Abstract
Non-coding RNAs (ncRNAs) are a class of functional RNAs that regulate gene expression in a post-transcriptional manner. NcRNAs include microRNAs, long non-coding RNAs and circular RNAs. They are highly expressed in the brain and are involved in the regulation of physiological and pathophysiological processes, including cerebral ischemic injury, neurodegeneration, neural development, and plasticity. Stroke is one of the leading causes of death and physical disability worldwide. Acute ischemic stroke (AIS) occurs when brain blood flow stops, and that stoppage results in reduced oxygen and glucose supply to cells in the brain. In this article, we review the latest progress on ncRNAs in relation to their implications in AIS, as well as their potential as diagnostic and prognostic biomarkers. We also review ncRNAs acting as possible therapeutic targets in future precision medicine. Finally, we conclude with a brief discussion of current challenges and future directions for ncRNAs studies in AIS, which may facilitate the translation of ncRNAs research into clinical practice to improve clinical outcome of AIS.
Keywords: Non-coding RNAs, ischemic stroke, cerebral ischemic injury, biomarker, therapeutic target
Introduction
Stroke is one of the leading causes of death and long-term disability, causing a high economic burden to society in both developed and developing countries1. Ischemic stroke, which accounts for 80% of all strokes, is the result of cerebral artery occlusion that decreases cerebral blood flow and causes rapid loss of brain functions2. The improvements in current treatments for cerebral ischemia are limited by many factors, particularly a narrow therapeutic window and an incomplete understanding of the cellular and molecular changes following acute ischemic stroke (AIS)3. Therefore, achieving an understanding of the pathogenesis and underlying mechanisms of cerebral ischemic injury is urgent, as it will help develop novel diagnostic and therapeutic targets for patients with AIS.
Noncoding RNAs (ncRNAs), a class of genetic, epigenetic and translational regulators, consists of microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs), each of which play important physiological and pathological roles by controlling transcription and translation4–6. NcRNAs are abundantly expressed in mammalian brains while recent studies show that cerebral ischemia alters ncRNAs expression profiles7–9. A growing number of studies have demonstrated that ncRNAs (especially miRNAs and lncRNAs) play a role in the pathogenic processes related to cerebral ischemia and post-stroke recovery3,10,11. These pathogenic processes include excitotoxicity, oxidative stress, neuroinflammation, and apoptosis, which can cause secondary brain damage and can impede functional recovery in patients with AIS12. However, literature on the circRNAs implicated in cerebral ischemic injury remains unknown.
MiRNAs, small molecules of 21–25 nucleotides in length, are a highly abundant and evolutionarily conservative class of endogenous ncRNAs. They inhibit translation and degrade the respective mRNA through imperfect or near perfect base pairing, mostly to the 3′ untranslated region (UTR) of target mRNAs13. LncRNAs, usually defined as having more than 200 nucleotides, are cell- and tissue-specific. They can be subclassified by their functionality, by the genomic location contained between gene coding regions (long intergenic ncRNAs), or by overlapping cording genes in either sense or anti-sense directions14. LncRNAs function as guides for chromatin-modifying-complexes or transcription factors in the nucleus15. Cytoplasm lncRNAs traditionally regulate the translation of mRNA by controlling mRNA stability or acting as competing endogenous RNA (ceRNA)16. CircRNAs (single-stranded and conserved RNA molecules) are formed by backsplicing of many primary RNA transcripts from which mRNAs are synthetized17. They are extremely stable and are not degraded by RNaseR, owing to the absence of defined 5′ and 3′ ends18. CircRNAs can control gene expression by various mechanisms, including functioning as ceRNA by sponging miRNA, forming ternary complexes with proteins, and encoding proteins19–21.
Advances in preclinical studies have established underlying mechanisms of cerebral ischemic injury resulting from dysregulation of ncRNAs, and have identified potential biomarkers and therapeutic targets to treat cerebral ischemia. However, to date, none of these advances have been successfully translated into clinical practice. The aim of this review is to provide a systemic description of the complex functions of ncRNAs in cerebral ischemia, and how these basic research findings could be translated into clinical practice. We discuss the functions and underlying molecular mechanisms of ncRNAs in AIS, and then explain the roles of ncRNAs as potential biomarkers. Next, we provide examples in which ncRNAs act as therapeutic targets, and then conclude with an outlook about how ncRNAs may impact on the prevention and treatment for AIS in the future.
Functions and Molecular Mechanisms of ncRNAs in Cerebral Ischemia
ncRNAs involved in nuclear factor kappa-B (NF-κB) signaling pathway
Inflammatory reaction is crucial to the pathogenesis of brain tissue damage in cerebral ischemia. Proinflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor alfa (TNF-α) are induced by molecules released from injured tissue, blood vessels, and necrotic cells in ischemic brain injury. This results in inflammation, leading to exacerbatation of primary brain damage22. The NF-κB signaling pathway, which regulates the expression of several genes involved in inflammatory responses, is activated by these cytokines 23. In non-stimulated conditions, NF-κB is sequestered in the cytoplasm through its interaction with κB inhibitor (IκB). In response to inflammatory signaling, IκB is phosphorylated by the IκB kinase (IKK) complex and then ubiquitylated by β-TRC, leading to its degradation through the proteasome. This contributes to the release of NF-κB. Next, it enters the nucleus and binds to its transcriptional targets including genes that encode pro-inflammatory cytokines, chemokines, and leukocyte adhesion molecules. The expression of inflammation-related signaling pathway molecules is regulated not only by transcription factors, but also by ncRNAs at the post-transcriptional level24.
The emerging evidence suggests that ncRNAs modulates many aspects of the canonical or noncanonical transcription factor NF-κB pathway (Table 1). This pathway is involved in various neurological pathologies underlying cerebral ischemic injury25. MiR-146a plays an important role in inhibiting NF-κB signaling through the targeting of tumor necrosis factor receptor associated factor 6 (TRAF6) and receptor-associated kinase-1 (IRAK1), two adaptor proteins that act upstream of the NF-κB pathway26. In aged-rat stroke models, the downregulation of miR-146a increases the expression of IRAK1, activating NF-κB in cerebral vasculature. This can exacerbate ischemic brain damage; monotherapy of tissue plasminogen activator (tPA) further amplifies these detrimental process; VELCADE, a potent proteasome inhibitor, inactivates NF-κB through the upregulation of miR-146a, resulting in post-stroke neuroprotection in aged rats27. MiR-155, an inflammation-related miRNA, is induced in mouse ischemic cerebral cortexes by middle cerebral artery occlusion (MCAO)28. MiR-155 promotes TNF-α and IL-1β expression by upregulating toll-like receptor 4 (TLR4) expression, and downregulating suppressor of cytokine signaling (SOCS1) and myeloid differentiation primary response 88 (MyD88) expression. This leads to the activation of NF-κB signaling in the ischemic cerebral tissue of MCAO mice and oxygen and glucose deprivation/reoxygenation (OGD/R)-treated BV2 cells; acetylbritannilactone exerts potent anti-inflammatory actions by suppressing miR-155 expression29. MiR-9 inhibits the expression of proinflammatory molecules by targeting NF-κB in rat models of MCAO30.
Table 1.
Emerging roles of ncRNAs on neuroinflammation and oxidative stress in AIS.
| NcRNAs | Targets | Mechanisms | Function of ncRNAs | Reference(s) |
|---|---|---|---|---|
| miR-146a | TRAF6, IRAK1 | Activate NF-κB in cerebral vasculature | Promote neuroinflammation | Zhang et al.27 |
| miR-155 | TLR4, SOCS1 and MyD88 | Promote TNF-α and IL-1β expression | Promote neuroinflammation | Wen et al.29 |
| miR-9 | NF-κB | Inhibit NF-κB, p65, TNF-α and IL-1β expression | Inhibit neuroinflammation | Liu et al.30 |
| lncRNAC2dat1 | CaMKIIδ | Activation of NF-κB signaling pathway | Promote neuroinflammation | Xu et al.31 |
| lncRNA ANRIL | VEGF | Enhance phosphorylation of IκB | Promote neuroinflammation | Zhang et al.32 |
| lncRNA Gm4419 | IκB, NF-κB | Increase the expression of IL-1β, IL-6 and TNF-α | Promote neuroinflammation | Wen et al.33 |
| miR-23a-3p | – | Reduce NO and 3-NT, increase MnSOD | Attenuate oxidative stress | Zhao et al.39 |
| miR-424 | Nrf2 | Decrease LDH leakage and MDA level, promote MnSOD activity | Inhibit oxidative stress | Liu et al.40 |
| miR-106b-5p | Mcl-1, Bcl-2 | Reduce LDH release and elevate SOD activity, decrease MDA content | Inhibit oxidative stress | Li et al.41 |
AIS: acute ischemic stroke, ncRNA, non-coding RNA; miRNA, microRNA; lncRNA, long non-coding RNA; TRAF6, tumor necrosis factor receptor associated factor 6; IRAK1, receptor-associated kinase-1; TLR4, Toll-like receptor 4; SOCS1, suppressor of cytokine signaling 1; MyD88, myeloid differentiation primary response 88; TNF-α, tumor necrosis factor alfa; IL-1β, interleukin-1β; NF-κB, nuclear factor kappa-B; CaMKIIδ, Calcium/Calmodulin-dependent Protein Kinase II Delta; VEGF, vascular endothelial growth factor; IκB, κB inhibitor; Nrf2, nuclear factor erythroid 2-related factor; LDH, lactate dehydrogenase; NO, nitric oxide; MDA, malondialdehyde; MnSOD, manganese superoxide dismutase; 3-NT, 3-nitrotyrosine; Mcl-1, myeloid cell leukemia-1; Bcl-2, B cell lymphoma-2.
