ABSTRACT
The utility of CareStartTM Malaria Pf/PAN (HRP2/pLDH) Ag Combo Test, in detecting non-endemic clinical malaria cases was evaluated in Sri Lanka, a country in prevention of re-establishment of malaria following elimination. RDT, microscopy and nested PCR were performed for 350 suspected malaria patients recruited prospectively. There were 173 PCR confirmed malaria patients and 177 PCR negative subjects. Plasmodium falciparum amounted to 48% of infections with 44% P. vivax, 6% P. ovale and 2% P. malariae. Performance characteristics of RDTs and microscopy were compared with nested PCR. Sensitivity and specificity of RDT with 95% confidence intervals (CI) were as follows: any malaria infection 95.95% (CI = 91.84–98.36) and 94.92% (CI = 90.57–97.65); P. falciparum 100% (CI = 95.65–100) and 97.00% (CI = 94.18–98.70) and other species 92.22% (CI = 84.63–96.82) and 99.62% (97.88–99.99) respectively. A significant difference between sensitivities of HRP2 (100%, CI = 95.65–100) and pan pLDH line (68.67%, CI = 57.56–78.41) was seen for P. falciparum, parasite densities less than 1000 parasites/microliter being detected only by HRP2. Sensitivity and specificity of microscopy with 95% CI were as follows: any malaria infection, 94.22% (CI = 89.63–97.19) and 99.44% (CI = 96.89–99.99); P. falciparum 89.16% (CI = 80.40–94.90) and 99.63% (CI = 97.94–99.99); other species 98.89% (CI = 93.96–99.97) and 100% (CI = 98.59–100) respectively. The low sensitivity of pan specific pLDH for P. falciparum, P. ovale and P. malariae should be taken in to consideration when using this RDT as a point of care test when and wherever microscopy facilities are not readily available. Considering the low sensitivity of microscopy for P. falciparum, it is preferable to perform both tests, when malaria is highly suspected.
KEYWORDS: CareStartTM, malaria rapid diagnostic test, Sri Lanka, prevention of re-establishment of malaria
Introduction
With the renewed global interest in malaria elimination in recent years, many malaria endemic countries are aiming to eliminate the disease. Since the year 2000, 18 countries and territories have been declared as no longer endemic or have reported zero indigenous malaria cases [1]. Sri Lanka, a tropical country, was certified as a malaria free country by World Health Organization (WHO) in 2016, but due to high receptivity and vulnerability, there is a risk of reintroduction of the disease [2]. Imported malaria cases continue to be reported in Sri Lanka with 57 cases reported in 2017. With Anopheles culicifacies being reported in most parts of the country, and the recent detection of Anopheles stephensi in the Northern region [3], early detection of imported malaria cases is essential to prevent the reintroduction of malaria to the country.
Based on the WHO recommendations for prompt parasitological confirmation by microscopy and/or Rapid Diagnostic Tests (RDTs) for all patients with suspected malaria [4] and the WHO policy recommendation on malaria diagnostics in low transmission settings [5], RDTs are being increasingly used in many malaria endemic countries, especially in areas and countries that are attempting to eliminate the disease.
Operational characteristics of RDTs have been studied under varying disease endemic conditions [6–9]. RDTs have a detection threshold of 100 parasites/microliter, which is also generally accepted to be the level of detection of field level microscopy [10]. At present RDTs are evaluated by the WHO against P. vivax and P. falciparum at a range of 200–2000 parasites/microliter [11] and the sensitivities of RDTs for P. ovale and P. malariae are known to be low [6,8]. False negative results are known to occur at low parasite densities directly due to low levels of targets antigens, at higher parasite densities due to prozone effect [12] and due to genetic deletions of the target antigen [13] irrespective of the parasite density. Cross reactions with other disease conditions [14] and persisting antigenemia [7,9] may also affect the result.
Since the year 2009, guidelines on selecting the appropriate RDT have been provided by the WHO. These depend on the parasite species in a particular setting, intensity, in-country experience and ease of use [11]. Based on these guidelines Sri Lanka has been using three-band RDTs that detect both Plasmodium falciparum and other Plasmodium species without differentiation, subject to confirmation by quality assured microscopy. Since 2014, any discrepancy between microscopy and RDT is resolved by Polymerase Chain Reaction (PCR).
Studies have not been carried out to determine the use of RDTs for routine diagnosis of malaria in the prevention of re-establishment phase in countries such as Sri Lanka with high vulnerability and receptivity. Considering the importance of detecting imported malaria cases as early as possible to prevent reestablishment of malaria in the country, this study was conducted to evaluate the efficacy of RDT to detect imported malaria cases considering the nested PCR as the reference standard.
