Abstract
OBJECTIVE
To study the extent to which BMI after pregnancy adds to the elevated risk of postpregnancy type 2 diabetes in women with a history of hypertensive disorders of pregnancy (HDP) (preeclampsia or gestational hypertension).
RESEARCH DESIGN AND METHODS
We used data from the Nurses’ Health Study II, a prospective cohort study. In women aged 45–54 years without prior gestational diabetes mellitus, we investigated the interaction between BMI and HDP history on the risk of type 2 diabetes. For clinical and public health relevance, we focused on additive interaction. The main outcome measure was the relative excess risk due to interaction calculated from multivariable Cox proportional hazards models using normal weight as the reference group.
RESULTS
In total, 6,563 (11.7%) of 56,159 participants had a history of HDP and 1,341 women developed type 2 diabetes during 436,333 person-years. BMI was a strong risk factor for type 2 diabetes regardless of HDP history. However, there was evidence of an additive interaction between BMI and HDP for the risk of type 2 diabetes (P = 0.004). The attributable proportion of risk due to the interaction ranged from 0.12 (95% CI −0.22, 0.46) in women who were overweight to 0.36 (95% CI 0.13, 0.59) in women with obesity class I.
CONCLUSIONS
Maintaining a healthy weight may be of even greater importance in women with a history of HDP, compared with other women with a history of only normotensive pregnancies, to reduce midlife risk of type 2 diabetes.
Introduction
During the decades following pregnancy, women with hypertensive disorders of pregnancy (HDP) (preeclampsia and gestational hypertension) experience greater weight gain and are at twice the risk of type 2 diabetes compared with women without a history of HDP (1,2). Consequently, a history of HDP could also be clinically relevant beyond the obstetric setting to improve prevention of cardiometabolic disorders through targeted screening and early intervention (3). Current recommendations suggest that women with a history of gestational diabetes mellitus should be screened for type 2 diabetes at least once every 3 years (4). By contrast, for women with a history of HDP the American College of Obstetrics and Gynecology recommends a healthy lifestyle but does not specify the frequency of postpartum follow-up or screening (5). We have previously shown that women with a history of HDP may additionally benefit from maintaining a healthy weight, as postpregnancy BMI modifies the association between HDP history and incident chronic hypertension (6). However, it is currently unknown whether maintaining a healthy BMI is of greater importance in the prevention of type 2 diabetes for women with a history of HDP compared with women without such a history.
In this study, we investigated the interaction (effect modification) between history of HDP and BMI after pregnancy and on the risk of type 2 diabetes in parous women without a history of gestational diabetes mellitus. To do so, we investigated the association between postpregnancy BMI and type 2 diabetes by HDP history and then compared the separate and joint effects of BMI and HDP history on the risk of developing type 2 diabetes. For clinical and public health relevance, we mainly focused on the additive interaction of joint effects (7). With respect to risk of type 2 diabetes, we hypothesized that a healthy weight postpregnancy would be even more important for women with a history of HDP relative to parous women with a history of only normotensive pregnancies.
Research Design and Methods
We used data from the Nurses’ Health Study II, a U.S.-based prospective cohort study of 116,429 female nurses aged 25–42 years when enrolled in 1989. Participants were free of known cancers (excluding benign skin cancer) at recruitment and were sent questionnaires on lifestyle and health biennially. The study was approved by the Institutional Review Board at Brigham and Women’s Hospital. Completion of the self-administered questionnaire was considered informed consent.
Study Sample
For the purpose of this analysis, follow-up was between 1991—the 1st year of dietary data collection and the 1st year participants reached age 45 years—and the return of the 2013 questionnaire. We restricted follow-up to age 45–54 years to balance clinical relevance and analytical assumptions, mainly because the association between pregnancy complications and cardiometabolic disease is attenuated with age (see below). One participant had missing date of birth and was excluded. In 2009, 76,839 participants provided detailed information on their full reproductive history. Of these responders, we further excluded 13,253 nulliparous women and 463 women with physician-diagnosed diabetes or self-reported antidiabetes medication use before 1991. Additionally, 2,598 women were excluded because they had a history of gestational diabetes mellitus in 1991 and 443 women were excluded because they developed type 2 diabetes after 1991 but before age 45 years. We also excluded 3,923 women for whom one or more exclusion criteria pertained during the follow-up period. Women were typically excluded because of being underweight (<18.5 kg/m2), having a BMI >50 kg/m2, or having missing data (see below). In total, 56,159 parous women contributed to this analysis.
