Abstract
Angiopoietin-1 (Ang1) mediates vascular maturation and immune response. Diabetes decreases Ang1 expression and disrupts Ang1/Tie2 signaling activity. Vasculotide is an Ang1 mimetic peptide, and has anti-inflammatory effects. In this study, we test the hypothesis that vasculotide treatment induces neuroprotection and decreases inflammation after stroke in type 1 diabetic (T1DM) rats. T1DM rats were subjected to embolic middle cerebral artery occlusion (MCAo) and treated with: 1) phosphate buffered saline (PBS); 2) vasculotide (3µg/kg, i.p. injection) administered half an hour prior to MCAo and at 8 and 24 hours after MCAo. Rats were sacrificed at 48 h after MCAo. Neurological function, infarct volume, hemorrhage, blood brain barrier (BBB) permeability and neuroinflammation were measured. Vasculotide treatment of T1DM-MCAo rats significantly improves functional outcome, decreases infarct volume and BBB permeability, but does not decrease brain hemorrhagic transformation compared with PBS-treated T1DM-MCAo rats. In the ischemic brain, Vasculotide treatment significantly decreases apoptosis, number of cleaved-caspase-3 positive cells, the expression of monocyte chemotactic protein-1 (MCP-1) and tumor necrosis factor (TNF-α). Western blot analysis shows that vasculotide significantly decreases expression of receptor for advanced glycation end products (RAGE), MCP-1 and TNF-α in the ischemic brain compared with T1DM-MCAo rats. Vasculotide treatment in cultured primary cortical neurons (PCN) significantly decreases TLR4 expression compared with control. Decreased neuroinflammation and reduced BBB leakage may contribute, at least in part, to vasculotide-induced neuroprotective effects after stroke in T1DM rats.
Keywords: diabetes mellitus, stroke, neuroprotection, angiopoietin-1, vasculotide, neuroinflammation
Introduction
Ischemic stroke is a prominent cause of death and long-term disability in adults, and is often accompanied by hefty medical and social costs. Diabetes mellitus is a chronic, lifelong, global health concern, that elevates multifold the risk of ischemic stroke1. Co-morbidity of stroke and diabetes worsens prognosis, and patients are prone to developing vascular disorders and stroke recurrence2,3. In addition to inducing metabolic abnormalities, diabetes induces extensive microvascular and macrovascular damage, which contributes to higher morbidity after stroke2,3. Diabetes decreases Angiopoietin-1 (Ang1) expression, disrupts Ang1/Tie2 signaling activity, and impairs Ang1-mediated vascular remodeling and immune responses4,5. In mice with diabetes, decreased Ang1 has been implicated to increase blood brain barrier (BBB) disruption and brain hemorrhagic transformation after stroke6. Ang1 is an endothelial growth factor, and is essential for endothelial cell survival and function7. Ang1 is a primary physiological ligand for tyrosine kinase, Tie2, and Ang1 mediates key steps of angiogenesis such as migration and adhesion of endothelial cells, and vessel maturation7,8. In the ischemic brain, Ang1 improves oxygen and nutrition availability to ischemic tissue via enhancement of angiogenesis and vascular development9,10.
Vasculotide is an Ang1 mimetic peptide that activates and/or promotes Tie2 function11. An in vivo study using a murine abdominal sepsis model has demonstrated that systemic administration of vasculotide induces long-lasting Tie2 activation, decreases mortality and exerts protection against microvascular dysfunction12. In stroke patients, significant reduction of plasma Ang1 level at the time of hospitalization was associated with poor functional outcomes at 3 months after stroke13. In rodents subject to diabetic stroke, exacerbated functional deficits have been attributed to a significant reduction of Ang-1 expression and increase of inflammatory factors in the ischemic brain, while treatments increasing Ang1 have been associated with improved functional outcome and decreased neuroinflammation in the ischemic brain6,8,14. In a mouse stroke model, stroke treatment using recombinant adenoviruses expressing Ang1 significantly decreases BBB permeability and reduces infarct volume14. Several studies have previously reported that there is a differential response to stroke treatments between diabetic and non-diabetic subjects15,16. In this study, we investigate the effectiveness of vasculotide as a neuroprotective agent for stroke in type 1 diabetes mellitus (T1DM) rats.
Ang1 has also been shown to exert anti-inflammatory effects and protect endothelial cell permeability against inflammatory factors17. Among the several up-regulated inflammatory mediators after stroke are included monocyte chemotactic protein-1 (MCP-1) and tumor necrosis factor-α (TNF-α)18,19. MCP-1 is an inflammatory chemokine that is expressed in neurons, astrocytes, and endothelial cells in response to ischemia, and regulates the migration and infiltration of monocytes and macrophages to the core of injured brain tissue18,20. TNF-α is a cytokine associated with proinflammatory responses post stroke21,22. TNF-α expression increases significantly post stroke in the ischemic core of experimental stroke animals, as well as in the serum and cerebrospinal fluid of stroke patients, and correlates with stroke severity22,23. Up regulation of TNF-α has been associated with increased BBB permeability19 and, as expected, inhibition of TNF-α activity reduces infarct volume and improves neurological outcome after stroke24. Therefore, decreasing neuroinflammation after stroke is important to promote neuroprotective effects and prevent additional damage to the injured brain. In this study, we investigated whether vasculotide treatment decreases proinflammatory factors and exerts neuroprotective effects after stroke in T1DM rats.
