Abstract
Cerebral small vessel disease (CSVD) is composed of several diseases affecting the small arteries, arterioles, venules, and capillaries of the brain, and refers to several pathological processes and etiologies. Neuroimaging features of CSVD include recent small subcortical infarcts, lacunes, white matter hyperintensities, perivascular spaces, microbleeds, and brain atrophy. The main clinical manifestations of CSVD include stroke, cognitive decline, dementia, psychiatric disorders, abnormal gait, and urinary incontinence. Currently, there are no specific preventive or therapeutic measures to improve this condition. In this review, we will discuss the pathophysiology, clinical aspects, neuroimaging, progress of research to treat and prevent CSVD and current treatment of this disease.
Keywords: Cerebral small vessel disease, arteriolosclerosis, cerebral amyloid angiopathy, neuroimaging, MRI, stroke syndrome, dementia
Introduction
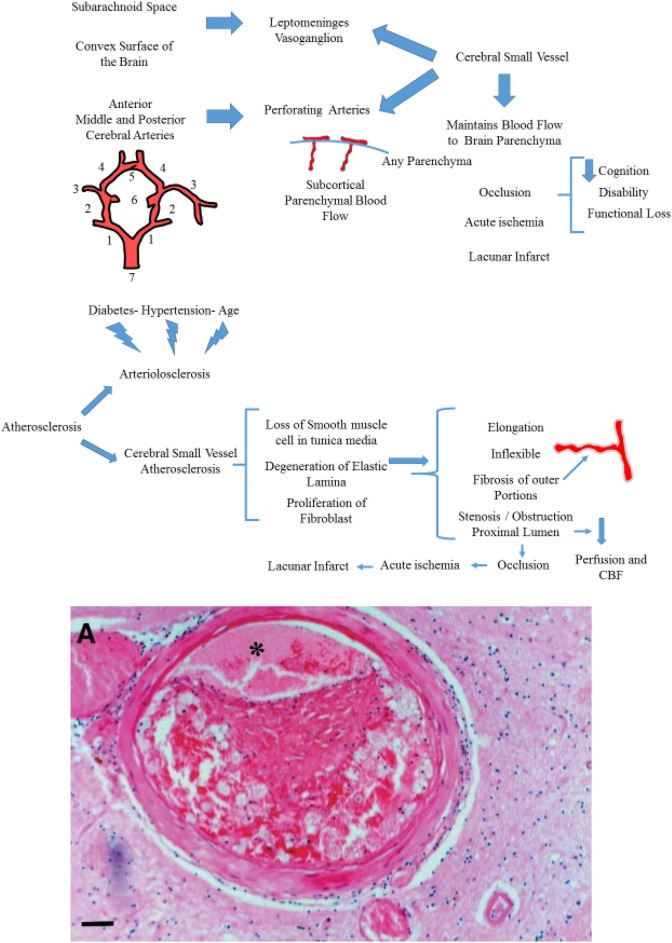
Cerebral small vessels comprise two components. First, the leptomeninges vasoganglion, which is derived from subarachnoid space covering, and the convex surface of brain. Second, perforating arteries are derived from anterior, middle, posterior cerebral arteries that supply the subcortical parenchyma (Fig. 1). The cerebral small vessels are crucial to maintenance of adequate blood flow to the sub-surface brain structure. They include small arteries, arterioles, venules, and capillaries which are commonly sized at 50–400 µm1,2.
Fig. 1.
Cerebral small vessel and its comprised compartments. (1): Posterior cerebral artery (2): Posterior communicating artery (3): Middle cerebral artery (4): Anterior cerebral artery (5): Anterior communicating artery (6): Internal carotid artery (7): Basilar artery. Small vessel atherosclerosis. (A). Eccentric atherosclerotic plaque in a perforating vessel in the putamen, causing significant narrowing of the lumen (asterisk).
Cerebral small vessel disease (CSVD) is a generic term that refers to intracranial vascular disease based on various pathological and neurological processes, as well as a syndrome referring to different clinical manifestations and neuroimaging features caused by the structural changes of vascular and brain parenchyma. Small vessel disease accounts for up to 25% of all ischemic strokes3 but also put patients at twice the risk for these conditions4. In addition, CSVD is a leading cause of functional loss, disability and cognitive decline in the elderly. Neuroimaging development allows increased understanding of CSVD. Thus, a comprehensive knowledge of its pathophysiologic mechanism, neuroimaging, and clinical features is imperative to further study on possible preventive and therapeutic measures.
Pathogenesis and Classification
The pathophysiologic mechanisms of CSVD are not yet clear. The European small brain vascular disease expert group puts forward the classification of CSVD based on cerebrovascular pathologic changes as the following: Arteriolosclerosis, sporadic and hereditary cerebral amyloid angiopathy, inherited or genetic small vessel diseases distinct from cerebral amyloid angiopathy, inflammatory and immunologically-mediated small vessel diseases, venous collagenosis, and other small vessel diseases such as post-radiation angiopathy1. These various pathologic changes cited by the European expert group do not only result in damage of brain parenchyma including neuronal apoptosis, diffuse axonal injury, demyelination and loss of oligodendrocytes, but also result in a series of symptoms and unusual neuroimaging findings.
CSVD is thought to result in reduced cerebral blood flow, impaired cerebral autoregulation and increased blood–brain barrier (BBB) permeability. However, the molecular mechanisms underlying CSVD are incompletely understood. Recent studies in monogenic forms of small vessel disease (SVD), such as cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), and ‘sporadic’ SVD have shed light on possible disease mechanisms in CSVD. Proteomic and biochemical studies in post-mortem monogenic CSVD patients, as well as in animal models of monogenic disease have suggested that disease pathways are shared between different types of monogenic disease, often involving the impairment of extracellular matrix (ECM) function. In addition, genetic studies in ‘sporadic’ CSVD have also shown that the disease is highly heritable, particularly among young-onset stroke patients, and that common variants in monogenic disease genes may contribute to disease processes in some CSVD subtypes5.
Arteriolosclerosis
Among the pathologic changes involved in CSVD, the two most common are arteriolosclerosis and cerebral small vascular atherosclerosis. Arteriolosclerosis, a vascular risk-factor-related SVD, is known to be age-related, and is the most common small vessel alteration in aged brains. The severity of arteriolosclerosis increases with aging and is exacerbated by hypertension and diabetes (Fig. 2)5. Thus, arteriolosclerosis is also named hypertensive SVD6. Cerebral small vascular atherosclerosis, particularly in arterioles smaller than 50 µm in diameter, is characterized by a loss of smooth muscle cells from the tunica media, degeneration of internal elastic lamina, proliferation of fibroblasts (Fig. 2), deposits of fibro-hyaline material and collagens, thickening of the vessel wall, formation of microatheroma, and narrowing of the lumen1. With these changes, the vessels become elongated, tortuous and inflexible (Fig. 2). In addition, wall damage causes distension of its outer portions due to fibrosis, that is microaneurysm, and the stenosis or obstruction of proximal lumen7. Ultimately, impaired autoregulation of involved small vessel results in reduced cerebral blood flow (CBF) and chronic cerebral hypoperfusion6. The occlusion of arterial lumen leads to acute ischemia, causing lacunar infarction (Figs. 2 and 3). Whereas, critical stenosis and hypoperfusion involving multiple small arterioles, mainly in deep white matter, lead to incomplete ischemia which are visualized as White Matter Hyperintensities (WMH) on neuroimaging8. The two pathophysiological pathways above can often overlap, so lacunes and white matter lesions often coexist in the same patient. Kuwabara and colleagues found a 25% decreases in CBF in patients with both Alzheimer’s and Binswanger’s diseases, with the use of positron emission tomography with oxygen-15-labelled water9. Conventional risk factors such as hypertension diabetes, smoking, high homocysteine concentrations, obesity, and dyslipidemia have been considered to lead to arteriolosclerosis. In addition, hematological disorders, infection, and hereditary diseases are increasingly recognized in various studies10,11.