Materials and methods
Study setting and participants
This study was conducted from April 2014 to December 2017 at the Central Laboratory of the Anti Malaria Campaign (AMC) Headquarters (HQ), Colombo, Sri Lanka. Since eliminating indegenous malaria in November 2012, all malaria cases reported in the country are imported cases, the majority of them being diagnosed in the Western Province. The Central Laboratory of the AMC functions as the referral centre for diagnosis and confirmation of malaria infections in the country and has diagnosed over 90% of the imported cases reported in the country during the study period.
Study population
When a suspected malaria patient seeks treatment, (usually for a febrile illness), from a health care institute in Sri Lanka, the treating physician may request for a malaria diagnostic test. Alternatively, the patient may request for a malaria diagnostic test from the treating physician. This mode of detection is termed as passive case detection (PCD). The study population comprised patients with clinical symptoms and/or signs of malaria in whom a confirmatory laboratory diagnostic test had been requested by clinicians from the Central laboratory of the AMC. Patients were prospectively recruited into the study after obtaining their informed consent.
Due to the low prevalence of malaria during the period from 2014 to 2017, all PCR confirmed malaria patients were included consecutively. Simple random sampling was used to select the PCR negative patients to be recruited. A total of 350 patients (173 Plasmodium positive and 177 Plasmodium negative) diagnosed by the reference test were included.
Ethics and protocol
The participants were referred to the Central Laboratory of the Anti Malaria Campaign for diagnosis of malaria by a Medical Officer. Informed consent was obtained from the individual to be included for this study. In the case of a minor, consent was obtained from the parents or guardian. The study design was in compliance with the STARD guidelines for presentation of diagnostic studies [15]. Ethical approval for this study was obtained from the Ethics Review Committee of the Sri Lanka Medical Association (ERC/13–053).
Sample collection
Finger prick blood was collected under aseptic conditions from the selected study participants. According to the routine procedure approximately two and six microliters of blood was collected to a glass slide for the preparation of thin and thick blood smears respectively. Five microliters of finger prick blood was then used for the RDT. Approximately 125 microliters of blood was also collected to a Flinders Technology Associates filter paper card (FTA®) for molecular assays. Samples collected for molecular assays were coded to perform the assays in a blinded manner.
Diagnostic test procedures
Rapid diagnostic test (RDTs)
CareStartTM Malaria Pf/PAN (HRP2/pLDH) Ag Combo Test, product G0131, the Rapid Diagnostic Test Kit currently used in government healthcare institutions was considered as the index test for evaluation. This RDT has been selected for use in a Sri Lankan setting based on their performance in WHO malaria RDT product testing and the target antigens detected (HRP 2 and pan–specific pLDH).
RDTs were performed on the finger prick blood collected and interpreted according to the manufacturer’s instructions and the Standard Operating Procedures prepared by the Anti Malaria Campaign. Briefly, five microliters of blood collected to the pipette provided with the test kit was added to the sample well. This was followed by the addition of two drops of assay buffer solution to the buffer well. The test result was interpreted in 20 minutes. Invalid RDTs (without the control band) were repeated. Individual band reactivity (HRP2 and pan-specific pLDH) was recorded for all positive test results.
Microscopy
Thick and thin blood smears were prepared and stained with 10% Giemsa stain for 10 minutes. Parasite density was calculated in the positive smears by counting the number of asexual parasites per 200 leukocytes (or per 500, if the count is < 100 asexual parasites/200 leukocytes), assuming a leukocyte count of 8,000/microliter [16].
Polymerase chain reaction (PCR)
To confirm the presence or absence of parasites and parasite species, all samples were analysed by nested PCR assay according to standardized protocols and published methods [17]. The nested PCR assay was performed at the AMC Central Laboratory. The assays were performed in a blinded manner using coded samples.
Data management and statistical analysis
Test outcomes and clinical data were collected on laboratory record forms and later entered in to a Microsoft Excel data base. Data was subsequently cleaned and analysed using SPSS Statistics software. RDT and microscopy performance was calculated compared with matched nested PCR, (the reference standard) with 95% confidence intervals (CI) for the following values: Sensitivity, specificity, positive likelihood ratio, negative likelihood ratio.
Results
Demographic and parasitological results
Of the patients routinely tested for malaria between April 2014 and December 2017, a total of 350 individuals were included in this study. The age of the study participants ranged from 1–73 years. Majority of participants were males (81%). A history of a recent visit to a malaria endemic country was given by 89%. The basic demographic information is given in Table 1.
Table 1.