LncRNAs are also involved in the regulation of neuro-inflammation in ischemic brain injury. Several lncRNAs are active in NF-κB signaling pathway in cerebral ischemic injury. LncRNAC2dat1 regulates Calcium/Calmodulin-dependent Protein Kinase II Delta (CaMKIIδ) expression via activation of NF-κB signaling pathway, and promotes neuronal survival in murine models of focal cerebral ischemia31. The overexpression of lncRNA ANRIL activates NF-κB signaling pathway by enhancing phosphorylation of IκB. This upregulates vascular endothelial growth factor (VEGF) and promotes angiogenesis in diabetes mellitus, combined with cerebral infarction in rat models32. The abnormal upregulation of LncRNA Gm4419 causes neuroinflammation injury during OGD/R through IκB phosphorylation and NF-κB activation, which increases the expression of proinflammatory cytokines such as IL-1β, IL-6 and TNF-α in OGD/R-treated microglial cells33.
ncRNAs involved in oxidative stress
Oxidative stress, an imbalance between upregulated expression of reactive oxygen species (ROS) and reactive nitrogen species (RNS) and missing antioxidants, contributes to secondary brain damage after cerebral ischemia, via oxidative modifications of proteins, lipids, and DNA34. Both ischemia and reperfusion elevate the production of ROS, such as superoxide anion, hydrogen peroxide (H2O2), hydroxyl radical, and singlet oxygen35,36. Increased ROS levels injure neurons and glia by promoting mitochondrial dysfunction, the activation of inflammatory signaling, calpain activation, and apoptosis37. This determines the infarct volume and the recovery of neurological function following cerebral ischemia. Emerging evidence has indicated that ncRNAs play an important role in regulating the balance between oxidants and antioxidants in experimental stroke models in vivo and in vitro38.
MiRNAs either enhance or attenuate oxidative stress damage in animal models of ischemia/reperfusion (I/R) (Table 1). MiR-23a-3p reduces H2O2 induced lactate dehydrogenase (LDH) leakage and production of nitric oxide (NO) and 3-nitrotyrosine (3-NT). It also decreases the expression of caspase-3 in neuro-2a cells. MiR-23a-3p reduces peroxidative production NO and 3-NT, and increases the expression of manganese superoxide dismutase (SOD) through intra-cerebroventricular injection of miR-23a-3p angomir. This leads to dramatic decrease cerebral infarction volume in mouse MCAO models39. Similarly, miR-424 inhibits oxidative stress to protect neurons against focal cerebral I/R injury in mice. In vitro miR-424 precludes H2O2-induced injury to neurons by decreasing LDH leakage and malondialdehyde (MDA) level. It also promotes manganese SOD activity. Meanwhile, the function of miR-424 suppressing oxidative stress is reversed by nuclear factor erythroid 2-related factor (Nrf2) knockdown and SOD inhibitor treatment. Intra-cerebroventricular injection of miR-424 angomir can reduce cerebral infarction volume and inhibit neuronal apoptosis after I/R, partially through keeping balance between oxidants and anti-oxidants in mouse cortexes40. MiR-106b-5p increases in serum of patients with AIS, which is associated with poor prognosis. MiR-106b-5p inhibitor upregulates the myeloid cell leukemia-1(Mcl-1) and B cell lymphoma-2 (Bcl-2) expression, guarding against glutamate-induced apoptosis and oxidative stress injury, as evidenced by reduced LDH release and elevated SOD activity in PC12 cells. MiR-106b-5p antagomir treatment significantly improves outcome in the rat MCAO models. This includes decreased neurological deficit scores, infarct volumes, and neuronal damage. Furthermore, it significantly decreases MDA content, restores SOD activity, upregulates the expression of Mcl-1 and Bcl-2, and downregulates Bax expression in the rat ischemic cortexes41.
Although accumulating evidence has shown miRNAs to be essential mediators for post-transcriptional gene regulating in cerebral ischemia-related oxidative stress injury, the pathogenic mechanisms underlying the effects of lncRNAs and circRNAs in oxidative stress damage following cerebral ischemia have yet to be explored. A series of lncRNAs, such as LincRNA-p21, LncRNA-RoR, LncRNA-JADE, H19, and ANRIL, are involved in oxidative stress, which is associated with DNA damage in cancer42. There will be increasing evidence that both lncRNAs and circRNAs are considered as an important class of ncRNAs in the response to, and control of pathological events leading to oxidative stress injury following ischemic stroke.
ncRNAs involved in angiogenesis
Angiogenesis plays an important role in vascular angiogenic remodeling and neurofunctional recovery after stroke43,44. It is controlled by many key angiogenic factors in the brain. Cerebral neovascularization can allow for increased cerebral blood flow, which augments the amount of oxygen and nutrients delivered to ischemic brain tissues. Inducing angiogenesis via various treatments that target angiogenic factors appears to be a useful approach for stroke in experimental study. Accumulating evidence has shown that ncRNAs are essential mediators of both vascular endothelial cell biology and angiogenesis.
VEGF, a classical pro-angiogenic factor, plays a critical role in angiogenesis, and it increases following cerebral ischemia45. The administration of vascular endothelial growth factor A (VEGF-A) appears to enhance microvessel density in the ischemic penumbra of animal model, thus promoting post ischemic angiogenesis46. Therefore, exploring the mechanisms involved in regulation of VEGF-A after cerebral ischemia is crucial. MiR-140-5p levels are downregulated more than twofold, while the expression of VEGF-A is dramatically increased in rat cerebral tissue following MCAO. Furthermore, MiR-140-5p binds to the 3′ UTR of VEGF-A, thus leading to the negative regulation of VEGF-A proteins. In an in vitro model, miR-140-5p exerts an inhibitory effect on angiogenesis after ischemia, partially through targeting VEGF-A47. The Notch signaling pathway plays an important part in the progression and homeostasis of the vasculature, as well as in regulating the behavior of endothelial and smooth muscle cells48. MiR-137 has been shown to increase the endothelial progenitor cell proliferation and angiogenesis by targeting nuclear receptor subfamily 4 group A member 2 (NR4A2) through the Notch signaling pathway in a mouse model of cerebral ischemia49. Similarly, a hypoxia-induced upregulation of miR-210 can activate the Notch signaling pathway, which may enhance angiogenesis after cerebral ischemia50. MiR-377, miR-150, miR-107, and miR-210 contribute to angiogenesis after ischemic stroke via targeting VEGF51–54.
Antisense ncRNA in the INK4 locus (ANRIL), a 3.8-kb lncRNA, is involved in many biological processes including cell growth and proliferation55. ANRIL increases VEGF expression and enhances angiogenesis by activating the NF-κB signal pathway in diabetes mellitus combined with cerebral infarction rats32. MiR-153-3p levels decrease, whereas hypoxia inducible factor-1α (HIF-1α) and its downstream targets (VEGF-A and Notch1) are upregulated in rat permanent MCAO models. Hypoxia induces the expression of lncRNA HIF1A-AS2 in human umbilical vein endothelial cells (HUVECs). Furthermore, lncRNA HIF1A-AS2 increases HIF-1α expression by sponging miR-153-3p, and HIF1A-AS2 facilitates HUVECs viability, migration ability, and tube formation. Consequently, HIF1A-AS2 promotes angiogenesis in hypoxia through activating HIF-1α/VEGFA/Notch1 cascades by sponging miR-153-3p in HUVECs16. The expression of LncRNA MEG3 and NOX4 in the rat brain microvascular endothelial cells are increased after OGD/R. The knockdown of MEG3 protects brain microvascular endothelial cells against OGD/R-induced apoptosis by decreasing NOX4 and p53 expression, and reducing intracellular ROS. It also enhances HIF-1a and VEGF expression. Furthermore, p53 promotes NOX4 expression by directly binding in the promoters of NOX4. This result indicates that MEG3 mediates angiogenesis after cerebral infarction via regulating p53/NOX4 axis56.
ncRNAs involved in microglial polarization
Microglia are brain-resident innate immune cells that function as drivers of the neuroinflammatory response that results in secondary neuronal injury. When cerebral ischemia occurs, microglia are activated to secrete both harmful and neuroprotective cytokines/chemokines. The balance of the two opposite mediators determines the destiny of injured neurons in the focal ischemic brain area. The activated peripheral macrophages are usually divided into classic phenotype and alternative phenotype, also called M1 and M257. Microglia show a similar dynamic phenotypic response to either pathogenic or cytokine stimulation just like peripheral macrophages, ranging from an M1-like pro-inflammatory activation phenotype to the M2-like alternative activation phenotype58.M1-like microglia produce pro-inflammatory molecules, such as TNF-α, IL-6, and IL-12, which exacerbate neuronal damage; M2-like microglia secrete anti-inflammatory cytokines that play a role in neuroprotection59.
Increasing evidence has shown that miRNAs regulate the inflammatory activation of microglia (Table 2). MiR-424 levels decrease in the plasma of patients with AIS and in the mouse brain tissues at 4, 8, and 24 h after MCAO ischemia. Both cerebral infarction size and brain edema are lessened after overexpression of miR-424 in a mouse MCAO model. Furthermore, in vitro experiments have found that miR-424 suppressed neuronal apoptosis and microglial activation. This study implied that miR-424 protects against ischemic cerebral damage by inhibiting the activation of microglia by post-transcriptional regulating the expression of proteins involved in cell cycle. This includes CCND1, CDC25A, and CDK660. The knockdown of miR-377 promotes angiogenesis and inhibits microglial activation by targeting VEGF and early growth response protein 2 (EGR2), thus alleviating ischemic brain injury in rat MCAO models and microglial cells51. In a study, the integrated expression profiles of mRNA and miRNA have been depicted in polarized primary murine microglia through microarrays and bioinformatics analysis. MiR-689, miR-124, and miR-155 were shown as predictors for pro-inflammatory pathways and M1-like activation phenotypes. MiR-711 and miR-145 were found as the predictors mediating anti-inflammatory signals and M2-like activation phenotypes58.
Table 2.
