Abstract
Stroke is one of the most devastating diseases worldwide. In recent years, a great number of studies have focused on the effects of microRNAs (miRNAs) on stroke and the results demonstrated that the expressions of miRNAs are associated with the prognosis of stroke. In the present study, we review relevant articles regarding miRNAs and stroke and will explain the complex link between both. The miRNAs participate extensively in the pathophysiology following the stroke, including apoptosis, neuroinflammation, oxidative stress, blood–brain barrier (BBB) disruption and brain edema. The information about the stroke–miRNA system may be helpful for therapeutic and diagnostic methods in stroke treatment.
Keywords: microRNA, stroke, cerebral ischemia, intracerebral hemorrhage, subarachnoid hemorrhage
Introduction
Stroke is one of the most devastating diseases, ranking as the second leading cause of death worldwide1,2. The World Health Organization (WHO) defines stroke as ‘rapidly developing clinical signs of focal or global disturbance of cerebral function, with symptoms lasting for at least 24 hours, or leading to death with no apparent cause other than vascular origin’3. According to the WHO, approximately 15 million people suffer from strokes around the world, of which 5.5 million people die from them annually4. The incidence of stroke, especially in developing countries, is increasing and poses a great societal burden5.
Stroke can be divided into two categories: ischemic stroke and hemorrhagic stroke. The core incident of stroke is the interruption of blood supply to the brain tissues, which further causes the shortage of oxygen and nutrients and leads to brain injuries. The pathological mechanisms in the process of stroke and neuroprotective effects of various drugs have been comprehensively studied throughout the years, which consists of cellular apoptosis, inflammation, oxidative stress, and brain edema6,7. However, no drugs with specific efficacy are currently available.
In recent years, hundreds of genes have been found to be associated with the pathogenesis of stroke, but only a few were fully shown to influence its susceptibility. In addition, many studies focused on the effects of microRNAs (miRNAs) on stroke, as previous studies demonstrated that the expression of miRNA was related to the prognosis of this condition8–10.
miRNAs are characterized by single-stranded non-coding (nc)RNA, formed with 20–24 nucleotides11. The initial forms are primary miRNA transcripts (pri-miRNAs), which are transcribed from genomic DNA under the help of RNA polymerase II12. Then, the pri-miRNAs are transformed to functional secondary structures that contain stem-loops consisting of inverted repeats12,13. Precursor miRNAs (pre-miRNAs) leave from the pri-miRNAs which are cleaved by the endonuclease Drosha (also known as ribonuclease III)14,15. Next, pre-miRNAs are exported into the cytoplasm by exportin-5 with a Ran-GTP-dependent mechanism. Finally, pre-miRNAs are cleaved by the RNase III enzyme (Dicer) to become mature miRNAs16.
miRNA was first reported by Lee and colleagues in Caenorhabditis elegans (C. elegans) in 199317. It exhibits its functions via mRNA degradation or translational repression18. The function of miRNA was initially identified in original tissue samples. Recently, the discovery of circulating fetal nucleic acids in maternal plasma19 as well as detecting circulating extracellular miRNAs in the blood20 suggests a broad opportunity for developing the use of circulating miRNAs as biomarkers for non-invasive molecular diagnostics. Circulating miRNAs remain rather stable even after suffering from rugged conditions, such as extreme temperatures, strong acids or alkalis, and long-term preservation21,22. In addition, the functions of miRNAs are also involved in the modulation of a variety of cellular processes and pathophysiology such as neuronal survival, differentiation, metabolism, death/apoptosis, cerebrovascular diseases and cancers23–28.
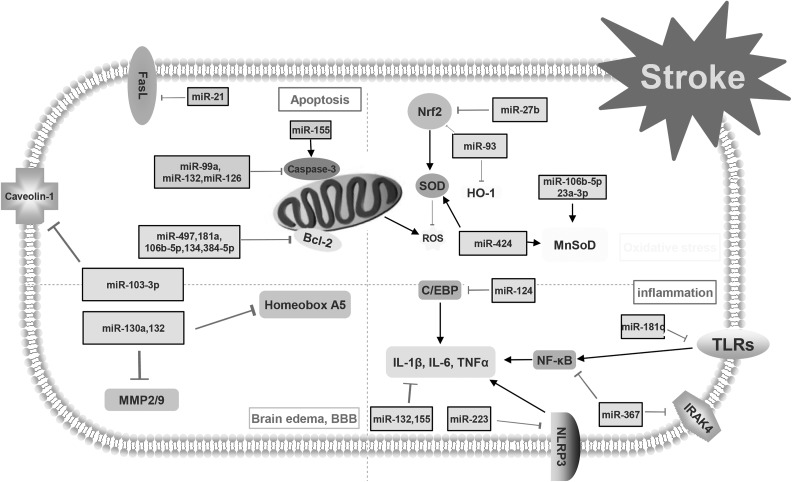
miRNAs have been reported to play key roles in the regulation of molecular processes after stroke29–32. The present study displays the complex relationship between miRNAs and stroke (Fig. 1). Therapeutic and diagnostic methods in stroke treatment may benefit from information about the stroke–miRNA system.
Fig 1.
This figure demonstrates the mechanisms of post-stroke pathophysiology. Apoptosis, neuroinflammation, oxidative stress and blood–brain barrier (BBB) disruption with brain edema are the main avenues that research tries to focus on to decrease the consequences of stroke. Different types of microRNAs and their targets are involved in the pathophysiology of stroke.
C/EBP: CCAAT/enhancer binding protein; FasL: Fas ligand; HO-1: hemeoxygenase-1; MMP: matrix metalloproteinase; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; ROS: reactive oxygen species; SOD: superoxide dismutase; TLR: Toll-like receptor.
Origin of miRNA Identified in Patients with Stroke
The functions of miRNA were initially identified in original tissue samples. Most of the studies focused on the relationships of miRNAs in brain tissues with underlying mechanisms of stroke, including cellular apoptosis, neuroinflammation, and oxidative stress33–36. In recent years, research has focused on the profiles of circulatory miRNAs. Whole blood and plasma could both be adopted as sources of circulatory miRNAs (Table 1). Tan and colleagues firstly evaluated the expression of miRNAs between healthy individuals and individuals diagnosed with cerebral ischemia using microarray analysis of blood specimens and selective TaqMan Quantitative polymerase chain reaction (qPCR) of miRNAs37. The function of miR-25, miR-125b-2, miR-125b-627, miR-125b-27a, miR-125b-488, miR-145 has been explored in blood in patients with cerebral ischemia. These miRNAs might be important biomarkers for diagnosis and treatment of stroke. In addition, Wang and colleagues studied circulating miRNAs in plasma from patients with acute stroke and those with non-stroke diseases to test whether circulating miRNAs in plasma could serve as biomarkers in diagnosing and treating stroke38. Overall, some miRNAs have been identified to be promising peripheral biomarkers, including miR-106b-5p, miR-126, miR-30a, miR-4306, let-7b38,39. Wang and colleagues demonstrated that high level of miR-130a in serum contributed to serious brain edema and poor prognosis after acute intracerebral hemorrhage (ICH)40. Wang and colleagues compared the expression of circulating microRNAs in blood and hematoma samples. The results showed that the levels of 59 miRNAs obviously decreased after ICH and the Hsa-miR-21-5p were obviously downregulated in both peripheral blood and hematoma samples after ICH41.
