Abstract
Background:
Reactive oxygen species and reactive nitrogen species, which are collective-ly called reactive oxygen-nitrogen species, are inevitable by-products of cellular metabolic redox reac-tions, such as oxidative phosphorylation in the mitochondrial respiratory chain, phagocytosis, reac-tions of biotransformation of exogenous and endogenous substrate in endoplasmic reticulum, eico-sanoid synthesis, and redox reactions in the presence of metal with variable valence. Among medici-nal plants, there is growing interest in Crocus Sativus L. It is a perennial, stemless herb, belonging to Iridaceae family, cultivated in various countries such as Greece, Italy, Spain, Israel, Morocco, Tur-key, Iran, India, China, Egypt and Mexico.
Objective:
The present study aims to address the anti-toxicant role of Crocus Sativus L. in the case of cardiovascular disease and its role towards the cardioprotective role of Crocus Sativus L.
Materials and
Methods:
An electronic literature search was conducted by the two authors from 1993 to August 2017. Original articles and systematic reviews (with or without meta-analysis), as well as case reports were selected. Titles and abstracts of papers were screened by a third reviewer to deter-mine whether they met the eligibility criteria, and full texts of the selected articles were retrieved.
Results:
Our review has indicated that scientific literature confirms the role of Crocus Sativus L. as a cardiovascular-protective agent. The literature review showed that Saffron is a potent cardiovascular-protective agent with a plethora of applications ranging from ischemia-reperfusion injury, diabetes and hypertension to hyperlipidemia.
Conclusion:
Literature findings represented in current review herald promising results for using Crocus Sativus L. and/or its active constituents as a cardiovascular-protective agent and in particular, Crocus Sativus L. manifests beneficial results against ischemia-reperfusion injury, hypertension, hy-perlipidemia and diabetes
Keywords: Saffron, crocin, crocetin, safranal, oxidative stress, cardiovascular disease, cardiovascular protection
1. Introduction
Reactive Oxygen Species (ROS) and reactive nitrogen species (RNS), which are collectively called Reactive Oxygen-Nitrogen Species (RONS), are inevitable by-products of cellular metabolic redox reactions, generated in the mitochondrial respiratory chain, endoplasmic reticulum, peroxisomes, and other cellular components. ROS are produced through enzymatic processes, which involve NADPH (nicotinamide adenine dinucleotide phosphate) oxidase, NADPH-like enzymes (NOX) xanthine oxidase, uncoupled Endothelial Nitric Oxide Synthase (eNOS), cytochrome P450 enzymes, Lipoxygenase (LOX), and Cyclooxygenase (COX) and non-enzymatic reactions, such as a mitochondrial respiratory chain. Hydroxyl (OH•), superoxide (O2•−), Nitric Oxide (NO•), Nitrogen Dioxide (NO2•), peroxyl (RO2•), alkoxyl (RO-) and lipid peroxyl (LOO•) are the most common ROS and RNS molecules associated with aging and related degenerations. Other oxygen and nitrogenous by-products, such as Hydrogen Peroxide (H2O2), Ozone (O3), Singlet Oxygen (1O2), Hypochlorous Acid (HOCl), Nitrous Acid (HNO2), and Peroxynitrite (ONOO•) are mediators of free radical reactions, as are either oxidizing agents or easily converted into radicals. RONS are very reactive molecules due to the presence of unpaired valence shell electrons or non-static bonds, and their regulation is vital for reducing tissue damage [1-8].
The formation of ROS occurs due to the natural consequence of aerobic metabolism and is integral for maintaining tissue oxygen homeostasis. It has been estimated that 2- 3% of oxygen consumption is diverted to production of ROS. However, under normal conditions, the redox state of a cell is kept within a narrow range. ROS are not simply a pernicious product of a defective system, but at moderate concentrations have an important role in core physiologic processes, such as vasodilation, synaptic plasticity, clotting, effective immune defence and glucose uptake by skeletal muscles. Also, ROS can act as secondary messengers in intracellular pathways, inducing cell senescence and apoptosis, whereas low levels of ROS may favour cell proliferation, differentiation and migration [7, 9].
Oxidative stress arises due to disturbed equilibrium between ROS generation and elimination processes, such as overproduction of ROS via mitochondrial dysfunction and inactivation of respiratory-chain enzymes, e.g., NADH-cytochrome c reductase and succinate -cytochrome c reductase, respiratory burst and augmented activity of various oxidases during environmental stress (smoking, pesticides, UV radiation, heat exposure, ionizing radiation, chemotherapy) or inflammatory stimulus and/or loss of antioxidant defences. Oxidative stress has deleterious cellular effects, implicated in oxidation of macromolecules, such as proteins, lipids, carbohydrates and DNA, thus irreversibly destroys them and alters their function, leading to loss of enzymatic activities, DNA damage, inhibition of protein synthesis, deregulation of ion transport and cell death. Radicals react with all components of the DNA molecule, including purine and pyrimidine bases and the deoxyribose backbone, leading to permanent modification of genetic material.
ROS reacts with the double bonds of the long-chain, Polyunsaturated Fatty Acids (PUFAS) of cell membranes to generate lipid peroxidation products, such as Oxidized Phosphatidylcholine (OxPC). Cutting of oxidized PUFAs forms a,b-unsaturated aldehyde cleavage residues, such as Malondialdehyde (MDA), 4-hydroxynonenal (HNE), 4-oxo-2-nonenal (ONE), and 2-propenal acrolein, whose mechanism produces serious side effects associated with oxidative stress. For example, 4-HNE potentially alters the phospholipid asymmetry of the cell membrane, leading to decreased fluidity and impairment of the membranes’ functionality. Also, lipid peroxidation can inactivate membrane-bound receptors, modulating non-specific permeability to ions and suspend enzyme activity, e.g. mitochondrial enzymes, disrupting mitochondrial energetic and ROS generation. Lipid peroxidation can also potentially produce reactive aldehydes, which can incorporate into proteins to produce carbonyl derivatives. Carbonylated proteins are more hydrophobic and resistant to proteolysis, allowing accumulation of non- functional proteins. Thus, free radical-induced lipid peroxidation serves to proliferate and enhance oxidant-mediated damage.
Protein oxidation is a process which can take place at several protein sites. Such include the amino group, the carboxyl group, or the side chains of the protein that subsequently alter the functional properties of the amino acid sequence. The side chains of the amino acid residues of proteins, in particular, cysteine and methionine residues of proteins are susceptible to oxidation. Oxidation of cysteine residues can lead to a reversible formation of mixed disulphides between protein thiol groups (-SH) and low molecular weight thiols, in particular, GSH (S-glutathiolation). Oxidative alterations of proteins enhance their vulnerability to proteolytic degradation by proteasomes.
Advanced Glycation End products (AGEs) are considered to be relatively unstable, reactive compounds formed by the non-enzymatic reaction of glucose and other glycating substances, derived from both glucose and increased fatty acid oxidation (e.g. dicarbonyls such as 3- deoxyglucosone, methylglyoxal and glyoxal) alongside to the amino groups of proteins, lipids, and nucleic acids. Under certain conditions, such as oxidative stress due to hyperglycemia or hyperlipidaemia AGEs formation can take place at high levels exceeding normal levels. AGEs can change the structure and function of intracellular proteins, whereas some AGEs have an intrinsic catalytic oxidative capacity, through activation of NAD(P)H oxidase. Plasma proteins modified by AGE precursors bind to RAGE receptors (Receptor for Advanced Glycation End Products) on macrophages, adipocytes, vascular endothelial cells, vascular smooth muscle cells, podocytes, and mesangial cells. Activation of RAGE can damage mitochondrial proteins, perpetuating AGEs' oxidative effects and might induce the generation of intracellular ROS. The latter activate the redox-sensitive transcription factor NF-kB, which in turn modulates the expression of a variety of genes, associated with inflammation, Including Interleukin-6 (IL-6), Tumour Necrosis Factor- a (TNF-a), Intracellular Adhesion Molecule-I (ICAM-I), Vascular Adhesion Cell Molecule-I (VCAM-I), Monocyte Chemotactic Protein-I (MCP-I), PAI-I (Plasminogen activator inhibitor-1).
Also, ROS stimulate inflammatory responses, via activation of plasma membrane phospholipase A2 to form arachidonic acid, an important precursor for eicosanoids (e.g., thromboxane A2 and leukotriene B4), implicated in leukocyte activation and chemotaxis [3, 6, 10-18].
Metabolically active and growing cells and high-performance tissues, e.g. neurons and myocardial cells have a high demand for oxygen and generate substantial amounts of ROS, thus are most likely to be severely affected by ROS burden. Aging, cancer, chronic inflammatory and autoimmune diseases (diabetes, rheumatoid arthritis, lupus erythematosus, vasculitis), cardiovascular diseases (atherosclerosis, hypertension, ischemia/reperfusion injury, obesity), age-related macular degeneration, neurological disorders [Parkinson’s disease, Alzheimer’s disease, ALS (Amyotrophic lateral sclerosis), schizophrenia], fibrotic diseases (pulmonary and liver fibrosis, diabetic nephropathy) and infections (septic shock, hepatitis, HIV) clearly illustrate the impact of oxidative stress on human health [1-3, 5, 17].
To maintain physiological redox balance, cells have a battery of redundant endogenous, antioxidant defences regulated at the transcriptional level by Nrf2/ARE [Nuclear factor (erythroid-derived 2)-like 2]. The cellular defence against ROS injury is achieved by enzymatic [catalase, Superoxide Dismutases (SOD), and the enzymes of glutathione thioredoxin system, i.e. Glutathione Peroxidise (GPx), Glutathione Reductase (GR) and Glutathione-S-Transferase (GST)] and non-enzymatic [glutathione (GSH), α-tocopherol, vitamin C, urate, carotenoids, anthocyanins, flavonoids, polyphenols] free radical scavenging systems. Catalases catalyse the conversion of H2O2 into water and oxygen in the presence of iron or manganese cofactors. SOD catalyses the breakdown of O2•− anions into oxygen and H2O2, in the presence of cooper/zinc in the cytosol and manganese in mitochondria. The GSH system provides the cell with multiple detoxification defences, catalysing the conjugation of GSH to a variety of endogenous electrophilic compounds, e.g. H2O2 and other hydroperoxides, using selenium as the cofactor, and xenobiotics. The non-enzymatic group contains a number of antioxidants which neutralize oxidative agents and are acquired from dietary sources. Cells also produce antioxidants that chelate and/or bind to redox metals, such as melatonin (N-acetyl-5- methoxytryptamine), ubiquinol (coenzyme Q), and GSH synthesized from the amino acids L-cysteine, L-glutamic acid, and glycine. Sulfhydryl groups (-SH) are highly reactive constituents of protein molecules and important scavengers of ROS, implicated in cell division, blood coagulation, maintenance of protein systems, and enzymatic activation, including antioxidant enzymes [1, 2, 4-6, 8, 17, 19].
Considering the pivotal role of oxidative stress in cellular damage, the manipulation of ROS levels may represent a promising treatment option to retard the process of aging, prevent or alleviate the symptoms of age-associated degenerative diseases. In this regard, carotenoids and retinoids have attracted wide attention as promising sources of pharmaceuticals with low toxicity for the prevention and treatment of a broad spectrum of diseases, due to their antioxidant, anti-inflammatory, and immunomodulatory effects. The most abundant carotenoids in human serum are β-carotene (vitamin A precursors), α-carotene, β-cryptoxanthin, lycopene, lutein and zeaxanthin. Indeed, vitamin A can either be obtained from preformed vitamin A, such as retinol and retinyl esters or from provitamin A carotenoids (α-carotene, β-carotene, β-cryptoxanthin), which are converted to vitamin A in the body. Nevertheless, their toxicity and storage in adipose tissue remain important limiting factors for their usage at high therapeutic doses. On the other hand, carotenoids contained in saffron are water-soluble substances, thus spreading easily throughout the body and reaching every tissue, whereas the excess is excreted and not stored in tissues, such as adipose tissue or liver. Indeed, saffron is regarded as a valuable plant source for drug development, endowed with pleiotropic health-promoting effects. In this review, we focused on the capacity of saffron extracts and its components, to attenuate oxidative stress, providing a potential means in prevention or treatment of several chronic diseases [2-6, 17, 20].
2. Chemistry of Crocus SATIVUS L
Among medicinal plants, there is growing interest in Crocus sativus L., known for its various pharmacological properties for over 3,600 years ago. It is a perennial, stemless herb, belonging to Iridaceae family, the line of Liliaceae, cultivated in various countries such as Greece, Italy, Spain, Israel, Morocco, Turkey, Iran, India, China, Egypt and Mexico [21, 22].
Saffron, the golden spice, is the flower’s dried stigma and it is known for its medicinal benefits against a host of health disorders, as it possesses anticancer, genoprotective, antioxidant, anti-inflammatory, antidiabetic, antiatherosclerotic, hypolipidaemic, hypotensive, hepatoprotective, antidepressant, anticonvulsant, anxiolytic and hypnotic effects, it improves learning, cognitive performance and memory impairment, even in Alzheimer's disease [22-46].