Ascertainment of Type 2 Diabetes
Participants who reported physician-diagnosed type 2 diabetes on the biennial questionnaire were sent a supplemental questionnaire to confirm the diagnosis. Type 2 diabetes was defined according to the American Diabetes Association 1998 criteria as at least one of the following: one or more classic symptoms (excessive thirst, polyuria, unintentional weight loss, hunger) and fasting plasma glucose concentration ≥126 mg/dL (7.0 mmol/L) or random plasma glucose ≥200 mg/dL (11.1 mmol/L), no symptoms but two or more elevated plasma glucose concentrations on more than one occasion or plasma glucose ≥200 mg/dL (11.1 mmol/L) after an oral glucose load, or hypoglycemic medication use (insulin or oral hypoglycemic agent). Between 1991 and 1998, the threshold for fasting plasma glucose was >140 mg/dL (7.8 mmol/L) according to the National Diabetes Data Group guidelines (8).
Ascertainment of HDP and Covariates
Women who reported a pregnancy complicated by either preeclampsia or gestational hypertension on the 2009 reproductive history questionnaire were defined as having a history of HDP from the year of the reported pregnancy. The quality of maternal recall of HDP does not appear to be uniformly affected by time since pregnancy (9).
In 1989, women retrospectively reported their weight at age 18 years and reported their current height. On each subsequent questionnaire, women reported their current weight. Self-report of height and weight has been shown to correspond to clinical measurements in nurse participants (10,11). We calculated BMI as (weight in kg)/(height in m2) and parameterized BMI into four categories: normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), obesity class I (30.0–34.9 kg/m2), and obesity class II–III (≥35.0 kg/m2) (12).
Participants self-reported race/ethnicity in 1989. Physical activity was updated every 4–6 years and based on total leisure-time physical activity per week with specific activities characterized by METs in quartiles (13). Self-reported diet was collected via a food frequency questionnaire every 4 years (14). We categorized participants into quartiles according to their compliance with the established Dietary Approaches to Stop Hypertension (DASH) guidelines (15), which are associated with risk of type 2 diabetes (16).
Statistical Analyses
Participants were eligible to contribute to the analysis from age 45 years until report of incident type 2 diabetes, age 55 years, death, a reported diagnosis of gestational diabetes mellitus or secondary diabetes or last returned questionnaire—whichever came first. Women entered the analysis the first time that they returned a biennial questionnaire at or after age 45 years. We used multivariable Cox proportional hazards models to estimate hazard ratios (HRs) for the association between BMI and type 2 diabetes by history of HDP in women aged 45–54 years. These models included HDP, BMI, and their interaction terms.
In the first stage of adjustment, we adjusted for race/ethnicity, age, and parity. Our main model additionally included BMI at age 18 years, menopausal status, parental history of diabetes, and updated DASH diet, physical activity, alcohol intake, and smoking. As not all relevant variables are collected each questionnaire cycle, we allowed for carryforward of variables to subsequent follow-up periods. If missing during a certain period of follow-up, BMI was carried forward for up to two cycles. Model covariates were generally carried forward for four cycles if missing. We used likelihood ratio tests, comparing models with and without interaction terms with age, to check the proportional hazards assumption (P > 0.05).
