TO THE EDITOR: Epstein-Barr virus (EBV)-positive mucocutaneous ulcer (MCU) is a recently documented condition that occurs in association with various forms of immunosuppression representing a unique, indolent form of EBV-driven B-cell lymphoproliferative disorder. It characteristically presents as isolated, well-delineated ulcers that respond well to conservative therapeutic intervention. It is usually located in the oropharyngeal area. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma), a low-grade B-cell lymphoma, can present with tonsillar enlargement in the oropharyngeal area. Here, we report a case of EBV-positive MCU that collided with MALT lymphoma in the palatine tonsils of an immunocompetent 49-year-old man with a history of gastric MALT lymphoma in complete remission. To our knowledge, a collision case of EBV-positive MCU and a palatine tonsillar MALT lymphoma relapse of gastric lymphoma has not yet been described.
Epstein-Barr virus (EBV)-positive mucocutaneous ulcer (MCU) is a newly documented condition occurring in the elderly or iatrogenically immunocompromised patients as published in the WHO classification of tumors of hematopoietic and lymphoid tissues in 2017 [1]. Lesions in the oral cavity, gastrointestinal tract, and skin have been described. They present as isolated, well-delineated ulcers that respond well to conservative therapeutic intervention despite large and atypical EBV-positive B-cell infiltration. Due to their indolent clinical behavior and excellent long-term prognosis, an understanding of the clinical and histomorphologic features should help to distinguish between EBV-positive MCU from ulcerations in the oral cavity caused by other high-grade lymphomas.
As one of the B-cell neoplasms showing indolent clinical course, extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) is well known and can be localized along the aerodigestive tract. Histologically, neoplastic marginal zone B-cells are clustered throughout the mucosal tissues [2]. In the oral cavity, they tend to present as a protruding mass with chronic inflammatory processes such as sialadenitis and Sjogren's syndrome [3]. Here, we report a case of EBV-positive MCU in the palatine tonsils arising in the setting of MALT lymphoma in an immunocompetent 49-year-old man with a history of gastric MALT lymphoma in complete remission. To the best of our knowledge, this has not yet been described in such a unique clinical setting.
Case
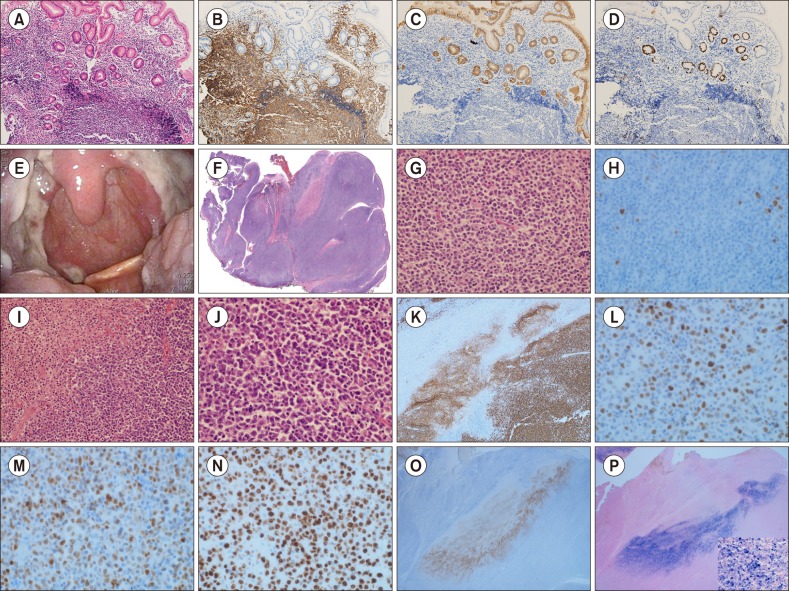
A 49-year-old male in complete remission (CR) for 2 years after gastric MALT lymphoma was admitted to Chungnam National University Hospital with complaints of oropharyngeal fullness with intermittent dysphagia lasting for a month. Due to the low initial stage of his gastric lesion (Fig. 1A–D), he was treated with conventional medication for Helicobacter pylori (H. pylori) eradication and achieved CR status in the previous two years. Notable weight loss and cervical lymphadenopathy were not observed. On clinical examination, two 3.5-cm tender, nonindurated, protruded, and enlarged palatine tonsils, partly covered with purulent exudate, were identified bilaterally (Fig. 1E). Examination of the rest of his mouth and extraoral examination were unremarkable. His laboratory test results were normal, and his anti-HIV antibody test was negative. In view of the persistent nature of the lesion and the patient's medical history, bilateral tonsillectomy was subsequently performed. Histologic assessment showed a shallow mucosal ulcer along the tonsillar surface with a focal necrotizing reaction. Under the mucosa, marked lymphoid proliferation forming a mass-growing and nodular pattern was noted. This lesion consisted of monotonous neoplastic lymphocytic cells with small to medium-sized slightly irregular nuclei with vaguely preserved remnant follicles. These neoplastic cells revealed moderately dispersed chromatin and inconspicuous nucleoli resembling those of centrocytes with a monocytoid appearance; these characteristics were consistent with MALT lymphoma, considering the patient's history of gastric lymphoma (Fig. 1F, G). Interestingly, another dense lymphoid infiltrate was noted in the ulcer base. The infiltrate was composed of large and