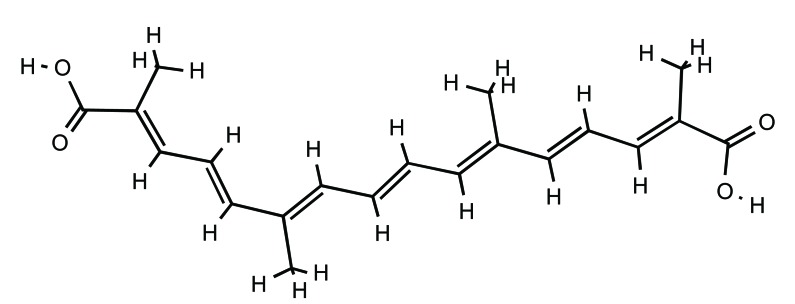
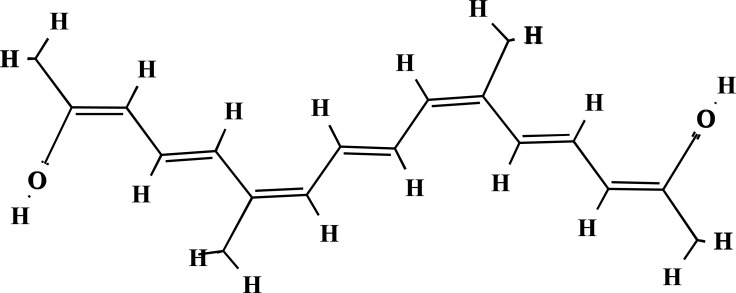
The plant is characterized by its three long red stigmas, joined by the style, three yellow stamens and six purple tepals. Saffron, has a distinct colour, flavour and smell. It contains more than 150 volatile and non-volatile compounds. The volatiles consists of more than 34 components which are mainly terpenes, terpene alcohols and their esters. Non-volatiles include crocins (Fig. 1), together with carotenes, crocetin (Fig. 2), picrocrocin (Fig. 3) and safranal (Fig. 4) [21, 47].
Saffron has three principal active substances (i) crocins (8’-diapocarotene-8,8’-dioic acid), which are mainly responsible for the red pigmentation of stigmas, (ii) picrocrocin (C16H26O7), mainly responsible for its distinctly bitter flavour, and (iii) safranal (2,6,6-trimethylcyclohexane-1,3-dien-1-carboxaldehyde) (C10H14O), the main component of the essential oil that gives the characteristic odour to saffron.
Picrocrocin is a monoterpene glycoside of the aglycone 4-hydroxy-2,6,6-trimethyl-1-carboxaldehyde-1-cyclohexene (HTCC). It is the degradation product of the zeaxanthin carotenoid and a precursor of safranal. Picrocrocin is the second most frequent component, accounting for approximately 1-13% of saffron’s dry mass. During the dehydration (thus drying) process of saffron, elevated temperatures and/or the action of glycosidases convert picrocrocin to safranal.
Safranal is an aromatic monoterpen aldehyde, which is formed by natural deglycosylation of picrocrocin. It is the most abundant (30-70%) of total volatiles and 0.001–0.006% of dry matter of saffron.
The crocin family is hydrophilic carotenoids that are either mono- or di-glycosyl esters of a polyene dicarboxylic acid, crocetin (2,6,11,15-tetramethylhexadeca-2,4,6,8,10,12, 14-heptaenedioic acid; C20H24O4), in which D-glucose and/or D-gentiobiose occur as carbohydrate residues. Ιn saffron all crocin derivatives, except crocin-1, occur as pairs of cis-trans isomers, among which trans-crocins 3 and 4 are the most abundant. Crocin derivatives in saffron are trans-crocetin (β-D-gentiobiosyl) ester, trans-crocetin di-(β-D-glucosyl) ester, trans-crocetin (β-D-glucosyl)-(β-D-gentiobiosyl)bester, cis-crocetin (β-D-glucosyl)-(β-D-gentiobiosyl) ester, trans-crocetin di-(β-D-gentiobiosyl) ester and cis-crocetin di-(β-D-gentiobiosyl) ester. The presence of sugar moieties attached to the terminal carboxyl (-COOH) groups of the crocetin skeleton plays a role in the penetration of cell membranes.Crocetin (8,8-diapo-8,8’-carotenoic acid) is an amphiphilic natural carotenoid that presents high solubility in organic solvents [24, 47-51].
Other constituents include carotenoids (lycopene, alpha-, beta-, gamma-carotene, zeaxanthin, phytoene, phytofluene, mangicrocin and xanthone-carotenoid glycosidic conjugate), phenolic and flavonoid compounds. Good quality saffron contains roughly 30% crocin, 5-15% picrocrocin, and usually up to 2.5% volatile compounds, including safranal [19, 20, 22, 24, 47-56].
3. Toxicity of Crocus SATIVUS L and its major constituents
Saffron has been considered a safe substance by FDA since 2012 and according to WHO, daily doses of up to 1.5 g are safe. Besides, it has been consumed for thousands of years as spice and food colorant, and for its medicinal qualities The LD50 of a 95% ethanolic extract of the stigmas was >600 mg/kg in mice, whereas no haematological or biochemical toxic effects were recorded after intragastric administration of up to 50 mg/kg in mice. On the contrary, intraperitoneal injection of ethanolic extract in Wistar rats at doses 0.35, 0.70 and 1.05 g/kg for 2 weeks affected haematological and biochemical parameters, e.g., dose-independent reduction in Haemoglobin (Hb), Haematocrit Levels (HCT) and total RBC count and dose-dependent increase in total WBC count, ALT, serum urea nitrogen (BUN), uric acid and Creatinine (Cr). Histological examination of liver and kidneys tissue specimens showed mild to severe hepatic and renal tissue injuries, thus supporting the previous findings. Similar results were observed in another study in which BALB/c mice were administered high doses of saffron orally (4000 and 5000 mg/kg). However, in another report, intraperitoneal injection of Saffron Petal Extract (SPE) for 14 days in mice did not affect blood parameters, immune system, and spleen histology, even in doses up to 450 mg/kg, with the exception of an increase in IgG levels [23, 57-62].
Oral or intraperitoneal administration of crocin in mice at doses up to 3 g/kg did not induce any mortality after 24 and 48 hours. Also, intraperitoneal administration of crocin (50, 100 and 200 mg/kg) once a week for four weeks in mice did not affect any biochemical parameters, MDA and GSH liver content. Also, intraperitoneal injection of crocin (180 mg/kg/day) for 21 days resulted in an increase in platelets and Cr, reduction in weight, food intake and alveolar size and atrophy of minor myosin light chain. The safety of crocin in doses up to 180 mg/kg was ascertained in mice, as exposure did not result in any considerable pathological lesions in heart, liver, spleen kidney and [61, 63].
Intraperitoneal exposure to safranal of male mice, female mice and male Wistar rats showed that LD50 values were 1.48, 1.88 and 1.50 ml/kg, respectively, whereas after oral administration the previous values were calculated 21.42, 11.42 and 5.53 ml/kg, respectively. The difference in LD50 values could be attributed to first pass metabolism and detoxification, lower absorption and differences in distribution, following oral administration. Evaluation of haematological parameters revealed a significant decrement in Hb, HCT, RBC counts, and platelets and increase in Lactic Acid Dehydrogenase (LDH) and BUN. Histological studies indicated that safranal did not have any toxic effect on the heart, liver and spleen. However, pathological changes were seen in the kidney and lung [61, 62, 64, 65].
Crocus sativus stigma tablets (200 and 400 mg) were evaluated for short-term safety and tolerability in healthy adult volunteers. Saffron diminished slightly some haematological parameters, e.g., Hb, HCT, RBC and platelets and increased sodium, BUN and Cr, but these alterations were clinically insignificant. In another study, administration of saffron tablets (200 or 400 mg/ day, for 7 days) did not impair coagulation or anticoagulation system [28, 61, 66, 67]. In patients with schizophrenia capsules of Saffron Aqueous Extract (SAE) and crocin were well tolerated in doses of 15 mg twice daily for 12 weeks [68]. Administrations of crocin tablets (20 mg) in healthy volunteers for one month decreased Partial Thromboplastin Time (PTT), amylase and WBC, but was not complicated with damage to any major organ during the trial.
Saffron at doses of 5 g/day or more can cause serious adverse reactions, whereas overdose (12-20 g/day) may be fatal. Probable adverse reactions of saffron include loss of appetite, insomnia, nausea, vomiting, marked thrombocytopenia, and abnormalities of blood clotting (localized skin haemorrhages, bleeding from the nose, lips and eyelids, uterine bleeding, bloody diarrhoea, haematuria), vertigo, numbness and yellowing of the skin sclera, or mucosas, due to accumulation of its coloured constituents. It is contraindicated during pregnancy as it may induce uterine contractions and/or bleeding in doses > 10 g/day [61].
With regards to the previous data saffron and its major components exhibit a relatively safe and normal profile, even in healthy volunteers, as according to the toxicity classification, substances with a LD50 value within the range of 1–5 g/kg are considered practically low-toxic and substances with LD50 >5 g/kg may be considered practically non-toxic [69].
4. Antioxidant capacity of saffron, and its main constituents
Saffron (Fig. 5) methanol extract solutions at a concentration above 2000 ppm displayed high antioxidant activity of about 40-50%, as assessed by DPPH (1,1-diphenyl-2- picryl-hydrazyl; C18H12N5O6) free radical scavenging activity. Saffron’s antioxidant potential could be attributed to its phenolic and flavonoid content, with gallic acid (Fig. 6) and pyrogallol (Fig. 7) being identified as the major phenolic and flavonoid compounds, respectively. Phenolics and flavonoids are highly effective scavengers of most types of oxidizing molecules [20].
DPPH free radical scavenging activity and FRAP (ferric reducing power activity) assays revealed that free radical scavenging activity of methanolic saffron stigma extract was stronger than that of the boiling water extract, followed by the ethanol stigma extract, due to differences in total phenolics and flavonoids. Antioxidant activity increased in a dose-dependent manner. However, it was lower than the antioxidant properties of antioxidant standards, such as BHT and α- tocopherol.
At a concentration of 300 μg/mL the scavenging activity of saffron extracts and standards on free radicals were found to be in an ascending order from α- tocopherol to BHT (butylated hydroxytoluene), to methanol, to boiling water and finally to ethanol. The IC50 of α- tocopherol, BHT, and methanol boiling water and ethanol extract was calculated to be 89.77, 60.39, 210.79, 255.44, and 299.44 μg/mL, respectively [70]. Moreover, the comparison between ethanolic and water extracts of saffron revealed that the ethanolic extract had the highest gallic acid content (67.62 mg /g), thus DPPH and FRAP assays verified that the ethanolic extract exerted the strongest antioxidant capacity [71].
The main bioactive constituents of saffron crocins and safranal also reduce the overload of oxidative stress. In fact, crocins showed profound concentration-dependent antioxidant capacity, as at concentrations of 500 and 1000 ppm DPPH radical scavenging activity was calculated at 48% and 64%, respectively, whereas safranal’s at concentrations of 500 ppm was found 34% [52].
In another research crocetin (CRT) and to a lesser extent Dimethylocrocetin (DMCRT) also exhibited significant antioxidant properties, comparable to those of well-known antioxidants BHT and Trolox, a water-soluble form of vitamin E. The IC50 values of CRT, DMCRT, BHT and Trolox were 17.8, 40, 5.2 and 5.3 μg/mL. The results were in accordance with another study, in which IC50 values of CRT, DMCRT and safranal were calculated 18 ± 1 μg/mL, 40 μg /mL and 95 ± 1 μg /mL, respectively [72]. The antioxidant activity in CRT is dose-dependent, whereas, in DMCRT, it reaches a certain point and then it starts to diminish. The superior free radical scavenging activity of CRT in comparison to DMCRT could be attributed to structural differences between the two carotenoids. Although they have the same length of conjugated double bonds, they differ in the presence of a hydroxyl moiety of the carboxylic group in CRT which reacts more easily with free radicals. On the contrary, DMCRT has a methyl ester group at the end of the unsaturated hydrocarbon chain, rendering DMCRT a less effective antioxidant [72, 73]. Also, the presence of sugar moieties, attached to the terminal -COOH groups of the CRT backbone allows extensive distribution and penetration of CRT through lipid bilayers of cell membranes [74].
5. Antioxidant activity of saffron, and its main constituents, in terms of protection against cardiovascular diseases
5.1. Protection Against Ischemia-reperfusion Injury
Ischemia-reperfusion Injury (IRI) or reoxygenation injury, is the tissue damage caused when tissue blood flow is restored (re+perfusion), after a period of ischemia or lack of oxygen (anoxia or hypoxia). The mechanisms contributing to the pathogenesis of IRI are multifactorial and highly integrated, including cytosolic calcium accumulation, massive production of RNOS, endoplasmic reticulum stress, mitochondrial dysfunction, depletion of Adenosine 5′-triphosphate (ATP), induction of apoptotic and autophagic pathways, neutrophil infiltration, complement activation, inflammatory response and cellular hypoxanthine overload during ischemia, and its conversion by xanthine oxidase, during reperfusion [12, 75, 76].
5.1.1. Protection Against Renal Ischemia-reperfusion Injury
Renal IRI is complicated with vascular endothelium injury, interstitial inflammation, and tubular damages. During renal ischemia, endothelium of renal blood vessels up-regulates the expression of adhesive molecules, e.g. intercellular adhesion molecule-1 (ICAM-1), P- selectin, and E- selectin, leading to the adhesion of leukocytes, platelets, and red blood cells. The significant increase in the cellular redox status during renal IR was assessed by Thiobarbituric Acid Reactive Species (TBARS) levels, which measure levels of MDA the end product of lipid peroxidation. The decrease in total antioxidant capacity was assessed by FRAP and total thiol concentration in kidney homogenate tissue samples. Sulfhydryl groups are depleted following ischemic insult. The previous compromised renal function, thus elevating plasma Cr and BUN.
