ABSTRACT
American visceral leishmaniasis (VL) is a vector-borne disease transmitted by some species of phlebotomine sandflies from the genus Lutzomyia. This neglected tropical zoonosis shows increasing urbanization process, since the end of the 1980s. After the emergence of foci of the disease in urban areas, VL has assumed an important role in public health. Although VL is widely prevalent in several parts of the world, diagnosing the illness is still difficult. We present a case of a 12-year-old girl with a history of recurrent fever, anorexia, cachexia, chronic fatigue, weight loss, left palpebral unilateral edema, persistent cough and pancytopenia. A diagnosis of VL was performed using a reference immunochromatographic rapid test. Identification of the infecting protozoan was directly obtained by PCR of bone marrow. The patient responded favorably to treatment using liposomal amphotericin B. This is the first report of human visceral leishmaniasis in the city of Lavras in the South of Minas Gerais State. This first report of VL highlighted the need of maintenance of permanent surveillance and control programs in the city of Lavras, including the active search of sandflies, human and canine cases. The current situation of Lavras should also be taken as an alert to other near cities where favorable eco-epidemiological conditions may exist.
KEYWORDS: Leishmaniasis, Neglected diseases, Vector-borne disease
INTRODUCTION
American visceral leishmaniasis (VL) is a vector-borne disease transmitted by some species of phlebotomine sandflies from the genus Lutzomyia 1 . VL is endemic in at least 98 tropical and subtropical countries on five continents 2 . This neglected tropical disease is the most severe clinical form of leishmaniasis, and it has an estimated incidence of 500,000 new cases and 60,000 deaths per year 3 . Brazil is among the six countries that harbor over 90% of the cases worldwide 4 .
VL is characterized by irregular bouts of fever, weight loss, enlargement of the spleen and liver, and anaemia 5 . The disease is fatal if untreated with a fatality rate in developing countries that can reach 100% within two years 6 . In this way, early diagnosis is considered an essential component of VL control.
After the emergence of foci of the disease in urban areas, visceral leishmaniasis (VL) has assumed an important role in public health 7 . The first autochthonous case of visceral leishmaniasis in Minas Gerais State was detected in Belo Horizonte in 1959. In the last 59 years, the disease has spread to other regions of the State as the number of human and canine cases of VL has substantially increased, suggesting an expansion in the rate of transmission of the disease.
In January 2017, the first autochthonous case of human visceral leishmaniasis was diagnosed in the Southern region of Minas Gerais State, which elevated to 226 the number of municipalities with reported cases of human VL.
Lavras is a city in Southern Minas Gerais State, Brazil located at an altitude of 919 m (21°14′43”S 44°59′59”W). Lavras has a population of roughly 99,229 inhabitants and the total area of the city is 564.7 km2.
CASE REPORT
A 12-year-old healthy female patient was hospitalized in December 2016 with a one-month history of recurrent fever, anorexia, cachexia, chronic fatigue, weight loss, left palpebral unilateral edema, persistent cough and pancytopenia. Physical examination revealed that she was hypochromic, icteric (++/4), with normal bowel sound, and had a mild splenomegaly. Abdominal examination was otherwise normal. Blood laboratory assessments are presented in Table 1. During the period of one month, the patient was hospitalized and her clinical status slowly progressed to a wasting disease.
Table 1. Laboratory data at admission to the inpatient unit, during and after treatment with liposomal amphotericin B.
| Reference values | Beggining of treatment | Day 1 aet | Day 15 aet | |
|---|---|---|---|---|
| Hemoglobin (g/dL) | 12-16 | 9.4 | 9.1 | 8.9 |
| Hematocrit (%) | 36-48 | 31.1 | 28.3 | 32.1 |
| Red blood cells (1012/L) | 4-5.8 | 3.89 | 3.7 | 3.95 |
| Total White blood cells (/mm3) | 4-11 | 2.6 | 3 | 4 |
| Band neutrophils (/mm3) | 0-3 | 6 | 4 | 20 |
| segmented neutrophils (/mm3) | 40-75 | 53 | 28 | 63 |
| Platelet (/mm3) | 150-450 | 174 | 100 | 330 |
| Glucose (mg/dL) | 60-99 | 361 | 79 | 110 |
| AST (U/L) | < 36 | 643 | 1614 | 22 |
| ALT (U/L) | 19-44 | 472 | 804 | 25 |
AST - aspartate transaminase; ALT - alanine transaminase; Aet - after the end of treatment
There was no serological evidence of HIV infection, hepatitis C, hepatitis B, liver disease or alcohol abuse. However, the patient presented a severe metabolic decompensation due to diabetes.
