Abstract
Human Papillomavirus (HPV) infection is the most common sexually transmitted infection and is associated with the development of cervical cancer. The purpose of this report is to provide the literature evidences on selecting the HPV vaccine for national immunization program (NIP) in Korea. To complete these tasks, we reviewed domestic and foreign literature on the current status of HPV infection, efficacy and effectiveness of HPV vaccine, safety of vaccine and cost effectiveness analysis of vaccination business. Given that the median age of first sexual intercourse is continuing to fall, this may have serious implications for HPV infection and cervical cancer incidence at the age of 20s. The World Health Organization recommends that the HPV vaccination should be included in the NIP being implemented in each country. Both the bivalent and quadrivalent vaccines have a 90% or greater preventive efficacy on cervical intraepithelial lesion 2–3 and cervical cancer by the HPV 16 or HPV 18. In the future, if HPV vaccination rate as part of NIP increases, it is expected that the incidence of HPV infection, genital warts, and cervical precancerous lesions will be decreased in the vaccination age group. Therefore, in order to increase the HPV vaccination rate at this point in Korea, social consensus and efforts such as the introduction and promotion of HPV vaccine to the NIP according to appropriate cost-effectiveness analysis are required.
Keywords: Human Papillomavirus Infection, National Immunization Program, Vaccination, Cervical Cancer
Graphical Abstract
INTRODUCTION
Human papillomavirus (HPV) is a DNA virus with more than 150 species, of which more than 40 are known to be able to infect human mucosal surfaces through sexual contact. Among these, HPV 16 and HPV 18 are known as major viruses related to the mechanism of carcinogenesis in various malignant diseases such as cervical cancer, vaginal cancer, vulvar cancer, anal cancer, and head and neck cancer.1 There are about 500,000 cases of cervical cancer worldwide every year.2 In addition, there are more than 40,000 cases of vulvar cancer and vaginal cancer worldwide each year. HPV can also infect men. Anal cancer has more than 90,000 cases every year in both men and women, and other types 6 and 11 are the main causes of genital warts in both genders.
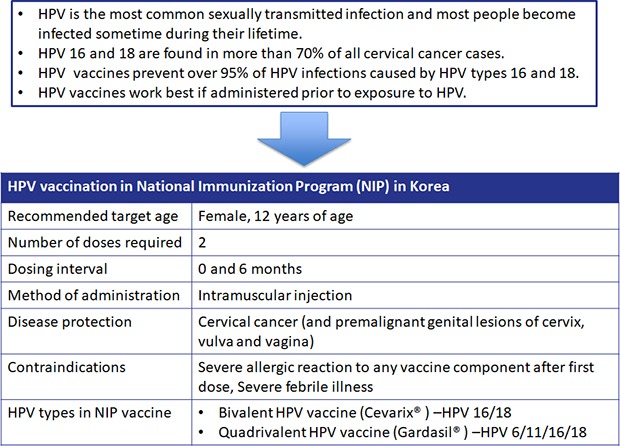
HPV infection is the leading cause of cervical cancer, and HPV infection is found in more than 99.7% of cervical cancer patients.3 The most frequent HPV subtypes in patients with cervical cancer in Korea are 16, 18, 58, 33, and 52. Among them, HPV 16 and 18 are found in more than 70% of cervical cancer patients.4 According to the National Cancer Census Report of Korea in 2017, the incidence of cervical cancer in 2015 was 14.1 per 100,000 women (age standardization incidence was 10.8) and ranked 7th among all women's cancers in Korea.5 Cervical cancer is the second most common women's cancer mortality among women worldwide (15–44 years), which is fatal enough to kill one patient every two minutes.6
The current cervical cancer vaccine, which has been introduced into clinical practice, is actually a vaccine against HPV infection. There are three types of vaccines: bivalent vaccine (Cervarix; GlaxoSmithKline, London, UK), quadrivalent vaccine, and 9-valent vaccine (Gardasil; Merck & Co., Kenilworth, NJ, USA). The bivalent vaccine has been approved by the Australian Government in 2007 and is currently on the market with the approval of the Korea Food and Drug Administration (KFDA) in 2008. The quadrivalent vaccine was approved by the US Food and Drug Administration in June 2006 and it was approved by the KFDA in June 2007 and is currently on the market (Table 1). Recently, the 9–valent HPV vaccine was approved by the KFDA in January 2016. The 9–valent HPV vaccine extends coverage to HPV 31, 33, 45, 52, and 58, which is expected to offer more protection by preventing infection and disease associated with 5 additional high-risk HPV.
Table 1. Characteristics of bivalent and quadrivalent HPV vaccine.
| Characteristics | Bivalent | Quadrivalent |
|---|---|---|
| Manufacturer | GlaxoSmithKline | Merck & Co. Inc. |
| VLP type | HPV 16/18 | HPV 6/11/16/18 |
| Vaccine composition (L1 protein), µg | 20/20 | 20/40/40/20 |
| Producer cells | Trichoplusia ni (Hi-5) insect cell line infected with L1 recombinant baculovirus | Saccharomyces cerevisiae (baker's yeast) expressing L1 |
| Adjuvant | ASO4: | AAHS: |
| 500 µg aluminum hydroxide | 225 µg amorphous aluminum hydroxyphosphate sulfate | |
| 50 µg 3-O-deacylated-4′-monophosphoryl lipid A | ||
| Dose and injection schedule | 0.5 mL at 0, 1, 6 mon | 0.5 mL at 0, 2, 6 mon |
| Route administration | Intramuscular injection | Intramuscular injection |
| Licensed | Female, 9–25 yr | Female, 9–26 yr |
| Male, 9–26 yr |
HPV = human papillomavirus, VLP = virus-like particle, ASO4 = aluminum hydroxide with 3-deacylated monophosphoryl lipid A, AAHS = amorphous aluminum hydroxyphosphate sulfate.
Both the bivalent and quadrivalent vaccines have been shown to have high safety and efficacy as a result of phase 3 trials. Long-term follow-up of 5 years after immunization also showed high effect. Both vaccines are recommended for women aged 9–25 (26) years, with 3-dose schedule (0, 1, and 6 months for bivalent vaccines and 0, 2, and 6 months for quadrivalent vaccines). It is best to inoculate before starting sexual activity. A recent clinical study of 9–13 (14) aged girls showed that the antibody titers after two vaccinations (0, 6 months) were not lower than the antibody titers after three vaccinations of 15–26 years old women. Therefore, the World Health Organization (WHO) has recently recommended 2-dose schedule at this age and emphasized that the HPV vaccine should be included in the country's National Immunization Program (NIP). Currently, HPV vaccines are managed as NIPs in 84 countries by September 2017. In Korea, the bivalent and quadrivalent HPV vaccines have been included in the NIP since June 2016, which is administered to 12 years old girl with 2-dose schedule.
In order to implement the NIP of HPV vaccine in Korea, it is necessary to select the appropriate vaccine to be used for the project, and to examine the vaccination subjects, and the number of vaccinations. The purpose of this report was to provide a theoretical basis for the decision to introduce a vaccine based on literature review through objective data collection on the current status of HPV related disease in Korea, vaccine prevention effect, safety and cost effectiveness of NIP.