Materials and Methods
All experiments were conducted in accordance with the standards and procedures of the American Council on Animal Care and Institutional Animal Care and Use Committee of Henry Ford Health System.
Diabetes induction
Streptozotocin dose dependently damages pancreatic β cells and is used widely to induce T1DM in rodents25,26. Adult male Wistar rats (225–250 g, 8–12 weeks old, Charles River, San Diego, CA, USA) were given a single intraperitoneal (i.p.) injection of Streptozotocin (60 mg/kg, Enzo, Farmingdale, NY, USA) to induce T1DM. Rats with fasting blood glucose >300 mg/dl at 2 weeks after Streptozotocin injection were considered diabetic and were subjected to embolic middle cerebral artery occlusion (MCAo).
MCAo model and experimental groups
Right embolic MCAo was performed on anesthetized T1DM rats as previously described27. Rats were randomized to two groups (n = 7/group): 1) Phosphate buffered saline (PBS) treatment for vehicle control; 2) vasculotide treatment (3 µg/kg, i.p. injection) initiated half an hour prior to MCAo and at 8 and 24 h after MCAo. A battery of functional tests was conducted and rats were sacrificed at 48 h after MCAo for immunostaining quantification analysis. The dose was estimated based on prior publications that have demonstrated that administering 500ng/mice via i.p. injection exerts protection against murine models of severe influenza28.
Neurological functional tests
All functional tests were performed by an investigator blinded to the experimental groups. Neurological functional outcome was evaluated using a battery of functional tests including foot-fault, and a modified neurological severity score (mNSS) evaluation before MCAo, and at 2, 24 and 48 h after MCAo. Briefly, mNSS is a composite of motor, sensory, balance and reflex tests29. The absence of a tested reflex or abnormal response is scored as 1 point; neurological function is scored between 0 and 18, with 0 indicating no deficits and 18 indicating maximum deficits. In the foot-fault test30, animals are placed on an elevated grid floor (45 cm × 30 cm), 2.5 cm higher than a solid base floor, with 2.5 cm × 2.5 cm diameter grid spacing. While the animal is moving on the wire frame using their paws, a fall or slip through a grid opening due to an inaccurate forelimb placement is recorded as a foot-fault. Data are presented as the percentage of foot-faults of the left paw over a 100 forelimb movements.
Immunohistochemistry
Transcardial perfusion with saline followed by perfusion and immersion in 4% paraformaldehyde was used to fix brains. The brains were then embedded in paraffin and a standard block was obtained from the center of the lesion (bregma –1 mm to +1 mm). A series of 6 μm thick sections were cut from the block. Seven hematoxylin and eosin (H&E) stained coronal sections of tissue were used for lesion volume calculation and presented as a percentage of lesion compared with the contralateral hemisphere. Brain hemorrhage was measured using H&E staining under light microscopy. The percentage areas of petechial and gross hemorrhage were measured in each histological section and summed.
Brain coronal tissue sections were prepared and antibody against TNF-α (1:200; Abcam, Cambridge, MA, USA), MCP-1 (1:100, Abcam, Cambridge, MA, USA) and Cleaved Caspase-3 (1:200, Cell Signaling Technology, Danvers, MA, USA) were employed. For measuring apoptosis, terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) was performed using a commercial kit (Apoptag Kit, S7100, Chemicon, Temecula, CA, USA). Control experiments consisted of staining brain coronal tissue sections as outlined above, but non-immune serum was substituted for the primary antibody.
Quantification analysis
An investigator blinded to the experimental groups performed all immunostaining quantification analysis. For quantitative measurements, five slides from each brain, with each slide containing six to eight fields from the ischemic lesion were digitized under a 20× objective (Olympus BX40) using a 3-CCD color video camera (DXC-970MD, Sony, Tokyo, Japan) interfaced with an MCID image analysis system (Imaging Research, St. Catharines, ON, Canada). The positive areas or positive cell numbers were then measured.
BBB disruption quantification measurements
To measure BBB leakage, an additional set (n = 4/group) of T1DM-MCAo rats treated with PBS or vasculotide were injected intravenously with 2% Evans blue dye 4 h before euthanasia (48 h after MCAO). Evans blue dye fluorescence intensity was measured using a microplate fluorescence reader (excitation 620 nm and emission 680 nm).
Western blot assay
Protein was isolated from samples using Trizol (Invitrogen, Carlsbad, CA, USA) following standard protocol. Protein concentration was measured using the BCA kit (Thermo Scientific, Waltham, MA, USA). Antibodies against MCP-1 (1:1000, Abcam, Cambridge, MA, USA), RAGE (receptor for advanced glycation end products, 1:500, R&D Systems, Minneapolis, MN, USA), TNF-α (1:1000, Abcam, Cambridge, MA, USA) and anti-β-actin (1:10000; Abcam, Cambridge, MA, USA) were employed for western blot measurements.