Fig. 2.
The relationship between conditions such as diabetes, and hypertension influencing arteriolosclerosis. In addition, the figure shows how cerebral small vessel atherosclerosis is characterized. A series of changes will lead to lacunar infarct.
CBF: cerebral blood flow.
Fig. 3.
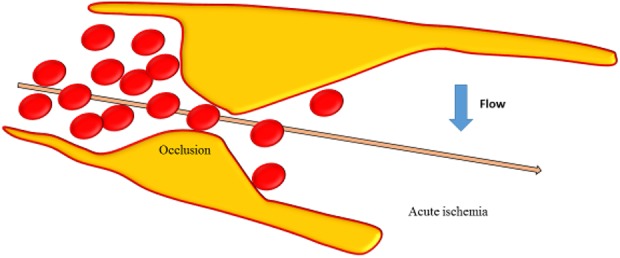
Occlusion of the vessel lumen is represented and acute ischemia due to decreased flow in the vessel occurred.
Cerebral Amyloid Angiopathy
Cerebral amyloid angiopathy (CAA) is the dominant cause of lobar intracerebral hemorrhage. Not only does it result in stroke and cognitive impairment in a significant proportion of older patients, but it is an important component of the senile plaques found in patients with Alzheimer’s disease12,13. CAA is characterized by the progressive accumulation of congophilic, immunoreactive, amyloid protein in the walls of small-to-medium-sized arteries and arterioles predominantly located in the leptomeningeal space, cortex, and, to a lesser extent, in the capillaries and veins1. A characteristic ‘double-barrel’ lumen of involved vessels is seen under light microscopy in leptomeninges and parenchyma due to the splitting of the internal elastic lamina caused by the deposition of hyaline material in the vessel wall. The thickening vessel walls stained with Congo red, and with thioflavin S and appear green birefringent under polarized light and fluoresce under ultraviolet light, respectively1,12.
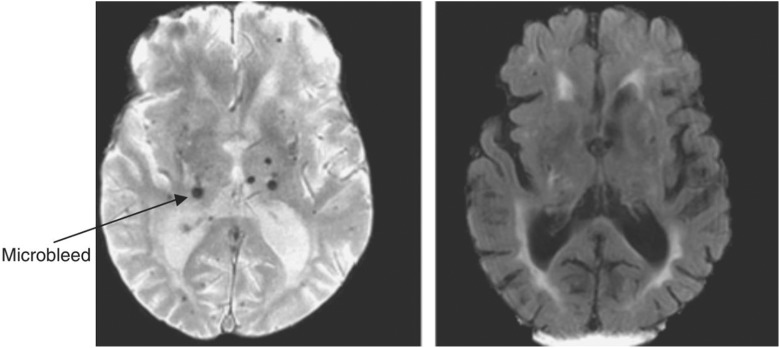
CAA appears in almost all elderly patients with dementia, and accounts for about 64% to 84.9% of the elderly without dementia14–16. Its distribution in the brain parenchyma is mainly on the region of hippocampus and cortex17, which may have a correlation with cognitive impairments caused by CAA, without clear potential causative mechanisms known to date. Many studies indicate that diffuse brain microbleeds, micro-infarcts, hypoperfusion, and white matter hypoxia caused by vessel changes associated with CAA may be responsible for cognitive decline and dementia, independent of Alzheimer’s disease and Lewy body pathology (Fig. 6)16,18–21.
Fig 6.
Gradient echo magnetic resonance imaging (left image) in a patient with small vessel disease and lacunar stroke showing microbleeds (arrowed) in the subcortical region not seen on fluid-attenuated inversion recovery (right image).
Patel B, Markus HS. Magnetic resonance imaging in cerebral small vessel disease and its use as a surrogate disease marker. Int J Stroke. 2011 Feb;6(1):47–59.
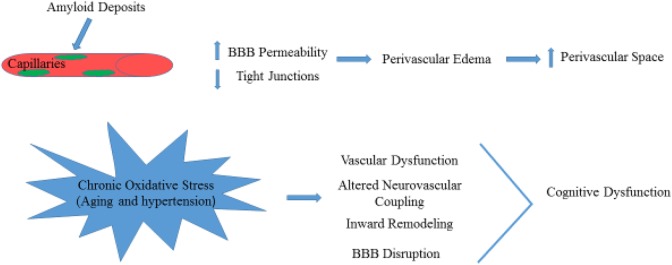
Amyloid-beta protein accumulation in capillaries affects BBB integrity, which leads to a loss of tight junction proteins and increased BBB permeability (Fig. 4)22. Then, perivascular edema and extravasation of toxic plasma components caused by the disruption of BBB contributes to localized damage to brain parenchyma and enlarged perivascular spaces (Fig. 4)4,23. Extensive white matter lesions can be found in patients with CAA, which carry an increased risk of warfarin-related intracerebral hemorrhage after ischemic stroke24.
Fig. 4.
The increase of amyloid deposits in the perivascular space consequently leads to increase in BBB permeability and decrease in tight junctions. The schematic effects of chronic oxidative stress in aging is shown.
BBB: Blood–brain barrier.
CAA is not only a biomarker of cognition impairment, but also found in some rare genetically transmitted diseases25 such as Down’s syndrome26. Probably, CAA is an expression of some systematic amyloid deposition diseases, including hereditary amyloidosis, transthyretin familial amyloid polyneuropathy27,28, plasma cell dyscrasia29, and myogenic disease30.
Long-term and chronic oxidative stress in aging, and hypertension lead to cerebral vascular dysfunction including impaired neurovascular coupling, inward remodeling, rarefaction, and BBB disruption, which result in brain injury and cognitive dysfunction (Fig. 4)31. In the inherited or genetic SVDs such as Fabry’s disease and CADASIL32,33, there is still a controversy about the mechanisms of brain injury. Moore and colleagues reported that the deposition of globotriaosylceramide (Gb3) leads to altered vascular reactivity, resulting in increased blood flow and metabolic vulnerability in the deep white matter. These findings are contrary to chronic cerebral hypoperfusion found in other studies34–36.