CURRENT STATUS OF HPV INFECTION IN KOREA
Increasing rate of HPV infection rate in young age
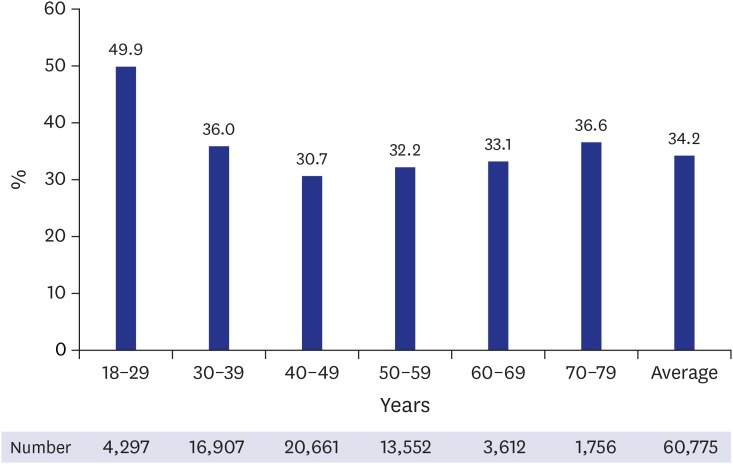
The Korean Society of Gynecologic Oncology (KSGO) analyzed 60,775 women aged 18–79 years in Korea from 2006 to 2011.7 As a result, 34.2% (20,787) of the subjects were infected with HPV. Of the women, 17.5% (10,628) had high-risk types of HPV that could develop into malignant tumors such as cervical cancer, and 16.7% (10,159) were infected with low-risk types of HPV causing genital warts. According to the HPV infection rate by age group, it was the highest (49.9%) in 18–29 years old and after that, it showed the U-shape which increased again to 36.6% in 70–79 years old (Fig. 1).
Fig. 1. Human papillomavirus infection rate by age, Korea gynecologic oncology group 2012.
The high HPV infection rates in sexually active young women, followed by a decrease in HPV infection rates in middle-aged women have been observed worldwide. Approximately 75%–80% of individuals, either men or women, are estimated to be HPV positive at least once in their lifetime.8 Although HPV infection does not necessarily progress to cervical cancer, persistent HPV infections can lead to serious conditions including cervical cancer and cervical intraepithelial neoplasia (CIN).
Increasing rate of young cervical cancer patients
According to data from the Ministry of Education, the Ministry of Health and Welfare, and the Korea Centers for Disease Control and Prevention (KCDC) in 2014, 3,282 high and middle school students who started sexual experience showed that their first sexual experience age was 13.1 years. This is faster than the 13.6 years of 2005.9 As the age of first sexual experience declines, younger girls are more likely to be exposed to HPV infection, leading to a higher incidence of cervical cancer in their 20s. According to recent domestic studies, the proportion of young women under 35 years of age is increasing among all cervical cancer patients. The percentage of patients under the age of 35 with cervical cancer increased from 6% in 1990–1992 to 11.3% in 2005–2006.10
HPV infection-related disease burden
Cervical cancer and genital warts that are caused by HPV are not only psychological but also economically burdensome to patients. In the burden of domestic HPV-related diseases, the cost of disease including cervical cancer, precancerous lesion of cervix, genital warts are about 179 billion Korean Won (KRW) every year. The costs of HPV-related disease were estimated to be 35.6 billion KRW for cervical cancer, 39.8 billion KRW for death from cervical cancer, 2.4 billion KRW for carcinoma in situ (CIS), 370 million KRW for CIN, and 1.8 billion KRW for genital warts.11 The social, economic and national consequences of HPV-related diseases, which are a serious threat to the health of women, are so serious that research has been conducted to suggest the burden of HPV infection and policy directions. In particular, the HPV vaccine is a primary preventive measure for increasing the immunity against HPV infection and ultimately preventing the development of cervical cancer, which can be an effective cervical cancer prevention and management method together with a Pap test.
BACKGROUND FOR THE INTRODUCTION OF HPV VACCINES INTO THE NIP BY COUNTRY
The WHO emphasizes that the HPV vaccine support program should be included in the NIPs being implemented in each country, and since the announcement of the WHO statement the vaccination rate is increasing internationally. Fifty-one of the 64 countries had introduced only quadrivalent HPV vaccine in their NIPs, only bivalent HPV vaccine in 14 countries and both the bivalent and quadrivalent vaccines in 19 countries (Table 2).
Table 2. Introduction of HPV vaccines into the NIP by country.
| Programs | Australia | France | Germany | UK | Denmark | Sweden | USA | Japan |
|---|---|---|---|---|---|---|---|---|
| NIP introduction (male) | 2007 (2013) | 2007 | 2007 | 2008 | 2009 | 2012 | 2006 (2011) | 2013 |
| Routine vaccination | 12–13 | 11 | 12–17 | 12–13 | 12 | 10–12 | 11–12 | 12–16 |
| Catch-up vaccination | 13–26 | 12–20 | - | 13–17 | 13–15 | 13–17 | 13–26 | - |
| NIP vaccine | 2HPV | 2HPV | 2HPV | 4HPV | 9HPV | 2HPV | 9HPV | 2HPV |
| 4HPV | 4HPV | 4HPV | 4HPV | 4HPV | ||||
| 9HPV | 9HPV | 9HPV | 9HPV |
HPV = human papillomavirus, NIP = national immunization program.
Quadrivalent vaccine inoculation as NIP
In Denmark and Australia, where quadrivalent HPV vaccine was introduced as a NIP, there was a significant decrease in CIN or genital warts. Australia has introduced a quadrivalent vaccine as a NIP for women aged 12–26 years from 2007. As a result, genital warts decreased by 89.7% in women under 21 years old compared to before vaccination, and genital warts decreased by about 87.3% in men under 21 years old who were not vaccinated because of herd immunity.12 In Denmark, 247,313 women vaccinated with the quadrivalent HPV vaccine in 2006 resulted in a significantly lower risk of cervical lesions. Especially in women born between 1993 and 1994, the risk of CIN 3 was reduced by up to 80% compared to unvaccinated women.13 The US government has introduced the HPV vaccine into the NIP since 2006. The infection rates of HPV 6, 11, 16, and 18 were reduced by 56% in 2007–2010 compared to 2003–2006 before the introduction of the quadrivalent HPV vaccination program. In addition, the infection rates of HPV 16 and 18 also decreased by 50% compared to the same period.14
Bivalent vaccine inoculation as NIP
In a periodic sample survey following the introduction of NIP in Scotland, vaccination with bivalent vaccine reduced the prevalence of HPV 16 and 18, and cross protection against non-vaccine carcinogenic HPV including HPV 31 and 33 was confirmed. In addition, regardless of the presence or absence of HPV infection before vaccination, the CIN 3 diagnosis was 55% lower in the vaccinated group than in the non-vaccinated group and statistically significant. In the UK, cervical cancer vaccination program for 12–13 years old girls were started using bivalent vaccine in 2008. Catch-up vaccination was also performed for women under 18 years old. In the UK, the prevalence of HPV infection surveyed from 2008 to 2014 showed that the vaccination program significantly reduced HPV 16 and 18 infection in young women. In 2012, the NIP in UK switched to the quadrivalent vaccine, which additionally protects against HPV 6 and 11.
Background for the introduction of HPV vaccines into the NIP
On July 2015, Denmark introduced a bivalent HPV vaccine into its 2016 NIP based on an overall review of the efficacy, adverse reactions, side effects, and price of the vaccine. The efficacy of the vaccine (93%) was assessed against all cancer-causing strains of HPV as well as HPV 16 and 18. In the Netherlands, while a quadrivalent vaccine showed a greater protective effect against genital warts, a bivalent vaccine was selected for the NIP since the primary goal for selection was to prevent cervical cancer and the bivalent vaccine was assessed to be more cost-effective than the quadrivalent vaccine.15 Further, in Hungary, Belgium, and Singapore, a bivalent vaccine was selected for its cost-effectiveness, and in South Africa, a bivalent vaccine that showed high immunogenicity was selected due to the country's high HIV infection rates. In the case of the UK, more focus was placed on the prevention of genital warts, and based on a highly effective cervical cancer screening program, a bivalent vaccine was switched to a quadrivalent vaccine in 2012.16
FINAL RESEARCH CONTENTS AND METHODS
For the introduction of HPV vaccine to the NIP, selection of vaccines, the vaccination subjects, and the number of vaccinations, we collected objective data on the status of HPV infection, prevention effect of vaccines, and safety. In addition, literature surveys were conducted fully reflecting the opinions of the KCDC.