Ischemic mice pretreated with crocin (50, 100, 200 and 400 mg/kg, intraperitoneally) demonstrated a dose-dependent inhibition in the expression of TNF-α and ICAM-1, a considerable reduction in lymphocyte infiltration and kidney TBARS levels (i.e. from 85.8 ± 5.4 to 20.9 ± 1.5 nmol/g tissue, at the dose of 400 mg/kg) and elevation in antioxidant power (FRAP value increased from 2.98 ± 0.11 to 4.15 ± 0.16 micromol/g tissue, at the dose of 400 mg/kg and total thiol pool increased from 0.38 ± 0.03 to 0.62 ± 0.03 mM, at the dose of 200 mg/kg). However, in another study, crocin was not able to restore FRAP levels [77]. Similar results were observed for the group that were administrated macerated aqueous extract of saffron (5, 20 and 80 mg/kg, intraperitoneally) prior to induction of ischemia; as lipid peroxidation products diminished (i.e. from 85.8 ± 5.4 to 15.9 ± 2.6 nmol/g tissue at the dose of 80 mg/kg) and antioxidant power increased (i.e. from 2.98 ± 0.11 to 5.97 ± 0.56 micromol/g tissue at the dose of 80 mg/kg). However, the saffron extract failed to adequately replenish total thiol groups following IRI [37]. In another study, pre-treatment with saffron extract (5, 10, and 20 mg/kg, intraperitoneally) failed to restore FRAP values in renal tissue, after IRI and BUN concentrations. On the other hand, it decreased plasma Cr, MDA content, TNF-α and ICAM-1 expression and leukocyte infiltration in a dose-dependent manner [78, 79].
5.1.2. Protection Against Myocardium Ischemia-reperfusion Injury
Isolated rabbit hearts, when submitted to IR, demonstrated a decline in Left Ventricular Pressure (LVP), heart rate and coronary flow and an increase in Left Ventricular End Diastolic Pressure (LVEDP). Saffron treatment prior to ischemia slightly ameliorated the recovery, whereas when given at reperfusion it significantly improved hemodynamic parameters and cardiac performance.
The redox status of heart cells can affect its excitability by modulating ion channels. Increased production of ROS during reperfusion directly activates SarcKATP (sarcolemmal-ATP-dependent potassium) channels and changes the inactivation kinetics of L-type calcium channels. SarcKATP channels activation reduces the action potential duration and effective refractory period, which in turn is implicated with cardiac arrhythmias. Electrophysiological recordings revealed a pronounced anti-arrhythmic effect in both premature ventricle contraction and ventricle tachycardia/fibrillation in saffron treated animals, in parallel to preservation of the contractile proteins α- actin, troponin C and T in the myocardium.
During reperfusion, SOD activity decreased and lipid peroxidation increased; the previous effects were attenuated when animals were treated with saffron during the first minutes of reperfusion. The outcome was not significant when saffron was administered prior to ischemia. Treatment with saffron extract before the ischemic period could have a protective effect as a membrane-stabilizing agent, whereas, during reperfusion, saffron maintained or bolstered the endogenous antioxidant defence system at near normal levels, decreasing ROS accumulation and Ca2+ influx.
IR decreased the phosphorylation of multiple components of the PI3K (Phosphatidylinositol-4,5-bisphosphate 3-kinase)/AKT/mTOR (mechanistic target of rapamycin / mammalian target of rapamycin)/4EBP1 (Eukaryotic translation initiation factor 4E-binding protein 1) survival pathway, including mTOR (Ser2448) and 4EBP1 (Ser 65/Thr 70), and activated p38 MAPK (mitogen-activated protein kinases), inhibiting cell survival and inducing apoptosis of cardiomyocytes. On the other hand, saffron promoted cell survival and growth, via maintaining the phosphorylation of mTOR pathway components and inhibiting p38 MAPK. Crocin elicited the same pathways in order to rescue cardiomyocytes from autophagy [80-83].
The previous findings corroborate the results of another study on the cardioprotective properties of crocin on reperfusion-induced arrhythmias in an IR mice model. IRI significantly decreased SOD activity and GSH content and augmented MDA levels of the heart muscle, whereas mice exposed to crocin exhibited a remarkable increase in catalase activity of heart tissue. However, crocin did not affect SOD, GSH and MDA levels. From a clinical perspective, crocin significantly reduced VEBs (premature ventricular beat), VT (ventricular tachycardia) episodes and VF (ventricular fibrillation) incidence [84].
CRT pre-treatment alleviated myocardial IRI, as inferred by decreased infarct size percentage, inhibition of ST -segment elevation, which is associated with myocardial blood flow and oxygen tension and decreased serum LDH and CK-MB (creatinine kinase), which are indexes of myocardial injury, as the enzymes are implicated in energy metabolism of cardiomyocytes. It also modulated oxidative stress indexes, e.g. increased cardiac Cu and Zn- SOD1 and decreased MDA content and MDA5 (Melanoma Differentiation-Associated protein 5) expression. MDA5 protein promotes the production of MDA, whereas SOD1 alterations are implicated in the development of heart failure. Moreover, CRT altered inflammation markers, e.g. up-regulated anti-inflammatory IL-10 activity and decreased pro-inflammatory cytokines TNF-α, IL-1β and IL-6 and anti-apoptotic markers, e.g. suppressed Bax expression and elevated Bcl-2 (B-cell lymphoma 2), NO content and eNOS activity. Bcl-2 is expressed on the outer membrane of the mitochondria, obstructing mPTP (mitochondrial permeability transition pore) opening and Ca2+ overload, hence preventing apoptosis. MPTP bridges the inner and outer mitochondrial membrane and allows the cytoplasmic release of soluble proteins, such as cytochrome c, Apoptosis-inducing Factor (AIF), and Smac (second mitochondria-derived activator of caspases) / Diablo homolog. On the contrary, Bax is a cytoplasmic protein which can induce the opening of mPTP or directly impair the outer membrane of the mitochondria, eventually activating caspase cascade and promoting apoptosis. Similarly, NO can lead to the inhibition of mPTP opening and eNOS can mediate its expression. Histopathological examination revealed that IRI-damaged myocardium suffered from cardiomyocyte membrane damage with marked oedema, disorganization of mitochondria with swollen and disrupted cristae, chromatin condensation, cytoplasmic vacuoles, transverse striation loss, extensive myonecrosis, and inflammatory cell infiltration. Pre-treatment with CRT resulted in significant structural improvement [85-87].
The protective effect of CRT on isoproterenol-induced acute myocardial ischemia was demonstrated to be exerted via inhibition of Rho/ROCK (Rho-associated protein kinase) /NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) pathway. The pathway is involved in pro-inflammatory molecule generation, cell adhesion, cell cycle control, contractility of vascular smooth muscle cells, gene transcription and endothelial NO production. CRT pre-treatment down-regulated the protein expressions of Rho GTPase, ROCK1, ROCK2 and phosphorylations of IκB (nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor) kinase (IKK) and NF-κBp65 (transcription factor p65/ nuclear factor NF-kappa-B p65 subunit). Rho family of GTPases is a family of small signalling G proteins, whose activity is mainly regulated by guanine nucleotide exchange factor (GEFs), GTPase-activating Proteins (GAPs) and guanine nucleotide dissociation inhibitors (GDIs). GEFs activate Rho proteins by catalysing the dissociation of GDP (guanosine diphosphate) for GTP (guanosine -5'-triphosphate). GAPs control the ability of the GTPase to hydrolyse GTP to GDP, controlling the rate of movement from the active to the inactive conformation. GDIs form a large complex with Rho protein, helping to prevent diffusion within the membrane and into the cytosol, allowing tight spatial control of Rho activation. Xanthine oxidase catalyses the synthesis of guanosine monophosphate (GMP), which is finally converted to GTP. Hence, inhibition of xanthine oxidase activity by CRT is attributed to the containing of Rho -GEF domain.
Also, the enzyme is a form of xanthine oxidoreductase, which catalyses the oxidation of hypoxanthine to xanthine and can further catalyse the oxidation of xanthine to uric acid, generating H2O2. The enzyme under some circumstances can generate O2−, the strong one-electron oxidant carbonate radical anion and peroxynitrite. The formation of ROS could be attributed to the cellular hypoxanthine overload during ischemia, and its conversion by xanthine oxidase, during reperfusion. Hence, inhibition of xanthine oxidase activity by CRT could lead to amelioration of oxidative stress during myocardial IR. ROCKs (ROCK1 and ROCK2) serine/ threonine kinases are the target effector molecules of Rho and are widely expressed in cardiovascular smooth muscle. During isoproterenol – induced acute myocardial ischemia IκB is phosphorylated at serine residues by IKK, leading to degradation by proteasomes, thus enhancing NF-κB-driven transcriptional activity. Phosphorylation of NF-κBp65 is also a crucial post-translational modification required for NF-κB activation, observed after isoproterenol – induced acute myocardial ischemia. Hence, inhibition of phosphorylation of IκΒ and NF-κBp65 by CRT could exert protective effects on hypoxic cardiomyocytes by ameliorating myocardial inflammation and oxidative stress pathways [87].
Safranal also countered myocardial IRI, as it significantly decreased infarct size, and improved left ventricular functions and the overall hemodynamic status of the myocardium. Histopathological and ultrastructural findings corroborated the previous positive functional outcomes, as demonstrated by the preservation of myocardial architecture and the decrease in inflammatory cells and oedema. Safranal exerted anti-inflammatory properties, as exhibited by a significant decline in TNF-a levels and anti-apoptotic features, as demonstrated by up-regulation of Bcl-2 expression and down-regulation of Bax and caspase-3 expression. Also, safranal restored cardiac injury markers (LDH and CK-MB) and the antioxidant capacity of myocardium, as assumed by an up-regulation of antioxidant enzymes (GPx and SOD) and down-regulation of pro-oxidant proteins (Nox4) and lipid peroxidation in the myocardium.
IR myocardium injury is partially dependent on NO deficiency, as NO in the presence of ROS combines with NO/tyrosine residues to produce nitrotyrosine. NO regulates myocardial contractility and vasodilation. Hence, IR myocardium insult could be attenuated via activation of eNOS and augmentation of NO levels. Also, IR-induced activation of TNF-a/IKK-β (inhibitor of nuclear factor kappa-B kinase subunit beta)/NF-kB amplifies oxidative stress, lipid peroxidation and disruption of sarcolemmal integrity via increasing Nox4 and decreasing SOD.
The cytoprotective effects of safranal were mediated via activation of Akt/GSK-3b (glycogen synthase kinase-3b) /eNOS and suppression of TNF-a/IKK-β/NF-kb /caspase-3 pathways in IR-challenged myocardium. Enhanced phosphorylation of Akt/GSK-3b detains central inflammatory transcriptional pathways and apoptosis, favours cardiomyocyte proliferation and protects against cardiac remodelling via actuating sarcoplasmic reticulum Ca2+ -ATPase2a (SERCA2a), leading to diminished cytosolic Ca2+ overload and improved contractile function. Also, Akt phosphorylates and activates eNOS [86].
5.1.3. Protection Against Cerebral Ischemia-reperfusion Injury
Stroke is an important cause of mortality and long-lasting disability in adults. Neurons are profoundly susceptible to glucose and oxygen deprivation and the extent of the damage depends on the nature and duration of the ischemic insult, the location of the cells relative to the infarcted area and the neuronal subtype affected.
Several interlinked mechanisms of neuronal injury in stroke have been suggested, including:
Overstimulation of glutamate receptors, after abnormal release of excitatory neurotransmitters, and reduced uptake and metabolism of glutamate by glial cells. Glutamate is the most prominent excitatory neurotransmitter in the mammalian Central Nervous System (CNS), acting through both ionotropic receptors (iGluRs), e.g. N-methyl-D- aspartate (NMDA), kainite and 2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl) proprionate (AMPA) receptors and metabotropic receptors (mGluRs), e.g. mGluRs 1-8.
Disruption of Ca2+ homeostasis. Excessive Ca2+ influx through glutamate receptors and release from intracellular pools during ischemia exceed the capacity of calcium regulatory mechanisms, hence leading to activation of calcium-dependent enzymes, which damage directly cell structures. These enzymes include nitric oxide synthase, cyclooxygenase, phospholipase A2, and calpain 1.
Oxidative stress, which triggers neuronal cell death and NF-κB-mediated expression of pro-inflammatory genes. After neuronal ischemia, within minutes of reperfusion, there is a burst of ROS generation which resolves within minutes to hours, following the time course of mitochondrial Ca2+ overload, a known cause of increased mitochondrial •O−2 production through the electron transport chain.
Mitochondrial dysfunction and structural alterations and mitochondrial-initiated cell death pathway. The opening of MPTP results in mitochondrial swelling, inner membrane depolarization, uncoupling of oxidative phosphorylation, increased •O−2 production, the outflow of matrix Ca2+ and release of intermembrane space proteins, e.g. cytochrome c and AIF that activate apoptotic effector caspases.
Generation of products of Free Fatty Acid (FFA) metabolism like arachidonic acid, thromboxane and leukotrienes, which favours cerebral oedema, vasoconstriction, and platelet aggregation.
Initiation of inflammatory cascades, e.g. pro-inflammatory cytokines [(IL-1b, IL-6, IL-8, TNF-α, and TGF-β (Transforming growth factor beta)], adhesion molecules (selectins, integrins, and immunoglobulin superfamily molecules), eicasanoids and inducible neuronal NOS (nNOS), via an interplay between endothelial cells, astrocytes, microglial cells and leukocytes (granulocytes, monocyte/macrophages and lymphocytes) [88, 89].
Acute cerebral ischemia in male Wistar rats severely compromised neurobehavioral activity, as assessed by grip strength, spontaneous motor activity, and motor coordination. Saffron (100 mg/mL/kg peros) treatment prior to induction of ischemia enhanced motor performance significantly. Saffron restored the antioxidant capacity of neuronal cells, such as GSH, GPx, GR, GST, SOD and catalase activities and countered MDA generation. Saffron also up-regulated the activity of Na + -K + ATPase, the inhibition of which is a fundamental event in neuronal IRI and decreased glutamate and aspartate content [90].
