Abstract
OBJECTIVE
Type 1 diabetes (T1DM) is associated with other autoimmune diseases (AIDs), which may have serious health consequences. The epidemiology of AIDs in T1DM is not well defined in adults with T1DM. In this cross-sectional cohort study, we sought to characterize the incident ages and prevalence of AIDs in adults with T1DM across a wide age spectrum.
RESEARCH DESIGN AND METHODS
A total of 1,212 adults seen at the Washington University Diabetes Center from 2011 to 2018 provided informed consent for the collection of their age, sex, race, and disease onset data. We performed paired association analyses based on age at onset of T1DM. Multivariate logistic regression was used to evaluate the independent effects of sex, race, T1DM age of onset, and T1DM duration on the prevalence of an additional AID.
RESULTS
Mean ± SD age of T1DM onset was 21.2 ± 14.4 years. AID incidence and prevalence increased with age. Female sex strongly predicted AID risk. The most prevalent T1DM-associated AIDs were thyroid disease, collagen vascular diseases, and pernicious anemia. T1DM age of onset and T1DM duration predicted AID risk. Patients with late-onset T1DM after 30 years of age had higher risks of developing additional AIDs compared with patients with younger T1DM onset.
CONCLUSIONS
The prevalence of AIDs in patients with T1DM increases with age and female sex. Later onset of T1DM is an independent and significant risk factor for developing additional AIDs. Individuals who are diagnosed with T1DM at older ages, particularly women, should be monitored for other autoimmune conditions.
Introduction
Type 1 diabetes (T1DM) is a common autoimmune disease (AID) that affects at least 30 million people worldwide (1,2). Its rising incidence is driven by the interplay between individual genetics and environmental triggers (3,4). T1DM is characterized by autoimmune destruction of pancreatic islet β-cells, resulting in insulin deficiency and necessitating lifelong hormone replacement therapy. Classically described as a disease of childhood, T1DM is increasingly diagnosed in adults (5–7), and with longer life expectancy for all patients with T1DM, the overall prevalence of T1DM in adults has risen substantially. Although tremendous attention is devoted to screening for and management of diabetes-related microvascular and macrovascular complications in T1DM, less attention has been paid to the characterization of AIDs and related immune deficiency disorders. These additional AIDs add to the complexity of diabetes management and disease burden for patients with T1DM.
Although overall AID prevalence is estimated to be 4–9% in the general population (8,9), the risk is markedly increased in individuals with established autoimmunity (10,11). Despite frequent AID associations in T1DM, the epidemiology of T1DM-related AIDs has been examined more extensively in young age-groups (1,10,12–14). Even adult T1DM studies tend to focus on those aged <30 years (15–17). Additionally, many studies have only reported AIDs affecting endocrine glands and have not included the broader spectrum of both organ-specific and systemic AIDs. Certain AIDs in T1DM have been underreported, particularly neurological diseases and immune deficiency disorders (11,18,19). Thus, we have a limited understanding of the lifetime risk of AIDs in adults with T1DM. These gaps of knowledge lessen our ability to anticipate the development of AIDs in this at-risk population.
In this study, we sought to determine the prevalence and risk factors associated with concomitant AIDs in >1,000 adults with T1DM. In addition to age, sex, and race demographics, we collected age of onset data for T1DM and for all additional AIDs. We report the prevalence of both organ-specific and systemic AIDs in persons with T1DM across a wide age spectrum and examine the relationship of AIDs with age, race, sex, age of T1DM onset, and T1DM duration. We discuss the implications of these findings for AID screening and highlight T1DM age of onset as a new predictor for AID development in later adulthood. By defining the prevalence of AIDs in adults with T1DM, we aim for a better understanding of shared pathogenesis of these autoimmune disorders that will inform future study, health planning, and preventive strategies.
Research Design and Methods
This study was approved by the Washington University Human Research Protection Office. Among a total of ∼1,500 patients with T1DM seen at the Washington University Diabetes Center between 2011 and 2017, 1,212 individuals gave consent and comprised the study. Written, verbal, or electronic consent was obtained from all participants, and a standardized interview and survey were conducted by physicians or trained coordinators, including questions about patient date of birth, sex, race, age of onset of T1DM, types of concomitant autoimmune and immune deficiency diseases, and ages of onset for each disease. All patient charts were reviewed for accuracy of self-reported survey data and to obtain additional information including pertinent medical history, laboratories, and office notes from other specialists. Although the bulk of survey data were accurate per patient report (example: presence and age of onset of vitiligo), occasional diagnoses were changed after chart review (example: a patient reported hypothyroidism and was found to have a history of Graves disease and thyroid ablation). A small number of patients contributed records from outside sources. Overall, <5% of data points were gleaned or changed as a result of chart review. Exclusion criteria included type 2 diabetes, monogenic diabetes, diabetes related to pancreatic disease, and checkpoint inhibitor-induced T1DM. Patients with T1DM were treated by multiple practitioners, and there was no standard screening protocol for AIDs or immune deficiency disorders. No diagnostic, antibody, or genetic tests were performed for the study.
