Abstract
Recurrent malignant gliomas (RMGs) are difficult to control, and no standard protocol has been established for their treatment. At our institute, we have often treated RMGs by tumor-selective particle radiation called boron neutron capture therapy (BNCT). However, despite the cell-selectivity of BNCT, brain radiation necrosis (BRN) may develop and cause severe neurological complications and sometimes death. This is partly due to the full-dose X-ray treatments usually given earlier in the treatment course. To overcome BRN following BNCT, recent studies have used bevacizumab (BV). We herein used extended BV treatment beginning just after BNCT to confer protection against or ameliorate BRN, and evaluated; the feasibility, efficacy, and BRN control of this combination treatment. Seven patients with RMGs (grade 3 and 4 cases) were treated with BNCT between June 2013 and May 2014, followed by successive BV treatments. They were followed-up to December 2017. Median overall survival (OS) and progression-free survival (PFS) after combination treatment were 15.1 and 5.4 months, respectively. In one case, uncontrollable brain edema occurred and ultimately led to death after BV was interrupted due to meningitis. In two other cases, symptomatic aggravation of BRN occurred after interruption of BV treatment. No BRN was observed during the observation period in the other cases. Common terminology criteria for adverse events grade 2 and 3 proteinuria occurred in two cases and necessitated the interruption of BV treatments. Boron neutron capture therapy followed by BV treatments well-prevented or well-controlled BRN with prolonged OS and acceptable incidence of adverse events in our patients with RMG.
Keywords: bevacizumab, boron neutron capture therapy, brain radiation necrosis, recurrent malignant glioma
Introduction
The prognosis of recurrent malignant gliomas (RMGs) is poor, and no standard treatment has been established.1) Since 2002 at our institute, we have applied a form of tumor-selective particle radiation called, boron neutron capture therapy (BNCT), for patients with RMGs and observed favorable survival outcomes.2,3) BNCT is a biochemically targeted radiotherapy based on the nuclear capture and fission reactions that occur when non-radioactive boron-10, which is a constituent of natural elemental boron, is irradiated with low-energy thermal neutrons to yield high-linear-energy transfer alpha particles and recoiling lithium-7 nuclei. These particles are released within a very short range such as 9 μm, and therefore the cytotoxic effects are confined to within boron-10-containing cells.4)
Boron-10-containing compounds can be accumulated selectively in tumor cells by several mechanisms. For example, boronophenylalanine (BPA) is selectively and preferentially accumulated in tumor cells by amino acid transporters through their increased metabolism of amino acids compared to normal cells. Even with this novel and selective particle radiation therapy, radiation damage—chiefly brain radiation necrosis (BRN) and symptomatic pseudoprogression (psPD)—often occurs.5,6) Radiation damage is especially likely in RMG cases, because full-dose X-ray treatment (XRT) is generally part of the treatment history in such cases.
In a previous study analyzing human BRN surgical specimens, we discovered that the edema in BRN is caused by overexpression of vascular endothelial growth factor (VEGF) in reactive astrocytes.7) Bevacizumab (BV), an anti-VEGF antibody, has recently been used for the treatment of symptomatic BRN.8–10) Based on these findings, we have recently used BV in an attempt to control the symptomatic BRN and the psPD encountered after BNCT for RMGs.5,11)
Recently, several reports have examined the use of re-irradiation for RMG with addition of BV to confer protection against radiation injury12,13) and to enhance the efficacy of irradiation by overcoming the hypoxia-mediated mechanisms of radio-resistance.14) Here, we introduce the preliminary results of our use of BNCT for re-irradiation of RMG with BV added just after BNCT and continued for as long as possible. In this study, we estimate the feasibility and efficacy of this treatment.
Materials and Methods
Patient background
From June 2013 to May 2014, we treated seven cases of RMG using reactor-based BNCT. All patients had undergone full-dose XRT at the initial treatment. Patients’ demographics are documented in Table 1; these included WHO grade (histology), age, class of recursive portioning analysis (RPA) for RMG as advocated by Carson et al. in a 2007 article in the Journal of Clinical Oncology.1) Patients were followed-up until the end of December 2017.
Table 1.