The neuroprotective effects of crocin were similar to saffron extracts. Administration of crocin intraperitoneally to mice at the onset of ischemia and at doses 30, 60, and 120 mg/kg significantly decreased infarct volume by 64%, 74%, and 73%, respectively, hence improving the neurologic outcome. Crocin at doses of 60 and 120 mg/kg were equally effective, indicating a plateau in the response. Crocin had a favourable effect on brain oedema. When administered at a dose of 60 mg/kg 1 hour before, at the start, or 1 hour after ischemia, it reduced brain oedema by 48%, 52%, and 51%, respectively. However, when given 3 or 6 hours after ischemia, it had no significant impact on brain oedema. The researchers postulated that the previous effects were attributed to the antioxidant capacity of crocin, as reflected by restoration of the activity of antioxidant enzymes, such as SOD and GPx in the brain cortex, and the parallel reduction in MDA cellular pool. The anti-oedematous effects of crocin were partly due to the inhibition of TNF-a synthesis, which damages cerebral endothelial cells, disrupting blood-brain barrier and increasing its permeability and the reduced production of pro-inflammatory cytokines, such as IL-1b and IL-6.
In a similar research, crocin administration diminished oxidative stress, NO content and peroxynitrite formation on cortical microvascular homogenates, as opposed to the transient global cerebral IR during which all markers of oxidative stress were increased. Elevated NO, mainly produced by eNOs, and peroxynitrite are cytotoxic and could be complicated with disruption of microvascular integrity, substantial microvilli loss and breakdown of the blood-brain barrier, hence leading to cerebral haemorrhage and oedema. During reperfusion NO can diffuse and react with •O−2 generated in end-feet and endothelia to form peroxynitrite around microvessels. In this study, crocin’s antioxidant and neuroprotective properties were attributed to modulation in GRK2 (G protein-coupled receptor kinase 2) subcellular distribution, ERK (extracellular signal-regulated kinase) phosphorylation and MMP-9 (matrix metalloproteinase-9) expression in cortical microvessels. More specifically, crocin inhibited GRK2 translocation from the cytosol to the membrane, thus down-regulating membrane GRK2 expression and increasing cytosol GRK2 expression. GRK2 and GRK3 mediate signals which have an important role in vascular functional recovery in ischemic injury, ageing and hypertension. Evidence suggests that membrane GRK2 levels can be increased by cytokines and oxidative stress. Crocin also inhibited ERK1/2 phosphorylation and MMP-9 expression. Phosphorylation and activation of ERK during reperfusion localize in areas of increased •O−2 generation. MMPs/ matrixins are calcium-dependent zinc-containing endopeptidases which collectively mediate degradation of all kinds of extracellular matrix proteins, e.g. collagen and laminins in basal lamina, disrupting the integrity of the vascular wall [91, 92].
Safranal was also able to ameliorate cerebral IRI, due to oxidative stress. Mice exposed to safranal intraperitoneally (72.75 mg/kg, 145.5 mg/kg, 363.75 mg/kg, 727.5 mg/kg) prior to reperfusion exhibited significant restoration of the antioxidant capacity of hippocampus, as assessed by FRAP and total –SH content, both of which were compromised during cerebral IR. The previous findings explain the significant and dose-dependent decline of MDA levels in hippocampus of rats receiving safranal [36].
Neuronally differentiated Pheochromocytoma Cells (PC-12) exposed to stress stimuli such as serum-free and hypoxic conditions, exhibited the characteristic morphology of necrotic and/or apoptotic cells, whereas re-oxygenation after the hypoxic stimulus activated caspases - 3 and lipid peroxidation. Treatment of cells with picrocrocin and dicrocin had little or no effect on cell morphology and activation of caspase-3. On the other hand, crocin and tricrocin prevented serum deprivation- and hypoxia-induced apoptotic morphological changes, caspase-3 activation and lipid peroxidation. Also, all crocins replenished intracellular GSH content in serum-deprived and hypoxic PC12 cells by up-regulating γ-GCS expression. Retinoic acid, a structurally related retinoid molecule did not have any positive effect on PC-12 cells [93].
5.2. Protection Against Diabetic Complications
Glycaemia mediated-ROS can be generated by both enzymatic and non-enzymatic pathways. The enzymatic pathways involve activation of NADPH oxidase, cytochrome P-450 (CYTP450), cyclooxygenase, lipoxygenase, xanthine oxidase, and Myeloperoxidase (MPO). Conversely, the non-enzymatic pathways include mitochondrial Electron Transport Chain (mETC) deficiencies, AGEs formation and interaction with RAGE receptors, glucose autooxidation, transition-metal catalysed Fenton reactions and polyol (sorbitol) pathway.
Subsequently, the overproduction of ROS in diabetes actuates major pathways, involved in the pathogenesis of diabetic complications, such as (i) Increased formation of AGEs and expression of RAGE receptors, the interaction of which triggers downstream inflammatory signalling pathways activation (e.g., NF-κB), resulting in production of pro-inflammatory cytokines, such as TNF-α and IL-6, (ii) Polyol pathway increased flux of glycolytic metabolites (sorbitol, fructose), (iii) Activation of Protein Kinase C (PKC) isoforms-α, -β and –δ via diacylglycerol (DAG), circulating cytokines, growth factors, endothelin-1, angiotensin II and free fatty acids, and (iv) Over-activity of the hexosamine pathway, in which glucose is converted to glucosamine-6-phosphate by L-glutamine: D-fructose-6-phosphate amidotransferase (GFAT), finally leading to the enzymatic glycosylation of several proteins, such as transcription factors, regulating the expression of pro-inflammatory and pro-coagulant genes. Also, diabetes directly inactivates two critical antiatherosclerotic enzymes of vascular endothelium, eNOS and prostacyclin synthase, involved in NO and prostacyclin biosynthesis, respectively. NO and prostacyclins are potent vasodilators and inhibitors of platelet aggregation. Nitric Oxide Synthases (NOSs), convert L-arginine to L-citrulline and NO. There are two isoforms of NOS in endothelial cells, endothelial NOS (eNOS) and inducible NOS (iNOS). Rapid and reversible deformities in mitochondria are an early and causal factor necessary for hyperglycemia ROS-induced injury [7, 14, 18, 94-98].
Dysfunction of endothelial cells is considered a major cause of vascular complications in diabetes. CRT was found to prevent AGEs-mediated cell apoptosis in Bovine Aortic Endothelial Cells (BEC). AGEs can selectively trigger apoptosis in endothelial cells in a time-dependent manner, probably due to an increase of intracellular Ca2+ concentration, DNA fragmentation in the S phase of cell cycle, mitochondrial membrane damage and decrease in mitochondrial membrane potential, leading to massive ROS release, implicating them in diabetes-associated vascular complications. CRT prevented AGEs-induced BEC apoptosis, by attenuating AGEs mediated increase of ROS, DNA fragmentation and elevation of intracellular Ca2+ concentration. The antioxidant properties of CRT on BEC cells were demonstrated in another study, in which CRT increased the activity of SOD and decreased MDA content.
AGEs stimulate expression of adhesion molecules in endothelial cells such as ICAM-1, E-selectin and VCAM-1. ICAM-1 is a transmembrane glycoprotein, expressed on endothelial cells and cell of the immune system. ICAM-1 possesses binding sites for a number of immune-associated ligands. Notably, ICAM-1 binds to Leukocyte Function-Associated Antigen (LFA-1), a receptor found on activated leukocytes, to Macrophage Adhesion ligand-1 (Mac-1) and to fibrinogen, facilitating transmigration of leukocytes across vascular endothelia in processes, such as extravasation and inflammation. Once leukocytes adhere to endothelial cells, the process may trigger inflammatory pathways in endothelial cells, thus amplifying inflammatory mechanisms in the diabetic vascular wall and accelerating development of atherosclerosis. Pre-incubation with CRT also reduced leukocyte adherence rate to BEC induced by AGEs in vitro and down-regulated the expression of ICAM-1 and MMP [99, 100].
CRT inhibited high glucose-induced apoptosis via PI3K/Akt/eNOS pathway in Human Umbilical Vein Endothelial Cells (HUVECs). High glucose attenuated activation of Akt and endothelial eNOS, whereas CRT increased phosphorylation and activation of Akt, followed by up-regulation of eNOS and NO generation, hence preventing ROS-induced endothelial cell injury [101].
VSMCs are the cellular component of the vascular middle layer and their migration from the media to the intima is implicated in diabetic vascular complications, such as atherosclerosis. Exposure of Vascular Smooth Muscle Cells (VSMCs) to AGEs increased about two-fold their migration, whereas pre-incubation with CRT or RAGE antibody prior to exposure to AGEs inhibited migration induced by AGEs via RAGE-dependent signalling pathway. RAGE expression after stimulation with AGEs increased significantly, whereas pre-treatment with CRT reduced expression of endogenous RAGE. Also, CRT reduced the expression of MMP-2 and MMP-9, which are secreted by VSMCs to mediate the degradation or remodelling of the Extracellular Matrix (ECM), promoting their migration.
Diabetic vascular complications are regarded as an inflammatory disease. CRT exerted a beneficial effect on AGEs-mediated inflammatory response, as depicted by abrogation of secretion from VSMCs of inflammatory mediators, such as TNF-α and IL-6. TNF- α and IL-6 activate receptor and transcription factors [e.g., NF-κB, Toll-Like Receptors (TLRs), c-Jun amino-terminal kinase, and RAGE), leading to systemic migration of VSMCs and endothelial dysfunction, all hallmarks of vascular diabetic complications. The previous results demonstrate that suppression of VSMCs migration might be one of the important pathways by which CRT attenuates diabetic macrovascular injury [102].
Glucose-induced neurotoxicity in PC12 cells was reversed with saffron extract (5 and 25 mg/ml) and crocin (10 and 50 μM) pre-treatment. Indeed, glucose-mediated neuronal apoptosis, through ROS generation, reaching a maximal effect after 96 hours, whereas saffron extract and crocin protected from ROS-induced cytotoxicity [58].
5.3. Protection Against Hyperlipidaemia and Atherosclerosis
The pathogenesis of atherosclerosis involves endothelium dysfunction, infiltration of monocytes and their activation into macrophages and smooth muscle cell proliferation, whereas Oxidative modification of Low-Density Lipoprotein (Ox-LDL) plays an important role in the initiation and progression of atherosclerosis. Vascular endothelial cells are vulnerable to injury caused by ROS, as they are in contact with the constituents of blood, especially activated leucocytes which release massive amounts of ROS. Damaged endothelial cells facilitate adhesion of platelets and other blood cells to the vascular wall initiating atherogenesis. Ox-LDL activates endothelial cells, altering the functional and structural integrity of the endothelial barrier, hence leading to increased permeability of the endothelium, and stimulating the expression of adhesion molecules on the endothelial surface, allowing monocytes/macrophages to attach and infiltrate the sub-endothelial space. Subsequently, OxLDL is taken up by the scavenger receptors of monocytes/macrophages, leading to the formation of lipid-laden macrophages (foam cells) cells which are essential components of fatty streaks and fibrous plaques. OxLDL is responsible for smooth muscle cell proliferation and migration. Since Ox-LDL and the progression of atherosclerosis are interlinked processes, the antioxidants, which decrease the level of Ox-LDL, could be administered as potential anti-atherogenic agents.
Constitutive generation of NO by the endothelial cells contributes to the vasodilatory tone, plays an important role in renal and systemic vascular resistance and tissue perfusion, interferes with platelet adhesion, retards cellular proliferation and serves as a neurotransmitter, an immune effector, an antioxidant, and a free radical. An increasing number of studies suggest that OxLDL which accumulate in the arterial wall in hypercholesterolemia may inhibit the NO-mediated endothelium-dependent vasorelaxation or reduce NO production through down-regulation of eNOS expression. NO deficiency could promote arteriosclerosis by facilitating cell migration and proliferation within blood vessel walls. Also, loss of the antithrombotic activity of NO on platelets facilitates the formation of microthrombi and the release of thrombogenic, proinflammatory, and mitogenic platelet factors [103].
The antioxidant properties of saffron and crocin were evaluated in Albino Wistar rats in conjunction with their hypolipidaemic effect. It is well established that hyperlipidaemia causes cellular production and plasma elevation of ROS. Rats on high-fat diet when treated with saffron (25, 50, 100 mg/kg) or crocin (4.84, 9.69, and 19.38 mg/kg) demonstrated significant decrease in serum triglycerides, total cholesterol and MDA and increase in SOD and catalase activity and total antioxidant capacity, as assessed by FRAP and total –SH in liver tissue homogenate. Also, saffron and crocin prevented the elevation of GPx, GSH, and oxidized glutathione (GSSG), due to high-fat diet. GPx activity leads to detoxification of H2O2. Increased plasma GPx activities could be related to lysis of erythrocytes, due to oxidative stress and inadequate GPx activity. The hypolipidaemic properties of saffron could be linked with the presence of flavonoids [20, 104].
Diabetic dyslipidaemia, characterized by high plasma triglyceride and lipoprotein (a) [Lp(a)] levels, increased concentrations of small dense LDL-cholesterol particles and low HDL, is a major risk factor for cardiovascular diseases in diabetic patients. The previous lipidaemic profile is extremely atherogenic and thrombogenic. Also, patients with diabetes have decreased plasma adiponectin concentrations, an adipocytokine secreted by adipose tissue. Adiponectin has a major role in metabolic homeostasis, as it enhances insulin sensitivity by lowering glucose levels, independently of plasma insulin levels, and reducing plasma concentration of free fatty acids and tissue triglyceride content. Furthermore, plasma adiponectin is negatively correlated with plasma triglycerides and LDL and positively correlated with HDL. Adiponectin is also an antioxidant which is inversely correlated with oxidative stress and inflammation. Paradoxically, in diabetes adiponectin is decreased which in association with oxidative stress and inflammatory responses are key elements in development and progression of diabetes complications [105, 106]. The significant hypoglycaemic, hypolipidaemic and anti-atherogenic benefits of saffron hydroalcoholic or aqueous extracts were highlighted in an animal model of diabetes. More specifically, serum levels of fasting blood glucose, triglycerides, VLDL and Lp(a) decreased significantly in treated groups. Also, saffron, especially the hydroalcoholic extract ameliorated hyperglycemia-mediated oxidative stress, as depicted by reduced lipid peroxidation and increased antioxidant capacity [107].
