Abstract
OBJECTIVE
Obese youth without diabetes with monophasic oral glucose tolerance test (OGTT) glucose response curves have lower insulin sensitivity and impaired β-cell function compared with those with biphasic curves. The OGTT glucose response curve has not been studied in youth-onset type 2 diabetes. Here we test the hypothesis that the OGTT glucose response curve at randomization in youth in the TODAY (Treatment Options for Type 2 Diabetes in Adolescents and Youth) study forecasts heightened glycemic failure rates and accelerated decline in β-cell function.
RESEARCH DESIGN AND METHODS
OGTTs (n = 662) performed at randomization were categorized as monophasic, biphasic, or incessant increase. Demographics, insulin sensitivity (1/fasting insulin), C-peptide index (△C30/△G30), and β-cell function relative to insulin sensitivity (oral disposition index [oDI]) were compared among the three groups.
RESULTS
At randomization, 21.7% had incessant increase, 68.6% monophasic, and 9.7% biphasic glucose response curves. The incessant increase group had similar insulin sensitivity but significantly lower C-peptide index and lower oDI, despite similar diabetes duration, compared with the other two groups. Glycemic failure rates were higher in the incessant increase group (58.3%) versus the monophasic group (42.3%) versus the biphasic group (39.1%) (P < 0.0001). The 6-month decline in C-peptide index (32.8% vs. 18.1% vs. 13.2%) and oDI (32.2% vs. 11.6% vs. 9.1%) was greatest in incessant increase versus monophasic and biphasic with no difference in insulin sensitivity.
CONCLUSIONS
In the TODAY study cohort, an incessant increase in the OGTT glucose response curve at randomization reflects reduced β-cell function and foretells increased glycemic failure rates with accelerated deterioration in β-cell function independent of diabetes duration and treatment assignment compared with monophasic and biphasic curves. The shape of the OGTT glucose response curve could be a metabolic biomarker prognosticating the response to therapy in youth with type 2 diabetes.
Introduction
Studies in adults and youth show that the shape of the glucose response curve during an oral glucose tolerance test (OGTT) identifies physiologically distinct groups of individuals with abnormalities in insulin secretion and insulin sensitivity. Subjects with a monophasic OGTT glucose response curve (i.e., a gradual increase in glucose concentrations between 30 and 90 min until a peak is reached followed by a subsequent decrease in glucose of ≥4.5 mg/dL) (1,2) have lower insulin sensitivity and decreased β-cell function compared with subjects with a biphasic OGTT glucose response curve (i.e., a second rise of glucose concentration of ≥4.5 mg/dL after the first decline in glucose) (1,2). Moreover, adults with monophasic OGTT have an increased risk of future impaired fasting glucose (IFG) (3) and type 2 diabetes (4). A small percentage of individuals with an OGTT glucose response curve that does not fit either are designated as “unclassified.” This latter category is rare in pediatrics, ranging from 1% to 12% depending on the study (2,5,6). In adults, this category, in which plasma glucose concentration continues to increase after 60 min and remains elevated at 120 min, identifies individuals with impaired glucose tolerance (IGT) (7), deficient insulin secretion, and muscle insulin resistance (7).
Data from 287 obese adolescents without diabetes demonstrate that 56.8% have a monophasic OGTT glucose response curve, 39.7% have a biphasic glucose response curve, and 3.5% have a gradual continuous rise or an incessant increase. Those with a monophasic curve compared with the biphasic curve have significantly lower in vivo insulin sensitivity and impaired β-cell function relative to insulin sensitivity, measured by the hyperinsulinemic-euglycemic and the hyperglycemic clamps, respectively (8). Because there are no studies of the OGTT glucose response curve in youth with type 2 diabetes and only one in Japanese adults (9), the proportion of the different glucose curve patterns, how they relate to the pathophysiological alterations of type 2 diabetes, and how they might be associated with the response to treatment are unknown.
The purpose of the current analysis of the TODAY (Treatment Options for Type 2 Diabetes in Adolescents and Youth) study data was to test the hypothesis that the shape of the OGTT glucose response curve at randomization 1) reflects β-cell function, 2) predicts glycemic failure, and 3) portends deterioration in β-cell function in youth with type 2 diabetes. The specific objectives were as follows: 1) to examine insulin sensitivity and β-cell function relative to insulin sensitivity according to the OGTT glucose response curve at randomization; 2) to investigate the relationship of the OGTT glucose response curve at randomization to glycemic failure rates and the rate of decline in β-cell function over time; and 3) to assess the change in the OGTT glucose response curve patterns after 6 months of randomization in each of the three TODAY study treatment arms (metformin alone, metformin plus rosiglitazone, and metformin plus intensive lifestyle) to determine whether any one treatment is better than the other in lessening the pathophysiological derangements that are characteristic of the OGTT glucose response curve pattern.
Research Design and Methods
A detailed description of the TODAY protocol (clinical trial reg. no. NCT00081328, www.ClinicalTrials.gov) and the primary outcome results have been published (10–12). Briefly, eligible participants in the TODAY study were 10 to <18 years old, had had diabetes (defined by American Diabetes Association criteria) for <2 years (median duration 8 months), were overweight or obese (BMI ≥85th and ≥95th percentile, respectively), and were islet cell antibody negative and C-peptide positive. After screening, eligible participants entered a 2- to 6-month run-in period to wean from nonstudy diabetes medications including insulin, to tolerate metformin up to a dose of 1,000 mg twice daily but not <1,000 mg/day, to attain an HbA1c level of <8.0% (64 mmol/mol) for at least 2 months while receiving metformin alone, and to demonstrate adherence to study medications and visit attendance (11,12). After the run-in phase, 699 youths were randomly assigned to receive metformin monotherapy, metformin plus rosiglitazone, or metformin plus lifestyle intervention (10,12). Demographic, anthropometric, and metabolic data were collected at randomization (11). HbA1c level was measured at screening, randomization, and at every study visit thereafter. Glycemic failure was defined as a sustained elevation in HbA1c of ≥8% (≥64 mmol/mol) over a 6-month period or the inability to wean from temporary insulin therapy within 3 months of acute metabolic decompensation (10). OGTTs were performed after a 10- to 14-h overnight fast at randomization, 6 months, 24 months, and annually thereafter. OGTT blood samples were obtained at 0, 30, 60, 90, and 120 min for measurement of glucose, insulin, and C-peptide levels (13).