Clinical data of individual patients in this series
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | |
|---|---|---|---|---|---|---|---|
| Age at BNCT | 68 | 66 | 36 | 37 | 62 | 57 | 57 |
| Gender | Male | Male | Male | Male | Male | Male | Male |
| WHO grade (histology) | IV (GBM) | IV (GBM) | III (AOA) | III (AO) | IV (GBM) | IV (GBM) | III (AOA) |
| RPA class | 7 | 3 | 2 | 1 | 7 | 7 | 3 |
| BRN while BV treatment | No | No | No | No | No | No | No |
| Cause of death | Tumor progression | Tumor progression | Tumor progression | Alive | Tumor progression | Meningitis | Alive |
| mOS in JCO# (Month) | 4.9 | 3.8 | 17.2 | 25.7 | 4.9 | 4.9 | 3.8 |
| OS (Month) | 15.1 | 7.5 | 38 | 53 (Censored) | 11.1 | 4.4 | 43 (Censored) |
| PFS (Month) | 3.6 | 5.4 | 19.5 | 53 (No progression) | 5 | Not applicable | 43 (No progression) |
| BNCT dose (Gy-equiv.) | |||||||
| Tumor mini | 36.8 | 29.9 | 42.6 | 60.4 | 27.6 | 43.8 | 63.9 |
| Tumor max | 56.3 | 69.2 | 97.5 | 96.5 | 54.4 | 96.2 | 151 |
| Normal max | 8.92 | 10.5 | 9.68 | 10.1 | 11.2 | 13 | 12 |
| Courses of BV | 19 | 9 | 55 | 32 | 20 | 2 | 65 |
| Adverse events | Proteinuria grade 1 | No | Proteinuria grade 2 | Proteinuria grade 1 | No | Meningitis | Proteinuria grade 3 |
BRN: brain radiation necrosis, BV: bevacizumab, OS: overall survival, PFS: progression free survival is determined by RANO criteria appeared in J Clin Oncol,20) RPA class: recursive portioning analysis class is determined by the criteria advocated by Carson et al.,1)
Median overall survival for the corresponding RPA class, described in J Clin Oncol 2007. See the detail in the text and reference number 1.
Clinical regimen of BNCT
This protocol was approved by the Ethical Committee of Osaka Medical College with the authorization number 1386. After confirmation of the recurrence of the original lesions on magnetic resonance imaging (MRI), the patients underwent BPA–positron emission tomography (PET) analysis to assess the distribution of BPA.15,16) The lesion/normal brain (L/N) ratio of BPA uptake can be estimated from this BPA–PET, and dose planning was performed according to the L/N ratio, as described previously.2,17) BPA was purchased from Interpharma Praha, a.s., (Prague, Czech Republic) and administered over a 2-h period (200 mg/kg/h) just prior to and during the neutron irradiation (100 mg/kg/h), as described previously.18) Based on the PET-based simulation described above and blood boron concentration in each patient, we selected a neutron irradiation time that would keep the peak brain dose below 11.0 Gy-equiv. (Gray-equivalent) in cases 1–4. After confirmation of the safety of this approach in these four cases, the neutron irradiation time was increased to keep the peak brain dose below 13.0 Gy-equiv. in cases 5–7. Here, Gy-equiv. corresponds to the biologically equivalent X-ray dose that would have equivalent effects on tumors and on the normal brain.
BV treatments
The BV treatments were initiated at 2–6 weeks after BNCT and continued until the tumor progression or occurrence of several adverse events (AE) such as continuous grade 3 proteinuria (common terminology criteria for adverse events: CTCAE ver. 4.0), or until discontinuation at the wish of the patient. The dose of BV was principally 10 mg/kg biweekly, but was decreased as appropriate due to AE.