Crocin or CRT, when administered (25, 50, 100 mg/kg/day) in an atherosclerosis model, which was established by feeding hyperlipidaemic diet for 9 weeks (14% lard, l6% groundnut oil, 1% cholesterol, 79% commercial basal diet) to male quails, both exerted hypolipidaemic properties and inhibited the formation of aortic plaque. Hyperlipidaemic diet increased significantly serum levels of triglycerides, total cholesterol, LDL-C and VLDL, whereas treatment with crocin and CRT deterred the previous increases. The treatment of quails with crocin and CRT protected against hyperlipidaemia diet-induced oxidative stress, as reflected by elevation of MDA levels and depletion of NO levels. Quails fed on hyperlipidaemic diet showed significant histopathological changes on the inner surface aorta, indicative of advanced atherosclerosis, such as smooth muscle cell proliferation, mixed foam macrophages and fibroblast-like cells with lipid droplets. Crocin and CRT markedly improved fibromuscular lesions and the formation of atherosclerosis, probably due to decreasing the accumulation of macrophage-derived foam cells [103, 108].
In another atherosclerosis model which was established by feeding hyperlipidaemic diet for 8 weeks to rabbits, high lipid diet was complicated with severe hypercholesterolemia and atherosclerotic lesions in thoracic aortas, together with profound up-regulation of VCAM-1 expression, followed by increased activation in aortas of NFκB, a redox-sensitive transcription factor essential for VCAM-1 expression, and significant elevation of TBARS and Ox-LDL, two important indices of oxidative stress. In contrast, supplementation with CRT markedly reduced the progression of atherosclerotic lesions and plasma levels of Ox-LDL, decreased NFκΒ activation, VCAM-1 expression and reduced aortic content of TBARS and macrophages/foam cells, whereas plasma total antioxidant capacity and SOD activity were increased. However, in this study plasma lipids level remained unchanged in CRT-treated animals. The anti-atherosclerotic effect of CRT could be partially attributed to inhibition of NFκΒ activation and VCAM-1 expression, a cytokine-inducible member of immunoglobulin gene superfamily, implicated in atherogenesis by promoting firm adhesion of monocytes to vascular endothelium, and subsequent transmigration into the sub-endothelial space. Of note, in humans, focal expression of VCAM-1 was detected mainly in atherosclerotic plaques and elevated plasma VCAM-1 was demonstrated in patients with hypolipoproteinaemia [109, 110].
Another mechanism by which CRT might halt hypercholesterolemia-induced endothelial dysfunction is restoring and up-regulating eNOS expression, in a dose-dependent manner. There is substantial evidence that ox-LDL is implicated in endothelial dysfunction via down-regulating eNOS expression and NO generation, hence contributing to impairment of endothelium-dependent relaxation and initiation and progression of atherosclerosis [111].
The protective properties of crocin against Ox-LDL cellular deleterious effects were demonstrated in several in vitro studies. Bovine aortic endothelial cells incubated with Ox-LDL showed a marked increase in the activity of LDH and inhibition of eNOS activity, leading to depleted NO in culture media. Treatment with crocin prior to Ox-LDL exposure protected against the Ox-LDL-induced elevation of LDH and depletion of NO. Also, Ox-LDL induced concentration-dependent endothelial cells apoptosis, whereas pre-treatment with crocin decreased the rate of endothelial cells apoptosis. Peritoneal macrophages, when cultured with Ox-LDL, demonstrated increased total free cholesterol and cholesteryl ester content in a concentration-dependent mode, whereas crocin inhibited their accumulation. The previous results indicate that crocin could inhibit the formation of foam cells induced by Ox-LDL.
As mentioned previously, excessive proliferation of Vascular Smooth Muscle Cells (VSMCs), leading to vascular intimal hyperplasia is a critical process during the formation of atherosclerotic plaques and restenosis after angioplasty. BOVINE AORTIC SMOOTH MUSCLE CELLS (BASMCs) proliferation was induced by Ox-LDL, and crocin inhibited the proliferation index elevation in a concentration-dependent manner. Also, when bovine smooth muscle aortic cells were treated with Ox-LDL and in the presence of extracellular Ca2+ exhibited marked increase in intracellular Ca2+, which is an important second messenger regulating a variety of cellular processes, including smooth muscle cell proliferation, apoptosis and gene expression On the contrary, crocin blocked the influx of extracellular Ca2+ in the presence of Ox-LDL. Also, it inhibited the release of the endoplasmic reticulum Ca2+ pool by blocking the activity of ryanodine-sensitive Ca2+ channels.
The antiproliferative effects of CRT on BASMCs were confirmed in another research and were partly associated with its antioxidant properties. More specifically, CRT inhibited AngII (angiotensin II) -induced cell-cycle progression and proliferation by arresting cells in the G0/G1 phase and decreasing the percentages of BASMCs entering the S and G2/M phases. Consistently, CRT suppressed AngII-induced ERK1/2 phosphorylation, activation and nuclear translocation and the expression of its downstream effector c-fos. Ang II acts through the Gq protein-coupled AT1 receptor, inducing significant ERK1/2 activation in VSMCs, which is requisite event in VSMCs mitogenic stimulation and growth. ERK1/2 functions as an integrator for mitogenic signals originating from several distinct cell surface receptors, such as receptor tyrosine kinases and G protein-coupled receptors, by phosphorylating a variety of substrates including transcriptional factors and kinases. c-Fos is a transcription factor which forms heterodimer with c-Jun, resulting in the formation of AP-1 (activator protein-1) complex. The latter binds DNA at AP-1 specific sites at the promoter and enhancer regions of target genes, modifying gene expression. The importance of c-fos in biological context has been depicted in several cellular processes, including cell proliferation, differentiation, survival and motility, cellular response to hypoxia and angiogenesis. An additional research demonstrated that inhibition of Ang II-induced ERK1/2 activation via CRT pre-treatment was associated with attenuation of Ang II-induced intracellular Ca2+ mobilization and extracellular Ca2+ influx via L-type Ca2+ channels.
Ang II also promotes the generation of ROS by stimulation of the activity of a membrane-associated NADH/ NADPH oxidase, which is the most important source of O2-• in VSMCs. Accumulating evidence demonstrates that ROS generation is essential for AngII-elicited activation of ERK1/2, depicting that ERK1/2 activation is a downstream effector of a redox-sensitive signalling pathway. Hence, a potential approach for the manipulation of vascular diseases, involving excessive proliferation of VSMCs, should include antioxidant therapy. The current research demonstrated that AngII increased ROS generation in BASMCs, whereas pre-incubation with CRT markedly reduced AngII-induced intracellular ROS generation and in parallel increased SOD activity [112-116].
Exposure of BEC to oxidative stress induced by incubation with H2O2 was complicated with cellular apoptosis, as demonstrated by cell shrinkage, condensation of nuclei, membrane blebbing and formation of apoptotic body. Endothelial cell apoptosis has an important role in the initiation and progression of atherosclerosis. Treatment with crocin increased endothelial resistance towards ROS damaging effects in a dose-dependent manner. The cytoprotective effects of crocin against H2O2 were attributed to increased expression of Bcl-2 and parallel reduction of Bax, leading to an increased ratio of Bcl-2/Bax. Bcl-2 reduces both the release of Ca2+ from the endoplasmic reticulum and Ca2+ influx from extracellular space. The intracellular Ca2+ has an important role in apoptotic signal transduction hence Bcl-2 overexpression might protect cells from Ca2+-induces apoptotic stimuli. Also, Bcl-2 may have a more direct effect on preventing apoptosis, by inhibiting activation of caspase cascade (inhibits the loss of mitochondrial membrane potential and the release of cytochrome c from mitochondria) and stabilizing the biomembranes. Interestingly, the treatment of cells with crocin alone had little effect on the value of Bcl-2/Bax ratio [103, 112, 117-119].
6. DISCUSSION
Saffron has been described in the catalogues of medicinal plants of the European pharmacopoeia since the 16th century [120]. It has been used as a compound in a great variety of remedies during the medieval times, yet it has been neglected with the advent of synthetic chemistry [120]. However, in recent years there is a growing interest towards the properties of saffron and its applications in human health. Several reports have dealt with the effects of saffron in human homeostasis and in a variety of physiological systems including the cardiovascular. It is well documented that crocins have a beneficial effect on human health. In a recent clinical trial, it has been reported that the administration of 100mg/day of crocin on metabolic syndrome patients succeeded the reduction of important metabolic factors such as cholesterol and triglycerides as compared to the control group [121]. Another interesting report has found that the administration of 100mg/day saffron had anti-inflammatory effects in patients with metabolic syndrome similarly reducing metabolic factors such as cholesterol, triglycerides, cytokines, fasting blood sugar etc. [122]. These results were confirmed in animal models as well, where similar experiments showed that saffron had advantageous effects on the cardiovascular system of rats [123] and rabbits [81]. As aforementioned in the previous sections, saffron manifested also an indirect cardiovascular protective effect, which included cardioprotection against doxorubicin toxicity [81, 124, 125].
The main finding from all these studies, as well as, those that we have examined during our review is that the constitutive administration of saffron, especially as a dietary supplement, has beneficial effects on several physiological systems and in particular in the cardiovascular system. In particular, most studies converge to the administration of 100mg/day as a beneficial dosage for the advantageous effects of saffron, yet this is a subject of further investigation since most clinical trials included a small number of subjects.
CONCLUSION
Saffron appears to be a promising medicinal plant for the treatment of several human health conditions. Especially, up-to-date knowledge shows that saffron consumption as a dietary supplement significantly improves metabolic as well as cardiovascular factors. The common denominator in saffron properties is its regular dietary consumption. All experimental and clinical trials converge to the assumption that a regular dosage of saffron, as a dietary supplement, is required in order to obtain its beneficial outcome. Yet, saffron properties still remain to be elucidated since more studies and clinical trials are required.
Fig. (1).
Structure of crocin (PubChem ID: 5281233).
Fig. (2).
Structure of crocetin (PubChem ID: 5281232).
Fig. (3).
Structure of picrocrocin (PubChem ID: 130796).
Fig. (4).
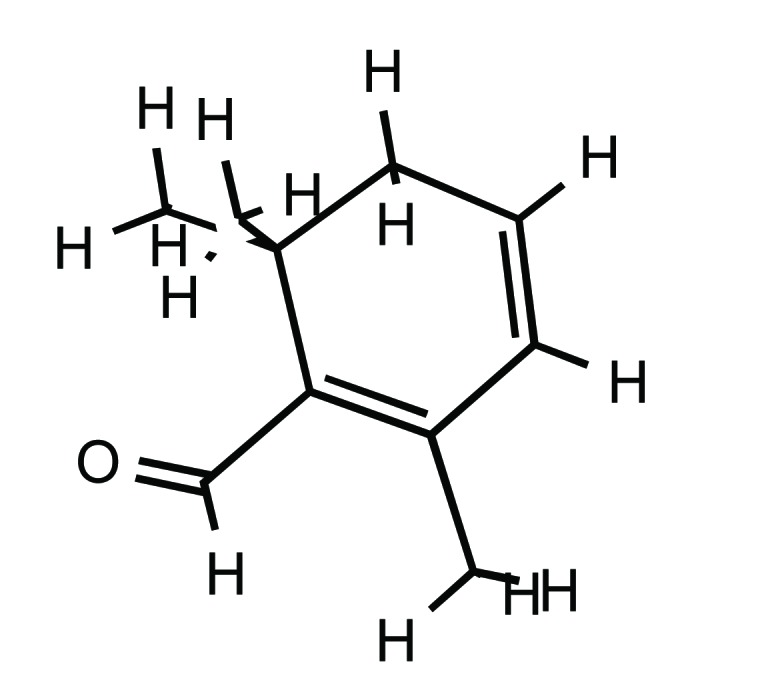
Structure of safranal (PubChem ID: 61041).
Fig. (5).
Structure of Saffron oil extract (PubChem ID: 6450930).
Fig. (6).
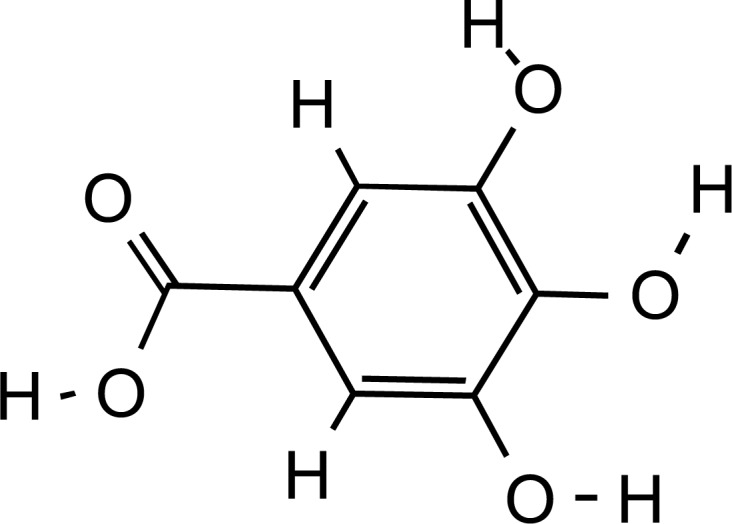
Structure of gallic acid, main phenolic constituent of Saffron (PubChem ID: 370).
