Abstract
Exogenous stem cell therapy (SCT) has been recognized recently as a promising neuroregenerative strategy to augment recovery in stroke survivors. Mesenchymal stem cells (MSCs) are the primary source of stem cells used in the majority of both pre-clinical and clinical studies in stroke. In the absence of evidence-based guidelines on the use of SCT in stroke patients, understanding the progress of MSC research across published studies will assist researchers and clinicians in better achieving success in translating research. We conducted a systematic review on published literature using MSCs in both pre-clinical studies and clinical trials between 2008 and 2017 using the public databases PubMed and Ovid Medline, and the clinical trial registry (www.clinicaltrials.gov). A total of 78 pre-clinical studies and eight clinical studies were identified. While majority of the pre-clinical and clinical studies demonstrated statistically significant effects, the clinical significance of these findings was still unclear. Effect sizes could not be measured mainly due to reporting issues in pre-clinical studies, thus limiting our ability to compare results across studies quantitatively. The overall quality of both pre-clinical and clinical studies was sub-optimal. By conducting a systematic review of both pre-clinical and clinical studies on MSCs therapy in stroke, we assessed the quality of current evidence and identified several issues and gaps in translating animal studies to human trials. Addressing these issues and incorporating changes into future animal studies and human trials may lead to better success of stem cells-based therapeutics in the near future.
Keywords: mesenchymal stem cell, stroke, recovery, pre-clinical science, clinical science
Introduction
Although interventions for early reperfusion such as intravenous thrombolysis and endovascular revascularization have shown significant benefit in stroke patients, stroke remains a leading cause of long-term disability worldwide1,2. Recently, stem cell therapy using different cell types (e.g., mesenchymal stem cells (MSCs)3–5, bone marrow mononuclear cells6–8, and neural stem cells9–11) has emerged as a promising regenerative treatment for stroke survivors with residual deficits. MSCs are multipotent adult stem cells characterized by the potential for easy isolation and amplification, low immunogenicity, and paracrine and immunomodulatory function. MSCs have been widely investigated in both experimental and clinical stroke. In addition to repairing injured tissue or replacing the lost neurons after stroke, MSCs may modulate the microenvironment of the damaged brain tissue toward a more regenerative and less inflammatory milieu12–17.
Several factors could influence outcomes after MSCs transplantation in the pre-clinical and clinical studies including donor cells (cell type, safety, autologous or allogeneic, cell dose), host factors (patient clinico-demographic characteristics, stroke severity, subtype, and lesion location), time from stroke (acute, sub-acute, or chronic), delivery route (intravenous, direct transplant, endovascular approach), and the outcome measures used to address to assess outcomes (behavioral outcomes and imaging assessment)16,18,19. These key determinants of success of MSCs transplant in stroke need to be carefully validated and confirmed in pre-clinical stroke models that allow for a more controlled environment to optimize these variables as a first step before proper design of future trials. Collaborative efforts, such as the Stem cell Therapies as an Emerging Paradigm in Stroke (STEPS) committee, have emerged to create a common and rigorous platform for pre-clinical investigations on MSCs transplantation in stroke. They highlighted an urgent need for a well-characterized cell population, dose-response studies, and tests in different models of at least two species, before applying it to stroke patients20–22. Those recommendations are in line with the Stroke Treatment Academic Industry Roundtable (STAIR) recommendations for pre-clinical stroke research23. Despite promising outcomes from many pre-clinical studies, success in human clinical trials has not been claimed to date24–27. We conducted a qualitative and quantitative systematic review of literature in both pre-clinical and clinical stroke investigating the efficacy of MSCs transplanting in improving outcomes.
Materials and Methods
Search Strategies
For pre-clinical studies, we manually searched professional journals between 2008 and 2017 in the PubMed and Ovid Medline databases. We used the following search strategy: (mesenchymal OR mesenchymal stem cell OR mesenchymal stromal cell) AND (stroke OR cerebrovascular OR middle cerebral artery OR MCA OR anterior cerebral artery OR ACA). We also reviewed secondary references. We excluded studies with the hemorrhagic stroke model, non-English studies, or if the MSCs therapy involved additional active components such as gene modification or combined with any another treatment24. Two authors independently abstracted all data from any eligible publication, according to a standard protocol. Discrepancies were resolved by discussion.
For clinical studies, we utilized the following search strategies to identify articles published in English language in PubMed, Ovid Medline and Stroke Trial Registry (www.clinicaltrial.gov) between 2008 and 2017: (mesenchymal stem cells OR mesenchymal stromal cells) AND (stroke OR cerebrovascular) AND Humans AND Clinical Trial. We further screened articles for relevance based on the title and abstract content.