Patient follow-up and study endpoints
Patients were followed-up by bimonthly Gd-enhanced and fluid-attenuated inversion recovery magnetic resonance imaging (FLAIR MRI). When the lesions became enlarged or new lesions appeared on the follow-up MRI, we applied BPA–PET to evaluate the tumor activity in selected cases,11) if the PET machine time was available, to distinguish the pathology as tumor progression or BRN or psPD, as shown in Table 1. BRN was judged by repetitive PET or transient aggravation on MRI during interruption of BV treatment, together with recovery of the aggravation by resumption of BV treatment. Worsening of the FLAIR and/or T1-Gd image on MRI even with the continuation of BV treatments was judged as tumor progression. If the follow-up PET showed the increased L/N ratio in comparison with the baseline PET, the lesion was judged as tumor recurrence. If the L/N ratio in follow-up PET showed decreased activity in comparison with baseline L/N ratio, the lesion was judged as BRN.19)
The feasibility (occurrence of any AE), efficacy (overall survival (OS)) and progression free survival (PFS), and control of BRN were evaluated.
RPA classification
To objectively evaluate the survival benefit of this treatment, we classified our BNCT cases according to Carson’s RPA classification as follows1): RPA class 1, not GBM (initial histology), KPS ≥ 80, frontal only (tumor location); RPA class 2, not GBM, KPS ≥ 80, not frontal only; RPA class 3, not GBM, 60 ≤ KPS ≤ 70; RPA class 4, GBM, age < 50, KPS ≥ 90; RPA class 5, GBM, age < 50, 60 ≤ KPS ≤ 80; RPA class 6, GBM, age ≥ 50, no steroid use; RPA class 7, GBM, age ≥ 50, steroid use. Table 1 shows the RPA classification for each of our cases treated by BNCT.
Results
Each of the endpoints (OS, PFS, AE and control of BRN), the corresponding median OS (mOS) described in Carson’s RPA classification, the BNCT-related minimum and maximum dose for tumor and maximum dose for normal brain tissue, and the courses of BV treatments and causes of death are summarized in Table 1.
Overall and progression free survival
Two of the seven patients were still alive at the end of the observation period (December 2017). mOS and PFS after the BNCT and BV treatments were 15.1 and 5.4 months, respectively. With regard to OS, only one case (Case 6) showed a shorter survival time in comparison with the reported mOS classified with the corresponding Carson’s RPA. The other six cases showed longer survival times than those in the classification. Even with the application of RANO criteria, it is rather difficult to assess PFS due to the modification of MRI made to account for the influence of BV.20) We lost four cases due to tumor progression and one case (Case 6) due to uncontrollable brain edema.
Control of BRN
Brain radiation necrosis may have been present in three cases (Cases 4, 6 and 7). In cases 4 and 6, BV treatments were discontinued due to severe meningitis (Table 1) and financial difficulties, respectively. Case 7 showed the aggravation of brain edema and slight enhancement during the discontinuance of BV treatments, which seemed to be BRN (see the detail in the case presentation below). Another case (Case 1) experienced transient aggravation suggestive of psPD on MRI, 1 month after BNCT. In the other three cases, there was no incidence of BRN or psPD, as judged by follow-up PET imaging or the clinical course, as defined above.
Adverse events
All cases showed CTCAE grade 2 alopecia which is usually observed in BNCT alone. As shown in Table 1, proteinuria was the most commonly observed AE during BV treatment. In the present study, CTCAE grade 2 and 3 proteinuria were observed in two individual cases (Cases 3 and 7) and grade 1 proteinuria was observed in two cases (Cases 1 and 4).
Instructive case presentations
Case 3:
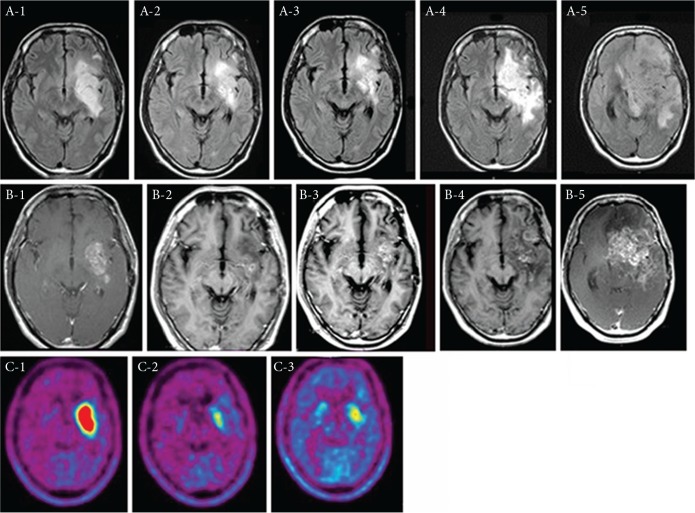
A 36-year-old male with recurrent anaplastic oligo-astrocytoma. RPA was classified as class 2. Prior to BNCT and BV treatment, he complained of intractable seizure. After the treatment the seizure could be well-controlled and the patient exhibited improvement on MRI. Repeated BPA–PET analysis yielded L/N ratios of 5.0, 1.9 and 2.1 just prior to, 7 months after, and 13 months after BNCT, respectively, as shown in Fig. 1.21–23) Unfortunately, MRI was aggravated as shown in the Fig. 1A-4 and B-4, and the patient died of tumor progression 38 months after BNCT. The patient received a total of 55 courses of BV. The prominent AE were general fatigue and proteinuria (CTCAE grade 2).