Basic Demographic Characteristics of study population.
| Demographic Characteristics | PCR |
Total (n = 350) |
|||||
|---|---|---|---|---|---|---|---|
| Positive (n = 173) |
Negative (n = 177) |
||||||
| n | % | n | % | n | % | ||
| Gender | Male | 159 | 92 | 123 | 69 | 282 | 81 |
| Female | 14 | 8 | 54 | 31 | 68 | 19 | |
| Age (Years) | Median | 37 | 41 | 38 | |||
| Range | 1–66 | 13–73 | 1–73 | ||||
| History of travel | Yes | 173 | 56 | 139 | 44 | 312 | 89 |
| No | 0 | 0 | 38 | 100 | 38 | 11 | |
Diagnostic test results
Of the 350 samples tested for malaria, 173 individuals (49.6%) were positive by nested PCR. Assuming a parasite density of 1 parasite per microliter for microscopically negative infections, this included 83 P. falciparum (48%) with a parasite density range of 1–864,000 parasites/µL, 77 P. vivax (44%) with a parasite density range of 1–49,655 parasites/µL, 10 P. ovale (6%) with a parasite density range of 250–12,500 parasites/µL and three P. malariae (2%) cases with a parasite density range of 1,260–17,453 parasites/µL. RDTs performed better than microscopy in detecting P. falciparum cases. While all P. falciparum cases were detected by RDTs, (69% by both HRP2 and pan-pLDH test bands and31% by HRP2 test band), microscopy detected only 89% of P. falciparum cases. No mixed infections were diagnosed. All the true positive HRP2 and pLDH test lines were due to P. falciparum mono infections. Among the non-falciparum infections, RDT detected 97% of P. vivax, 70% of P. ovale and 33% of P. malariae infections. Microscopy detected 99% of P. vivax, all P. ovale (one P. ovale case was reported as P. vivax) and all P. malariae infections. A comparison of the results of microscopy and RDT with the results of nested PCR are given in Figure 1 and Table 2.
Figure 1.
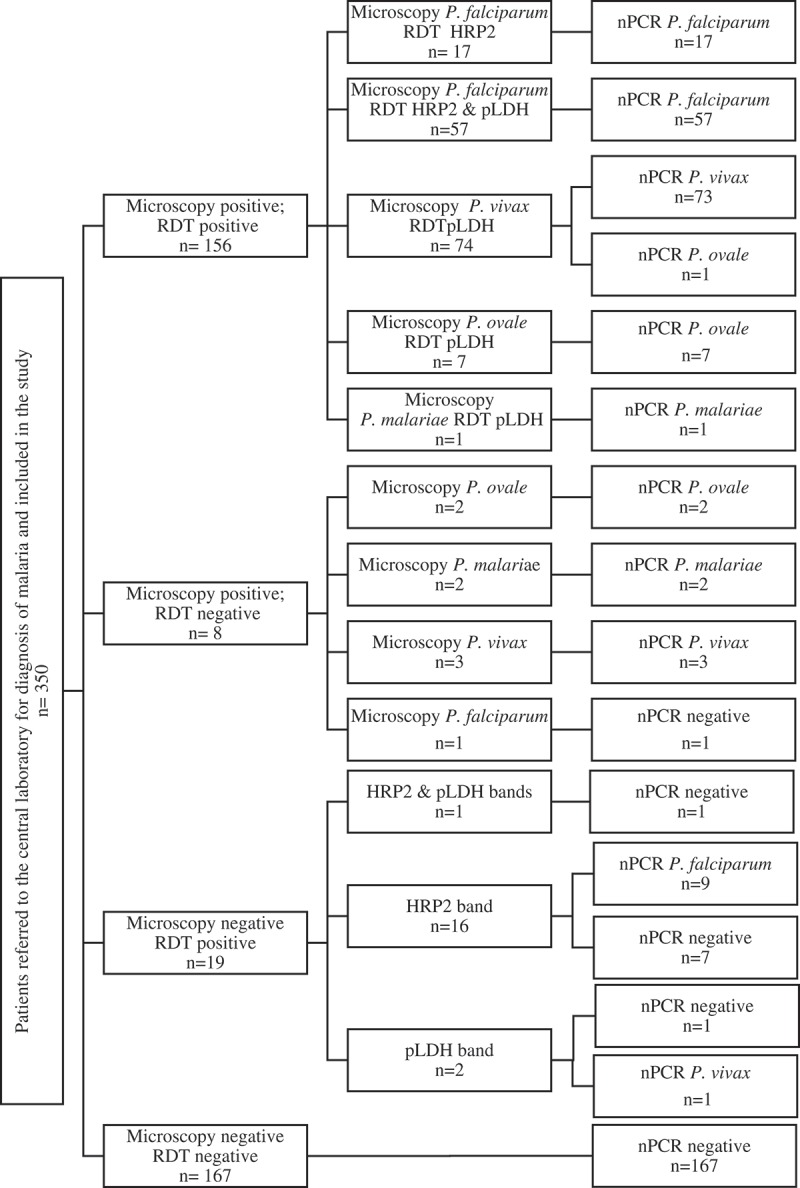
Positivity of RDT, microscopy and PCR among the patients recruited.