Assays and Calculations
All assays, including for HbA1c (high-performance liquid chromatography), C-peptide (two-site immunoenzymatic assay), and insulin (double-antibody radioimmunoassay) were performed at the TODAY study central laboratory (Northwest Lipid Research Laboratory, University of Washington, Seattle, WA) as previously described (12,13).
Surrogate markers of insulin sensitivity, β-cell function, and oral disposition index (oDI), a measure of β-cell function relative to insulin sensitivity, were calculated (13–16). Briefly, insulin sensitivity was calculated as 1/fasting insulin (1/IF) (13–15), C-peptide index (△C30/△G30) as the ratio of the incremental C-peptide and glucose responses over the first 30 min of the OGTT (13,14), and oDI as the product of insulin sensitivity multiplied by the C-peptide index (1/IF × △C30/△G30) (13,14,16). As previously reported (14), we used the C-peptide index as a measure of insulin secretion (△C30/△G30) because some participants had received insulin prior to screening/enrollment in the TODAY study, which could potentially result in circulating insulin antibodies interfering with the insulin assay. In addition, differences in insulin clearance in different racial/ethnic groups could confound the circulating insulin data (14).
Classification of OGTT Glucose Response Curve
The shape of the OGTT glucose response curve of each TODAY study participant was classified at baseline (randomization) into three categories (1,2,8). A monophasic curve was defined as a gradual increase in blood glucose between 30 and 90 min until a peak was reached followed by a subsequent decline of ≥4.5 mg/dL. A biphasic curve was defined as a rise of blood glucose to a peak followed by a fall (as in the monophasic curve), but then followed by a second rise of ≥4.5 mg/dL. An incessant increase curve was defined as a continuing gradual increase in blood glucose during the 2 h of the OGTT without a fall of ≥4.5 mg/dL. These definitions were also applied to classify month 6 OGTT curves.
Statistical Methods
Subjects included in this article (n = 662) are those who had undergone an OGTT at baseline. Their baseline demographic and metabolic characteristics were similar to those described for the entire randomized TODAY study cohort (n = 699). Outliers, suspected nonfasting values, and values for C-peptide index of ≤0 were set to missing for analysis purposes. Of the 2,383 C-peptide index values obtained over the 48 months of follow-up, 61 (2.6%) were ≤0. Although mathematically possible, such values were judged biologically implausible and were treated as missing values, similar to our approach in prior TODAY study publications (13,14). These improbable responses were observed in 16 subjects (average of one per subject), of whom 6 had a response ≤0 at baseline necessitating their exclusion from the longitudinal analyses. Decline of subjects over time at each time point because of participant dropout, nonfasting state, or nonattendance at a scheduled study visit was as follows: 4% at month 6; 13% at month 24; 24% at month 36; and 29% at month 48. There were no differences in baseline OGTT glucose response curves between those with available data versus those without (i.e., with missing data) at each of the time points (all P values >0.05).
Data are presented as the mean and SD or percentage. Variables with a skewed distribution were log transformed as appropriate. At baseline, quantitative and categorical characteristics were compared between OGTT glucose response curve groups using F tests and χ2 tests, respectively. If the overall test was significant, pairwise comparisons were performed. Baseline differences in the metabolic parameters were assessed before and after adjustment for baseline age, sex, race-ethnicity, and waist circumference.
Comparison of glycemic failure rates by OGTT glucose response curve pattern were analyzed using survival curve methodology with log-rank tests. At baseline, all TODAY study youth were free of glycemic failure and not receiving insulin per study protocol. Survival curves were run separately for each group and plotted by baseline OGTT glucose response curve as well as by month 6 OGTT glucose response curves. Figures based on month 6 OGTT glucose response curves excluded participants who failed to maintain glycemic control in the first 6 months of the study (i.e., they had already reached the study end point; n = 136) or who were lost to follow-up early and did not have an OGTT at that visit (n = 2). Log-rank tests were used to compare OGTT glucose response curve groups before and after adjustment for treatment assignment.
Longitudinal data for insulin sensitivity, insulin secretion, and β-cell function, out to and including month 48, after which the group numbers were too small for meaningful statistical analysis, were analyzed using generalized linear mixed models to estimate the mean levels of each of the parameters over repeated time points within glucose curve groups (SAS PROC MIXED). Models examining differences in OGTT glucose response curve groups in insulin sensitivity, insulin secretion, and β-cell function over time were adjusted for time (TODAY study visit month 0–48), treatment group, baseline age, baseline BMI (and separately replacing baseline BMI for change from baseline), and diabetes duration, and included a term of the interaction of time with group. The models assumed an unstructured covariance structure as it resulted in a better model fit (smallest Akaike information criterion value). Because of the skewness of the data, longitudinal analyses were performed on log-transformed values. Data shown in the figures are model-adjusted geometric means ± SE asymmetric limits (obtained as the exp [mean ± SE] of the log values). Longitudinal analyses performed on the log values allowed model-derived estimates to be presented in terms of the percentage of change over time. The mean percentage of change from baseline to 6 months was computed, and the average rate of change from 6 to 48 months was estimated from linear contrast of the model-estimated means over time (13). Analyses were performed using SAS for Windows (version 9.4; SAS Institute Inc., Cary, NC). All analyses were considered exploratory, with statistical significance defined as P values of <0.05 and no adjustment for multiple testing.