Procedure for conducting research
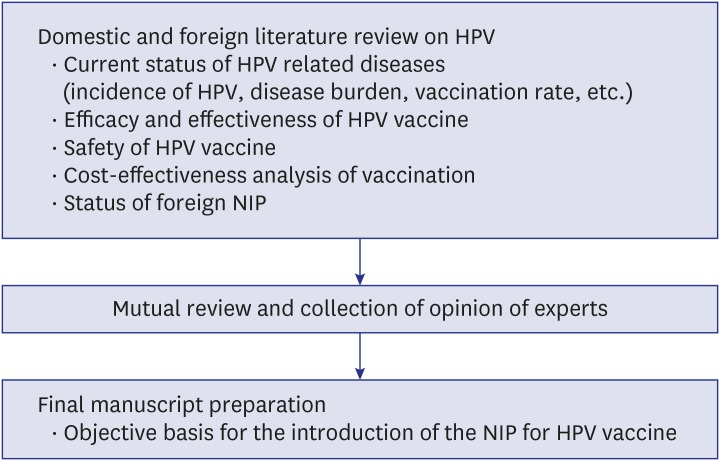
We reviewed domestic and foreign literature on the current status of HPV related diseases (incidence of HPV, disease burden, vaccination rate, etc.), efficacy and effectiveness of vaccine, safety of vaccine, and cost-effectiveness analysis of vaccination business. After reviewing the contents of the literature review, the manuscripts were prepared by mutual review among the joint researchers, and in particular, the manuscript length, the manuscript format, and the manuscript content were discussed. The contents of the guidelines were unified and the opinions of experts were collected and corrected. A final report was prepared to provide an objective basis for the introduction of the NIP for HPV vaccines (Fig. 2).
Fig. 2. Research progress diagram.
HPV = human papillomavirus, NIP = national immunization program.
Research methods
A review of the literature has identified the latest evidence on the clinical efficacy and safety of HPV vaccines. We searched KoreaMed, Cochrane Library, MEDLINE, and EMBASE for the relevant literature. The scope of the literature searches was from the period of searchable sites to the end of November 2015. We used the following Medical Subject Heading (MeSH) search terms: “papillomavirus vaccine,” “papillomavirus vaccination,” “HPV vaccine,” “HPV vaccination,” “program evaluation,” “population surveillance,” “sentinel surveillance,” “incidence,” “prevalence,” “papillomavirus infection,” “condylomata acuminata,” “anogenital warts,” “CIN,” “cervical dysplasia,” “uterine cervical neoplasm,” and “HPV related diseases.” Based on the criteria of the study, two reviewers independently conducted literature selection and exclusion. First, the title and abstract were selected and excluded, and even if only one of the reviewers selected, the original text was searched. Second, the original text was confirmed and final selection and exclusion were decided. Other data on the disease burden, incidence of HPV-related diseases and vaccination rate were collected through search of national academic research service report and search of the National Statistical Office data and articles. Key findings include the status of HPV-related domestic and foreign conditions (incidence of HPV, disease burden, vaccination rate, etc.), efficacy and effect of vaccination, stability of vaccination, cost effectiveness of vaccination program, and current status of foreign NIP.
HPV-RELATED DOMESTIC AND FOREIGN STATUS
Domestic cervical cancer incidence rate
The incidence of cervical cancer has been steadily decreasing although it has been ranked among the first to second cancer among Korean woman patients. According to the data released by the Korea Central Cancer Registry in 2017, 214,701 cancers occurred in Korea in 2015. Among them, cervical cancer excluding CIS is 3,582 cases in 2015, which is 1.6% of total cancer incidence. The crude incidence rate was 14.2 per 100,000 population, ranking 7th among women cancers.5 The age standardization incidence per 100,000 population by year also decreased from 18.6 in 1999 to 10.8 in 2015. Age standardization mortality of cervical cancer in 2013 was 3.5 per 100,000 population, ranking ninth in women's cancer deaths and in 2016, the number of cervical cancer deaths was 897 and the mortality rate was 3.5 per 100,000 population.17 With regard to the incidence and mortality rate of cervical cancer, the incidence was slightly lower than in 1999 but the mortality rate did not change significantly.5,17
Foreign cervical cancer incidence rate
When the age standardization incidence estimates for 2012 are compared internationally, the incidence in Korea is lower than the incidence in Japan, but it is higher than that in developed countries such as the US and UK. According to the International Agency of Research on Cancer (IARC), the cancer incidence rate for 2012 is 9.5 per 100,000 population, slightly lower than that in Japan, and higher than that in the US and the UK (Table 3). Cervical cancer is the fourth most common cancer in the world, accounting for 7.9% of woman cancer cases worldwide. It is estimated that 528,000 cases of cervical cancer have occurred each year in 2012. Although the incidence of cervical cancer has been steadily declining in most developed countries due to the development of screening and treatment techniques in the last 30 years, there has been no significant or even increased trend in developing countries.
Table 3. Age-standardized incidence rates of cervical cancer in 2012 by countries.
| Countries | Korea | Japan | USA | UK | Australia |
|---|---|---|---|---|---|
| Age-standardized ratea | 9.5 | 10.9 | 6.6 | 7.1 | 5.5 |
aPer 100,000 standard population.
Incidence rate of domestic genital warts
Genital warts are associated with HPV 6 and 11 among low-risk viruses. HPV 6 and 11 are found in more than 90% of genital warts. According to the statistics of the KCDC in 2014, the number of reported genital warts caused by HPV among sexually transmitted infectious diseases increased annually from 281 (men 187, women 94) in 2001 to 2,197 (men 1,396, women 801) in 2014, showing a 7.8-fold increase.18
Distribution of HPV types in Korea
The prevalence of HPV infection in Korea is estimated to be about 10%–15%, especially at younger ages.19,20 In particular, 38.8% of the women with sexual experience were infected with HPV, which was higher than that of general women with 15.2%. Bae et al.4 conducted a meta-analysis of 18 studies (13,842 cases) that reported HPV infection rates in Korea between 1995 and 2007, and reported that, in the normal cervix, the HPV infection rate was 20.4% and the high-risk HPV infection rate was 16.7%. The HPV infection rate in the atypical squamous cells of undetermined significance and CIN 1 was 63.2% and the high-risk HPV infection rate was 56.3%. The HPV infection rate in CIN 2, 3, or CIS patients was 85.6% and the high-risk HPV infection rate was 83.7%. Among patients with cervical cancer, HPV infection rate was 88.3%, and that of high-risk HPV infection was 84.6%. The highest infection rate among HPV types was HPV 16 followed by HPV 58 and HPV 18.4 When we compare the results of East Asian studies to which Korea belongs, the results of Asia as a whole showed a similar tendency up to the fifth rank of more affected type. In an analysis of HPV infection rates by strain in cervical precancerous lesions and cancers, HPV 16 was the most prevalent strain found in cervical diseases in Korea, HPV 16 and HPV 18 were detected in 65.1% of all cervical cancer cases, and 5 of the major high-risk HPV strains (16, 18, 58, 33, 52) were detected in 80.8% of cervical cancers.