We calculated the additive interaction between BMI and history of HDP for the risk of developing type 2 diabetes by comparing the separate and joint effects of BMI and HDP history on type 2 diabetes incidence. To do so, we used effect estimates from the main Cox model in which women with normal BMI and no history of HDP constituted the reference group. To investigate the additive interaction of BMI and history of HDP, we estimated the relative excess risk due to interaction (RERI) (>0 supports additive interaction), attributable proportion, and synergy index (17) across categories of BMI using a previously described macro (18). The reference group for all comparisons was women with normal BMI and no history of HDP (i.e., women with the lowest a priori risk of type 2 diabetes). We also calculated the additive interaction of BMI as a continuous variable and HDP history on the risk of type 2 diabetes. As recommended (17), we also calculated the multiplicative interaction between BMI and history of HDP. Analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Results
In total, 56,159 women contributed 436,333 person-years and 1,341 women were diagnosed with type 2 diabetes during follow-up. Table 1 shows the characteristics of women at entry into the analysis by HDP history. At the start of follow-up, 11.7% (6,563) of women reported a history of HDP. Descriptively, women with a history of HDP had higher BMI and were more likely to report parental history of diabetes compared with women with only normotensive pregnancies. Supplementary Fig. 1 shows crude cumulative incidence curves for type 2 diabetes by BMI and HDP status between ages 45 and 55 years. In women with normal weight, the incidence of type 2 diabetes between ages 45 and 55 years was low regardless of HDP history. In all BMI groups, women with a history of HDP appeared to have a higher cumulative incidence of type 2 diabetes.
Table 1.
Descriptive characteristics of the study sample at study entry by history of HDP
| Variable | No HDP (n = 49,596) | HDP (n = 6,563) |
|---|---|---|
| Age, years, mean (SD) | 46.3 (1.1) | 46.3 (1.2) |
| History of preeclampsia in any pregnancy* | n/a | 4,325 (65.9) |
| History of gestational hypertension in any pregnancy* | n/a | 3,736 (56.9) |
| BMI at age 18 years, kg/m2, mean (SD)† | 20.9 (2.8) | 21.7 (3.3) |
| Parental history of diabetes | 14,709 (29.7) | 2,112 (32.2) |
| Current BMI, kg/m2, mean (SD) | 25.9 (5.3) | 28.7 (6.4) |
| Current BMI, kg/m2 | ||
| 18.5–24.9 | 26,577 (53.6) | 2,261 (34.5) |
| 25.0–29.9 | 13,688 (27.6) | 1,949 (29.7) |
| 30.0–34.9 | 5,868 (11.8) | 1,224 (18.7) |
| 35.0–50.0 | 3,463 (7.0) | 1,129 (17.2) |
| Physical activity, METs/week | ||
| Fourth quartile (high activity) | 12,273 (24.7) | 1,455 (22.2) |
| Third quartile | 12,411 (25.0) | 1,547 (23.6) |
| Second quartile | 12,533 (25.3) | 1,713 (26.1) |
| First quartile (low activity) | 12,379 (25.0) | 1,848 (28.2) |
| DASH diet score | ||
| Fourth quartile (high adherence) | 10,917 (22.0) | 1,316 (20.1) |
| Third quartile | 13,380 (27.0) | 1,786 (27.2) |
| Second quartile | 12,439 (25.1) | 1,693 (25.8) |
| First quartile (low adherence) | 12,860 (25.9) | 1,768 (26.9) |
| Race/ethnicity | ||
| White/Caucasian | 46,232 (93.2) | 6,157 (93.8) |
| African American | 547 (1.1) | 83 (1.3) |
| Latina | 620 (1.3) | 84 (1.3) |
| Asian | 656 (1.3) | 52 (0.8) |
| Other | 806 (1.6) | 93 (1.4) |
| Missing | 735 (1.5) | 94 (1.4) |
| Alcohol intake | ||
| No intake | 19,180 (38.7) | 2,832 (43.2) |
| 1–15 g/day | 27,078 (54.6) | 3,347 (51.0) |
| >15 g/day | 3,338 (6.7) | 384 (5.9) |
| Smoking status | ||
| Never | 32,832 (66.2) | 4,308 (65.6) |
| Past | 12,627 (25.5) | 1,716 (26.1) |
| Current | 4,137 (8.3) | 539 (8.2) |
| Parity | ||
| 1 birth | 8,250 (16.6) | 1,223 (18.6) |
| 2 births | 24,293 (49.0) | 3,137 (47.8) |
| 3 or more births | 17,053 (34.4) | 2,203 (33.6) |
| Menopausal status | ||
| Premenopause | 41,853 (84.4) | 5,354 (81.6) |
| Postmenopause | 6,340 (12.8) | 995 (15.2) |
| Unknown | 1,403 (2.8) | 214 (3.3) |
Data are n (%) unless otherwise indicated. n/a, not applicable.