NcRNAs involved in the inflammatory activation of microglia in AIS.
| NcRNAs | Targets | Mechanisms | Inflammatory activation of microglia | Reference(s) |
|---|---|---|---|---|
| miR-424 | CCND1, CDC25A, and CDK6 | Regulate cell cycle | Inhibit | Zhao et al.60 |
| miR-377 | VEGF and EGR2 | – | Promote | Fan et al.51 |
| miR-689, miR-124, and miR-155 | – | – | Promote | Freilich et al.58 |
| miR-771 and miR-145 | – | – | Inhibit | Freilich et al.58 |
| lncRNA SNHG14 | PLA2G4A | Sponge miR-145-5p | Promote | Qi et al.61 |
| lncRNA Gm4419 | IκB, NF-κB | Increase the expression of IL-1β, IL-6 and TNF-α | Promote | Wen et al.33 |
| lncRNA H19 | HDAC1 | – | Promote | Wang et al.62 |
AIS: acute ischemic stroke, ncRNA, non-coding RNA; miRNA, microRNA; lncRNA, long non-coding RNA; CCND1, cyclin D1; CDC25A, cell division cycle 25 homolog A; CDK6, cyclin-dependent kinase 6; VEGF, vascular endothelial growth factor; EGR2, growth response protein 2; PLA2G4A, cytosolic phospholipase A2; HDAC1, histone deacetylase 1.
Little is known about the lncRNAs and circRNAs in inflammatory activation of microglia (Table 2). LncRNA SNHG14 is highly expressed in ischemic brain tissues of mouse MCAO models and BV-2 cells after OGD treatment. LncRNA SNHG14 promotes microglial cells activation by upregulating the expression of cytosolic phospholipase A2(PLA2G4A) via sponging miR-145-5p, and contributes to an increase in TNF-a and NO production as well as neuronal apoptosis in cerebral infarction61. LncRNA Gm4419, which has emerged as a critical mediator in the NF-κB signaling pathway activation via IκBα phosphorylation, is induced during OGD/R injury to microglial cells. It promotes production of pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-1β, leading to OGD/R injury as cell apoptosis and death33. The knockdown of H19, a classic lncRNA that is highly expressed in the plasma of patients with AIS and mouse MCAO models, blocks OGD/R-induced M1 microglial polarization to decrease the expression of TNF-α and CD11b. It also upregulates the production of human arginase-1(Arg-1) and CD206. Moreover, H19 knockdown reverses the OGD/R-induced increasing expression of HDAC1 and the decrease of acetyl-histone H3 and acetyl-histone H4. Conversely, the upregulation of HDAC1 eliminates the function of H19 knockdown. H19 promotes neuroinflammation by stimulating histone deacetylase 1(HDAC1)-dependent M1 microglial polarization62.
ncRNAs involved in the blood–brain barrier permeability
The blood–brain barrier (BBB) is composed of cerebral endothelial cells, astrocytes, pericytes, the basement membrane, and tight junctions. Maintaining BBB integrity is crucial for brain homeostasis because it limits entry of circulating leukocytes, blood-borne molecules, and toxic substance into the brain. Brain endothelial cells malfunction after cerebral ischemia contributes to BBB breakdown and enhances its permeability. This promotes neuroinflammation and exacerbates cerebral ischemic damage. NcRNAs are highly expressed in cerebral endothelial cells, where they play a critical role in maintaining the normal functions of cerebral vascular. Dysregulation of ncRNAs activation is significantly related to the pathophysiology of cerebral vascular endothelium in the brain response to ischemic stimulation (Table 3).
Table 3.
NcRNAs involved in the BBB integrity in AIS.
| NcRNAs | Targets | Mechanisms | BBB integrity | Reference(s) |
|---|---|---|---|---|
| miR-130a | Homeobox A5 | Induce monolayer permeability | Break | Wang et al.63 |
| miR-15a | Bcl-2 | Protect endothelial cells | Stable | Yin et al.64 |
| miR-34a | cytochrome c | Reduce mitochondrial function and disrupt tight junctions ZO-1 | Disrupt | Bukeirat et al.65 |
| Let-7 and miR-98 | – | Inhibit the production of CCL2 and CCL5 | Protect | Rom et al.66 |
| miR-155 | – | Inhibit the expression of tight junction proteins | Break | Lopez-Ramirez et al.67 |
| lncRNA Malat1 | ULK2 | Sponge miR-26b | Stable | Li et al.68 |
| circRNA DLGAP4 | miR-143 | Regulate endothelial-mesenchymal transition | Preserve | Bai et al.11 |
AIS: acute ischemic stroke, BBB: blood–brain barrier, ncRNA, non-coding RNA; miRNA, microRNA; lncRNA, long non-coding RNA; circRNA, circular RNA; Bcl-2, B cell lymphoma-2; ULK2, unc51 like kinase 2.
MiR-130a predominantly expresses in brain microvascular endothelial cells and increases after I/R in rat MCAO models. The administration of antagomir-130a can contribute to alleviated brain edema, lower BBB permeability, reduced infarct volume, and improved neurologic function. MiR-130a overexpression increases ischemia-induced monolayer permeability in vitro by targeting Homeobox A5. Therefore, MiR-130a mediates brain ischemia-induced BBB dysfunction by inhibiting Homeobox A5 expression63. Peroxisome proliferator-activated receptor delta protects endothelial cells against cerebral ischemia induced-damage through suppressing bcl-2 expression by increasing miR-15a levels in mice. This leads to stable BBB integrity64. Overexpression of miR-34a reduces mitochondrial function, consisting of impairing mitochondrial oxidative phosphorylation. It also reduces ATP production by negatively regulating cytochrome c, which increases BBB permeability and disrupts tight junctions ZO-1 in brain microvascular endothelial cells65. Let-7 and miR-98 prevent BBB breakdown by inhibiting the production of pro-inflammatory cytokines, including chemokine (C-C motif) ligand 2(CCL2) and chemokine (C-C motif) ligand 5(CCL5) CCL5, under neuroinflammatory conditions in vitro66. MiR-155 negatively regulates BBB function during neuroinflammation by suppressing the expression of proteins related to tight junctions in a post-transcriptional manner67.
LncRNAs and circRNAs have recently emerged as key regulators of brain disorders. However, the studies of their roles in BBB dysfunction is scarce. LncRNA Malat1 levels increases after I/R or OGD/R treatment, and exerts a protective role in endothelial cells against cerebral ischemic damage. It promotes brain microvascular endothelial cells autophagy and survival by sponging miR-26b and upregulating unc-51 like kinase 2 (ULK2) expression. Therefore, LncRNA Malat1 can prevent BBB disruption by protecting brain microvascular endothelial cells against OGD/R injury68. Circular RNA DLGAP4 expression is reduced in the plasma of patients with AIS, and has also been shown to be reduced in a mouse MCAO model. The upregulation of circRNA DLGAP4 improves neurological functions, decreases infarct volumes, and prevents BBB dysfunction in mouse transient MCAO models. It also inhibits endothelial-mesenchymal transition that can induce BBB permeability by regulating tight junction proteins and mesenchymal cell marker expression. Moreover, circRNA DLGAP4 sponges miR-143 and inhibit tight junction proteins expression. These results reveal that circRNA DLGAP4 preserves BBB integrity through negatively regulating endothelial-mesenchymal transition by sponging miR-143 and improves outcome in ischemic stroke11.
ncRNAs involved in neuroprotection
Cerebral ischemia triggers changes in the ncRNAs profiles and is a concomitant altered expression of proteins involved in neuroprotection in rodents and humans9,69–72. Many studies have explored the change of these ncRNAs after stroke and are related to neuroprotection in order to gain new biomarkers and therapeutic targets.
Both upregulation and downregulation of a special miRNA exert neuroprotection effects against cerebral ischemic damage, including reduced ischemic infarction, improved neurological deficit and anti-apoptosis of neurons in rodent I/R models. Intravenous injections of miR-155 inhibitor improves blood flow and microvascular integrity, which has promoted neurological function recovery in mouse MCAO models73. MiR-134 levels rise in primary culture neuronal cells and mouse brain tissue after OGD/R and MCAO, respectively. The knockdown of miR-134 reduce neurons death and apoptosis via upregulating heat shock protein A12B expression in a post-transcriptional manner. This mediates neuroprotection against OGD/R induced neurons injury74. Similarly, the inhibition of miR-181b in mouse brains following cerebral ischemia drives neuroprotection against ischemic damage by negatively regulating heat shock protein A5 and ubiquitin carboxyl-terminal hydrolase isozyme L1 protein levels75. AntagomiR-613, a specific inhibitor of miR-613, exerts neuroprotective function against OGD/R injury in neuronal cells, probably by upregulating Sphingosine kinase 2 expression76.
Conversely, the systemic upregulation of some miRNAs also plays a neuroprotective role against I/R damage to the brain by negatively regulating target genes expression. Increasing miR-215 expression not only suppresses cell apoptosis and autophagy in vitro, but also decreases ischemic infarction volume and improves neurological deficits in mouse MCAO models. MiR-215 mimics achieve neuroprotective function against ischemic damage by negatively regulating NF-κB activator 1/IL-17 receptor A signaling77. MiR-128-3p can target the 3’ UTR of p38α, which is a pro-apoptotic protein and reported to be downregulated 2 h after cerebral ischemia, leading to reduced p38α protein expression. MicroRNA-128-3p protects mice against brain ischemic injury by inhibiting p38α mitogen activated protein kinase activation78. Previous studies have indicated that electroacupuncture, fastigial nucleus stimulation, and vagus nerve stimulation promote neuroprotection through induction of the microRNA in cerebral ischemic animal models, respectively79–81. Some molecules, such as cytosine-phosphate-guanine, insulin-like growth factor-1, natural vitamin E α-tocotrienol, and lithium induce neuroprotection in cerebral ischemia by regulating some specific miRNAs82–85.
Dysregulation of lncRNAs can result in brain pathologies, including primary brain tumors, neurodegeneration, neuroimmunological disorders, and psychiatric diseases86. Alterations of lncRNAs expression profiles has been found in the plasma of patients with AIS and in the brain tissues of mouse MCAO models8,87. LncMalat1, a metastasis-associated lung adenocarcinoma transcript, plays an important anti-apoptotic and anti-inflammatory role in brain microvasculature. It does so by binding to Bim and E-selectin, and inhibiting their expression in mouse brain microvascular endothelial cells inducing by OGD/R and in experimental stroke mouse models. This study suggests that LncMalat1 plays a crucial protective role in ischemic stroke10. LncMalat1 is highly expressed in vitro and in vivo mimics of cerebral ischemia conditions. The knockdown of Malat1 attenuates neuronal cells death through inhibiting beclin1-dependent autophagy by regulating miR-30a expression88.
