Abstract
Spontaneous intracerebral hemorrhage (ICH) is one type of the most devastating cerebrovascular diseases worldwide, which causes high morbidity and mortality. However, efficient treatment is still lacking. Stem cell therapy has shown good neuroprotective and neurorestorative effect in ICH and is a promising treatment. In this study, our aim was to review the therapeutic effects, strategies, related mechanisms and safety issues of various types of stem cell for ICH treatment. Numerous studies had demonstrated the therapeutic effects of diverse stem cell types in ICH. The potential mechanisms include tissue repair and replacement, neurotrophy, promotion of neurogenesis and angiogenesis, anti-apoptosis, immunoregulation and anti-inflammation and so forth. The microenvironment of the central nervous system (CNS) can also influence the effects of stem cell therapy. The detailed therapeutic strategies for ICH treatment such as cell type, the number of cells, time window, and the routes of medication delivery, varied greatly among different studies and had not been determined. Moreover, the safety issues of stem cell therapy for ICH should not be ignored. Stem cell therapy showed good therapeutic effect in ICH, making it a promising treatment. However, safety should be carefully evaluated, and more clinical trials are required before stem cell therapy can be extensively applied to clinical use.
Keywords: intracerebral hemorrhage, stem cell therapy, neuroprotective effect, mechanism, safety
Introduction
Spontaneous intracerebral hemorrhage (ICH) is one type of the most devastating cerebrovascular disease worldwide, which accounts for 15% of all strokes1. ICH shows high morbidity and mortality. The incidence of ICH is about 0.1–0.2% in the general population and is even higher in elderly people, among which the mortality rate is extremely high, with a death rate of almost 30–50%. Survivors inevitably suffer from long-term and severe neurologic impairment despite multiple treatment approaches2. Based on the current data, the prognosis of ICH is extremely poor. There are various risk factors contributing to the onset of ICH, which include coagulation dysfunction, amyloidosis, vasculitis, drug abuse, and genetic factors3. However, the most important risk factor inducing ICH is hypertension, constituting about 60% of all ICH cases4.
The pathological mechanism of ICH comprises two parts: the primary and the secondary injuries. The first type is the occupying effect and the mechanical damage to adjacent brain tissue resulting from the hematoma. In the meantime, toxic effects of the blood and the decomposed products of blood cells such as enzymes, hemoglobin, and iron ions result in a more severe secondary injury. The secondary injury involves diverse molecular, cellular and biochemical responses induced by the primary injury; typically, inflammation, apoptosis, lipid peroxidation, free radical damage, and glutamate excitotoxicity, to name but a few5.
Nowadays, the available treatments for ICH include surgery, the control of intracranial hypertension and blood pressure, the alleviation of cerebral edema, supportive care, and rehabilitation. However, only limited effectiveness of intervention is currently demonstrated6. The novel alternatives or efficacious methods for treating ICH are in demand. Stem cell therapy, as a promising approach, has thus aroused considerable interest in researchers worldwide.
Stem cells (SCs) refer to a type of cell that have the potential to proliferate, self-renew, and differentiate into a variety of functional cells in a certain condition7. According to the developmental stages, SCs can be divided into two broad types: embryonic SCs (ESCs), which are isolated from the inner cell mass of blastocysts, and adult SCs (ASCs), also known as somatic SCs (SSCs), which can be found in various adult tissues, including neural SCs (NSCs), hematopoietic SCs (HSCs), mesenchymal SCs (MSCs), epidermal SCs, and so forth. According to the differentiative potential, the SCs can be divided into three categories: totipotent SCs, pluripotent SCs (PSCs) and unipotent SCs. The attempts to treat human diseases using SCs have been in existence for several decades. One of the most mature and general applications is the human SC transplantation therapy for multiple malignant or benign hematological diseases, which shows a great clinical value8. Furthermore, the treatment of neurological diseases by means of SC therapy, such as ischemic stroke, traumatic brain injury, and subarachnoid hemorrhage, had been developing rapidly in recent years and showed promising results9–11.
Currently, a growing number of studies have been conducted on SC treatment for ICH, not only in animal experiments, but also in clinical trials, which presented favorable curative effects, and potentials in saving the damaged brain tissue and promoting functional recovery. MSCs, NSCs, ESCs, HSCs and induced PSCs (iPSCs) are the most common types of SC in research and having application in ICH treatment.
The possible therapeutic mechanisms of SCs in ICH treatment involve multiple factors that have been studied for many years. One of the most important mechanisms is that SC transplantation repairs or replaces the destroyed nerve cells and tissues, including neurons and glial cells, which helps to ensure the integrity of nerve conduction pathways, thus rebuilding neurological functions12. Moreover, at the molecular level, incorporated SCs are capable of providing neurotrophic factors through paracrine signaling to develop neurotrophic effects. In addition, SCs can help to reduce ICH-induced secondary injuries including apoptosis, inflammation, and blood–brain barrier (BBB) destruction, and to promote angiogenesis and neurogenesis; therefore, the neuroprotection, together with the neurorestoration, is manifested in SC therapy13,14.
Despite the extensive studies of cell replacement in neurodegenerative disorders and ischemic stroke having been established already, studies on ICH have just come into being in the last decade. In this study, we aimed to provide a detailed review on the neuroprotective effects of SCs and the potential mechanisms for treating ICH, which is expected to benefit the application of SC therapy to ICH management in the near future.
Commonly Used Stem Cell Types for Intracerebral Hemorrhage Treatment
Mesenchymal Stem Cells
MSCs are a kind of PSC which originate from early developing mesoderm, having the potency to self-renew and differentiate into many cell types. MSCs were first found in bone marrow; however, they could also be isolated from adipose tissue15, umbilical cord blood16, peripheral blood17, and other tissues. In specific situations, MSCs can differentiate into adipose tissue, bone, cartilage, muscle, tendon, ligament, nerves, liver, myocardium, or endothelial cells, both in vivo and in vitro. Either cultured or cryopreserved MSCs have multidirectional differentiation potential that can be used as ideal seed cells for tissue and/or organ repair caused by aging or disease.
The most commonly applied MSCs in clinical practice are bone marrow (BM) MSCs, human umbilical cord (HUC) MSCs, and adipose-derived (AD) MSCs. BM-MSCs are frequently reported to have therapeutic effects on diverse diseases including stroke, possibly because on the one hand, they can be easily acquired from the host to avoid the transplant rejection18; on the other hand, it has been demonstrated by researchers that the transplanted BM-MSCs are able to pass through the BBB without disrupting the structure19. They have displayed the ability to migrate to the injured area, differentiating into neurons or neuron-like cells20–22, thus developing the effects of neurorestoration via secreting various neurotrophic factors23. There were lots of studies reporting that BM-MSCs could ameliorate neurological deficits and BBB dysfunction, as well as improve the recovery of neurological function in ICH rats24–30. Yang et al. reported administration of BM-MSCs overexpressing glial-cell-derived neurotrophic factor (GDNF) displays better neuroprotective effects in a rat model of ICH31. Cui et al. found that BM-MSCs transplantation could attenuate neurological deficits and promote axonal regeneration through increasing GAP-43 expression in ICH32. Moreover, Feng et al. demonstrated the beneficial effects of BM-MSCs in a primate model33.
Another kind of widely applied MSC is HUC-MSCs that have been used for the treatment of various neurological diseases including ICH in animal models and patients34–36. HUC-MSCs can be easily acquired in large quantities. Zhang et al. suggested the minimally invasive hematoma aspiration combined with HUC-MSC transplantation might be more effective in reducing neural damage and improving neural functions37.
ADMSCs are isolated from adipose tissue in recent years. Adipose tissue has several advantageous characteristics such as being accessible, abundant, and reliable for cell isolation for the purpose of regenerative applications with minimum damage to human body38. Studies showed that ADMSCs can stably proliferate and have low apoptotic rate in vitro, which makes them suitable for large-scale cultivation. In addition to the induced differentiation, ADMSCs can also differentiate to specific mature cells by co-culture with the mature somatic cell39. The most important application of ADMSCs is the repair of tissue defect and the tissue engineering. However, there is a lot of research focusing on the treatment effects of ADMSCs on diverse diseases including ICH. Chen et al. injected rat ADMSCs into the lateral cerebral ventricle of ICH rats and found them differentiating into neuron-like and astrocyte-like cells around the hematoma; simultaneously, the level of vascular endothelial growth factor (VEGF) and the score of neural function were both increased40. Yang et al. injected human ADMSCs into the femoral vein of ICH rat, which also showed significant functional improvement despite the divergence between two species and the different routes of drug administration41. Kim et al. also released the similar results. What’s more, ADMSC transplantation in the ICH model could alleviate long-term brain degeneration42. Above all, MSC transplantation may become a viable alternative for the treatment of ICH.
Embryonic Stem Cells
ESCs are a class of highly undifferentiated cells isolated from early embryos or the original gonads. They have the characteristics including limitless proliferation, self-renewal, and multidirectional differentiation in vitro. The ESCs were firstly isolated and reported by Evans and Kaufman in vivo43. Both in vitro and in vivo, ESCs can be induced to differentiate into almost all cell types including neurons and glial cells, which makes them one of the most promising SCs in treating central nervous system diseases44,45. Induced by all-trans retinoic acid (ATRA), some of ESCs will express neuron-specific antigens, while the others may have glial-specific antigens. Some neuron-like cells even showed enzyme activity involving acetylcholinesterase or glutamic acid decarboxylase46. A few studies have been established, focusing mainly on the treatment effects of ESC-derived neural cells on ICH. Nonaka et al. found that ATRA-treated ESCs injected intracerebroventricularly were transformed into neurons and glial cells around the hematoma cavity in the brain, producing neuroprotective and neurorestorative effects47. However, they could not enter the hematoma cavity to remedy the neuronal tissue defects. The related molecular mechanisms of cell migration and cytokine regulation are still unknown, and need further exploration. Leaving aside this incomprehension, the application of ESCs may come into conflict with the ethics, which will impose a limit on the clinical use.