*Categories not mutually exclusive.
†Missing = 445 (0.8%).
Association Between Postpregnancy BMI and Type 2 Diabetes by History of HDP
Table 2 shows the association between BMI after pregnancy and development of type 2 diabetes by HDP history. There were strong dose-response associations of BMI with diabetes risk regardless of whether women had a history of HDP. For example, among women with a history of HDP, women who were overweight had a fourfold (95% CI 1.91, 7.67) increased rate of type 2 diabetes compared with women who were normal weight; this HR increased to 14 (95% CI 7.50, 27.80) for women who had obesity class I.
Table 2.
Incidence rates and HRs for type 2 diabetes according to BMI by history of HDP in parous middle-aged women without history of gestational diabetes mellitus
| Incidence rates | |||||
|---|---|---|---|---|---|
| Normal weight (18.5–24.9 kg/m2) | Overweight (25.0–29.9 kg/m2) | Obesity class I (30.0–34.9 kg/m2) | Obesity class II–III (≥35.0 kg/m2) | Total | |
| Crude incidence | |||||
| No HDP | |||||
| Events/person-years* | 60/193,169 | 221/113,891 | 313/50,566 | 443/29,306 | 1,037/386,932 |
| Incidence per 1,000 person-years | 0.31 | 1.9 | 6.2 | 15.1 | 2.7 |
| History of HDP | |||||
| Events/person-years* | 10/15,573 | 40/15,360 | 96/9,934 | 158/8,534 | 304/49,401 |
| Incidence per 1,000 person-years |
0.64 |
2.6 |
9.7 |
18.5 |
6.2 |
| HR (95% CI) |
|||||
| Normal weight (18.5–24.9 kg/m2) | Overweight (25.0–29.9 kg/m2) | Obesity class I (30.0–34.9 kg/m2) | Obesity class II–III (≥35.0 kg/m2) | Ptrend† | |
|---|---|---|---|---|---|
| Basic Cox regression model | |||||
| No HDP‡ | 1.00 (Ref) | 6.18 (4.65, 8.23) | 19.60 (14.85, 25.87) | 46.94 (35.79, 61.56) | <0.001 |
| History of HDP‡ | 1.00 (Ref)§ | 3.96 (1.98, 7.92) | 14.73 (7.67, 28.28) | 29.60 (15.60, 56.17) | <0.001 |
| Main Cox regression model | |||||
| No HDP‡ | 1.00 (Ref) | 5.89 (4.42, 7.85) | 18.20 (13.72, 24.13) | 44.6 (33.56, 59.25) | <0.001 |
| History of HDP‡ | 1.00 (Ref)§ | 3.83 (1.91, 7.67) | 14.44 (7.50, 27.80) | 28.78 (15.06, 54.98) | <0.001 |
Ref, reference group. Women categorized as underweight (BMI <18.5 kg/m2) or with BMI >50 kg/m2 are excluded. Basic models include adjustment for age, race/ethnicity (white, Latina, African American, Asian, or other), and parity (1, 2, or ≥3 births). Main models additionally include DASH diet score (quartiles), physical activity (quartiles), BMI at age 18 years, smoking (nonsmoker, current smoker, or former smoker), alcohol intake (none, 1–15 g/day, or >15 g/day), menopausal status (premenopausal, postmenopausal, or unknown), parental history of diabetes (yes/no), and interaction terms between HDP and BMI categories.
*Rounded to year.
†Continuous BMI included in the models instead of categorical BMI.
‡Separate Cox proportional hazards models by HDP history.