Data Extraction
We followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis: The PRISMA Statement28. In pre-clinical studies we extracted details of experimental design from each manuscript. Study quality was assessed according to the STAIR guidelines, including (1) publication in a peer-reviewed journal; (2) statement confirming compliance with animal welfare requirements; (3) avoided neuroprotective anesthetics; (4) statements describing control of temperature; (5) random treatment assignment; (6) allocation concealment; (7) blinded outcome assessment; (8) inclusion of a sample-size calculation; (9) use of animals with relevant comorbidities; and (10) inclusion of a statement declaring presence or absence of any conflicts of interest23. One point was given for each criterion reported. Potential score ranges from 0 to 10, with higher scores indicating greater methodological rigor. From each study, we extracted data including source of MSCs, administration routes, immunogenicity, animal species, animal stroke model, time in relation to stroke, transplanted cell doses, number of animals in each study, behavioral outcomes, surrogate outcomes (i.e., infarct volume) and outcomes at molecular level. When a manuscript reported multiple time points, we only extracted the outcomes at 14 days after cell transplantation. If no data were available at day 14, the final assessment in the study was included. When a manuscript reported several treatment groups, each treatment group and control group was extracted separately as if the groups were from different studies.
For clinical studies, we also extracted data including cell type, administration route, cell doses, study design, characteristics of the study population (mean age of subjects, stroke type, and time from stroke), sample size, outcomes, and adverse events. We used PEDro score to measure the methodological quality of clinical trials29.
Results
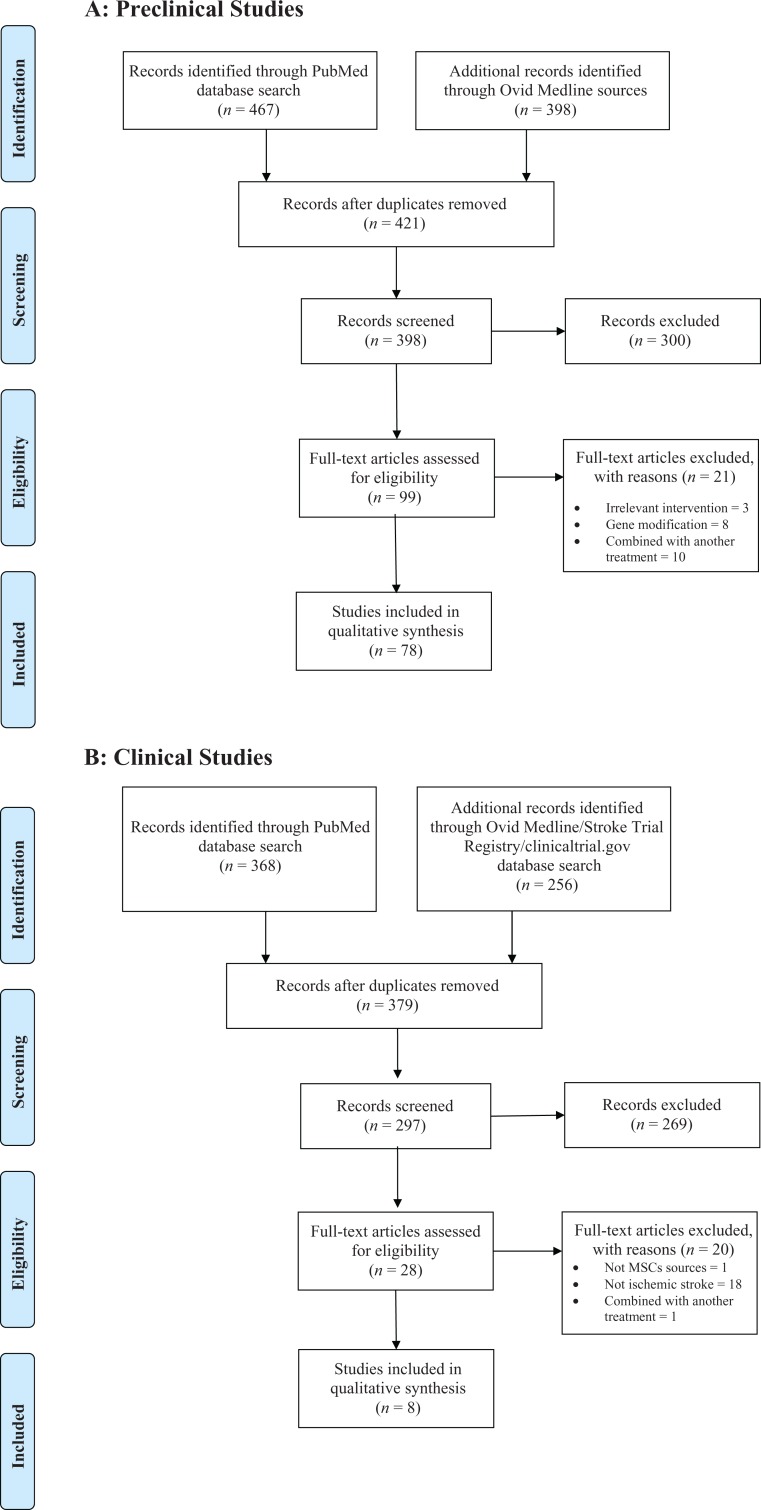
In pre-clinical research, a total of 78 studies were identified (Fig. 1a; Supplemental Table 1a). The median STAIR Score across the 78 studies was 5.5 (range 3–8; Table 1). Among clinical trials, eight studies were identified and included (Fig. 1b; Supplemental Table 1b). Only three studies had a control arm. The PEDro scores for the three human trials were 5, 5, and 8 (Table 2).