Fig. 1.
Periodic change of MRI and BPA–PET in Case 3. The upper panel (A-1 to A-5) shows FLAIR MRI studies. The middle panel (B-1 to B-5) shows Gd-T1 MRI studies. The lower panel (C-1 to C-3) shows F-BPA–PET studies. The lesion to normal brain (L/N) ratio of the enhanced tumor is 5.0 (C-1), 2.2 (C-2), and 1.7 (C-3). A-1, B-1, and C-1; prior to BNCT. A-2, B-2, and C-2; 7 months after BNCT. A-3, B-3, and C-3; 13 months after BNCT. A-4 and B-4; 26.5 months after BNCT. A-5 and B-5; 33 months after BNCT. This is the representative tumor recurrence appeared in this treatment regimen. BV treatment can control the BRN but even after BNCT some tumor recurred. The tumor recurrence with the maintenance BV treatments seems to be aggressive as reported.41–43)
Case 7:
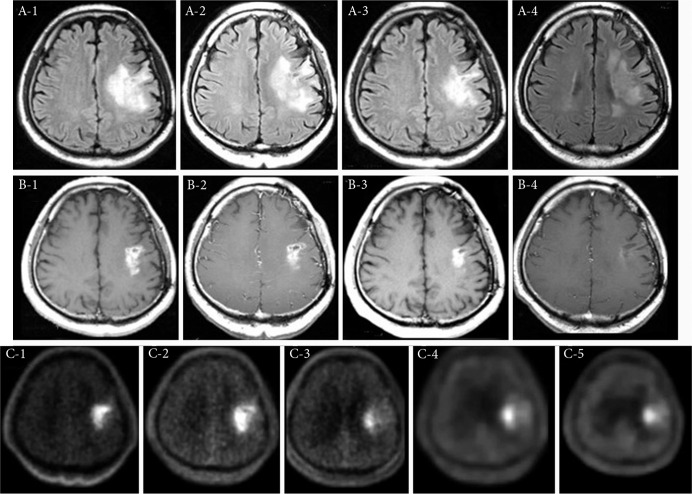
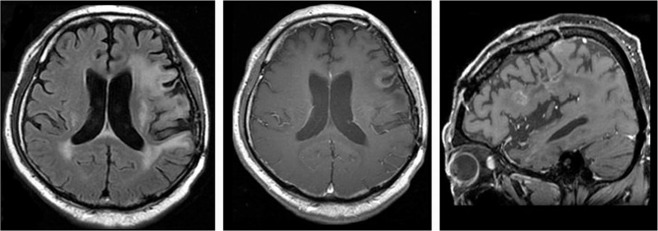
A 57-year-old male with re-recurrent anaplastic oligo-astrocytoma. RPA was classified as class 3, because he lost his job as a carpenter due to disease progression. He underwent 60 Gy XRT at the first recurrence, but unfortunately this had no effect on the recurrent tumor. The L/N ratios of BPA–PET prior to XRT and BNCT were 2.9 and 3.7, respectively. BNCT was administered at 6 months after XRT. BNCT and subsequent BV well-controlled his tumor. The L/N ratios of BPA–PET studies at 1, 3.5, and 12 months after BNCT were 2.2, 2.1 and 1.8, respectively, as shown in Fig. 2. He returned to his job 3 months after BNCT. He underwent 58 courses of BV. The prominent AE was proteinuria (CTCAE grade 3), which necessitated an occasional interruption of BV. When 5 months had passed after discontinuance of BV administration (38 months after BNCT) because of proteinuria, his right hemiparesis and dysarthria worsened and his convulsions increased. Based on MRI studies, we attributed these developments to symptomatic BRN (Fig. 3).