Table 1.
The miRNAs in the blood and CSF of stroke patients.
| Upregulation | Downregulation | References | |
|---|---|---|---|
| Whole blood | hsa-let-7e, miR-1184, -1246, -1261, -1275, -1285, -1290, -181a, -25*, -513a-5p, -550, -602, -665, -891a, -933, -939, -923 | hsa-let-7f, miR-126, -1259, -142-3p, -15b, -186, -519e, -768-5p | 37 |
| Plasma | hsa-miR-106b-5P, hsa-miR-4306, miR-30a, miR-126, hsa-let-7i-3p, hsa-miR-296-5p | hsa-miR-320e, hsa-miR-320d, let-7b, other 78 miRNAs were downregulated | 38–40 |
| CSF | hsa-miR-217, hsa-miR-9-5p, hsa-miR-338-3p, hsa-miR-204-5p, hsa-miR-34c-5p, miR-135a-5p, miR-219a-5p, miR-34b-5p, miR-92a-1-5p, miR-138-1-3p, miR-34b-3p, miR-33a-5p, miR-99a-3p, miR-338-5p, miR-519a-3p, miR-490-3p, miR-518e-3p, miR-138-2-3p | hsa-miR-19a-5p, miR-208b-3p, miR-493-3p, miR-301b, miR-219a-1-3p, miR-200a-5p, miR-126-5p | 41 |
CSF cerebrospinal fluid; miRNA: microRNA.
In addition, other studies evaluated the expression profiles of miRNAs in cerebrospinal fluid (CSF) after stroke. Iwuchukwu and colleagues evaluated the miRNA profiles in cerebrospinal fluid of patients with ICH. Their results showed that 25 miRNAs display significant fold changes. The highly expressed miR-204-5p (2075-fold), miR-125b-5p (108-fold), miR-9-5p (299-fold), miR-338-3p (146-fold), miR-187-3p (21-fold), and miR-9-3p (42-fold) in CSF were associated with the regulation of matrix metalloproteinase-9 (MMP-9), interleukin (IL)-1b, IL-6, occludin, and selectin E, among others. miRNA profiles in CSF were physiologically contiguous with profiles in brain extracellular fluid42. However, only a small number of studies have been reviewed. This area of stroke research would benefit from more studies investigating the function of miRNAs in CSF of patients with stroke.
The Role of miRNAs in Stroke
The expression of miRNAs is activated in stroke and plays an important role in the regulation of prognosis of patients with stroke. The biological functions of miRNAs are quite complex and should be discussed within a specific condition. On one hand, activation of miRNAs could contribute to attenuating neurological deficits. miRNA 21, miR-99a and miR-497 were reported to reduce the ischemic volume and protect neuronal cells from apoptosis, thus improving the neurological functions in rat and in vitro model of ischemic stroke43–45. Except for the functions mentioned above, overexpression of miR-424 and miR-let-7c-5p could also suppress the activation of microglia in cerebral ischemia46. As for hemorrhagic stroke, overexpression of miR-132, miR-126, miR-103 and miR-367 could attenuate neurobehavioral and neuropathological changes via improving BBB integrity, suppressing neuroinflammation and reducing neuronal apoptosis47–50. Additionally, miR-210 overexpression could promote angiogenesis and neurogenesis in mouse brain and help to repair the injured brain tissues51. On the other hand, the role of miRNA could also be negative. Liu and colleagues found that silencing miR-155 expression could increase the proliferation, migration, and tube formation ability of human brain micro-vessel endothelial cells via decreasing cellular apoptosis and reactive oxygen species (ROS) production52. As for hemorrhagic stroke, Xu and colleagues found that miR-27b inhibition could alleviate neurological deficits via suppressing neuroinflammation and reducing cell death. In addition, miR-155 and miR-124 were reported to play roles in the polarization of macrophages53. miRNA was also involved in the regulation of synaptic plasticity. miR-134 could help to remodel neuronal structures via repressing the translation of Limk1-mRNA, a protein kinase that influences dendritic spine development54.
However, some types of miRNA were reported to exert multiple functions. For example, Xie and colleagues showed that miR-181a could significantly improve cellular survival in vitro by suppressing inflammation responses in monocytes and macrophages55. In the report of Moon, however, it was demonstrated that the inhibition of miR-181a could reduce forebrain ischemia-induced neuronal apoptosis56.
Underlying Mechanisms of the Regulation of miRNAs in stroke
miRNAs and Apoptosis or Cell Death
Apoptosis is one type of cell death characterized by energy dependence and programmed cell death57. The term ‘apoptosis’ was first described by Kerry and colleagues58. Apoptosis is of vital importance to normal physiological metabolism and growth and development. It maintains hemostasis by scavenging aging or damaged cells, and regulates the immune system by removing defective and excessive cells59,60. However, uncontrolled apoptosis may result in various pathological processes of different diseases including cancers, Alzheimer’s disease, and stroke61,62. The apoptosis process is initially triggered either by the extrinsic or intrinsic pathway. The extrinsic pathway is conducted via the activation of cell surface death receptors, including tumor necrosis factor (TNF)-α, Fas and TNF-related apoptosis-inducing ligand receptors, while the intrinsic pathway is related to the mitochondrial signaling pathway63,64. Stroke induces a mass influx of Ca2+ into the cell, which leads to the release of mitochondrial cytochrome c (Cytc) or apoptosis-inducing factor (AIF)65. The released Cytc binds to the apoptotic protease-activating factor-1 and procaspase-9 to form an apoptosome which activates caspase-9 and subsequently, caspase-3, leading to the damage of nDNA and finally to cell death. What’s more, AIF is translocated to the nucleus and induces large-scale (50 kb) DNA fragmentation and cell death in a caspase-independent manner66.
Many studies have demonstrated that the expression of miRNA could modulate post-stroke neuronal survival by regulating the levels of target genes67,68. Liu and colleagues reported that miR-298 was upregulated in both brain and blood samples in both experimental models of cerebral ischemia and ICH69.
miR-21 is reported to be a potent antiapoptotic factor in biological systems. Buller and colleagues evaluated the expression level of miR-21 in vivo and in vitro. The results demonstrated that the level of miR-21 significantly increases after ischemic stroke through suppressing neuronal cell death through reducing Fas ligand (FasL)G, an important cell death-inducing ligand43. miR-155 was also reported to exert important roles in modulating cellular apoptosis by regulating caspase-3 expression. Knockdown of miR-155 significantly reduced apoptosis of brain micro-vessel endothelial cells52. miR-99a was reported to not only inhibit the activation of pro-caspase-3 and the expression of caspase-3, but also reduce neuronal apoptosis after ischemic stroke. Moreover, following cerebral Ischemia/Reperfusion (I/R), miR-99a reduced neuronal damage via regulation of the cell cycle and cellular apoptosis, which implied that miR-99a could be used as a new therapeutic agent targeting neuronal cell cycle re-entry following ischemic stroke44. In addition, the bcl-2 family plays a key role in the regulation of apoptosis. miR-497, miR-181a, miR-106b-5p, miR134, miR-384-5p were all reported to increase apoptosis by decreasing the level of bcl-2 proteins45,55,56,70,71.