Neuroimaging Features of CSVD
Due to mild clinical symptoms or lower mortality than common stroke, the onset of CSVD was frequently neglected. Thus, neuroimaging has become an important tool in diagnosing CSVD and the silent neurovascular disease, especially at early stage. Neuroimaging of CSVD primarily involves visualizing recent small subcortical infarcts, lacunar infarct, WMH, microbleeds, enlarged perivascular spaces, and brain atrophy1 (Fig. 6).
Recent Small Subcortical Infarcts
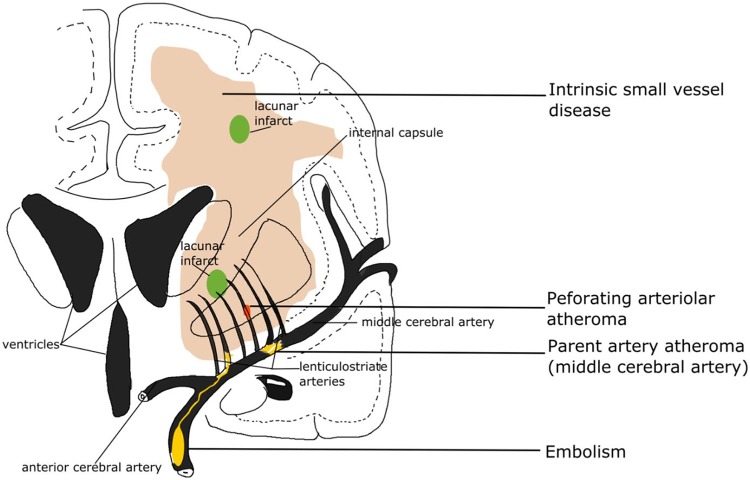
Recent small subcortical infarcts are considered to result from acute severe ischemia of a single perforating artery, and are lesions occurring in the territory of one perforating arteriole within the previous few weeks, with neuroimaging evidence or clinical symptoms of recent infarction (Fig. 5)37. Recent infarcts are regarded as round or ovoid lesions less than 20 mm in maximal diameter in the white matter, basal ganglia, or brainstem. They present hyperintense on diffusion-weighted imaging (DWI), hypointense on the apparent diffusion coefficient map, and either normal or hyperintense to a normal brain on fluid-attenuated inversion recovery (FLAIR)/T2 imaging, and with less hyperintensity than cerebrospinal fluid on T238,39. However, a number that can reach 50% of patients with acute stroke may appear with no responsible lesions on computed tomography (CT) and magnetic resonance imaging (MRI). DWI is the most sensitive sequence for acute ischemic lesions, allowing detection of acute ischemia within the first few hours after stroke onset. With the increasing recognition of recent small subcortical infarcts, Gattringer and colleagues recommended the new term ‘recent small subcortical infarct’ instead of lacunar infarct40. From recent small subcortical infarct to cavity formation, there are a number of morphological changes, including volume and diameter reductions, occurring within the first 90 days of onset39,41. In the development of recent small subcortical infarcts, more than one-third (39%) of the lesions recovered with no residual cavities, and 40% of the infarct lesions were located adjacent or fused in preexisting white matter lesions39. It is reported that the incidence of cavity formation after recent small subcortical infarcts is in the broad range of 28–94%41–44. There are three outcomes from recent small subcortical infarcts: (1) A common lacuna and/or (2) white matter hyperintensity without apparent cavitation on T2-weighted sequences, and (3) less common fates that disappearing with little visible consequence on conventional MRI.
Fig. 5.
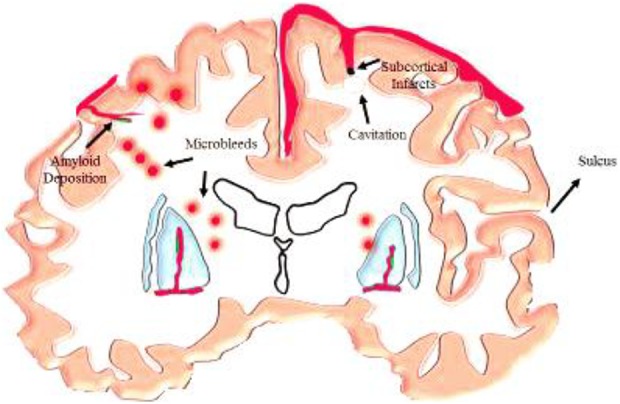
Subcortical infarcts resulting from acute severe ischemia. It also shows microbleeds occurring in the cerebral cortex and gray/white matter junction due to deposition of amyloid. Cavitation is also shown as a result of acute severe ischemia.
Lacunar Infarct
Lacunar stroke accounts for up to a quarter of all acute ischemic strokes. it is a small fluid-filled cavity that was thought to mark the healed stage of a small deep brain infarct. In neuroimaging, a lacuna is a round or ovoid, subcortical, fluid-filled cavity with similar signal to cerebrospinal fluid (CSF) (Fig. 5). It measures between 3–15 mm in diameter, which is consistent with a previous acute small deep brain infarct or hemorrhage in the territory of one perforating arteriole37. Lacunar infarcts are typically located in the basal ganglia, internal capsule, thalamus, corona radiata, centrum semiovale (CSO), and brainstem. Poirier and colleagues divided the lacunas into three subtypes based on the formation: Subtype I lacunas are secondary to old lacunar infarction; subtype II lacunas secondary to old hemorrhagic lesions; subtype Ⅲ lacunas are secondary to enlarged perivascular spaces. Herve and colleagues classified the lacunar lesions by three-dimensional MRI reconstruction, according to their shapes into four types: Slab, stick, multiple components, or ovoid/spheroid, then proposing that most of lacunar infarcts (83%) were ovoid or spheroid. Infarct lesions manifest isolated, adjacent to or fused into white matter hyperintensity38. Moreau and colleagues found that lacunas almost always present at 90 days after acute lacunar infarction and appear as a central CSF-like hypointensity with or without a surrounding border of hyperintensity on FLAIR sequence but only CSF-like hypointensity and hyperintensity on T1-weighted and T2-weighted, respectively. Moreover, the sensitivity of FLAIR for cavitation was greatly lower than for T1-weighted sequences41.
White Matter Hyperintensities
The white matter is the most vulnerable region to suffer hypoxia/hypoperfusion due to the watershed effect. WMH are supposed to be comprehensive expressions including disturbances of small blood vessels, breakdown of the BBB, small infarcts in the white matter, glial activation, loss of oligodendrocytes, and demyelination caused by chronic diffuse hypoperfusion or reduced cerebral blood flow1,31. Bilateral, mostly symmetrical hyperintensities on T2-weighted and FLAIR MRI are characteristic features of white matter lesions accompanied with some hypointensity different from CSF on T1-weighted MRI and low density on cerebral CT in most older individuals with or without cognition decline. In addition to white matter, the hyperintensity lesions are also located in subcortical gray matter structures, such as basal ganglia, and brainstem37,45. However, whether the hyperintensities of gray matter and brainstem should be adopted into WMH is controversial37,46. To differ WMH from other lesions, such as lacuna and atrophy, and then research their specific role on dementia, diffusion tensor MRI (DT-MRI), and magnetization transfer MRI (MT-MRI) were increasingly used to give a quantitative information on the state of the brain’s white matter.