HPV seroprevalence
According to studies on the seroprevalence of HPV, which could reveal the current or past HPV infection history, the seroprevalence of HPV was 15%–20%. In a 2009 report on 1,094 Korean women aged 9–59 years, the overall seroprevalence of HPV 16 and 18 was 8.7% and the prevalence was highest at 13.4% in the 25–29 year old age group, decreased to 7.6% in the 30–39 year old age group, and increased again to 10.9% in the 40–49 year old age group; the seroprevalence of HPV 16 and 18 was 7.4% and 2.7%, respectively.21
HPV vaccination rate
The KSGO has recommended a quadrivalent vaccine to 9–26 years old women including catch-up vaccination and 9–15 years old boys. The bivalent vaccine was recommended to a 10–25 years old women including catch-up vaccination. The KCDC recommends that the optimal age of vaccination for both vaccines should be between the ages of 11 and 12, taking into account the age of first sexual experience. In a survey of HPV vaccination rates in Asia, the rate of vaccination among teenage girls and adult women in Korea, Malaysia, Taiwan, and Thailand in 2008 was reported to be less than 4%. The vaccination rate of Korea was 12% for woman college students in some areas of Seoul, 6% for woman college students in Daegu and Gyeongbuk provinces, and 2% for high school students in Busan (Table 4).22,23,24 According to the 2013 National Immunization Survey, the HPV vaccination rate was 12.6% for 19–59 years old. By age, 28.7%, 15.9%, and 4.6% were for 19–26 years old, 27–39 years old and 40–59 years old, respectively.25 Although the general public's perception and vaccination rate may be higher than before, HPV vaccination rates are still low because of the low chance of getting access to cervical cancer or vaccines and low knowledge about HPV vaccination.
Table 4. HPV vaccination rate in Korea.
| Studies | Subjects | Vaccination rate, % (No.) |
|---|---|---|
| Lee et al.48 | Female high school students | 2.2 (9/404) |
| Yum et al.23 | Female university students | 3.1 (18/572) |
| Bang et al.24 | Female university students | 12.0 (24/200) |
| Lee and Park22 | Female university students | 5.5 (43/777) |
HPV = human papillomavirus.
EFFICACY OF HPV VACCINE
According to the WHO guidelines, the immunological correlates of protection against the vaccine is not known for the HPV vaccine, and the most definitive clinical indicator, cervical cancer diagnosis, takes more than 10 years to develop. Therefore, the use of CIN 2–3 and adenocarcinoma in situ (AIS) as a clinical indicator is recommended for studies on the effects of vaccines and long-term persistence. The representative clinical trials evaluating the efficacy of quadrivalent and bivalent vaccines are FUTURE (The Women United to Unilaterally Reduce Endo/Ectocervical Disease) I, II, The Papilloma Trial against Cancer in Young Adults (PATRICIA; HPV008) and Costa-Rica vaccine trial (CVT) as multinational Phase III clinical studies (Tables 5 and 6).26,27,28,29
Table 5. Characteristics of phase III efficacy studies in young women.
| Title | FUTURE I | FUTURE II | PATRICIA | CVT |
|---|---|---|---|---|
| Vaccine | Gardasil® | Gardasil® | Cevarix® | Cevarix® |
| Funding source | Merck & Co., Inc. | Merck & Co., Inc. | GlaxoSmithKline | National Cancer Institute |
| No. of study sites | 62 | 90 | 135 | 7 |
| Countries included | 16 | 13 | 14 | 1 |
| No. of enrolled | 5,455 | 12,167 | 18,644 | 7,466 |
| Duration of trial, yr | 4 | 4 | 4 | 4 |
| Age, yr | 16–24 | 15–26 | 15–25 | 18–25 |
| Exclusion criteria | Pregnancy, history of abnormal Pap smear or genital warts | Pregnancy, history of abnormal Pap smear | Pregnancy, breastfeeding, history of colposcopy, autoimmune disease or immunodeficiency | Pregnancy, breastfeeding, history immunosuppression, hysterectomy, hepatitis A vaccination |
| Method | Anogenital examination, cervicovaginal sampling (Pap smear), anogenital swabs (for HPV), initial serology | Anogenital examination, cervicovaginal sampling (Pap smear), anogenital swabs, initial serology | Gynecological examination, cervical sampling (for HPV), cervical liquid-based cytology, blood sampling | Initial interview, pelvic exam, cervical secretion, cervical cell sampling (cervix brush), blood sampling |
| Primary endpoint | Incident HPV 6, 11, 16, 18-associated genital warts, CIN 1-3, VIN 1-3, VaIN 1-3, AIS and cervical, vaginal or vulvar cancer | Incident HPV 16, 18-associated CIN 2-3, AIS or cervical cancer | Incident HPV 16, 18-associated CIN 2+ | Incident 12-month persistent HPV 16, 18 infection |
FUTURE = the women united to unilaterally reduce endo/ectocervical disease, PATRICIA = the papilloma trial against cancer in young adults, CVT = Costa Rica HPV trial, HPV = human papillomavirus, CIN = cervical intraepithelial neoplasia, VIN/VaIN = vulvar/vaginal intraepithelial neoplasia, AIS = adenocarcinoma in situ.
Table 6. Comparison of efficacy of HPV vaccines.
| Vaccines (clinical trials) | Bivalent (PATRICIA), % | Quadrivalent, % (FUTURE I & II) | ||
|---|---|---|---|---|
| Genital disease related to the vaccine specific type | ||||
| ATP/PPEa | ||||
| CIN 2+ | 94.9 | 100 (CIN 2) | ||
| CIN 3+ | 91.7 | 96.8 (CIN 3) | ||
| AIS | 100 | 100 | ||
| TVC-naive/mITTb | ||||
| CIN 2+ | 99.0 | 100 (CIN 2) | ||
| CIN 3+ | 100 | 100 (CIN 3) | ||
| AIS | 100 | 100 | ||
| TVC/ITTc | ||||
| CIN 2+ | 60.7 | 54.8 (CIN 2) | ||
| CIN 3+ | 45.7 | 45.1 (CIN 3) | ||
| AIS | 70.0 | 60.0 | ||
| Genital disease irrespective of HPV type | ||||
| TVC/ITTc | ||||
| CIN 2+ | 33.1 | 19.3 (CIN 2) | ||
| CIN 3+ | 45.6 | 16.4 (CIN 3) | ||
| AIS | 76.9 | 62.5 | ||
| TVC-naive/mITTb | ||||
| CIN 2+ | 64.9 | 42.9 (CIN 2) | ||
| CIN 3+ | 93.2 | 43.0 (CIN 3) | ||
| AIS | 100 | 100 | ||
HPV = human papillomavirus, PATRICIA = the papilloma trial against cancer in young adults, FUTURE = the women united to unilaterally reduce endo/ectocervical disease, ATP = according to protocol, PPE = per-protocol efficacy, CIN = cervical intraepithelial neoplasia, AIS = adenocarcinoma in situ, TVC = total vaccine cohort, ITT = intention-to-treat.
aReceived 3 doses, seronegative at baseline and DNA-negative to the vaccine specific type, normal or low grade cervical cytology at baseline, no protocol violation; bReceived ≤ 1 dose, baseline HPV DNA negative, seronegative for the vaccine specific type, and cytology negative; cReceived ≤ 1 dose, regardless of compliance, enrollment cytology, HPV-DNA or HPV serology status.