Following hemorrhagic stroke, miR-132 was reported to exert a neuroprotective effect by decreasing neuronal death in ICH mice. The mice overexpressing miR-132 were less likely to suffer from neurological deficits47. Overexpression of miR-126 with lentivirus exhibits a protective role in ICH and antiapoptotic effects via downregulating the level of casepase-348. In addition, Wang and colleagues showed that miR-103-3p was obviously upregulated in the experimental model of subarachnoid hemorrhage. miR-103-3p exerted its neuroprotective effect by reducing the neuronal death via decreasing caveolin-149.
miRNAs and Neuroinflammation
Inflammation is a complex immune response following an injury. Under normal conditions, inflammation helps to scavenge necrotic cells or tissues, and initiates the tissue repair process72. However, excessive activation of immune responses is harmful to the organisms and can cause injury73. Recent studies have showed that neuroinflammation was a key factor in determining prognosis after stroke50,74–76. Either ischemic or hemorrhagic stroke triggers the activation of microglial and the releasing of inflammatory factors77,78. The activation of microglial and subsequent inflammatory factors, such as TNF-α contribute to the progression of brain injury53,79–80. Additionally, there are also some peripherally-derived cytokines produced and secreted by natural killer cells, mononuclear phagocytes, T-lymphocytes and polymorpho-nuclear leukocytes which participate in neuroinflammation after stroke81–82.
Many miRNAs target several genes participating in the regulation of neuroinflammation83–85. Zhao and colleagues demonstrated that lentiviral overexpression of miR-424 could significantly decrease brain injury after ischemic stroke through suppressing microglia activity46. In addition, miR-let-7c-5p was also reported to exert neuroprotection against neuroinflammation after ischemic stroke by inhibiting the activation of microglial and translational repression of caspase-386. miR-124 is almost exclusively expressed in the central nervous system and referred to as ‘brain-specific miRNA’87–88. In 2009, Laterza and colleagues showed that miR-124 was upregulated in plasma after brain injury (induced by middle cerebral artery occlusion)88. Ponomarev and colleagues found that miR-124 directly inhibited CCAAT/enhancer-binding protein alpha (C/EBP-α) and its downstream factor PU.1, promoting microglia quiescence. It also suppressed experimental autoimmune encephalomyelitis (EAE) by deactivating macrophages. In addition, Toll-like receptors (TLRs) are also reported to play important roles in neuroinflammation after stroke89–91. Zhang and colleagues showed that miR-181c suppressed the expression of TLR4 by binding to its 3’UTR, therefore reducing the level of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and the production of downstream proinflammatory factors, which could be regarded as one potential therapeutic target for the treatment of ischemic stroke92.
As for hemorrhagic stroke, the mice with miR132 overexpression had better prognosis compared with the control group. miR132 overexpression suppressed the activation of microglia and the expression of proinflammatory cytokines47. In the experimental model of ICH, Yuan and colleagues demonstrated that miR-367 inhibited the level of IRAK4 through directly binding its 3’-untranslated region. In addition, miR-367 also suppressed the activation of NF-κB and the production of its downstream proinflammatory factors. miR-223 was reported to downregulate NLRP3 and inhibit inflammation through caspase-1 and IL-1beta, thus to improve neurological functions93.
The role of miRNAs in the modulation of microglia and microphage polarization is also important for its anti-inflammatory effects in the brain. Some studies reported that miR-155 promotes skewing toward the M1 phenotype by targeting M2-associated genes. miR-155 clearly targeted multiple genes associated with the M2 phenotype and reduced the expression of M2-asociated proinflammatory factors such as Arg-1, IL-10, IL13Rα1 and CD206 94,95.
MicroRNA and Oxidative Stress
The oxidative stress, which is mainly caused by the overbalance of pro-oxidants (ROS/RNS) and/or deficiency of antioxidant systems in cells, takes part in the pathogenesis of many diseases96–98. The formation of free radicals and ROS during stroke involves several mechanisms, including high stimulation of N-methyl-D-aspartic acid (NMDA) glutamate receptors99, Ca2+ overload, mitochondrial dysfunction100–102, and neuronal nitric oxide synthase (nNOS) activation103. Oxidative damage is a basic mechanism of brain injury after stroke. The brain is vulnerable when exposed to oxidative stress because of its highly oxygenated characteristics, with high levels of peroxi-disable lipids, low levels of antioxidants, and high iron content104. Several antioxidant and detoxifying enzymes such as glutathione peroxidase, superoxide dismutase (SOD), glutathione reductase, and glutathione-S-transferase, which maintain redox homeostasis in the brain, have been widely studied105,106. Nuclear factor erythroid-2-related factor-2 (Nrf2) was reported to have neuroprotective effects against brain injuries after stroke, such as hydrogen peroxide (H2O2) exposure, Ca2+ overload situations, and oxidative glutamate excitotoxicity via activation of antioxidant response elements107.
It was reported that 85 miRNAs could regulate the level of Nrf2 mRNA108. miR-93 was reported to inhibit the expression of Nrf2 and hemeoxygenase-1 (HO-1) after ischemic stroke109. In addition, for cerebral ischemia, miR-424 could decrease infarct volume via reducing the level of ROS and malondialdehyde in the cortex, and increasing manganese SOD (MnSOD) as well as extracellular SOD. Similarly, miR-424 could significantly reduce H2O2-induced injury in neuronal cultures, increase cell viability and MnSOD activity and decrease the level of lactate dehydrogenase leakage and malondialdehyde110. In addition, miR-106b-5p and miR-23a-3p could exert a neuroprotective effect against post-ischemic oxidative damages by increasing the expression of MnSOD111–112. In addition, miR-145 could inhibit the expression of SOD2 after ischemic stroke113.
Xu and colleagues showed that the inhibition of miR-27b could alleviate brain injury and upregulate the expression of Nrf2, Hmox1, SOD1 and Nqo1 after ICH via the Nrf2/ARE pathway114. The gene could be a potential therapeutic target in treating ICH. However, limited studies have been reported regarding the anti-oxidative effects of miRNAs in hemorrhagic stroke. Further research should be carried out to explore the roles of miRNAs in hemorrhagic stroke.
miRNA and Brain Edema
BBB is a continuous, non-fenestrated system which regulates the movement of many particles and cells, such as ion, toxins, and inflammatory cells. Any factors disrupting the BBB would deteriorate the condition of neurological diseases, including stoke, traumatic brain injury and neurodegenerative diseases115–116. The most common complication of BBB disturbance is vasogenic brain edema, which is reported to be related to the early expression of matrix metalloproteinase (MMP)-2,9 after brain injury117–118.