Hematopoietic Stem Cells
HSCs, also known as hematopoietic PSCs (HPSCs), are a group of primitive hematopoietic cells derived from hematopoietic tissues including bone marrow, embryonic liver, peripheral blood, and umbilical cord blood. HSCs are one of the most important components of blood, serving as the initial cells of various blood cells48. Till et al. confirmed the existence of HSCs in the spleen of mice in 1961 for the first time49. HSCs appear in the yolk sac at the second week of development; after birth, the bone marrow becomes the main source of HSCs. The cell surface markers of human HSCs are CD34, together with CD133 and CD90, excluding CD3850. HSC transplantation plays an important role in the treatment of a variety of diseases, especially the hematological ones.
Recently, an increasing number of studies have focused on the therapeutic effect of HSCs on ICH. Sobrino et al. studied the level of circulating HSCs after ICH and found that the higher level of HSCs was associated with the better functional outcome at three months after ICH51. Previous studies have demonstrated that the granulocyte-colony stimulating factor (G-CSF) can mobilize HSCs to the ischemic lesion52 and the transplantation of HSCs could improve the outcome of stroke in a rat model53. Based on the above research, England et al. used G-CSF to mobilize HSCs into the circulation and found they could promote functional recovery from not only ischemic stroke but also ICH54. They further discovered that the labeled HSCs gathered on the brain injury site to exert neurorestoration effects, which was consistent with the animal experiment55. With the convenient acquisition of cells in large numbers, HSCs may as well be broadly used in clinical practice.
Neural Stem Cells
NSCs are a class of SCs that present in the nervous system with the ability to split and self-renew and the potential to differentiate into neurons or glial cells56. The concept was first proposed by Reynolds et al. in 1992. He isolated a group of cells from the striatum of adult mouse, which showed the ability to proliferate in vitro and the potency of multiple differentiation57. According to the differentiation potential, NSCs can be additionally divided into neuroectodermal cells (neural tube epithelium), neuroblasts (primitive nerve cells) and neural precursors. According to the location, NSCs can be divided into neural crest SCs and CNS SCs58.
Some studies have demonstrated that NSC transplantation can promote functional recovery in ICH rats59–61. Transplantation of genetically modified NSCs with some genes overexpressed can even enhance their function of ameliorating the ICH62–66. NSCs were mainly acquired from the embryo or the fetus, which was called exogenous NSCs. For a long time, it had been generally acknowledged that no NSC existed in the adult nervous systems. However, an increasing number of studies have refuted this acknowledgement and further confirmed the fact that NSCs actually exist in the subventricular zone (SVZ) and subgranular zone of the dentate gyrus, which are then named endogenous NSCs67. The endogenous NSCs are silent under normal circumstances; however, they can be activated in many pathological conditions, migrate to the injury sites to contribute to neurorestoration. It was reported that endogenous NSCs were activated in the brain of experimental ICH rat and helped the neurons to achieve self-repair68. Yu et al. found the up-regulation of hypoxia-inducible factor-1 alpha (HIF-1α) gene could promote the proliferation, migration, and differentiation of endogenous NSCs, thus contributing to neurofunctional recovery from ICH69. Even physical exercise was noted to be able to enhance the survival and migration of NSCs after ICH70. All of the above implied the neuroplasticity of NSCs, which may be utilized to facilitate the regeneration of NSCs. The NSCs possess low immunogenicity and high histocompatibility with brain tissue, which makes them an available alternative to treating ICH.
Induced Pluripotent Stem Cells
The iPSCs were first found by two Japanese scholars in 2006. They transferred four transcription factors (Oct4, Sox2, Klf4, and c-Myc) into differentiated somatic cells using viral vectors, so that they could be reprogrammed into a cell type similar to ESCs. By being introduced with exogenous genes, somatic cells can dedifferentiate into PSCs, which are called iPSCs71. In September 2014, a Japanese patient with polypoidal choroidal vasculopathy was the first patient in the world to be transplanted with autologous iPSCs72.
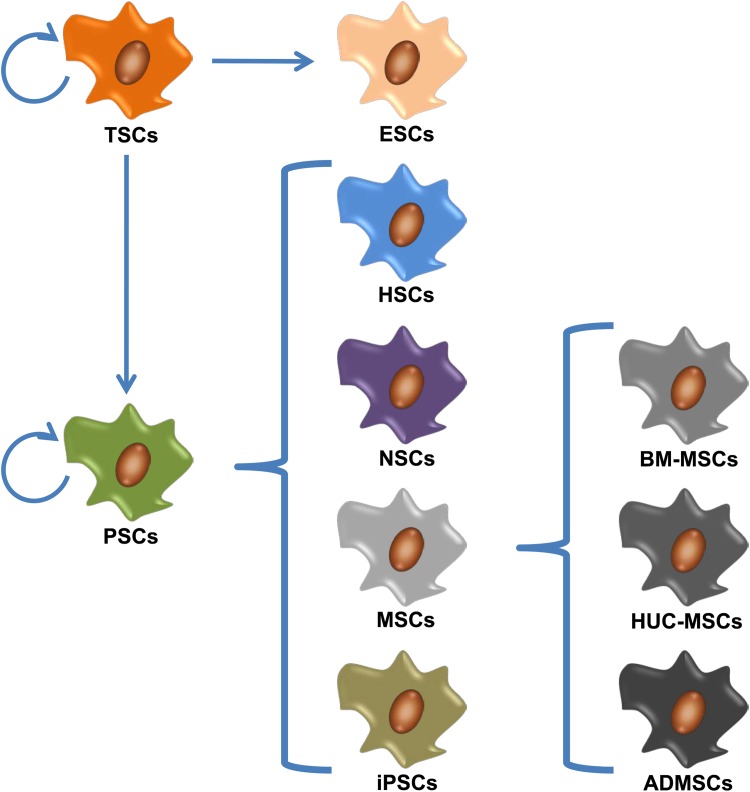
However, iPSC treatment of ICH remains at the experimental stage. Three consecutive reports from Qin et al. discussed the treatment effects of iPSCs for ICH rats. They observed good therapeutic effects of iPSCs in rat ICH model73–75. The iPSCs could differentiate into neuroepithelium-like/neuroepithelioid SCs and neural cells, which could also secrete neurotrophic factors. Functional improvement may be partially due to neuronal supplementation, anti-inflammation and neurotrophic factors. The iPSC technology is a major breakthrough in the field of SC research. It avoids the ethical issues and solves the problem of immune rejection in SC transplantation, which helps to make it much closer to clinical application. However, the problem of low efficiency of reprogramming still needs to be overcome before the extensive applications. The commonly used SC types for ICH treatment were summarized in Fig. 1 and Table 1.
Fig. 1.
Commonly used stem cell types for intracerebral hemorrhage (ICH) treatment.
Embryonic stem cells (ESCs) are a kind of totipotent stem cell (TSC) for treating ICH.
Mesenchymal stem cells (MSCs), neural stem cells (NSCs), induced pluripotent stem cells (iPSCs) and hematopoietic stem cells (HSCs) are the most common types of pluripotent stem cell (PSC) for treating ICH. MSCs include bone marrow mesenchymal stem cells (BM-MSCs), human umbilical cord-mesenchymal stem cells (HUC-MSCs) and adipose-derived mesenchymal stem cells (ADMSCs).
Table 1.
Summary of studies concerning transplantation of commonly used stem cell types for ICH treatment.
