1365-2958.2012.08057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ogunniyi AD, Mahdi LK, Jennings MP, McEwan AG, McDevitt CA, Van der Hoek MB, Bagley CJ, Hoffmann P, Gould KA, Paton JC. 2010. Central role of manganese in regulation of stress responses, physiology, and metabolism in Streptococcus pneumoniae. J Bacteriol 192:4489–4497. doi: 10.1128/JB.00064-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vitko NP, Grosser MR, Khatri D, Lance TR, Richardson AR. 2016. Expanded glucose import capability affords Staphylococcus aureus optimized glycolytic flux during infection. mBio 7:e00296-16. doi: 10.1128/mBio.00296-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cech P, Lehrer RI. 1984. Phagolysosomal pH of human neutrophils. Blood 63:88–95. [PubMed] [Google Scholar]
- 62.Papp-Wallace KM, Maguire ME. 2006. Manganese transport and the role of manganese in virulence. Annu Rev Microbiol 60:187–209. doi: 10.1146/annurev.micro.60.080805.142149. [DOI] [PubMed] [Google Scholar]
- 63.Kehres DG, Maguire ME. 2003. Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria. FEMS Microbiol Rev 27:263–290. doi: 10.1016/S0168-6445(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 64.Puri S, Hohle TH, O'Brian MR. 2010. Control of bacterial iron homeostasis by manganese. Proc Natl Acad Sci U S A 107:10691–10695. doi: 10.1073/pnas.1002342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Juttukonda LJ, Skaar EP. 2015. Manganese homeostasis and utilization in pathogenic bacteria. Mol Microbiol 97:216–228. doi: 10.1111/mmi.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boyer E, Bergevin I, Malo D, Gros P, Cellier MF. 2002. Acquisition of Mn(II) in addition to Fe(II) is required for full virulence of Salmonella enterica serovar Typhimurium. Infect Immun 70:6032–6042. doi: 10.1128/IAI.70.11.6032-6042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fetherston JD, Mier I Jr, Truszczynska H, Perry RD. 2012. The Yfe and Feo transporters are involved in microaerobic growth and virulence of Yersinia pestis in bubonic plague. Infect Immun 80:3880–3891. doi: 10.1128/IAI.00086-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dintilhac A, Alloing G, Granadel C, Claverys JP. 1997. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol Microbiol 25:727–739. doi: 10.1046/j.1365-2958.1997.5111879.x. [DOI] [PubMed] [Google Scholar]
- 69.Kehres DG, Janakiraman A, Slauch JM, Maguire ME. 2002. SitABCD is the alkaline Mn(2+) transporter of Salmonella enterica serovar Typhimurium. J Bacteriol 184:3159–3166. doi: 10.1128/JB.184.12.3159-3166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zaharik ML, Cullen VL, Fung AM, Libby SJ, Kujat Choy SL, Coburn B, Kehres DG, Maguire ME, Fang FC, Finlay BB. 2004. The Salmonella enterica serovar typhimurium divalent cation transport systems MntH and SitABCD are essential for virulence in an Nramp1G169 murine typhoid model. Infect Immun 72:5522–5525. doi: 10.1128/IAI.72.9.5522-5525.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deutscher J, Francke C, Postma PW. 2006. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev 70:939–1031. doi: 10.1128/MMBR.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liebeke M, Dorries K, Zuhlke D, Bernhardt J, Fuchs S, Pane-Farre J, Engelmann S, Volker U, Bode R, Dandekar T, Lindequist U, Hecker M, Lalk M. 2011. A metabolomics and proteomics study of the adaptation of Staphylococcus aureus to glucose starvation. Mol Biosyst 7:1241–1253. doi: 10.1039/c0mb00315h. [DOI] [PubMed] [Google Scholar]
- 73.Gotz A, Goebel W. 2010. Glucose and glucose 6-phosphate as carbon sources in extra- and intracellular growth of enteroinvasive Escherichia coli and Salmonella enterica. Microbiology 156:1176–1187. doi: 10.1099/mic.0.034744-0. [DOI] [PubMed] [Google Scholar]
- 74.Inada T, Kimata K, Aiba H. 1996. Mechanism responsible for glucose-lactose diauxie in Escherichia coli: challenge to the cAMP model. Genes Cells 1:293–301. doi: 10.1046/j.1365-2443.1996.24025.x. [DOI] [PubMed] [Google Scholar]