Fig. 1.
PRISMA flow diagram.
Table 1.
Quality Check of Pre-Clinical Studies Using STAIR Guideline.
| Quality Score criterion | Number of Studies Meeting Criterion (%) |
|---|---|
| Published in peer-reviewed journal | 100 |
| Statement confirming compliance w/ animal welfare requirements | 99 |
| Avoided neuroprotective anesthetics | 81 |
| Control of temperature | 62 |
| Random treatment assignment | 60 |
| Allocation concealment | 56 |
| Conflict of interest statement | 57 |
| Blinded outcomes | 34 |
| Animals with comorbidities | 5 |
| Sample-size calculation | 1 |
* Mean of STAIR Score: 5.5; range 3–8.
Table 2.
Quality Check of Human Studies Using PEDro Score.
| Criterion | Bhasin 2011 |
Bhasin 2013 |
Lee 2010 |
|---|---|---|---|
| 1. Eligibility criteria were specified | 1 | 1 | 1 |
| 2. Subjects were randomly allocated to groups (in a crossover study, subjects were randomly allocated an order in which treatments were received) | 0 | 0 | 1 |
| 3. Allocation was concealed | 0 | 0 | 0 |
| 4.The groups were similar at baseline regarding the most important prognostic indicators | 1 | 1 | 1 |
| 5. There was blinding of all subjects | 0 | 0 | 0 |
| 6.There was blinding of all therapists who administered the therapy | 0 | 0 | 1 |
| 7. There was blinding of all assessors who measured at least one key outcome | 0 | 0 | 1 |
| 8. Measures of at least one key outcome were obtained from more than 85% of the subjects initially allocated to groups | 1 | 1 | 1 |
| 9. All subjects for whom outcome measures were available received the treatment or control condition as allocated or, where this was not the case, data for at least one key outcome was analyzed by “intention to treat” | 0 | 0 | 1 |
| 10. The results of between-group statistical comparisons are reported for at least one key outcome | 1 | 1 | 1 |
| 11. The study provides both point measures and measures of variability for at least one key outcome | 1 | 1 | 0 |
| TOTAL | 5 | 5 | 8 |
Study Characteristics
In pre-clinical studies, 41 studies used MSCs sourced from human, 33 studies from rat, two studies from mouse, and one study from dog, and there were two studies with MSC source unstated. With respect to route of cell administration, 47 studies used intravenous (IV) injection, 18 studies used intracerebral (IC) injection, 18 studies used intra-arterial (IA) injection, one study used intrathecal injection (IT), and one study used intranasal administration. Regarding the timing of MSCs transplantation in relation to stroke onset time, there were 21 studies that were within 8 hours, 32 studies at 24 hours, 27 studies between 24 hours to 7 days, and four studies after 7 days. The administration doses range from 1 ×104 to 1 ×107 cells. Only one pre-clinical study used autologous cells (Table 3).
Table 3.
Summary of Characteristics of Included Studies.