| Stem cells types | References | Animal/human studies | Sample size | Routes of administration | Time of administration (post ICH) | Number of cells | Efficacy (behavioral recovery) | Earliest effective time (post ICH) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| TSCs | ESCs | Nonaka et al.47 | Rat | Sham: 5; treatment: 10 | Intraventricular | 7 days | 105 | NA | NA | |
| PSCs | MSCs | BM-MSCs | Cui et al.32 | Rat | Control: 15; treatment: 15 | Intravenous | 1 and 24 hours | 5 × 106 | Decreased the NSS scores | 3 days |
| Chang et al.36 | Human | Control: 8; treatment: 7 | Intra-cavity | 2 and 3 weeks | 1.8 × 108 | Decreased the NIHSS, mRS scores and increased the mBI scores | 3 months | |||
| Wang et al.24 | Rat | Sham: 12; control: 24; treatment: 24 | Intravenous | NA | 1 × 106 | Decreased the mNSS and MLPT scores | 14 days | |||
| Sun et al.82 | Mouse | NA | Intranasal | 3 and 7 days | 1 × 106 | Decreased the mNSS scores; improvement in the adhesive removal, rotarod and open field tests | 14 days | |||
| Zhu et al.119 | Human | Control: 96; treatment: 110 | Intracerebral, intrathecal | 5.5 days and 4 weeks | NA | Decreased the NIHSS scores and Rankin Scale; increased the Barthel scores | 6 months | |||
| Chen et al.25 | Rat | NA | Intravenous | 2 hours | 5 × 106 | Decreased the mNSS scores | 3 days | |||
| Vaquero et al.78 | Rat | Control: 20; treatment: 20 | Intracerebral | 2 months | 5 × 106 | Improved in rotarod test and VTB tests | 4 months | |||
| Bao et al.93 | Rat | Control: 67; treatment: 65 | Intracerebral | 24 hours | 2 × 105 | Decreased the mNSS scores | 3 days | |||
| Liang et al.27 | Rat | Sham: 8; control: 16; treatment:16 | Intracerebral | 24 hours | 1 × 106 | Decreased the MLPT scores and improved in the vibrissae-elicited forelimb-placing test | 7 days | |||
| Wang et al.95 | Rat | Control: 6; treatment:6 | Intravenous | 1 hour | 1 × 106 | Decreased the mNSS scores | 7 days | |||
| Otero et al.90 | Rat | Control: 48; treatment: 48 | Intracerebral | 2 hours | 2 × 106 | NA | NA | |||
| Yang et al.31 | Rat | Control: 10; treatment:20 | Intracerebral | 3 days | 5 × 105 | Decreased the mNSS scores | 7 days | |||
| Bhasin et al.120–122 | Human | Control: 1; treatment:2 | Intravenous | 9.6 months | 5–6 × 107 | Decreased the Ashworth Tone Grade Scale scores; increased the mBI scores, FM scores and MRC grade, but no statistical difference | 11.6 months | |||
| Otero et al.87 | Rat | Control: 10; treatment: 10 | Intracerebral | 2 months | 5 × 106 | Decreased the mNSS scores; improved in the rotarod and VTB tests, and locomotor activity | 3 months | |||
| Feng et al.33 | Monkey | Control: 8; treatment:16 | Intracerebral | 1 week or 4 weeks | 1–5 × 106 | Decreased the neurologic deficit scores | 2 or 5 weeks | |||
| Otero et al.26 | Rat | Control: 10; treatment:10 | Intracerebral | 3 days | 2 × 106 | Decreased the mNSS scores and improved in the rotarod test | 3 weeks | |||
| Seyfried et al.30 | Rat | Control: 9; treatment:18 | Intravenous | 24 hours | 0.5–1 × 106 | Decreased the mNSS scores and improved in corner-turn test | 7 days | |||
| Seyfried et al.29 | Rat | Control: 18; treatment :18 | intra-arterial | 24 hours | 1 × 106 | Decreased the mNSS scores and improved in corner-turn test | 7 days | |||
| Nagai et al.22 | Mouse | Control: 4; treatment: 14 | Intracerebral | 7 days | 2 × 105 | Improved in rotarod test | 8 days | |||
| Seyfried et al.28 | Rat | Control: 27; treatment: 27 | Intracerebral | 24 hours | 3, 5 and 8 × 106 | Decreased mNSS scores and improvement in corner-turn test | 7 days | |||
| Zhang et al.80 | Rat | Blank: 5; sham: 20; control: 20; treatment: 60 | Intra-arterial, intravenous and intraventricular | 1, 3, 5 and 7 days | 2 × 106 | Improvement in the beam-walking test (except the intravenous group) | 24 hours | |||
| HUC-MSCs | Xie et al.34 | Rat | Sham: 10; control: 16; treatment: 36 | Intracerebral, intravenous | NA | 2 × 105 (IC), 2 × 106 (IV) | Decreased mNSS scores | 7 days | ||
| Chang et al.36 | Human | Control: 8; treatment: 9 | Intra-cavity | 2 and 3 weeks | 1.8 × 108 | Decreased NIHSS, mRS scores and increased mBI scores | 3 months | |||
| Zhang et al.37 | Rat | Control: 60; treatment: 60 | Intracerebral | 6 hours | 1 × 105 | Decreased mNSS scores | 24 hours | |||
| Nan et al.35 | Rat | NA | Intravenous | 24 hours | 2.4–3.2 × 106 | Improvement in limb-placement, stepping and body-swing tests | 7 days | |||
| ADMSCs | Chen et al.40 | Rat | Control: 40; treatment: 40 | Intraventricular | 2 days | 2–4 × 105 | Improvement in Zea Longa 5-grade scale | 3 days | ||
| Yang et al.41 | Rat | Control: 6; treatment: 9 | Intravenous | 24 hours | 1 × 106 | Decreased mNSS scores | 7 days | |||
| Kim et al.42 | Rat | Control: 16; treatment: 16 | Intravenous | 24 hours | 3 × 106 | Decreased MLPT scores | 28 days | |||
| HSCs | NA* | |||||||||
| NSCs | Gao et al.98 | Rat | Sham: 20; control: 48; treatment: 26 | Intracerebral | 3 hours | 5 × 105 | Decrease the mNSS scores | 3 days | ||
| Wakai et al.62 | Mouse | NA | Intracerebral | 3 days | 1 × 105 | Improve in cylinder and corner-turn tests | 21 days | |||
| Xue et al.123 | Human | Control: 20; treatment: 20 | Intrathecal | 1 week, twice per week, a total of four times | 4 × 108 | Decrease the NIHSS scores | 2 weeks | |||
| Wang et al.61 | Rat | Control: 26; treatment: 13 | Intracerebral | 3 days | 1 × 106 | Decrease the MLPT scores | 24 days | |||
| Lee et al.63 | Mouse | Control: 28; treatment: 61 | Intracerebral | 7 days | 2 × 105 | Improve in rotarod test and decrease the MLPT scores | 8 days | |||
| Lee et al.65 | Mouse | Control: 20; treatment: 18 | Intracerebral | 7 days | 2 × 105 | Improve in rotarod test and decrease the MLPT scores | 5 weeks | |||
| Lee et al.64 | Mouse | Control: 18; treatment: 39 | Intracerebral | 7 days | 2 × 105 | Improve in rotarod test and decrease the MLPT scores | 8 days | |||
| Lee et al.99 | Rat | Sham: 30; control: 30; treatment: 120 | Intravenous, intracerebral | 2 or 24 hours | 5 × 106 (IV), 1 × 106 (IC) | Decrease the MLPT scores | 24 hours | |||
| Lee et al.60 | Mouse | Control: 30; treatment: 31 | intracerebral | 7 days | 2 × 105 | Improve in rotarod test and decrease the MLPT scores | 21 days | |||
| Lee et al.66 | Mouse | Control: 20; treatment: 50 | intracerebral | 7 days | 2 × 105 | Improve in rotarod test and decrease the MLPT scores | 8 days | |||
| Li et al.88 | Rat | Control: 8; treatment: 40 | intra-arterial | 2, 7, 14, 21 or 28 days | 4 × 106 | Improve in rotarod,beam walking,limb placing and spontaneous cycling tests | 28 days | |||
| An et al.110 | Rat | NA | Intracerebral | 3 days | 3 × 106 | NA | NA | |||
| Jeong et al.59 | Rat | Control: 13; treatment: 12 | Intravenous | 24 hours | 5 × 106 | Improve in rotarod test and decrease the MLPT scores | 14 days | |||
| iPSCs | Qin et al.75 | Rat | Sham: 30; control: 63; treatment: 59 | Intracerebral | 6 hours | 1 × 106 | Decrease the MLPT scores | 14 days | ||
| Qin et al.73 | Rat | NA | Intracerebral | 24 hours | 2 × 106 | Decrease the mNSS and MLPT scores | 14 days | |||
| Qin et al.74 | Rat | Sham: 12; control: 24; treatment: 12 | Intracerebral | 24 hours | 1 × 106 | Decrease the mNSS and MLPT scores | 14 days | |||
* There are no reports on directly-administered HSCs for ICH treatment.
ICH: intracerebral hemorrhage; TSCs: totipotent stem cells; ESCs: embryonic stem cells; PSCs: pluripotent stem cells; MSCs: mesenchymal stem cells; NSCs: neural stem cells; iPSCs: induced pluripotent stem cells; HSCs: hematopoietic stem cells; BM-MSCs: bone marrow mesenchymal stem cells; HUC-MSCs: human umbilical cord-mesenchymal stem cells; ADMSCs: adipose-derived mesenchymal stem cells; IV: intravenous; IC: intracerebral; NA: not available; NSS: Neurological Severity Score; mNSS: modified NSS; NIHSS: National Institutes of Health Stroke Scale; mRS: modified Rankin Scale (mRS); mBI: modified Barthel; MLPT: modified limb-placing test; VTB: video-tracking box; FM: Fugl Meyer; MRC: Medical Research Council.
The Therapeutic Strategies Involving Stem Cells
The Therapeutic Modalities
SC transplantation mainly has two methods. One is the transplantation of proper SCs directly into the body, in which the internal environment and specific signal molecules will guide these SCs towards differentiation into the desired mature cells, thus exerting the necessary functions. The other method is to isolate, cultivate, purify and amplify a certain kind of SC, and induce them to differentiate into the cells with desired functions in vitro so that these mature cells can be implanted into the human body for treatment. The appropriate combination of both the techniques may produce the best effects to patients.
The Routes of Stem Cell Administration
The routes of SC delivery vary greatly in research, and comprise the intracerebral, intraventricular, intranasal, intravenous, intrathecal, intra-subarachnoid space, intra-arterial, and intraperitoneal manners76.
Intraventricular/intra-subarachnoid/intrathecal transplantation provides an effective access for the SCs to pass through the BBB, making it possible for the implanted cells to migrate to the injured brain tissue via the cerebrospinal fluid (CSF) flow. However, entrance into the hematoma cavity for the SCs to fill the large deficit is extremely difficult to make. The mechanisms involved in the migration of transplanted cells are still unknown47. Intracerebrally delivered SCs may form cell clusters, impeding their further migration towards sites of the damaged brain, which compromises the effects and limits the application77. Vaquero et al. reported that the group receiving intracerebral transplantation of BM-MSCs embedded in a platelet-rich plasma scaffold afforded better functional improvement compared with the group receiving BM-MSCs in saline78. A cell transplantation pump can also be implanted into the brain to provide SCs continuously, though it has not been put into practice. However, these delivery routes will cause additional damages to the brain tissues of the patients.