atypical immunoblast-like cells with irregular nuclear contours, open chromatin, and prominent nucleoli (Fig. 1I, J). To explore these histologically different lymphocytic lesions, an immunohistochemical study was conducted, and the following strikingly contrasting expressions were identified: the nodular areas composed of small centrocyte-like neoplastic cells were positive for CD20 and CD79a and negative for CD30, BCL-2, BCL-6, MUM-1, CD10, Cyclin-D1, and EBER-ISH with a relatively low Ki-67 labeling index of 10% (Fig. 1H). In contrast to this expression, the large lymphoid cells and immunoblast-like cells were positive for CD20, CD79a, CD30, BCL-2, BCL-6, MUM-1, and Epstein-Barr virus encoded RNA in situ hybridization (EBER-ISH) and negative for CD3, CD10, and CD15. The Ki-67 labeling index was nearly 90% (Fig. 1K–P). Considering the unique clinical presentation and all these histologic findings, EBV-positive MCU composite relapsed gastric MALT lymphoma of the palatine tonsils was diagnosed. A systemic lymphoproliferative disorder could not be excluded, and the patient was referred for a hematological opinion. Medical imaging and blood investigations excluded a systemic lymphoproliferative disorder and any underlying cause of immunosuppression. However, a polymerase chain reaction for T-cell receptor gene rearrangements in our patient showed multiple prominent irregular patterns indicating restricted T-cell responses. At regular follow-ups, the patient remained asymptomatic and reported no further episodes of oral problems, and no remaining disease was found.
Fig. 1. Microscopic and immunohistochemical study findings of the patient's gastric MALT lymphoma in the past 2 years. (A) Gastric glands infiltrated by small monotonous lymphocytic cells (H&E, ×200). (B, C) Notable lymphoepithelial lesions demonstrated by the immunohistochemical stain for cytokeratin. (D) Neoplastic B-cells' low proliferative nature demonstrated by the Ki-67 labeling index. Gross and microscopic findings of the palatine tonsils. (E) Enlarged bilateral palatine tonsils covered with purulent exudate and showing shallow ulcer. (F) Low-magnification image showing diffusely effaced follicles with shallow ulcer along the surface epithelium. (G) Small- to medium-sized monotonous neoplastic cells in the nodular area showing irregular nuclei, dispersed chromatin, and inconspicuous nucleoli (H&E, ×400). (H) Low Ki-67 labeling index of neoplastic cells composing the nodular area supporting MALT lymphoma. (I) Ulcer base consisting of solid sheets of large, atypical immunoblast-like cells with many apoptotic bodies (H&E, ×100). (J) Immunoblast-like cells showing irregular nuclear contour, open chromatin, and prominent nucleoli (H&E, ×400). Immunohistochemical staining results of the large cell area. (K) Diffuse CD20-positive solid sheets of immunoblast-like cells in the ulcer base. (L) Bcl-6 positive. (M) MUM-1/IRF-4 positive. (N) High Ki-67 labeling index. (O) Striking demonstration of CD30 reactivity. (P) EBER-ISH reactivity.
DISCUSSION
EBV-positive MCU is a rare provisional condition listed in the 2017 update of the WHO's classification of lymphoid neoplasms [1]. It was first described by Dojcinov et al. [4] in a series of 16 elderly patients and 9 iatrogenic immunosuppressed patients. EBV-positive MCU presents with solitary localized areas of mucosal ulceration. The ulcer base is characterized by a superficial, well-circumscribed inflammatory infiltrate that is striking both for its density and its polymorphous nature [4]. B lymphocytes differ in size from small to intermediate with scattered larger immunoblastic forms. Many of these cells are atypical and have Hodgkin and Reed-Sternberg-like morphology that may be of concern to histopathologists. These B lymphocytes show co-expression of CD30 in keeping with an activated phenotype and are entirely positive for EBER-ISH [5,6]. Due to its good response to conservative measures, especially showing an indolent behavior and a self-limited clinical course, this condition should be distinguished from high-grade neoplastic B-cell proliferations, such as extranodal classical Hodgkin's lymphoma and EBV-associated diffuse large B-cell lymphoma, typically seen in elderly patients.