In the rat model of ICH, the expression of miR-130a was obviously upregulated in serum and perihematomal samples, which was consistent with the changes of brain edema after the induction of ICH. The application of miR-130a inhibitors could significantly attenuate brain edema, reduce BBB permeability, and improve neurological functions via increasing caveolin-1 and decreasing MMP-2/9. Moreover, Wang and colleagues also found the effects of miR-130a in rats with cerebral ischemia. Inhibition of miR-130a could alleviate brain edema, reduce infarct volume and BBB permeability, and enhance neurologic function by targeting Homeobox A5119. Similarly, Zhang and colleagues demonstrated that mice injected with lentivirus encoding miR-132 had significantly reduced brain edema and improved BBB integrity47. In the rat model of subarachnoid hemorrhage, miR-103-3p exerted its neuroprotective effects via increasing the level of caveolin-149.
miRNA and Neurogenesis
Endogenous neurogenesis, the process of self-repairing, is increased after stroke. Neurogenesis is necessary for the neurological recovery for patients with ischemic or hemorrhagic stroke120,121.
Neurotrophic factors are small polypeptide molecules, which take part in cell proliferation, differentiation, migration and development of the nervous system. Previous studies have shown that neurotrophic factors such as nerve growth factor, brain derived neurotrophic factor (BDNF), ciliary neurotrophic factor, and glial-derived neurotrophic factor and insulin-like growth factor-1 (IGF-1) could reduce neuronal death and brain injuries122–123.
In the experimental model of cerebral ischemia, miR-Let7f was found to provide IGF-1-like neuroprotection122. Moreover, inhibition of miR-134 could significantly attenuate ischemic injuries via improving the expression of BDNF and Bcl-2. miR-30-5p and miR-107 could also regulate the expression of BDNF and be potential therapeutic targets in neuroprotection124. What’s more, endogenous neural stem cells (NSCs) and neural precursor cells (NPCs) could be activated and migrate to the injured area125. miR-21 could regulate the function of NPCs via Wnt and transforming growth factor (TGF)-β signaling pathways. In addition, miR-34a negatively regulated NPC proliferation after cerebral ischemia126. Furthermore, miR-126 could improve the neurorestorative effects induced with umbilical cord blood cells after stroke, according to studies completed in type-2 diabetic mice by Chen and colleagues127.
miRNA and Angiogenesis
The focus during the treatment of stroke should not only be placed on the regeneration of neural cells, but should also on the supporting tissues. This includes blood vessels to increase angiogenesis after stroke. Zhang and colleagues studied the structural changes after stroke, and they found that vascular volume was increased from 3% prior to stroke to 6% at 90 days after stroke128. miRNAs that regulate the process of angiogenesis could be regarded as potential therapeutic targets in the treatment of ischemic stroke129. Zheng and colleagues found that miR-210 played a key role in promoting angiogenesis after cerebral ischemia, partly via increasing the level of vascular endothelial growth factor (VEGF). In addition, Zeng and colleagues also showed that miR-210 could induce vascular endothelial cell migration and tube formation under hypoxia in vitro51. In the rat model of ICH, Ma and colleagues demonstrated that miR-129-5p could inhibit the HMGB1-RAGE signaling pathway and thus restrain the revascularization130. Overexpression of miR126 protected against ICH, which may be involved in the process of angiogenesis via upregulating the protein levels of VEGF-A65. Thus, promotion of angiogenesis could be regarded as a promising therapeutic approach for stroke via pharmacological modulation of these miRNAs.
Conclusions
In this review, we have introduced the basic knowledge of stroke and miRNAs. In addition, we further explored available concrete mechanisms of miRNAs in the post-stroke pathophysiological process to guide us in better understanding the processes involved in stroke pathology, including apoptosis, neuroinflammation, oxidative stress, BBB integrity, brain edema, neurogenesis, and angiogenesis (Table 2). With improved understanding comes the potential for a new therapeutic agent. However, several problems should not be ignored. First, the expression profiles of miRNAs in varied samples may change depending on the disease state, making it difficult to find appropriate endogenous controls. Second, the studies regarding the miRNAs in patients with subarachnoid hemorrhage (SAH) were limited. Lastly, more studies should also be carried out to explore the functions of miRNAs in CSF. Finally, till now, most studies were limited to experimental models while clinical research was scarce. More clinical studies should be carried out based on clinical medical ethics.
Table 2.
The roles of different miRNA in stroke.
| Type of mechanisms | Type of diseases | miRNA involved | References |
|---|---|---|---|
| Apoptosis | ischemic stroke | miR-298, miR-21, miR-155, miR-99a, miR-106b-5p, miR-497, miR-181a, miR134, miR-384-5p | 55–63 |
| hemorrhagic stroke | miR-298, miR-132, miR126, miR-103-3p | 55,64–66 | |
| Neuroinflammation | ischemic stroke | miR-424, miR-let-7c-5p, miR-124, miR-181c, miR132 | 83–90 |
| hemorrhagic stroke | miR132, miR-367, miR-223 | 64,91 | |
| BBB disruption/brain edema | ischemic stroke | miR-130a | 115 |
| hemorrhagic stroke | miR-132, miR-103-3p | 64,66 | |
| Oxidative stress | ischemic stroke | miR-93, miR-424, miR-106b-5p, miR-23a-3p, miR-145 | 105–109 |
| hemorrhagic stroke | miR-27b | 110 | |
| Neurogenesis | ischemic stroke | miR-Let7f, miR-134, MiR-107, miR-30-5p, miR-21, miR-34a | 118,120–122 |
| hemorrhagic stroke | miR-126 | 123 | |
| Angiogenesis | ischemic stroke | miR-210 | 126 |
| hemorrhagic stroke | miR-129-5p, miR126 | 65,127 |
BBB: blood–brain barrier; miRNA: microRNA
Acknowledgments
Sheng Chen, Jingwei Zheng, Weilin Xu and Liansheng Gao have equally contributed to this work as co-first authors.
Footnotes
Ethical Approval: This study was approved by the review board of the second affiliated hospital of Zhejiang univeristy.
Statement of Human and Animal Rights: Statement of Human and Animal Rights is not applicable.
Statement of Informed Consent: Statement of Informed Consent is not applicable.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (81500992), Natural Science Foundation of Zhejiang(LQ16H090002), Medical and health key project of Zhejiang Province (2016RCA015), China Postdoctoral Science Foundation (2017M612010) and National Natural Science Foundation of China (81701144).