Table 2.
Microscopy and RDT positivity compared to nested PCR.
| Test result | P. falciparum | P. vivax | P. ovale | P. malariae | Total |
|---|---|---|---|---|---|
| Positive by nested PCR (%) | 83 (48%) | 77 (44%) | 10 (6%) | 3 (2%) | 173 (49.4%) |
| Density range (parasites/µL) | 1–864,000 | 1–49,655 | 250–12,500 | 1260–17,453 | 1–864,000 |
| Low density parasitaemia infections (%) | 16 (19%) | 3 (4%) | – | – | 19 (11%) |
| Positive by microscopy (%) | 74 (45%) | 76 (47%) | 9 (5%) | 3 (2%) | 164 (46.6%) |
| Density range (parasites/µL) | 16–864,000 | 16–49,655 | 250–12,500 | 1260–17,453 | 16–864,000 |
| % Low density infections detected | 44% | 67% | – | – | 47% |
| False positives (%) | 1 (0.3%) | 1 | – | – | 1 |
| False negatives (%) | 9 (2.6%) | 1 | 1 | – | 10 |
| Positive by RDT (%) | 83 (23.7%) | 74 (21.4%) | 7 (2%) | 1 (0.3%) | 166 (47.4%) |
| Density range (parasites/µL) | 1–864,000 | 1–49,655 | 850–12,500 | 1450 | 1–86,400 |
| % Low density infections detected | 100 % | 100 % | – | – | 100 % |
| False positives (%) | 8 (2.4%) | 1 (0.3%) | – | – | 9 |
| False negatives (%) | – | 2 (0.6%) | 3 (0.9%) | 2 (0.6%) | 7 |
Note: Species-wise results are based on species specific PCR assays. When considering positivity of PCR and microscopy for a particular species, even a positive result for another species was considered as negative. One P. ovale infection identified as P. vivax by microscopy was considered as a false positive for P. vivax and a false negative for P. ovale. Total positives were considered based on the genus specific nested PCR.
Relationship of parasite density with microscopy and RDT positivity
For all microscopically positive malaria infections, the parasite densities were calculated by microscopy. For the microscopy negative PCR positive (low density parasitaemia) infections, a parasite density of 1 parasite/µL was assigned. The relationship of the index test positivity with parasite densities for each species is given in Figure 2.
Figure 2.
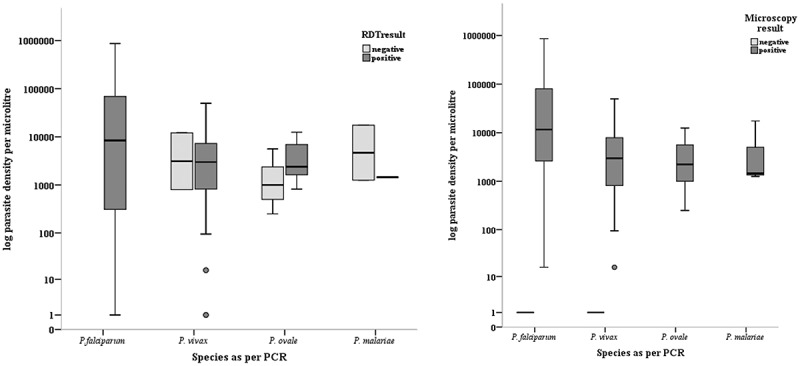
Microscopy and RDT positivity according to parasite density.
Note-Parasite density was based on microscopy. For microscopy negative, PCR confirmed malaria infections, a parasite density of one parasite per microliter was assigned.RDTs detected all the P. falciparum infections and all low density parasitaemia infections (16 P. falciparum and 3 P. vivax). Microscopy detected only nine of these low density parasitaemia infections (7 P. falciparum and 2 P. vivax).
There were 16 P. falciparum and 3 P. vivax infections with low density parasitaemia (< 100 parasites/µL). Microscopy detected only nine of these infections (7 P. falciparum and 2 P. vivax). However, all these infections were detected by the RDTs.
RDTs detected only one out of four P. ovale infections below a density of 1,000 parasites/µL. Out of three P. malariae infection only one was detected by RDT.
Detection of P. falciparum by HRP2 and pan specific pLDH test band according to the parasite density
The relationship between HRP2 and pan-pLDH test band reactivity, according to different parasite densities of P. falciparum was analysed. All P. falciparum infections below the parasite density of 1000 parasites/µL (including microscopy negatives) were detected only by HRP2 test band. This amounted to 29% of total P. falciparum infections. In two P. falciparum infection which had a parasite density within the range of 1001–10,000 and 10,001–100,000 parasites/µL, only the HRP2 test band was visible. Proportions of the P. falciparum infections detected by HRP2 and pan-pLDH antigens in RDTs disaggregated according to parasite density is given in Figure 3.