Clinical study of bivalent vaccine
The bivalent vaccine, through three efficacy trials, showed a consistent preventive efficacy of 61%–75% on cervical cancer-associated lesions above CIN 2, regardless of HPV type. These studies were performed on 15–25 years old women (HPV-non-infected) in four continents (PATRICIA study 64.9%; CVT 61.4%; Japan Phase 2 trial 73.9%). The PATRICIA study is a multinational clinical trial of 18,644 women aged 15–25 years, with no more than six lifetime sexual partners in 14 countries in Asia, Europe, and North and South America.30 CIN 2+ associated with HPV 16 and 18 were observed after an average follow-up of 35 months after vaccination. As a result, in the case of total vaccinated cohort (TVC)-naive vaccine, which had normal cytology and HPV DNA-negative and received at least one dose of vaccine, 98% had a preventive efficacy. The according to protocol cohort for efficacy (ATP-E) group who received 3 doses of vaccine and had normal or low-grade cytology at baseline had a preventive efficacy in 93%. Regardless of the HPV DNA results, 53% of the TVC patients who received at least one dose of vaccine had a preventive efficacy. The vaccine efficacy against CIN3 + associated with HPV 16 and 18 was 100%, 80%, and 34% in TVC-naive, ATP-E, and TVC group, respectively. In addition, the vaccine was 100% effective against persistent HPV infection for more than 12 months.
In the end of study analysis after 4 years of follow-up in the PATRICIA trial in 2012, the efficacy of HPV vaccine against CIN 3+ and AIS was reported: the efficacy of the vaccine against HPV 16 and 18–associated CIN 3+ was 100% in the TVC-naive group and 45.7% in the TVC group; vaccine efficacy against all CIN 3+ was 93.2% in the TVC-naive group and 45.6% in the TVC group; vaccine efficacy against all AIS was 100% in the TVC-naive group and 76.9% in the TVC group.30,31 CIN 3+ is an endpoint and proxy indicator that can predict invasive cervical cancer more accurately than CIN 2+, and the bivalent vaccine was able to prevent 93% of all cases of CIN 3+, irrespective of HPV genotype. Thus, even though the bivalent vaccine was developed against the HPV 16 and 18, it also has a general protective effect against other high-risk HPV strains. Further, consistent cross-protective vaccine efficacy against persistent infection and CIN 2+, was observed across cohorts for HPV 33, 31, 45, and 51. Vaccine efficacy against CIN 2+ associated with a total of 12 non-vaccine HPV strains, regardless of HPV 16 and 18 coinfection, was 46.8% in the ATP-E group, 56.2% in the TVC-naive group, and 34.2% in the TVC group. The corresponding values for CIN 3+ were 73.8%, 91.4%, and 47.5% in the 3 groups, respectively. Thus, cross-protective efficacy of the bivalent vaccine was seen against 4 oncogenic non-vaccine HPV strains (HPV 33, 31, 45, 51).32
The CVT is a randomized, double-blind, clinical trial study of 7,466 women aged 18–25 years old in two Costa Rica regions. Pap test was performed every year after vaccination. In cases of abnormal findings, colposcopic biopsy was performed to assess the incidence rate of histologic CIN 2–3 and to assess vaccine efficacy. HPV-negative women were included in the ATP group, and all randomly assigned women were included in the intention-to-treat (ITT) group. Vaccine efficacy against HPV 16 and 18 infections was 90.9% in the ATP group; 44.5% against HPV 31, 33, and 45; and 12.4% against all oncogenic HPV infections. While the protective efficacy of the vaccine against HPV 16 and 18 in the overall ITT group was 49.0%, it reached almost 100% after 4 years of follow-up.31,33 Although vaccine efficacy against HPV 16 and 18 in the ATP group was comparable among age groups, protective efficacy was greatest in young women and 79.8% in women without sexual experience. In women without previous exposure to HPV, vaccination was highly protective against HPV 16 and 18, and partial cross-protective efficacy was confirmed against HPV 31, 33, and 45.33
Clinical study of quadrivalent vaccine
In the FUTURE I and II clinical trials of a quadrivalent vaccine, vaccine efficacy was evaluated by analyzing the ITT group that included all randomly selected women regardless of their HPV infection status and cervical disorders, the modified intention-to-treat (mITT) group that included women who were uninfected by the target HPV genotypes as determined by serum or DNA analysis and had been vaccinated at least once, and the per-protocol (PP) group that included women who were uninfected by the target HPV genotypes as determined by serum or DNA analysis and had been vaccinated 3 times. FUTURE I had performed Pap test every year for 5,455 women aged 16–24 years old and observed the incidence of anogenital disease. As a result of the quadrivalent vaccine, 100% of the preventive effect of CIN 1, 2, 3 and AIS associated with 4 types of HPV 6, 11, 16 and 18 were observed.26 In the ITT group, vaccine efficacy was 73% against external genital disorders associated HPV 6, 11, 16, and 18 and 55% against cervical disorders; irrespective of the HPV strain, vaccine efficacy was 34% against external genital disorders and 20% against cervical disorders.26 In FUTURE II, 12,167 women subjects aged 15–26 years old were followed up with Pap and HPV test to observe the incidence of CIN 2, 3 and AIS, and 98% vaccination prevention effect was observed 3 years after the first vaccine was administered. In addition, vaccine efficacy was 17% against all high-grade cervical lesions of ITT group, irrespective of the HPV strain.27
Combining FUTURE I and II clinical trials, with 17,622 women aged 15–26 years, the protective efficacy of the vaccine was 100% against CIN 2, 3, and AIS in the mITT group.31,34 Upon vaccination with the quadrivalent vaccine, protective efficacy against vulvar intraepithelial neoplasia (VIN), and vaginal intraepithelial neoplasia (VaIN) grade 2 or greater was 100% in the PP group and 95% in the mITT group; protective efficacy against genital warts was 100% in the PP group and 96% in the mITT group.
The most important long-term persistence study of the quadrivalent HPV vaccine was the Nordic Study, which was followed up for 14 years in the Nordic countries by CIN 2, 3, and AIS associated with HPV 16 and 18. A long-term follow-up of approximately 2,000 women aged 16–23 years old participating in the FUTURE II study showed 100% vaccine efficacy for 10 years due to the absence of CIN 2–3, CIS caused by HPV 16, 18. However, there were no direct comparative clinical studies of the efficacy of the bivalent and quadrivalent vaccines, and the efficacy of the vaccine in each phase 3 trials may vary depending on the women population. Therefore, it is not known which vaccine is more effective in preventing cervical cancer. Through 3 doses of quadrivalent vaccine including HPV 6 and 11, the most common HPV strains that cause genital warts, it is possible to achieve high levels of protection against genital warts in both men and women and anogenital precancerous lesions in men aged 16–26 years. Such protection persisted for as long as 8 years. Among vaccinated individuals that were seronegative for the relevant vaccine-type HPV, high rates of seroconversion and high levels of anti-HPV antibodies to HPV 6/11-like particles were observed in women aged 9–45 years and men aged 9–26 years. According to research on vaccine efficacy, a quadrivalent vaccine can provide nearly 100% protection against HPV 6/11-associated genital warts and 83% protection against all genital warts in individuals without previous HPV infection.31
Cross protection of HPV vaccine
According to the PATRICIA study, the bivalent vaccine in the ATP-E group had the following effects against persistent HPV infection for more than 6 months: 77.5% (96.1% confidence interval [CI], 68.3–84.4) for HPV 31 (the most similar to HPV 16 type), 45.1% (96.1% CI, 21.7–61.9) for HPV 33 and 76.1% (96.1% CI, 59.1–86.7) for HPV 45 (the most similar to HPV 18).28 In the ATP-E group of the PATRICIA study, the efficacy of the bivalent vaccine against all 12 non-vaccine oncogenic HPV types (HPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68) and HPV 16, 18 (CIN2–associated lesions associated with 14 species) was 61.9% (96.1% CI, 46.7–73.2; P < 0.001). The effect of the bivalent vaccine on lesions of CIN 2+ was 30.4% (96.1% CI, 16.4–42.1) in the TVC group. The effect of bivalent vaccine against CIN 2+ in 11,641 patients with TVC-naive group was 70.2% (96.1% CI, 54.7–80.9). Similarly, in the CIN 3+ group, 33.4% in the TVC group and 87.0% in the TVC-naive group. In the ATP-E group, the composite efficacy against CIN 2+ associated with 12 non-vaccinated oncogenic HPVs was 54.0% (95% CI, 34.0–68.4). In the TVC-naive group, significant preventive efficacies were observed for 6 months of continuous infection and CIN 2+, and based on this, cross-protective effects against HPV 31, 33 and 45 were also demonstrated.