Statistical Analysis
Prevalence of additional AIDs was compared between males versus females and among races using a χ2 test and Fisher exact test when appropriate. T1DM ages of onset in patients with or without additional AIDs were compared using a Student t test. Associations between AID frequency (categorized as 0, 1, 2, 3, or >3) and T1DM age of onset were evaluated using Pearson correlation. Associations between sex and total AID burden were analyzed with the Mann-Whitney U test. Multivariate logistic regression was used to evaluate the independent effects of sex, race, age of T1DM onset, and T1DM duration on the prevalence of additional AIDs. Current age was not included in the multivariate model due to multicollinearity between current age and age of T1DM onset. Spearman correlation analysis showed a high degree of correlation between these two variables (ρ = 0.45886; P < 0.0001). A two-tailed P value of <0.05 was considered significant. SAS version 9.4 (2012; SAS Institute Inc., Cary, NC) was used for data analysis.
Results
Participants included 1,212 individuals with T1DM, age range 19–96 years, mean ± SD 46.8 ± 16.2 years, and median 46 years (Table 1). The majority of participants were aged ≥30 years (82.1%) at the time of this study, whereas 17.9% were <30 years of age. Participants were 51.8% female; 89.6% were white, 9.0% black, and 1.4% other race/ethnicity. Mean duration of T1DM for the entire cohort was 25.5 ± 15.0 years. Mean age of onset of T1DM was 21.2 ± 14.4 years, median 18.0 years, and range 1–78 years. A total of 35.1% of patients had at least one AID in addition to T1DM.
Table 1.
Cohort demographics
| Participants (n = 1,212) | No additional AID (n = 787) | Additional AID (n = 425) | P value | |
|---|---|---|---|---|
| Age (years), mean (SD) | 46.8 (16.2) | 44.3 (16.0) | 51.3 (15.7) | <0.0001 |
| Sex | <0.0001 | |||
| Men | 584 (48.2) | 444 (76.0) | 140 (24.0) | |
| Women | 628 (51.8) | 343 (54.6) | 285 (45.4) | |
| Race/ethnicity | 0.1264 | |||
| White | 1,086 (89.6) | 695 (64.0) | 391 (36.0) | |
| Black | 109 (9.0) | 79 (72.5) | 30 (27.5) | |
| Other | 17 (1.4) | 13 (76.5) | 4 (23.5) | |
| Age (years) | <0.0001 | |||
| ≤29 | 217 (17.9) | 174 (80.2) | 43 (19.8) | |
| 30–39 | 241 (19.9) | 176 (73.0) | 65 (27.0) | |
| 40–49 | 222 (18.3) | 146 (65.8) | 76 (34.2) | |
| 50–59 | 243 (20.1) | 139 (57.2) | 104 (42.8) | |
| ≥60 | 289 (23.8) | 152 (52.6) | 137 (47.4) | |
| Age of T1DM onset (years), mean (SD) | 21.2 (14.4) | 20.1 (13.7) | 23.4 (15.4) | 0.0001 |
Data are n (%) unless otherwise specified. P values are unadjusted.
AID Burden Increases With Age
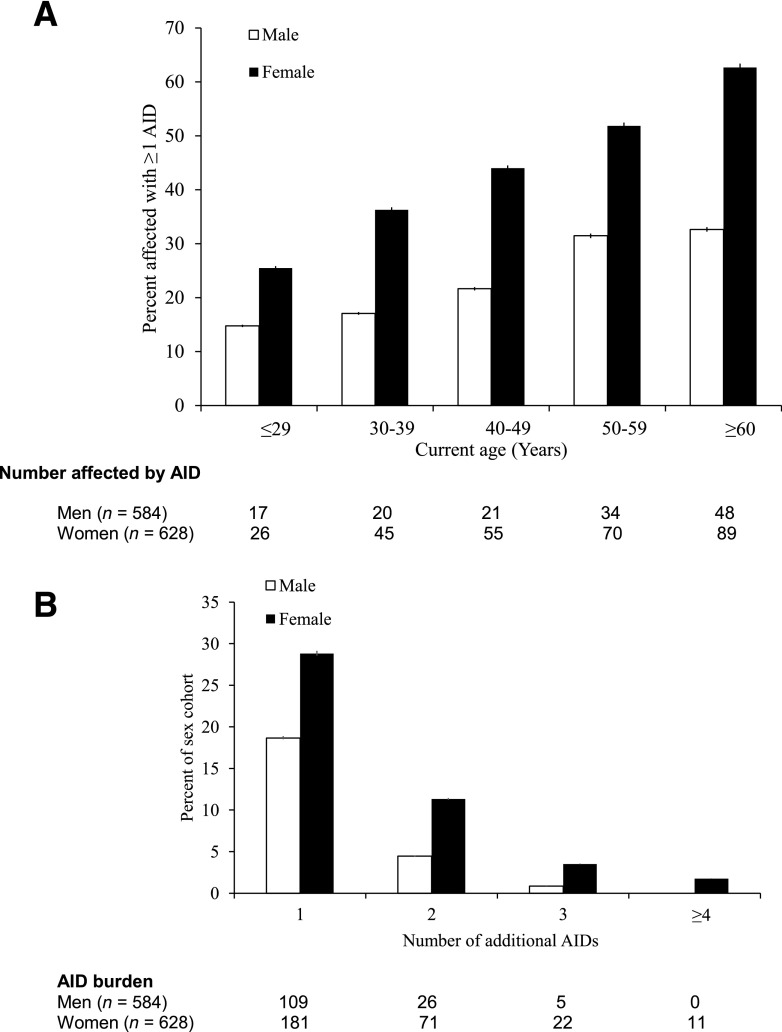
AID burden increased with age in both men and women (Table 1 and Fig. 1A). Whereas 19.8% of patients with T1DM aged ≤29 years had additional AID, this percentage steadily increased over the next age decades to 34.2% of those aged 40–49 years and 47.4% of those >60 years of age (Table 1). Mean current age for those without an additional AID was 44.3 ± 16.0, whereas the mean age for patients with an additional AID was 51.3 ± 15.7, suggesting an association of older age with accumulated AIDs (P < 0.0001) (Table 1). After adjusting for age of T1DM onset, sex, and race, a multivariate logistic regression model showed that participants with an age ≥60 years had the highest risk of having an additional AID compared with participants aged ≤29 years (odds ratio [OR] 3.70 [95% CI 2.35–5.84]; P < 0.0001) (Table 2).