LncRNA-N1LR levels are increased abruptly in focal ischemic cerebral tissues of mice subjected to MCAO. LncRNA-N1LR promotes cell cycle progression and cell proliferation, and also suppresses the apoptosis of N2a cells that are subjected to OGD/R. Furthermore, it reduces neuronal apoptosis and neural cell loss in mouse experimental ischemic models. LncRNA-N1LR enhances neuroprotective roles probably through the inhibition of p53 phosphorylation89. Experimental cerebral ischemia induces upregulation of LncRNA MEG3 expression, and recruits p53 into MEG3 complex. LncRNA MEG3 binds with the p53 DNA binding domain (DBD) consisting of amino acids 271–282, and this promotes p53-mediated transactivation and contributes to ischemic neuronal death. Treatment with the membrane-permeable peptide inhibitor Tat-p53-DBD271–282 selectively blocks MEG3-p53 complex, which significantly protects against ischemic neuron death. LncRNA MEG3 promotes neuronal cells death by interacting with p53 to mediate ischemic damage90. Neuron apoptosis is one of main reason for patients with AIS. LncRNA taurine-upregulated gene 1 functions as a neuron apoptosis promoter through increasing Bcl2l11 expression by sponging MiR-991.
ncRNA biomarkers
Recent studies have demonstrated altered ncRNAs expression profiles in bodily fluids of AIS patients and rodents cerebral ischemia model. This implies that ncRNAs could be useful biomarkers for diagnosis, disease severity and prognosis in patients with AIS9,92,93.
microRNA biomarkers
Circulating miRNAs have been suggested as potential diagnostic and prognostic biomarkers for AIS, and they may be widely use in future clinical practice (Table 4). A study of plasma samples of patients with AIS identifies that miR-125a-5p, miR-125b-5p, and miR-143-3p are upregulated compared with both healthy control subjects and patients with transient ischemic attack. Furthermore, miR-125b-5p and miR-143-3p return to control levels at day 2, while miR-125a-5p remains elevated at 3 months. These three miRNAs might have clinical utility as early diagnostic markers for AIS94. Similarly, another study has shown that the elevated serum expressions of miR-15a, miR-16, and miR-17-5p are correlated with AIS, and that miR-17-5p may be a significant and independent predictor for determining the presence of AIS95. MiR-221-3p and miR-382-5p levels were significantly downregulated in a study of the circulating serum of acute stroke patients, and might be usable as potential non-invasive biomarkers for the diagnosis96.
Table 4.
NcRNA biomarkers for AIS and their proposed clinical applications in humans.
| NcRNAs | Alteration | Sample source | Proposed clinical application | Reference(s) |
|---|---|---|---|---|
| miR-125a-5p | Up | Plasma | Diagnosis | Tiedt et al.94 |
| miR-143-3p | Up | Plasma | Diagnosis | Tiedt et al.94 |
| miR-125b-5p | Up | Plasma | Diagnosis | Tiedt et al.94 |
| miR-17-5p | Up | Serum | Diagnosis | Wu et al.95 |
| miR-382-5p | Down | Serum | Diagnosis | Wang et al.96 |
| miR-221-3p | Down | Serum | Diagnosis | Wang et al.96 |
| miR-150-5p | Down | Plasma | Prognosis | Scherrer et al.97 |
| miR-124-3p | Up | Plasma | Prognosis | Rainer et al.98 |
| miR-16 | Down | Plasma | Prognosis | Rainer et al.98 |
| miR-132 | Up | Plasma | Prognosis | Huang et al.99 |
| miR-223 | Up | Whole blood | Prognosis | Chen et al.102 |
| miR-29b | Down | White blood cells | Disease severity | Wang et al.104 |
| lncRNA NR_002332 | Up | Whole blood | – | Dykstra-Aiello et al.71 |
| lncRNA AJ131606 | Up | Whole blood | – | Dykstra-Aiello et al.71 |
| lncRNA f57-2 | Down | Whole blood | – | Dykstra-Aiello et al.71 |
| lncRNA C10 | Down | Whole blood | – | Dykstra-Aiello et al.71 |
| lncRNA MIAT | Up | Whole blood | Disease severity prognosis | Zhu et al.105 |
AIS: acute ischemic stroke, ncRNA, non-coding RNA; miRNA, microRNA; lncRNA, long non-coding RNA.
Some miRNAs may have value for estimating prognosis in patients with AIS. MiR-150-5p plasma levels are negatively associated with mortality within 90 days, improving risk classification beyond traditional risk factors in acute stroke patients97. In patients with AIS, plasma miR-124-3p concentrations are positively related with 3-month mortality. Higher plasma miR-16 concentrations are indicative patient survival, while lower plasma miR-16 concentrations are associated with poor clinical outcome at 3 months98. Furthermore, elevated serum miR-132 is a risk biomarker of post-stroke cognitive impairment, and angiogenesis is involved in post-stroke recovery99. In order to assess their correlations with risk and severity of acute stroke, a study detects 28 pro-angiogenesis and anti-angiogenesis miRNAs in plasma of patients with AIS compared with healthy controls. This indicates that circulating plasma miR-126, miR-130a, miR-222, miR-218, and miR-185 could serve as promising and independent biomarkers for risk of acute stroke, and miR-126, miR-378, miR-222, miR-101, miR-218, and miR-206 could be used for disease severity estimation of AIS100. The brain-enriched miRNAs of MiR-9-5p, miR-9-3p, miR-124-3p, and miR-128-3p are higher than the controls in cerebrospinal fluid from patients with a larger infarct volume101.
Circulating exosomal miR-223 from blood samples of patients with AIS is significantly higher than in the controls. Upregulation of exosomal miR-223 is associated with the occurrence of AIS, severity of stroke, and poor prognosis102. One study has demonstrated that miR-150 polymorphisms may be used as novel biomarkers for predicting the risk of AIS103. The expression of miR-29b is decreased in the white blood cells of humans and mice after cerebral ischemia, and negatively related to severity of stroke and brain infarct volume. Furthermore, the overexpression of miR-29b reduces BBB dysfunction through targeting of AQP-4104.
LncRNAs biomarkers
Like that of miRNAs, expression of lncRNAs also changes in peripheral blood after AIS (Table 4). LncRNA NR_002332 and lncRNA AJ131606 are upregulated while lncRNA C10 and lncRNA f57-2 are downregulated in a time-dependent manner in whole blood samples from patients with ischemic stroke71. LncRNA MIAT levels are significantly increased in blood samples of acute stroke patients and are correlated with the severity of stroke, high-sensitivity C-reactive protein, infarct volume, and 3-month poststroke outcome. The overexpression of lncRNA MIAT indicates poor prognosis. This lncRNA may be used as an independent prognostic marker of functional outcome and survival time for patients with ischemic stroke105. In mouse primary brain microvascular endothelial cells after OGD, LncRNA expressional signatures are detected by RNA sequencing technology. A total of 147 upregulated lncRNAs and 70 downregulated lncRNAs are found. LncRNA Snhg12, LncRNA Malat1, and LncRNA-OGD 1006 are the most highly upregulated lncRNAs, whereas LncRNA 281008D09Rik, LncRNA Peg13, and LncRNA-OGD 3916 are the most highly downregulated lncRNAs106.
Therapeutic targets
There are several common problems in AIS treatment, including the availability of only two approved approaches, short therapeutic window, and secondary brain damage. Furthermore, mechanical thrombectomy and intravenous thrombolysis has deleterious effects in some patients, such as neurotoxicity, ischemic reperfusion injury, and hemorrhagic transformation. Identifying new targets and improving the prognosis for patients with AIS is dependent on a deeper understanding of mechanisms of this disease. Recent studies have demonstrated that ncRNAs play a crucial role in the pathophysiological processes of neuroinflammation, oxidative stress, excitotoxicity, and apoptosis, thus contributing to cerebral ischemic injury. Drugs derived from ncRNAs have been shown to penetrate BBB and exert neuroprotection against brain ischemic injury in experimental animal stroke models107. Therefore, treatments which target ncRNAs or make use of ncRNA molecules might help to improve prognosis of AIS (Table 5).
Table 5.
NcRNAs-based treatment for AIS.
| Approach | Targets | Mechanisms | Outcome | Reference(s) |
|---|---|---|---|---|
| miR-1906 mimic | TLR4 | Inhibit neuroinflammation | Reduce infarct volumes and improve functional outcomes | Xu et al.108 |
| miR-17-92 cluster-enriched exosomes | PI3K/PKB/mechanistic target of rapamycin /GSK3β pathway | Promote neural plasticity | Promote functional recovery | Xin et al.107 |
| miR-122 mimic | Vcam1, Nos2, Pla2g2a | Reduce neurological deficits and brain infarction, and maintain vessel integrity | Improve prognosis | Liu et al.109 |
| miR-15a/16 -1 antagomir | Bcl-2, Bcl-w, IL-6, MCAP1, Vcam1 and TNFα | Anti-apoptotic, inhibit neuroinflammation | Ameliorate cerebral ischemic damage | Yang et al.110 |
| miR-383 antagomir | Peroxisome proliferator-activated receptor gamma | Inhibit oxidant stress | Attenuate focal ischemic brain injury | Pei et al.111 |
| miR-93 antagomir | Nrf2/HO-1 antioxidant pathway | Inhibit oxidant stress | Decrease cerebral infarction volume, neural apoptosis and NIHSS scores | Wang et al.112 |
| miR-106b-5p antagomir | Bcl2 and Mcl1 | Inhibit apoptosis and oxidative stress | Protect brain tissues against i/r injury | Li et al.41 |
| miR-155 inhibitor | Rheb | Maintain TJ integrity | Decrease brain tissue damage | Caballero-Garrido et al.73 |
| miR-181 antagomir | Bcl2 and Xiap | Anti-apoptotic, inhibit neuroinflammation | Alleviate injury and improve long-term behavioral recovery | Xu et al.113 |
| si-miRNA-30a | Beclin 1 | Enhance beclin 1 autophagy | Reduce cerebral ischemic injury | Wang et al.114 |
AIS: acute ischemic stroke, ncRNA, non-coding RNA; miRNA, microRNA; lncRNA, long non-coding RNA; TLR4, toll-like receptor 4; PKB, protein kinase B; GSK3β, glycogen synthase kinase 3β; Vcam1, vascular cell adhesion molecule 1; Nos2, nitric oxide synthase 2; Pla2g2a, phospholipase A2 group IIA; IL-6, interleukin 6; MCAP1, m-adenylyl cyclase associated protein 1; TNFα, tumor necrosis factor alfa; Nrf2, nuclear factor erythroid 2-related factor; HO-1, heme oxygenase-1; Mcl-1, myeloid cell leukemia-1; Bcl-2, B cell lymphoma-2; Rheb, Ras homolog enriched in brain; Xiap, X-linked inhibitor of apoptosis protein.