Microbleeds
Cerebral microbleeds (CMBs) are magnetic resonance (MR)-visible small (generally 2–5 mm in diameter, but up to 10 mm) areas of signal void caused by perivascular collections of hemosiderin deposits that are foci of past hemorrhages resulted from small vessels involved in CAA or arteriolosclerosis (Fig. 5)18,37. Small hypointense lesions appear on paramagnetic-sensitive MR sequences such as T2-weighted gradient-recalled echo (GRE) or susceptibility-weighted imaging sequences with a ‘blooming effect’ (larger or more conspicuous on GRE than on spin-echo MRI)18, and are generally not seen on CT, or on FLAIR, T1-weighted, or T2-weighted MR sequences. Microbleeds lesions, either round or oval in shape, are most commonly located in the cortico-subcortical junction, and deep gray or white matter in the cerebral hemispheres, brainstem, and cerebellum. Lesions were classified as three types according to location: lobar, deep and subtentorial CMBs47. Lobar microbleeds are especially associated with a decline in executive functions, information processing and memory function relative to a decline in motor speed associated with deep or subtentorial microbleeds48.
Enlarged Perivascular Spaces
Perivascular spaces, as well as Virchow–Robin spaces, are extensions of the extracerebral fluid-filled spaces that follow the typical course of a vessel as it goes through gray or white matter49. These spaces follow the path of perforating arteries including arteries, arterioles, veins, and venules. Given the different imaging planes, the lesions appear linear, round or ovoid CSF-like intensity on all MRI sequences, with the diameter generally smaller than 3 mm50. Enlarged perivascular spaces are predominantly located in the basal ganglia and CSO, and with increased signal intensity equal to cerebrospinal fluid on T2-weighted images, hypointensity on T1-weighted and occasionally hypointensity on FLAIR images without hyperintense rim to distinguish from old lacunar infarcts49.
Brain Atrophy
Brain atrophy indicates a lower brain volume on neuroimaging that is not related to definitive macroscopic focal injury such as trauma or infarction. Characteristic manifestations of atrophy are symmetrical or asymmetrical decreased total volume, increased ventricular volumes, enlarged superficial sulci, and decreased specific gray or white matter volumes. The hippocampus is an example on imaging examinations that include cranial CT or MRI that has characteristic manifestations of atrophy51,52. Brain atrophy frequently occurs together with WMH in elderly, and is greatly associated with cognitive decline, and dementia. Some investigators studied the association between brain atrophy and WMH and indicated that increased hyperintensities would accelerate brain atrophy. This was especially true by visualizing the loss of deep tissue demonstrated by an increase in ventricular size52,53.
Clinical Manifestation of CSVD
The clinical manifestations of CSVD vary depending on the specific cause of the disease, as well as the brain regions affected. Individuals may present sudden onset stroke symptoms, progressive cognitive deterioration, dementia, gait disorder, sphincter dysfunctions, and psychiatric disorders, etc.54–56.
Cognitive Decline and Dementia
SVD is thought to be among the main causes of vascular cognitive impairment, and is thought to account for about 45% of dementia cases, which is also associated with so-called silent lacunar infarcts which are asymptomatic infarcts with definite lesions on neuroimaging57–60. Cognitive decline caused by CSVD presents with executive dysfunctions, attention and memory decline, set-shifting disabilities, slower speed of information processing, decline of verbal fluency, and delayed recall. On the behavior area, symptoms showed apathic, mood disorder, depression and daily living disability1,54,56. Among others, some clinical features include sleep disorders, vertigo, tinnitus, and hearing disorder.
Neuropsychiatric Symptoms
Neuropsychiatric symptoms resulting from SVD mainly include hallucination, agitation, depression, anxiety, disinhibition, apathy, irritability, sleep disturbance, and appetite changes61. It is found that the presence of multiple cerebral microbleeds, particularly multiple lobar microbleeds, is associated with higher global neuropsychiatric burden, particularly with depression and disinhibition. Tang and colleagues reported that the emotional disinhibition was associated with CMBs, while the behavioral disinhibition symptoms are commonly found among patients with Alzheimer’s disease62.
Urinary disturbances are common in cerebral vascular pathology, which mainly include nocturia, incontinence, urinary frequency, and urgency. In the LADIS (Leukoaraiosis And Disability) study62, Poggesi and colleagues researched 639 individuals with age-related white matter changes (ARWMC) ranging from mild to severe, and reported that 70% of the participants complained of at least one urinary symptom. In addition, 60% suffered from nocturia, and approximately 20% reported urinary frequency, incontinence, and urgency. Urinary frequency and nocturia are more prevalent in men, whereas incontinence is more frequent in women. Urinary urgency is associated with the severity of ARWMC, while urinary frequency is only associated with the stroke history. In patients with Alzheimer’s disease, larger ARWMCs in volume were found to be associated with urinary incontinence.
Gait disturbance (GD), characterized by impairment of locomotion, equilibrium and gait ignition, is another common manifestation of CSVD63. Both White Matter Lesions (WMLs) and lacunar infarcts are independently associated with several gait parameters including a lower gait velocity, a shorter stride length and a reduced cadence64. De Laat and colleagues offered the first indication that microbleeds may be associated with GDs, independent of other coexisting markers of CSVD including white matter lesions and lacunar infarcts. They suggested that a higher number of microbleeds was associated with a shorter stride length, a lower gait velocity and a longer double-support time65.
Preventive and Therapeutic Measures of CSVD
The precise diagnosis of CSVD depends on neuropathological examination, which is difficult in clinical practice. Therefore, the clinical diagnosis bases on clinical features, neuroimaging of the brain parenchyma, and the ancillary use of other investigative techniques, such as ultrasonography (carotid and cardiac), combining with the risk factors. The prognosis is better than other strokes in the short term after onset, because the primary lesion is small, and the rate of recovery is often rapid.
Risk factors for lacunar infarcts include non-modifiable risk factors (age, regional distribution, sex) and acquired risk factors (hypertension, cigarette smoking, diabetes, atrial fibrillation, hyperhomocysteinemia, chronic kidney disease, high circulatory phosphate level and obesity)66–69. Controlling or removing risk factors is important for the prevention of CSVD. Hypertension is the most prevalent and important risk factor for stroke in general, as well as the most treatable factor. Many studies showed that lowering blood pressure reduces stroke70, and dementia or cognitive decline in patients who have had a stroke and in those without cerebrovascular disease. In a recent study, researchers drew a conclusion that targeting a systolic blood pressure of <120 mmHg, as compared with <140 mmHg, had a non-significant 11% lower incidence of stroke in patients at high risk for cardiovascular events but without diabetes71. Similarly, the patients whose intensive systolic blood pressure goal was <130 mmHg in the Secondary Prevention of Small Subcortical Strokes trial, and <120 mmHg in the ACCORD trial had a non-significant 19% and a significant 41% lower incidence of stroke, respectively, than the incidence with higher targets70. Statins, primarily used to lower low density lipoprotein cholesterol levels, have been reported as having cardiovascular benefits in recent years. Statins may improve vasomotor reserve capacity72 and cerebral endothelial function by inhibiting cerebral vascular superoxide production73, enhancing endothelial-derived Nitric Oxide bioavailability and decreasing oxidative stress, thus protecting against cerebral ischemia. In the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) study, patients with stroke or transient ischemic attack were treated by atorvastatin, and therefore had a significant reduction in stroke74 while high-dose atorvastatin (80 mg/d) may increase the incidence of hemorrhagic stroke75.