§The same model as for “no HDP” except that women with prior HDP and BMI 18.5–24.9 kg/m2 constitute the reference group.
Interaction Between Postpregnancy BMI and History of HDP on Risk of Type 2 Diabetes
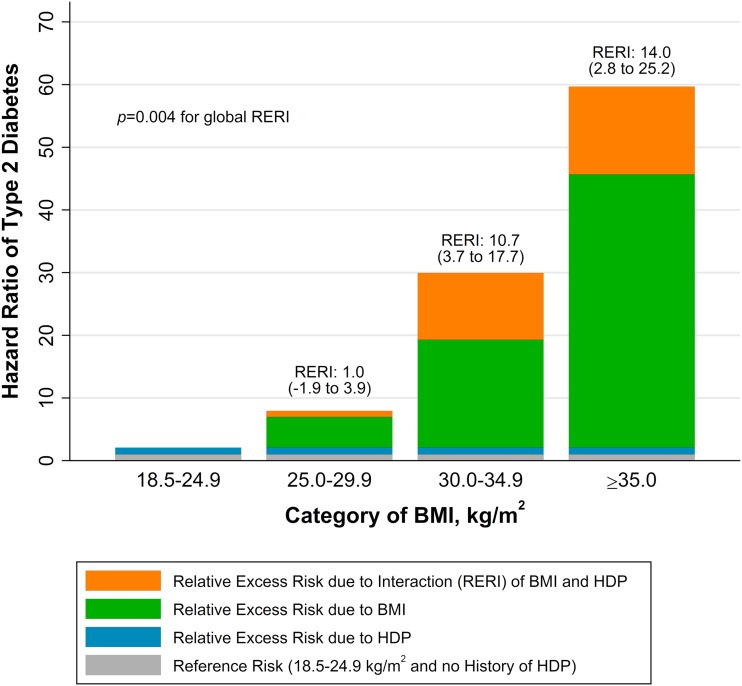
Women with normal weight and history of HDP were at twice the risk (HR 2.08 [95% CI 1.06, 4.06]) of type 2 diabetes compared with women with normal weight and no history of HDP. Figure 1 summarizes the results of the additive interaction analysis, in which the single effects of BMI and HDP history are compared with their joint effects. The HR of 1 for the reference group (normal BMI, no HDP history) is shown as gray bars. The relative excess risk due to HDP is represented by blue bars, the relative excess risk due to higher BMI by green bars, and their interaction defined as the RERI by orange bars. There was evidence of an additive interaction effect of higher BMI (P = 0.004 across categories of BMI) on the risk of type 2 diabetes with a history of HDP, especially in women with obesity. By contrast, there was no evidence of an interaction on the multiplicative scale (P = 0.37 across categories of BMI).
Figure 1.
The joint effect of BMI after pregnancy and history of HDP on the risk of type 2 diabetes in women without a history of gestational diabetes mellitus by BMI, presented as HRs partitioned into the relative excess risks due to BMI, HDP, and their interaction (RERI). For each category of BMI, we show the relative excess risk due to each risk factor (BMI or HDP) and their additive interaction (RERI); the latter is supported if RERI >0. For example, in women with BMI ≥35.0 kg/m2, RERI is calculated as follows: RERIBMI ≥35.0 = HRBMI ≥35.0, HDP − HRBMI ≥18.5–24.9, HDP − HRBMI ≥35.0, No HDP + 1. All HRs are adjusted for age, race/ethnicity, parity, diet, physical activity, BMI at age 18 years, menopausal status, alcohol, smoking, and parental history of diabetes.
In Table 3 we present additional additive interaction statistics, which support our main results. In women in the overweight category, the proportion of the risk of type 2 diabetes attributable to the interaction between BMI and HDP history was 0.12 (95% CI −0.22, 0.46). In women in the obesity class I category, this proportion was 0.36 (95% 0.13, 0.59), and in women in the obesity class II-III category, the corresponding proportion was 0.24 (0.05, 0.42). This means that 36% of the risk of type 2 diabetes in women with both history of HDP and postpregnancy obesity class I is potentially attributable to their interaction, or joint effect. The interaction analysis in which we used BMI as a continuous variable supported the main results. With a BMI of 25 kg/m2 and no history of HDP as the reference, there was a nominally significant additive interaction (RERI 0.11 [95% CI 0.06, 0.15]) for the risk of type 2 diabetes with 1 unit higher BMI.