Intravenous delivery of cells overcomes the above disadvantages and can afford the transfer of a large number of SCs. The MSCs in the treatment for ICH rats can probably migrate to the areas of the injured brain after the cells are infused into the femoral or tail vein of the rats28,41. In addition, G-CSF treatment can mobilize HSCs into the circulation and thus functioning through an intravenous route. However, the ability to pass through the biological barriers or the feasibility of delivering an adequate number of cells to the areas of the injured brain by this route is a controversial issue79. Zhang et al. reported that there was no improvement shown in the ICH rats that received intravenous injection of BM-MSCs80. The other type of vascular delivery is the intra-arterial route, where cells can be transferred direct to the injury site, and is more effective than the intravenous route81. However, occlusion and thrombosis are the biggest problems that affect the applications of these routes.
Another alternative route is via intranasal administration, which will not cause secondary injury. Sun et al. reported that intranasally administered hypoxia-preconditioned BM-MSCs could be detected in ICH brain tissues82 as early as 1 h after intranasal administration83. The anatomic characteristics might play an important role in the migration of SCs from olfactory epithelium to the damaged brain. However, the exact mechanisms have not been clarified84. In summary, all these routes have been shown as relatively safe with no major complication. The optimal route has not been confirmed. Many aspects, such as the type and number of SCs, the characteristics of the patients, and so forth should be taken into account, and more effective and safe routes need to be investigated.
The Number of Stem Cells Used for Treatment
Similar to the route of SC delivery, the amount of SCs for the treatment of ICH is also indeterminate, which depends on multiple aspects, including the type of SCs, the route of administration, and so forth. Most studies used the million-scale of cells for the treatment of ICH, which showed acceptable results85. It is self-evident that insufficient transplanted cells do not exert therapeutic effects, whereas an excess of cells may cause multiple complications including occlusion, thrombosis, and even the increased risk of tumor formation. The most appropriate amount of SCs for treating ICH still needs investigation before clinical use.
The Time Window of Stem Cell Therapy
Regarding the time window of SC therapy for ICH, different studies gave controversial results. On the one hand, most studies supported early treatment. Early delivery of SCs, especially in the first week can effectively reduce the secondary injury after ICH, including inflammation and apoptosis, which contributes to the functional recovery because the secondary injury most often occurs during the first week post ICH86. Furthermore, Zhang et al. discovered that MSC transplantation on the third day post ICH showed a better therapeutic effect compared with that on the first, fifth and seventh day post ICH80. On the other hand, some studies suggested delayed treatment would be beneficial for ICH. A secondary injury may be so severe during the first week post ICH that the transplanted SCs may be injured and even die. After the acute stage of ICH, SCs can replace the damaged tissues and rebuild neuronal function. Vaquero et al. found that intracerebral transplantation of BM-MSCs embedded in a platelet-rich plasma scaffold 2 months after ICH could also improve neurological function78. Otero et al. had similar findings87. In addition, the time of transplantation may also determine the fate of cell differentiation. Delivered SCs are prone to differentiate into astrocytes during the acute stage of ICH whereas neurons do so later on85. Li et al. found that the therapeutic effects of NSCs depended on the time of transplantation. Animals treated at 7 and 14 days after ICH had a higher percentage of neurons differentiated from NSCs and displayed the most significant functional recovery88.
In fact, different cell types with different routes of delivery can affect how the SCs reach the target and work, which will lead to different conclusions, thus there is no comparability with regard to these studies. More specific experiments focusing on the time window of SC delivery for ICH are needed.
Immunosuppressive Therapy
It is still uncertain whether the immunosuppressive therapy necessitates being accompanied by SC therapy. Most studies did not use immunosuppressive therapy after SC therapy, which showed no reject reaction. Only two studies used cyclosporine after allograft SC transplantation for ICH treatment35,47. Generally speaking, immunosuppressive therapy is unnecessary for autologous SC transplantation. No study confirmed the necessity of immunosuppressive therapy for allograft SC transplantation, which needs further exploration.
The Neuroprotective Mechanisms of Stem Cells for Intracerebral Hemorrhage
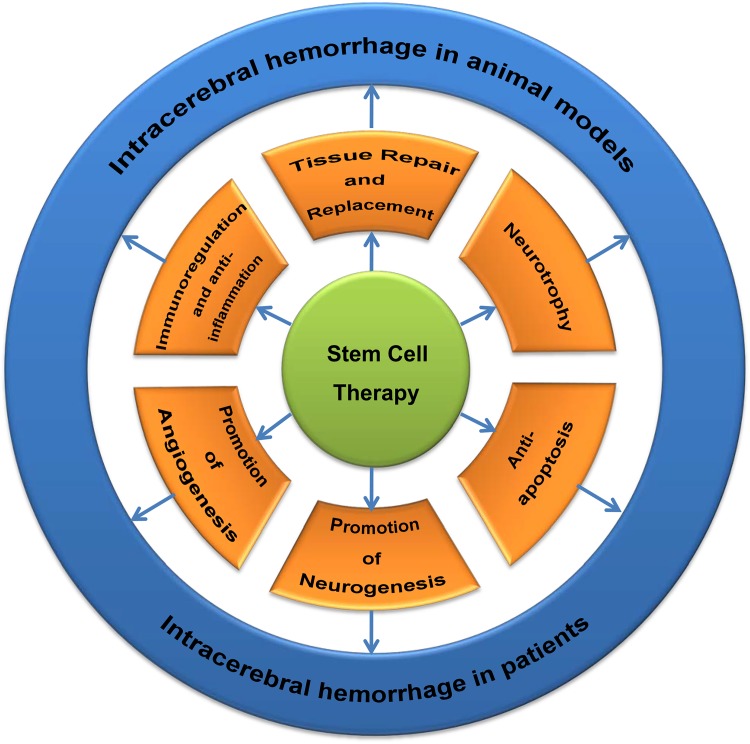
The neuroprotective mechanisms of SCs in ICH involve tissue repair and replacement, neurotrophy, promotion of neurogenesis and angiogenesis, anti-apoptosis, immunoregulation and anti-inflammation and so forth (Fig. 2).
Fig. 2.
The mechanisms of stem cell therapy for intracerebral hemorrhage (ICH) both in animal models and patients.
The neuroprotective mechanisms of stem cells in ICH involve in tissue repair and replacement, neurotrophy, anti-apoptosis, anti-inflammation, promotion of angiogenesis and neurogenesis.
Tissue Repair and Replacement
As mentioned above, under the influence of the internal environment and multiple nerve growth factors, many types of transplanted SCs can differentiate into the two most common functional cells after ICH, neurons and glial cells; they can migrate to the injury area to replace the damaged tissues and rebuild nerve conduction pathways. The differentiation directions are related to the time-point of transplantation. However, there are some studies finding the opposite. They found transplanted human amniotic MSCs could just survive at the perihematomal areas but did not differentiate into neurons or astrocytes13. These findings suggested two facts: one is that different types of SCs may have diverse capacities of differentiation after being transplanted into the ICH brain; the other is that the beneficial effects must be mediated through other mechanisms, which requires extensive investigation.
Neurotrophic Effects and Promotion of Neurogenesis
Neurogenesis plays an important role in brain injury repair and functional recovery after ICH, which would be beneficial for ICH treatment89. Studies showed that BM-MSC transplantation increased the amount of BrdU+, DCX+ and BrdU+/DCX+ cells in the SVZ and perihematomal areas, which suggested enhanced neurogenesis82. The reason may be explained by the production of neurotrophic factors. MSCs can secrete various cytokines such as brain-derived neurotrophic factor, GDNF, nerve growth factor, hepatocyte growth factor and G-CSF, which have the ability to promote neurogenesis34,90. Furthermore, MSCs can evoke the plasticity of damaged neurons and activate astrocytes to secrete neurotrophic factors91. The up-regulation of neurotrophic factors can also provide a favorable microenvironment for the survival of neuronal cells and the inhibition of cell death.
Promotion of Angiogenesis
The neurological function recovery lies on not only the neuronal cells, but also the microenvironment around them, which includes vascularity and extracellular matrix (ECM). Angiogenesis refers to the new vessel formation, which can help to repair tissues. Angiogenesis occurs during many brain insults including ICH, which becomes a critical therapeutic target92. Transplanted MSCs can secrete VEGF to promote vascular stabilization as well as decrease VEGF-induced BBB breakdown after ICH. Another mechanism suggested is that MSCs merge into the cerebral vasculature and significantly increase vascular density in the perihematomal areas93. The ECM works as the supporting component of neuronal tissues. The ECM component, including fibronectin, which is derived from MSCs, and the cell adhesion molecules such as integrin, cadherin, and selectin, can all promote neurorestoration and axonal regeneration18. Above all, various trophic factors and molecular components participate in the reconstruction of neurovascular unit, which promote functional improvement in combination.
Anti-Apoptosis Effects
Another widely studied mechanism of SC therapy for ICH is the anti-apoptosis. Apoptosis is involved in almost all neurological diseases including ICH, which is responsible for neurological functional recovery as well. Apoptotic cells are often detected by the techniques such as terminal deoxynucleotidyl transferase-mediated 2’-deoxyuridine 5’-triphosphate (dUTP) nick-end labeling staining (TUNEL), flow cytometry, immunohistochemistry and electron microscope. Yang et al. confirmed that TUNEL-positive cells were reduced after intravenous injection of human umbilical-tissue-derived cells, which was consistent with the improvement of motor function94. The changes of the expression of apoptosis-related proteins can also influence the apoptosis. Western blotting showed that the pro-apoptotic proteins such as p53, caspase-9, caspase-3, and Bax greatly decreased, while anti-apoptotic proteins such as Bcl-2 and the signaling molecules of cell survival like Akt1 and ERK-MAPK significantly increased in the ICH brains receiving the transplantation of SC compared with those without SC transplantation, which explained the functional recovery in ICH mice64,95.