Figure 3.
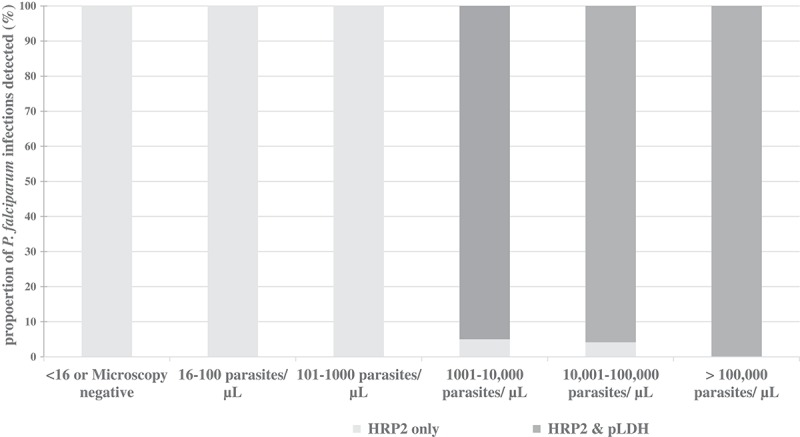
Proportion of P. falciparum infections detected byHRP2 and pan specific pLDH antigens in RDT disaggregated according to parasite density.
Performance characteristics of index tests
Performance characteristics of the RDTs and microscopy in detecting any malaria species, P. falciparum, non-falciparum species and P. vivax separately are given in Table 3. RDT had a sensitivity of 95.95% (CI = 91.84–98.36) and a specificity of 94.92% (CI = 90.57–97.65) in detecting a malaria infection. Sensitivity of microscopy was 94.22% (CI = 89.63–97.19) while specificity was 99.44% (CI = 96.89–99.99). When species–wise detection is considered, the lowest sensitivity of 68.67% (CI = 57.56–78.41) was observed in pan-pLDH test positivity for P. falciparum malaria infections. Microscopy had a sensitivity of 89.16% (CI = 80.40–94.90). Highest sensitivity (100%; CI = 95.65–100) was observed for detection of P. falciparum by RDTs. This was possible due to the high sensitivity of the HRP2 test band. Microscopy had the highest specificity of 100% (CI = 98.59–100) for detection of non-falciparum species. The sensitivity of RDT for detection of non-falciparum malaria infections was 92.22% (CI = 84.63–96.82). Values above 97% were observed for other sensitivities and specificities by microscopy and RDTs.
Table 3.
Performance characteristics of RDTs and microscopy with nested PCR as reference standard.
| Malaria infection/Species | Index test | Sensitivity % (95% CI) |
Specificity % (95% CI) |
Positive Likelihood Ratio (95% CI) |
Negative Likelihood Ratio (95% CI) |
|---|---|---|---|---|---|
| Any malaria infection | RDT | 95.95 (91.84–98.36) |
94.92 (90.57–97.65) |
18.87 (9.98–35.69) |
0.04 (0.02–0.09) |
| microscopy | 94.22 (89.63–97.19) |
99.44 (96.89–99.99) |
167.77 (23.61–1177.81) |
0.06 (0.03–0.11) |
|
| P. falciparum | RDT* | 100.00 (95.65–100.00) |
97.00 (94.18–98.70) |
33.38 (16.87–66.04) |
0 |
| pLDH | 68.67 (57.56–78.41) |
99.63 (97.93–99.99) |
183.36 (25.78–1303.98) |
0.31 (0.23–0.43) |
|
| microscopy | 89.16 (80.40–94.90) |
99.63 (97.94–99.99) |
238.94 (33.73–1692.55) |
0.11 (0.06–0.20) |
|
| P. vivax | RDT | 97.40 (90.93–99.68) |
99.63 (97.98–99.99) |
265.91 (37.58–1881.64) |
0.03 (0.01–0.10) |
| microscopy | 98.70 (92.98–99.97) |
99.63 (97.98–99.99) |
269.45 (38.09–1906.40) |
0.01 (0.0–0.09) |
|
| Non falciparum | RDT | 92.22 (84.63–96.82) |
99.62 (97.88–99.99) |
239.78 (33.87–1697.41) |
0.08 (0.04–0.16) |
| microscopy | 98.89 (93.96–99.97) |
100.00 (98.59–100.00) |
– | 0.01 (0.00–0.08) |
* HRP2 irrespective of presence of pan pLDH
False positivity rate of index tests
The false positive rate of RDTs was 5% (9/177). There were no incorrect species identifications, all false positives being among Plasmodium negative samples. There were seven false positives with HRP2 test line, one false positive with pLDH test line and one false positive with both test lines. The false positive rate of microscopy was 0.56% (1/177).