Fig. 2.
Periodic change of MRI in Case 7. The upper panel (A-1 to A-4) shows FLAIR MRI studies. The middle panel (B-1 to B-4) shows Gd-T1 MRI studies. The lower panel (C-1 to C-5) shows time course of BPA–PET imaging. A-1 and B-1 are prior to BNCT. A-2 and B-2 are 1 week after BNCT. A-3 and B-3 are 1 month after BNCT. A-4 and B-4 are 24 months after BNCT. Each time of point and L/N ratio of PET imaging are stated in case presentation in the text of Results. This is the illustrative case that BNCT can control tumor activity, while BV can control BRN successively and successfully for long time.
Fig. 3.
Aggravation of BRN in case 7 due to BV discontinuance. Five months after discontinuance of BV administration because of proteinuria (38 months after BNCT) in case 7. His right hemiparesis and dysarthria got worse and convulsions increased. We considered them as due to symptomatic BRN by follow-up MRI studies and clinical course.
Discussion
Malignant gliomas still represent one of the most aggressive and devastating tumors in neuro-oncology. In spite of extensive research, the treatment outcome is still not satisfying. At primary diagnosis, treatment commonly consists of neurosurgical resection, as radical as possible without resulting in marked morbidity, followed by post-operative radiotherapy. With this combination, overall survival rates of 9–12 months have been obtained.24,25) Recently, novel chemotherapy regimens have been added to this treatment combination. Radiochemotherapy with temozolomide has achieved a significant increase in overall survival times up to 14.6 months.26) Concomitant administration of ACNU/VM26 to radiotherapy could extend overall survival to 16.5 months.27) However, almost all GBM recur, with local failure being the most common pattern of recurrence.28) Therefore, local control is the most critical issue to help improve overall survival for patients with GBM.
In most patients, treatment options at the time of recurrence are limited. Chemotherapy is the most frequently applied treatment for recurrent gliomas. Radiotherapy as a treatment for recurrent gliomas is controversial. Commonly, full doses of irradiation have been applied post-operatively after primary diagnosis, and re-irradiation is associated with a higher risk of BRN, with toxicity outweighing its benefits.29) Even with tumor-selective particle radiation BNCT, BRN or symptomatic psPD are inevitable due to not only the preceding XRT but also BNCT which gives the peak dose of approximately 10–13 Gy-equiv. to the normal brain by single fraction irradiation, as in the present series.
In this small-scale preliminary clinical study, only one case (Case 6) showed an OS shorter than the corresponding mOS in Carson’s RPA classification. Two patients (Cases 4 and 7) are still alive without tumor progression. In these patients a rather high tumor minimum dose was prescribed (>60 Gy-equiv., as shown in Table 1), which may have been the reason for the excellent OS. The other six cases showed good control of BRN by this treatment protocol and showed an OS longer than the corresponding mOS in Carson’s RPA classification.
With regard to newly diagnosed GBM, BV was not found to contribute to the prolongation of OS in two large-scale phase 3 clinical trials.30,31) Also for recurrent GBM, BV showed no contribution to the prolongation of OS in the phase 3 EORTC 26101 trial,32) although promising results were obtained in a phase 2 clinical trial.33) In comparison with these clinical results, our preliminary study showed several potentially conflicting findings in regard to OS prolongation. Based on our study, BV was expected to contribute to the prolongation of OS through its synergy with BNCT. However, although there have been several retrospective reports of the role of BV in re-irradiation for RMG,34–37) none of them showed any apparent benefit of BV in the prolongation of OS for RMG in a re-irradiation setting. Further clarification is expected to come from the RTOG 1205/NCT01730950 trial, which was conducted to compare re-irradiation/BV with BV alone. As discussed above, BRN was well-controlled in the present study, with only one patient dying from severe brain edema because he could not be kept on BV treatments. Therefore, the prolonged OS in this series should be attributed to the synergy of BNCT and the control of BRN by BV.