Immunoregulative and Anti-Inflammatory Effects
At last, SCs also exert immunoregulative and anti-inflammatory effects on ICH. MSCs could develop immunomodulatory features in vitro, which was shown in animal model of cerebral ischemia96. In ischemic brain, MSCs reduced the numbers of Iba-1+ and ED1+ inflammatory cells97. In ICH animal models, transplanted neural SCs increased the regulatory T cells in the brain and peripheral blood but decreased the gamma–delta (γδ)T cells. Accordingly, the anti-inflammatory cytokines such as interleukin 4 (IL-4), IL-10, and transforming growth factor beta (TGF-β) were increased, and the pro-inflammatory cytokines such as IL-6 and interferon gamma (IFN-γ) were decreased98. Bao et al. transplanted Flk-1+ BM-MSCs into ICH brain, which resulted in the decreased activation of inflammatory cells in the perihematomal areas, as well as the reduction of inflammatory factors such as IL-1β, IL-2, IL-4, IL-6, and tumor necrosis factor alpha (TNF-α)93. Lee et al. reported early intravenous NSC injection could reduce inflammatory infiltrations in hemorrhagic stroke99. All of the above suggested SCs exert significant immunoregulative and anti-inflammatory effects for ICH treatment.
The Influence of the Central Nervous System Microenvironment on the Stem Cell Therapy
The microenvironment of the CNS plays an important role in the tissue repair. The neurovascular unit (NVU) refers to a complicated system that comprises neurons, glial cells, blood vessels and ECM which form the microenvironment of the CNS and is closely inter-related to the maintenance of its homeostasis100. The NVU functions to regulate the blood flow and the metabolism, modulate the exchange of substances across the BBB, provide trophic support and repair the injured neurons; it also contributes to the immune surveillance100. The brain tissue damage caused by ICH is thought to involve all cell and matrix components of the brain. The transplanted SCs seated in the microenvironment may receive multiple complex signals from the components of NVU. Damaged tissue and microenvironment can affect the survival, migration and differentiation of the transplanted SCs, thus influencing the effectiveness of SC therapy101,102.
ICH can cause the release of a variety of bioactive substances such as thrombin, erythrocyte lysate, excitatory amino acid, free radicals and nitric oxide due to ischemia and hypoxia, as well as the coagulation, dissolution and absorption of the hematoma103, which consequently leads to inactivation or even death of the endogenous or exogenous SCs. However, ischemia and hypoxia can induce both angiogenesis and the increase of related cytokines and their receptors in the brain, such as VEGF and basic fibroblast growth factor, which promote the angiogenesis104. New blood vessels can eliminate necrotic cell debris and toxic substances, as well as transport neurotrophic factors that induce SC proliferation, differentiation, migration and the neuronal regeneration105.
Glial cells are also important components of the NVU, which provide structural support for neurons, control neuronal activity through synapse formation, and may participate in the formation of local capillaries. Once activated, astrocytes can form protuberances and release a series of neurotrophic factors and growth factors to integrate the transplanted SCs and the host cells, thus exerting repairing effects106. Activated and proliferated microglia can help to remove the extracellular oxidative proteins and devour cell fragments, which provide a favorable microenvironment for the SCs to promote tissue repair107. Oligodendroglia forms myelin sheath in the CNS. Injured oligodendroglia is able to express myelin-associated inhibitor factor, which will inhibit the neuroregenerating effects of SCs108.
ECM is another important component of NVU, which is derived from both host cells and transplanted SCs and forms the supporting structure in the neuronal tissues. ECM in the microenvironment suck as fibronectin, integrin, cadherin and selectin can guide the proliferation, differentiation and migration of transplanted SCs and promote neurorestoration of SCs18. After ICH, the content of specific cytokines such as brain-derived growth factor, platelet-derived growth factor, insulin-like growth factor in the brain are significantly increased, which can improve the survival of the transplanted SCs. The combined application of these cytokines will greatly improve the therapeutic effect of SC transplantation.
Moreover, the diversity of the microenvironment could determine the multiple differentiations of the SCs109,110. As described before, the time of transplantation also determines the fate of cell differentiation, which may also be due to the different microenvironments.
Conversely, exogenous SCs may also alter the local environment to be more conducive to neuronal regeneration101. Otero-Ortega et al. reported that the exosomes derived from MSCs might work as paracrine effectors which were responsible for promoting neurovascular remodeling and functional recovery in an animal model of ICH111. Suda et al. found that autologous bone-marrow-derived mononuclear cells (MNCs) could protect the integrity of the NVU and alleviate inflammation and neurological deficits after ICH112.
The Safety of Stem Cell Therapy
As previously described, a growing number of experimental and clinical studies have suggested good curative effects of SC treatment for ICH. However, the safety and reliability of SC therapy should not be neglected. The administration of SCs may cause severe adverse reactions and/or complications, which impose the limitation to its applications. Some studies reported excess SCs and/or excessive infusion rate might cause occlusion of the blood vessels by thrombosis or embolism113,114. A few studies deemed that transplanted SCs could lead to some level of immune rejection and thus suggested concurrent immunosuppressive therapy35,47,115.
The most severe side effect of SC transplantation is the tumorigenicity and instability75. It had been reported that having NSCs directly implanted into adult rodents could form cell clusters in their brains47. Miura et al. found that murine BM-MSCs could spontaneously transform into malignant cells and form fibrosarcoma in vivo. The possible mechanisms may lie with the cumulative chromosomal abnormalities, gradual increase in telomerase activity, and the elevated expression of c-Myc116.
In addition, the SC transplantation can cause other side effects such as seizure, infection, hyperpyrexia and even death75,117. Nakamura et al. reported a rare case where the transplanted MSCs after ICH caused the formation of arteriovenous malformation a few years later118. The safety issues associated with SC therapy should be carefully evaluated and investigated thoroughly. Reducing the adverse effects may be more important than increasing the efficacy of SC treatment.
Conclusions and Perspective
Above all, SC therapy shows good therapeutic effects on ICH, making it a promising treatment for ICH. However, it is still in its infancy and there are some studies challenging this conclusion. Issues of concern include ethical conflicts, therapeutic effects, adverse reaction, complications, immune rejection, cell purification and the most important problem, tumorigenicity, which brings huge safety risks47. The detailed therapeutic strategies and mechanisms, together with the challenge of controlling its proliferation and differentiation properly are still undetermined and need further exploration. In addition, most studies have been conducted in animal models due to lack of clinical studies. A small number of clinical trials on SC transplantation in ICH patients have been conducted that give us optimistic results36,119–124. However, before application in clinical practice, SC therapy for ICH needs more animal experiments to assess the abovementioned challenges. Large-scaled and multicenter clinical trials are also required. It is believed that with the continuous evolution of SC technology, SC therapy will certainly achieve a great breakthrough for the clinical treatment of ICH.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Science Foundation of China (No. 81601003).