References
- 1. Heron M. Deaths: leading causes for 2004. Natl Vital Stat Rep. 2007;56:1–96. [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention (CDC). Prevalence of disabilities and associated health conditions among adults-United States, 1999. Morb Mortal Wkly Rep. 2001;50:120–125. [PubMed] [Google Scholar]
- 3. WHO MONICA Project Principal Investigators. The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. J Clin Epidemiol. 1988;41(2):105–114. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization. The World Health Report 2004: changing history. Geneva: World Health Organization; 2004. [Google Scholar]
- 5. Banerjee TK, Roy MK, Bhoi KK. Is stroke increasing in India-preventive measures that need to be implemented. J Indian Med Assoc. 2005;103(3):162–166. [PubMed] [Google Scholar]
- 6. Xu XH, Zhong Z. Disease modeling and drug screening for neurological diseases using human induced pluripotent stem cells. Acta Pharmacol Sin. 2013;34(6):755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yi BR, Kim SU, Choi KC. Development and application of neural stem cells for treating various human neurological diseases in animal models. Lab Anim Res. 2013;29(3):131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zheng HW, Wang YL, Lin JX, Li N, Zhao XQ, Liu GF, Liu LP, Jiao Y, Gu WK, Wang DZ, Wang YJ. Circulating MicroRNAs as potential risk biomarkers for hematoma enlargement after intracerebral hemorrhage. CNS Neurosci Ther. 2012;18(12):1003–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stylli SS, Adamides AA, Koldej RM, Luwor RB, Ritchie DS, Ziogas J, Kaye AH. miRNA expression profiling of cerebrospinal fluid in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2017;126(4):1131–1139. [DOI] [PubMed] [Google Scholar]
- 10. Powers CJ, Dickerson R, Zhang SW, Rink C, Roy S, Sen CK. Human cerebrospinal fluid microRNA: temporal changes following subarachnoid hemorrhage. Physiol Genomics. 2016;48(5):361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. [DOI] [PubMed] [Google Scholar]
- 12. Lee Y, Jeon K, Lee JT, Kim S, Kim VN. MicroRNA maturation: stepwise processing and subcellular localization. EMBO J. 2002;21:4663–4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zeng Y, Cullen BR. Sequence requirements for micro RNA processing and function in human cells. RNA. 2003;9:112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Basyuk E, Suavet F, Doglio A, Bordonné R, Bertrand E. Human let-7 stem-loop precursors harbor features of RNase III cleavage products. Nucleic Acids Res. 2003;31(22):6593–6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Han J, Lee Y, Yeom KH, Kim YK, Jin H, Kim VN. The Drosha–DGCR8 complex in primary microRNA processing. Genes Dev. 2004;18(24):3016–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432(7014):235–240. [DOI] [PubMed] [Google Scholar]
- 17. Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. [DOI] [PubMed] [Google Scholar]
- 18. Bhalala OG, Srikanth M, Kessler JA. The emerging roles of microRNAs in CNS injuries. Nat Rev Neurol. 2013;9(6):328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chim SS, Shing TK, Hung EC, Leung TY, Lau TK, Chiu RW, Lo YM. Detection and characterization of placental microRNAs in maternal plasma. Clin Chem. 2008;54(3):482–490. [DOI] [PubMed] [Google Scholar]
- 20. Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K, Banham AH, Pezzella F, Boultwood J, Wainscoat JS, Hatton CS, Harris AL. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Brit J Haematol. 2008;141(5):672–675. [DOI] [PubMed] [Google Scholar]
- 21. Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006. [DOI] [PubMed] [Google Scholar]
- 22. Tsui NB, Ng EK, Lo YM. Stability of endogenous and added RNA in blood specimens, serum, and plasma. Clin Chem. 2002;48(10):1647–1653. [PubMed] [Google Scholar]
- 23. Mocellin S, Pasquali S, Pilati P. Oncomirs: from tumor biology to molecularly targeted anticancer strategies. Mini Rev Med Chem. 2009;9(1):70–80. [DOI] [PubMed] [Google Scholar]
- 24. Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O, Brummelkamp TR, Fleming MD, Camargo FD. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature. 2008;451(7182):1125–1129. [DOI] [PubMed] [Google Scholar]
- 25. Aumiller V, Förstemann K. Roles of microRNAs beyond development-metabolism and neural plasticity. Biochim Biophys Acta. 2008;1779(11):692–696. [DOI] [PubMed] [Google Scholar]
- 26. Bushati N, Cohen SM. MicroRNAs in neurodegeneration. Curr Opin Cell Biol. 2008;18(3):292–96. [DOI] [PubMed] [Google Scholar]
- 27. Khoshnam SE, Winlow W, Farbood Y, Moghaddam HF, Farzaneh M. Emerging roles of micrornas in ischemic stroke: as possible therapeutic agents. J Stroke. 2017;19(2):166–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bourassa MW, Ratan RR. The interplay between microRNAs and histone deacetylases in neurological diseases. Neurochem Int. 2014;77:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke. 2008;39(3):959–966. [DOI] [PubMed] [Google Scholar]
- 30. Redell JB, Liu Y, Dash PK. Traumatic brain injury alters expression of hippocampal microRNAs: potential regulators of multiple pathophysiological processes. J Neurosci Res. 2009;87(6):1435–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kikkawa Y, Ogura T, Nakajima H, Ikeda T, Takeda R, Neki H, Kohyama S, Yamane F, Kurogi R, Amano T, Nakamizo A, Mizoguchi M, Kurita H. Altered Expression of MicroRNA-15a and Kruppel-Like Factor 4 in Cerebrospinal Fluid and Plasma After Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2017;108:909–916.e3. [DOI] [PubMed] [Google Scholar]
- 32. Uhlmann S, Mracsko E, Javidi E, Lamble S, Teixeira A, Hotz-Wagenblatt A, Glatting KH, Veltkamp R. Genome-wide analysis of the circulating miRNome after cerebral ischemia reveals a reperfusion-induced MicroRNA Cluster. Stroke. 2017;48(3):762–769. [DOI] [PubMed] [Google Scholar]