Primary cortical neuron cell culture and experimental groups
Primary cortical neurons (PCN) were harvested from embryonic day-18 Wistar rats (Charles River, Wilmington, MA, USA). Cultures were prepared according to a previously described procedure31,32. Briefly, embryos were removed, and the cerebral cortex dissected and dissociated by a combination of Ca2+- and Mg2+-free Hanks balance salt solution (HBSS) containing 0.125% trypsin digestion for 15 min, then mechanically triturated around 20 times. After the cells were harvested they were initially plated in DMEM with 5% fetal bovine serum (FBS) for 4 h, and then cultured in a medium consisting of neurobasal (Invitrogen, Carlsbad, CA, USA), 2% B-27 (Invitrogen, Carlsbad, CA, USA), 2 mM GlutaMax, and 1% antibiotic-antimycotic31,32 for a week prior to oxygen and glucose deprivation (OGD). The triturated cells were passed through a 40 µm cell strainer and counted to obtain a concentration of 3 × 107 cells/ml. Cells were then exposed to OGD for 2 h. Following OGD, PCN cells were cultured with high glucose (37.5 mM) and divided into two groups: 1) control group and 2) 10 nM vasculotide-treated group (n = 4/group). Cells were treated overnight and, on the following day, RNA was isolated with TRIzol following a standard protocol.
Real time polymerase chain reaction assay
Total RNA was isolated with TRIzol (Invitrogen, Carlsbad, CA, USA), following standard protocol. Total RNA (2 µg) was used to make cDNA (complementary DNA) using the M-MLV (Invitrogen) standard protocol. A 2 µl aliquot of this cDNA was then used to run a quantitative polymerase chain reaction (PCR) using the SYBR Green real-time PCR method. Quantitative PCR was performed on a ViiA 7 PCR instrument (Applied Biosystems, Foster City, CA, USA) using 3-stage program parameters provided by the manufacturer, as follows; 2 min at 50°C, 10 min at 95°C, and then 40 cycles of 15 s at 95°C and 1 min at 60°C. Each sample was tested in triplicate, and analysis of relative gene expression was collected using the 2–ΔΔCT method.
TLR4: Fwd: TCTAACTTCCCTCCTGAGATGG; Rev: ACTGGCTAGAGAGCAAGAGGAA
Statistical analysis
One-way analysis of variance (ANOVA) was used for the evaluation of functional outcome and histology, respectively. “Contrast/estimate” statement was used to test the group difference. If an overall treatment group effect was detected at P < 0.05, pair-wise comparisons were made. All data are presented as mean ± standard error (SE).
Results
Vasculotide treatment for stroke significantly improves neurological functional outcome and decreases infarct volume and BBB leakage in T1DM rats
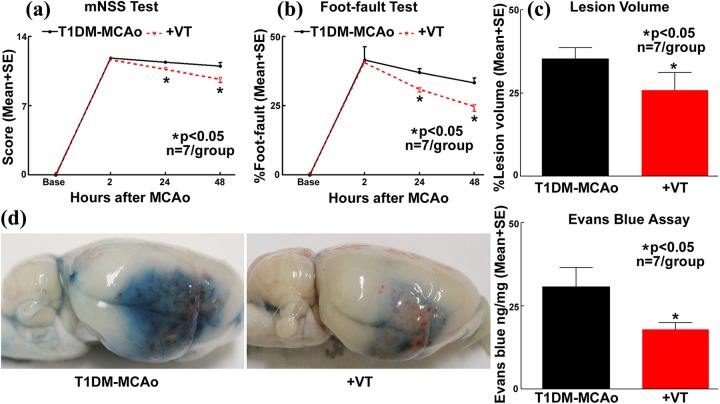
Vasculotide treatment in T1DM-MCAo rats significantly improves neurological functional outcome as indicated by mNSS and foot fault test at 24 and 48 h after stroke (Fig. 1a-b), decreases ischemic burden as indicated by decreased infarct volume (Fig. 1c), and decreases BBB disruption as evaluated by Evans blue dye extravasation assay (Fig. 1d) compared with PBS-treated T1DM stroke rats.
Fig. 1.
Vasculotide treatment improves functional outcome after stroke in T1DM rats. In T1DM stroke rats, vasculotide (VT) treatment significantly improves neurological functional outcome as indicated by a) mNSS and b) foot-fault tests; c) decreases lesion volume; and d) decreases BBB leakage compared with PBS-treated T1DM stroke rats.