| Pre-clinical Studies | No. of studies | Clinical Studies | No. of Studies |
|---|---|---|---|
| Total publications | 78 | Total publications | 8 |
| Having control group | 78 | Having control group | 3 |
| Source of MSCs | Source of MSCs | ||
| Human | 41 | Human bone marrow | 6 |
| Rat | 33 | Human umbilical cord | 2 |
| Mouse | 2 | ||
| Dog | 1 | ||
| No stated | 2 | ||
| Animal Species | |||
| Rat | 64 | ||
| Mouse | 9 | ||
| Rabbit | 2 | ||
| Dog | 1 | ||
| Primate | 2 | ||
| Cell doses | 1×104 ∼ 1 ×107 | Cell doses | 2.5×106 ∼ 1.6 ×108 |
| Administration Route | Administration Route | ||
| IV | 47 | IV | 5 |
| IA | 18 | IA | 0 |
| IC | 18 | IC | 3 |
| Intrathecal | 1 | Intrathecal | 0 |
| Nasal | 1 | Nasal | 0 |
| Other | 1 | Other | 0 |
| Time of cell administration* | Time of cell administration | ||
| 0–8 h | 21 | 0–7 d | 1 |
| h | 32 | >7 d–1 m | 1 |
| >24 h–1 wk | 27 | >1 m–3 m | 1 |
| >1 wk–60 d | 4 | >3 m | 5 |
| Cell Immunogenicity# | Cell Immunogenicity | ||
| Autologous | 1 | Autologous | 6 |
| Allogeneic | 35 | Allogeneic | 2 |
| Xenogeneic | 41 | Xenogeneic | 0 |
| unknown | 2 |
* several studies transplanted cells at different time points. # One study has two types of cell immunogenicity.
In pre-clinical studies, 23 studies included cell tracking for the MSC treatment. Five studies used superparamagnetic iron oxide formulation to label the MSCs, six studies used CM-DiI fluorescent dye, and 12 studies used a variety of methods (Supplemental Table 1a).
In term of cell source in the clinical studies, six studies used human bone marrow MSCs and two studies used umbilical cord MSCs. Six studies used autologous cell transplantation and the other two studies chose allogeneic cells. Five studies used IV injections and three employed IC injection. The cell doses ranged from 2.5 ×106 to 1.6 × 108 cells. The time from stroke onset was not uniform across studies (3 months post stroke in five studies, 7 days post stroke in one study, 7 days to 1 month in one study, and 1–3 months in one study) (Table 3).
Study Outcomes
Among the behavioral outcome measures used in the 78 pre-clinical studies, modified Neurological Severity Score (mNSS, 28/78), adhesive removal test (ART, 12/78), and rotarod test (18/78) were the most frequently used tests. Infarct volume (56/78) was frequently used as a surrogate measure. Among these outcome measures, 27 out of 28 studies showed positive mNSS improvement, 10 of 12 studies showed positive effect on ART, and 15 of 18 studies showed improved performance on the rotarod test. In 40 out of 56 studies, MSCs therapy reduced infarct volume compared with the control group (Table 4). There were a variety of outcomes at molecular levels, including chemokines associated with neurological recovery (CXCR4/SDF-1, CXCL-16, etc.) and protein markers (VEGF, BDNF, HGF, etc.) (Supplemental Table 1a).
Table 4.
Comparison of Pre-clinical and Clinical Studies Results.
| Pre-clinical Studies | Clinical Studies | ||||||
|---|---|---|---|---|---|---|---|
| Outcomes | Positive | Neutral | Total | Outcomes | Positive | Neutral | Total |
| mNSS | 27/28 | 1/28 | 28 | mRS | 1/1 | 0/1 | 1 |
| ART | 10/12 | 2/12 | 12 | BI | 0/2 | 2/2 | 2 |
| Rotarod test | 15/18 | 3/18 | 18 | FMMS | 0/2 | 2/2 | 2 |
| Infarct volume | 40/56 | 16/56 | 56 | Infarct volume | 0/0 | 0/0 | 0 |
* mNSS: modified Neurological Severity Score; ART: Adhesive Removal Test; mRS: modified Rankin Scale; BI: Bethel Index; FMMS: Fugl-Meyer Motor Scale.
In clinical studies several outcome measures were also used, including global impairment scale (National Institute of Health Stroke Scale, NIHSS) or motor impairment scale (Fugl-Meyer Motor Scale, FMMS) and global functional scales (Bethel index, BI and modified Rankin Scale, mRS). The majority of these human studies show statistically significant positive results on the different outcomes measures (Table 4). In three out of eight studies no adverse events were observed. Five studies noticed the following adverse events: seizure, recurrent vascular episode, headache, fever, infection, nausea, vomiting, mirror dizziness, depression, muscle spasticity, fatigue, drowsiness, etc. (Supplemental Table 1b).