All RDT false positives were negative by microscopy and nested PCR. Demographic and clinical characteristics of the patients with false positive RDT and microscopy results are shown in Table 4.
Table 4.
Demographic and clinical characteristics of patients with false positive RDT and/or microscopy results.
| Patient | Gender | Age (yrs) |
RDT result |
microscopy | History of overseas travel | History of malaria during overseas stay | Other causes |
|---|---|---|---|---|---|---|---|
| 1 | Male | 56 | HRP2 | Negative | Yes | Not known | - |
| 2 | Male | 30 | HRP2 | Negative | Yes | Not known | - |
| 3 | Male | 31 | HRP2 | Negative | Yes | Yes | - |
| 4 | Male | 42 | HRP2 | Negative | Yes | Yes | - |
| 5 | Male | 36 | HRP2 | Negative | Yes | Yes | - |
| 6 | Male | 52 | pLDH | Negative | Yes | No | Pancytopenia |
| 7 | Female | 48 | HRP2 | Negative | No | No | Dengue |
| 8 | Male | 38 | HRP2 | Negative | Yes | Yes | - |
| 9 | Female | 60 | HRP2 & pLDH | Negative | No | No | Non-Hodgkin’s lymphoma |
| 10 | Male | 58 | Negative | Positive | Yes | No | - |
Discussion
Quality assured RDTs and microscopy are the primary diagnostic tools for confirmation and management of cases of suspected clinical malaria in all epidemiological situations, including areas of low transmissions. The present study assessed the utility of malaria RDTs in diagnosing non-endemic clinical malaria cases in a setting of prevention of reestablishment of malaria in a country known for its high receptivity and vulnerability for the disease. The diagnostic accuracy of RDTs and microscopy were compared with nested PCR as the reference standard.
For detection of a malaria infection, the CarestartTM pf/pan combo RDTs showed a sensitivity and a specificity (95.95% and 94.92% respectively) comparable to microscopy (sensitivity of 94.22% and specificity of 99.44%). Compared to the studies done using the same brand of RDT, the sensitivity reported in this study is higher than the sensitivity reported from Uganda and Burkina Faso [18] with nested PCR as the reference standard, and in the China-Myanmar border, with microscopy corrected PCR as the reference standard [19]. Similar sensitivities and specificities were observed in Ethiopia using microscopy as the reference standard [20–22], while higher sensitivity (100%) among acute febrile patients clinically suspected of malaria have been reported in Ethiopia [23]. These differences in the sensitivity and specificity could be due to the differences in malaria species circulating in different study settings, observer variation or due to the actual true positives being missed by the reference test (PCR) as suggested in some studies [18]. Apart from the study done in a reference setting which has evaluated the sensitivity of CarestartTM RDT against P. falciparum, P. vivax, P. malariae and P. ovale [6], others have reported performance of CarestartTM RDT Pf/PAN combo either in P. falciparum predominant regions [18,20–23] or in P. falciparum and P. vivax coexisting regions [19]. Here we report the performance of CarestartTM RDT Pf/PAN combo in detecting all human malaria species. Another key difference between the above-mentioned studies and this one is that in the current study, the samples were obtained from symptomatic patients as compared to community surveys or routine testing in asymptomatic individuals. RDTs are recommended for diagnosis of malaria among symptomatic patients as they are likely to have higher parasite densities and hence, better performance of the RDT [24]. However, in this study, 19% of P. falciparum and 4% of P. vivax infections amounting to 11% of total positive cases were due to low density malaria infections. It is important to note that RDTs managed to detect all these cases.
In this study RDTs had a 100% sensitivity and a 97% specificity for detection of P. falciparum. This sensitivity is much higher than the sensitivity reported previously [6,19–21], while similar specificity [20] and higher values for specificity have been reported [19,21]. Thirty one percent of P. falciparum cases, including all low density parasitaemia were detected based on the presence of positive HRP2 test line and a negative pan-specific test line. This proportion detected by RDTs is higher than the 12.5% of P. falciparum infections that had been detected by the HRP2 test line only in a study done in a reference setting [6]. This could be due to the difference in the method of sample collection as samples archived over several years have been used in that study, whereas in the present study RDTs were performed on patients, as a point of care test.