Vasculotide treatment of stroke in T1DM rats decreases apoptosis and inflammatory factor expression
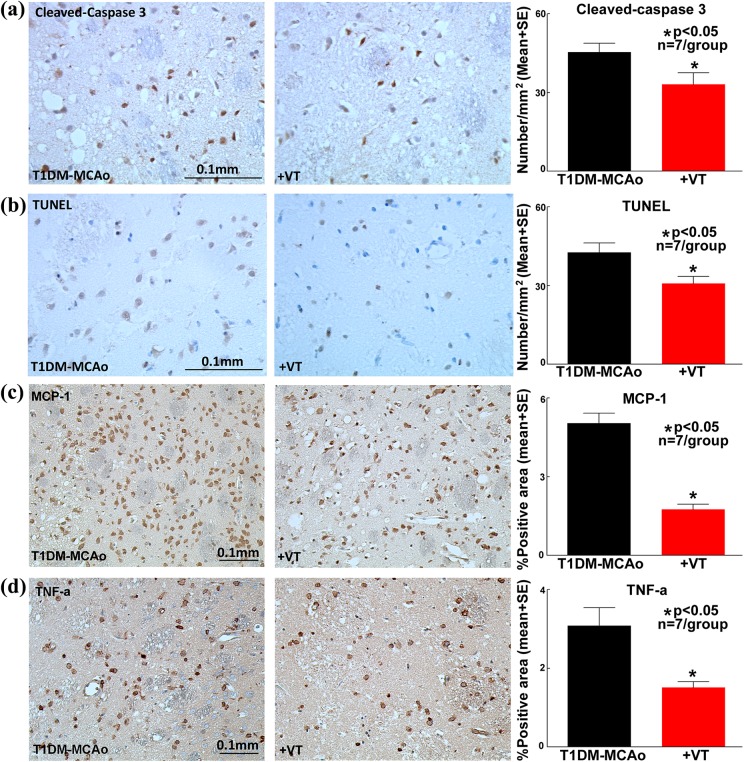
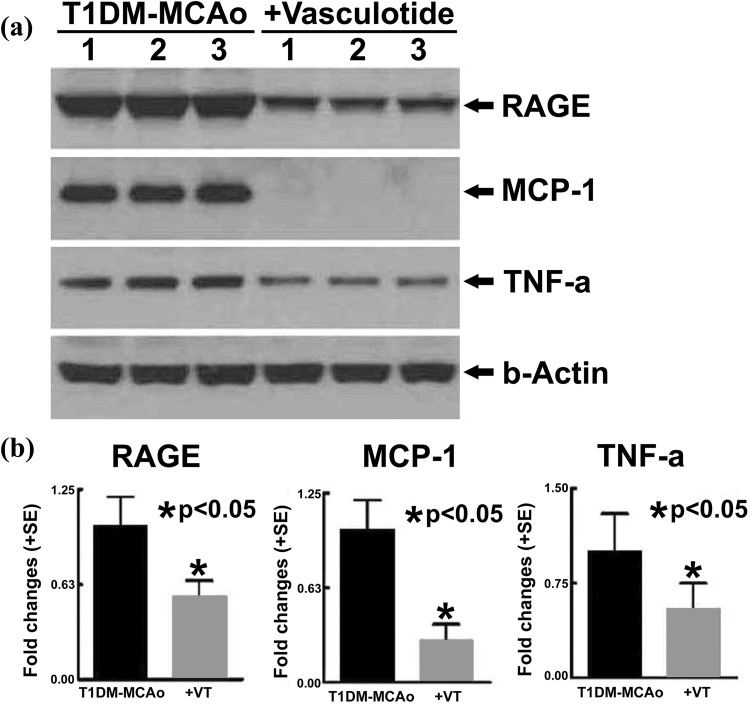
Immunostaining quantification analysis in the ischemic regions of brain shows that vasculotide treatment of stroke in T1DM rats significantly decreases the number of cleaved-caspase-3 positive cells (Fig. 2a) and TUNEL positive cells (Fig. 2b), in comparison with PBS-treated T1DM-MCAo rats. Based on caspase 3 activation or DNA degradation, cleaved-caspase3 and TUNEL staining are markers of damaged cells that have entered an apoptotic pathway, across all cell types. Therefore, it is difficult to identify the cell types that are labeled by these immunostaining in Fig. 2a-b. Vasculotide treatment of stroke in T1DM rats significantly decreases the expression of MCP-1 (Fig. 2c) and TNF-α (Fig. 2d) in comparison with PBS-treated T1DM-MCAo rats. Western blot analysis (Fig. 3a-b) shows that vasculotide treatment of stroke in T1DM rats significantly decreases the expression of RAGE, MCP-1 and TNF-α in the ischemic brain compared with PBS-treated T1DM-MCAo rats; thereby confirming immunohistochemical findings.
Fig. 2.
Vasculotide treatment decreases apoptosis and neuroinflammation after stroke in T1DM rats. In T1DM stroke rats, vasculotide (VT) treatment significantly decreases apoptosis in ischemic regions as indicated by a) cleaved-caspase 3 and b) TUNEL; and expression of inflammatory factors such as c) MCP-1 and d) TNF-α immunostaining and quantification analysis.
Fig. 3.
Vasculotide treatment decreases neuroinflammation after stroke in T1DM rats. Vasculotide (VT) treatment of stroke in T1DM rats decreases the expression of inflammatory factors RAGE, MCP-1 and TNF-α in the ischemic brain tissue as measured by a) western blot assay and b) quantification analysis.