Cross-protection effect analysis of the quadrivalent vaccine was performed on 17,622 women subjects, including the FUTURE I study and the FUTURE II study. Four years of follow-up observation was performed. Similar to the ATP groups, all women in the beginning stage of the study were analyzed only if they were negative for 14 HPV strains. HPV 31 and 45 infection was reduced by 40.3% by quadrivalent vaccine, and CIN 1–3/AIS were decreased by about 43.6%. HPV 31, 33, 45, 52, 58 infection and CIN 1–3/ AIS decreased by 25.0% and 29.2%, respectively. The reduction effect of CIN 2–3/AIS on 10 non-vaccine HPV types was 32.5% and the reduction effect of HPV 31 was great.35 Both vaccines showed some significant cross-protection against HPV types not included in the vaccine. However, there are controversies regarding the cross-prevention reaction of HPV vaccine due to various reasons.
Immunogenicity and duration of vaccine
The methods of measuring neutralizing antibodies of two HPV vaccines are different. Further, since there has still been little information about the lowest amount of neutralizing antibodies that can prevent CIN 2–3 or persistent HPV infection, it is difficult to resolve the controversy about the different abilities of the vaccines to induce antibody production, and the clinical relevance of such differences in immunogenicity is still unclear. Two review articles that analyzed the data of the 3-dose of bivalent and quadrivalent vaccine concluded that the serum antibody immediately reached the peak after the third vaccination. They also concluded that they entered the ballast at about 2 years and stabilized for at least 5 years after inoculation.36,37 In the case of bivalent vaccine, the immunogenicity and efficacy at 3 doses for infections and uterine lesions associated with HPV 16 and 18 were proven to be 8.4 and 9.4 years, respectively. In the case of the quadrivalent vaccine, immunogenicity data for 8 years after 3 doses showed that antibody titers to HPV 18 decreased after 4 years. On the other hand, the efficacy of HPV 16 and 18 associated with infection and uterine lesions persisted for up to 8 years.36 Long-term follow-up studies also showed that efficacy persisted for up to 8 years after vaccination.
Alternative dosing schedule of vaccine (2-dose)
The HPV vaccination in the teenager is economically positive because it saves the cost of vaccination completion and is more effective because it can be prevented before exposure to HPV infection. The American Academy of Pediatrics, the Centers for Disease Control, and the Advisory Committee on Immunization Practices recommend that women aged 11–12 years are vaccinated against cervical cancer. In a study of the number of vaccination, 3-dose vaccination groups of 15–25 years old of age with actual clinical effects in large-scale clinical trials were compared with 2-dose vaccination groups of 9–14 years old of age in case of bivalent vaccine. The 2-dose vaccination group of 9–14 years old reported comparable or superior antibody formation to the 3-dose vaccination group of 15–25 years old.38 In the case of quadrivalent vaccine, antibody formation in 9–13 years old girls with 2-dose vaccine is not inferior to the 3-dose group in 16–26 years old women who have been proven effective.39 Based on the results of this study, WHO also changed the HPV vaccination in October 2014 to 2-dose schedule for girls aged 9–13 years old.27,39 However, during the 2-dose vaccination, both vaccines were recommended to be given a second vaccination after 6 months followed by the first vaccination. On the other hand, at age 15 and over, both vaccines are recommended for 3-doses.
Cost-effectiveness analysis of HPV vaccine
The cost-effectiveness analysis of HPV vaccination using a cervical cancer screening test as comparison group has been performed in several countries. In Ireland, cost-effectiveness of the NIP of a 12 years old girl's bivalent vaccine (assuming an 80% inoculation rate) was shown to be cost-effective in the Irish healthcare environment with EUR 17,383/life year gained.40 Among the catch-up programmes, the first year program for 13–15 years old was the most cost-effective.41 Elbasha et al.42 showed that quadrivalent HPV vaccine was cost-effective at USD 2,964 per quality adjusted life year (QALY) gained when administered to a 12 years old girl in the US in comparison to the non-vaccinated group. In Japanese studies, the case of 12 years old girls receiving a quadrivalent HPV vaccine compared with no vaccination and the case of 12–24 years old girls with a catch-up vaccination compared to 12 years old girls were cost-effective.43 KCDC have studied the cost-effectiveness analysis of domestic HPV vaccination.41,44 Assuming that the cost of vaccine is 600,000 KRW for the 3 dose of vaccination, KCDC estimated cost of vaccines to be 190 billion KRW for 100% vaccination and 970 billion KRW for 50% vaccination for girls entering age 15.41 However, the number of cervical cancer that can be prevented by the vaccination in the age group vaccinated is estimated to be several thousand, and the benefit obtained by vaccination is estimated to be 120 billion KRW for 100% vaccination and 62 billion KRW for 50% vaccination. Therefore, it is estimated that the cost is higher than the benefit, so it is not economically feasible at the current vaccine price level.41 KCDC also estimated that the costs for vaccine in both scenarios were 139.1 billion KRW and 137.6 billion KRW, respectively.44 The benefits are estimated at 39.9 billion KRW and 41.4 billion KRW, respectively and the costs are much higher than the benefits.44 According to a cost-effectiveness study published in December 2012 by the National Evidence-based Healthcare Collaborating Agency, as a result of the economic analysis of the HPV vaccine against the vaccination of 12 years old girls, in the case of 3 doses of HPV vaccine, the incremental cost-utility ratio is 32 million KRW/QALY, which is not cost-effective considering Korea's cost-effectiveness standard is 20–30 million KRW. In the sensitivity analysis, the vaccination rate, the change in medical cost, and the mortality rate in patients with recurrence of cervical cancer did not significantly affect the outcome, but the vaccine price, vaccine effect, and discount rate significantly influenced the cost-effectiveness.45 In 2014, the WHO urged the government to reduce vaccination frequency by 2-dose schedule to emphasize the country's NIP, and the Korean government has reached the stage of discussing the HPV vaccine with the priority of the NIP. Based on the results of previous studies, the Korea Institute for Health and Social Affairs conducted a reanalysis in consideration of the recent environment and concluded that it is cost effective to introduce the HPV vaccination with 2–dose schedule to the NIP. In other words, it was found that cost-effective cost about 18 million KRW was required for 2 doses of vaccine, while in case of 3 doses of vaccination cost about 32 million KRW was required to survive 1 year in full health condition.