- 75.Nairn BL, Lonergan ZR, Wang J, Braymer JJ, Zhang Y, Calcutt MW, Lisher JP, Gilston BA, Chazin WJ, de Crecy-Lagard V, Giedroc DP, Skaar EP. 2016. The response of Acinetobacter baumannii to zinc starvation. Cell Host Microbe 19:826–836. doi: 10.1016/j.chom.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lipsky BA, Tabak YP, Johannes RS, Vo L, Hyde L, Weigelt JA. 2010. Skin and soft tissue infections in hospitalised patients with diabetes: culture isolates and risk factors associated with mortality, length of stay and cost. Diabetologia 53:914–923. doi: 10.1007/s00125-010-1672-5. [DOI] [PubMed] [Google Scholar]
- 77.Fowler VG Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, Corey GR, Spelman D, Bradley SF, Barsic B, Pappas PA, Anstrom KJ, Wray D, Fortes CQ, Anguera I, Athan E, Jones P, van der Meer JTM, Elliott TSJ, Levine DP, Bayer AS, ICE Investigators . 2005. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 293:3012–3021. doi: 10.1001/jama.293.24.3012. [DOI] [PubMed] [Google Scholar]
- 78.Kourany WM, Miro JM, Moreno A, Corey GR, Pappas PA, Abrutyn E, Hoen B, Habib G, Fowler VG Jr, Sexton DJ, Olaison L, Cabell CH, ICE MD Investigators . 2006. Influence of diabetes mellitus on the clinical manifestations and prognosis of infective endocarditis: a report from the International Collaboration on Endocarditis-Merged Database. Scand J Infect Dis 38:613–619. doi: 10.1080/00365540600617017. [DOI] [PubMed] [Google Scholar]
- 79.King JT Jr, Goulet JL, Perkal MF, Rosenthal RA. 2011. Glycemic control and infections in patients with diabetes undergoing noncardiac surgery. Ann Surg 253:158–165. doi: 10.1097/SLA.0b013e3181f9bb3a. [DOI] [PubMed] [Google Scholar]
- 80.Kornum JB, Thomsen RW, Riis A, Lervang HH, Schonheyder HC, Sorensen HT. 2008. Diabetes, glycemic control, and risk of hospitalization with pneumonia: a population-based case-control study. Diabetes Care 31:1541–1545. doi: 10.2337/dc08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kelliher JL, Radin JN, Grim KP, Parraga Solorzano PK, Degnan PH, Kehl-Fie TE. 2018. Acquisition of the phosphate transporter NptA enhances Staphylococcus aureus pathogenesis by improving phosphate uptake in divergent environments. Infect Immun 86:e00631-17. doi: 10.1128/IAI.00631-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Besold AN, Gilston BA, Radin JN, Ramsoomair C, Culbertson EM, Li CX, Cormack BP, Chazin WJ, Kehl-Fie TE, Culotta VC. 2017. The role of calprotectin in withholding zinc and copper from Candida albicans. Infect Immun 85:e00779-17. doi: 10.1128/IAI.00779-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grim KP, San Francisco B, Radin JN, Brazel EB, Kelliher JL, Parraga Solorzano PK, Kim PC, McDevitt CA, Kehl-Fie TE. 2017. The metallophore staphylopine enables Staphylococcus aureus to compete with the host for zinc and overcome nutritional immunity. mBio 8:e01281-17. doi: 10.1128/mBio.01281-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Andrews SC, Robinson AK, Rodríguez-Quiñones F. 2003. Bacterial iron homeostasis. FEMS Microbiol Rev 27:215–237. doi: 10.1016/S0168-6445(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 85.Remy L, Carrière M, Derré-Bobillot A, Martini C, Sanguinetti M, Borezée-Durant E. 2013. The Staphylococcus aureus Opp1 ABC transporter imports nickel and cobalt in zinc-depleted conditions and contributes to virulence. Mol Microbiol 87:730–743. doi: 10.1111/mmi.12126. [DOI] [PubMed] [Google Scholar]
- 86.McCord JM. 2001. Analysis of superoxide dismutase activity. Curr Protoc Toxicol Chapter 7:Unit 7.3. doi: 10.1002/0471140856.tx0703s00. [DOI] [PubMed] [Google Scholar]
- 87.Begg SL, Eijkelkamp BA, Luo Z, Counago RM, Morey JR, Maher MJ, Ong CL, McEwan AG, Kobe B, O'Mara ML, Paton JC, McDevitt CA. 2015. Dysregulation of transition metal ion homeostasis is the molecular basis for cadmium toxicity in Streptococcus pneumoniae. Nat Commun 6:6418. doi: 10.1038/ncomms7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Counago RM, Ween MP, Begg SL, Bajaj M, Zuegg J, O'Mara ML, Cooper MA, McEwan AG, Paton JC, Kobe B, McDevitt CA. 2014. Imperfect coordination chemistry facilitates metal ion release in the Psa permease. Nat Chem Biol 10:35–41. doi: 10.1038/nchembio.1382. [DOI] [PubMed] [Google Scholar]