MALT lymphoma is a non-Hodgkin lymphoma localized throughout the aerodigestive tract [7]. Specifically, the stomach (43%) is the most common primary site of MALT lymphoma, followed by the ocular adnexa (12%), lungs (10%), skin (9%), non-gastrointestinal tract (8%), salivary glands (6%), thyroid (6%), head and neck (3%), and breasts (2%) [8]. Histologically, MALT lymphoma is composed of heterogeneous small B-cells including centrocyte-like cells, monocytoid cells, plasmacytoid cells, small lymphocytes, scattered immunoblasts, and centroblast-like cells. Lymphoepithelial lesions are often observed. MALT lymphoma has the following immunophenotypes: CD20+, CD79a+, CD5−/+, CD10−, CD23−, CD43+/−, BCL6−, and MUM1−/+ [2]. It was hypothesized that the origin of this lymphoma arises from memory B-lymphocytes with the capacity to differentiate into marginal cells and plasma cells [9]. There is increasing evidence that MALT lymphoma may be associated with inflammatory chronic episodes, usually secondary to autoimmune or bacterial stimuli. The classic example is the association of H. pylori with chronic gastritis and gastric MALT lymphoma. Similar to MALT lymphoma, EBV-positive MCU characteristically presenting oral ulcer seemed to be associated with chronic inflammation. Mucosal trauma and persistent antigenic stimulation may initiate the activation of B lymphocytes with uncontrolled proliferation, which goes unchecked in cases of immunosuppression and where T-cell function is lost as is observed in several types of immunosuppression. This complex interplay of defective host immunity in the face of persistent chronic inflammation is what triggers and sustains the proliferation of EBV-infected lymphocytes [5].
A literature review by Zullo et al. [10] demonstrated a gastric MALT lymphoma relapse rate of 7.2% at the initial site in 994 patients after 3,253 patient-years of follow-up, and a MALT lymphoma at different sites has been reported showing a propensity for late relapse even decades later [11,12,13,14,15]. However, only a few studies have reported on tonsillar MALT lymphoma relapse or concurrent gastric lymphoma [11,12]. The gastrointestinal tract and Waldeyer's ring are considered part of the gut-associated lymphoid tissue, and their loco-relationship may be a reflection of the homing properties of MALT. Normal MALT B-cells leave the mucosa after antigenic stimulation through the efferent lymphatics and then travel through the mesenteric lymph nodes and thoracic duct to reach the systemic circulation [16]. These MALT B-cells then return or home back to the lamina propria as memory B-cells or plasma cells [16]. Neoplastic counterparts would be expected to show homing patterns similar to their precursors [17]. Consequently, when MALT lymphoma disseminates, they preferentially colonize other mucosal sites and organs of the MALT system, for example, conjunctival lymphoma spreading to the lungs and gastric lymphoma to the intestines [18].
In our unique case, the EBV-positive MCU and tonsillar MALT lymphoma due to relapsed gastric lymphoma explained by its homing phenomenon coincidently collided with the palatine tonsils, which are one of the well-known mucosal sites of frequent lymphoid malignancy due to their close association with the lymphoid-rich tissue of Waldeyer's ring. It could be speculated that gastric MALT lymphoma slowly relapsed in our patient's bilateral palatine tonsils, making them appear nodular, protruded, and enlarged, subsequently leaving them vulnerable to EBV-positive MCU development despite his immunocompetency. In summary, we report a case of EBV-positive MCU that collided with MALT lymphoma in the palatine tonsils of a 49-year-old immunocompetent man with a history of gastric MALT lymphoma who maintained CR. Most patients have either iatrogenic or senescent immunosuppression. However, our case demonstrated that mechanically enlarged tonsils caused by MALT lymphoma involvement also may evoke EBV-positive MCU despite patient's immunocompetency. This distinctive case may broaden the diagnostic clinical considerations currently recognized. Further investigations are necessary to discover whether other lymphoid malignancies interplay with or contribute to EBV-positive MCU.