Figure 1.
AID burden increases with age and female sex. A: Increased age is associated with additional AIDs in both men and women. ORs are reported in Table 2. B: Women are more likely than men to have multiple AIDs in addition to T1DM. Wilcoxon-Mann-Whitney P < 0.0001.
Table 2.
Late-onset T1DM predicts risk of additional AIDs
| Adjusted OR (95% CI) | P value | |
|---|---|---|
| Sex | ||
| Men | Reference | |
| Women | 2.75 (2.13–3.55) | <0.0001 |
| Race | ||
| White and other | Reference | |
| Black | 0.71 (0.45–1.12) | 0.1382 |
| Duration of T1DM (years) | ||
| ≤10 | Reference | |
| 11–20 | 1.11 (0.74–1.67) | 0.6144 |
| 21–30 | 1.66 (1.09–2.52) | 0.0184 |
| 31–40 | 1.66 (1.06–2.60) | 0.0281 |
| ≥41 | 3.08 (1.97–4.81) | <0.0001 |
| Age of T1DM onset (years) | ||
| ≤10 | Reference | |
| 11–20 | 1.30 (0.92–1.84) | 0.1318 |
| 21–30 | 1.55 (1.05–2.28) | 0.0274 |
| 31–40 | 2.36 (1.51–3.69) | 0.0002 |
| ≥41 | 3.42 (2.15–5.43) | <0.0001 |
| ≤29 | Reference | |
| ≥30 | 2.13 (1.59–2.85) | <0.0001 |
ORs, CIs, and P values were based on multivariate logistic regression analyses of odds of additional AIDs.
Prevalence of AID by Sex
Risk of developing additional AIDs with increasing age was more pronounced in women than men (Fig. 1A). Women were also more likely than men to develop multiple AIDs in addition to T1DM (P < 0.0001) (Fig. 1B). After adjusting for age of T1DM onset and race/ethnicity, multivariate logistic regression analysis showed that female sex predicts greater odds of developing an additional AID (OR 2.75 [95% CI 2.13–3.55]; P < 0.0001) (Table 2). Women were particularly prone to developing thyroid disease, pernicious anemia, vitiligo, rheumatoid arthritis, lupus, and neurological AID (all χ2 P < 0.01) (Table 3), as well as celiac disease and IgA deficiency (all χ2 P < 0.05) (Table 3). No AID showed statistically significant male predominance (all χ2 P > 0.05) (Table 3).
Table 3.