The most common and prominent additional AE in this treatment regimen, compared to BNCT alone, was proteinuria. In this series, the occurrence rate and the severity of proteinuria may have been related to the total BV dose. In the literature, there are only two reports about the relationship of the incidence of proteinuria to the total dose of BV. Hayman SR reported an apparent relationship between proteinuria and the cumulative dose of BV,38) while Slusarz39) did not recognize any relationship. There have been several reports concerning the distinctive relationship between a single dose of BV and proteinuria.40–43) We and other investigators reported that a regimen with smaller doses of BV (5 mg/kg, biweekly) still exhibited potent effects for BRN.9,10) Further clinical trials will thus be needed to examine the potential of such smaller doses to reduce the risk of proteinuria. In addition, the timing of the first BV treatment should start within a month, and hopefully within 2 weeks, after BNCT, because Case 1 showed symptomatic psPD at 1 month after BNCT, which was recovered with subsequent addition of BV.
Boron neutron capture therapy followed by BV treatments well-prevented or well-controlled BRN with prolonged OS and acceptable incidence of AE in our cohort of patients with RMG. Most interesting issue in this clinical study not only for authors but also for readers is BNCT with early administration of BV can show the superior clinical results for RMG especially in OS in comparison with BNCT alone or not. In the previous work,3) we showed BNCT without early BV intervention showed mOS as 10.8M (95% confidence interval (CI): 7.3–12.8M) and 9.1M (95% CI: 4.4–11.0M) for all RMG (n = 22) and poor risk RMG (RPA class 3 and 7) (n = 11), respectively. While in this current study we showed BNCT with early BV administration showed mOS as 15.1M (95% CI: 6.5–42.7M) and 11.1M (95% CI: −3.0–35.5M) for all RMG (n = 7) and for poor risk RMG (RPA class 3 and 7) (n = 5), respectively. At a glance, there are differences in OS both in all RMG and in poor risk RMG cases between BNCT with and without early BV treatments. However, there is no statistical significance between them with statistical analyses. Possibly small patient numbers and large variations in OS in this current study may be the cause of the lack of statistical significance. Based on these preliminary results, now we are performing a prospective clinical trial using BNCT and simultaneous BV treatments for poor prognosis patients with recurrent malignant glioma.
Footnotes
Funding
This work was partly supported by “Kenzo Suzuki Memorial Medical Science Applications Foundation”.
Conflicts of Interest Disclosure
The author declares that there are no conflicts of interest.
References
- 1).Carson KA, Grossman SA, Fisher JD, Shaw EG: Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials. J Clin Oncol 25: 2601–2606, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Miyatake S, Kawabata S, Kajimoto Y, et al. : Modified boron neutron capture therapy for malignant gliomas performed using epithermal neutron and two boron compounds with different accumulation mechanisms: an efficacy study based on findings on neuroimages. J Neurosurg 103: 1000–1009, 2005 [DOI] [PubMed] [Google Scholar]
- 3).Miyatake S, Kawabata S, Yokoyama K, et al. : Survival benefit of boron neutron capture therapy for recurrent malignant gliomas. J Neurooncol 91: 199–206, 2009 [DOI] [PubMed] [Google Scholar]
- 4).Barth RF, Vicente MG, Harling OK, et al. : Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat Oncol 7: 146, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Miyatake S, Furuse M, Kawabata S, et al. : Bevacizumab treatment of symptomatic pseudoprogression after boron neutron capture therapy for recurrent malignant gliomas. Report of 2 cases. Neuro-oncology 15: 650–655, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Miyatake S, Kawabata S, Nonoguchi N, et al. : Pseudoprogression in boron neutron capture therapy for malignant gliomas and meningiomas. Neuro-oncology 11: 430–436, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Nonoguchi N, Miyatake S, Fukumoto M, et al. : The distribution of vascular endothelial growth factor-producing cells in clinical radiation necrosis of the brain: pathological consideration of their potential roles. J Neurooncol 105: 423–431, 2011 [DOI] [PubMed] [Google Scholar]