Acknowledgments
This study was supported by a grant from Chungnam National University.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. Lyon, France: IARC Press; 2017. pp. 307–308. [Google Scholar]
- 2.Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. Lyon, France: IARC Press; 2017. pp. 259–262. [Google Scholar]
- 3.Merino F, Martinez CV, Zubillaga I, Aniceto GS, Ballestin C. MALT lymphoma occurring in the maxillofacial region: a review of the literature and case report. Oral Maxillofac Surg Case. 2017;3:70–75. [Google Scholar]
- 4.Dojcinov SD, Venkataraman G, Raffeld M, Pittaluga S, Jaffe ES. EBV positive mucocutaneous ulcer-a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010;34:405–417. doi: 10.1097/PAS.0b013e3181cf8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujita H, Nishikori M, Takaori-Kondo A, et al. A case of HIV-associated lymphoproliferative disease that was successfully treated with highly active antiretroviral therapy. Int J Hematol. 2010;91:692–698. doi: 10.1007/s12185-010-0542-8. [DOI] [PubMed] [Google Scholar]
- 6.Mahe E, Ross C, Sur M. Lymphoproliferative lesions in the setting of HIV infection: a five-year retrospective case series and review. Patholog Res Int. 2011;2011:618760. doi: 10.4061/2011/618760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983;52:1410–1416. doi: 10.1002/1097-0142(19831015)52:8<1410::aid-cncr2820520813>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Thieblemont C, Bastion Y, Berger F, et al. Mucosa-associated lymphoid tissue gastrointestinal and nongastrointestinal lymphoma behavior: analysis of 108 patients. J Clin Oncol. 1997;15:1624–1630. doi: 10.1200/JCO.1997.15.4.1624. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka T, Kitabatake K, Iino M, Goto K. Immunohistochemical comparison of CD5, lambda, and kappa expression in primary and recurrent buccal mucosa-associated lymphoid tissue (MALT) lymphomas. Diagn Pathol. 2011;6:82. doi: 10.1186/1746-1596-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zullo A, Hassan C, Cristofari F, et al. Effects of Helicobacter pylori eradication on early stage gastric mucosa-associated lymphoid tissue lymphoma. Clin Gastroenterol Hepatol. 2010;8:105–110. doi: 10.1016/j.cgh.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Lee JT, Paquette R, Sercarz JA, Wang MB. Mucosa-associated lymphoid tissue lymphoma of the lingual tonsil. Am J Otolaryngol. 2000;21:271–276. doi: 10.1053/ajot.2000.8382. [DOI] [PubMed] [Google Scholar]
- 12.Paulsen J, Lennert K. Low-grade B-cell lymphoma of mucosa-associated lymphoid tissue type in Waldeyer's ring. Histopathology. 1994;24:1–11. doi: 10.1111/j.1365-2559.1994.tb01265.x. [DOI] [PubMed] [Google Scholar]
- 13.Thieblemont C, Berger F, Dumontet C, et al. Mucosa-associated lymphoid tissue lymphoma is a disseminated disease in one third of 158 patients analyzed. Blood. 2000;95:802–806. [PubMed] [Google Scholar]
- 14.Raderer M, Vorbeck F, Formanek M, et al. Importance of extensive staging in patients with mucosa-associated lymphoid tissue (MALT)-type lymphoma. Br J Cancer. 2000;83:454–457. doi: 10.1054/bjoc.2000.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephen MR, Farquharson MA, Sharp RA, Jackson R. Sequential malt lymphomas of the stomach, small intestine, and gall bladder. J Clin Pathol. 1998;51:77–79. doi: 10.1136/jcp.51.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zucca E, Roggero E. Biology and treatment of MALT lymphoma: the state-of-the-art in 1996. A workshop at the 6th International Conference on Malignant Lymphoma. Mucosa-Associated Lymphoid Tissue. Ann Oncol. 1996;7:787–792. doi: 10.1093/oxfordjournals.annonc.a010756. [DOI] [PubMed] [Google Scholar]
- 17.Harris NL. Low-grade B-cell lymphoma of mucosa-associated lymphoid tissue and monocytoid B-cell lymphoma. Related entities that are distinct from other low-grade B-cell lymphomas. Arch Pathol Lab Med. 1993;117:771–775. [PubMed] [Google Scholar]
- 18.Isaacson PG, Spencer J. The biology of low grade MALT lymphoma. J Clin Pathol. 1995;48:395–397. doi: 10.1136/jcp.48.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]