Individual AID demographics
| AID | Total N (%) | Male | Female | Caucasian and other | Black |
|---|---|---|---|---|---|
| T1DM | 1,212 | 584 | 628 | 1,103 | 109 |
| Thyroid disease | 322 (26.6) | 106 (18.2)** | 216 (34.4)** | 301 (27.3) | 21 (19.4) |
| Hypothyroidism | 267 (22.0) | 89 (15.2)** | 178 (28.3)** | 255 (23.1)** | 12 (11.0)** |
| Hyperthyroidism | 61 (5.0) | 17 (2.9)** | 44 (7)** | 51 (4.6)* | 10 (9.2)* |
| Collagen vascular disease | 79 (6.5) | 21 (3.6)** | 58 (9.2)** | 69 (6.2) | 10 (9.2) |
| Rheumatoid arthritis | 34 (2.8) | 7 (1.2)** | 27 (4.3)** | 28 (2.5) | 6 (5.5) |
| Psoriasis | 25 (2.1) | 9 (1.5) | 16 (2.6) | 24 (2.2) | 1 (0.9) |
| MCTD | 4 (0.3) | 2 (0.3) | 2 (0.3) | 4 (0.4) | 0 (0) |
| JVRA | 4 (0.3) | 1 (0.2) | 3 (0.5) | 3 (0.3) | 1 (0.9) |
| Lupus | 12 (1.0) | 1 (0.2)** | 11 (1.8)** | 10 (0.9) | 2 (1.8) |
| Sjogren | 7 (0.6) | 1 (0.2) | 6 (1.0) | 6 (0.5) | 1 (0.9) |
| Granulomatosis with polyangiitis | 3 (0.2) | 0 (0) | 3 (0.5) | 2 (0.2) | 1 (0.9) |
| Scleroderma | 1 (0.1) | 0 (0) | 1 (0.2) | 1 (<0.1) | 0 (0) |
| Skin and hair disorders | 54 (4.5) | 16 (2.7)** | 38 (6.1)** | 47 (4.3) | 7 (6.4) |
| Vitiligo | 37 (3.1) | 8 (1.4)** | 29 (4.6)** | 35 (3.2) | 2 (1.8) |
| Alopecia | 18 (1.5) | 8 (1.4) | 10 (1.6) | 13 (1.2)* | 5 (4.6)* |
| Pernicious anemia | 55 (4.5) | 9 (1.5)** | 46 (7.3)** | 54 (4.9) | 1 (0.9) |
| Gastrointestinal AIDs | 45 (3.7) | 14 (2.4)* | 31 (5.0)* | 43 (3.9) | 2 (1.8) |
| Celiac disease | 31 (2.6) | 8 (1.4)* | 23 (3.7)* | 30 (2.7) | 1 (0.9) |
| Ulcerative colitis | 12 (1.0) | 4 (0.7) | 8 (1.3) | 11 (1.0) | 1 (0.9) |
| Crohn disease | 6 (0.5) | 4 (0.7) | 2 (0.3) | 5 (0.5) | 1 (0.9) |
| Immune deficiency disorders | 22 (1.8) | 4 (0.7)** | 18 (2.9)** | 21 (1.9) | 1 (0.9) |
| IgA deficiency | 12 (1.0) | 2 (0.3)* | 10 (1.6)* | 12 (1.1) | 0 (0) |
| Common variable immunodeficiency | 11 (0.9) | 2 (0.3) | 9 (1.4) | 10 (0.9) | 1 (0.9) |
| Neurological disease | 18 (1.5) | 3 (0.5)** | 15 (2.4)** | 12 (1.1)** | 6 (5.5)** |
| Moyamoya† | 3 (0.2) | 0 (0) | 3 (0.5) | 3 (0.3) | 0 (0) |
| Multiple sclerosis | 4 (0.3) | 0 (0) | 4 (0.6) | 1 (<0.1)** | 3 (2.8)** |
| Myasthenia gravis | 2 (0.2) | 1 (0.2) | 1 (0.2) | 1 (<0.1) | 1 (0.9) |
| Uveitis | 5 (0.4) | 0 (0) | 5 (0.8) | 4 (0.4) | 1 (0.9) |
| Autoimmune neuropathy (CIDP) | 2 (0.2) | 1 (0.2) | 1 (0.2) | 1 (<0.1) | 1 (0.9) |
| Stiff person syndrome | 3 (0.2) | 1 (0.2) | 2 (0.3) | 3 (0.3) | 0 (0) |
| Autoimmune liver disease | 7 (0.6) | 2 (0.3) | 5 (0.8) | 5 (0.5) | 2 (1.8) |
| Primary biliary cirrhosis | 3 (0.2) | 0 (0) | 3 (0.5) | 2 (0.2) | 1 (0.9) |
| Primary sclerosing cholangitis | 2 (0.2) | 2 (0.3) | 0 (0) | 2 (0.2) | 0 (0) |
| Autoimmune hepatitis | 2 (0.2) | 0 (0) | 2 (0.3) | 1 (<0.1) | 1 (0.9) |
| Premature ovarian failure | 9 (0.7) | 0 (0) | 9 (1.4) | 7 (0.6) | 2 (1.8) |
| Addison disease | 7 (0.6) | 3 (0.5) | 4 (0.6) | 7 (0.6) | 0 (0) |
Data are n (%). List arranged in descending order of disease prevalence. CIDP, chronic inflammatory demyelinating polyneuropathy; JVRA, juvenile rheumatoid arthritis; MCTD, mixed connective tissue disease.
*P < 0.05. **P < 0.01.
†Possible autoimmune etiology.
AID Burden Increases With T1DM Age of Onset and T1DM Duration
We explored the risk of AIDs with age of onset of T1DM and found that older age of T1DM onset was associated with higher AID risk. Mean age of onset of TIDM in patients with no additional AID was 20.1 years, whereas T1DM age of onset in patients with ≥1 AID was 23.4 years (Table 1). Compared with childhood-onset T1DM (≤10 years of age), the risk for additional AIDs is increased for T1DM onset at age 31–40 years (OR 2.36 [95% CI 1.51–3.69]; P = 0.0002) and ≥41 years (OR 3.42 [95% CI: 2.15–5.43]; P < 0.0001) (Table 2). Using 30 years of age as a separator, those with T1DM onset at ≥30 years had significantly higher risk of AIDs (OR 2.13 [95% CI 1.59–2.85]; P < 0.0001) (Table 2). The full breakdown of AID burden by T1DM onset age is shown in Supplementary Table 1. Duration of T1DM was also found to be a significant risk factor for additional AIDs, independent of age of T1DM onset. The highest risk was seen in those with >40 years of T1DM duration (OR 3.08 [95% CI 1.97–4.81]; P < 0.0001) (Table 2).