Vasculotide treatment of PCN significantly decreases inflammatory factor TLR4 expression compared with non-treated PCN controls
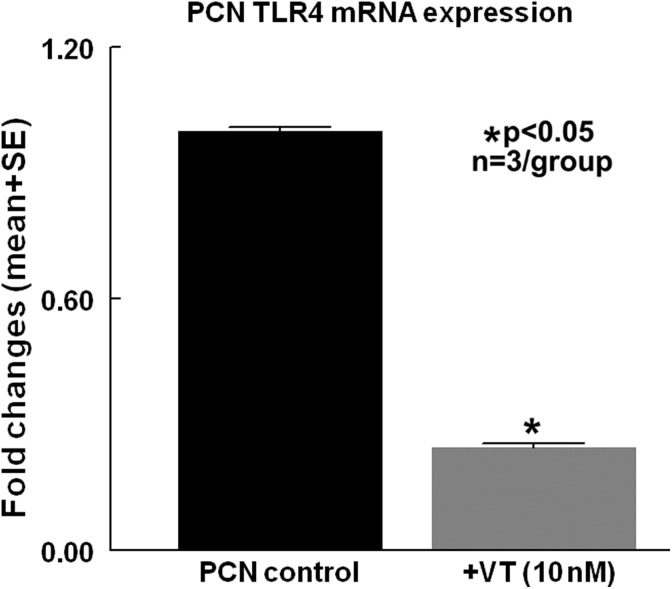
To test whether vasculotide regulates PCN inflammatory factor expression, PCN cell culture was performed. Figure 4c shows that PCNs that were subjected to OGD and high glucose conditions, and treated with vasculotide, exhibit significantly decreased TLR-4 gene expression compared with the non-treated control group.
Fig. 4.
Vasculotide treatment decreases neuroinflammation in cultured PCN. Vasculotide (VT) treatment significantly decreases inflammatory factor TLR4 gene expression in cultured primary cortical neuron (PCN) subject to 2 h of OGD and high glucose conditions, compared with non-treated controls.
Discussion
In this study, we show for the first time that vasculotide treatment of stroke in T1DM rats promotes neuroprotective effects such as reducing infarct volume, decreasing BBB disruption, and improving neurological functional outcome as well as reducing neuroinflammation.
Diabetes instigates and aggravates endothelial cell dysfunction resulting in increased vascular permeability after stroke2,33. We have employed a Streptozotocin-induced T1DM model in rats that is well documented25,26. We have demonstrated previously that the STZ-induced T1DM model decreases cerebral vascular perfusion, decreases cerebral artery diameter with increasing wall thickness, and increases the number of occluded cerebral arterioles compared with non-diabetic rats34. In addition, stroke in T1DM rats induced worse neurological outcome, vascular dysfunction and greater BBB leakage compared with stroke in non-diabetic rats15,34. BBB disruption after stroke is a key event that triggers neurovascular uncoupling and facilitates invasion of inflammatory factors, neurotoxins, and pathogens into the brain, contributing to adverse neurological outcome post stroke in the diabetic population33,35,36. Protecting BBB integrity has been associated with improved neurological function after brain injury, particularly in stroke and diabetic stroke8,37. In our study, we found that vasculotide treatment of T1DM stroke significantly decreases infarct volume and BBB leakage, which may contribute to improved neurological function after stroke.
Vasculotide is a synthetic Ang1 mimetic peptide. Ang1 is an angiogenic growth factor that activates Tie-2 to promote migration, sprouting, and survival of endothelial cells and recruitment of vascular smooth muscle cells and pericytes during vascular maturation36. The effects of Ang1 overexpression in the brain include increased vascularization and neuronal dendrite configuration, neurite outgrowth, and neuronal differentiation38. Apart from its role in angiogenic processes, Ang1 signaling also counteracts several inflammatory responses. A major gateway to the pathophysiological mechanisms of diabetic stroke is the activation of inflammatory responses that exert adverse effects on the progression of tissue damage and aggravate vascular damage39. Inflammatory processes are considered to be a leading cause of stroke-induced brain dysfunction, and diabetic stroke patients suffer from worse and prolonged BBB leakage15,33,40.
The neuroprotective effects of vasculotide in T1DM stroke rats may be due, at least in part, to a decrease in neuroinflammatory responses. MCP-1 has been demonstrated to be a potent monocyte chemotactic factor in several models of inflammation, and to promote monocyte/macrophage infiltration41. In patients with cardiovascular diseases such ischemic stroke and myocardial infarction, a two-fold increase in circulating MCP-1 levels has been reported42. In T2DM patients, MCP-1 is significantly increased in the circulation43, and is associated with diabetic complications such as diabetic nephropathy, diabetic retinopathy, etc44. In rats subjected to either transient or permanent MCAo, MCP-1 expression significantly increased in the ischemic hemisphere as early as 6 h, and peaked at 2 days after stroke45,46. TNF-α significantly increases in neurons in the ischemic cortex as early as 1–3 h post MCAo, peaks at 12 h, and remains elevated even at 5 days after stroke21. Given the temporal profiles of TNF-α and MCP-1, which increase early after MCAo, it is likely that they create a proinflammatory and hostile environment in the ischemic brain, which exacerbates tissue damage. In experimental stroke models, MCP-1 deficiency improves neurological functional outcome, reduces ischemic lesion volume, decreases the accumulation of phagocytic macrophages in the ischemic core and penumbra, reduces BBB disruption, and attenuates the expression of inflammatory factors47,48. Similarly, treating rodents subject to experimental stroke models with either TNF-α binding receptors or an inhibitor of TNF-α converting enzyme to block TNF-α activity, has several neuroprotective effects, including decreased infarction volume, leading to improved neurological function24,49. In our study, we found that treatment of stroke in T1DM rats with vasculotide significantly attenuates the expression of MCP-1 and TNF-α, which may be associated with vasculotide-induced neuroprotective effects after stroke.