Intravenous tissue plasminogen activator (t-PA) is the gold standard of treatment of acute ischemic stroke, but the role of t-PA in patients with lacunar infarction has been debated due to the different pathomechanisms compared with common stroke related to large-vessel changes and increasing risks of hemorrhage in patients with WMH or CMB. Some investigators have found that the increased risk of symptomatic Intra Cerebral Hemorrhage (ICH) attributable to CMB is small and unlikely to exceed the benefits of thrombolytic therapy76,77 while there is significantly higher risk of ICH in patients with multiple CMBs78,79. A higher number of microbleeds (>10 CMBs) is associated with a higher risk for symptomatic ICH after intravenous thrombolysis (IVT) when compared with patients with 0 to 10 CMBs or 1 to 10 CMBs on pretreatment MRI. Thus, high microbleed burden may be included in individual risk stratification scores predicting ICH risk following IVT for AIS80.
Anti-platelets are generally used in ischemia stroke. The Guidelines for management of ischemic stroke and transient ischemic attack in 2008 by the European Stroke Organization Executive Committee81. However, Lundström and colleagues reported that non-responsiveness to clopidogrel or clopidogrel resistance presented in the patients with CSVD, not carotid atherosclerosis, after minor ischemia stoke and transient ischemic attack, possibly because of glucose intolerance and insulin resistance82. In the Secondary Prevention of Small Subcortical Strokes (SPS3) trial, the results showed no benefit of dual antiplatelet treatment on the prevention of recurrent stroke, and there was no significant different rate of recurrence between the patients on dual therapy (2.5%) and those taking aspirin (2.7%)83. Pearce and colleagues reported that neither dual antiplatelet treatment nor the lower blood pressure target was associated with the difference in the change of cognitive function during up to 5 years of follow up83.
There are some other therapeutic measures except for the usual methods above. Homocysteine lowering using B-vitamins was proved to reduce WMH volume increment in those with severe baseline SVD84. In addition, vitamin E tocotrienols were recently found to attenuate the progression of WMH among healthy subjects with WMH85. In the study of spontaneously hypertensive stroke-prone rats, which is the best model to simulate human cerebrovascular disease, it had been reported that chronic spironolactone treatment increased tone and reactivity of cerebral vascular and alters vascular structure of the middle cerebral artery (MCA) by increasing the lumen and outer diameter, without any change in blood pressure. Thus, these changes may enhance the autoregulatory behavior of the MCA, therefore helping to protect the brain in the event of an ischemic stroke7. In a randomized double-blind trial in CADASIL, Dichgans and colleagues reported that donepezil does not result in cognition impairment at week 18; however, improvements were detected in executive function and processing speed, but there was no additional benefit of donepezil at week 24 compared with week 1885.
CSVD is a relatively homogeneous disease process and an important cause of stroke, cognitive decline, and age-related disability. Although there have been a large number of researches on CSVD, the mechanism of vascular pathology and brain injury is still not clear, and there are numerous controversies on the prevention and management. More attention and targeted efforts are needed to better define the clinical consequences of these diseases. The main difficulty of investigating CSVD is the frequent coexistence of different forms including white matter lesions, lacunar infarcts, and microbleeds. Furthermore, more clinic trails should be investigated to study the diagnostic criteria of CSVD and the preventive and therapeutic measures to reduce the burden of disability or dementia caused by CSVD, and animal models should be established to study the specific pathogenesis of different forms of CSVD.
Footnotes
Author Contribution: Qian Li and Yang Yang contributed equally to this manuscript.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: John H. Zhang  http://orcid.org/0000-0002-4319-4285
http://orcid.org/0000-0002-4319-4285
References
- 1. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689–701. [DOI] [PubMed] [Google Scholar]
- 2. Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci. 2012;13(5):336–349. [DOI] [PubMed] [Google Scholar]
- 3. Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of functional outcome, survival, and recurrence. Stroke. 2000;31(5):1062–1068. [DOI] [PubMed] [Google Scholar]
- 4. Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12(5):483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tan R, Traylor M, Rutten-Jacobs L, Markus H. New insights into mechanisms of small vessel disease stroke from genetics. Clin Sci (Lond). 2017;131(7):515–531. [DOI] [PubMed] [Google Scholar]
- 6. Ter Telgte A, van Leijsen EMC, Wiegertjes K, Klijn CJM, Tuladhar AM, de Leeuw FE. Cerebral small vessel disease: from a focal to a global perspective. Nat Rev Neurol. 2018;14(7):387–398. [DOI] [PubMed] [Google Scholar]
- 7. Kraft P, Schuhmann MK, Garz C, Jandke S, Urlaub D, Mencl S, Zernecke A, Heinze HJ, Carare RO, Kleinschnitz C, Schreiber S. Hypercholesterolemia induced cerebral small vessel disease. Plos One. 2017;12(8):e0182822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rigsby CS, Pollock DM, Dorrance AM. Spironolactone improves structure and increases tone in the cerebral vasculature of male spontaneously hypertensive stroke-prone rats. Microvasc Res. 2007;73(3):198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lammie GA. Hypertensive cerebral small vessel disease and stroke. Brain Pathol. 2002;12(3):358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rensma SP, van Sloten TT, Launer LJ, Stehouwer CDA. Cerebral small vessel disease and risk of incident stroke, dementia and depression, and all-cause mortality: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;90:164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blair GW, Hernandez MV, Thrippleton MJ, Doubal FN, Wardlaw JM. Advanced neuroimaging of cerebral small vessel disease. Curr Treat Options Cardiovasc Med. 2017;19(7):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gartner V, Eigentler TK. Pathogenesis of diabetic macro- and microangiopathy. Clin Nephrol. 2008;70(1):1–9. [DOI] [PubMed] [Google Scholar]