Results
Demographic and Metabolic Characteristics
At randomization, 21.7% of the TODAY study cohort had OGTTs categorized as incessant increase, 68.6% as monophasic, and 9.7% as biphasic (Table 1). Sex, race/ethnicity, duration of diagnosed diabetes, BMI, and BMI z scores were similar among the three OGTT glucose response curve groups. Age, Tanner stage, and waist circumference were significantly different among the three groups (Table 1). HbA1c, fasting glucose, and 2-h glucose levels were significantly different and higher in the incessant increase group, but fasting insulin and insulin sensitivity were not different among the three groups (Table 1). C-peptide index was lower in the incessant increase group, whereas oDI was higher in the biphasic group relative to the other groups (Table 1). After adjusting for baseline age, sex, race/ethnicity, and waist circumference, C-peptide index and oDI were significantly different in all three group pairwise comparisons (Table 1). When baseline OGTT glucose response curve categories were analyzed in each treatment group, metformin monotherapy, therapy with metformin plus rosiglitazone, and therapy with metformin plus intensive lifestyle intervention, there was no difference in curve type according to treatment group (Supplementary Table 1).
Table 1.
Demographic and metabolic characteristics of TODAY study participants (n = 662) by OGTT glucose response curve at baseline
| Incessant increase (n = 144; 21.7%) | Monophasic (n = 454; 68.6%) | Biphasic (n = 64; 9.7%) | Unadjusted P value* | Adjusted P value† | |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age at randomization (years) | 13.7 ± 2.1 | 14.1 ± 2.0 | 13.5 ± 2.2 | 0.0069ac | |
| Female (%) | 71.5 | 63.9 | 54.7 | NS | |
| Race-ethnicity (%) | |||||
| Non-Hispanic black | 37.4 | 32.8 | 39.7 | NS | |
| Hispanic | 40.3 | 44.8 | 41.4 | ||
| Non-Hispanic white | 22.3 | 22.3 | 19.0 | ||
| Pubertal stage (%) | |||||
| Stages 1–3 | 16.0 | 9.0 | 17.2 | 0.0112 | |
| Stages 4–5 | 84.0 | 91.0 | 82.8 | ||
| Time since diagnosis (months) | 8.0 ± 5.7 | 7.5 ± 5.6 | 9.4 ± 6.9 | NS | |
| Weight (kg) | 88.9 ± 23.9 | 97.6 ± 25.6 | 93.7 ± 23.7 | 0.0012a | |
| BMI (kg/m2) | 34.0 ± 8.2 | 35.2 ± 7.6 | 33.7 ± 7.2 | NS | |
| BMI z score | 2.1 ± 0.5 | 2.2 ± 0.5 | 2.2 ± 0.4 | NS | |
| Waist circumference (cm) | 105.3 ± 16.1 | 109.8 ± 16.9 | 106.7 ± 15.6 | 0.0125a | |
| Metabolic characteristics | |||||
| HbA1c (%) | 6.3 ± 0.8 | 6.0 ± 0.7 | 5.8 ± 0.7 | <0.0001abc | <0.0001ab |
| Fasting glucose (mg/dL) | 118.8 ± 30.4 | 109.8 ± 23.8 | 105.8 ± 22.1 | 0.0001ab | <0.0001ab |
| 2-h glucose (mg/dL) | 252.8 ± 64.4 | 192.1 ± 59.1 | 179.2 ± 55.4 | <0.0001ab | <0.0001ab |
| Fasting insulin (µU/mL)‡ | 30.4 ± 24.0 | 30.9 ± 20.5 | 31.0 ± 18.6 | NS | NS |
| Fasting C-peptide (ng/mL)‡ | 3.6 ± 1.5 | 3.9 ± 1.6 | 3.7 ± 1.4 | 0.0179a | NS |
| Insulin sensitivity [1/IF] (mL/µU)‡ | 0.053 ± 0.041 | 0.047 ± 0.037 | 0.048 ± 0.034 | NS | NS |
| C-peptide index [∆C30/∆G30] (ng/mL per mg/dL)‡ | 0.065 ± 0.073 | 0.081 ± 0.130 | 0.110 ± 0.076 | <0.0001abc | <0.0001abc |
| oDI [1/IF × ∆C30/∆G30]‡ | 0.003 ± 0.003 | 0.003 ± 0.007 | 0.005 ± 0.004 | 0.0007bc | 0.0003abc |
Continuous data are presented as the mean ± SD, and categorical data as indicated. NS, P > 0.05.
*Unadjusted P values were calculated from F tests for continuous variables and from χ2 tests for categorical variables.
†Adjusted P values were calculated from models adjusted for baseline age, sex, race/ethnicity, and waist circumference. Pairwise comparisons were performed when an overall difference by OGTT glucose response curve was found; significant comparisons (P < 0.05) between curve groups are indicated as follows: aincessant increase vs. monophasic, bincessant increase vs. biphasic, and cmonophasic vs. biphasic. Pairwise comparisons for insulin sensitivity, C-peptide index, and oDI were performed after adjusting for baseline age, sex, race/ethnicity, and waist circumference.
‡Variables were log transformed prior to testing.