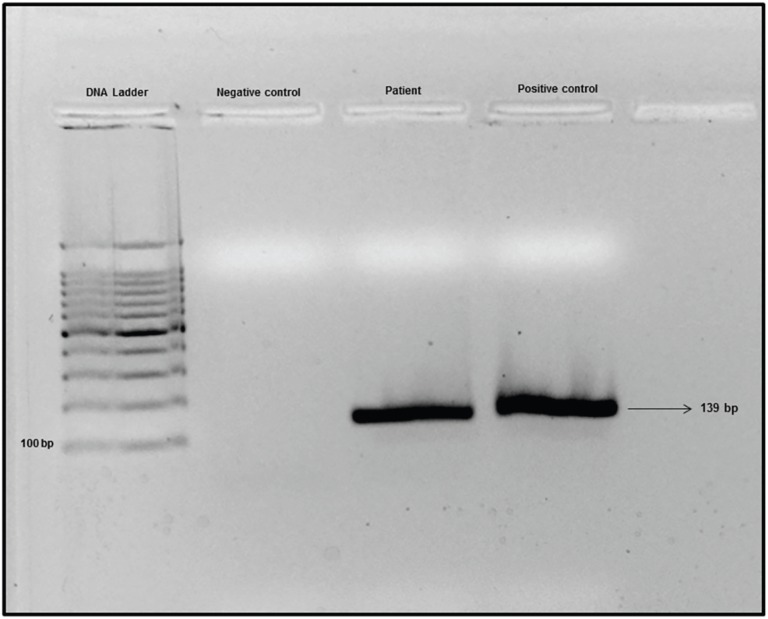
In the present case, 20 days have passed since the appearance of the first symptoms until the definitive diagnosis of VL using a reference immunochromatographic rapid test based on the rK39 antigen (IT-LEISH®, DiaMed Latino-America SA, Switzerland). Examination of the bone marrow aspirate revealed some structures that resembled Leishmania fragments. Identification of the infecting protozoan was directly obtained by PCR of the bone marrow sample using the primers 5-TTTTCTGGTCCCGCGGGTAGG-3 and 5-CCACCTGGCCTATTTTACACCA-3 (Figure 1).
Figure 1. Gel electrophoresis of PCR products. Lane 1, DNA ladder 100 bp. Lane 2, negative control. Lane 3, sample of patient. Lane 4, Leishmania infantum DNA - positive control.
Treatment was initiated in January 6th, 2017, with a daily regimen of 4 mg/kg/day of liposomal amphotericin B (LAMB - AmBisome®, United Medical, Brazil), during five consecutive days, totaling 20 ampoules or 1 g of liposomal amphotericin B. Glycemia was stabilized with the use of 4 UI of subcutaneous insulin (Humulin®, Eli Lilly do Brasil Ltda). The patient experienced an uneventful recovery and was discharged five days after the beginning of the use of LAMB, and is currently being followed as an outpatient, demonstrating clinical improvement. Laboratory exams performed on days 1 and 15 after treatment are presented in Table 1.
DISCUSSION
Human visceral leishmaniasis is usually preceded by canine cases 8 – 10 . In this way, the detection of new geographical areas of canine VL is a critical point for starting or improving the epidemiological surveillance of leishmaniasis 9 .
It is indisputable that if not suspected or left untreated, VL has devastating consequences. Therefore, it is of great clinical importance that health professionals be familiar with the clinical patterns of VL to avoid delayed or missed diagnosis 11 . Since the first confirmed case of canine VL in 2013, and the first report of Lutzomyia longipalpis in the municipality of Lavras in 2015 12 , several health education actions were implemented by the authors, who were aware of the importance of the disease and the early diagnosis and treatment. These actions were applied to health professionals, teachers of elementary and secondary education schools and throughout the community by the distribution of explanatory brochures, flipcharts, and lectures. Health education is considered an important tool in controlling leishmaniasis 13 . Of note, the delay and failure in the early diagnosis leading to health worsening, often evidence the negligence of the disease by health professionals 12 , 14 , 15 . In the present case, 20 days have passed since the appearance of the first symptoms until the definitive diagnosis. As such, health education actions might be important in the rapid diagnosis of the patient and consequently to improve the success of the early beginning of correct treatment.