- 13. Vinters HV. Cerebral amyloid angiopathy. A critical review. Stroke. 1987;18(2):311–324. [DOI] [PubMed] [Google Scholar]
- 14. Sacco RL. Lobar intracerebral hemorrhage. N Engl J Med. 2000;342(4):276–279. [DOI] [PubMed] [Google Scholar]
- 15. Thal DR, Ghebremedhin E, Orantes M, Wiestler OD. Vascular pathology in Alzheimer disease: correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J Neuropathol Exp Neurol. 2003;62(12):1287–1301. [DOI] [PubMed] [Google Scholar]
- 16. Zhang CE, Wong SM, van de Haar HJ, Staals J, Jansen JF, Jeukens CR, Hofman PA, van Oostenbrugge RJ, Backes WH. Blood-brain barrier leakage is more widespread in patients with cerebral small vessel disease. Neurology. 2017;88(5):426–432. [DOI] [PubMed] [Google Scholar]
- 17. Arvanitakis Z, Leurgans SE, Wang Z, Wilson RS, Bennett DA, Schneider JA. Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann Neurol. 2011;69(2):320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shi Y, Wardlaw JM. Update on cerebral small vessel disease: a dynamic whole-brain disease. Stroke Vasc Neurol. 2016;1(3):83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, Launer LJ, Van Buchem MA, Breteler MM, Microbleed Study G. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8(2):165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Soontornniyomkij V, Lynch MD, Mermash S, Pomakian J, Badkoobehi H, Clare R, Vinters HV. Cerebral microinfarcts associated with severe cerebral beta-amyloid angiopathy. Brain Pathol. 2010;20(2):459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salat DH, Smith EE, Tuch DS, Benner T, Pappu V, Schwab KM, Gurol ME, Rosas HD, Rosand J, Greenberg SM. White matter alterations in cerebral amyloid angiopathy measured by diffusion tensor imaging. Stroke. 2006;37(7):1759–1764. [DOI] [PubMed] [Google Scholar]
- 22. Holland CM, Smith EE, Csapo I, Gurol ME, Brylka DA, Killiany RJ, Blacker D, Albert MS, Guttmann CR, Greenberg SM. Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke. 2008;39(4):1127–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kraft P, Schuhmann MK, Garz C, Jandke S, Urlaub D, Mencl S, Zernecke A, Heinze HJ, Carare RO, Kleinschnitz C, Schreiber S. Amyloid-beta contributes to blood-brain barrier leakage in transgenic human amyloid precursor protein mice and in humans with cerebral amyloid angiopathy. Stroke 2012;43(2):514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taheri S, Gasparovic C, Huisa BN, Adair JC, Edmonds E, Prestopnik J, Grossetete M, Shah NJ, Wills J, Qualls C, Rosenberg GA. Blood-brain barrier permeability abnormalities in vascular cognitive impairment. Stroke. 2011;42(8):2158–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yamamoto Y, Craggs L, Baumann M, Kalimo H, Kalaria RN. Review: molecular genetics and pathology of hereditary small vessel diseases of the brain. Neuropathol Appl Neurobiol. 2011;37(1):94–113. [DOI] [PubMed] [Google Scholar]
- 26. Jastrzebski K, Kacperska MJ, Majos A, Grodzka M, Glabinski A. Hemorrhagic stroke, cerebral amyloid angiopathy, Down syndrome and the Boston criteria. Neurol Neurochir Pol. 2015;49(3):193–196. [DOI] [PubMed] [Google Scholar]
- 27. Nakagawa M, Sekijima Y, Yazaki M, Tojo K, Yoshinaga T, Doden T, Koyama J, Yanagisawa S, Ikeda S. Carpal tunnel syndrome: a common initial symptom of systemic wild-type ATTR (ATTRwt) amyloidosis. Amyloid. 2016;23(1):58–63. [DOI] [PubMed] [Google Scholar]
- 28. Conceicao I, Gonzalez-Duarte A, Obici L, Schmidt HH, Simoneau D, Ong ML, Amass L. “Red-flag” symptom clusters in transthyretin familial amyloid polyneuropathy. J Peripher Nerv Syst. 2016;21(1):5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baraldi O, Grandinetti V, Donati G, Comai G, Battaglino G, Cuna V, Capelli I, Sala E, La Manna G. Hematopoietic Cell and Renal Transplantation in Plasma Cell Dyscrasia Patients. Cell Transplant. 2016;25(6):995–1005. [DOI] [PubMed] [Google Scholar]
- 30. Tanabe H, Maki Y, Urabe S, Higuchi I, Obayashi K, Hokezu Y. Myopathy in a patient with systemic AA amyloidosis possibly induced by psoriasis vulgaris: an autopsy case. Muscle Nerve. 2015;52(6):1113–1117. [DOI] [PubMed] [Google Scholar]
- 31. De Silva TM, Miller AA. Cerebral small vessel disease: targeting oxidative stress as a novel therapeutic strategy? Front Pharmacol. 2016;7:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gatti JR, Zhang X, Korcari E, Lee SJ, Greenstone N, Dean JG, Maripudi S, Wang MM. Redistribution of mature smooth muscle markers in brain arteries in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Transl Stroke Res. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang X, Meng H, Blaivas M, Rushing EJ, Moore BE, Schwartz J, Lopes MB, Worrall BB, Wang MM. Von willebrand factor permeates small vessels in CADASIL and inhibits smooth muscle gene expression. Transl Stroke Res. 2012;3(1):138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moore DF, Altarescu G, Barker WC, Patronas NJ, Herscovitch P, Schiffmann R. White matter lesions in Fabry disease occur in ‘prior’ selectively hypometabolic and hyperperfused brain regions. Brain Res Bull. 2003;62(3):231–240. [DOI] [PubMed] [Google Scholar]
- 35. Hilz MJ, Kolodny EH, Brys M, Stemper B, Haendl T, Marthol H. Reduced cerebral blood flow velocity and impaired cerebral autoregulation in patients with Fabry disease. J Neurol. 2004;251(5):564–570. [DOI] [PubMed] [Google Scholar]
- 36. Itoh Y, Esaki T, Cook M, Qasba P, Shimoji K, Alroy J, Brady RO, Sokoloff L, Moore DF. Local and global cerebral blood flow and glucose utilization in the alpha-galactosidase A knockout mouse model of Fabry disease. J Neurochem. 2001;79(6):1217–1224. [DOI] [PubMed] [Google Scholar]