Glycemic Failure Rates in the Three OGTT Glucose Response Curve Groups
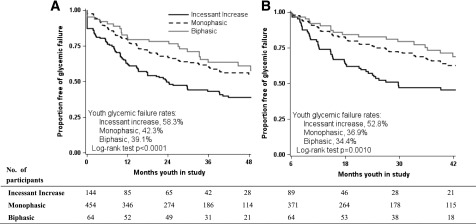
Glycemic failure rates according to baseline OGTT glucose response curves were significantly (P < 0.0001) different among the three groups, highest in the incessant increase group (58.3%), intermediate in the monophasic group (42.3%), and lowest in the biphasic group (39.1%) (Fig. 1A). These differences remained significant after adjustment for treatment group (adjusted log-rank P = 0.0036). Similar results were observed when glycemic failure rates were based on the shape of the month 6 OGTT glucose response curve: 52.8% in the incessant increase group, 36.9% in the monophasic group, and 34.4% in the biphasic group (P = 0.001) (Fig. 1B). Previously, we showed that HbA1c and oDI at randomization were determinants of glycemic failure in the TODAY study (13). However, adjusting glycemic failure rates for baseline HbA1c (P = 0.0416), baseline oDI (P = 0.0008), baseline fasting glucose (P = 0.0020), or baseline BMI (P = 0.0001) did not change the significant differences in failure rates among the three glucose curve groups. Because there was a significant difference in BMI change from baseline among the three groups over time (P = 0.0494), we replaced baseline BMI with BMI change from baseline in the model. This did not change the significant differences in failure rates (P < 0.0001).
Figure 1.
Survival curves and log-rank test results for freedom from glycemic failure in the TODAY study cohort by OGTT glucose response curve at baseline (A) and at month 6 (B). Data are shown for up to 48 months of follow-up (accounting for 95.7% of glycemic failure). Panel B excludes participants who failed to maintain glycemic control in the first 6 months of the study (n = 136 or 20.5%) or were lost to follow-up early and did not undergo an OGTT collected at month 6 (n = 2), resulting in a starting population of 524 youths free of glycemic failure at month 6.
When glycemic failure rates were analyzed within each treatment arm (Supplementary Fig. 1A–C), differences among OGTT glucose response curve groups were found in the metformin plus rosiglitazone arm (log-rank P = 0.0082), with glycemic failure rates highest in the incessant increase group (52.5%) and lowest in the biphasic group (30.4%) (Supplementary Fig. 1B). This was also the case in the metformin plus intensive lifestyle arm (P = 0.0101), with glycemic failure rates highest in the incessant increase group (61.2%) and lowest in the biphasic group (32.0%) (Supplementary Fig. 1C), but not in the metformin-alone arm (log-rank P = NS) (Supplementary Fig. 1A).
Temporal Patterns of Insulin Sensitivity, C-Peptide Index, and oDI in the Three OGTT Glucose Response Curve Groups
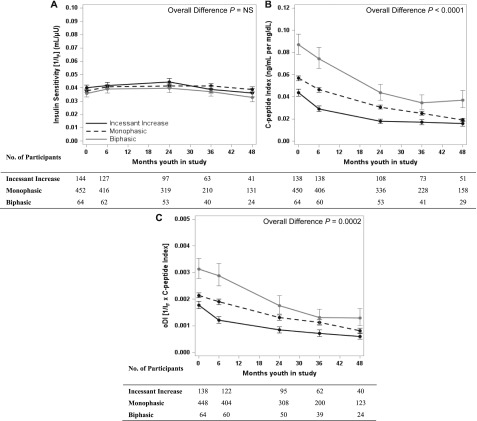
For the longitudinal analysis of insulin sensitivity, C-peptide index, and oDI, only patients with a baseline and follow-up evaluation of each outcome measure contributed data to the analyses in Fig. 2. These longitudinal models present data over 48 months of follow-up by baseline OGTT glucose response curve adjusted for treatment, baseline age, BMI, and diabetes duration. Although the temporal patterns of insulin sensitivity were similar among the three curve groups and remained stable over the 48 months (Fig. 2A), there was progressive decline in C-peptide index and oDI, which was significantly different among the three groups (Fig. 2B and C). Replacing baseline BMI in the model for BMI change from baseline did not affect the results.
Figure 2.
Temporal patterns of insulin sensitivity (A), C-peptide index (B), and C-peptide oDI (C) in the three baseline OGTT glucose response curve groups. Data are reported as model-adjusted geometric mean ± SE asymmetric limits (obtained as exp [mean ± SE of log values]) over 48 months of follow-up in the TODAY study, analyzed using log-transformed values. P values refer to the overall effect of baseline OGTT glucose response curve in the longitudinal models, adjusted for treatment group, baseline age and BMI, and diabetes duration.
Table 2 shows the short-term change, as the mean percentage change from baseline to 6 months, and the longer-term change, as the percentage per year from 6 to 48 months, for insulin sensitivity, C-peptide index, and oDI in each OGTT glucose response curve group. There was no difference among the three glucose curve groups with respect to the short-term or longer-term change in insulin sensitivity. However, in the first 6 months the incessant increase group had a significantly greater decline in C-peptide index and oDI compared with the other two groups, with no difference thereafter (6–48 months).
Table 2.