Table 3.
Statistics for additive interaction between postpregnancy BMI and history of HDP in multivariable-adjusted models of incident type 2 diabetes in parous women without history of gestational diabetes mellitus
| Additive interaction statistic | BMI category (kg/m2) |
|||
|---|---|---|---|---|
| Normal weight (18.5–24.9) | Overweight (25.0–29.9) | Obesity class I (30.0–34.9) | Obesity class II–III (≥35.0) | |
| RERI (95% CI), null = 0 | Reference* | 0.98 (−1.92, 3.87) | 10.69 (3.67, 17.70) | 14.02 (2.83, 25.22) |
| Attributable proportion (95% CI), null = 0† | Reference* | 0.12 (−0.22, 0.46) | 0.36 (0.13, 0.59) | 0.24 (0.05, 0.42) |
| Synergy index (95% CI), null = 1 | Reference* | 1.16 (0.72, 1.60) | 1.59 (1.34, 1.83) | 1.31 (1.12, 1.51) |
*The reference group is women with BMI 18.5–24.9 kg/m2 and no history of HDP.
†The proportion of risk that is attributable to the additive interaction between postpregnancy BMI and history of HDP.
Conclusions
In this study, we confirmed that high BMI is an important risk factor for incident type 2 diabetes in parous middle-aged women. Furthermore, our results suggest that the risk of type 2 diabetes was above and beyond that expected for women with both high BMI and a history of HDP. To the best of our knowledge, the American Diabetes Association has no clinical guidelines on how to best prevent and screen for diabetes following pregnancy among women with a history of HDP. As the guidelines from the American College of Obstetricians and Gynecologists (5) on screening women with a history of HDP for metabolic disease postpregnancy currently are agnostic on how to best identify those with high risk of type 2 diabetes, we believe our results are important and immediately relevant. Moreover, this study further supports that history of HDP is a marker of metabolic susceptibility (6,19), which may synergistically interact (7) with BMI in the years after pregnancy to further increase the risk of type 2 diabetes.
Information on history of pregnancy complications might have different clinical utility depending on the preventive setting and goals. In a setting in which women have comprehensive care throughout their reproductive years, it is important to acknowledge that women with pregnancy complications have a higher risk of type 2 diabetes and cardiovascular disease later in life and to potentially offer more frequent type 2 diabetes screening depending on BMI status. Another option is to use pregnancy complications to risk stratify parous women who are overweight or obese to identify candidates for a clinical intervention if resources cannot target all women.
Although this analysis suggests that women with a history of HDP have additional benefits of maintaining a healthy weight, it does not provide evidence that this can be translated to a clinically relevant risk reduction. A more adverse cardiometabolic phenotype in women with a history of HDP not only might increase the risk of type 2 diabetes at a certain BMI but also may reduce the effectiveness of lifestyle interventions to reduce BMI. However, when we previously studied the impact of diet and physical activity on the risk of chronic hypertension by HDP history, we did not find any evidence that women with HDP history benefitted less than women without HDP history (6). In this study of type 2 diabetes, we focused exclusively on BMI and did not study other lifestyle factors (e.g., diet or physical activity), as we were not powered to consider the interaction of these exposures.
As we included women during the age period 45–54 years, our results are directly applicable to women who are likely to have already completed childbearing. However, as preventing weight gain is easier than losing weight, the appropriate time period for controlling BMI and preventing overweight and obesity for women with a history of HDP is likely soon after the HDP pregnancy. Optimizing BMI between pregnancies also appears to be important to prevent obstetric complications (20).