MiRNAs might be potential targets because they are enriched in brain and are capable of regulating the expression of potentially deleterious genes in post-transcriptional manner after ischemic stroke. Meanwhile, miRNAs control the specific proteins expression that result in the neuroprotection and angiogenesis that promote recovery and repair mechanisms in acute stroke patients. Exogeneous miR-1906 uptake by astrocytes, microglia, and neurons is visualized by Cy3 labeling, which reduces infarct volumes and improves functional outcomes in mouse tMCAO models. MiR-1906 inhibits neuroinflammation after ischemic stroke by targeting TLR4108. Intravenously administered miR-17-92 cluster-enriched exosomes harvested from mesenchymal stem cells (MSCs) transfected with an miR-17-92 cluster plasmid promote neural plasticity and functional recovery after stroke through activating PI3K/PKB/mechanistic target of rapamycin/GSK3β pathway by targeting phosphatase and tensin homolog in rats107.
The expression of miR-122 in blood decreases in patients and rats following AIS. Intravenous injection of miR-122 mimic reduces neurological deficits and brain infarction, and maintains vessel integrity after MCAO via suppressing target genes expression in blood leukocytes, such as VCAM1, Nos2, and Pla2g2a109. Among current studies involved in targeted miRNA therapy, most focus on inhibition detrimental miRNAs by administrating miRNA antagomir or using drugs. Genetic deletion of the miR-15a/16 -1 cluster or intravenous delivery of miR-15a/16 -1 antagomir significantly ameliorates cerebral ischemic damage through both upregulation of antiapoptotic proteins (Bcl-2 and Bcl-w) and inhibition of proinflammatory molecules (IL-6, MCAP1, VCAM1, and TNF-α)110. The administration of miR-383 antagomir attenuates focal ischemic brain injury via directly downregulating peroxisome proliferator-activated receptor gamma expression111. Treatment with miR-93 antagomir decreases cerebral infarction volume, neural apoptosis and severity of stroke via activation of Nrf2/HO-1 antioxidant pathway112. A study has shown that miR-106b-5p antagomir significantly protects brain tissues against I/R injury by inhibiting apoptosis and oxidative stress in rat MCAO models41. Intravenous injections of a specific miR-155 inhibitor decreases brain tissue damage, supports brain microvasculature, and promotes neurofunctional recovery through maintaining the integrity of tight junction proteins by targeting Rheb in mouse MCAO models73. The administration of miR-181 antagomir via intra-cerebroventricular and intravenous routes alleviates cerebral ischemic injury and improves long-term behavioral recovery by inhibition of neuroinflammation and upregulation of anti-apoptotic proteins in mice after focal cerebral ischemia113. Interestingly, inhibition of miR-30a reduces cerebral ischemic injury through enhancing beclin 1-mediated autophagy114.
Cumulative studies have indicated that lncRNAs plays critical roles in the development and progression of ischemic stroke12,115,116. Although there has been no lncRNAs-based research on target therapy for ischemic stroke, lncRNAs that participate in the pathologic processes of ischemic stroke, might be useful as therapeutic targets.
Although the potential of ncRNAs in AIS therapy could lead to exciting future directions, many challenges remain. Such challenges are similar to those associated with ncRNAs-based treatments in cancer, including rapid degradation and clearance, poor cellular uptake, off-target effects, and immunogenicity117. Off target effects cannot be neglected, because they are the main reason for side effects and poor healing efficacy in miRNA-based therapy118–120; however, to date there is no research related to off target effects of miRNAs-based treatments in AIS. To solve this difficult problem, comprehensive research is needed in the future.
Future directions and conclusions
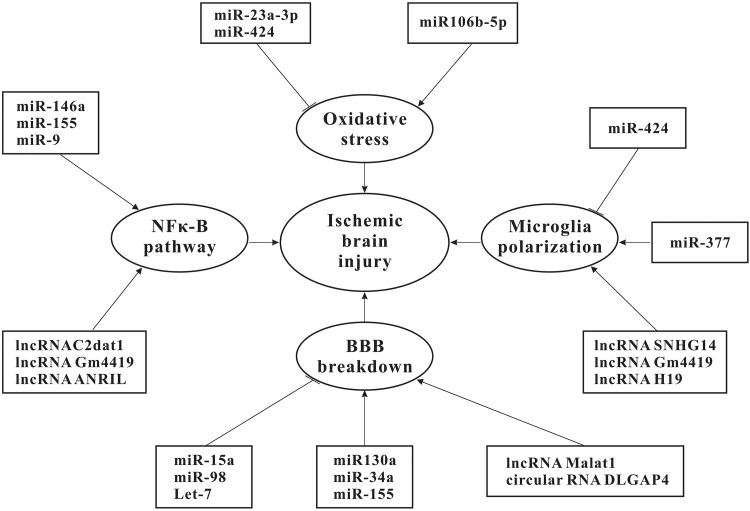
NcRNAs contribute to cerebral ischemic injury by regulating NFκ-B pathway activation, oxidative stress, microglial polarization, neuronal apoptosis, and BBB permeability (Fig. 1). On the contrary, ncRNAs promote functional recovery by enhancing angiogenesis, neurogenesis, and neuroprotection. Much research to date has been done on miRNAs, whereas the role of lncRNAs and circRNAs in ischemic stroke remains largely unknown. Further research will likely unearth these ncRNAs, which is also beneficial in helping us to better understand the mechanisms underlying brain ischemic damage. Most existing research linking ncRNAs to ischemic stroke has been conducted with rodent models of MCAO and in vitro OGD/R models. These experimental models will continue to be useful in defining the role of ncRNAs in AIS, either by using genetic manipulation or through the use of ncRNA antagomirs or agomirs.
Figure 1.
NcRNAs involved in ischemic brain injury. NcRNAs play a role in pathogenic processes related to ischemic cerebral injury. These pathogenic processes include the activation of NF-κB signaling pathway, oxidative stress, microglia activation and BBB breakdown. NF-κB, nuclear factor kappa-B; BBB, blood–brain barrier.
An increasing number of case-control studies have demonstrated that alterations in ncRNAs expression can be detected in serum, plasms, and throughout the bloodstreams of patients with ischemic stroke. Many ncRNAs have significantly high disease specificity. Furthermore, many ncRNAs are associated with several clinicopathological parameters of AIS, such as infarct volume, brain edema, severity of stroke, and clinical outcome. Therefore, in the future, ncRNAs may act as non-invasive biomarkers of diagnosis and prognosis for patients with AIS.
Although there has been rapid progress in miRNA biopharmaceutical research, ncRNAs-based treatments have not been used in clinical practice. It has been shown that miRNA mimics and inhibitors can penetrate BBB and protect against brain ischemic injury in animal models with AIS. Exosomes, liposomes and lentivirus might be used for carrying ncRNAs-based drugs and take them to the cerebral infarction area107,121,122. Hence, ncRNAs-derived therapy may be a novel adjunctive therapeutic strategy to improve outcome after intravenous thrombolysis and endovascular mechanical reperfusion for AIS.
Author Contribution: Z-SS participated in the design of the present study. All authors participated in the interpretation and collection of the data. S-WW wrote the initial manuscript. Z-SS revised the manuscript. All authors critically reviewed and edited the manuscript and approved the final version.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by a National Natural Science Foundation of China grant (81873752, 81720108014, 81371275), Science and Technology Planning Project of Guangzhou City (201704020166), and Fundamental Research Funds for Central Universities, Sun Yat-sen University (16ykjc12).