References
- 1. Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344(19):1450–1460. [DOI] [PubMed] [Google Scholar]
- 2. Inagawa T. What are the actual incidence and mortality rates of intracerebral hemorrhage? Neurosurgical Rev. 2002;25(4):237–246. [DOI] [PubMed] [Google Scholar]
- 3. Ariesen MJ, Claus SP, Rinkel GJ, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003;34(8):2060–2065. [DOI] [PubMed] [Google Scholar]
- 4. Brott T, Thalinger K, Hertzberg V. Hypertension as a risk factor for spontaneous intracerebral hemorrhage. Stroke. 1986;17(6):1078–1083. [DOI] [PubMed] [Google Scholar]
- 5. Zheng H, Chen C, Zhang J, Hu Z. Mechanism and therapy of brain edema after intracerebral hemorrhage. Cerebrovasc Dis. 2016;42(3-4):155–169. [DOI] [PubMed] [Google Scholar]
- 6. Morgenstern LB, Hemphill JC III, Anderson C, Becker K, Broderick JP, Connolly ES., Jr Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annual review of cell and developmental biology. 2001;17:387–403. [DOI] [PubMed] [Google Scholar]
- 8. Blommestein HM, Verelst SG, Huijgens PC, Blijlevens NM, Cornelissen JJ, Uyl-de Groot CA. Real-world costs of autologous and allogeneic stem cell transplantations for haematological diseases: a multicentre study. Annals of hematology. 2012;91(12):1945–1952. [DOI] [PubMed] [Google Scholar]
- 9. Reis C, Wilkinson M, Reis H, Akyol O, Gospodarev V, Araujo C. A look into stem cell therapy: exploring the options for treatment of ischemic stroke. Stem cells Int. 2017;2017:3267352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reis C, Gospodarev V, Reis H, Wilkinson M, Gaio J, Araujo C, Chen S, Zhang JH. Traumatic brain injury and stem cell: pathophysiology and update on recent treatment modalities. Stem Cells Int. 2017;2017:6392592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khalili MA, Sadeghian-Nodoushan F, Fesahat F, Mir-Esmaeili SM, Anvari M, Hekmati-Moghadam SH. Mesenchymal stem cells improved the ultrastructural morphology of cerebral tissues after subarachnoid hemorrhage in rats. Exp Neurobiol. 2014;23(1):77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu W, Zheng J, Gao L, Li T, Zhang J, Shao A. Neuroprotective Effects of Stem Cells in Ischemic Stroke. Stem Cells Int. 2017;2017:4653936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou H, Zhang H, Yan Z, Xu R. Transplantation of human amniotic mesenchymal stem cells promotes neurological recovery in an intracerebral hemorrhage rat model. Biochem Biophys Res Commun. 2016;475(2):202–208. [DOI] [PubMed] [Google Scholar]
- 14. Liao W, Zhong J, Yu J, Xie J, Liu Y, Du L, Yang S, Liu P, Xu J, Wang J, Han Z, Han ZC. Therapeutic benefit of human umbilical cord derived mesenchymal stromal cells in intracerebral hemorrhage rat: implications of anti-inflammation and angiogenesis. Cell Physiol Biochem. 2009;24(3-4):307–316. [DOI] [PubMed] [Google Scholar]
- 15. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. [DOI] [PubMed] [Google Scholar]
- 16. Erices AA, Allers CI, Conget PA, Rojas CV, Minguell JJ. Human cord blood-derived mesenchymal stem cells home and survive in the marrow of immunodeficient mice after systemic infusion. Cell Transplant. 2003;12(6):555–561. [DOI] [PubMed] [Google Scholar]
- 17. Villaron EM, Almeida J, Lopez-Holgado N, Alcoceba M, Sanchez-Abarca LI, Sanchez-Guijo FM, Alberca M, Pérez-Simon JA, San Miguel JF, Del Cañizo MC. Mesenchymal stem cells are present in peripheral blood and can engraft after allogeneic hematopoietic stem cell transplantation. Haematologica. 2004;89(12):1421–1427. [PubMed] [Google Scholar]
- 18. Seo JH, Cho SR. Neurorestoration induced by mesenchymal stem cells: potential therapeutic mechanisms for clinical trials. Yonsei Med J. 2012;53(6):1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96(19):10711–10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bae KS, Park JB, Kim HS, Kim DS, Park DJ, Kang SJ. Neuron-like differentiation of bone marrow-derived mesenchymal stem cells. Yonsei Med J. 2011;52(3):401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wislet-Gendebien S, Hans G, Leprince P, Rigo JM, Moonen G, Rogister B. Plasticity of cultured mesenchymal stem cells: switch from nestin-positive to excitable neuron-like phenotype. Stem Cells. 2005;23(3):392–402. [DOI] [PubMed] [Google Scholar]
- 22. Nagai A, Kim WK, Lee HJ, Jeong HS, Kim KS, Hong SH, Park IH, Kim SU. Multilineage potential of stable human mesenchymal stem cell line derived from fetal marrow. PLoS One. 2007;2(12):e1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H, Kocsis JD. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain. 2006;129(Pt 10):2734–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang C, Fei Y, Xu C, Zhao Y, Pan Y. Bone marrow mesenchymal stem cells ameliorate neurological deficits and blood-brain barrier dysfunction after intracerebral hemorrhage in spontaneously hypertensive rats. Int J Clin Exp Pathol. 2015;8(5):4715–4724. [PMC free article] [PubMed] [Google Scholar]
- 25. Chen M, Li X, Zhang X, He X, Lai L, Liu Y, Zhu G, Li W, Li H, Fang Q, Wang Z, Duan C. The inhibitory effect of mesenchymal stem cell on blood-brain barrier disruption following intracerebral hemorrhage in rats: contribution of TSG-6. J Neuroinflammation. 2015;12:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Otero L, Bonilla C, Aguayo C, Zurita M, Vaquero J. Intralesional administration of allogeneic bone marrow stromal cells reduces functional deficits after intracerebral hemorrhage. Histol Histopathol. 2010;25(4):453–461. [DOI] [PubMed] [Google Scholar]
- 27. Liang H, Yin Y, Lin T, Guan D, Ma B, Li C, Wang Y, Zhang X. Transplantation of bone marrow stromal cells enhances nerve regeneration of the corticospinal tract and improves recovery of neurological functions in a collagenase-induced rat model of intracerebral hemorrhage. Mol Cells. 2013;36(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seyfried D, Ding J, Han Y, Li Y, Chen J, Chopp M. Effects of intravenous administration of human bone marrow stromal cells after intracerebral hemorrhage in rats. J Neurosurg. 2006;104(2):313–318. [DOI] [PubMed] [Google Scholar]
- 29. Seyfried DM, Han Y, Yang D, Ding J, Savant-Bhonsale S, Shukairy MS, Chopp M. Mannitol enhances delivery of marrow stromal cells to the brain after experimental intracerebral hemorrhage. Brain Res. 2008;1224:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seyfried DM, Han Y, Yang D, Ding J, Shen LH, Savant-Bhonsale S, Chopp M. Localization of bone marrow stromal cells to the injury site after intracerebral hemorrhage in rats. J Neurosurg. 2010;112(2):329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang C, Zhou L, Gao X, Chen B, Tu J, Sun H, Liu X, He J, Liu J, Yuan Q. Neuroprotective effects of bone marrow stem cells overexpressing glial cell line-derived neurotrophic factor on rats with intracerebral hemorrhage and neurons exposed to hypoxia/reoxygenation. Neurosurg. 2011;68(3):691–704. [DOI] [PubMed] [Google Scholar]
- 32. Cui J, Cui C, Cui Y, Li R, Sheng H, Jiang X, Tian Y, Wang K, Gao J. Bone marrow mesenchymal stem cell transplantation increases GAP-43 expression via ERK1/2 and PI3K/Akt pathways in intracerebral hemorrhage. Cellular Physiol Biochem. 2017;42(1):137–144. [DOI] [PubMed] [Google Scholar]
- 33. Feng M, Zhu H, Zhu Z, Wei J, Lu S, Li Q, Zhang N, Li G, Li F, Ma W, An Y, Zhao RC, Qin C, Wang R. Serial 18F-FDG PET demonstrates benefit of human mesenchymal stem cells in treatment of intracerebral hematoma: a translational study in a primate model. J Nuclear Med. 2011;52(1):90–97. [DOI] [PubMed] [Google Scholar]
- 34. Xie J, Wang B, Wang L, Dong F, Bai G, Liu Y. Intracerebral and intravenous transplantation represents a favorable approach for application of human umbilical cord mesenchymal stromal cells in intracerebral hemorrhage rats. Med Sci Monit. 2016;22:3552–3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nan Z, Grande A, Sanberg CD, Sanberg PR, Low WC. Infusion of human umbilical cord blood ameliorates neurologic deficits in rats with hemorrhagic brain injury. Ann N Y Acad Sci. 2005;1049:84–96. [DOI] [PubMed] [Google Scholar]
- 36. Chang Z, Mao G, Sun L, Ao Q, Gu Y, Liu Y. Cell therapy for cerebral hemorrhage: Five year follow-up report. Exp Ther Med. 2016;12(6):3535–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Q, Shang X, Hao M, Zheng M, Li Y, Liang Z, Cui Y, Liu Z. Effects of human umbilical cord mesenchymal stem cell transplantation combined with minimally invasive hematoma aspiration on intracerebral hemorrhage in rats. Am J Translat Res. 2015;7(11):2176–2786. [PMC free article] [PubMed] [Google Scholar]
- 38. Gimble JM, Guilak F, Bunnell BA. Clinical and preclinical translation of cell-based therapies using adipose tissue-derived cells. Stem Cell Res Ther. 2010;1(2):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maurin AC, Chavassieux PM, Frappart L, Delmas PD, Serre CM, Meunier PJ. Influence of mature adipocytes on osteoblast proliferation in human primary cocultures. Bone. 2000;26(5):485–489. [DOI] [PubMed] [Google Scholar]