High mobility group box 1 (HMGB1) is an inflammatory mediator secreted upon injury by immune cells or injured cells, and it binds to its receptors TLR4 and RAGE. In our previous studies, we have reported a significant increase of HMGB1 and RAGE expression post stroke in the ischemic brain of T1DM rats50. Proinflammatory factors such as TLR4 and RAGE are significantly increased after diabetic stroke and have been implicated in aggravated tissue injury50,51. Increased RAGE expression after stroke in DM animals has been associated with inflammation in the ischemic brain and correlated with poor neurological functional outcome50. Ang1 significantly decreases the expression of inflammatory factor RAGE in cultured brain endothelial cells8. We found that vasculotide treatment of stroke in T1DM rats significantly decreases the expression of inflammatory factors such as MCP-1, RAGE, and TNF-α in the ischemic brain and TLR4 in cultured PCN subject to high glucose and OGD conditions. Thus, vasculotide treatment may exert neuroprotective effects by regulating post stroke inflammatory responses.
Limitations
This is a proof-of-concept study in which we show that vasculotide induces neuroprotective effects after stroke in T1DM rats, which may, in part (directly or indirectly), be attributed to reduction of neuroinflammation. This study employs vasculotide as a pre-stroke treatment. To minimize translation hurdles of therapies from pre-clinical testing to the clinic52, future studies on post-stroke treatment with wide therapeutic window and testing the long-term effects of vasculotide treatment for T1DM stroke are warranted. Causal mechanistic studies are missing and future using a co-culture system to get insight into the mechanisms of vasculotide-induced neuroprotective and anti-inflammatory effects and future studies are warranted.
Conclusions
Vasculotide treatment promotes neuroprotective effects after stroke in T1DM rats and improves neurological function outcome compared with PBS-treated T1DM stroke rats. Protection of BBB integrity and decrease of neuroinflammatory responses may contribute to vasculotide-induced neuroprotective effects observed after stroke in T1DM rats.
Acknowledgments
The authors sincerely thank Cynthia Roberts, Qinge Lu and Sutapa Santra for their technical assistance.
Footnotes
Ethical Approval: All experiments were conducted in accordance with the standards and procedures of the American Council on Animal Care and Institutional Animal Care and Use Committee of Henry Ford Health System.
Statement of Human and Animal Rights: All experiments were conducted in accordance with the standards and procedures of the American Council on Animal Care and Institutional Animal Care and Use Committee of Henry Ford Health System.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institute of Neurological Disorders and Stroke R01 NS083078-01A1 (JC), RO1 NS099030-01 (JC), R01NS097747 (JC), American Heart Association grant17POST33410580 (PV).
References
- 1. Idris I, Thomson GA, Sharma JC. Diabetes mellitus and stroke. Int J Clin Pract. 2006;60(1):48–56. [DOI] [PubMed] [Google Scholar]
- 2. Yong M, Kaste M. Dynamic of hyperglycemia as a predictor of stroke outcome in the ECASS-II trial. Stroke. 2008;39(10):2749–2755. [DOI] [PubMed] [Google Scholar]
- 3. Ergul A, Hafez S, Fouda A, Fagan SC. Impact of comorbidities on acute injury and recovery in preclinical stroke research: focus on hypertension and diabetes. Transl Stroke Res. 2016;7(4):248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abdelsaid M, Prakash R, Li W, Coucha M, Hafez S, Johnson MH, Fagan SC, Ergul A. Metformin treatment in the period after stroke prevents nitrative stress and restores angiogenic signaling in the brain in diabetes. Diabetes. 2015;64(5):1804–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zacharek A, Chen J, Li A, Cui X, Li Y, Roberts C, Feng Y, Gao Q, Chopp M. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab. 2007;27(10):1684–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cui X, Chopp M, Zacharek A, Ye X, Roberts C, Chen J. Angiopoietin/Tie2 pathway mediates type 2 diabetes induced vascular damage after cerebral stroke. Neurobiol Dis. 2011;43(1):285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87(7):1171–1180. [DOI] [PubMed] [Google Scholar]
- 8. Yan T, Venkat P, Ye X, Chopp M, Zacharek A, Ning R, Cui Y, Roberts C, Kuzmin-Nichols N, Sanberg CD, Chen J. HUCBCs increase angiopoietin 1 and induce neurorestorative effects after stroke in T1DM rats. CNS Neurosci Ther. 2014;20(10):935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett. 2013;328(1):18–26. [DOI] [PubMed] [Google Scholar]