References
- 1. Hu X, De Silva TM, Chen J, Faraci FM. Cerebral vascular disease and neurovascular injury in ischemic stroke. Circ Res. 2017;120(3):449–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beal CC. Gender and stroke symptoms: a review of the current literature. J Neurosci Nurs. 2010;42(2):80–87. [PubMed] [Google Scholar]
- 3. Khoshnam SE, Winlow W, Farbood Y, Moghaddam HF, Farzaneh M. Emerging roles of microRNAs in ischemic stroke: as possible therapeutic agents. J Stroke. 2017;19(2):166–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iyengar BR, Choudhary A, Sarangdhar MA, Venkatesh KV, Gadgil CJ, Pillai B. Non-coding RNA interact to regulate neuronal development and function. Front Cell Neurosci. 2014;8:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kitagawa M, Kitagawa K, Kotake Y, Niida H, Ohhata T. Cell cycle regulation by long non-coding RNAs. Cell Mol Life Sci. 2013;70(24):4785–4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495(7441):333–338. [DOI] [PubMed] [Google Scholar]
- 7. Dharap A, Bowen K, Place R, Li LC, Vemuganti R. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab. 2009;29(4):675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dharap A, Nakka VP, Vemuganti R. Effect of focal ischemia on long noncoding RNAs. Stroke. 2012;43(10):2800–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mehta SL, Pandi G, Vemuganti R. Circular RNA expression profiles alter significantly in mouse brain after transient focal ischemia. Stroke 2017;48(9):2541–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang X, Tang X, Liu K, Hamblin MH, Yin KJ. Long noncoding RNA Malat1 regulates cerebrovascular pathologies in ischemic stroke. J Neurosci. 2017;37(7):1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bai Y, Zhang Y, Han B, Yang L, Chen X, Huang R, Wu F, Chao J, Liu P, Hu G, Zhang JH, Yao H. Circular RNA DLGAP4 ameliorates ischemic stroke outcomes by targeting miR-143 to regulate endothelial-mesenchymal transition associated with blood–brain barrier integrity. J Neurosci. 2018;38(1):32–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vemuganti R. All’s well that transcribes well: non-coding RNAs and post-stroke brain damage. Neurochem Int. 2013;63(5):438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu J. Control of protein synthesis and mRNA degradation by microRNAs. Curr Opin Cell Biol. 2008;20(2):214–221. [DOI] [PubMed] [Google Scholar]
- 14. Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861–874. [DOI] [PubMed] [Google Scholar]
- 15. Klingenberg M, Matsuda A, Diederichs S, Patel T. Non-coding RNA in hepatocellular carcinoma: mechanisms, biomarkers and therapeutic targets. J Hepatol. 2017;67(3):603–618. [DOI] [PubMed] [Google Scholar]
- 16. Li L, Wang M, Mei Z, Cao W, Yang Y, Wang Y, Wen A. LncRNAs HIF1A-AS2 facilitates the up-regulation of HIF-1alpha by sponging to miR-153-3p, whereby promoting angiogenesis in HUVECs in hypoxia. Biomed Pharmacother. 2017;96:165–172. [DOI] [PubMed] [Google Scholar]
- 17. Rybak-Wolf A, Stottmeister C, Glažar P, Jens M, Pino N, Giusti S, Hanan M, Behm M, Bartok O, Ashwal-Fluss R, Herzog M, Schreyer L, Papavasileiou P, Ivanov A, Öhman M, Refojo D, Kadener S, Rajewsky N. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell. 2015;58(5):870–885. [DOI] [PubMed] [Google Scholar]
- 18. Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19(2):141–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang R, Zhang Y, Han B, Bai Y, Zhou R, Gan G, Chao J, Hu G, Yao H. Circular RNA HIPK2 regulates astrocyte activation via cooperation of autophagy and ER stress by targeting MIR124-2HG. Autophagy. 2017;13(10):1722–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Du WW, Yang W, Liu E, Yang Z, Dhaliwal P, Yang BB. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016;44(6):2846–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang Y, Gao X, Zhang M, Yan S, Sun C, Xiao F, Huang N, Yang X, Zhao K, Zhou H, Huang S, Xie B, Zhang N. Novel role of FBXW7 circular RNA in repressing glioma tumorigenesis. J Natl Cancer Inst. 2018;110(3):304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 2010;87(5):779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang Y, Zhou H, Yang Y, Li W, Zhou M, Zeng Z, Xiong W, Wu M, Huang H, Zhou Y, Peng C, Huang C, Li X, Li G. Lipopolysaccharide (LPS) regulates TLR4 signal transduction in nasopharynx epithelial cell line 5-8F via NFkappaB and MAPKs signaling pathways. Mol Immunol. 2007;44(5):984–992. [DOI] [PubMed] [Google Scholar]
- 24. Cheng HS, Njock MS, Khyzha N, Dang LT, Fish JE. Noncoding RNAs regulate NF-kappaB signaling to modulate blood vessel inflammation. Front Genet. 2014;5:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ouyang YB, Stary CM, Yang GY, Giffard R. MicroRNAs: innovative targets for cerebral ischemia and stroke. Curr Drug Targets. 2013;14(1):90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng HS, Sivachandran N, Lau A, Boudreau E, Zhao JL, Baltimore D, Delgado-Olguin P, Cybulsky MI, Fish JE. MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol Med. 2013;5(7):1017–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang L, Chopp M, Liu X, Teng H, Tang T, Kassis H, Zhang ZG. Combination therapy with VELCADE and tissue plasminogen activator is neuroprotective in aged rats after stroke and targets microRNA-146a and the toll-like receptor signaling pathway. Arterioscler Thromb Vasc Biol. 2012;32(8):1856–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hunsberger JG, Fessler EB, Wang Z, Elkahloun AG, Chuang DM. Post-insult valproic acid-regulated microRNAs: potential targets for cerebral ischemia. Am J Transl Res. 2012;4(3):316–332. [PMC free article] [PubMed] [Google Scholar]
- 29. Wen Y, Zhang X, Dong L, Zhao J, Zhang C, Zhu C. Acetylbritannilactone modulates MicroRNA-155-mediated inflammatory response in ischemic cerebral tissues. Mol Med. 2015;21:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu W, Wang X, Zheng Y, Shang G, Huang J, Tao J, Chen L. Electroacupuncture inhibits inflammatory injury by targeting the miR-9-mediated NF-kappaB signaling pathway following ischemic stroke. Mol Med Rep. 2016;13(2):1618–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu Q, Deng F, Xing Z, Wu Z, Cen B, Xu S, Zhao Z, Nepomuceno R, Bhuiyan MI, Sun D, Wang QJ, Ji A. Long non-coding RNA C2dat1 regulates CaMKIIdelta expression to promote neuronal survival through the NF-kappaB signaling pathway following cerebral ischemia. Cell Death Dis. 2016;7:e2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang B, Wang D, Ji TF, Shi L, Yu JL. Overexpression of lncRNA ANRIL up-regulates VEGF expression and promotes angiogenesis of diabetes mellitus combined with cerebral infarction by activating NF-kappaB signaling pathway in a rat model. Oncotarget. 2017;8(10):17347–17359. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33. Wen Y, Yu Y, Fu X. LncRNA Gm4419 contributes to OGD/R injury of cerebral microglial cells via IkappaB phosphorylation and NF-kappaB activation. Biochem Biophys Res Commun. 2017;487(4):923–929. [DOI] [PubMed] [Google Scholar]
- 34. Guldiken B, Demir M, Guldiken S, Turgut N, Turgut B, Tugrul A. Oxidative stress and total antioxidant capacity in diabetic and nondiabetic acute ischemic stroke patients. Clin Appl Thromb Hemost. 2009;15(6):695–700. [DOI] [PubMed] [Google Scholar]
- 35. Radak D, Resanovic I, Isenovic ER. Link between oxidative stress and acute brain ischemia. Angiology. 2014;65(8):667–676. [DOI] [PubMed] [Google Scholar]
- 36. Jung JE, Karatas H, Liu Y, Yalcin A, Montaner J, Lo EH, van Leyen K. STAT-dependent upregulation of 12/15-lipoxygenase contributes to neuronal injury after stroke. J Cereb Blood Flow Metab. 2015;35(12):2043–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW, Chuang YC. Roles of oxidative stress, apoptosis, PGC-1alpha and mitochondrial biogenesis in cerebral ischemia. Int J Mol Sci. 2011;12(10):7199–7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li G, Morris-Blanco KC, Lopez MS, Yang T, Zhao H, Vemuganti R, Luo Y. Impact of microRNAs on ischemic stroke: from pre- to post-disease. Prog Neurobiol. 2018;163–164:59–78. [DOI] [PubMed] [Google Scholar]
- 39. Zhao H, Tao Z, Wang R, Liu P, Yan F, Li J, Zhang C, Ji X, Luo Y. MicroRNA-23a-3p attenuates oxidative stress injury in a mouse model of focal cerebral ischemia-reperfusion. Brain Res. 2014;1592:65–72. [DOI] [PubMed] [Google Scholar]
- 40. Liu P, Zhao H, Wang R, Wang P, Tao Z, Gao L, Yan F, Liu X, Yu S, Ji X, Luo Y. MicroRNA-424 protects against focal cerebral ischemia and reperfusion injury in mice by suppressing oxidative stress. Stroke. 2015;46(2):513–519. [DOI] [PubMed] [Google Scholar]
- 41. Li P, Shen M, Gao F, Wu J, Zhang J, Teng F, Zhang C. An antagomir to microRNA-106b-5p ameliorates cerebral ischemia and reperfusion injury in rats via inhibiting apoptosis and oxidative stress. Mol Neurobiol. 2017;54(4):2901–2921. [DOI] [PubMed] [Google Scholar]
- 42. Kim C, Kang D, Lee EK, Lee JS. Long noncoding RNAs and RNA-binding proteins in oxidative stress, cellular senescence, and age-related diseases. Oxid Med Cell Longev. 2017;2017:2062384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8(5):491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yin KJ, Hamblin M, Chen YE. Angiogenesis-regulating microRNAs and ischemic stroke. Curr Vasc Pharmacol. 2015;13(3):352–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M, Petit E, Risau W. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156(3):965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. VEGF enhances angiogenesis and promotes blood–brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106(7):829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sun J, Tao S, Liu L, Guo D, Xia Z, Huang M. MiR1405p regulates angiogenesis following ischemic stroke by targeting VEGFA. Mol Med Rep. 2016;13(5):4499–4505. [DOI] [PubMed] [Google Scholar]