- 40. Chen J, Tang YX, Liu YM, Chen J, Hu XQ, Liu N, Wang SX, Zhang Y, Zeng WG, Ni HJ, Zhao B, Chen YF, Tang ZP. Transplantation of adipose-derived stem cells is associated with neural differentiation and functional improvement in a rat model of intracerebral hemorrhage. CNS Neurosci Ther. 2012;18(10):847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang KL, Lee JT, Pang CY, Lee TY, Chen SP, Liew HK, Chen SY, Chen TY, Lin PY. Human adipose-derived stem cells for the treatment of intracerebral hemorrhage in rats via femoral intravenous injection. Cell Mol Biol Lett. 2012;17(3):376–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim JM, Lee ST, Chu K, Jung KH, Song EC, Kim SJ, Park DK, Kang KM, Hyung Hong N, Park HK, Won CH, Kim KH, Kim M, Kun Lee S, Roh JK. Systemic transplantation of human adipose stem cells attenuated cerebral inflammation and degeneration in a hemorrhagic stroke model. Brain Res. 2007;1183:43–50. [DOI] [PubMed] [Google Scholar]
- 43. Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–156. [DOI] [PubMed] [Google Scholar]
- 44. Martello G, Smith A. The nature of embryonic stem cells. Annu Rev Cell Dev Biol. 2014;30:647–675. [DOI] [PubMed] [Google Scholar]
- 45. Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Dev Biol. 1995;168(2):342–357. [DOI] [PubMed] [Google Scholar]
- 46. Fraichard A, Chassande O, Bilbaut G, Dehay C, Savatier P, Samarut J. In vitro differentiation of embryonic stem cells into glial cells and functional neurons. J Cell Sci. 1995;108(Pt 10):3181–3188. [DOI] [PubMed] [Google Scholar]
- 47. Nonaka M, Yoshikawa M, Nishimura F, Yokota H, Kimura H, Hirabayashi H, Nakase H, Ishizaka S, Wanaka A, Sakaki T. Intraventricular transplantation of embryonic stem cell-derived neural stem cells in intracerebral hemorrhage rats. Neurol Res. 2004;26(3):265–272. [DOI] [PubMed] [Google Scholar]
- 48. Panch SR, Szymanski J, Savani BN, Stroncek DF. Sources of Hematopoietic Stem and Progenitor Cells and Methods to Optimize Yields for Clinical Cell Therapy. Biol Blood Marrow Transplant. 2017;23(8):1241–1249. [DOI] [PubMed] [Google Scholar]
- 49. Till JE, Mc CE. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiation Res. 1961;14:213–222. [PubMed] [Google Scholar]
- 50. Calloni R, Cordero EA, Henriques JA, Bonatto D. Reviewing and updating the major molecular markers for stem cells. Stem Cells Dev. 2013;22(9):1455–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sobrino T, Arias S, Perez-Mato M, Agulla J, Brea D, Rodriguez-Yanez M, Castillo J. Cd34+ progenitor cells likely are involved in the good functional recovery after intracerebral hemorrhage in humans. J Neurosci Res. 2011;89(7):979–985. [DOI] [PubMed] [Google Scholar]
- 52. Jendelova P, Herynek V, Urdzikova L, Glogarova K, Rahmatova S, Fales I, Andersson B, Procházka P, Zamecník J, Eckschlager T, Kobylka P, Hájek M, Syková E. Magnetic resonance tracking of human CD34+ progenitor cells separated by means of immunomagnetic selection and transplanted into injured rat brain. Cell Transplant. 2005;14(4):173–182. [DOI] [PubMed] [Google Scholar]
- 53. Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–1011. [DOI] [PubMed] [Google Scholar]
- 54. England TJ, Abaei M, Auer DP, Lowe J, Jones DR, Sare G, Walker M, Bath PM. Granulocyte-colony stimulating factor for mobilizing bone marrow stem cells in subacute stroke: the stem cell trial of recovery enhancement after stroke 2 randomized controlled trial. Stroke. 2012;43(2):405–411. [DOI] [PubMed] [Google Scholar]
- 55. Willing AE, Lixian J, Milliken M, Poulos S, Zigova T, Song S, Hart C, Sanchez-Ramos J, Sanberg PR. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J Neurosci Res. 2003;73(3):296–307. [DOI] [PubMed] [Google Scholar]
- 56. McKay R. Stem cells in the central nervous system. Science. 1997;276(5309):66–71. [DOI] [PubMed] [Google Scholar]
- 57. Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–1710. [DOI] [PubMed] [Google Scholar]
- 58. Rao M. Stem and precursor cells in the nervous system. J Neurotrauma. 2004;21(4):415–427. [DOI] [PubMed] [Google Scholar]
- 59. Jeong SW, Chu K, Jung KH, Kim SU, Kim M, Roh JK. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke. 2003;34(9):2258–2263. [DOI] [PubMed] [Google Scholar]
- 60. Lee HJ, Kim KS, Kim EJ, Choi HB, Lee KH, Park IH, Ko Y, Jeong SW, Kim SU. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells. 2007;25(5):1204–1212. [DOI] [PubMed] [Google Scholar]
- 61. Wang Z, Cui C, Li Q, Zhou S, Fu J, Wang X, Zhuge Q. Intracerebral transplantation of foetal neural stem cells improves brain dysfunction induced by intracerebral haemorrhage stroke in mice. J Cell Mol Med. 2011;15(12):2624–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wakai T, Sakata H, Narasimhan P, Yoshioka H, Kinouchi H, Chan PH. Transplantation of neural stem cells that overexpress SOD1 enhances amelioration of intracerebral hemorrhage in mice. J Cereb Blood Flow Metab. 2014;34(3):441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lee HJ, Lim IJ, Lee MC, Kim SU. Human neural stem cells genetically modified to overexpress brain-derived neurotrophic factor promote functional recovery and neuroprotection in a mouse stroke model. J Neurosci Res. 2010;88(15):3282–3294. [DOI] [PubMed] [Google Scholar]
- 64. Lee HJ, Park IH, Kim HJ, Kim SU. Human neural stem cells overexpressing glial cell line-derived neurotrophic factor in experimental cerebral hemorrhage. Gene Ther. 2009;16(9):1066–1076. [DOI] [PubMed] [Google Scholar]
- 65. Lee HJ, Kim MK, Kim HJ, Kim SU. Human neural stem cells genetically modified to overexpress Akt1 provide neuroprotection and functional improvement in mouse stroke model. PLoS One. 2009;4(5):e5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee HJ, Kim KS, Park IH, Kim SU. Human neural stem cells over-expressing VEGF provide neuroprotection, angiogenesis and functional recovery in mouse stroke model. PLoS One. 2007;2(1):e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tang T, Li XQ, Wu H, Luo JK, Zhang HX, Luo TL. Activation of endogenous neural stem cells in experimental intracerebral hemorrhagic rat brains. Chinese Med J. 2004;117(9):1342–1347. [PubMed] [Google Scholar]
- 69. Yu Z, Chen LF, Tang L, Hu CL. Effects of recombinant adenovirus-mediated hypoxia-inducible factor-1alpha gene on proliferation and differentiation of endogenous neural stem cells in rats following intracerebral hemorrhage. Asian Pacific J Trop Med. 2013;6(10):762–767. [DOI] [PubMed] [Google Scholar]
- 70. Jin J, Kang HM, Park C. Voluntary exercise enhances survival and migration of neural progenitor cells after intracerebral haemorrhage in mice. Brain Injury. 2010;24(3):533–540. [DOI] [PubMed] [Google Scholar]
- 71. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. [DOI] [PubMed] [Google Scholar]
- 72. Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;376(11):1038–1046. [DOI] [PubMed] [Google Scholar]
- 73. Qin J, Song B, Zhang H, Wang Y, Wang N, Ji Y, Qi J, Chandra A, Yang B, Zhang Y, Gong G, Xu Y. Transplantation of human neuro-epithelial-like stem cells derived from induced pluripotent stem cells improves neurological function in rats with experimental intracerebral hemorrhage. Neurosci Lett. 2013;548:95–100. [DOI] [PubMed] [Google Scholar]
- 74. Qin J, Gong G, Sun S, Qi J, Zhang H, Wang Y, Wang N, Wang QM, Ji Y, Gao Y, Shi C, Yang B, Zhang Y, Song B, Xu Y. Functional recovery after transplantation of induced pluripotent stem cells in a rat hemorrhagic stroke model. Neurosci Lett. 2013;554:70–75. [DOI] [PubMed] [Google Scholar]
- 75. Qin J, Ma X, Qi H, Song B, Wang Y, Wen X, Wang QM, Sun S, Li Y, Zhang R, Liu X, Hou H, Gong G, Xu Y. Transplantation of induced pluripotent stem cells alleviates cerebral inflammation and neural damage in hemorrhagic stroke. PLoS One. 2015;10(6):e0129881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rodriguez-Frutos B, Otero-Ortega L, Gutierrez-Fernandez M, Fuentes B, Ramos-Cejudo J, Diez-Tejedor E. Stem cell therapy and administration routes after stroke. Transl Stroke Res. 2016;7(5):378–387. [DOI] [PubMed] [Google Scholar]
- 77. Wictorin K, Brundin P, Gustavii B, Lindvall O, Bjorklund A. Reformation of long axon pathways in adult rat central nervous system by human forebrain neuroblasts. Nature. 1990;347(6293):556–568. [DOI] [PubMed] [Google Scholar]
- 78. Vaquero J, Otero L, Bonilla C, Aguayo C, Rico MA, Rodriguez A, Zurita M. Cell therapy with bone marrow stromal cells after intracerebral hemorrhage: impact of platelet-rich plasma scaffolds. Cytotherapy. 2013;15(1):33–43. [DOI] [PubMed] [Google Scholar]
- 79. Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39(2):573–576. [DOI] [PubMed] [Google Scholar]
- 80. Zhang H, Huang Z, Xu Y, Zhang S. Differentiation and neurological benefit of the mesenchymal stem cells transplanted into the rat brain following intracerebral hemorrhage. Neurol Res. 2006;28(1):104–112. [DOI] [PubMed] [Google Scholar]
- 81. Li Y, Chen J, Wang L, Lu M, Chopp M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 2001;56(12):1666–1672. [DOI] [PubMed] [Google Scholar]
- 82. Sun J, Wei ZZ, Gu X, Zhang JY, Zhang Y, Li J, Wei L. Intranasal delivery of hypoxia-preconditioned bone marrow-derived mesenchymal stem cells enhanced regenerative effects after intracerebral hemorrhagic stroke in mice. Exp Neurol. 2015;272:78–87. [DOI] [PubMed] [Google Scholar]
- 83. Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64(7):614–628. [DOI] [PubMed] [Google Scholar]