- 10. Beck H, Plate KH. Angiogenesis after cerebral ischemia. Acta Neuropathol. 2009;117(5):481–496. [DOI] [PubMed] [Google Scholar]
- 11. Van Slyke P, Alami J, Martin D, Kuliszewski M, Leong-Poi H, Sefton MV, Dumont D. Acceleration of diabetic wound healing by an angiopoietin peptide mimetic. Tissue Eng Part A. 2009;15(6):1269–1280. [DOI] [PubMed] [Google Scholar]
- 12. Kumpers P, Gueler F, David S, Van Slyke P, Dumont DJ, Park JK, Bockmeyer CL, Parikh SM, Pavenstadt H, Haller H, Shushakova N. The synthetic Tie2 agonist peptide vasculotide protects against vascular leakage and reduces mortality in murine abdominal sepsis. Crit Care. 2011;15(5):R261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Golledge J, Clancy P, Maguire J, Lincz L, Koblar S, McEvoy M, Attia J, Levi C, Sturm J, Almeida OP, Yeap BB, Flicker L, Norman PE, Hankey GJ. Plasma angiopoietin-1 is lower after ischemic stroke and associated with major disability but not stroke incidence. Stroke. 2014;45(4):1064–1068. [DOI] [PubMed] [Google Scholar]
- 14. Zhang ZG, Zhang L, Croll SD, Chopp M. Angiopoietin-1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience. 2002;113(3):683–687. [DOI] [PubMed] [Google Scholar]
- 15. Chen J, Ye X, Yan T, Zhang C, Yang XP, Cui X, Cui Y, Zacharek A, Roberts C, Liu X, Dai X, Lu M, Chopp M. Adverse effects of bone marrow stromal cell treatment of stroke in diabetic rats. Stroke. 2011;42(12):3551–3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fan X, Qiu J, Yu Z, Dai H, Singhal AB, Lo EH, Wang X. A rat model of studying tissue-type plasminogen activator thrombolysis in ischemic stroke with diabetes. Stroke. 2012;43(2):567–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pizurki L, Zhou Z, Glynos K, Roussos C, Papapetropoulos A. Angiopoietin-1 inhibits endothelial permeability, neutrophil adherence and IL-8 production. Br J Pharmacol. 2003;139(2):329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Che X, Ye W, Panga L, Wu D-C, Yang G-Y. Monocyte chemoattractant protein-1 expressed in neurons and astrocytes during focal ischemia in mice. Brain Research. 2001;902(2):171–177. [DOI] [PubMed] [Google Scholar]
- 19. Dobbie MS, Hurst RD, Klein NJ, Surtees RA. Upregulation of intercellular adhesion molecule-1 expression on human endothelial cells by tumour necrosis factor-alpha in an in vitro model of the blood-brain barrier. Brain Res. 1999;830(2):330–336. [DOI] [PubMed] [Google Scholar]
- 20. Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29(6):313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, Feuerstein GZ. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke. 1994;25(7):1481–1488. [DOI] [PubMed] [Google Scholar]
- 22. Pan W, Kastin AJ. Tumor necrosis factor and stroke: role of the blood-brain barrier. Prog Neurobiol. 2007;83(6):363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zaremba J, Losy J. Early TNF-alpha levels correlate with ischaemic stroke severity. Acta Neurol Scand. 2001;104(5):288–295. [DOI] [PubMed] [Google Scholar]
- 24. Nawashiro H, Martin D, Hallenbeck JM. Inhibition of tumor necrosis factor and amelioration of brain infarction in mice. J Cereb Blood Flow Metab. 1997;17(2):229–232. [DOI] [PubMed] [Google Scholar]
- 25. Wu KK, Huan Y. Streptozotocin-induced diabetic models in mice and rats Current Protocols in Pharmacology. John Wiley & Sons, Inc; 2008;40(1):5.47.1–5.47.14. [DOI] [PubMed] [Google Scholar]
- 26. Wu J, Yan LJ. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic beta cell glucotoxicity. Diabetes Metab Syndr Obes. 2015;8:181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang L, Zhang RL, Jiang Q, Ding G, Chopp M, Zhang ZG. Focal embolic cerebral ischemia in the rat. Nat Protoc. 2015;10(4):539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sugiyama MG, Armstrong SM, Wang C, Hwang D, Leong-Poi H, Advani A, Advani S, Zhang H, Szaszi K, Tabuchi A, Kuebler WM, Van Slyke P, Dumont DJ, Lee WL. The Tie2-agonist Vasculotide rescues mice from influenza virus infection. Sci Rep. 2015;5:11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–1011. [DOI] [PubMed] [Google Scholar]
- 30. Rogers DC, Campbell CA, Stretton JL, Mackay KB. Correlation between motor impairment and infarct volume after permanent and transient middle cerebral artery occlusion in the rat. Stroke. 1997;28(10):2060–2065; discussion 2066. [DOI] [PubMed] [Google Scholar]