According to the Brazilian Ministry of Health, the primary treatment of visceral leishmaniasis should be with Glucantime® 11 . However, in the reported case, due to a severe metabolic decompensation related to diabetes, the treatment was conducted using the LAMB, resulting in a rapid resolution of symptoms. Five days after the beginning of the use of LAMB, the patient was discharged. Fifteen days after treatment, all hematological parameters returned to normal. Romero et al. 16 , during the assessment of efficacy and safety of the currently recommended treatments for VL in Brazil, point towards a recommendation for the use of LAMB as the first line treatment for VL in Latin American countries. LAMB presents an acceptable efficacy profile, lower toxicity and shorter administration time when compared to Glucantime. Results on the safety and efficacy of LAMB were also shown by other groups in Europe 17 – 19 .
In this study, the patient presented jaundice (++/4). Although jaundice and ascites are considered rare features in VL 20 , other studies have demonstrated that different degrees of jaundice has been observed as an usual clinical presentation, particularly in children 21 , 22 .
After this first report of human VL, other four cases were diagnosed in the city of Lavras in 2017: a 36-year-old man, a 1-year-old child, a 38-year-old woman and a 3-year-old child. Furthermore, four cases of tegumentary leishmaniasis were also diagnosed in 2017. These reports highlighted the need of maintenance of permanent surveillance and control programs in the city of Lavras, including the active search of sandflies, human and canine leishmaniasis cases. The current situation of Lavras should also be taken as an alert to other near cities where favorable eco-epidemiological conditions may exist.
ACKNOWLEDGMENTS
We wish to thank the Health Surveillance, Epidemiological Surveillance and Environmental Surveillance of the city of Lavras for their support and for providing the patient's data.
REFERENCES
- 1.Bauzer LG, Souza NA, Maingon RD, Peixoto AA. Lutzomyia longipalpis in Brazil: a complex or a single species? A minireview. Mem Instituto Oswaldo Cruz. 2007;102:1–12. doi: 10.1590/s0074-02762007000100001. [DOI] [PubMed] [Google Scholar]
- 2.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belo VS, Werneck GL, Barbosa DS, Simões TC, Nascimento BW, Silva ES, et al. Factors associated with visceral leishmaniasis in the Americas: a systematic review and meta-analysis. Plos Negl Trop Dis. 2013;7:e2182. doi: 10.1371/journal.pntd.0002182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sousa-Gomes ML, Romero GA, Werneck GL. Visceral leishmaniasis and HIV/AIDS in Brazil: are we aware enough? Plos Negl Tropic Dis. 2017;11:e0005772. doi: 10.1371/journal.pntd.0005772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Leishmaniasis. [[cited 2018 July 19]]. Available from: http://who.int/leishmaniasis/en/
- 6.Bora D. Epidemiology of visceral leishmaniasis in India. Natl Med J India. 1999;12:62–68. [PubMed] [Google Scholar]
- 7.Marzochi MC, Coutinho SG, Souza WJ, Toledo LM, Grimaldi G, Júnior, Momen H, et al. Canine visceral leishmaniasis in Rio de Janeiro, Brazil: clinical, parasitological, therapeutical and epidemiological findings. Mem Inst Oswaldo Cruz. 1985;80:349–357. doi: 10.1590/s0074-02761985000300012. [DOI] [PubMed] [Google Scholar]
- 8.Werneck GL. Forum: geographic spread and urbanization of visceral leishmaniasis in Brazil. Introduction. Cad Saude Publica. 2008;24:2937–2940. doi: 10.1590/s0102-311x2008001200023. [DOI] [PubMed] [Google Scholar]
- 9.Quinnell RJ, Courtenay O. Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitology. 2009;136:1915–1934. doi: 10.1017/S0031182009991156. [DOI] [PubMed] [Google Scholar]
- 10.Brasil. Ministério da Saúde Secretaria de Vigilância em Saúde. Casos confirmados de leishmaniose visceral, Brasil, Grandes Regiões e Unidades Federadas: 1990 a 2013. [[cited 2018 July 30]]. Available from: http://portalarquivos2.saude.gov.br/images/pdf/2014/setembro/09/LV-Casos.pdf.