Safety of HPV vaccine
The WHO Global Advisory Committee on Vaccine Safety (GACVS) regularly reviews the safety of HPV vaccines. The committee reviewed postmarketing surveys and manufacturer data from the US, Australia, and Japan. Data provided by all sources continues to be positive about the safety of the two vaccines. GACVS concluded in March 2014 that the two HPV vaccines maintained an excellent safety profile.46,47 First, to explain the local reaction, injection site pain is very common in both bivalent and quadrivalent vaccine reports, with up to 80% reporting. About 6% of severe pain (spontaneous pain or pain that interferes with normal activity) is reported. In the placebo-controlled clinical trial prior to approval for the quadrivalent vaccine, injection site reactions were pain (84%), erythema (less than 25%), and edema (25%). The pain was more common in the vaccinated group compared to both placebo (pain 49%) and aluminum placebo (pain 75%). Local adverse events were similar in bivalent HPV vaccination. As a result of reviewing the safety of HPV vaccines, both vaccines were associated with a relatively high incidence of injection site reactions, particularly pain, but these responses were short and disappeared spontaneously. Second, to explain systemic reaction, in pre-approval clinical trials of the quadrivalent vaccine, systemic adverse events were observed for the first 15 days after vaccination. The adverse reactions that occurred in more than 1% of all cases were only fever. This was more frequent than placebo (10.1% and 8.4%, respectively). Systemic adverse events were observed from 7 to 30 days after vaccination. There were no clinically significant differences in the quadrivalent versus bivalent vaccine with respect to the onset of new chronic diseases such as the emergence of new autoimmune diseases. After more than four years after the bivalent vaccine was approved, the safety of the vaccine did not reveal the pattern or trend of potential immune-mediated disease. Facial nerve palsy and confirmed Guillain-Barré syndrome were also comparable to those expected in the entire population. The available information on the safety and immunogenicity of HPV vaccines against immunization or HIV infections is limited, but HPV vaccination appears to be safe for these groups. The safety and efficacy of the HPV vaccine for children under the age of nine has not been proven.
CONCLUSION
In view of the domestic and foreign HPV vaccination status, compared to the countries designated as NIP, the vaccination rate of HPV vaccine is low in Korea. Studies have shown that when 2 doses of HPV vaccine for girl students aged 9–14 years old (0, 6 months) is compared to 3 doses for girl students aged 9–14 years old (0, 1 or 2, 6 months) or 3 doses for women 15–24 years old, it is not inferior in immunogenicity. Accordingly, the WHO recommended the latest recommendations for HPV vaccine in October 2014, reducing the number of HPV vaccinations for girls under 14 years from three to two doses. It also emphasized that the HPV vaccine should be included in the country's NIP.
As a result of analysis of economic efficiency related to cervical cancer prevention effect of HPV vaccine in 2014, 3–dose vaccination in 12 years-old girls was not cost effective to be introduced into the NIP. However, HPV vaccine was cost effective in the 2 doses of vaccination standard. In other words, the 3 doses of vaccination were not cost effective because it required about 32 million KRW to survive for one year in complete health condition. On the other hand, it was estimated that costing about 18 million KRW for 2 doses of vaccination.
Both the bivalent vaccine and the quadrivalent vaccine have a 90% or greater preventive efficacy on CIN 2–3 and cervical cancer incidence by the HPV 16, 18 in relation to the efficacy and economic viability of the HPV vaccine. In particular, both vaccines reported nearly 100% prevention of CIN 3 + by HPV 16 and 18. There were no direct comparative clinical studies on the efficacy of the two vaccines. It is not known which vaccine is more effective in the prevention of cervical cancer, since the effect of the vaccine according to each phase 3 study may vary depending on the woman population. The safety of HPV vaccines has been thoroughly reviewed in large-scale clinical trials and post-marketing surveys in WHO-affiliated GACVS, and no significant adverse events have been observed except local reactions at the inoculation site. The choice of HPV vaccine should be based on a number of factors, including the extent of HPV-related public health problems (cervical cancer, other genital organs, genital warts) and the population approved for vaccination, depending on the current state of the country. In the future, if HPV vaccine is introduced into the NIP, it is expected that the incidence of HPV infection, genital warts, and cervical precancerous lesions will be decreased in the vaccination age group. Prevention of HPV-related diseases including cervical cancer through cervical cancer screening and HPV vaccination is an important task in the field of social health today. In some countries where HPV vaccine coverage is high, there are already visible effects such as reduction of HPV infection rate, genital warts, and cervical precancerous lesion. Therefore, in order to increase the vaccination rate of HPV vaccine at this point in Korea, social consensus and efforts such as the introduction of the NIP according to appropriate cost-effectiveness analysis are required.
Footnotes
Funding: This work was supported by a grant from the Korea Centers for Disease Control and Prevention (2016P3200100).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Kim MA, Kim JH.
- Data curation: Kim MA, Han GH.
- Investigation: Kim MA, Han GH.
- Writing - original draft: Kim MA.
- Writing - review & editing: Kim JH, Seo K.
References
- 1.National Cancer Institute (US) HPV and cancer. Bethesda, MD: National Cancer Institute; [Updated 2015]. [Accessed June 16, 2016]. http://www.cancer.gov/cancertopics/factsheet/Risk/HPV/print. [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.Bae JH, Lee SJ, Kim CJ, Hur SY, Park YG, Lee WC, et al. Human papillomavirus (HPV) type distribution in Korean women: a meta-analysis. J Microbiol Biotechnol. 2008;18(4):788–794. [PubMed] [Google Scholar]
- 5.Korea Central Cancer Registry, National Cancer Center. Annual report of cancer statistics in Korea in 2015. [Updated 2018]. [Accessed June 16, 2016]. http://ncc.re.kr/cancerStatsView.ncc?bbsnum=438&searchKey=total&searchValue=&pageNum=1.
- 6.Bruni L, Barrionuevo-Rosas L, Albero G, Aldea M, Serrano B, Valencia S, et al. ICO Information Centre on HPV and Cancer (HPV Information Centre). Human papillomavirus and related diseases in the world. Summary report. [Updated 2016]. [Accessed July 14, 2017]. http://www.hpvcentre.net/statistics/reports/XWX.pdf.