- 37. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Okazaki S, Hornberger E, Griebe M, Gass A, Hennerici MG, Szabo K. MRI characteristics of the evolution of supratentorial recent small subcortical infarcts. Front Neurol. 2015;6:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Potter GM, Doubal FN, Jackson CA, Chappell FM, Sudlow CL, Dennis MS, Wardlaw JM. Enlarged perivascular spaces and cerebral small vessel disease. Int J Stroke. 2015;10(3):376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gattringer T, Eppinger S, Pinter D, Pirpamer L, Berghold A, Wunsch G, Ropele S, Wardlaw JM, Enzinger C, Fazekas F. Morphological MRI characteristics of recent small subcortical infarcts. Int J Stroke. 2015;10(7):1037–1043. [DOI] [PubMed] [Google Scholar]
- 41. Moreau F, Patel S, Lauzon ML, McCreary CR, Goyal M, Frayne R, Demchuk AM, Coutts SB, Smith EE. Cavitation after acute symptomatic lacunar stroke depends on time, location, and MRI sequence. Stroke. 2012;43(7):1837–1842. [DOI] [PubMed] [Google Scholar]
- 42. Loos CM, Staals J, Wardlaw JM, van Oostenbrugge RJ. Cavitation of deep lacunar infarcts in patients with first-ever lacunar stroke: a 2-year follow-up study with MR. Stroke. 2012;43(8):2245–2247. [DOI] [PubMed] [Google Scholar]
- 43. Koch S, McClendon MS, Bhatia R. Imaging evolution of acute lacunar infarction: leukoariosis or lacune? Neurology. 2011;77(11):1091–1095. [DOI] [PubMed] [Google Scholar]
- 44. Potter GM, Doubal FN, Jackson CA, Chappell FM, Sudlow CL, Dennis MS, Wardlaw JM. Counting cavitating lacunes underestimates the burden of lacunar infarction. Stroke. 2010;41(2):267–272. [DOI] [PubMed] [Google Scholar]
- 45. Schmidt R, Grazer A, Enzinger C, Ropele S, Homayoon N, Pluta-Fuerst A, Schwingenschuh P, Katschnig P, Cavalieri M, Schmidt H, Langkammer C, Ebner F, Fazekas F. MRI-detected white matter lesions: do they really matter? J Neural Transm (Vienna). 2011;118(5):673–681. [DOI] [PubMed] [Google Scholar]
- 46. Bastin ME, Clayden JD, Pattie A, Gerrish IF, Wardlaw JM, Deary IJ. Diffusion tensor and magnetization transfer MRI measurements of periventricular white matter hyperintensities in old age. Neurobiol Aging. 2009;30(1):125–136. [DOI] [PubMed] [Google Scholar]
- 47. Cordonnier C, Potter GM, Jackson CA, Doubal F, Keir S, Sudlow CL, Wardlaw JM, Al-Shahi Salman R. improving interrater agreement about brain microbleeds: development of the Brain Observer MicroBleed Scale (BOMBS). Stroke. 2009;40(1):94–99. [DOI] [PubMed] [Google Scholar]
- 48. Moulin S, Labreuche J, Bombois S, Rossi C, Boulouis G, Henon H, Duhamel A, Leys D, Cordonnier C. Dementia risk after spontaneous intracerebral haemorrhage: a prospective cohort study. Lancet Neurol. 2016;15(8):820–829. [DOI] [PubMed] [Google Scholar]
- 49. Aribisala BS, Valdes Hernandez MC, Royle NA, Morris Z, Munoz Maniega S, Bastin ME, Deary IJ, Wardlaw JM. Brain atrophy associations with white matter lesions in the ageing brain: the Lothian Birth Cohort 1936. Eur Radiol. 2013;23(4):1084–1092. [DOI] [PubMed] [Google Scholar]
- 50. Riba-Llena I, Nafría C, Mundet X, López-Rueda A, Fernández-Cortiñas I, Jarca CI, Jiménez-Balado J, Domingo M, Tovar JL, Orfila F, Pujadas F, Álvarez-Sabín J, Maisterra O, Montaner J, Delgado P. Assessment of enlarged perivascular spaces and their relation to target organ damage and mild cognitive impairment in patients with hypertension. Eur J Neurol. 2016;23(6):1044–1050. [DOI] [PubMed] [Google Scholar]
- 51. Jokinen H, Lipsanen J, Schmidt R, Fazekas F, Gouw AA, van der Flier WM, Barkhof F, Madureira S, Verdelho A, Ferro JM, Wallin A, Pantoni L, Inzitari D, Erkinjuntti T; LADIS Study Group. Brain atrophy accelerates cognitive decline in cerebral small vessel disease: the LADIS study. Neurology. 2012;78(22):1785–1792. [DOI] [PubMed] [Google Scholar]
- 52. Muller M, Appelman AP, van der Graaf Y, Vincken KL, Mali WP, Geerlings MI. Brain atrophy and cognition: interaction with cerebrovascular pathology? Neurobiol Aging. 2011;32(5):885–893. [DOI] [PubMed] [Google Scholar]
- 53. Mok V, Wong KK, Xiong Y, Wong A, Schmidt R, Chu W, Hu X, Leung EY, Chen S, Chen Y, Tang WK, Chen X, Ho CL, Wong KS, Wong ST. Cortical and frontal atrophy are associated with cognitive impairment in age-related confluent white-matter lesion. J Neurol Neurosurg Psychiatry. 2011;82(1):52–57. [DOI] [PubMed] [Google Scholar]
- 54. de Laat KF, Tuladhar AM, van Norden AG, Norris DG, Zwiers MP, de Leeuw FE. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain. 2011;134(Pt 1):73–83. [DOI] [PubMed] [Google Scholar]
- 55. van der Flier WM, van Straaten EC, Barkhof F, Verdelho A, Madureira S, Pantoni L, Inzitari D, Erkinjuntti T, Crisby M, Waldemar G, Schmidt R, Fazekas F, Scheltens P. Small vessel disease and general cognitive function in nondisabled elderly: the LADIS study. Stroke. 2005;36(10):2116–2120. [DOI] [PubMed] [Google Scholar]
- 56. Del Bene A, Makin SD, Doubal FN, Inzitari D, Wardlaw JM. Variation in risk factors for recent small subcortical infarcts with infarct size, shape, and location. Stroke. 2013;44(11):3000–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(9):2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xu X, Chan QL, Hilal S, Goh WK, Ikram MK, Wong TY, Cheng CY, Chen CL, Venketasubramanian N. Cerebral microbleeds and neuropsychiatric symptoms in an elderly Asian cohort. J Neurol Neurosurg Psychiatry. 2017;88(1):7–11. [DOI] [PubMed] [Google Scholar]
- 59. Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6(7):611–619. [DOI] [PubMed] [Google Scholar]
- 60. Yang Y, Kimura-Ohba S, Thompson J, Rosenberg GA. Rodent models of vascular cognitive impairment. Transl Stroke Res. 2016;7(5):407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tang WK, Chen YK, Lu JY, Mok VC, Xiang YT, Ungvari GS, Ahuja AT, Wong KS. Microbleeds and post-stroke emotional lability. J Neurol Neurosurg Psychiatry. 2009;80(10):1082–1086. [DOI] [PubMed] [Google Scholar]
- 62. Poggesi A, Pracucci G, Chabriat H, Erkinjuntti T, Fazekas F, Verdelho A, Hennerici M, Langhorne P, O’Brien J, Scheltens P, Visser MC, Crisby M, Waldemar G, Wallin A, Inzitari D, Pantoni L; Leukoaraiosis And DISability Study Group. Urinary complaints in nondisabled elderly people with age-related white matter changes: the Leukoaraiosis And DISability (LADIS) Study. J Am Geriatr Soc. 2008;56(9):1638–1643. [DOI] [PubMed] [Google Scholar]
- 63. Iseki K, Hanakawa T, Hashikawa K, Tomimoto H, Nankaku M, Yamauchi H, Hallett M, Fukuyama H. Gait disturbance associated with white matter changes: a gait analysis and blood flow study. Neuroimage. 2010;49(2):1659–1666. [DOI] [PubMed] [Google Scholar]