Prevalence of AID by Race/Ethnicity
Multivariate logistic regression showed that blacks were less likely than whites to develop an additional AID after adjusting for sex and T1DM age of onset (OR 0.71 [95% CI 0.45–1.12]; P = 0.1382) (Table 2). Among 29 total AID types, hypothyroidism and pernicious anemia were significantly more prevalent in whites than blacks (χ2 P < 0.01) (Table 3). Hyperthyroidism, alopecia, and multiple sclerosis occurred more frequently in blacks than whites (χ2 P < 0.05) (Table 3).
Prevalence of AID by Disease Type
AIDs spanned a spectrum, with 29 discrete AIDs incurred by patients with T1DM in our study cohort (Table 3). Thyroid diseases were the most common and affected 26.6% (n = 322) of participants, including 22.0% with hypothyroidism (n = 267) and 5.0% with hyperthyroidism (n = 61). Also of particularly high representation were autoimmune collagen vascular diseases (6.5%; n = 79), skin and hair disorders (4.5%; n = 54), and pernicious anemia (4.5%; n = 55). Gastrointestinal AIDs (3.7%; n = 45), immune deficiencies (1.8%; n = 22), and neurological diseases (1.5%; n = 18) were less common. Individual disease prevalence for some of the most common AIDs was within expected range as previously reported in patients with T1DM: rheumatoid arthritis (2.8% Washington University cohort vs. 2.5% previously reported [20,21]), pernicious anemia (4.5% vs. 3–5% [22,23]), inflammatory bowel disease (1.5% vs. 1.5% [24]), and Addison disease (0.6% vs. 0.3–1.6% [12,25]). In contrast, celiac disease, which typically peaks in the young, was slightly underrepresented in our study (2.6% vs. 3.8–6.4% [11,26,27]).
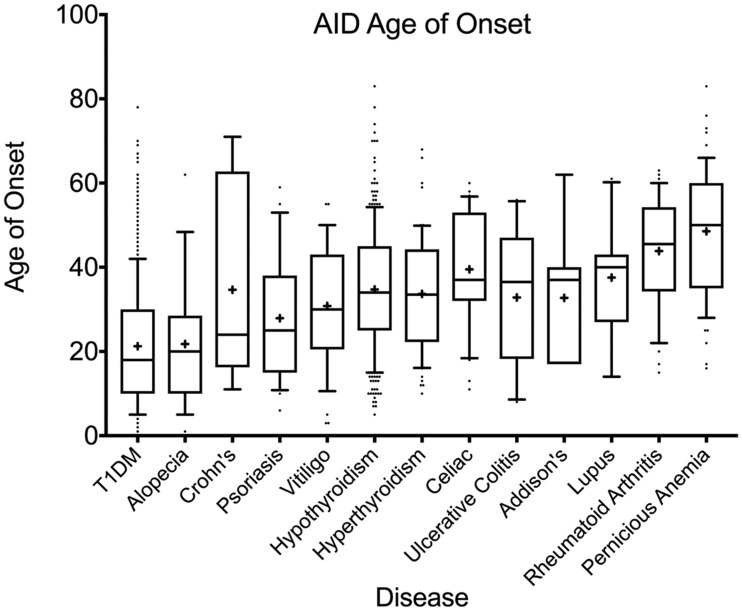
AID Age of Onset
Ages of onset of the most prevalent AIDs are shown by whisker plot in Fig. 2, whereas the full list is available in Supplementary Table 2. Among the 29 AIDs, only three diseases (juvenile rheumatoid arthritis, scleroderma, and autoimmune hepatitis) occurred at a younger mean age than T1DM, at 3.5, 19.0, and 19.5 years, respectively, though case number was low for all three. Other AIDs were diagnosed at ages ≥22 years and most after 30 years of age. Mean age of diagnosis of hypothyroidism (34.7 years) was more than a decade after the mean age of onset of T1DM (21.2 years). Even celiac, a disease that often presents in childhood, was diagnosed across a broad age range, with a surprisingly late mean age of onset of 39.5 years in this cohort. Diseases with the latest age of onset included pernicious anemia (mean ± SD 48.5 ± 15.1 years), primary biliary cirrhosis (52.7 ± 7.6 years), and Sjogren (57.6 ± 9.2 years).
Figure 2.
Age of onset of AIDs in relation to T1DM. Top 13 most common AIDs in this study population, in order of median age of onset. Boxes are 25th–75th percentiles with median indicated by line and mean indicated by “+”; whiskers are 10–90th percentiles.