- 8).Furuse M, Kawabata S, Kuroiwa T, Miyatake S: Repeated treatments with bevacizumab for recurrent radiation necrosis in patients with malignant brain tumors: a report of 2 cases. J Neurooncol 102: 471–475, 2011 [DOI] [PubMed] [Google Scholar]
- 9).Furuse M, Nonoguchi N, Kuroiwa T, et al. : A prospective, multicentre, single-arm clinical trial of bevacizumab for patients with surgically untreatable, symptomatic brain radiation necrosis†. Neurooncol Pract 3: 272–280, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Levin VA, Bidaut L, Hou P, et al. : Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys 79: 1487–1495, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Miyatake S, Kawabata S, Hiramatsu R, Furuse M, Kuroiwa T, Suzuki M: Boron neutron capture therapy with bevacizumab may prolong the survival of recurrent malignant glioma patients: four cases. Radiat Oncol 9: 6, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Perez-Torres CJ, Yuan L, Schmidt RE, et al. : Specificity of vascular endothelial growth factor treatment for radiation necrosis. Radiother Oncol 117: 382–385, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Schnell O, Thorsteinsdottir J, Fleischmann DF, et al. : Re-irradiation strategies in combination with bevacizumab for recurrent malignant glioma. J Neurooncol 130: 591–599, 2016 [DOI] [PubMed] [Google Scholar]
- 14).Anderson JC, Duarte CW, Welaya K, et al. : Kinomic exploration of temozolomide and radiation resistance in glioblastoma multiforme xenolines. Radiother Oncol 111: 468–474, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Imahori Y, Ueda S, Ohmori Y, et al. : Positron emission tomography-based boron neutron capture therapy using boronophenylalanine for high-grade gliomas: part I. Clin Cancer Res 4: 1825–1832, 1998 [PubMed] [Google Scholar]
- 16).Imahori Y, Ueda S, Ohmori Y, et al. : Positron emission tomography-based boron neutron capture therapy using boronophenylalanine for high-grade gliomas: part II. Clin Cancer Res 4: 1833–1841, 1998 [PubMed] [Google Scholar]
- 17).Kawabata S, Miyatake S, Kajimoto Y, et al. : The early successful treatment of glioblastoma patients with modified boron neutron capture therapy. Report of two cases. J Neurooncol 65: 159–165, 2003 [DOI] [PubMed] [Google Scholar]
- 18).Kawabata S, Hiramatsu R, Kuroiwa T, Ono K, Miyatake S: Boron neutron capture therapy for recurrent high-grade meningiomas. J Neurosurg 119: 837–844, 2013 [DOI] [PubMed] [Google Scholar]
- 19).Miyashita M, Miyatake S, Imahori Y, et al. : Evaluation of fluoride-labeled boronophenylalanine-PET imaging for the study of radiation effects in patients with glioblastomas. J Neurooncol 89: 239–246, 2008 [DOI] [PubMed] [Google Scholar]
- 20).Wen PY, Macdonald DR, Reardon DA, et al. : Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28: 1963–1972, 2010 [DOI] [PubMed] [Google Scholar]
- 21).Han SJ, Rolston JD, Molinaro AM, et al. : Phase II trial of 7 days on/7 days off temozolmide for recurrent high-grade glioma. Neuro-oncology 16: 1255–1262, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Omuro A, Chan TA, Abrey LE, et al. : Phase II trial of continuous low-dose temozolomide for patients with recurrent malignant glioma. Neuro-oncology 15: 242–250, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Mrugala MM, Engelhard HH, Dinh Tran D, et al. : Clinical practice experience with NovoTTF-100ATM system for glioblastoma: The Patient Registry Dataset (PRiDe). Semin Oncol 41 Suppl 6: S4–S13, 2014 [DOI] [PubMed] [Google Scholar]