Fig. (7).
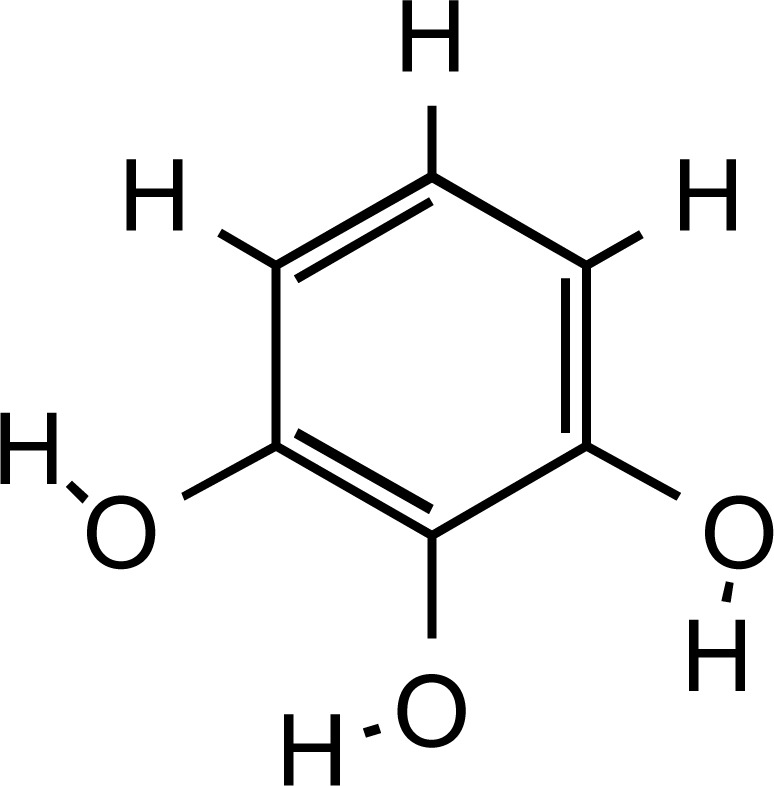
Structure of pyrogallol, main flavonoid constituent of Saffron (PubChem ID: 1057).
Acknowledgements
Declared none.
LIST OF ABBREVIATIONS
- 1O2
Singlet Oxygen
- 4EBP1
Eukaryotic Translation Initiation Factor 4E-binding Protein 1
- 6-OHDA
6- hydroxydopamine
- AchE
Acetylcholinesterase
- ADH
Alcohol Dehydrogenase
- AFB1-FABY
Aflatoxin B1 Formaminopyrimidine
- AFB1-N7-Gua
8,9-dihydroxy-8-(N7 guanyl)-9-hydroxy Aflatoxin B1
- AFB1-SG
8- (S-glutathionyl)-9-hydroxy-8,9-dihydro-AFB1
- AFP
Alpha-fetoprotein
- AGEs
Advanced Glycation end Products
- AIF
Apoptosis-inducing Factor
- ALP
Alkaline Phosphatase
- ALS
Amyotrophic Lateral Sclerosis
- ALT
Alanine Aminotransferase
- AMPA
2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl) proprionate
- AP sites
Apurinic/Apyrimidinic Sites
- APDG
N-alkylpurine-DNA Glycosylases
- AST
Aspartate Aminotransferase
- ATP
Adenosine 5′-triphosphate
- Aβ
Αmyloid β
- B(a)p
Benzo(a)pyrene
- Bcl-2
B-cell lymphoma 2
- Bcl-xL
B-cell Lymphoma-extra Large
- BEC
Bovine Aortic Endothelial Cells
- BER
Base Excision Repair
- BHT
Butylated Hydroxytoluene
- BUN
Urea Nitrogen
- CCl4
Carbon Tetrachloride
- CD
Circular Dichroism
- CK-MB
Creatinine Kinase-MB
- CNS
Central Nervous System
- COX
Cyclooxygenase
- COX-2
Cyclooxygenase-2
- Cr
Creatinine
- CRT
Crocetin
- ctDNA
DNA
- CYTP450
Cytochrome P-450
- DAG
Diacylglycerol
- DEN
Diethylnitrosamine
- DMBA
7-12 Dimethylbenz(a)anthracene
- DMCRT
Dimethylocrocetin
- DOPAC
3,4-dihydroxyphenylacetic acid
- DOX
Doxorubicin
- DOXol
Doxorubicinol
- DPPH
1,1-diphenyl-2- picryl-hydrazyl
- ECM
Extracellular Matrix
- eNOS/ NOS3
Endothelial Nitric Oxide Synthase / Nitric Oxide Synthase 3
- ERK
Extracellular Signal-regulated Kinase
- ETC
Electron Transport Chain
- FFA
Free Fatty Acid
- FRAP
Ferric Reducing Power Activity
- G6PD
Glucose-6-phosphate Dehydrogenase
- GAPs
GTPase-activating Proteins
- Gclc
Glutamate-cysteine Ligase Catalytic
- GCLM
Glutamate-cysteine Ligase Modifier
- GDIs
GDP Dissociation Inhibitors
- GDP
Guanosine Diphosphate
- GEFs
Guanine Nucleotide Exchange Factor
- GFAT
Glucosamine-6-phosphate by L-glutamine: D-fructose-6-phosphate Amidotransferase
- GFR
Glomerular Filtration Rate
- GGT
Gamma-glutamyl Transferase
- GMP
Guanosine Monophosphate
- GPx
Glutathione Peroxidise
- GR
Glutathione Reductase
- GRK2
G protein-coupled Receptor Kinase 2
- GSH
Glutathione
- GSK-3b
Glycogen Synthase Kinase-3b
- GSSG
Oxidized Glutathione
- GST
Glutathione-S-transferase
- GTP
Guanosine -5'-triphosphate
- H2O2
Hydrogen Peroxide
- Hb
Haemoglobin
- HCT
Hematocrit
- HMOX1
Heme Oxygenase-1
- HNE
4-hydroxynonenal
- HNO2
Nitrous Acid
- HOCl
Hypochlorous Acid
- HTCC
4-hydroxy-2,6,6-trimethyl-1-carboxaldehyde-1-cyclohexene
- HUVECs
Human Umbilical Vein Endothelial Cells
- HVA
Homovanillic Acid
- ICAM-1
Intracellular Adhesion Molecule-1
- ICAM-1
Intercellular Adhesion Molecule-1
- IDH
Isocitrate Dehydrogenase
- iGluRs
Ionotropic Receptors
- IKK
IκB Kinase
- IKK-β
Inhibitor of Nuclear Factor Kappa-B Kinase Subunit Beta
- IL-
Interleukin
- IL-6
Interleukin-6
- IR
Ischemia Reperfusion
- IRI
Ischemia-reperfusion Injury
- IRP-1
Iron Regulatory Protein 1/ Aconitase-1
- IκB kinase
Nuclear Factor of Kappa Light Polypeptide Gene Enhancer in B-cells Inhibitor
- JNK
c-Jun N-terminal Kinase
- KSR
Kinase Suppressors of Ras
- LDH
Lactic Acid Dehydrogenase
- LDL
Low Density Lipoprotein
- LFA-1
Leukocyte Function Associated Antigen
- LOO•
Lipid Peroxyl
- LOX
Lipoxygenase
- LV
Left Ventricular
- LVEDP
Left Ventricular end Diastolic Pressure
- LVP
Left Ventricular Pressure
- Mac-1
Macrophage Adhesion Ligand-1
- MAO
Monoamine Oxidase
- MAPK
Mitogen-activated Protein Kinases
- MCP-1
Monocyte Chemotactic Protein-1
- MDA
Malondialdehyde
- MDA5
Melanoma Differentiation-Associated Protein 5
- MDH
Malate Dehydrogenase
- mETC
Mitochondrial Electron Transport Chain
- mGluRs
Metabotropic Receptors
- MMP
Matrix Metalloproteinase
- MMS
Methyl Methanesulfonate
- MPO
Myeloperoxidase
- mPTP
Mitochondrial Permeability Transition Pore
- Mrps
Multidrug Resistance-associated Proteins
- mTOR
Mechanistic Target of Rapamycin / Mammalian Target of Rapamycin
- MTP
Mitochondrial Transmembrane Potential
- NADPH
Nicotinamide Adenine Dinucleotide Phosphate
- NFT
Νeurofibrillary Tangles
- NF-κB
Nuclear Factor Kappa-light-chain-enhancer of Activated B Cells
- NF-κBp65
Transcription Factor p65/ Nuclear Factor NF-kappa-B p65 Subunit
- NMDA
N-methyl-D- Aspartate
- NMDARs
N-methyl-D-aspartate-type Glutamate Receptors
- nNOS
Neuronal NOS
- NO
Nitric Oxide
- NO•
Nitric Oxide
- NO2•
Nitrogen Dioxide
- NOS
Nitric Oxide Synthase
- NOSs
Nitric oxide Synthases
- NOX
Nadph-like Enzymes
- Nqo1
NAD(P)H Quinone Oxidoreductase 1
- Nrf2/ARE
Nuclear Factor (erythroid-derived 2)-like 2
- nSMase
Neutral Sphingomyelinase
- O2•−
Superoxide
- O3
Ozone
- OH•
Hydroxyl-radical
- ONE
4-oxo-2-nonenal
- ONOO•
Peroxynitrite
- Ox-LDL
Oxidized Low Density Lipoprotein
- OxPC
Phosphatidylcholine
- PAI-1
Plasminogen Activator Inhibitor-1
- PARK7 /DJ-1
Parkinson Disease Protein 7/ Protein Deglycase DJ-1
- PARP
Poly (ADP-ribose) Polymerase
- PCC
Protein Carbonyl Content
- PI3K
Phosphatidylinositol-4,5-bisphosphate 3-kinase
- PINK1
PTEN-induced Putative Kinase 1
- PKB
Protein Kinase B
- PKC
Protein Kinase C
- PP1
Protein Phosphatase 1
- PP2A
Protein Phosphatase 2A
- ppm
Parts Per Million
- PQ
Paraquat
- PTT
Partial Thromboplastin Time
- PUFAs
Polyunsaturated Fatty Acids
- RAGE
Receptor for Advanced Glycation End Products
- RBC
Red Blood Cell Count
- RNS
Reactive Nitrogen Species
- RO-
Alkoxyl
- RO2•
Peroxyl
- ROCK
Rho-associated Protein Kinase
- RONS
Reactive Oxygen Nitrogen Species
- ROS
Reactive Oxygen Species
- ROT
Rotenone
- SAE
Saffron Aqueous Extract
- SarcKATP
Sarcolemmal-ATP-dependent Potassium
- SDH
Succinate Dehydrogenase
- SDH
Sorbitol Dehydrogenase
- SERCA2a
Sarcoplasmic Reticulum Ca2+ -ATPase2a
- –SH
Thiol /Sulfhydryl Groups
- SMAC
Second Mitochondria-derived Activator of Caspases
- SOD
Superoxide Dismutase
- SPE
Saffron Petal Extract
- SRXN1
Sulfiredoxin 1
- TBARS
Thiobarbituric Acid Reactive Species
- TGF-β
Transforming Growth Factor Beta
- TLRs
Toll-like Receptors
- TNF-a
Tumor Necrosis Factor- a
- TNF-α
Tumour Necrosis Factor-alpha
- tRNA
Transfer RNA
- TXNRD1
Thioredoxin Reductase 1
- UCH-L1
Ubiquitin Carboxy-terminal Hydrolase L1
- UGT
UDP-glucuronosyltransferase
- VCAM-1
Vascular Adhesion Cell Molecule-1
- VEBs
Premature Ventricular Beat
- VF
Ventricular Fibrillation
- VLDL
Very Low Density Lipoprotein
- VSMCs
Vascular Smooth Muscle Cells
- VT
Ventricular tachycardia
- WBC
White Blood Cells
- X/XO
Xanthine-xanthine Oxidase
- γ-GCS
γ-glutamyl-cysteinyl Synthase
Consent for Publication
Not applicable.
Conflict of Interest
The author declares no conflict of interest, financial or otherwise.