- 64. de Laat KF, van Norden AG, Gons RA, van Oudheusden LJ, van Uden IW, Bloem BR, Zwiers MP, de Leeuw FE. Gait in elderly with cerebral small vessel disease. Stroke. 2010;41(8):1652–1658. [DOI] [PubMed] [Google Scholar]
- 65. de Laat KF, van den Berg HA, van Norden AG, Gons RA, Olde Rikkert MG, de Leeuw FE. Microbleeds are independently related to gait disturbances in elderly individuals with cerebral small vessel disease. Stroke. 2011;42(2):494–497. [DOI] [PubMed] [Google Scholar]
- 66. Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, Culebras A, Degraba TJ, Gorelick PB, Guyton JR, Hart RG, Howard G, Kelly-Hayes M, Nixon JV, Sacco RL; American Heart Association/American Stroke Association Stroke Council; Atheroscleroti. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37(6):1583–1633. [DOI] [PubMed] [Google Scholar]
- 67. Norrving B. Long-term prognosis after lacunar infarction. Lancet Neurol. 2003;2(4):238–245. [DOI] [PubMed] [Google Scholar]
- 68. Lau WL, Huisa BN, Fisher M. The cerebrovascular-chronic kidney disease connection: perspectives and mechanisms. Transl Stroke Res. 2017;8(1):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chung CP, Peng LN, Chou KH, Liu LK, Lee WJ, Lin CP, Chen LK, Wang PN. High circulatory phosphate level is associated with cerebral small-vessel diseases. Transl Stroke Res. 2018. [DOI] [PubMed] [Google Scholar]
- 70. Group SPSS, Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, Pearce LA, Pergola PE, Szychowski JM. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382(9891):507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Group SR Wright JT Jr., Williamson JD Whelton PK Snyder JK Sink KM Rocco MV Reboussin DM Rahman M Oparil S and others . A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sander K, Hof U, Poppert H, Conrad B, Sander D. Improved cerebral vasoreactivity after statin administration in healthy adults. J Neuroimaging. 2005;15(3):266–270. [DOI] [PubMed] [Google Scholar]
- 73. Erdos B, Snipes JA, Tulbert CD, Katakam P, Miller AW, Busija DW. Rosuvastatin improves cerebrovascular function in Zucker obese rats by inhibiting NAD(P)H oxidase-dependent superoxide production. Am J Physiol Heart Circ Physiol. 2006;290(3):H1264–H1270. [DOI] [PubMed] [Google Scholar]
- 74. Amarenco P1, Benavente O, Goldstein LB, Callahan A, 3rd, Sillesen H, Hennerici MG, Gilbert S, Rudolph AE, Simunovic L, Zivin JA, Welch KM; Stroke Prevention by Aggressive Reduction in Cholesterol Levels Investigators. Results of the stroke prevention by aggressive reduction in cholesterol levels (SPARCL) trial by stroke subtypes. Stroke. 2009;40(4):1405–1409. [DOI] [PubMed] [Google Scholar]
- 75. Amarenco P, Bogousslavsky J, Callahan A, 3rd, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA; Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549–559. [DOI] [PubMed] [Google Scholar]
- 76. Charidimou A, Kakar P, Fox Z, Werring DJ. Cerebral microbleeds and the risk of intracerebral haemorrhage after thrombolysis for acute ischaemic stroke: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2013;84(3):277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fiehler J, Albers GW, Boulanger JM, Derex L, Gass A, Hjort N, Kim JS, Liebeskind DS, Neumann-Haefelin T, Pedraza S, Rother J, Rothwell P, Rovira A, Schellinger PD, Trenkler J; MR STROKE Group. Bleeding risk analysis in stroke imaging before thromboLysis (BRASIL): pooled analysis of T2*-weighted magnetic resonance imaging data from 570 patients. Stroke. 2007;38(10):2738–2744. [DOI] [PubMed] [Google Scholar]
- 78. Dannenberg S, Scheitz JF, Rozanski M, Erdur H, Brunecker P, Werring DJ, Fiebach JB, Nolte CH. Number of cerebral microbleeds and risk of intracerebral hemorrhage after intravenous thrombolysis. Stroke. 2014;45(10):2900–2905. [DOI] [PubMed] [Google Scholar]
- 79. Akhtar N, Salam A, Kamran S, D’Souza A, Imam Y, Own A, ElSotouhy A, Vattoth S, Bourke P, Bhutta Z, Joseph S, Santos M, Khan RA, Shuaib A. Pre-existing small vessel disease in patients with acute stroke from the middle east, southeast asia, and philippines. Transl Stroke Res. 2018;9(3):274–282. [DOI] [PubMed] [Google Scholar]
- 80. Tsivgoulis G, Zand R, Katsanos AH, Turc G, Nolte CH, Jung S, Cordonnier C, Fiebach JB, Scheitz JF, Klinger-Gratz PP, Oppenheim C, Goyal N, Safouris A, Mattle HP, Alexandrov AW, Schellinger PD, Alexandrov AV. Risk of symptomatic intracerebral hemorrhage after intravenous thrombolysis in patients with acute ischemic stroke and high cerebral microbleed burden: a meta-analysis. JAMA Neurol. 2016;73(6):675–683. [DOI] [PubMed] [Google Scholar]
- 81. European Stroke Organisation Executive C, Committee ESOW. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25(5):457–507. [DOI] [PubMed] [Google Scholar]
- 82. Lundstrom A, Wallen H, von Arbin M, Jorneskog G, Gigante B, Hoeg Dembrower K, Laurencikas E, Laska AC. Clopidogrel resistance after minor ischemic stroke or transient ischemic attack is associated with radiological cerebral small-vessel disease. J Stroke Cerebrovasc Dis. 2015;24(10):2348–2357. [DOI] [PubMed] [Google Scholar]
- 83. Pearce LA, McClure LA, Anderson DC, Jacova C, Sharma M, Hart RG, Benavente OR, Investigators SPS. Effects of long-term blood pressure lowering and dual antiplatelet treatment on cognitive function in patients with recent lacunar stroke: a secondary analysis from the SPS3 randomised trial. Lancet Neurol. 2014;13(12):1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cavalieri M, Schmidt R, Chen C, Mok V, de Freitas GR, Song S, Yi Q, Ropele S, Grazer A, Homayoon N, Enzinger C, Loh K, Wong KS, Wong A, Xiong Y, Chang HM, Wong MC, Fazekas F, Eikelboom JW, Hankey GJ; VITATOPS Trial Study Group. B vitamins and magnetic resonance imaging-detected ischemic brain lesions in patients with recent transient ischemic attack or stroke: the VITAmins TO Prevent Stroke (VITATOPS) MRI-substudy. Stroke. 2012;43(12):3266–3270. [DOI] [PubMed] [Google Scholar]
- 85. Dichgans M, Markus HS, Salloway S, Verkkoniemi A, Moline M, Wang Q, Posner H, Chabriat HS. Donepezil in patients with subcortical vascular cognitive impairment: a randomised double-blind trial in CADASIL. Lancet Neurol. 2008;7(4):310–318. [DOI] [PubMed] [Google Scholar]