- 84. Chapman CD, Frey WH, 2nd, Craft S, Danielyan L, Hallschmid M, Schioth HB, Benedict C. Intranasal treatment of central nervous system dysfunction in humans. Pharm Res. 2013;30(10):2475–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Andres RH, Guzman R, Ducray AD, Mordasini P, Gera A, Barth A, Widmer HR, Steinberg GK. Cell replacement therapy for intracerebral hemorrhage. Neurosurg Focus. 2008;24(3-4):E16. [DOI] [PubMed] [Google Scholar]
- 86. Gong C, Boulis N, Qian J, Turner DE, Hoff JT, Keep RF. Intracerebral hemorrhage-induced neuronal death. Neurosurgery. 2001;48(4):875–882; discussion 882–883. [DOI] [PubMed] [Google Scholar]
- 87. Otero L, Zurita M, Bonilla C, Aguayo C, Vela A, Rico MA, Vaquero J. Late transplantation of allogeneic bone marrow stromal cells improves neurologic deficits subsequent to intracerebral hemorrhage. Cytotherapy. 2011;13(5):562–571. [DOI] [PubMed] [Google Scholar]
- 88. Li F, Liu Y, Zhu S, Wang X, Yang H, Liu C, Zhang Y, Zhang Z. Therapeutic time window and effect of intracarotid neural stem cells transplantation for intracerebral hemorrhage. Neuroreport. 2007;18(10):1019–1023. [DOI] [PubMed] [Google Scholar]
- 89. Yang D, Han Y, Zhang J, Ding C, Anagli J, Seyfried DM. Improvement in recovery after experimental intracerebral hemorrhage using a selective cathepsin B and L inhibitor. J Neurosurg. 2011;114(4):1110–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Otero L, Zurita M, Bonilla C, Aguayo C, Rico MA, Rodriguez A, Vaquero J. Allogeneic bone marrow stromal cell transplantation after cerebral hemorrhage achieves cell transdifferentiation and modulates endogenous neurogenesis. Cytotherapy. 2012;14(1):34–44. [DOI] [PubMed] [Google Scholar]
- 91. Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59(4):514–523. [DOI] [PubMed] [Google Scholar]
- 92. Tang T, Liu XJ, Zhang ZQ, Zhou HJ, Luo JK, Huang JF, Yang QD, Li XQ. Cerebral angiogenesis after collagenase-induced intracerebral hemorrhage in rats. Brain Res. 2007;1175:134–142. [DOI] [PubMed] [Google Scholar]
- 93. Bao XJ, Liu FY, Lu S, Han Q, Feng M, Wei JJ, Yang QD, Li XQ. Transplantation of Flk-1+ human bone marrow-derived mesenchymal stem cells promotes behavioral recovery and anti-inflammatory and angiogenesis effects in an intracerebral hemorrhage rat model. Int J Mol Med. 2013;31(5):1087–1096. [DOI] [PubMed] [Google Scholar]
- 94. Yang D, Han Y, Zhang J, Seyda A, Chopp M, Seyfried DM. Therapeutic effect of human umbilical tissue-derived cell treatment in rats with experimental intracerebral hemorrhage. Brain Res. 2012;1444:1–10. [DOI] [PubMed] [Google Scholar]
- 95. Wang SP, Wang ZH, Peng DY, Li SM, Wang H, Wang XH. Therapeutic effect of mesenchymal stem cells in rats with intracerebral hemorrhage: reduced apoptosis and enhanced neuroprotection. Mol Med Rep. 2012;6(4):848–854. [DOI] [PubMed] [Google Scholar]
- 96. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nature Rev Immunol. 2008;8(9):726–736. [DOI] [PubMed] [Google Scholar]
- 97. Kim SS, Yoo SW, Park TS, Ahn SC, Jeong HS, Kim JW, Huh Y, Lee JE, Lee SY, Lee YD, Suh-Kim H. Neural induction with neurogenin1 increases the therapeutic effects of mesenchymal stem cells in the ischemic brain. Stem Cells. 2008;26(9):2217–2228. [DOI] [PubMed] [Google Scholar]
- 98. Gao L, Lu Q, Huang LJ, Ruan LH, Yang JJ, Huang WL, ZhuGe WS, Zhang YL, Fu B, Jin KL, ZhuGe QC. Transplanted neural stem cells modulate regulatory T, gamma-delta T cells and corresponding cytokines after intracerebral hemorrhage in rats. Int J Mol Sci. 2014;15(3):4431–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM, Hong NH, Kim JH, Ban JJ, Park HK, Kim SU, Park CG, Lee SK, Kim M, Roh JK. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008;131(Pt 3):616–629. [DOI] [PubMed] [Google Scholar]
- 100. Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathologica. 2010;120(3):287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kim H, Cooke MJ, Shoichet MS. Creating permissive microenvironments for stem cell transplantation into the central nervous system. Trends Biotechnol. 2012;30(1):55–63. [DOI] [PubMed] [Google Scholar]
- 102. Dooley D, Vidal P, Hendrix S. Immunopharmacological intervention for successful neural stem cell therapy: new perspectives in CNS neurogenesis and repair. Pharmacol Ther. 2014;141(1):21–31. [DOI] [PubMed] [Google Scholar]
- 103. Jiang Y, Wu J, Keep RF, Hua Y, Hoff JT, Xi G. Hypoxia-inducible factor-1alpha accumulation in the brain after experimental intracerebral hemorrhage. J Cereb Blood Flow Metab. 2002;22(6):689–696. [DOI] [PubMed] [Google Scholar]
- 104. Tsai PT, Ohab JJ, Kertesz N, Groszer M, Matter C, Gao J, Liu X, Wu H, Carmichael ST. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J Neurosci. 2006;26(4):1269–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Investig. 2004;114(3):330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Pataky DM, Borisoff JF, Fernandes KJ, Tetzlaff W, Steeves JD. Fibroblast growth factor treatment produces differential effects on survival and neurite outgrowth from identified bulbospinal neurons in vitro. Ex Neurol. 2000;163(2):357–372. [DOI] [PubMed] [Google Scholar]
- 107. Stolzing A, Wengner A, Grune T. Degradation of oxidized extracellular proteins by microglia. Arch Biochem Biophys. 2002;400(2):171–179. [DOI] [PubMed] [Google Scholar]
- 108. Park JB, Yiu G, Kaneko S, Wang J, Chang J, He XL, Garcia KC, He Z. A TNF. receptor family member, TROY, is a coreceptor with Nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron. 2005;45(3):345–351. [DOI] [PubMed] [Google Scholar]
- 109. Veizovic T, Beech JS, Stroemer RP, Watson WP, Hodges H. Resolution of stroke deficits following contralateral grafts of conditionally immortal neuroepithelial stem cells. Stroke. 2001;32(4):1012–1019. [DOI] [PubMed] [Google Scholar]
- 110. An YH, Wang HY, Gao ZX, Wang ZC. Differentiation of rat neural stem cells and its relationship with environment. Biomed Environ Sci. 2004;17(1):1–7. [PubMed] [Google Scholar]
- 111. Otero-Ortega L, Gomez de Frutos MC, Laso-Garcia F, Rodriguez-Frutos B, Medina-Gutierrez E, Lopez JA, Vázquez J, Díez-Tejedor E, Gutiérrez-Fernández M. Exosomes promote restoration after an experimental animal model of intracerebral hemorrhage. J Cereb Blood Flow Metab. 2017:271678X17708917. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 112. Suda S, Yang B, Schaar K, Xi X, Pido J, Parsha K. Autologous bone marrow mononuclear cells exert broad effects on short- and long-term biological and functional outcomes in rodents with intracerebral hemorrhage. Stem Cell Dev. 2015;24(23):2756–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Banerjee S, Williamson D, Habib N, Gordon M, Chataway J. Human stem cell therapy in ischaemic stroke: a review. Age Ageing. 2011;40(1):7–13. [DOI] [PubMed] [Google Scholar]
- 114. Cui LL, Kerkela E, Bakreen A, Nitzsche F, Andrzejewska A, Nowakowski A. The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cell Res Ther. 2015;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Barker RA, Widner H. Immune problems in central nervous system cell therapy. NeuroRx. 2004;1(4):472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Miura M, Miura Y, Padilla-Nash HM, Molinolo AA, Fu B, Patel V, Seo BM, Sonoyama W, Zheng JJ, Baker CC, Chen W, Ried T, Shi S. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24(4):1095–1103. [DOI] [PubMed] [Google Scholar]
- 117. Jeong H, Yim HW, Cho YS, Kim YI, Jeong SN, Kim HB. Efficacy and safety of stem cell therapies for patients with stroke: a systematic review and single arm meta-analysis. Int J Stem Cell. 2014;7(2):63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Nakamura M, Samii A, Lang JM, Gotz F, Samii M, Krauss JK. De novo arteriovenous malformation growth secondary to implantation of genetically modified allogeneic mesenchymal stem cells in the brain. Neurosurgery. 2016;78(4):E596–E600. [DOI] [PubMed] [Google Scholar]
- 119. Zhu J, Xiao Y, Li Z, Han F, Xiao T, Zhang Z, Geng F. Efficacy of surgery combined with autologous bone marrow stromal cell transplantation for treatment of intracerebral hemorrhage. Stem Cell Int. 2015;2015:318269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bhasin A, Srivastava MV, Kumaran SS, Mohanty S, Bhatia R, Bose S, Gaikwad S, Garg A, Airan B. Autologous mesenchymal stem cells in chronic stroke. Cerebrovasc Dis Extra. 2011;1(1):93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bhasin A, Srivastava MV, Mohanty S, Bhatia R, Kumaran SS, Bose S. Stem cell therapy: a clinical trial of stroke. Clin Neurol Neurosurg. 2013;115(7):1003–1008. [DOI] [PubMed] [Google Scholar]
- 122. Bhasin A, Kumaran SS, Bhatia R, Mohanty S, Srivastava MVP. Safety and feasibility of autologous mesenchymal stem cell transplantation in chronic stroke in Indian patients. A four-year follow up. J Stem Cells Regen Med. 2017;13(1):14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Xue YZ, Li XX, Li L, Pang SL, Yao JG, Hao PL. Curative effect and safety of intrathecal transplantation of neural stem cells for the treatment of cerebral hemorrhage. Genet Mol Research. 2014;13(4):8294–8300. [DOI] [PubMed] [Google Scholar]
- 124. Shimamura N, Mtsuda N, Ktayama K, Kakuta K, Katagai T, Naraoka M, Ohkuma H. Stem cell therapies for intracerebral hemorrhages. Curr Drug Deliv. 2017;14(6):758–765. [DOI] [PubMed] [Google Scholar]