Discussion
Our systematic review of literature in both pre-clinical and clinical research reveals several issues and gaps between the pre-clinical and clinical studies. Significantly more pre-clinical studies than human studies were conducted in the past decade on use of MSCs for stroke recovery. While the majority of pre-clinical studies are positive, we have to interpret these results cautiously. The quality of pre-clinical studies, to some extent, is sub-optimal based on the quality assessments. For example, only one study out of 78 justified the sample-size calculation in the manuscript30. The average number of animals per arm in the pre-clinical studies is about 12, and the study with the largest sample size is 93 (51 in the treatment group and 42 in the control group)31. This raises concerns that these studies are underpowered and the risk of making type II error could be high. Half of the studies have allocation concealment and only one-third of the studies assessed outcomes in a blinded fashion while selection bias and ascertainment bias cannot be ruled out in these circumstances. Another concern is that only 5% of studies used animals with comorbidities, suggesting the animal stroke models may not mimic human stroke models (which frequently have comorbidities such as hypertension, dyslipidemia, or diabetes). Following STAIR’s recommendations23 would improve the rigor and reproducibility of future studies on this topic. In addition, behavioral outcomes are often restricted to the mNSS scoring system, which is a crude measure of outcomes and may not capture the post-stroke motor recovery induced by MSCs32; however, the behavioral test selection should be minimally affected by repeated testing or by the appearance of compensatory strategies, and the use of more optimal batteries of motor and cognitive tasks including assessment of forearm laterality, fine motor skills on reaching or handling tasks, motor coordination on ladder tasks, assessment of grip strength, spatial learning and memory tasks, and others, may be required to better reflect the effect of intervention on specific aspects of post-stroke recovery. Various outcomes at molecular levels were also used in pre-clinical studies but their value appears to be minimal.
In the clinical arena, well-designed multi-center studies with large sample size on MSCs transplantation in stroke are still lacking. Of the published trials reviewed only three studies were conducted with placebo control and these investigations were still at the proof-of-concept stage. Overall, the sample size of these studies is relatively small. For example, Lee et al. conducted the largest study with 52 subjects (36 in the control arm and 16 in the treatment arm), but the study was conducted in an open-label, unblinded fashion5. Although this study demonstrated long-term 5-year safety and possible beneficial effects of autologous MSCs transplantation, the data need to be further tested in a phase II study in a blinded fashion. None of the evaluated human studies included patient-centered outcomes measuring quality of life, although they did use both impairment scales (i.e., NIHSS or FMMS) and/or functional outcomes (i.e., mRS or BI). The field is in need of a large, adequately powered, well-designed, phase II multi-center clinical trial to systematically assess the safety and preliminary efficacy of MSCs in stroke survivors.
Regarding safety, the majority of animal studies did not systematically assess and report adverse events. Only two dedicated animal studies investigated the safety of IA injection approaches33,34. Janowski et al. reported frequent occurrence of strokes due to microemboli when injecting cells at a higher dose (2×106) but not at a lower dose (1×106) in rats33. Similarly, Yavagal et al. concluded the maximum tolerated dose for the IA approach is 1×105 in rats (relatively lower than Janowski’s study)34. This approach has not yet been tested in humans (only IV and IC approaches have been investigated). The reported adverse events observed in human trials include seizure, headache, fever, infection, nausea, vomiting, depression, muscle spasticity, fatigue, local pain, drowsiness, and so forth3,5,10,35,36. The top three reported adverse events are headache (19/89, 21.3%), fever (7/89, 7.8%), and seizures (2/89, 2.2%). No brain tumors have been reported though one study did report a benign skin lesion (eccrine poroma), but the causation was not established5. It is not clear whether the incidence of adverse events associated with MSCs is higher or lower than other types of stem cell. Such comparison is needed to better understand the safety profiles of stem cell transplantation. The total number of subjects included in the eight human trials is quite small with 89 subjects, and long-term safety as well as rare serious adverse events can only be detected with a larger sample size.
While the majority of pre-clinical studies (73/78) used rats and mice, there were two proof-of-concept studies using non-human primates37,38. For example, in Li et al.’s proof-of-concept study37, eight Macaca fascicularis were randomized to a low-dose group (1×106 cells, n = 3), a high-dose group (5 × 106 cells, n = 3), and a control group (n = 2). Human bone-marrow-derived MSCs (hBMSCs) were transplanted intracranially around the ischemic lesions at 7 days after ischemia, and both groups demonstrated that hBMSCs treatment exerted neuroprotective and anti-apoptotic effects while also inhibiting astroglial reactivity on cerebral ischemia with upregulated expression of IL-10 level in the peri-ischemia region37.