REFERENCES
- 1.Ozcan A., Ogun M., Gowder S.J.T. Biochemistry of reactive oxygen and nitrogen species. In basic principles and clinical significance of oxidative stress. Rijeka: InTech; 2015. [Google Scholar]
- 2.Ahmadinejad F., Geir Moeller S., Hashemzadeh-Chaleshtori M., et al. Molecular mechanisms behind free radical scavengers function against oxidative stress. Antioxidants. 2017;6(3):51. doi: 10.3390/antiox6030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valko M., Leibfritz D., Moncol J., et al. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Ye Z.W., Zhang J., Townsend D.M., et al. Oxidative stress, redox regulation and diseases of cellular differentiation. Biochim. Biophys. Acta. 2015;1850(8):1607–1621. doi: 10.1016/j.bbagen.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lushchak V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014;224:164–175. doi: 10.1016/j.cbi.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Busik J.V., Mohr S., Grant M.B. Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes. 2008;57(7):1952–1965. doi: 10.2337/db07-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andreoli T.E. Free radicals and oxidative stress. Am. J. Med. 2000;108(8):650–651. doi: 10.1016/s0002-9343(00)00418-6. [DOI] [PubMed] [Google Scholar]
- 9.Reczek C.R., Chandel N.S. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 2015;33:8–13. doi: 10.1016/j.ceb.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adibhatla R.M., Hatcher J.F. Lipid oxidation and peroxidation in CNS health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2010;12(1):125–169. doi: 10.1089/ars.2009.2668. [DOI] [PubMed] [Google Scholar]
- 11.Bar-Or D., Bar-Or R., Rael L.T., et al. Oxidative stress in severe acute illness. Redox Biol. 2015;4:340–345. doi: 10.1016/j.redox.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collard C.D., Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology. 2001;94(6):1133–1138. doi: 10.1097/00000542-200106000-00030. [DOI] [PubMed] [Google Scholar]
- 13.Fakhruddin S., Alanazi W., Jackson K.E. Diabetes-induced reactive oxygen species: mechanism of their generation and role in renal injury. J. Diabetes Res. 2017;2017:8379327. doi: 10.1155/2017/8379327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circ. Res. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jnaneshwari S., Hemshekhar M., Santhosh M.S., et al. Crocin, a dietary colorant, mitigates cyclophosphamide-induced organ toxicity by modulating antioxidant status and inflammatory cytokines. J. Pharm. Pharmacol. 2013;65(4):604–614. doi: 10.1111/jphp.12016. [DOI] [PubMed] [Google Scholar]
- 16.Massaad C.A. Neuronal and vascular oxidative stress in Alzheimer’s disease. Curr. Neuropharmacol. 2011;9(4):662–673. doi: 10.2174/157015911798376244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thapa A., Carroll N.J. Dietary modulation of oxidative stress in Alzheimer’s disease. Int. J. Mol. Sci. 2017;18(7):E1583. doi: 10.3390/ijms18071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan L.J. Pathogenesis of chronic hyperglycemia: from reductive stress to oxidative stress. J. Diabetes Res. 2014;2014:137919. doi: 10.1155/2014/137919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bathaie S.Z., Mousavi S.Z. New applications and mechanisms of action of saffron and its important ingredients. Crit. Rev. Food Sci. Nutr. 2010;50(8):761–786. doi: 10.1080/10408390902773003. [DOI] [PubMed] [Google Scholar]
- 20.Rahaiee S., Moini S., Hashemi M., et al. Evaluation of antioxidant activities of bioactive compounds and various extracts obtained from saffron (Crocus sativus L.): A review. J. Food Sci. Technol. 2015;52(4):1881–1888. doi: 10.1007/s13197-013-1238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel S., Sarwat M., Khan T.H. Mechanism behind the anti-tumour potential of saffron (Crocus sativus L.): The molecular perspective. Crit. Rev. Oncol. Hematol. 2017;115:27–35. doi: 10.1016/j.critrevonc.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Christodoulou E., Kadoglou N.P., Kostomitsopoulos N., et al. Saffron: A natural product with potential pharmaceutical applications. J. Pharm. Pharmacol. 2015;67(12):1634–1649. doi: 10.1111/jphp.12456. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt M., Betti G., Hensel A. Saffron in phytotherapy: Pharmacology and clinical uses. Wien. Med. Wochenschr. 2007;157(13-14):315–319. doi: 10.1007/s10354-007-0428-4. [DOI] [PubMed] [Google Scholar]
- 24.Alavizadeh S.H., Hosseinzadeh H. Bioactivity assessment and toxicity of crocin: A comprehensive review. Food Chem. Toxicol. 2014;64:65–80. doi: 10.1016/j.fct.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Amin B., Abnous K., Motamedshariaty V., et al. Attenuation of oxidative stress, inflammation and apoptosis by ethanolic and aqueous extracts of Crocus sativus L. stigma after chronic constriction injury of rats. An. Acad. Bras. Cienc. 2014;86(4):1821–1832. doi: 10.1590/0001-3765201420140067. [DOI] [PubMed] [Google Scholar]
- 26.Amin B., Malekzadeh M., Heidari M.R., et al. Effect of Crocus sativus extracts and its active constituent safranal on the harmaline-induced tremor in mice. Iran. J. Basic Med. Sci. 2015;18(5):449–458. [PMC free article] [PubMed] [Google Scholar]
- 27.Amin B., Nakhsaz A., Hosseinzadeh H. Evaluation of the antidepressant-like effects of acute and sub-acute administration of crocin and crocetin in mice. Avicenna J. Phytomed. 2015;5(5):458–468. [PMC free article] [PubMed] [Google Scholar]
- 28.Ayatollahi H., Javan A.O., Khajedaluee M., et al. Effect of Crocus sativus L. (saffron) on coagulation and anticoagulation systems in healthy volunteers. Phytother. Res. 2014;28(4):539–543. doi: 10.1002/ptr.5021. [DOI] [PubMed] [Google Scholar]
- 29.Azimi P., Ghiasvand R., Feizi A., et al. Effect of cinnamon, cardamom, saffron and ginger consumption on blood pressure and a marker of endothelial function in patients with type 2 diabetes mellitus: A randomized controlled clinical trial. Blood Press. 2016;25(3):133–140. doi: 10.3109/08037051.2015.1111020. [DOI] [PubMed] [Google Scholar]
- 30.Ghasemi T., Abnous K., Vahdati F., et al. Antidepressant effect of Crocus sativus aqueous extract and its effect on CREB, BDNF, and VGF transcript and protein levels in rat hippocampus. Drug Res. (Stuttg.) 2015;65(7):337–343. doi: 10.1055/s-0034-1371876. [DOI] [PubMed] [Google Scholar]
- 31.Hosseinzadeh H. Saffron: A herbal medicine of third millennium. Jundishapur J. Nat. Pharm. Prod. 2014;9(1):1–2. doi: 10.17795/jjnpp-16700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosseinzadeh H., Ghenaati J. Evaluation of the antitussive effect of stigma and petals of saffron (Crocus sativus) and its components, safranal and crocin in guinea pigs. Fitoterapia. 2006;77(6):446–448. doi: 10.1016/j.fitote.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Hosseinzadeh H., Jahanian Z. Effect of Crocus sativus L. (saffron) stigma and its constituents, crocin and safranal, on morphine withdrawal syndrome in mice. Phytother. Res. 2010;24(5):726–730. doi: 10.1002/ptr.3011. [DOI] [PubMed] [Google Scholar]
- 34.Hosseinzadeh H., Modaghegh M.H., Saffari Z. Crocus sativus L. (Saffron) extract and its active constituents (crocin and safranal) on ischemia-reperfusion in rat skeletal muscle. Evid. Based Complement. Alternat. Med. 2009;6(3):343–350. doi: 10.1093/ecam/nem125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosseinzadeh H., Noraei N.B. Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituents, crocin and safranal, in mice. Phytother. Res. 2009;23(6):768–774. doi: 10.1002/ptr.2597. [DOI] [PubMed] [Google Scholar]
- 36.Hosseinzadeh H., Sadeghnia H.R. Safranal, a constituent of Crocus sativus (saffron), attenuated cerebral ischemia induced oxidative damage in rat hippocampus. J. Pharm. Pharm. Sci. 2005;8(3):394–399. [PubMed] [Google Scholar]
- 37.Hosseinzadeh H., Sadeghnia H.R., Ziaee T., et al. Protective effect of aqueous saffron extract (Crocus sativus L.) and crocin, its active constituent, on renal ischemia-reperfusion-induced oxidative damage in rats. J. Pharm. Pharm. Sci. 2005;8(3):387–393. [PubMed] [Google Scholar]
- 38.Hosseinzadeh H., Younesi H.M. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002;2:7. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imenshahidi M., Hosseinzadeh H., Javadpour Y. Hypotensive effect of aqueous saffron extract (Crocus sativus L.) and its constituents, safranal and crocin, in normotensive and hypertensive rats. Phytother. Res. 2010;24(7):990–994. doi: 10.1002/ptr.3044. [DOI] [PubMed] [Google Scholar]
- 40.Imenshahidi M., Razavi B.M., Faal A., et al. The effect of chronic administration of saffron (Crocus sativus) stigma aqueous extract on systolic blood pressure in rats. Jundishapur J. Nat. Pharm. Prod. 2013;8(4):175–179. doi: 10.17795/jjnpp-12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khorasany A.R., Hosseinzadeh H. Therapeutic effects of saffron (Crocus sativus L.) in digestive disorders: A review. Iran. J. Basic Med. Sci. 2016;19(5):455–469. [PMC free article] [PubMed] [Google Scholar]
- 42.Mehdizadeh R., Parizadeh M.R., Khooei A.R., et al. Cardioprotective effect of saffron extract and safranal in isoproterenol-induced myocardial infarction in wistar rats. Iran. J. Basic Med. Sci. 2013;16(1):56–63. [PMC free article] [PubMed] [Google Scholar]
- 43.Moshiri M., Vahabzadeh M., Hosseinzadeh H. Clinical applications of saffron (Crocus sativus) and its constituents: A review. Drug Res. (Stuttg.) 2015;65(6):287–295. doi: 10.1055/s-0034-1375681. [DOI] [PubMed] [Google Scholar]
- 44.Razavi B.M., Hosseinzadeh H. Saffron as an antidote or a protective agent against natural or chemical toxicities. Daru. 2015;23:31. doi: 10.1186/s40199-015-0112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vahdati Hassani F., Naseri V., Razavi B.M., et al. Antidepressant effects of crocin and its effects on transcript and protein levels of CREB, BDNF, and VGF in rat hippocampus. Daru. 2014;22(1):16. doi: 10.1186/2008-2231-22-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pitsikas N., Zisopoulou S., Tarantilis P.A., et al. Effects of the active constituents of Crocus sativus L., crocins on recognition and spatial rats’ memory. Behav. Brain Res. 2007;183(2):141–146. doi: 10.1016/j.bbr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Winterhalter P., Straubinger M. Saffron renewed interest in an ancient spice. Food Rev. Int. 2000;16(1):39–59. [Google Scholar]
- 48.Liakopoulou-Kyriakides M., Kyriakidis D.A. Croscus sativus-biological active constitutents. Stud Nat Prod Chem. 2002;26:293–312. [Google Scholar]
- 49.Bolhassani A., Khavari A., Bathaie S.Z. Saffron and natural carotenoids: Biochemical activities and anti-tumor effects. Biochim. Biophys. Acta. 2014;1845(1):20–30. doi: 10.1016/j.bbcan.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Caballero-Ortega H., Pereda-Miranda R., Abdullaev F.I. HPLC quantification of major active components from 11 different saffron (Crocus sativus L.) sources. Food Chem. 2007;100(3):1126–1131. [Google Scholar]
- 51.Giaccio M. Crocetin from saffron: An active component of an ancient spice. Crit. Rev. Food Sci. Nutr. 2004;44(3):155–172. doi: 10.1080/10408690490441433. [DOI] [PubMed] [Google Scholar]
- 52.Assimopoulou A.N., Sinakos Z., Papageorgiou V.P. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother. Res. 2005;19(11):997–1000. doi: 10.1002/ptr.1749. [DOI] [PubMed] [Google Scholar]
- 53.Tarantilis P.A., Tsoupras G., Polissiou M. Determination of saffron (Crocus sativus L.) components in crude plant extract using high-performance liquid chromatography-UV-visible photodiode-array detection-mass spectrometry. J. Chromatogr. A. 1995;699(1-2):107–118. doi: 10.1016/0021-9673(95)00044-n. [DOI] [PubMed] [Google Scholar]
- 54.Rahaiee S., Hashemi M., Shojaosadati S.A., et al. Nanoparticles based on crocin loaded chitosan-alginate biopolymers: Antioxidant activities, bioavailability and anticancer properties. Int. J. Biol. Macromol. 2017;99:401–408. doi: 10.1016/j.ijbiomac.2017.02.095. [DOI] [PubMed] [Google Scholar]
- 55.Gutheil W.G., Reed G., Ray A., et al. Crocetin: An agent derived from saffron for prevention and therapy for cancer. Curr. Pharm. Biotechnol. 2012;13(1):173–179. doi: 10.2174/138920112798868566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rezaee R., Hosseinzadeh H. Safranal: From an aromatic natural product to a rewarding pharmacological agent. Iran. J. Basic Med. Sci. 2013;16(1):12–26. [PMC free article] [PubMed] [Google Scholar]
- 57.Shati A.A., Elsaid F.G., Hafez E.E. Biochemical and molecular aspects of aluminium chloride-induced neurotoxicity in mice and the protective role of Crocus sativus L. extraction and honey syrup. Neuroscience. 2011;175:66–74. doi: 10.1016/j.neuroscience.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 58.Mousavi S.H., Tayarani N.Z., Parsaee H. Protective effect of saffron extract and crocin on reactive oxygen species-mediated high glucose-induced toxicity in PC12 cells. Cell. Mol. Neurobiol. 2010;30(2):185–191. doi: 10.1007/s10571-009-9441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feizzadeh B., Afshari J.T., Rakhshandeh H., et al. Cytotoxic effect of saffron stigma aqueous extract on human transitional cell carcinoma and mouse fibroblast. Urol. J. 2008;5(3):161–167. [PubMed] [Google Scholar]