Conclusions
This study examines an adult cohort with T1DM, asking three questions: 1) what is the prevalence of autoimmune conditions in adults with T1DM, 2) when do they develop in relation to T1DM onset, and 3) what factors are related to the development of concomitant AIDs? Previous surveys of AID prevalence in T1DM have focused on children and young adults, providing only a partial view of the lifetime risk of AIDs in association with T1DM. These studies also tend not to report age of onset of T1DM, blurring the distinction between early- and later-onset T1DM (1,11). These issues were rectified in our study. Our cohort had a high incidence of adult-onset T1DM and high overall prevalence of AIDs. Previous studies in young T1DM cohorts have estimated 33–36% of individuals at T1DM onset or soon thereafter to exhibit markers of nonislet autoimmunity, but only half had clinical disease (10,12). In our cohort with older median age and longer T1DM duration, 35.1% carried diagnoses of comorbid AIDs, a figure that would have been higher had those with positive antibodies but clinically silent disease been counted. The majority of individual AIDs had prevalence similar to that in previously studied T1DM cohorts with comparable age and ethnicity. Thus, the high AID incidence in our study is not due to overrepresentation of select AIDs but to capture of a wide array of diseases including neurological and immune deficiency disorders.
Yet this age-dependent increase in AID prevalence is not simply explained by accrued AIDs over time. Rather, at least two other factors are at play: age itself is a risk factor for autoimmunity (28–30), and the timing of T1DM onset as well as T1DM duration were predictors of AID burden (11). Greater AID occurrence has been observed in those diagnosed with T1DM later in childhood in a pediatric study (16). Our data set extends this finding to the adult population, showing that later-onset T1DM (aged ≥30 years) is an independent risk factor for concomitant AIDs, with an OR of 2.13 compared with earlier T1DM onset. An age of ≥30 years was chosen as the cutoff because it is a more stringent definition of adult-onset T1DM and because this group exhibits distinct patterns of antibody positivity and clinical characteristics (5,31,32). In our study, 30 years of age indeed appears to be an inflection point, after which we observe a linear increase in the OR of developing AIDs, suggesting that the older the individual at the time of T1DM diagnosis, the greater his or her risk for autoimmunity. In light of recent reports of T1DM being increasingly diagnosed in adulthood (5–7), our study demonstrates a compound increase in T1DM-associated AIDs as a function of T1DM onset age. This finding is important for both understanding disease mechanism and improving clinical surveillance, and it should alert clinicians to the necessity of autoimmune screening in susceptible individuals.
To identify subpopulations most at risk for T1DM-associated AIDs, we examined the relationship between AID frequency and sex, race, and other modifiers. T1DM is unique among AIDs in that it affects women and men equally. Yet in our cohort of roughly equal men and women with underlying T1DM, women outnumber men in 24 out of 29 AID categories and are at 2.75 times greater risk than men to acquire additional AIDs, a result strikingly similar to previous population-based estimates (33). In contrast, the impact of race/ethnicity on AID development is disease specific. In autoimmune thyroid disease, Hashimoto thyroiditis was more often seen in whites, whereas Graves disease was more common in blacks. Higher percentages of blacks were also affected with skin and hair disorders (alopecia), collagen vascular disease (rheumatoid arthritis and systemic lupus erythematosus), and neurological disease (multiple sclerosis). HLA as the primary genetic factor in the development of organ-specific autoimmunity may well underlie the heterogeneity that we observe among race/ethnic groups and those with unique AID cluster patterns. Certain genome-wide association study single nucleotide polymorphisms, depending on the individual’s HLA background, may also modify T1DM age of onset and polyglandular autoimmunity (34,35). Due to study limitations, genetics were not evaluated in our patients.
Strengths of this study include its comprehensive cataloging of AIDs and capture of age of onset of both T1DM and all of the concomitant AIDs. We observed a broad range of T1DM age of onset and many other AIDs. Certain AIDs such as thyroid disease, collagen vascular disease, pernicious anemia, immune deficiency disorders, and neurological diseases developed a decade or more after T1DM onset. Exceptions included skin and hair disorders, particularly alopecia, which tends to occur before or concurrently with T1DM. Interestingly, the ages of onset among AIDs do not necessarily predict clinical outcome. Although early-onset systemic lupus erythematosus may predict more active disease, other diseases such as thyroid disease, rheumatoid arthritis, or multiple sclerosis tend to evolve differently, and severity is not always influenced by age of onset. Nonetheless, knowing when to anticipate the development of AIDs and understanding their early-stage symptoms will help physicians to diagnose these conditions sooner, which may improve outcomes.
The limitations of this study are that it was performed at a single center, which as a tertiary referral center may have attracted more complex patients and thus higher AID caseload. In contrast, the lack of standardized screening protocols may have contributed to an underestimate of AID prevalence. Autoantibody testing was done as indicated for clinical diagnosis, but was not done for screening purposes due to its low positive predictive value, particularly in the less common AIDs. Mortality data were not collected, and the known higher mortality among those with earlier T1DM onset (36,37) could have lowered our AID detection rate in younger-onset patients. Another limitation was the lack of genetic testing due to study design. As HLA typing becomes more rapid and cost-effective, we would consider including it in future studies to gain valuable mechanistic information.