Changes in measures of insulin sensitivity, C-peptide index, and C-peptide oDI from randomization to 6 months and rates of change among means from 6 months to 4 years based on a longitudinal model adjusted for baseline factors
| Factor | Incessant increase | Monophasic | Biphasic | Incessant increase vs. monophasic P value | Incessant increase vs. biphasic P value | Monophasic vs. biphasic P value |
|---|---|---|---|---|---|---|
| Insulin sensitivity [1/IF] (mL/µU) | ||||||
| N | 144 | 452 | 64 | |||
| Mean % change from 0 to 6 months | 2.7 [−2.5, 7.9] | 6.7 [3.7, 9.7] | 6.4 [−1.3, 14.2] | NS | NS | NS |
| Rate of change from 6 months to 4 years (% per year) | −14.8 [−20.5, −9.1] | −10.8 [−14.1, −7.5] | −15.6 [−23.1, −8.2] | NS | NS | NS |
| C-peptide Index [∆C30/∆G30] (ng/mL per mg/dL) | ||||||
| N | 138 | 450 | 64 | |||
| Mean % change from 0 to 6 months | −32.8 [−37.5, −28.1] | −18.1 [−21.2, −15.0] | −13.2 [−21.8, −4.7] | 0.0220 | 0.0151 | NS |
| Rate of change from 6 months to 4 years (% per year) | −35.5 [−42.2, −28.8] | −46.1 [−49.2, −42.9] | −40.5 [−48.9, −32.2] | NS | NS | NS |
| oDI [1/IF × ∆C30/∆G30] | ||||||
| N | 138 | 448 | 64 | |||
| Mean % change from 0 to 6 months | −32.2 [−38.3, −26.1] | −11.6 [−15.9, −7.3] | −9.1 [−20.6, 2.5] | 0.0135 | 0.0148 | NS |
| Rate of change from 6 months to 4 years (% per year) | −43.1 [−50.4, −35.7] | −47.8 [−51.6, −44.0] | −47.9 [−56.7, −39.2] | NS | NS | NS |
Data are reported as the percentage change from baseline and mean rates of change (percent per year) [95% CI]. P values from models based on the log-transformed value adjusted for treatment group, baseline age and BMI, and diabetes duration. NS, P > 0.05.
OGTT Glucose Response Curve Patterns After 6 Months of Randomization
Six months after randomization, the percentage of participants in each of the three curve categories was similar to baseline (20.3% incessant increase, 68.6% monophasic, and 11.1% biphasic) (Supplementary Table 2). There was no significant shift from one curve pattern to another with all three treatment groups combined or in each treatment group separately (Supplementary Table 3). Participants in the metformin plus rosiglitazone arm, who had lower glycemic failure rates than the metformin alone or metformin plus intensive lifestyle arm (10), did not show higher rates of improvement in the glucose curve pattern from incessant increase to the other two patterns (50.0%) compared with the metformin alone (68.0%) or metformin plus intensive lifestyle arm (63.4%, P = NS) (Supplementary Table 3).
Conclusions
The present investigation of OGTT glucose response curves in the TODAY study cohort of youth with type 2 diabetes demonstrates the following: 1) that the shape of the OGTT glucose response curve at randomization reflects β-cell function, which is worse in the incessant increase group; 2) that glycemic failure rates were highest in youth with a baseline incessant increase OGTT glucose response curve compared with the monophasic and biphasic groups; 3) that in the first 6 months, β-cell function deteriorated fastest in youth with an incessant increase curve at randomization; and 4) that treatment assignment was not associated with changes, either favorable or unfavorable, in the OGTT glucose response curve.
Studies in individuals without diabetes show that the monophasic response curve is the dominant phenotype, with prevalence ranging from 57% to 84% in adults at high risk for diabetes (1,4,17–19) and from 35% to 69% in obese youth at risk for type 2 diabetes (2,5,6,8) or youth at risk for type 1 diabetes (20). In the TODAY study, the most frequent OGTT glucose response curve was monophasic at 68.6%, with a high prevalence of the incessant increase pattern at 21.7%, followed by biphasic at 9.7%. Part of the reason for the higher incessant increase category is that the majority of reported studies excluded curve patterns that did not fit the monophasic or biphasic categories. A limited study in the Japanese literature of elderly adults with diabetes and a fasting glucose concentration of <140 mg/dL reported monophasic prevalence at 66.7%, biphasic prevalence at 13.3%, and “upward” at 20.0%. In subjects with a fasting glucose concentration of >140 mg/dL, the prevalence of monophasic and “upward” was higher, and none were biphasic, implying that with deteriorating glycemia the prevalence of the most favorable curve pattern declines and the least favorable curve pattern escalates (9). These data are consistent with the TODAY study showing higher rates of incessant increase in youth with diabetes. An analysis of 287 obese youths without diabetes with normal glucose tolerance, IGT, and IFG revealed a lower frequency of incessant increase (3.5%) and a higher frequency of biphasic curves (39.7%) (8). However, youths with IGT compared with normal glucose tolerance had a higher prevalence of incessant increase (16% vs. 4%, respectively) (5). This is not surprising since incessant increase and monophasic curve patterns reflect more severe alterations in the pathophysiology of type 2 diabetes than the biphasic curve patterns (2,4,5,7,8,17,19). In adults, an incessant increase OGTT glucose response curve is characteristic of individuals with IGT, muscle insulin resistance, and impaired β-cell function (7). Longitudinal data in adults without diabetes demonstrate increased risk of future IFG in individuals with a monophasic curve at baseline, in those with stable monophasic morphology over time, and in those whose patterns change from biphasic to monophasic (3). Moreover, in adults with prediabetes and a monophasic glucose response, the conversion rate to type 2 diabetes over 7–8 years was nearly double the rate in those with biphasic response despite similar fasting and 2-h plasma glucose concentrations (4).