- 24).Walker MD, Gehan EA: Clinical studies in malignant gliomas and their treatment with the nitrosoureas. Cancer Treat Rep 60: 713–716, 1976 [PubMed] [Google Scholar]
- 25).Combs SE, Gutwein S, Schulz-Ertner D, et al. : Temozolomide combined with irradiation as postoperative treatment of primary glioblastoma multiforme. Phase I/II study. Strahlenther Onkol 181: 372–377, 2005 [DOI] [PubMed] [Google Scholar]
- 26).Stupp R, Mason WP, van den Bent MJ, et al. : Concomitant and adjuvant temozolomide (TMZ) and radiotherapy (RT) for newly diagnosed glioblastoma multiforme (GBM). Conclusive results of a randomized phase III trial by the EORTC Brain & RT Groups and NCIC Clinical Trials Group. 2004 [Google Scholar]
- 27).Weller M, Müller B, Koch R, Bamberg M, Krauseneck P, Neuro-Oncology Working Group of the German Cancer Society : Neuro-oncology Working Group 01 trial of nimustine plus teniposide versus nimustine plus cytarabine chemotherapy in addition to involved-field radiotherapy in the first-line treatment of malignant glioma. J Clin Oncol 21: 3276–3284, 2003 [DOI] [PubMed] [Google Scholar]
- 28).Sneed PK, Gutin PH, Larson DA, et al. : Patterns of recurrence of glioblastoma multiforme after external irradiation followed by implant boost. Int J Radiat Oncol Biol Phys 29: 719–727, 1994 [DOI] [PubMed] [Google Scholar]
- 29).Brandes AA, Pasetto LM, Monfardini S: New drugs in recurrent high grade gliomas. Anticancer Res 20: 1913–1920, 2000 [PubMed] [Google Scholar]
- 30).Chinot OL, Wick W, Mason W, et al. : Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370: 709–722, 2014 [DOI] [PubMed] [Google Scholar]
- 31).Gilbert MR, Dignam JJ, Armstrong TS, et al. : A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370: 699–708, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Wick W, Gorlia T, Bendszus M, et al. : Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med 377: 1954–1963, 2017 [DOI] [PubMed] [Google Scholar]
- 33).Taal W, Oosterkamp HM, Walenkamp AM, et al. : Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol 15: 943–953, 2014 [DOI] [PubMed] [Google Scholar]
- 34).Flieger M, Ganswindt U, Schwarz SB, et al. : Re-irradiation and bevacizumab in recurrent high-grade glioma: an effective treatment option. J Neurooncol 117: 337–345, 2014 [DOI] [PubMed] [Google Scholar]
- 35).Gutin PH, Iwamoto FM, Beal K, et al. : Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 75: 156–163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Hundsberger T, Brügge D, Putora PM, Weder P, Weber J, Plasswilm L: Re-irradiation with and without bevacizumab as salvage therapy for recurrent or progressive high-grade gliomas. J Neurooncol 112: 133–139, 2013 [DOI] [PubMed] [Google Scholar]
- 37).Niyazi M, Ganswindt U, Schwarz SB, et al. : Irradiation and bevacizumab in high-grade glioma retreatment settings. Int J Radiat Oncol Biol Phys 82: 67–76, 2012 [DOI] [PubMed] [Google Scholar]
- 38).Hayman SR, Calle JC, Jatoi A, et al. : Urinary podocyte excretion and proteinuria in patients treated with antivascular endothelial growth factor therapy for solid tumor malignancies. Oncology 86: 271–278, 2014 [DOI] [PubMed] [Google Scholar]
- 39).Slusarz KM, Merker VL, Muzikansky A, Francis SA, Plotkin SR: Long-term toxicity of bevacizumab therapy in neurofibromatosis 2 patients. Cancer Chemother Pharmacol 73: 1197–1204, 2014 [DOI] [PubMed] [Google Scholar]
- 40).Izzedine H, Massard C, Spano JP, Goldwasser F, Khayat D, Soria JC: VEGF signalling inhibition-induced proteinuria: mechanisms, significance and management. Eur J Cancer 46: 439–448, 2010 [DOI] [PubMed] [Google Scholar]
- 41).Johnson DH, Fehrenbacher L, Novotny WF, et al. : Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 22: 2184–2191, 2004 [DOI] [PubMed] [Google Scholar]
- 42).Yang JC, Haworth L, Sherry RM, et al. : A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349: 427–434, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Zhu X, Wu S, Dahut WL, Parikh CR: Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis 49: 186–193, 2007 [DOI] [PubMed] [Google Scholar]