It is recommended that women with a history of gestational diabetes mellitus be regularly screened for type 2 diabetes postpregnancy (4). Given that gestational diabetes mellitus is strongly associated with type 2 diabetes, and gestational diabetes mellitus increases the risk of HDP, we excluded women with a history of gestational diabetes mellitus from our analysis. In doing so, we mimic the clinical situation in which decisions regarding additional type 2 diabetes screening would need to be made for women with HDP but without gestational diabetes mellitus.
There is mounting evidence that there is a strong association between pregnancy complications and later risk of cardiometabolic disease (21). Still, there appears to be an overall lack of awareness among internists of this association (22,23). As health care visits constitute opportunities for prevention, routinely collected data on pregnancy complications could potentially be used to better predict later disease development. In this context, it is notable that a recent U.S.-based study reported current weight to be a potentially important barrier for health care utilization in women (24).
The richly characterized data set with confirmed end points allows for adjustment of important confounders, and the large sample size and extensive follow-up time allow us to present an interaction analysis requiring high statistical power. In the analysis, we also allow for the updating of participants’ BMI every other year throughout follow-up. However, our results hinge on methodological assumptions that are important but not easily verifiable, e.g., no substantial residual confounding or bias. For the purpose of this study, we believe these assumptions to reasonably hold true, but it is important that the observational data that we present are followed by appropriately powered trials. For example, it is unlikely that all participants were screened for gestational diabetes mellitus with an oral glucose tolerance test during pregnancy, and though we do not think that this is a major methodological concern, conducting a structured and prospective investigation from the time of pregnancy could largely exclude this possibility altogether. For correct interpretation of our results, it is important to appreciate the different implications of additive and multiplicative interaction. Here, we focus on the additive interaction owing to its relevance for public health interpretation (25) and potential indication of mechanistic synergism within a sufficient cause framework (7). The generalizability of our results to women of other ethnicities may be limited, as our study sample includes predominantly white women. However, we do not believe that there are any strong reasons that the presented results would be qualitatively different in other racial/ethnic groups.
Future studies should focus on clinical interventions to aid in weight reduction or reducing long-term weight gain in women with a history of HDP. For facilitation of clinical implementation, it is also important to evaluate the cost-effectiveness of such efforts. The strong association between reproductive outcomes and later chronic health also affirms reproductive care as an integral part of women’s health care, not easily separated from other medical services. Additionally, to better inform policy and prevent cardiometabolic disease in women it is important to better understand how risk factors on the individual level, such as those focused on in this study, interact with factors and barriers on higher levels, such as family structure, neighborhood, and socioeconomic status.
In summary, this study suggests that maintaining a healthy weight is especially important for prevention of type 2 diabetes in women with a history of HDP. Consequently, interventions targeting weight might be of additional importance in parous women with such pregnancy complication history.
Supplementary Material
Article Information
Funding. S.T. is supported by an international postdoctoral grant from Vetenskapsrådet (the Swedish Research Council). Additional project funding was provided by Bundy Academy (Lund, Sweden), Folksam Research Foundation, the Swedish Heart and Lung Association, Anna Lisa and Sven Erik Lundgren’s Foundation, and Hulda and E Conrad Mossfelt’s Foundation. J.J.S. and L.J.T. are supported by National Heart, Lung, and Blood Institute grant T32HL098048 and L.J.T. also by grant F31HL131222. The Nurses’ Health Study II cohort is supported by a National Institutes of Health infrastructure grant (UM1 CA176726).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. S.T. performed the statistical analysis. S.T. with input from J.J.S., L.J.T., F.B.H., P.W.F., and J.W.R.-E. drafted the manuscript. S.T., F.B.H., P.W.F., and J.W.R.-E. acquired funding for the study or data collection. S.T. and J.W.R.-E. with input from J.J.S., L.J.T., and P.W.F. planned the research. S.T., J.J.S., L.J.T., F.B.H., P.W.F., and J.W.R.-E. approved the final draft. S.T. and J.W.R.-E. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the Society for Epidemiologic Research Annual Meeting, Baltimore, MD, 19–22 June 2018.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-1532/-/DC1.