To our knowledge, this is the first investigation of the OGTT glucose response curve in youth with established type 2 diabetes. In this TODAY study cohort, the shape of the OGTT glucose response curve at baseline mirrored β-cell function. Those with an incessant increase curve had lower values for C-peptide index and lower oDI compared with the monophasic and biphasic groups. Because the prevalence of incessant increase in youths without diabetes is low, data are available primarily for monophasic and biphasic groups. Obese youths with a monophasic OGTT glucose response curve compared with the biphasic group had lower in vivo hepatic and peripheral insulin sensitivity, lack of compensatory increase in first- and second-phase insulin secretion, and impaired disposition index, all measured by the clamp method (8). Consistent with this study, the monophasic group in the TODAY study had worse β-cell function than the biphasic group, but in contrast there was no difference in insulin sensitivity among the three curve groups. This is most likely due to the severe insulin resistance in youths with type 2 diabetes (21) or to using an estimate of insulin sensitivity instead of the highly sensitive clamp method. Studies of Latino youths without diabetes (2), Caucasian adolescents (5), and obese girls (6), using OGTT-derived estimates of insulin sensitivity and insulin secretion, demonstrate that the monophasic glucose response curve harbors an amplified metabolic risk profile for type 2 diabetes compared with biphasic or more complex shapes. In youths at risk for type 1 diabetes, the monophasic curve group had lower OGTT C-peptide index values compared with the biphasic group and a higher cumulative incidence of type 1 diabetes (20).
Considering that the different OGTT glucose response curves indicate a differential risk for β-cell impairment, we postulated that youths with incessant increase in OGTT glucose response curve at randomization would have significantly higher glycemic failure rates with an accelerated decline in β-cell function. Indeed, this was the case. Almost 60% of youths with an incessant increase OGTT glucose response curve at randomization failed to maintain glycemic control compared with 42.3% in the monophasic group and 39.1% in the biphasic group. Moreover, although temporal patterns in insulin sensitivity among the three curve groups were not different and remained stable over the 48 months of the analysis, C-peptide index and β-cell function declined precipitously by ∼30% in the incessant increase group in the first 6 months compared with the other groups (Fig. 2 and Table 2). Thereafter, the rate of decline was not different among the groups. When the three curve groups were analyzed in each treatment arm separately, the failure rate among the three curve groups did not differ in the metformin monotherapy arm but did in the metformin plus rosiglitazone arm and the metformin plus lifestyle intervention arm, and it was worse in the incessant increase arm (Supplementary Fig. 1). One could speculate that when a weak insulin sensitizer such as metformin is used, the shape of the glucose response curve and its reflection of β-cell function may not provide any prognostic value for therapeutic response. On the other hand, when a more potent insulin sensitizer, such as rosiglitazone, is used, the combination of insulin sensitization together with a less impaired β-cell function, as reflected in the biphasic and/or monophasic curves, may lead to a better therapeutic outcome. A potential clinical advantage of the OGTT glucose response curve is that it provides additional information above and beyond other measures of β-cell function that predict prognosis, because the differences in glycemic failure rates among the three glucose curve groups remained significant after adjusting for baseline oDI, HbA1c, fasting glucose, and BMI, as well as BMI change from baseline.
Longitudinal data in nondiabetic adults demonstrated that the shape of the OGTT glucose response curve remained stable over a period of 3 years (3). Likewise, the shape of the OGTT glucose response curve in TODAY study youths remained stable between baseline and 6 months (Supplementary Table 2). Furthermore, there was no difference among the three treatment groups with respect to the shifts in glucose response curves either in a favorable or an unfavorable direction (Supplementary Table 3).
The strengths of the present investigation include the following: 1) a first-time examination of the OGTT glucose response curve in a large cohort of youths with established and well-characterized type 2 diabetes; 2) assessment of the relationship between the glucose response curve and alterations in insulin sensitivity and β-cell function; 3) evaluation of glycemic failure rates related to OGTT glucose response curves at randomization; and 4) examination of the rate of decline of β-cell function based on OGTT glucose response curve pattern at baseline. Potential limitations are that the OGTT glucose response curve at each time point was determined by a single OGTT, which may have limited reproducibility in youth (22). A recent study in adults found inadequate reproducibility of the OGTT glucose response curve (23), but such data do not exist in pediatrics. An additional weakness is that youths at screening in the TODAY study were receiving a variety of treatments that could have potentially modified their OGTT glucose response curve. Moreover, the standardized run-in period for metformin therapy of at least 2 months prior to randomization may have altered their OGTT glucose response curve. However, there was no relationship between HbA1c level at screening (2–6 months before randomization) and OGTT glucose response curve at randomization (data not shown). Last, we could only use surrogate estimates of insulin sensitivity and β-cell function because using the clamp method was not possible in such a large cohort.
In summary, the current study is the first to demonstrate that in youths with established type 2 diabetes, the incessant increase OGTT glucose response curve reflects reduced β-cell function at randomization and foretells increased glycemic failure rates with accelerated deterioration in β-cell function independent of diabetes duration and treatment assignment compared with monophasic and biphasic curves. It remains to be determined 1) whether in youths with type 2 diabetes, the OGTT glucose response curve could serve as a metabolic biomarker prognosticating response to therapy and 2) which of the different metabolic biomarkers, OGTT glucose response patterns or time to glucose peak, elegantly described very recently in adults without diabetes in the EGIR-RISC (the European Group for the Study of Insulin Resistance: Relationship between Insulin Sensitivity and Cardiovascular Disease Risk) study (24), could serve as a metabolic biomarker prognosticating response to therapy.
Supplementary Material
Article Information
Acknowledgments. The authors thank the American Indian partners associated with the clinical center located at the University of Oklahoma Health Sciences Center, including members of the Absentee Shawnee Tribe, Cherokee Nation, Chickasaw Nation, Choctaw Nation of Oklahoma, and Oklahoma City Area Indian Health Service for participation and guidance. Materials developed and used for the TODAY standard diabetes education program and the intensive lifestyle intervention program are available to the public at https://today.bsc.gwu.edu/.