Our study provides evidence that T1DM age of onset is an independent predictor of disease comorbidities. This leads to a critical question: is the association of T1DM with other AIDs simply correlative, or does first having T1DM as an index disease change the immune phenotype and predispose the individual to having more or less AID? We speculate that late-onset T1DM may represent an immunologically distinct disease from classic childhood-onset T1DM. This leads to a second question: what are the modifiable factors underlying multisystem autoimmunity, and how might one target prevention or treatment? Trials of immunomodulatory therapy have shown promise in slowing T1DM progression and in preserving β-cell function. Longitudinal follow-up of these study populations who have received early disease-modifying therapy may shed light on whether reversing nascent islet autoimmunity can alter other disease risk later in life. The worldwide incidence of autoimmune disorders is on the rise. Clustering of AIDs in individuals is common, but determining specific disease risks will require more studies that span the age spectrum and provide genetic information. Future investigations would benefit from immune genotyping and phenotyping to understand the etiopathology and natural history of AIDs, with the potential to transform disease diagnosis and management.
Supplementary Material
Article Information
Acknowledgments. The authors thank Mary Jane Clifton, Carol Recklein, and Garrett Pagano (all from Washington University School of Medicine in St. Louis) for administrative and data collection support. The authors also thank Dr. Philip Miller, Professor of Biostatistics at Washington University School of Medicine in St. Louis, for helpful discussions of data analysis and the clinicians and patients in the Washington University Diabetes Center (St. Louis, MO) for the support and participation.
Funding. This work was supported by the Washington University Diabetes Research Center through National Institutes of Health grant P30-DK-020579 (to J.B.M.), Doris Duke Charitable Foundation grant 2015215 (to J.W.H.), a Foundation for Barnes-Jewish Hospital grant (to G.S.T.), and National Institutes of Health training grant T32-DK-007120 (to J.W.H., Y.K.B., M.S., C.R.K., K.J., and V.B.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.W.H., Y.K.B., M.S., P.J., C.R.K., K.J., D.N., V.B., O.J.J., E.C.B., G.S.T., and J.B.M. participated in the data collection, reviewed the manuscript, and contributed to its revision. J.W.H., Y.K.B., and J.B.M. designed the study and wrote the manuscript. Y.K.B. performed data analysis with input from J.W.H. and J.B.M. J.W.H. and J.B.M. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at ENDO 2018, the Endocrine Society’s annual meeting, Chicago, IL, 17–20 March 2018.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-1157/-/DC1.
References
- 1.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al.; SEARCH for Diabetes in Youth Study . Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med 2017;376:1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am 2010;39:481–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G; EURODIAB Study Group . Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet 2009;373:2027–2033 [DOI] [PubMed] [Google Scholar]
- 4.Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet 2016;387:2340–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, Hattersley AT. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol 2018;6:122–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabbah E, Savola K, Ebeling T, et al. . Genetic, autoimmune, and clinical characteristics of childhood- and adult-onset type 1 diabetes. Diabetes Care 2000;23:1326–1332 [DOI] [PubMed] [Google Scholar]
- 7.Diaz-Valencia PA, Bougnères P, Valleron A-J. Global epidemiology of type 1 diabetes in young adults and adults: a systematic review. BMC Public Health 2015;15:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper GS, Bynum MLK, Somers EC. Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J Autoimmun 2009;33:197–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayter SM, Cook MC. Updated assessment of the prevalence, spectrum and case definition of autoimmune disease. Autoimmun Rev 2012;11:754–765 [DOI] [PubMed] [Google Scholar]
- 10.Triolo TM, Armstrong TK, McFann K, et al. . Additional autoimmune disease found in 33% of patients at type 1 diabetes onset. Diabetes Care 2011;34:1211–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes JW, Riddlesworth TD, DiMeglio LA, Miller KM, Rickels MR, McGill JB; T1D Exchange Clinic Network . Autoimmune diseases in children and adults with type 1 diabetes from the T1D Exchange Clinic Registry. J Clin Endocrinol Metab 2016;101:4931–4937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barker JM, Yu J, Yu L, et al. . Autoantibody “subspecificity” in type 1 diabetes: risk for organ-specific autoimmunity clusters in distinct groups. Diabetes Care 2005;28:850–855 [DOI] [PubMed] [Google Scholar]