- 11.Silva GA, Boechat TO, Ferry FR, Pinto JF, Azevedo MC, Carvalho RS, et al. First case of autochthonous human visceral leishmaniasis in the urban center of Rio de Janeiro: case report. Rev Inst Med Trop Sao Paulo. 2014;56:81–84. doi: 10.1590/S0036-46652014000100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barçante TA, Botelho MC, Freitas HF, Soares GD, Barçante JM. First report of the main vector of visceral leishmaniasis in America, Lutzomyia longipalpis (Lutz, Neiva, 1912) (Diptera: Psychodidae: Phlebotominae), in southern Minas Gerais State, Brazil. J Vector Ecol. 2015;40:412–414. doi: 10.1111/jvec.12182. [DOI] [PubMed] [Google Scholar]
- 13.Brasil, Ministério da Saúde . Secretaria de Vigilância em Saúde. Departamento de Vigilância das Doenças Transmissíveis. Manual de vigilância da leishmaniose tegumentar. Brasília: Ministério da Saúde; 2017. [[cited 2018 July 30]]. Available from: http://bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_leishmaniose_tegumentar.pdf. [Google Scholar]
- 14.Oyama J, Ferreira FB, Conter CC, Lera-Nonose DS, Ramos-Milaré AC, Venazzi EA, et al. American tegumentary leishmaniasis: diagnostic and treatment challenges in a clinical case. Rev Inst Med Trop Sao Paulo. 2018;60:e3. doi: 10.1590/S1678-9946201860003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva GA, Eyer-Silva WA, Magalhães MC, Ferry FR, Pinto JF, Azevedo MC, et al. A novel case of human visceral leishmaniasis from the urban area of the city of Rio de Janeiro: autochthonous or imported from Spain? Rev Inst Med Trop Sao Paulo. 2017;59:e11. doi: 10.1590/S1678-9946201759011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero GA, Costa DL, Costa CH, Almeida RP, Melo EV, Carvalho SF, et al. Efficacy and safety of available treatments for visceral leishmaniasis in Brazil: a multicenter, randomized, open label trial. Plos Negl Trop Dis. 2017;11:e0005706. doi: 10.1371/journal.pntd.0005706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.di Martino L, Davidson RN, Giacchino R, Scotti S, Raimondi F, Casragnola E, et al. Treatment of visceral leishmaniasis in children with liposomal amphotericin B. J Pediatr. 1997;131:271–277. doi: 10.1016/s0022-3476(97)70165-3. [DOI] [PubMed] [Google Scholar]
- 18.Davidson RN, di Martino L, Gradoni L, Giacchino R, Gaeta GB, Pempinello R, et al. Short-course treatment of visceral leishmaniasis with liposomal amphotericin B (AmBisome) Clin Infect Dis. 1996;22:938–943. doi: 10.1093/clinids/22.6.938. [DOI] [PubMed] [Google Scholar]
- 19.Syriopoulou V, Daikos GL, Theodoridou M, Pavlopoulou I, Manolaki AG, Sereti E, et al. Two doses of a lipid formulation of amphotericin B for the treatment of Mediterranean visceral leishmaniasis. Clin Infect Dis. 2003;36:560–566. doi: 10.1086/367843. [DOI] [PubMed] [Google Scholar]
- 20.Mamoon AB, Chowdhury ZA, Jahan K, Begum N, Chowdhury SA, Masum MA. Seroprevalence of kala-azar and its clinical presentation in a rural community of Bangladesh. Orion. 2005;22:291–293. [Google Scholar]
- 21.Prakash A, Singh NP, Sridhara G, Malhotra V, Makhija A, Garg D, et al. Visceral leishmaniasis masquerading as chronic liver disease. J Assoc Physicians India. 2006;54:893–894. [PubMed] [Google Scholar]
- 22.Rashid AM, Mamun AA, Rasul CH, Asrafuzzaman MD, Hossain M, Rahman MM. Jaundice in pediatric visceral leishmaniasis (kala-azar) patients. J Med. 2007;8:14–16. [Google Scholar]