The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the respective Tribes and the Indian Health Service.
Funding. The TODAY Study Group thanks the following companies for donations in support of the study: Becton, Dickinson and Company; Bristol-Myers Squibb; Eli Lilly and Company; GlaxoSmithKline; LifeScan, Inc.; Pfizer; and Sanofi. This work was completed with funding from National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health grants U01-DK-61212, U01-DK-61230, U01-DK-61239, U01-DK-61242, and U01-DK-61254; from National Center for Research Resources General Clinical Research Centers Program grants M01-RR-00036 (Washington University School of Medicine in St. Louis), M01-RR-00069 (University of Colorado Denver), M01-RR-00084 (Children’s Hospital of Pittsburgh), M01-RR-01066 (Massachusetts General Hospital), and M01-RR-14467 (University of Oklahoma Health Sciences Center); and from the National Center for Research Resources Clinical and Translational Science Awards grants UL1-RR-024134 (Children’s Hospital of Philadelphia), UL1-RR-024153 (Children’s Hospital of Pittsburgh), UL1-RR-024992 (Washington University in St. Louis), UL1-RR-025758 (Massachusetts General Hospital), and UL1-RR-025780 (University of Colorado Denver).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. S.A. planned the study scheme; developed the manuscript design, data analyses, and data interpretation; and wrote the manuscript. L.E.g. contributed to data management, statistical data analysis and interpretation, and the writing of the manuscript. J.Y.K. contributed to the study plan, manuscript design, data analyses and interpretation, and editing of the manuscript. F.B., C.C., H.M.I., L.E.L.K., L.L., J.B.T., and N.H.W. contributed to the study and manuscript design, data collection and interpretation, and editing of the manuscript. L.E.g. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 77th Scientific Sessions of the American Diabetes Association, San Diego, CA, 9–13 June 2017.
Footnotes
Clinical trial reg. no. NCT00081328, clinicaltrials.gov
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-1122/-/DC1.
A complete list of the TODAY Study Group is included in the online Supplementary Data.
Contributor Information
Collaborators: TODAY Study Group, S. McKay, M. Haymond, B. Anderson, C. Bush, S. Gunn, H. Holden, S.M. Jones, G. Jeha, S. McGirk, S. Thamotharan, L. Cuttler, E. Abrams, T. Casey, W. Dahms, C. Ievers-Landis, B. Kaminski, M. Koontz, S. MacLeish, P. McGuigan, S. Narasimhan, M. Geffner, V. Barraza, N. Chang, B. Conrad, D. Dreimane, S. Estrada, L. Fisher, E. Fleury-Milfort, S. Hernandez, B. Hollen, F. Kaufman, E. Law, V. Mansilla, D. Miller, C. Muñoz, R. Ortiz, A. Ward, K. Wexler, Y.K. Xu, P. Yasuda, L. Levitt Katz, R. Berkowitz, S. Boyd, B. Johnson, J. Kaplan, C. Keating, C. Lassiter, T. Lipman, G. McGinley, H. McKnight, B. Schwartzman, S. Willi, S. Arslanian, F. Bacha, S. Foster, B. Galvin, T. Hannon, A. Kriska, I. Libman, M. Marcus, K. Porter, T. Songer, E. Venditti, R. Goland, D. Gallagher, P. Kringas, N. Leibel, D. Ng, M. Ovalles, D. Seidman, L. Laffel, A. Goebel-Fabbri, M. Hall, L. Higgins, J. Keady, M. Malloy, K. Milaszewski, L. Rasbach, D.M. Nathan, A. Angelescu, L. Bissett, C. Ciccarelli, L. Delahanty, V. Goldman, O. Hardy, M. Larkin, L. Levitsky, R. McEachern, D. Norman, D. Nwosu, S. Park-Bennett, D. Richards, N. Sherry, B. Steiner, S. Tollefsen, S. Carnes, D. Dempsher, D. Flomo, T. Whelan, B. Wolff, R. Weinstock, D. Bowerman, S. Bristol, J. Bulger, J. Hartsig, R. Izquierdo, J. Kearns, R. Saletsky, P. Trief, P. Zeitler, N. Abramson, A. Bradhurst, N. Celona-Jacobs, J. Higgins, M. Kelsey, G. Klingensmith, K. Nadeau, T. Witten, K. Copeland, E. Boss, R. Brown, J. Chadwick, L. Chalmers, S. Chernausek, A. Hebensperger, C. Macha, R. Newgent, A. Nordyke, D. Olson, T. Poulsen, L. Pratt, J. Preske, J. Schanuel, S. Sternlof, J. Lynch, N. Amodei, R. Barajas, C. Cody, D. Hale, J. Hernandez, C. Ibarra, E. Morales, S. Rivera, G. Rupert, A. Wauters, N. White, A. Arbeláez, D. Flomo, J. Jones, T. Jones, M. Sadler, M. Tanner, A. Timpson, R. Welch, S. Caprio, M. Grey, C. Guandalini, S. Lavietes, P. Rose, A. Syme, W. Tamborlane, K. Hirst, S. Edelstein, P. Feit, N. Grover, C. Long, L. Pyle, B. Linder, S.M. Marcovina, J. Harting, J. Shepherd, B. Fan, L. Marquez, M. Sherman, J. Wang, M. Nichols, E. Mayer-Davis, Y. Liu, J. Lima, S. Gidding, J. Puccella, E. Ricketts, R. Danis, A. Domalpally, A. Goulding, S. Neill, P. Vargo, D. Wilfley, D. Aldrich-Rasche, K. Franklin, C. Massmann, D. O’Brien, J. Patterson, T. Tibbs, D. Van Buren, M. Palmert, R. Ratner, D. Dremaine, and J. Silverstein
References
- 1.Tschritter O, Fritsche A, Shirkavand F, Machicao F, Häring H, Stumvoll M. Assessing the shape of the glucose curve during an oral glucose tolerance test. Diabetes Care 2003;26:1026–1033 [DOI] [PubMed] [Google Scholar]