- 48. Fouillade C, Monet-Lepretre M, Baron-Menguy C, Joutel A. Notch signalling in smooth muscle cells during development and disease. Cardiovasc Res. 2012;95(2):138–146. [DOI] [PubMed] [Google Scholar]
- 49. Liu XL, Wang G, Song W, Yang WX, Hua J, Lyu L. MicroRNA-137 promotes endothelial progenitor cell proliferation and angiogenesis in cerebral ischemic stroke mice by targeting NR4A2 through the Notch pathway. J Cell Physiol. 2018;233(7):5255–5266. [DOI] [PubMed] [Google Scholar]
- 50. Lou YL, Guo F, Liu F, Gao FL, Zhang PQ, Niu X, Guo SC, Yin JH, Wang Y, Deng ZF. MiR-210 activates notch signaling pathway in angiogenesis induced by cerebral ischemia. Mol Cell Biochem. 2012;370(1–2):45–51. [DOI] [PubMed] [Google Scholar]
- 51. Fan Y, Ding S, Sun Y, Zhao B, Pan Y, Wan J. MiR-377 regulates inflammation and angiogenesis in rats after cerebral ischemic injury. J Cell Biochem. 2018;119(1):327–337. [DOI] [PubMed] [Google Scholar]
- 52. He QW, Li Q, Jin HJ, Zhi F, Suraj B, Zhu YY, Xia YP, Mao L, Chen XL, Hu B. MiR-150 regulates poststroke cerebral angiogenesis via vascular endothelial growth factor in rats. CNS Neurosci Ther 2016;22(6):507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li Y, Mao L, Gao Y, Baral S, Zhou Y, Hu B. MicroRNA-107 contributes to post-stroke angiogenesis by targeting Dicer-1. Sci Rep. 2015;5:13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zeng L, He X, Wang Y, Tang Y, Zheng C, Cai H, Liu J, Fu Y, Yang GY. MicroRNA-210 overexpression induces angiogenesis and neurogenesis in the normal adult mouse brain. Gene Ther. 2014;21(1):37–43. [DOI] [PubMed] [Google Scholar]
- 55. Hua L, Wang CY, Yao KH, Chen JT, Zhang JJ, Ma WL. High expression of long non-coding RNA ANRIL is associated with poor prognosis in hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8(3):3076–3082. [PMC free article] [PubMed] [Google Scholar]
- 56. Zhan R, Xu K, Pan J, Xu Q, Xu S, Shen J. Long noncoding RNA MEG3 mediated angiogenesis after cerebral infarction through regulating p53/NOX4 axis. Biochem Biophys Res Commun. 2017;490(3):700–706. [DOI] [PubMed] [Google Scholar]
- 57. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Freilich RW, Woodbury ME, Ikezu T. Integrated expression profiles of mRNA and miRNA in polarized primary murine microglia. PLoS One. 2013;8(11):e79416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhao SC, Ma LS, Chu ZH, Xu H, Wu WQ, Liu F. Regulation of microglial activation in stroke. Acta Pharmacol Sin. 2017;38(4):445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhao H, Wang J, Gao L, Wang R, Liu X, Gao Z, Tao Z, Xu C, Song J, Ji X, Luo Y. MiRNA-424 protects against permanent focal cerebral ischemia injury in mice involving suppressing microglia activation. Stroke. 2013;44(6):1706–1713. [DOI] [PubMed] [Google Scholar]
- 61. Qi X, Shao M, Sun H, Shen Y, Meng D, Huo W. Long non-coding RNA SNHG14 promotes microglia activation by regulating miR-145-5p/PLA2G4A in cerebral infarction. Neuroscience. 2017;348:98–106. [DOI] [PubMed] [Google Scholar]
- 62. Wang J, Zhao H, Fan Z, Li G, Ma Q, Tao Z, Wang R, Feng J, Luo Y. Long noncoding RNA H19 promotes neuroinflammation in ischemic stroke by driving histone deacetylase 1-dependent M1 microglial polarization. Stroke. 2017;48(8):2211–2221. [DOI] [PubMed] [Google Scholar]
- 63. Wang Y, Wang MD, Xia YP, Gao Y, Zhu YY, Chen SC, Mao L, He QW, Yue ZY, Hu B. MicroRNA-130a regulates cerebral ischemia-induced blood–brain barrier permeability by targeting Homeobox A5. FASEB J. 2018;32(2):935–944. [DOI] [PubMed] [Google Scholar]
- 64. Yin KJ, Deng Z, Hamblin M, Xiang Y, Huang H, Zhang J, Jiang X, Wang Y, Chen YE. Peroxisome proliferator-activated receptor delta regulation of miR-15a in ischemia-induced cerebral vascular endothelial injury. J Neurosci. 2010;30(18):6398–6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bukeirat M, Sarkar SN, Hu H, Quintana DD, Simpkins JW, Ren X. MiR-34a regulates blood–brain barrier permeability and mitochondrial function by targeting cytochrome c. J Cereb Blood Flow Metab. 2016;36(2):387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rom S, Dykstra H, Zuluaga-Ramirez V, Reichenbach NL, Persidsky Y. MiR-98 and let-7g* protect the blood–brain barrier under neuroinflammatory conditions. J Cereb Blood Flow Metab. 2015;35(12):1957–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lopez-Ramirez MA, Wu D, Pryce G, Simpson JE, Reijerkerk A, King-Robson J, Kay O, de Vries HE, Hirst MC, Sharrack B, Baker D, Male DK, Michael GJ, Romero IA. MicroRNA-155 negatively affects blood–brain barrier function during neuroinflammation. FASEB J. 2014;28(6):2551–2565. [DOI] [PubMed] [Google Scholar]
- 68. Li Z, Li J, Tang N. Long noncoding RNA Malat1 is a potent autophagy inducer protecting brain microvascular endothelial cells against oxygen-glucose deprivation/reoxygenation-induced injury by sponging miR-26b and upregulating ULK2 expression. Neuroscience. 2017;354:1–10. [DOI] [PubMed] [Google Scholar]
- 69. Tan KS, Armugam A, Sepramaniam S, Lim KY, Setyowati KD, Wang CW, Jeyaseelan K. Expression profile of microRNAs in young stroke patients. PLoS One. 2009;4(11):e7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bell JD, Cho JE, Giffard RG. MicroRNA changes in preconditioning-induced neuroprotection. Transl Stroke Res. 2017;8(6):585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dykstra-Aiello C, Jickling GC, Ander BP, Shroff N, Zhan X, Liu D, Hull H, Orantia M, Stamova BS, Sharp FR. Altered expression of long noncoding RNAs in blood after ischemic stroke and proximity to putative stroke risk Loci. Stroke. 2016;47(12):2896–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liu C, Zhang C, Yang J, Geng X, Du H, Ji X, Zhao H. Screening circular RNA expression patterns following focal cerebral ischemia in mice. Oncotarget. 2017;8(49):86535–86547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Caballero-Garrido E, Pena-Philippides JC, Lordkipanidze T, Bragin D, Yang Y, Erhardt EB, Roitbak T. In vivo inhibition of miR-155 promotes recovery after experimental mouse stroke. J Neurosci. 2015;35(36):12446–12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chi W, Meng F, Li Y, Li P, Wang G, Cheng H, Han S, Li J. Impact of microRNA-134 on neural cell survival against ischemic injury in primary cultured neuronal cells and mouse brain with ischemic stroke by targeting HSPA12B. Brain Res. 2014;1592:22–33. [DOI] [PubMed] [Google Scholar]
- 75. Peng Z, Li J, Li Y, Yang X, Feng S, Han S. Downregulation of miR-181b in mouse brain following ischemic stroke induces neuroprotection against ischemic injury through targeting heat shock protein A5 and ubiquitin carboxyl-terminal hydrolase isozyme L1. J Neurosci Res. 2013;91(10):1349–1362. [DOI] [PubMed] [Google Scholar]
- 76. Di G, Wang Z, Wang W, Cheng F, Liu H. AntagomiR-613 protects neuronal cells from oxygen glucose deprivation/re-oxygenation via increasing SphK2 expression. Biochem Biophys Res Commun. 2017;493(1):188–194. [DOI] [PubMed] [Google Scholar]
- 77. Sun H, Zhong D, Jin J, Liu Q, Wang H, Li G. Upregulation of miR-215 exerts neuroprotection effects against ischemic injury via negative regulation of Act1/IL-17RA signaling. Neurosci Lett. 2018;662:233–241. [DOI] [PubMed] [Google Scholar]
- 78. Mao G, Ren P, Wang G, Yan F, Zhang Y. MicroRNA-128-3p protects mouse against cerebral ischemia through reducing p38alpha mitogen-activated protein kinase activity. J Mol Neurosci. 2017;61(2):152–158. [DOI] [PubMed] [Google Scholar]
- 79. Zhou H, Yang C, Bai F, Ma Z, Wang J, Wang F, Li F, Wang Q, Xiong L. Electroacupuncture alleviates brain damage through targeting of neuronal calcium sensor 1 by miR-191a-5p after ischemic stroke. Rejuvenation Res. 2017;20(6):492–505. [DOI] [PubMed] [Google Scholar]
- 80. Pang XM, Liu JL, Li JP, Huang LG, Zhang L, Xiang HY, Feng LB, Chen CY, Li SH, Su SY. Fastigial nucleus stimulation regulates neuroprotection via induction of a novel microRNA, rno-miR-676 -1, in middle cerebral artery occlusion rats. J Neurochem. 2015;133(6):926–934. [DOI] [PubMed] [Google Scholar]
- 81. Jiang Y, Li L, Tan X, Liu B, Zhang Y, Li C. MiR-210 mediates vagus nerve stimulation-induced antioxidant stress and anti-apoptosis reactions following cerebral ischemia/reperfusion injury in rats. J Neurochem. 2015;134(1):173–181. [DOI] [PubMed] [Google Scholar]