- 33. Zhang L, Dong LY, Li YJ, Hong Z, Wei WS. The microRNA miR-181c controls microglia-mediated neuronal apoptosis by suppressing tumor necrosis factor. J Neuroinflammation. 2012;9:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wen Y, Zhang X, Dong L, Zhao J, Zhang C, Zhu C. Acetyl-britannilactone modulates microRNA-155-mediated inflammatory response in ischemic cerebral tissues. Mol Med. 2015;21:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martinez B, Peplow PV. Blood microRNAs as potential diagnostic markers for hemorrhagic stroke. Neural Regen Res. 2017;12(1):13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Banerjee S, Xie N, Cui H, Tan Z, Yang S, Icyuz M, Abraham E, Liu G. MicroRNA let-7c regulates macrophage polarization. J Immunol. 2013;190(12):6542–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tan KS, Armugam A, Sepramaniam S, Lim KY, Setyowati KD, Wang CW, Jeyaseelan K. Expression profile of MicroRNAs in young stroke patients. PLoS One. 2009;4(11):e7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang W, Sun G, Zhang L, Shi L, Zeng Y. Circulating microRNAs as novel potential biomarkers for early diagnosis of acute stroke in humans. J Stroke Cerebrovasc Dis. 2014;23(10):2607–2613. [DOI] [PubMed] [Google Scholar]
- 39. Long G, Wang F, Li H, Yin Z, Sandip C, Lou Y, Wang Y, Chen C, Wang DW. Circulating miR-30a, miR-126 and let-7b as biomarker for ischemic stroke in humans. BMC Neurol. 2013;13:178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang MD, Wang Y, Xia YP, Dai JW, Gao L, Wang SQ, et al. High serum MiR-130a levels are associated with severe perihematomal edema and predict adverse outcome in acute ICH. Mol Neurobiol. 2016; 53(2):1310–21. [DOI] [PubMed] [Google Scholar]
- 41. Wang J, Zhu Y, Jin F, Tang L, He Z, He Z. Differential expression of circulating microRNAs in blood and haematoma samples from patients with intracerebral haemorrhage. J Int Med Res. 2016;44(3):419–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Iwuchukwu I, Nguyen D, Sulaiman W. MicroRNA Profile in cerebrospinal fluid and plasma of patients with spontaneous intracerebral hemorrhage. CNS Neurosci Ther. 2016;22(12):1015–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ben Buller, Xianshuang Liu, Xinli Wang, Rui Lan Zhang, Li Zhang, Ann Hozeska-Solgot, Michael Chopp, Zheng Gang Zhang. miR-21 protects neurons from ischemic death. FEBS J. 2010;277(20):4299–4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tao Z, Zhao H, Wang R, Liu P, Yan F, Zhang C, Ji X, Luo Y. Neuroprotective effect of microRNA-99a against focal cerebral ischemia-reperfusion injury in mice. J Neurol Sci. 2015;355(1–2):113–119. [DOI] [PubMed] [Google Scholar]
- 45. Yin KJ, Deng Z, Huang H, Hamblin M, Xie C, Zhang J, Chen YE. miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol Dis. 2010;38(1):17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhao H, Wang J, Gao L, Wang R, Liu X, Gao Z, Tao Z, Xu C, Song J, Ji X, Luo Y. MiRNA-424 protects against permanent focal cerebral ischemia injury in mice involving suppressing microglia activation. Stroke. 2013;44:1706–1713. [DOI] [PubMed] [Google Scholar]
- 47. Zhang Y, Han B, He Y, Li D, Ma X, Liu Q, Hao J. MicroRNA-132 attenuates neurobehavioral and neuropathological changes associated with intracerebral hemorrhage in mice. Neurochem Int. 2017;107:182–190. [DOI] [PubMed] [Google Scholar]
- 48. Kong F, Zhou J, Zhou W, Guo Y, Li G, Yang L. Protective role of microRNA-126 in intracerebral hemorrhage. Mol Med Rep. 2017;15(3):1419–1425. [DOI] [PubMed] [Google Scholar]
- 49. Wang LM, Xie Y, Xu LL, Ye RD, Liu XF. Target-regulated caveolin-1 by miR-103 improves neurological deficits following subarachnoid hemorrhage. Chin J Geriatr Heart Brain Vessel Dis. 2016;18(5):531–534. [Google Scholar]
- 50. Yuan B, Shen H, Lin L, Su T, Zhong L, Yang Z. MicroRNA367 negatively regulates the inflammatory response of microglia by targeting IRAK4 in intracerebral hemorrhage. J Neuroinflammation. 2015;12(9):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. C Zeng L, He X, Wang Y, Tang Y, Zheng C, Cai H, Liu J, Wang Y, Fu Y, Yang GY. MicroRNA-210 overexpression induces angiogenesis and neurogenesis in the normal adult mouse brain. Gene Ther. 2014;21(1):37–43. [DOI] [PubMed] [Google Scholar]
- 52. Liu Y, Pan Q, Zhao Y, He C, Bi K, Chen Y, Zhao B, Chen Y, Ma X. MicroRNA-155 regulates ROS production, no generation, apoptosis and multiple functions of human brain microvessel endothelial cells under physiological and pathological conditions. J Cell Biochem. 2015;116(12):2870–2881. [DOI] [PubMed] [Google Scholar]
- 53. Yin M, Chen Z, Ouyang Y, Zhang H, Wan Z, Wang H, Wu W, Yin X. Thrombin-induced, TNFR-dependent miR-181c downregulation promotes MLL1 and NF-κB target gene expression in human microglia. J Neuroinflammation. 2017;14(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439(7074):283–289. [DOI] [PubMed] [Google Scholar]
- 55. Xie W, Li M, Xu N, Lv Q, Huang N, He J, Zhang Y. Mir-181a regulates inflammation responses in monocytes and macrophages. PLoS One. 2013;8:e58639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moon JM, Xu L, Giffard RG. Inhibition of microRNA-181 reduces forebrain ischemia-induced neuronal loss. J Cereb Blood Flow Metab. 2013;33(12):1976–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Meier P, Finch A, Evan G. Apoptosis in development. Nature. 2000;407(6805):796–801. [DOI] [PubMed] [Google Scholar]
- 60. Rathmell JC, Thompson CB. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell. 2002;109:97–107. [DOI] [PubMed] [Google Scholar]
- 61. Reed JC. Apoptosis-based therapies. Nat Rev Drug Discov. 2002;1:111–121. [DOI] [PubMed] [Google Scholar]
- 62. Shao A, Wang Z, Wu H, Dong X, Li Y, Tu S, Tang J, Zhao M, Zhang J, Hong Y. Enhancement of autophagy by histone deacetylase inhibitor trichostatin: an ameliorates neuronal apoptosis after subarachnoid hemorrhage in rats. Mol Neurobiol. 2016;53(1):18–27. [DOI] [PubMed] [Google Scholar]
- 63. Adams JM. Ways of dying: multiple pathways to apoptosis. Genes Dev. 2003;17(20):2481–2495. [DOI] [PubMed] [Google Scholar]
- 64. Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. [DOI] [PubMed] [Google Scholar]
- 65. Culmsee C, Zhu C, Landshamer S, Becattini B, Wagner E, Pellecchia M, Blomgren K, Plesnila N. Apoptosis-inducing factor triggered by poly (ADP-ribose) polymerase and Bid mediates neuronal cell death after oxygen-glucose deprivation and focal cerebral ischemia. J Neurosci. 2005;25(44):10262–10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40(5):e331–e339. [DOI] [PubMed] [Google Scholar]
- 67. Peng Z, Li J, Li Y, Yang X, Feng S, Han S, Li J. Downregulation of miR-181b in mouse brain following ischemic stroke induces neuroprotection against ischemic injury through targeting heat shock protein A5 and ubiquitin carboxyl-terminal hydrolase isozyme L-1. J Neurosci Res. 2013;91:1349–1362. [DOI] [PubMed] [Google Scholar]