While three meta-analyses were conducted with published pre-clinical stem cell studies, the majority of values for mean and standard error were obtained via quasi-quantitative methods, mostly on highly magnified images using the line length measuring tool in PowerPoint, which leaves room for errors24–26. Because of the reporting issues in the pre-clinical studies, we were only able to calculate effect size from 9/78 (11.5%) manuscripts, which significantly limited our ability to quantitatively summarize and compare results from these studies. Similarly, in the clinical studies, we were not able to conduct a meta-analysis, because of the very small number of published studies available. As a result, we could only conduct qualitative assessment rather than quantitative appraisal of the included manuscripts. Regardless, we made several observations: (1) Although most of the pre-clinical and clinical studies are positive, the effect size is not measurable. In addition, statistical significance does not necessarily translate to minimal clinically important differences (MCID)39; (2) Various outcomes were used in both pre-clinical and clinical studies, which need to be consolidated, if possible, for better cross-study comparison. For example, infarct volume was used in 2/3 of animal studies as a surrogate measure, but its value as a surrogate measure in human stroke population has not been established; (3) The quality of both pre-clinical and clinical studies needs to be significantly improved to have greater scientific rigor in order to have better success in translation. For example, following the Consolidated Standards Of Reporting Trials (CONSORT) statement would be a good practice for researchers to prepare manuscripts for clinical trials, to minimize study bias and to facilitate critical appraisal40.
Our study is not free from limitations. We only included published manuscripts in the English language, and it is possible that we omitted appropriate manuscripts published in other languages, which may diminish the comprehensiveness of this review. The inability to conduct a meta-analysis on behavioral and molecular outcomes (i.e., calculating effect size) because of reporting issues limits our ability to quantitatively compare results across pre-clinical and clinical studies.
Conclusion
By conducting a systematic review of both pre-clinical and clinical research on MSCs transplantation, we have identified several critical issues and gaps in translating MSCs research to human stroke survivors. Addressing these concerns at the pre-clinical level and optimizing pre-clinical studies is critical to increase the odds of success in future human clinical trials.
Supplemental Material
Supplemental Material, Supplemental_Table1b_clinical_References_final for Mesenchymal Stem Cells Therapy in Stroke: A Systematic Review of Literature in Pre-Clinical and Clinical Research by Haiqing Zheng, Bin Zhang, Pratik Y. Chhatbar, Yi Dong, Ali Alawieh, Forrest Lowe, Xiquan Hu, and Wuwei Feng in Cell Transplantation
Supplemental Material
Supplemental Material, Supplemental_Table_1a_References_Revision_trackchanges for Mesenchymal Stem Cells Therapy in Stroke: A Systematic Review of Literature in Pre-Clinical and Clinical Research by Haiqing Zheng, Bin Zhang, Pratik Y. Chhatbar, Yi Dong, Ali Alawieh, Forrest Lowe, Xiquan Hu, and Wuwei Feng in Cell Transplantation
Acknowledgments
Dr. Zheng would like to acknowledge his grant support from National Natural Science Foundation of China (81572228) and Natural Science Foundation of Guangdong Province (2015A030313015); Dr. Hu would like to acknowledge his grant support from National Natural Science Foundation of China (81672261).
Author Contribution: Haiqing Zheng and Bin Zhang contributed equally to this manuscript.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
Reference
- 1. Kim JT, Fonarow GC, Smith EE, Reeves MJ, Navalkele DD, Grotta JC, Grau-Sepulveda MV, Hernandez AF, Peterson ED, Schwamm LH, Saver JL. Treatment with tissue plasminogen activator in the golden hour and the shape of the 4.5-hour time-benefit curve in the national united states get with the guidelines-stroke population. Circulation. 2017;135(2):128–139. [DOI] [PubMed] [Google Scholar]
- 2. Asadi H, Williams D, Thornton J. Changing management of acute ischaemic stroke: the new treatments and emerging role of endovascular therapy. Curr Treat Options Neurol. 2016;18(5):20. [DOI] [PubMed] [Google Scholar]
- 3. Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, Kim AS, Johnson JN, Bates D, King B, Case C, McGrogan M, Yankee EW, Schwartz NE. Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke. 2016;47(7):1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57(6):874–882. [DOI] [PubMed] [Google Scholar]