- 31. Taylor AM, Blurton-Jones M, Rhee SW, Cribbs DH, Cotman CW, Jeon NL. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat Methods. 2005;2(8):599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ueno Y, Chopp M, Zhang L, Buller B, Liu Z, Lehman NL, Liu XS, Zhang Y, Roberts C, Zhang ZG. Axonal outgrowth and dendritic plasticity in the cortical peri-infarct area after experimental stroke. Stroke. 2012;43(8):2221–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Venkat P, Chopp M, Chen J. Blood–brain barrier disruption, vascular impairment, and ischemia/reperfusion damage in diabetic stroke. J Am Heart Assoc. 2017;6(6):e005819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yan T, Chopp M, Ning R, Zacharek A, Roberts C, Chen J. Intracranial aneurysm formation in type-one diabetes rats. PLoS One. 2013;8(7):e67949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Venkat P, Chopp M, Chen J. New insights into coupling and uncoupling of cerebral blood flow and metabolism in the brain. Croat Med J. 2016;57(3):223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen J, Venkat P, Zacharek A, Chopp M. Neurorestorative therapy for stroke. Front Hum Neurosci. 2014;8:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reeson P, Tennant KA, Gerrow K, Wang J, Weiser Novak S, Thompson K, Lockhart KL, Holmes A, Nahirney PC, Brown CE. Delayed inhibition of VEGF signaling after stroke attenuates blood-brain barrier breakdown and improves functional recovery in a comorbidity-dependent manner. J Neurosci. 2015;35(13):5128–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen X, Fu W, Tung CE, Ward NL. Angiopoietin-1 induces neurite outgrowth of PC12 cells in a Tie2-independent, beta1-integrin-dependent manner. Neurosci Res. 2009;64(4):348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van den Oever IA, Raterman HG, Nurmohamed MT, Simsek S. Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediators Inflamm. 2010;2010:792393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Belayev L, Busto R, Zhao W, Ginsberg MD. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996;739(1–2):88–96. [DOI] [PubMed] [Google Scholar]
- 41. Koyanagi M, Egashira K, Kitamoto S, Ni W, Shimokawa H, Takeya M, Yoshimura T, Takeshita A. Role of monocyte chemoattractant protein-1 in cardiovascular remodeling induced by chronic blockade of nitric oxide synthesis. Circulation. 2000;102(18):2243–2248. [DOI] [PubMed] [Google Scholar]
- 42. Arakelyan A, Petrkova J, Hermanova Z, Boyajyan A, Lukl J, Petrek M. Serum levels of the MCP-1 chemokine in patients with ischemic stroke and myocardial infarction. Mediators Inflamm. 2005;2005(3):175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Simeoni E, Hoffmann MM, Winkelmann BR, Ruiz J, Fleury S, Boehm BO, Marz W, Vassalli G. Association between the A-2518G polymorphism in the monocyte chemoattractant protein-1 gene and insulin resistance and Type 2 diabetes mellitus. Diabetologia. 2004;47(9):1574–1580. [DOI] [PubMed] [Google Scholar]
- 44. Panee J. Monocyte chemoattractant protein 1 (MCP-1) in obesity and diabetes. Cytokine. 2012;60(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yamagami S, Tamura M, Hayashi M, Endo N, Tanabe H, Katsuura Y, Komoriya K. Differential production of MCP-1 and cytokine-induced neutrophil chemoattractant in the ischemic brain after transient focal ischemia in rats. J Leukoc Biol. 1999;65(6):744–749. [DOI] [PubMed] [Google Scholar]
- 46. Wang X, Yue TL, Barone FC, Feuerstein GZ. Monocyte chemoattractant protein-1 messenger RNA expression in rat ischemic cortex. Stroke. 1995;26(4):661–665; discussion 665–666. [DOI] [PubMed] [Google Scholar]
- 47. Strecker JK, Minnerup J, Schutte-Nutgen K, Gess B, Schabitz WR, Schilling M. Monocyte chemoattractant protein-1-deficiency results in altered blood-brain barrier breakdown after experimental stroke. Stroke. 2013;44(9):2536–2544. [DOI] [PubMed] [Google Scholar]
- 48. Hughes PM, Allegrini PR, Rudin M, Perry VH, Mir AK, Wiessner C. Monocyte chemoattractant protein-1 deficiency is protective in a murine stroke model. J Cereb Blood Flow Metab. 2002;22(3):308–317. [DOI] [PubMed] [Google Scholar]
- 49. Wang X, Feuerstein GZ, Xu L, Wang H, Schumacher WA, Ogletree ML, Taub R, Duan JJ, Decicco CP, Liu RQ. Inhibition of tumor necrosis factor-alpha-converting enzyme by a selective antagonist protects brain from focal ischemic injury in rats. Mol Pharmacol. 2004;65(4):890–896. [DOI] [PubMed] [Google Scholar]
- 50. Ye X, Chopp M, Liu X, Zacharek A, Cui X, Yan T, Roberts C, Chen J. Niaspan reduces high-mobility group box 1/receptor for advanced glycation endproducts after stroke in type-1 diabetic rats. Neuroscience. 2011;190:339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108(7):949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jolkkonen J, Kwakkel G. Translational hurdles in stroke recovery studies. Transl Stroke Res. 2016;7(4):331–342. [DOI] [PubMed] [Google Scholar]