- 68. Cardoso AL, Guedes JR, Pereira de Almeida L, Pedroso de Lima MC. miR-155 modulates microglia-mediated immune response by down-regulating SOCS-1 and promoting cytokine and nitric oxide production. Immunology. 2012;135:73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Liu DZ, Tian Y, Ander BP, Xu H, Stamova BS, Zhan X, Turner RJ, Jickling G, Sharp FR. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J Cereb Blood Flow Metab. 2010;30(1):92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhai F, Zhang X, Guan Y, Yang X, Li Y, Song G, Guan L. Expression profiles of microRNAs after focal cerebral ischemia/reperfusion injury in rats. Neural Regen Res. 2012;7:917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Huang W, Liu X, Cao J, Meng F, Li M, Chen B, hang J. Mir-134 regulates ischemia/reperfusion injury-induced neuronal cell death by regulating CREB signaling. J Mol Neurosci. 2015;55:821–829. [DOI] [PubMed] [Google Scholar]
- 72. Turrin NP, Rivest S. Molecular and cellular immune mediators of neuroprotection. Mol. Neurobiol. 2006;34(3):221–242. [DOI] [PubMed] [Google Scholar]
- 73. Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol. Neurobiol. 2010;41(2–3):242–247. [DOI] [PubMed] [Google Scholar]
- 74. Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67(2):181–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chamorro A, Hallenbeck J. The harms and benefits of inflammatory and immune responses in vascular disease. Stroke. 2006;37(2):291–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ponomarev ED, Veremeyko T, Weiner HL. MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS. Glia. 2013;61(1):91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Amantea D, Nappi G, Bernardi G, Bagetta G, Corasaniti MT. Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J. 2009;276:13–26. [DOI] [PubMed] [Google Scholar]
- 78. Bazzoni F, Rossato M, Fabbri M, Gaudiosi D, Mirolo M, Mori L, Tamassia N, Mantovani A, Cassatella MA, Locati M. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc Natl Acad Sci USA. 2009;106(13):5282–5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mrak RE, Griffin WS. Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging. 2005;26:349–354. [DOI] [PubMed] [Google Scholar]
- 80. Eikelenboom P, Rozemuller AJ, Hoozemans JJ, Veerhuis R, van Gool WA. Neuroinflammation and Alzheimer disease: clinical and therapeutic implications. Alzheimer Dis Assoc Dis. 2000;14(Suppl. 1):S54–S61. [DOI] [PubMed] [Google Scholar]
- 81. Ferrarese C, Mascarucci P, Zoia C, Cavarretta R, Frigo M, Begni B, Sarinella F, Frattola L, De Simoni MG. Increased cytokine release from peripheral blood cells after acute stroke. J Cereb Blood Flow Metab. 1999;19(9):1004–1009. [DOI] [PubMed] [Google Scholar]
- 82. Bache S, Rasmussen R, Rossing M, Laigaard FP, Nielsen FC, Møller K. MicroRNA Changes in cerebrospinal fluid after subarachnoid hemorrhage. Stroke. 2017;48(9):2391–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Saugstad JA. MicroRNAs as effectors of brain function with roles in ischemia and injury, neuroprotection, and neurodegeneration. J Cereb Blood Flow Metab. 2010;30(9):1564–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tan JR1, Koo YX, Kaur P, Liu F, Armugam A, Wong PT, Jeyaseelan K. microRNAs in stroke pathogenesis. Curr Mol Med. 2011;11(2):76–92. [DOI] [PubMed] [Google Scholar]
- 85. Guo D, Liu J, Wang W, Hao F, Sun X, Wu X, Bu P, Zhang Y, Liu Y, Liu F, Zhang Q, Jiang F. Alteration in abundance and compartmentalization of inflammation-related miRNAs in plasma after intracerebral hemorrhage. Stroke. 2013;44(6):1739–1742. [DOI] [PubMed] [Google Scholar]
- 86. Ni J, Wang X, Chen S, Liu H, Wang Y, Xu X, Cheng J, Jia J, Zhen X. MicroRNA let-7c-5p protects against cerebral ischemia injury via mechanisms involving the inhibition of microglia activation. Brain Behav Immun. 2015;49:75–85. [DOI] [PubMed] [Google Scholar]
- 87. Meza-Sosa KF, Pedraza-Alva G, Perez-Martinez L. MicroRNAs: key triggers of neuronal cell fate. Front Cell Neurosci. 2014;8:175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Laterza OF, Lim L, Garrett-Engele PW, Vlasakova K, Muniappa N, Tanaka WK, Johnson JM, Sina JF, Fare TL, Sistare FD, Glaab WE. Plasma microRNAs as sensitive and specific biomarkers of tissue injury. Clin Chem. 2009;55:1977–1983. [DOI] [PubMed] [Google Scholar]
- 89. Weinstein JR, Koerner IP, Möller T. Microglia in ischemic brain injury. Future Neurol. 2010;5:227–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immun. 2010;11(5):373–384. [DOI] [PubMed] [Google Scholar]
- 91. Karikó K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279:12542–12550. [DOI] [PubMed] [Google Scholar]
- 92. Zhang L, Li YJ, Wu XY, Hong Z, Wei WS. MicroRNA-181c negatively regulates the inflammatory response in oxygen-glucose-deprived microglia by targeting Toll-like receptor-4. J Neurochem. 2015;132:713–723. [DOI] [PubMed] [Google Scholar]
- 93. Yang Z, Zhong L, Xian R, Yuan B. MicroRNA-223 regulates inflammation and brain injury via feedback to NLRP3 inflammasome after intracerebral hemorrhage. Mol Immunol. 2015;65(2):267–276. [DOI] [PubMed] [Google Scholar]
- 94. Martinez-Nunez RT, Louafi F, Sanchez-Elsner T. The interleukin 13 (IL-13) pathway in human macrophages is modulated by microRNA-155 via direct targeting of interleukin 13 receptor alpha1 (IL13Ralpha1). J Biol Chem. 2011;286(3):1786–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ruffell D, Mourkioti F, Gambardella A, Kirstetter P, Lopez RG, Rosenthal N, Nerlov C. A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci USA. 2009;106(41):17475–17480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gasche Y, Copin JC, Sugawara T, Fujimura M, Chan PH. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood–brain barrier disruption after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:1393–1400. [DOI] [PubMed] [Google Scholar]
- 97. Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. [DOI] [PubMed] [Google Scholar]
- 98. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, elser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. [DOI] [PubMed] [Google Scholar]
- 99. Cherubini A, Ruggiero C, Polidori MC, Mecocci P. Potential markers of oxidative stress in stroke. Free Radical Bio Med. 2005;39:841–852. [DOI] [PubMed] [Google Scholar]
- 100. Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262(5134):689–695. [DOI] [PubMed] [Google Scholar]
- 101. Cuzzocrea S, Riley DP, Caputi AP, Salvemini D. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol Rev. 2001;53:135–159. [PubMed] [Google Scholar]
- 102. Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. NMDA-dependent superoxide production and neurotoxicity. Nature. 1993;364(6437):535–537. [DOI] [PubMed] [Google Scholar]