Compared to the sensitivity of HRP2 (100%), pan-specific pLDH test line had a low sensitivity (68.67 %). When considering the parasite density, all P. falciparum cases below a parasite density of 1000 parasites per microliter had only a HRP2 positive test line while there were two high density P. falciparum infections that were also detected only by HRP2 test line. If HRP2 test line was not there, all malaria infections below 1000 parasite/µL would have been missed. Low positivity rates of the pan-pLDH test line against P. falciparum are known with a relationship between antigen concentration and band positivity [25]. The performance of RDTs are known to vary even among lots and products [24,25]. This has a serious implication in the context of gene deletion of pfHRP2 that has been reported in more than 10 countries [26]. Considering the fact that many imported cases reported in Sri Lanka are from regions where the HRP2 gene deletions have been recently detected, the low sensitivity of pan-specific pLDH may have a serious implication in use of the current RDTs in the case detection. Given the fact that Sri Lanka is a country that has eliminated indigenous malaria and with high receptivity risk, the continuous influx of travellers from those regions where the pf HRP2/3 gene deletion is prevalent will be a major concern (e.g. tourists, gem traders, UN Peace Keeping Missions). This low sensitivity of pan-specific pLDH has to be taken in to consideration when RDTs are used to test suspected malaria patients with a history of travel to such areas.
In addition RDTs detecting pLDH are more sensitive to tropical conditions than RDTs detecting HRP2 and may show poor performance when exposed to excess heat and humidity during improper transport and storage. Therefore, quality control of procured RDTs is essential to minimize false negative pLDH results.
The 97% specificity for detection of P. falciparum is high compared to other studies. In a sub-Saharan African context, the use of 3 test line RDTs have proved to increase specificity of the test [7,9] and the presence of persisting HRP2 antigenemia even after treatment is known to result in false positives reducing the specificity of RDTs [5,9]. Zero or very low numbers of false positives and false negatives are important characteristics when selecting the most appropriate RDT for a particular country. In the POR phase with no endogenous malaria transmission occurring, the presence of HRP2 antigenemia within the population is limited (treated patients), resulting in fewer number of false positives seen.
RDTs claiming to provide ‘pan’-specific detection of malaria are expected to detect all known pathogenic species of Plasmodium [8]. The performance characteristics of the pan-specific pLDH in this RDT varied among different species. The highest sensitivity of 97.4 % was observed for P. vivax, which is a much higher sensitivity than previously reported [6]. For P falciparum, it was lower than acceptable sensitivity (68.67%). As discussed, this could be due to differences in the target antigen concentrations and the analytical sensitivity or limit of detection of the pLDH [24,26,27]. Furthermore, studies have shown that even at the same parasite density, the antigen concentrations can vary widely, and average concentrations of pLDH are higher in P. vivax than in P. falciparum [24]. In the WHO product testing of malaria RDTs, the RDTs are currently validated only against P. falciparum and P. vivax, with the sensitivity and specificity for other species being low [11]. Only a few P. ovale and P. malariae positive patients were present among the study participants, which limited the evaluation of RDT against those parasite species. However, as previously reported [6] the unacceptable low test positivity of pan-pLDH for P. ovale and P. malariae (which applies for all 3-band RDTs) was also observed in this study, with four out of seven false negatives being for those species. This limitation should be considered when using this RDT as a point of care test whenever microscopy facilities are not available.
Conclusions
CarestartTM pf/pan combo test performed well in the POR setting and showed good performance for detection of P. falciparum and P. vivax. The combination of HRP2 test line has significantly improved the performance of RDT in detecting P. falciparum. The low sensitivity of pan specific pLDH for P. falciparum, P. ovale and P. malariae should be considered when using this RDT as a point-of-care test whenever microscopy facilities are not available, especially in instances where HRP2 gene deletion is a possibility. It is recommended that RDTs are used in conjunction with microscopy and not as a substitute and that both tests are performed when malaria is highly suspected and when one test is negative.