- 82. Vartanian KB, Mitchell HD, Stevens SL, Conrad VK, McDermott JE, Stenzel-Poore MP. CpG preconditioning regulates miRNA expression that modulates genomic reprogramming associated with neuroprotection against ischemic injury. J Cereb Blood Flow Metab. 2015;35(2):257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bake S, Selvamani A, Cherry J, Sohrabji F. Blood brain barrier and neuroinflammation are critical targets of IGF-1-mediated neuroprotection in stroke for middle-aged female rats. PLoS One. 2014;9(3):e91427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Park HA, Kubicki N, Gnyawali S, Chan YC, Roy S, Khanna S, Sen CK. Natural vitamin E alpha-tocotrienol protects against ischemic stroke by induction of multidrug resistance-associated protein 1. Stroke. 2011;42(8):2308–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Doeppner TR, Kaltwasser B, Sanchez-Mendoza EH, Caglayan AB, Bahr M, Hermann DM. Lithium-induced neuroprotection in stroke involves increased miR-124 expression, reduced RE1-silencing transcription factor abundance and decreased protein deubiquitination by GSK3beta inhibition-independent pathways. J Cereb Blood Flow Metab. 2017;37(3):914–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Qureshi IA, Mehler MF. Emerging roles of non-coding RNAs in brain evolution, development, plasticity and disease. Nat Rev Neurosci. 2012;13(8):528–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li H, Wu Y, Suo G, Shen F, Zhen Y, Chen X, Lv H. Profiling neuron-autonomous lncRNA changes upon ischemia/reperfusion injury. Biochem Biophys Res Commun. 2018;495(1):104–109. [DOI] [PubMed] [Google Scholar]
- 88. Guo D, Ma J, Yan L, Li T, Li Z, Han X, Shui S. Down-regulation of lncRNA MALAT1 attenuates neuronal cell death through suppressing beclin1-dependent autophagy by regulating mir-30a in cerebral ischemic stroke. Cell Physiol Biochem. 2017;43(1):182–194. [DOI] [PubMed] [Google Scholar]
- 89. Wu Z, Wu P, Zuo X, Yu N, Qin Y, Xu Q, He S, Cen B, Liao W, Ji A. LncRNA-N1LR enhances neuroprotection against ischemic stroke probably by inhibiting p53 phosphorylation. Mol Neurobiol. 2017;54(10):7670–7685. [DOI] [PubMed] [Google Scholar]
- 90. Yan H, Yuan J, Gao L, Rao J, Hu J. Long noncoding RNA MEG3 activation of p53 mediates ischemic neuronal death in stroke. Neuroscience. 2016;337:191–199. [DOI] [PubMed] [Google Scholar]
- 91. Chen S, Wang M, Yang H, Mao L, He Q, Jin H, Ye ZM, Luo XY, Xia YP, Hu B. LncRNA TUG1 sponges microRNA-9 to promote neurons apoptosis by up-regulated Bcl2l11 under ischemia. Biochem Biophys Res Commun. 2017;485(1):167–173. [DOI] [PubMed] [Google Scholar]
- 92. Dewdney B, Trollope A, Moxon J, Thomas Manapurathe D, Biros E, Golledge J. Circulating microRNAs as biomarkers for acute ischemic stroke: a systematic review. J Stroke Cerebrovasc Dis. 2018;27(3):522–530. [DOI] [PubMed] [Google Scholar]
- 93. Takuma A, Abe A, Saito Y, Nito C, Ueda M, Ishimaru Y, Harada H, Abe K, Kimura K, Asakura T. Gene expression analysis of the effect of ischemic infarction in whole blood. Int J Mol Sci. 2017;18(11):E2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tiedt S, Prestel M, Malik R, Schieferdecker N, Duering M, Kautzky V, Stoycheva I, Böck J, Northoff BH, Klein M, Dorn F, Krohn K, Teupser D, Liesz A, Plesnila N, Holdt LM, Dichgans M. RNA-Seq identifies circulating miR-125a-5p, miR-125b-5p, and miR-143-3p as potential biomarkers for acute ischemic stroke. Circ Res. 2017;121(8):970–980. [DOI] [PubMed] [Google Scholar]
- 95. Wu J, Du K, Lu X. Elevated expressions of serum miR-15a, miR-16, and miR-17-5p are associated with acute ischemic stroke. Int J Clin Exp Med. 2015;8(11):21071–21079. [PMC free article] [PubMed] [Google Scholar]
- 96. Wang Y, Ma Z, Kan P, Zhang B. The diagnostic value of serum miRNA-221-3p, miRNA-382-5p, and miRNA-4271 in ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26(5):1055–1060. [DOI] [PubMed] [Google Scholar]
- 97. Scherrer N, Fays F, Mueller B, Luft A, Fluri F, Christ-Crain M, Devaux Y, Katan M. MicroRNA 150-5p improves risk classification for mortality within 90 days after acute ischemic stroke. J Stroke. 2017;19(3):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rainer TH, Leung LY, Chan CPY, Leung YK, Abrigo JM, Wang D, Graham CA. Plasma miR-124-3p and miR-16 concentrations as prognostic markers in acute stroke. Clin Biochem. 2016;49(9):663–668. [DOI] [PubMed] [Google Scholar]
- 99. Huang S, Zhao J, Huang D, Zhuo L, Liao S, Jiang Z. Serum miR-132 is a risk marker of post-stroke cognitive impairment. Neurosci Lett. 2016;615:102–106. [DOI] [PubMed] [Google Scholar]
- 100. Jin F, Xing J. Circulating pro-angiogenic and anti-angiogenic microRNA expressions in patients with acute ischemic stroke and their association with disease severity. Neurol Sci. 2017;38(11):2015–2023. [DOI] [PubMed] [Google Scholar]
- 101. Sorensen SS, Nygaard AB, Carlsen AL, Heegaard NHH, Bak M, Christensen T. Elevation of brain-enriched miRNAs in cerebrospinal fluid of patients with acute ischemic stroke. Biomark Res. 2017;5:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Chen Y, Song Y, Huang J, Qu M, Zhang Y, Geng J, Zhang Z, Liu J, Yang GY. Increased circulating exosomal miRNA-223 is associated with acute ischemic stroke. Front Neurol. 2017;8:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Choi GH, Ko KH, Kim JO, Kim J, Oh SH, Han IB, Cho KG, Kim OJ, Bae J, Kim NK. Association of miR-34a, miR-130a, miR-150 and miR-155 polymorphisms with the risk of ischemic stroke. Int J Mol Med. 2016;38(1):345–356. [DOI] [PubMed] [Google Scholar]
- 104. Wang Y, Huang J, Ma Y, Tang G, Liu Y, Chen X, Zhang Z, Zeng L, Ouyang YB, Yang GY. MicroRNA-29b is a therapeutic target in cerebral ischemia associated with aquaporin 4. J Cereb Blood Flow Metab. 2015;35(12):1977–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhu M, Li N, Luo P, Jing W, Wen X, Liang C, Tu J. Peripheral blood leukocyte expression of lncRNA MIAT and its diagnostic and prognostic value in ischemic stroke. J Stroke Cerebrovasc Dis. 2018;27(2):326–337. [DOI] [PubMed] [Google Scholar]
- 106. Zhang J, Yuan L, Zhang X, Hamblin MH, Zhu T, Meng F, Li Y, Chen YE, Yin KJ. Altered long non-coding RNA transcriptomic profiles in brain microvascular endothelium after cerebral ischemia. Exp Neurol 2016;277:162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Xin H, Katakowski M, Wang F, Qian JY, Liu XS, Ali MM, Buller B, Zhang ZG, Chopp M. MicroRNA cluster miR-17-92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke. 2017;48(3):747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Xu X, Wen Z, Zhao N, Wang F, Gao J, Jiang Y, Liu X. MicroRNA-1906, a novel regulator of toll-like receptor 4, ameliorates ischemic injury after experimental stroke in mice. J Neurosci. 2017;37(43):10498–10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Liu da Z, Jickling GC, Ander BP, Hull H, Zhan X, Cox C, Shroff N, Dykstra-Aiello C, Stamova B, Sharp FR. Elevating microRNA-122 in blood improves outcomes after temporary middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 2016;36(8):1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yang X, Tang X, Sun P, Shi Y, Liu K, Hassan SH, Stetler RA, Chen J, Yin KJ. MicroRNA-15a/16 -1 antagomir ameliorates ischemic brain injury in experimental stroke. Stroke. 2017;48(7):1941–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Pei L, Meng S, Yu W, Wang Q, Song F, Ma L. Inhibition of microRNA-383 ameliorates injury after focal cerebral ischemia via targeting PPARgamma. Cell Physiol Biochem. 2016;39(4):1339–1346. [DOI] [PubMed] [Google Scholar]
- 112. Wang P, Liang X, Lu Y, Zhao X, Liang J. MicroRNA-93 downregulation ameliorates cerebral ischemic injury through the Nrf2/HO-1 defense pathway. Neurochem Res. 2016;41(10):2627–2635. [DOI] [PubMed] [Google Scholar]
- 113. Xu LJ, Ouyang YB, Xiong X, Stary CM, Giffard RG. Post-stroke treatment with miR-181 antagomir reduces injury and improves long-term behavioral recovery in mice after focal cerebral ischemia. Exp Neurol. 2015;264:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wang P, Liang J, Li Y, Li J, Yang X, Zhang X, Han S, Li S. Down-regulation of miRNA-30a alleviates cerebral ischemic injury through enhancing beclin 1-mediated autophagy. Neurochem Res. 2014;39(7):1279–1291. [DOI] [PubMed] [Google Scholar]
- 115. Bhattarai S, Pontarelli F, Prendergast E, Dharap A. Discovery of novel stroke-responsive lncRNAs in the mouse cortex using genome-wide RNA-seq. Neurobiol Dis. 2017;108:204–212. [DOI] [PubMed] [Google Scholar]
- 116. Wang J, Cao B, Han D, Sun M, Feng J. Long non-coding RNA H19 induces cerebral ischemia reperfusion injury via activation of autophagy. Aging Dis. 2017;8(1):71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Liu Y, Wang J. Therapeutic potentials of noncoding RNAs: targeted delivery of ncRNAs in cancer cells. Adv Exp Med Biol. 2016;927:429–458. [DOI] [PubMed] [Google Scholar]
- 118. Sarvestani ST, Stunden HJ, Behlke MA, Forster SC, McCoy CE, Tate MD, Ferrand J, Lennox KA, Latz E, Williams BR, Gantier MP. Sequence-dependent off-target inhibition of TLR7/8 sensing by synthetic microRNA inhibitors. Nucleic Acids Res. 2015;43(2):1177–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Cao H, Yu W, Li X, Wang J, Gao S, Holton NE, Eliason S, Sharp T, Amendt BA. A new plasmid-based microRNA inhibitor system that inhibits microRNA families in transgenic mice and cells: a potential new therapeutic reagent. Gene Ther. 2016;23(6):527–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Karlsen TA, Brinchmann JE. Liposome delivery of microRNA-145 to mesenchymal stem cells leads to immunological off-target effects mediated by RIG-I. Mol Ther. 2013;21(6):1169–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Moon JM, Xu L, Giffard RG. Inhibition of microRNA-181 reduces forebrain ischemia-induced neuronal loss. J Cereb Blood Flow Metab. 2013;33(12):1976–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Zeng LL, He XS, Liu JR, Zheng CB, Wang YT, Yang GY. Lentivirus-mediated overexpression of microRNA-210 improves long-term outcomes after focal cerebral ischemia in mice. CNS Neurosci Ther. 2016;22(12):961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]