- 103. Piantadosi CA, Zhang J. Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke. 1996;27:327–331; discussion 332. [DOI] [PubMed] [Google Scholar]
- 104. Saeed SA, Shad KF, Saleem T, Javed F, Khan MU. Some new prospects in the understanding of the molecular basis of the pathogenesis of stroke. Exp Brain Res. 2007;182:1–10. [DOI] [PubMed] [Google Scholar]
- 105. Gariballa SE, Hutchin TP, Sinclair AJ. Antioxidant capacity after acute ischaemic stroke. QJM. 2002;95(10):685–690. [DOI] [PubMed] [Google Scholar]
- 106. Spranger M, Krempien S, Schwab S, Donneberg S, Hacke W. Superoxide dismutase activity in serum of patients with acute cerebral ischemic injury. Correlation with clinical course and infarct size. Stroke. 1997;28:2425–2428. [DOI] [PubMed] [Google Scholar]
- 107. Johnson JA, Johnson DA, Kraft AD, Calkins MJ, Jakel RJ, Vargas MR, Chen PC. The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann NY Acad Sci. 2008;1147:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Papp D, Lenti K, Módos D, Fazekas D, Dúl Z, Türei D, Földvári-Nagy L, Nussinov R, Csermely P, Korcsmáros T. The NRF2-related interactome and regulome contain multifunctional proteins and fine-tuned autoregulatory loops. FEBS Lett. 2012;586(13):1795–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wang P, Liang X, Lu Y, Zhao X, Liang J. MicroRNA-93 downregulation ameliorates cerebral ischemic injury through the Nrf2/HO-1 defense pathway. Neurochem Res. 2016;41:2627–2635. [DOI] [PubMed] [Google Scholar]
- 110. Liu P, Zhao H, Wang R, Wang P, Tao Z, Gao L, Yan F, Liu X, Yu S, Ji X, Luo Y. MicroRNA-424 protects against focal cerebral ischemia and reperfusion injury in mice by suppressing oxidative stress. Stroke. 2015;46:513–519. [DOI] [PubMed] [Google Scholar]
- 111. Li P, Shen M, Gao F, Wu J, Zhang J, Teng F, Zhang C. An antagomir to microRNA-106b-5p ameliorates cerebral ischemia and reperfusion injury in rats via inhibiting apoptosis and oxidative stress. Mol Neurobiol. 2017;54(4):2901–2921. [DOI] [PubMed] [Google Scholar]
- 112. Zhao H, Tao Z, Wang R, Liu P, Yan F, Li J, Zhang C, Ji X, Luo Y. MicroRNA-23a-3p attenuates oxidative stress injury in a mouse model of focal cerebral ischemia-reperfusion. Brain Res. 2014;1592:65–72. [DOI] [PubMed] [Google Scholar]
- 113. Dharap A, Bowen K, Place R, Li LC, Vemuganti R. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab. 2009;29:675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Xu W, Li F, Liu Z, Xu Z, Sun B, Cao J, Liu Y. MicroRNA-27b inhibition promotes Nrf2/ARE pathway activation and alleviates intracerebral hemorrhage-induced brain injury. Oncotarget. 2017;8(41):70669–70684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Liu YC, Lee YD, Wang HL, Liao KH, Chen KB, Poon KS, Pan YL, Lai TW. Anesthesia-Induced hypothermia attenuates early-phase blood–brain barrier disruption but not infarct volume following cerebral ischemia. PLoS One. 2017;12(1):e0170682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Alhadidi Q, Sayeed MS, Shah Z. The Interplay between Cofilin and Phospho-cofilin: Its Role in Maintaining Blood–Brain Barrier Integrity. CNS Neurol Disord Drug Targets. 2017;16(3):279–290. [DOI] [PubMed] [Google Scholar]
- 117. Fujimura M, Gasche Y, Morita-Fujimura Y, Massengale J, Kawase M, Chan PH. Early appearance of activated matrix metalloproteinase-9 and blood–brain barrier disruption in mice after focal cerebral ischemia and reperfusion. Brain Res. 1999;842:92–100. [DOI] [PubMed] [Google Scholar]
- 118. Gasche Y, Fujimura M, Morita-Fujimura Y, Copin JC, Kawase M, Massengale J, Chan PH. Early appearance of activated matrix metalloproteinase-9 after focal cerebral ischemia in mice: a possible role in blood–brain barrier dysfunction. J Cereb Blood Flow Metab. 1999;19:1020–1028. [DOI] [PubMed] [Google Scholar]
- 119. Wang Y, Wang MD, Xia YP, Gao Y, Zhu YY, Chen SC, Mao L, He QW, Yue ZY, Hu B. MicroRNA-130a regulates cerebral ischemia-induced blood–brain barrier permeability by targeting Homeobox A5. FASEB J. 2018;32(2):935–944. [DOI] [PubMed] [Google Scholar]
- 120. Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. [DOI] [PubMed] [Google Scholar]
- 121. Zhao Y, Wei ZZ, Zhang JY, Zhang Y, Won S, Sun J, Yu SP, Li J, Wei L. GSK-3β Inhibition induced neuroprotection, regeneration, and functional recovery after intracerebral hemorrhagic stroke. Cell Transplant. 2017;26(3):395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Cheng YD, Al-Khoury L, Zivin JA. Neuroprotection for ischemic stroke: two decades of success and failure. NeuroRx. 2004;1:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Li M, Zhang J. Circulating MicroRNAs: potential and emerging biomarkers for diagnosis of cardiovascular and cerebrovascular diseases. Biomed Res Int. 2015;2015:730535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mellios N, Huang HS, Grigorenko A, Rogaev E, Akbarian S. A set of differentially expressed miRNAs, including miR-30a-5p, act as post-transcriptional inhibitors of BDNF in prefrontal cortex. Hum Mol Genet. 2008;17:3030–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, Ho KL, Morshead C, Chopp M. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–448. [DOI] [PubMed] [Google Scholar]
- 126. Liu FJ, Lim KY, Kaur P, Sepramaniam S, Armugam A, Wong PT, Jeyaseelan K. MicroRNAs involved in regulating spontaneous recovery in embolic stroke model. PLoS One. 2013;8:e66393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Chen J, Ning R, Zacharek A, Cui C, Cui X, Yan T, Venkat P, Zhang Y, Chopp M. MiR-126 Contributes to human umbilical cord blood cell-induced neurorestorative effects after stroke in type-2 Diabetic Mice. Stem Cells. 2016;34(1):102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Zhang RL, Chopp M, Roberts C, Liu X, Wei M, Nejad-Davarani SP, Wang X, Zhang ZG. Stroke increases neural stem cells and angiogenesis in the neurogenic niche of the adult mouse. PLoS One. 2014;9(12):e113972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wu F, Yang Z, Li G. Role of specific microRNAs for endothelial function and angiogenesis. Biochem Biophys Res Commun. 2009;386:549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ma XL, Li SY, Shang F. Effect of microRNA-129-5p targeting HMGB1-RAGE signaling pathway on revascularization in a collagenase-induced intracerebral hemorrhage rat model. Biomed Pharmacother. 2017;93:238–244. [DOI] [PubMed] [Google Scholar]