Funding Statement
This work was supported by financial assistance from the National Science Foundation [Grant No: RG/2014/HS/03].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].World Health Organization World malaria report 2017. Geneva: World Health Organization; 2017. [Google Scholar]
- [2].World Health Organization Eliminating malaria. (reference number WHO/HTM/GMP/2016.3). Geneva: World Health Organization; 2016. [Google Scholar]
- [3].Dharmasiri AG, Perera AY, Harishchandra J, et al. First record of Anopheles stephensi in Sri Lanka: a potential challenge for prevention of malaria reintroduction. Malaria J. 2017;16:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].World Health Organization Guidelines for the treatment of malaria. 3rd ed. Geneva: World Health Organization; 2015. [Google Scholar]
- [5].World Health Organization WHO policy recommendation on malaria diagnostics in low transmission settings. (reference number WHO/HTM/GMP/MPAC.2014.4). Geneva: World Health Organization; 2014. [Google Scholar]
- [6].Maltha J, Gillet P, Bottieau E, et al. Evaluation of a rapid diagnostic test (CareStart™ Malaria HRP-2/pLDH (Pf/pan) Combo Test) for the diagnosis of malaria in a reference setting. Malaria J. 2010;9:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hawkes M, Conroy AL, Opoka RO, et al. Use of a three-band HRP2/pLDH combination rapid diagnostic test increases diagnostic specificity for falciparum malaria in Ugandan children. Malaria J. 2014;13:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].de Laval F, Oliver M, Rapp C, et al. The challenge of diagnosing Plasmodium ovale malaria in travelers: report of six clustered cases in French soldiers returning from West Africa. Malaria J. 2010;9:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fogg C, Twesigye R, Batwala V, et al. Assessment of three new parasite lactate dehydrogenase (pan-pLDH) tests for diagnosis of uncomplicated malaria. Trans Royal Soc Trop Med Hyg. 2008;102:25–31. [DOI] [PubMed] [Google Scholar]
- [10].Mosha JF, Sturrock HJ, Greenhouse B, et al. Epidemiology of subpatent Plasmodium falciparum infection: implications for detection of hotspots with imperfect diagnostics. Malaria J. 2013;12:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cunningham J, Gatton ML, Kolaxzinski K. Malaria rapid diagnostic test performance: Results of WHO product testing of malaria RDTs: Round 7 (2015–2016). Geneva: World Health Organization; 2017.
- [12].Gillet P, Scheirlinck A, Stokx J, et al. Prozone in malaria rapid diagnostics tests: how many cases are missed? Malaria J. 2011;10:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cheng Q, Gatton ML, Barnwell J, et al. Plasmodium falciparum parasites lacking histidine-rich protein 2 and 3: a review and recommendations for accurate reporting. Malaria J. 2014;13:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lee JH, Jang JW, Cho CH, et al. False-positive results for rapid diagnostic tests for malaria in patients with rheumatoid factor. J Clin Microbiology. 2014;52:3784–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. Radiology. 2015;277:826–832. [DOI] [PubMed] [Google Scholar]
- [16].World Health Organization Bench aids for the diagnosis of malaria infections. Geneva: World Health Organization; 2009. [Google Scholar]
- [17].Snounou G, Singh B.. Nested PCR analysis of Plasmodium parasites In: Malaria methods and protocols. Totowa, New Jersey: Humana Press; 2002. p. 189–203. [DOI] [PubMed] [Google Scholar]
- [18].Kyabayinze DJ, Zongo I, Cunningham J, et al. HRP2 and pLDH-based rapid diagnostic tests, expert microscopy, and PCR for detection of malaria infection during pregnancy and at delivery in areas of varied transmission: a prospective cohort study in Burkina Faso and Uganda. PloS One. 2016;11:e0156954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xiaodong S, Tambo E, Chun W, et al. Diagnostic performance of CareStart™ malaria HRP2/pLDH (Pf/pan) combo test versus standard microscopy on falciparum and vivax malaria between China-Myanmar endemic borders. Malaria J. 2013;12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Moges B, Amare B, Belyhun Y, et al. Comparison of CareStart™ HRP2/pLDH COMBO rapid malaria test with light microscopy in north-west Ethiopia. Malaria J. 2012;11:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Feleke DG, Tarko S, Hadush H. Performance comparison of CareStart™ HRP2/pLDH combo rapid malaria test with light microscopy in north-western Tigray, Ethiopia: a cross-sectional study. BMC Infect Dis. 2017;17:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Woyessa A, Deressa W, Ali A, et al. Evaluation of CareStart™ malaria Pf/Pv combo test for Plasmodium falciparum and Plasmodium vivax malaria diagnosis in Butajira area, south-central Ethiopia. Malaria J. 2013;12:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Feleke SM, Animut A, Belay M. Prevalence of malaria among acute febrile patients clinically suspected of having malaria in the Zeway Health Center, Ethiopia. Jpn J Infect Dis. 2015;68:55–59. [DOI] [PubMed] [Google Scholar]
- [24].World Health Organization Parasitological confirmation of malaria diagnosis: report of a WHO technical consultation. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- [25].World Health Organization Update on Plasmodium falciparum hrp2/3 gene deletions. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- [26].Gatton ML, Rees-Channer RR, Glenn J, et al. Pan-Plasmodium band sensitivity for Plasmodium falciparum detection in combination malaria rapid diagnostic tests and implications for clinical management. Malaria J. 2015;14:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jimenez A, Rees-Channer RR, Perera R, et al. Analytical sensitivity of current best-in-class malaria rapid diagnostic tests. Malaria J. 2017;16:128. [DOI] [PMC free article] [PubMed] [Google Scholar]