- 13.Lu M-C, Chang S-C, Huang K-Y, Koo M, Lai N-S. Higher risk of thyroid disorders in young patients with type 1 diabetes: a 12-year nationwide, population-based, retrospective cohort study. PLoS One 2016;11:e0152168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozhakhmetova A, Wyatt RC, Caygill C, et al. . A quarter of patients with type 1 diabetes have co-existing non-islet autoimmunity: the findings of a UK population-based family study. Clin Exp Immunol 2018;192:251–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorham ED, Garland FC, Barrett-Connor E, Garland CF, Wingard DL, Pugh WM. Incidence of insulin-dependent diabetes mellitus in young adults: experience of 1,587,630 US Navy enlisted personnel. Am J Epidemiol 1993;138:984–987 [DOI] [PubMed] [Google Scholar]
- 16.Wägner AM, Santana A, Herńndez M, Wiebe JC, Nóvoa J, Mauricio D; T1DGC6 . Predictors of associated autoimmune diseases in families with type 1 diabetes: results from the Type 1 Diabetes Genetics Consortium. Diabetes Metab Res Rev 2011;27:493–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menke A, Orchard TJ, Imperatore G, Bullard KM, Mayer-Davis E, Cowie CC. The prevalence of type 1 diabetes in the United States. Epidemiology 2013;24:773–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greco D, Maggio F. Selective immunoglobulin a deficiency in type 1 diabetes mellitus: a prevalence study in Western Sicily (Italy). Diabetes Metab J 2015;39:132–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes JW, Wyckoff JA, Hollander AS, Derdeyn CP, McGill JB. Moyamoya syndrome causing stroke in young women with type 1 diabetes. J Diabetes Complications 2016;30:1640–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao KP, Gunnarsson M, Källberg H, et al. . Specific association of type 1 diabetes mellitus with anti-cyclic citrullinated peptide-positive rheumatoid arthritis. Arthritis Rheum 2009;60:653–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunter TM, Boytsov NN, Zhang X, Schroeder K, Michaud K, Araujo AB. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004-2014. Rheumatol Int 2017;37:1551–1557 [DOI] [PubMed] [Google Scholar]
- 22.Perros P, Singh RK, Ludlam CA, Frier BM. Prevalence of pernicious anaemia in patients with type 1 diabetes mellitus and autoimmune thyroid disease. Diabet Med 2000;17:749–751 [DOI] [PubMed] [Google Scholar]
- 23.De Block CEM, De Leeuw IH, Van Gaal LF. Autoimmune gastritis in type 1 diabetes: a clinically oriented review. J Clin Endocrinol Metab 2008;93:363–371 [DOI] [PubMed] [Google Scholar]
- 24.Leeds JS, Hopper AD, Hadjivassiliou M, Tesfaye S, Sanders DS. Inflammatory bowel disease is more common in type 1 diabetes mellitus. Gut 2011;60(Suppl. 1):A208 [Google Scholar]
- 25.Chantzichristos D, Persson A, Eliasson B, et al. . Incidence, prevalence and seasonal onset variation of Addison’s disease among persons with type 1 diabetes mellitus: nationwide, matched cohort studies. Eur J Endocrinol 2018;178:115–122 [DOI] [PubMed] [Google Scholar]
- 26.Mahmud FH, Murray JA, Kudva YC, et al. . Celiac disease in type 1 diabetes mellitus in a North American community: prevalence, serologic screening, and clinical features. Mayo Clin Proc 2005;80:1429–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeMelo EN, McDonald C, Saibil F, Marcon MA, Mahmud FH. Celiac disease and type 1 diabetes in adults: is this a high-risk group for screening? Can J Diabetes 2015;39:513–519 [DOI] [PubMed] [Google Scholar]
- 28.Kyvik KO, Nystrom L, Gorus F, et al. . The epidemiology of type 1 diabetes mellitus is not the same in young adults as in children. Diabetologia 2004;47:377–384 [DOI] [PubMed] [Google Scholar]
- 29.Goronzy JJ, Weyand CM. Immune aging and autoimmunity. Cell Mol Life Sci 2012;69:1615–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vadasz Z, Haj T, Kessel A, Toubi E. Age-related autoimmunity. BMC Med 2013;11:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mølbak AG, Christau B, Marner B, Borch-Johnsen K, Nerup J. Incidence of insulin-dependent diabetes mellitus in age groups over 30 years in Denmark. Diabet Med 1994;11:650–655 [DOI] [PubMed] [Google Scholar]
- 32.Bruno G, Runzo C, Cavallo-Perin P, et al.; Piedmont Study Group for Diabetes Epidemiology . Incidence of type 1 and type 2 diabetes in adults aged 30-49 years: the population-based registry in the province of Turin, Italy. Diabetes Care 2005;28:2613–2619 [DOI] [PubMed] [Google Scholar]
- 33.Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol 1997;84:223–243 [DOI] [PubMed] [Google Scholar]
- 34.Redondo MJ, Geyer S, Steck AK, et al. . TCF7L2 genetic variants contribute to phenotypic heterogeneity of type 1 diabetes. Diabetes Care 2018;41:311–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Houcken J, Degenhart C, Bender K, König J, Frommer L, Kahaly GJ. PTPN22 and CTLA-4 polymorphisms are associated with polyglandular autoimmunity. J Clin Endocrinol Metab 2018;103:1977–1984 [DOI] [PubMed] [Google Scholar]
- 36.Gagnum V, Stene LC, Jenssen TG, et al. . Causes of death in childhood-onset type 1 diabetes: long-term follow-up. Diabet Med 2017;34:56–63 [DOI] [PubMed] [Google Scholar]
- 37.Morgan E, Cardwell CR, Black CJ, McCance DR, Patterson CC. Excess mortality in type 1 diabetes diagnosed in childhood and adolescence: a systematic review of population-based cohorts. Acta Diabetol 2015;52:801–807 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.