References
- 1.Feig DS, Shah BR, Lipscombe LL, et al. Preeclampsia as a risk factor for diabetes: a population-based cohort study. PLoS Med 2013;10:e1001425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension 2009;53:944–951 [DOI] [PubMed] [Google Scholar]
- 3.Catov JM, Bairey-Merz N, Rich-Edwards J. Cardiovascular health during pregnancy: future health implications for mothers. Curr Epidemiol Rep 2017;4:232–238 [Google Scholar]
- 4.American Diabetes Association Management of diabetes in pregnancy. Sec. 12. In Standards of Medical Care in Diabetes—2015. Diabetes Care 2015;38(Suppl.):S77–S79 [DOI] [PubMed] [Google Scholar]
- 5.American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy . Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122–1131 [DOI] [PubMed] [Google Scholar]
- 6.Timpka S, Stuart JJ, Tanz LJ, Rimm EB, Franks PW, Rich-Edwards JW. Lifestyle in progression from hypertensive disorders of pregnancy to chronic hypertension in Nurses’ Health Study II: observational cohort study. BMJ 2017;358:j3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.VanderWeele TJ, Knol MJ. A tutorial on interaction. Epidemiol Methods 2014;3:33–72 [Google Scholar]
- 8.National Diabetes Data Group Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–1057 [DOI] [PubMed] [Google Scholar]
- 9.Stuart JJ, Bairey Merz CN, Berga SL, et al. Maternal recall of hypertensive disorders in pregnancy: a systematic review. J Womens Health (Larchmt) 2013;22:37–47 [DOI] [PubMed] [Google Scholar]
- 10.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990;1:466–473 [DOI] [PubMed] [Google Scholar]
- 11.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord 1995;19:570–572 [PubMed] [Google Scholar]
- 12.World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation. Geneva, Switzerland, World Health Org., 2000 (Tech. Rep. Ser., no. 894) [PubMed] [Google Scholar]
- 13.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 1994;23:991–999 [DOI] [PubMed] [Google Scholar]
- 14.Yuan C, Spiegelman D, Rimm EB, et al. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol 2017;185:570–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tobias DK, Hu FB, Chavarro J, Rosner B, Mozaffarian D, Zhang C. Healthful dietary patterns and type 2 diabetes mellitus risk among women with a history of gestational diabetes mellitus. Arch Intern Med 2012;172:1566–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jannasch F, Kröger J, Schulze MB. Dietary patterns and type 2 diabetes: a systematic literature review and meta-analysis of prospective studies. J Nutr 2017;147:1174–1182 [DOI] [PubMed] [Google Scholar]
- 17.Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol 2012;41:514–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol 2007;17:227–236 [DOI] [PubMed] [Google Scholar]
- 19.Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ 2002;325:157–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cnattingius S, Villamor E. Weight change between successive pregnancies and risks of stillbirth and infant mortality: a nationwide cohort study. Lancet 2016;387:558–565 [DOI] [PubMed] [Google Scholar]
- 21.Rich-Edwards JW, Fraser A, Lawlor DA, Catov JM. Pregnancy characteristics and women’s future cardiovascular health: an underused opportunity to improve women’s health? Epidemiol Rev 2014;36:57–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young B, Hacker MR, Rana S. Physicians’ knowledge of future vascular disease in women with preeclampsia. Hypertens Pregnancy 2012;31:50–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkins-Haug L, Celi A, Thomas A, Frolkis J, Seely EW. Recognition by women’s health care providers of long-term cardiovascular disease risk after preeclampsia. Obstet Gynecol 2015;125:1287–1292 [DOI] [PubMed] [Google Scholar]
- 24.Bairey Merz CN, Andersen H, Sprague E, et al. Knowledge, attitudes, and beliefs regarding cardiovascular disease in women: the Women’s Heart Alliance. J Am Coll Cardiol 2017;70:123–132 [DOI] [PubMed] [Google Scholar]
- 25.Rothman KJ, Greenland S, Walker AM. Concepts of interaction. Am J Epidemiol 1980;112:467–470 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.