- 2.Kim JY, Coletta DK, Mandarino LJ, Shaibi GQ. Glucose response curve and type 2 diabetes risk in Latino adolescents. Diabetes Care 2012;35:1925–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manco M, Nolfe G, Pataky Z, et al. Shape of the OGTT glucose curve and risk of impaired glucose metabolism in the EGIR-RISC cohort. Metabolism 2017;70:42–50 [DOI] [PubMed] [Google Scholar]
- 4.Abdul-Ghani MA, Lyssenko V, Tuomi T, Defronzo RA, Groop L. The shape of plasma glucose concentration curve during OGTT predicts future risk of type 2 diabetes. Diabetes Metab Res Rev 2010;26:280–286 [DOI] [PubMed] [Google Scholar]
- 5.Nolfe G, Spreghini MR, Sforza RW, Morino G, Manco M. Beyond the morphology of the glucose curve following an oral glucose tolerance test in obese youth. Eur J Endocrinol 2012;166:107–114 [DOI] [PubMed] [Google Scholar]
- 6.Bervoets L, Mewis A, Massa G. The shape of the plasma glucose curve during an oral glucose tolerance test as an indicator of beta cell function and insulin sensitivity in end-pubertal obese girls. Horm Metab Res 2015;47:445–451 [DOI] [PubMed] [Google Scholar]
- 7.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006;55:1430–1435 [DOI] [PubMed] [Google Scholar]
- 8.Kim JY, Michaliszyn SF, Nasr A, et al. The shape of the glucose response curve during an oral glucose tolerance test heralds biomarkers of type 2 diabetes risk in obese youth. Diabetes Care 2016;39:1431–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchigami M, Nakano H, Oba K, Metori S. Oral glucose tolerance test using a continuous blood sampling technique for analysis of the blood glucose curve. Nihon Ronen Igakkai Zasshi 1994;31:518–524 [in Japanese] [DOI] [PubMed] [Google Scholar]
- 10.Zeitler P, Hirst K, Pyle L, et al.; TODAY Study Group . A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Copeland KC, Zeitler P, Geffner M, et al.; TODAY Study Group . Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab 2011;96:159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeitler P, Epstein L, Grey M, et al.; TODAY Study Group . Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes 2007;8:74–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arslanian S, Pyle L, Payan M, et al.; TODAY Study Group . Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care 2013;36:1749–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arslanian S, El ghormli L, Bacha F, et al.; TODAY Study Group . Adiponectin, insulin sensitivity, β-cell function, and racial/ethnic disparity in treatment failure rates in TODAY. Diabetes Care 2017;40:85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George L, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Surrogate estimates of insulin sensitivity in obese youth along the spectrum of glucose tolerance from normal to prediabetes to diabetes. J Clin Endocrinol Metab 2011;96:2136–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sjaarda LG, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Oral disposition index in obese youth from normal to prediabetes to diabetes: relationship to clamp disposition index. J Pediatr 2012;161:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanauchi M, Kimura K, Kanauchi K, Saito Y. Beta-cell function and insulin sensitivity contribute to the shape of plasma glucose curve during an oral glucose tolerance test in non-diabetic individuals. Int J Clin Pract 2005;59:427–432 [DOI] [PubMed] [Google Scholar]
- 18.Trujillo-Arriaga HM, Román-Ramos R. Fitting and evaluating the glucose curve during a quasi continuous sampled oral glucose tolerance test. Comput Biol Med 2008;38:185–195 [DOI] [PubMed] [Google Scholar]
- 19.Tura A, Morbiducci U, Sbrignadello S, Winhofer Y, Pacini G, Kautzky-Willer A. Shape of glucose, insulin, C-peptide curves during a 3-h oral glucose tolerance test: any relationship with the degree of glucose tolerance? Am J Physiol Regul Integr Comp Physiol 2011;300:R941–R948 [DOI] [PubMed] [Google Scholar]
- 20.Ismail HM, Xu P, Libman IM, et al.; Type 1 Diabetes TrialNet Study Group . The shape of the glucose concentration curve during an oral glucose tolerance test predicts risk for type 1 diabetes. Diabetologia 2018;61:84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gungor N, Bacha F, Saad R, Janosky J, Arslanian S. Youth type 2 diabetes: insulin resistance, beta-cell failure, or both? Diabetes Care 2005;28:638–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Libman IM, Barinas-Mitchell E, Bartucci A, Robertson R, Arslanian S. Reproducibility of the oral glucose tolerance test in overweight children. J Clin Endocrinol Metab 2008;93:4231–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kramer CK, Vuksan V, Choi H, Zinman B, Retnakaran R. Emerging parameters of the insulin and glucose response on the oral glucose tolerance test: reproducibility and implications for glucose homeostasis in individuals with and without diabetes. Diabetes Res Clin Pract 2014;105:88–95 [DOI] [PubMed] [Google Scholar]
- 24.Hulman A, Witte DR, Vistisen D, et al. Pathophysiological characteristics underlying different glucose response curves: a latent class trajectory analysis from the prospective EGIR-RISC study. Diabetes Care 2018;41:1740–1748 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.