- 5. Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28(6):1099–1106. [DOI] [PubMed] [Google Scholar]
- 6. Savitz SI, Misra V, Kasam M, Juneja H, Cox CS, Jr, Alderman S, Aisiku I, Kar S, Gee A, Grotta JC. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol. 2011;70(1):59–69. [DOI] [PubMed] [Google Scholar]
- 7. Prasad K, Sharma A, Garg A, Mohanty S, Bhatnagar S, Johri S, Singh KK, Nair V, Sarkar RS, Gorthi SP, Hassan KM, Prabhakar S, Marwaha N, Khandelwal N, Misra UK, Kalita J, Nityanand S. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: a multicentric, randomized trial. Stroke. 2014;45(12):3618–3624. [DOI] [PubMed] [Google Scholar]
- 8. Taguchi A, Sakai C, Soma T, Kasahara Y, Stern DM, Kajimoto K, Ihara M, Daimon T, Yamahara K, Doi K, Kohara N, Nishimura H, Matsuyama T, Naritomi H, Sakai N, Nagatsuka K. Intravenous autologous bone marrow mononuclear cell transplantation for stroke: phase1/2a clinical trial in a homogeneous group of stroke patients. Stem Cells Dev. 2015;24(19):2207–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kalladka D, Sinden J, Pollock K, Haig C, McLean J, Smith W, McConnachie A, Santosh C, Bath PM, Dunn L, Muir KW. Human neural stem cells in patients with chronic ischaemic stroke (pisces): a phase 1, first-in-man study. Lancet. 2016;388(10046):787–796. [DOI] [PubMed] [Google Scholar]
- 10. Qiao LY, Huang FJ, Zhao M, Xie JH, Shi J, Wang J, Lin XZ, Zuo H, Wang YL, Geng TC. A two-year follow-up study of cotransplantation with neural stem/progenitor cells and mesenchymal stromal cells in ischemic stroke patients. Cell Transplant. 2014;23(Suppl 1):S65–S72. [DOI] [PubMed] [Google Scholar]
- 11. Kondziolka D, Wechsler L, Goldstein S, Meltzer C, Thulborn KR, Gebel J, Jannetta P, DeCesare S, Elder EM, McGrogan M, Reitman MA, Bynum L. Transplantation of cultured human neuronal cells for patients with stroke. Neurology. 2000;55(4):565–569. [DOI] [PubMed] [Google Scholar]
- 12. Wang Y, Ji X, Leak RK, Chen F, Cao G. Stem cell therapies in age-related neurodegenerative diseases and stroke. Ageing Res Rev. 2017;34:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doeppner TR, Herz J, Gorgens A, Schlechter J, Ludwig AK, Radtke S, de Miroschedji K, Horn PA, Giebel B, Hermann DM. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med. 2015;4(10):1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xin H, Katakowski M, Wang F, Qian JY, Liu XS, Ali MM, Buller B, Zhang ZG, Chopp M. Microrna cluster mir-17-92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke. 2017;48(3):747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Q, Zhao Y, Xu Y, Chen Z, Liu N, Ke C, Liu B, Wu W. Sodium ferulate and n-butylidenephthalate combined with bone marrow stromal cells (bmscs) improve the therapeutic effects of angiogenesis and neurogenesis after rat focal cerebral ischemia. J Transl Med. 2016;14(1):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wei L, Wei ZZ, Jiang MQ, Mohamad O, Yu SP. Stem cell transplantation therapy for multifaceted therapeutic benefits after stroke. Prog Neurobiol. 2017;157:49–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yan T, Venkat P, Chopp M, Zacharek A, Ning R, Roberts C, Zhang Y, Lu M, Chen J. Neurorestorative responses to delayed human mesenchymal stromal cells treatment of stroke in type 2 diabetic rats. Stroke. 2016;47(11):2850–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abe K, Yamashita T, Takizawa S, Kuroda S, Kinouchi H, Kawahara N. Stem cell therapy for cerebral ischemia: from basic science to clinical applications. J Cereb Blood Flow Metab. 2012;32(7):1317–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vahidy FS, Rahbar MH, Zhu H, Rowan PJ, Bambhroliya AB, Savitz SI. Systematic review and meta-analysis of bone marrow-derived mononuclear cells in animal models of ischemic stroke. Stroke. 2016;47(6):1632–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Savitz SI, Chopp M, Deans R, Carmichael T, Phinney D, Wechsler L. Stem cell therapy as an emerging paradigm for stroke (STEPS) II. Stroke. 2011;42(3):825–829. [DOI] [PubMed] [Google Scholar]
- 21. Savitz SI, Cramer SC, Wechsler L. Stem cells as an emerging paradigm in stroke 3: enhancing the development of clinical trials. Stroke. 2014;45(2):634–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Mello RF, Santos Ide S, Alencar AP, Bensenor IM, Lotufo PA, Goulart AC. Major depression as a predictor of poor long-term survival in a brazilian stroke cohort (study of stroke mortality and morbidity in adults) emma study. J Stroke Cerebrovasc Dis. 2016;25(3):618–625. [DOI] [PubMed] [Google Scholar]