- 7.Lee EH, Um TH, Chi HS, Hong YJ, Cha YJ. Prevalence and distribution of human papillomavirus infection in Korean women as determined by restriction fragment mass polymorphism assay. J Korean Med Sci. 2012;27(9):1091–1097. doi: 10.3346/jkms.2012.27.9.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver BA. Epidemiology and natural history of genital human papillomavirus infection. J Am Osteopath Assoc. 2006;106(3) Suppl 1:S2–S8. [PubMed] [Google Scholar]
- 9.Ministry of Health and Welfare, Korea Centers for Disease Control and Prevention, Ministry of Education. The 10th Korea Youth Risk Behavior Web-based Survey. Cheongju: Korea Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 10.Han CH, Cho HJ, Lee SJ, Bae JH, Bae SN, Namkoong SE, et al. The increasing frequency of cervical cancer in Korean women under 35. Cancer Res Treat. 2008;40(1):1–5. doi: 10.4143/crt.2008.40.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choe YJ, Han OP, Cho H, Bae GR, Chun BC, Kim JH, et al. Prioritization of the introduction of new vaccines to the national immunization program in the Republic of Korea. Vaccine. 2014;32(46):6049–6053. doi: 10.1016/j.vaccine.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Read TR, Hocking JS, Chen MY, Donovan B, Bradshaw CS, Fairley CK. The near disappearance of genital warts in young women 4 years after commencing a national human papillomavirus (HPV) vaccination programme. Sex Transm Infect. 2011;87(7):544–547. doi: 10.1136/sextrans-2011-050234. [DOI] [PubMed] [Google Scholar]
- 13.Baldur-Felskov B, Dehlendorff C, Munk C, Kjaer SK. Early impact of human papillomavirus vaccination on cervical neoplasia--nationwide follow-up of young Danish women. J Natl Cancer Inst. 2014;106(3):djt460. doi: 10.1093/jnci/djt460. [DOI] [PubMed] [Google Scholar]
- 14.Markowitz LE, Hariri S, Lin C, Dunne EF, Steinau M, McQuillan G, et al. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis. 2013;208(3):385–393. doi: 10.1093/infdis/jit192. [DOI] [PubMed] [Google Scholar]
- 15.Westra TA, Stirbu-Wagner I, Dorsman S, Tutuhatunewa ED, de Vrij EL, Nijman HW, et al. Inclusion of the benefits of enhanced cross-protection against cervical cancer and prevention of genital warts in the cost-effectiveness analysis of human papillomavirus vaccination in the Netherlands. BMC Infect Dis. 2013;13(1):75. doi: 10.1186/1471-2334-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Public Health England. Human Papillomavirus (HPV) Vaccine Coverage in England, 2008/09 to 2013/14: a Review of the Full Six Years of the Three-Dose Schedule. London: Public Health England; 2015. [Google Scholar]
- 17.Statistics Korea. 2016 Annual Report on the Causes of Death Statistics. Daejeon: Statistics Korea; 2016. [Google Scholar]
- 18.Korea Centers for Disease Control and Prevention. 2014 Infectious Diseases Surveillance Yearbook. Cheongju: Korea Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 19.Shin HR, Franceschi S, Vaccarella S, Roh JW, Ju YH, Oh JK, et al. Prevalence and determinants of genital infection with papillomavirus, in female and male university students in Busan, South Korea. J Infect Dis. 2004;190(3):468–476. doi: 10.1086/421279. [DOI] [PubMed] [Google Scholar]
- 20.Shin HR, Lee DH, Herrero R, Smith JS, Vaccarella S, Hong SH, et al. Prevalence of human papillomavirus infection in women in Busan, South Korea. Int J Cancer. 2003;103(3):413–421. doi: 10.1002/ijc.10825. [DOI] [PubMed] [Google Scholar]
- 21.Kim MA, Oh JK, Chay DB, Park DC, Kim SM, Kang ES, et al. Prevalence and seroprevalence of high-risk human papillomavirus infection. Obstet Gynecol. 2010;116(4):932–940. doi: 10.1097/AOG.0b013e3181edbeba. [DOI] [PubMed] [Google Scholar]
- 22.Lee EJ, Park JS. Knowledge about cervical cancer, health beliefs and human papillomavirus vaccination rate in Female university students. J Korean Onol Nurs. 2011;11(1):65–73. doi: 10.4069/kjwhn.2011.17.4.346. [DOI] [PubMed] [Google Scholar]
- 23.Yum JH, Jeong HS, Lee DW, Park KH, Kim NL. Factors Influencing Willingness for human papilloma virus (HPV) vaccination in female students at one university. Korean J Health Promot. 2011;11(2):100–105. [Google Scholar]
- 24.Bang KS, Sung S, Koo B, Kim M, Kim Y, Kim J, et al. Female university students' HPV-related knowledge and influencing factors on HPV vaccination. J Korean Oncol Nurs. 2011;11(3):186–192. [Google Scholar]
- 25.Korea Centers for Disease Control and Prevention. 2013 National Immunization Survey in South Korea. Cheongju: Korea Centers for Disease Control and Prevention; 2013. [Google Scholar]
- 26.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 27.FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 28.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369(9580):2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 29.Herrero R, Hildesheim A, Rodríguez AC, Wacholder S, Bratti C, Solomon D, et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine. 2008;26(37):4795–4808. doi: 10.1016/j.vaccine.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsagué X, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13(1):89–99. doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 31.Schiller JT, Castellsagué X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(Suppl 5):F123–F138. doi: 10.1016/j.vaccine.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wheeler CM, Castellsagué X, Garland SM, Szarewski A, Paavonen J, Naud P, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13(1):100–110. doi: 10.1016/S1470-2045(11)70287-X. [DOI] [PubMed] [Google Scholar]
- 33.Herrero R, Wacholder S, Rodríguez AC, Solomon D, González P, Kreimer AR, et al. Prevention of persistent human papillomavirus infection by an HPV16/18 vaccine: a community-based randomized clinical trial in Guanacaste, Costa Rica. Cancer Discov. 2011;1(5):408–419. doi: 10.1158/2159-8290.CD-11-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.FUTURE II Study Group. Prophylactic efficacy of a quadrivalent human papillomavirus (HPV) vaccine in women with virological evidence of HPV infection. J Infect Dis. 2007;196(10):1438–1446. doi: 10.1086/522864. [DOI] [PubMed] [Google Scholar]
- 35.Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis. 2009;199(7):926–935. doi: 10.1086/597307. [DOI] [PubMed] [Google Scholar]
- 36.Erickson BK, Landers EE, Huh WK. Update on vaccination clinical trials for HPV-related disease. Clin Ther. 2014;36(1):8–16. doi: 10.1016/j.clinthera.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Romanowski B. Long term protection against cervical infection with the human papillomavirus: review of currently available vaccines. Hum Vaccin. 2011;7(2):161–169. doi: 10.4161/hv.7.2.13690. [DOI] [PubMed] [Google Scholar]
- 38.Romanowski B, Schwarz TF, Ferguson LM, Ferguson M, Peters K, Dionne M, et al. Immune response to the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose or 3-dose schedule up to 4 years after vaccination: results from a randomized study. Hum Vaccin Immunother. 2014;10(5):1155–1165. doi: 10.4161/hv.28022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dobson SR, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA. 2013;309(17):1793–1802. doi: 10.1001/jama.2013.1625. [DOI] [PubMed] [Google Scholar]
- 40.Health Information and Quality Authority (IE) The Role of Human Papillomavirus Vaccines in Reducing the Risk of Cervical Cancer in Ireland. Dublin: Health Information and Quality Authority; 2008. [Google Scholar]
- 41.Korea Centers for Disease Control and Prevention. Burden of Human Papillomavirus Infection Related Diseases. Cheongju: Korea Centers for Disease Control and Prevention; 2007. [Google Scholar]
- 42.Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine. 2010;28(42):6858–6867. doi: 10.1016/j.vaccine.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 43.Yamabe K, Singhal PK, Abe M, Dasbach EJ, Elbasha EH. The cost-effectiveness analysis of a quadrivalent human papillomavirus vaccine (6/11/16/18) for females in Japan. Value Health Reg Issues. 2013;2(1):92–97. doi: 10.1016/j.vhri.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Korea Centers for Disease Control and Prevention. Prioritization of Introduction to the National Immunization Program. Cheongju: Korea Centers for Disease Control and Prevention; 2010. [Google Scholar]
- 45.National Evidence-based Healthcare Collaborating Agency (KR) Economic Evaluation of HPV Vaccination. Seoul: National Evidence-based Healthcare Collaborating Agency; 2013. [Google Scholar]
- 46.World Health Organization, Global Advisory Committee on Vaccine Safety. Update on HPV vaccines. [Updated 2013]. [Accessed July 14, 2017]. http://www.who.int/vaccine_safety/committee/topics/hpv/June_2017/en/
- 47.World Health Organization, Global Advisory Committee on Vaccine Safety. Statement on the continued safety of HPV vaccination. [Updated 2014]. [Accessed July 14, 2017]. http://www.who.int/vaccine_safety/committee/topics/hpv/GACVS_Statement_HPV_12_Mar_2014.pdf?ua=1.
- 48.Lee YE, Park JS, Choi EJ. The exact state of female high school students' knowledge about cervical cancer, human papillomavirus vaccination-related health belief and vaccination rate. J Korean Soc Matern Child Health. 2013;17(1):27–37. [Google Scholar]