- 23. Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40(6):2244–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vu Q, Xie K, Eckert M, Zhao W, Cramer SC. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology. 2014;82(14):1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Janowski M, Walczak P, Date I. Intravenous route of cell delivery for treatment of neurological disorders: a meta-analysis of preclinical results. Stem Cells Dev. 2010;19(1):5–16. [DOI] [PubMed] [Google Scholar]
- 26. Lees JS, Sena ES, Egan KJ, Antonic A, Koblar SA, Howells DW, Macleod MR. Stem cell-based therapy for experimental stroke: a systematic review and meta-analysis. Int J Stroke. 2012;7(7):582–588. [DOI] [PubMed] [Google Scholar]
- 27. Wu Q, Wang Y, Demaerschalk BM, Ghimire S, Wellik KE, Qu W. Bone marrow stromal cell therapy for ischemic stroke: a meta-analysis of randomized control animal trials. Int J Stroke. 2017;12(3):273–284. [DOI] [PubMed] [Google Scholar]
- 28. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blobaum P. Physiotherapy evidence database (pedro). J Med Libr Assoc. 2006;94(4):477–478. [Google Scholar]
- 30. Vibhuti Khan R, Sharma A, Jain S, Mohanty S, Prasad K. Intra-arterial transplantation of human bone marrow mesenchymal stem cells (hMMMSCs) improves behavioral deficits and alters gene expression in rodent stroke model. J Neurochem. 2017;143(6):722–735. [DOI] [PubMed] [Google Scholar]
- 31. Tang G, Liu Y, Zhang Z, Lu Y, Wang Y, Huang J, Li Y, Chen X, Gu X, Wang Y, Yang GY. Mesenchymal stem cells maintain blood-brain barrier integrity by inhibiting aquaporin-4 upregulation after cerebral ischemia. Stem Cells. 2014;32(12):3150–3162. [DOI] [PubMed] [Google Scholar]
- 32. Boltze J, Lukomska B, Jolkkonen J; MEMS–IRBI consortium. Mesenchymal stromal cells in stroke: improvement of motor recovery or functional compensation? J Cereb Blood Flow Metab. 2014;34(8):1420–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Janowski M, Lyczek A, Engels C, Xu J, Lukomska B, Bulte JW, Walczak P. Cell size and velocity of injection are major determinants of the safety of intracarotid stem cell transplantation. J Cerebral Blood Flow Metab. 2013;33(6):921–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yavagal DR, Lin B, Raval AP, Garza PS, Dong C, Zhao W, Rangel EB, McNiece I, Rundek T, Sacco RL, Perez-Pinzon M, Hare JM. Efficacy and dose-dependent safety of intra-arterial delivery of mesenchymal stem cells in a rodent stroke model. PLoS One. 2014;9(5):e93735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R, Waxman SG, Kocsis JD. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134(Pt 6):1790–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Suarez-Monteagudo C, Hernandez-Ramirez P, Alvarez-Gonzalez L, Garcia-Maeso I, de la Cuetara-Bernal K, Castillo-Diaz L, Bringas-Vega ML, Martinez-Aching G, Morales-Chacon LM, Baez-Martin MM, Sanchez-Catasus C, Carballo-Barreda M, Rodriguez-Rojas R, Gomez-Fernandez L, Alberti-Amador E, Macias-Abraham C, Balea ED, Rosales LC, Del Valle Perez L, Ferrer BB, Gonzalez RM, Bergado JA. Autologous bone marrow stem cell neurotransplantation in stroke patients. An open study. Restor Neurol Neurosci. 2009;27(3):151–161. [DOI] [PubMed] [Google Scholar]
- 37. Li J, Zhu H, Liu Y, Li Q, Lu S, Feng M, Xu Y, Huang L, Ma C, An Y, Zhao RC, Wang R, Qin C. Human mesenchymal stem cell transplantation protects against cerebral ischemic injury and upregulates interleukin-10 expression in macacafascicularis. Brain Res. 2010;1334:65–72. [DOI] [PubMed] [Google Scholar]
- 38. Sasaki M, Honmou O, Radtke C, Kocsis JD. Development of a middle cerebral artery occlusion model in the nonhuman primate and a safety study of i.v. infusion of human mesenchymal stem cells. PLoS One. 2011;6(10):e26577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Copay AG, Subach BR, Glassman SD, Polly DW, Jr, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541–546. [DOI] [PubMed] [Google Scholar]
- 40. Schulz KF, Altman DG, Moher D. Consort 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340(7748):698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Supplemental_Table1b_clinical_References_final for Mesenchymal Stem Cells Therapy in Stroke: A Systematic Review of Literature in Pre-Clinical and Clinical Research by Haiqing Zheng, Bin Zhang, Pratik Y. Chhatbar, Yi Dong, Ali Alawieh, Forrest Lowe, Xiquan Hu, and Wuwei Feng in Cell Transplantation
Supplemental Material, Supplemental_Table_1a_References_Revision_trackchanges for Mesenchymal Stem Cells Therapy in Stroke: A Systematic Review of Literature in Pre-Clinical and Clinical Research by Haiqing Zheng, Bin Zhang, Pratik Y. Chhatbar, Yi Dong, Ali Alawieh, Forrest Lowe, Xiquan Hu, and Wuwei Feng in Cell Transplantation