- 60.Babaei A., Arshami J., Haghparast A., et al. Effects of saffron (Crocus sativus) petal ethanolic extract on hematology, antibody response, and spleen histology in rats. Avicenna J. Phytomed. 2014;4(2):103–109. [PMC free article] [PubMed] [Google Scholar]
- 61.Bostan H.B., Mehri S., Hosseinzadeh H. Toxicology effects of saffron and its constituents: A review. Iran. J. Basic Med. Sci. 2017;20(2):110–121. doi: 10.22038/ijbms.2017.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Milajerdi A., Djafarian K., Hosseini B. The toxicity of saffron (Crocus sativus L.) and its constituents against normal and cancer cells. J. Nutr. Intermed. Metab. 2016;3:23–32. [Google Scholar]
- 63.Hosseinzadeh H., Abootorabi A., Sadeghnia H.R. Protective effect of Crocus sativus stigma extract and crocin (trans-crocin 4) on methyl methanesulfonate-induced DNA damage in mice organs. DNA Cell Biol. 2008;27(12):657–664. doi: 10.1089/dna.2008.0767. [DOI] [PubMed] [Google Scholar]
- 64.Hosseinzadeh H., Sadeghi Shakib S., Khadem Sameni A., et al. Acute and subacute toxicity of safranal, a constituent of saffron, in mice and rats. Iran. J. Pharm. Res. 2013;12(1):93–99. [PMC free article] [PubMed] [Google Scholar]
- 65.Abdullaev F.I., Riveron-Negrete L., Caballero-Ortega H., et al. Use of in vitro assays to assess the potential antigenotoxic and cytotoxic effects of saffron (Crocus sativus L.). Toxicol. In Vitro. 2003;17(5-6):731–736. doi: 10.1016/s0887-2333(03)00098-5. [DOI] [PubMed] [Google Scholar]
- 66.Modaghegh M.H., Shahabian M., Esmaeili H.A., et al. Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytomedicine. 2008;15(12):1032–1037. doi: 10.1016/j.phymed.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 67.Mohamadpour A.H., Ayati Z., Parizadeh M.R., et al. Safety Evaluation of crocin (a constituent of saffron) tablets in healthy volunteers. Iran. J. Basic Med. Sci. 2013;16(1):39–46. [PMC free article] [PubMed] [Google Scholar]
- 68.Mousavi B., Bathaie S.Z., Fadai F., et al. Safety evaluation of saffron stigma (Crocus sativus L.) aqueous extract and crocin in patients with schizophrenia. Avicenna J. Phytomed. 2015;5(5):413–419. [PMC free article] [PubMed] [Google Scholar]
- 69.Finley J.W., Gao S. A Perspective on Crocus sativus L. (saffron) constituent crocin: A potent water-soluble antioxidant and potential therapy for Alzheimer’s disease. J. Agric. Food Chem. 2017;65(5):1005–1020. doi: 10.1021/acs.jafc.6b04398. [DOI] [PubMed] [Google Scholar]
- 70.Karimi E., Oskoueian E., Hendra R., et al. Evaluation of Crocus sativus L. stigma phenolic and flavonoid compounds and its antioxidant activity. Molecules. 2010;15(9):6244–6256. doi: 10.3390/molecules15096244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Amin A., Hamza A.A., Bajbouj K., et al. Saffron: A potential candidate for a novel anticancer drug against hepatocellular carcinoma. Hepatology. 2011;54(3):857–867. doi: 10.1002/hep.24433. [DOI] [PubMed] [Google Scholar]
- 72.Kanakis C.D., Tarantilis P.A., Pappas C., et al. An overview of structural features of DNA and RNA complexes with saffron compounds: Models and antioxidant activity. J. Photochem. Photobiol. B. 2009;95(3):204–212. doi: 10.1016/j.jphotobiol.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 73.Kanakis C.D., Tarantilis P.A., Tajmir-Riahi H.A., et al. Crocetin, dimethylcrocetin, and safranal bind human serum albumin: Stability and antioxidative properties. J. Agric. Food Chem. 2007;55(3):970–977. doi: 10.1021/jf062638l. [DOI] [PubMed] [Google Scholar]
- 74.Ordoudi S.A., Befani C.D., Nenadis N., et al. Further examination of antiradical properties of Crocus sativus stigmas extract rich in crocins. J. Agric. Food Chem. 2009;57(8):3080–3086. doi: 10.1021/jf804041g. [DOI] [PubMed] [Google Scholar]
- 75.Kalogeris T., Baines C.P., Krenz M., et al. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 2012;298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karaman A., Turkmen E., Gursul C., et al. Prevention of renal ischemia/reperfusion-induced injury in rats by leflunomide. Int. J. Urol. 2006;13(11):1434–1441. doi: 10.1111/j.1442-2042.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- 77.Yamashita J., Kita S., Iwamoto T., et al. Attenuation of ischemia/reperfusion-induced renal injury in mice deficient in Na+/Ca2+ exchanger. J. Pharmacol. Exp. Ther. 2003;304(1):284–293. doi: 10.1124/jpet.102.039024. [DOI] [PubMed] [Google Scholar]
- 78.Yarijani Z.M., Pourmotabbed A., Pourmotabbed T., et al. Crocin has anti-inflammatory and protective effects in ischemia-reperfusion induced renal injuries. Iran. J. Basic Med. Sci. 2017;20(7):753–759. doi: 10.22038/IJBMS.2017.9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mahmoudzadeh L., Najafi H., Ashtiyani S.C., et al. Anti-inflammatory and protective effects of saffron extract in ischaemia/reperfusion-induced acute kidney injury. Nephrology (Carlton) 2017;22(10):748–754. doi: 10.1111/nep.12849. [DOI] [PubMed] [Google Scholar]
- 80.Nader M., Chahine N., Salem C., et al. Saffron (Crocus sativus) pretreatment confers cardioprotection against ischemia-reperfusion injuries in isolated rabbit heart. J. Physiol. Biochem. 2016;72(4):711–719. doi: 10.1007/s13105-016-0510-8. [DOI] [PubMed] [Google Scholar]
- 81.Chahine N., Makhlouf H., Duca L., et al. Cardioprotective effect of saffron extracts against acute doxorubicin toxicity in isolated rabbit hearts submitted to ischemia-reperfusion injury. Z. Naturforsch. C. 2014;69(11-12):459–470. doi: 10.5560/znc.2014-0124. [DOI] [PubMed] [Google Scholar]
- 82.Joukar S., Ghasemipour-Afshar E., Sheibani M., et al. Protective effects of saffron (Crocus sativus) against lethal ventricular arrhythmias induced by heart reperfusion in rat: a potential anti-arrhythmic agent. Pharm. Biol. 2013;51(7):836–843. doi: 10.3109/13880209.2013.767362. [DOI] [PubMed] [Google Scholar]
- 83.Zeng C., Li H., Fan Z., et al. Crocin-elicited autophagy rescues myocardial ischemia/reperfusion injury via paradoxical mechanisms. Am. J. Chin. Med. 2016;44(3):515–530. doi: 10.1142/S0192415X16500282. [DOI] [PubMed] [Google Scholar]
- 84.Jahanbakhsh Z., Rasoulian B., Jafari M., et al. Protective effect of crocin against reperfusion-induced cardiac arrhythmias in anaesthetized rats. EXCLI J. 2012;11:20–29. [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Y., Sun J., Liu C., et al. Protective effects of crocetin pretreatment on myocardial injury in an ischemia/reperfusion rat model. Eur. J. Pharmacol. 2014;741:290–296. doi: 10.1016/j.ejphar.2014.07.052. [DOI] [PubMed] [Google Scholar]
- 86.Bharti S., Golechha M., Kumari S., et al. Akt/GSK-3beta/eNOS phosphorylation arbitrates safranal-induced myocardial protection against ischemia-reperfusion injury in rats. Eur. J. Nutr. 2012;51(6):719–727. doi: 10.1007/s00394-011-0251-y. [DOI] [PubMed] [Google Scholar]
- 87.Huang Z., Nan C., Wang H., et al. Crocetin ester improves myocardial ischemia via Rho/ROCK/NF-kB pathway. Int. Immunopharmacol. 2016;38:186–193. doi: 10.1016/j.intimp.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 88.Neumar R.W. Molecular mechanisms of ischemic neuronal injury. Ann. Emerg. Med. 2000;36(5):483–506. doi: 10.1067/mem.2000.110995. [DOI] [PubMed] [Google Scholar]
- 89.Taoufik E., Probert L. Ischemic neuronal damage. Curr. Pharm. Des. 2008;14(33):3565–3573. doi: 10.2174/138161208786848748. [DOI] [PubMed] [Google Scholar]
- 90.Saleem S., Ahmad M., Ahmad A.S., et al. Effect of Saffron (Crocus sativus) on neurobehavioral and neurochemical changes in cerebral ischemia in rats. J. Med. Food. 2006;9(2):246–253. doi: 10.1089/jmf.2006.9.246. [DOI] [PubMed] [Google Scholar]
- 91.Vakili A., Einali M.R., Bandegi A.R. Protective effect of crocin against cerebral ischemia in a dose-dependent manner in a rat model of ischemic stroke. J. Stroke Cerebrovasc. Dis. 2014;23(1):106–113. doi: 10.1016/j.jstrokecerebrovasdis.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 92.Zheng Y.Q., Liu J.X., Wang J.N., et al. Effects of crocin on reperfusion-induced oxidative/nitrative injury to cerebral microvessels after global cerebral ischemia. Brain Res. 2007;1138:86–94. doi: 10.1016/j.brainres.2006.12.064. [DOI] [PubMed] [Google Scholar]
- 93.Ochiai T., Shimeno H., Mishima K., et al. Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo. Biochim. Biophys. Acta. 2007;1770(4):578–584. doi: 10.1016/j.bbagen.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 94.Teresa Vanessa F., Annamaria P., Zuo P., Franco F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr. Pharm. Des. 2013;19(32):5695–5703. doi: 10.2174/1381612811319320005. [DOI] [PubMed] [Google Scholar]
- 95.Yu T., Robotham J.L., Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc. Natl. Acad. Sci. USA. 2006;103(8):2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schulze P.C., Yoshioka J., Takahashi T., et al. Hyperglycemia Promotes Oxidative Stress through Inhibition of Thioredoxin Function by Thioredoxin-interacting Protein. 2004. pp. 30369–30374. [DOI] [PubMed] [Google Scholar]
- 97.Fakhruddin S., Alanazi W., Jackson K.E. Diabetes-Induced Reactive Oxygen Species: Mechanism of Their Generation and Role in Renal Injury. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rolo A.P., Palmeira C.M. Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicol. Appl. Pharmacol. 2006;212(2):167–178. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 99.Xiang M., Yang M., Zhou C., et al. Crocetin prevents AGEs-induced vascular endothelial cell apoptosis. Pharmacol. Res. 2006;54(4):268–274. doi: 10.1016/j.phrs.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 100.Xiang M., Qian Z.Y., Zhou C.H., et al. Crocetin inhibits leukocyte adherence to vascular endothelial cells induced by AGEs. J. Ethnopharmacol. 2006;107(1):25–31. doi: 10.1016/j.jep.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 101.Meng L., Cui L. Inhibitory effects of crocetin on high glucose-induced apoptosis in cultured human umbilical vein endothelial cells and its mechanism. Arch. Pharm. Res. 2008;31(3):357–363. doi: 10.1007/s12272-001-1164-y. [DOI] [PubMed] [Google Scholar]
- 102.Xiang M., Yang R., Zhang Y., et al. Effect of crocetin on vascular smooth muscle cells migration induced by advanced glycosylation end products. Microvasc. Res. 2017;112:30–36. doi: 10.1016/j.mvr.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 103.He S.Y., Qian Z.Y., Tang F.T., et al. Effect of crocin on experimental atherosclerosis in quails and its mechanisms. Life Sci. 2005;77(8):907–921. doi: 10.1016/j.lfs.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 104.Asdaq S.M., Inamdar M.N. Potential of Crocus sativus (saffron) and its constituent, crocin, as hypolipidemic and antioxidant in rats. Appl. Biochem. Biotechnol. 2010;162(2):358–372. doi: 10.1007/s12010-009-8740-7. [DOI] [PubMed] [Google Scholar]
- 105.Lihn A.S., Pedersen S.B., Richelsen B. Adiponectin: Action, regulation and association to insulin sensitivity. Obes. Rev. 2005;6(1):13–21. doi: 10.1111/j.1467-789X.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 106.Whitehead J.P., Richards A.A., Hickman I.J., et al. Adiponectin--a key adipokine in the metabolic syndrome. Diabetes Obes. Metab. 2006;8(3):264–280. doi: 10.1111/j.1463-1326.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- 107.Hemmati M., Zohoori E., Mehrpour O., et al. Anti-atherogenic potential of jujube, saffron and barberry: Anti-diabetic and antioxidant actions. EXCLI J. 2015;14:908–915. doi: 10.17179/excli2015-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.He S.Y., Qian Z.Y., Wen N., et al. Influence of Crocetin on experimental atherosclerosis in hyperlipidamic-diet quails. Eur. J. Pharmacol. 2007;554(2-3):191–195. doi: 10.1016/j.ejphar.2006.09.071. [DOI] [PubMed] [Google Scholar]
- 109.Zheng S., Qian Z., Sheng L., et al. Crocetin attenuates atherosclerosis in hyperlipidemic rabbits through inhibition of LDL oxidation. J. Cardiovasc. Pharmacol. 2006;47(1):70–76. doi: 10.1097/01.fjc.0000194686.11712.02. [DOI] [PubMed] [Google Scholar]
- 110.Zheng S., Qian Z., Tang F., et al. Suppression of vascular cell adhesion molecule-1 expression by crocetin contributes to attenuation of atherosclerosis in hypercholesterolemic rabbits. Biochem. Pharmacol. 2005;70(8):1192–1199. doi: 10.1016/j.bcp.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 111.Tang F.T., Qian Z.Y., Liu P.Q., et al. Crocetin improves endothelium-dependent relaxation of thoracic aorta in hypercholesterolemic rabbit by increasing eNOS activity. Biochem. Pharmacol. 2006;72(5):558–565. doi: 10.1016/j.bcp.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 112.Zheng S., Qian Z., Wen N., et al. Crocetin suppresses angiotensin II-induced vascular smooth-muscle cell proliferation through inhibition of ERK1/2 activation and cell-cycle progression. J. Cardiovasc. Pharmacol. 2007;50(5):519–525. doi: 10.1097/FJC.0b013e31813c114e. [DOI] [PubMed] [Google Scholar]
- 113.Zhou C.H., Qian Z.Y., Zheng S.G., et al. ERK1/2 pathway is involved in the inhibitory effect of crocetin on angiotensin II-induced vascular smooth muscle cell proliferation. Eur. J. Pharmacol. 2006;535(1-3):61–68. doi: 10.1016/j.ejphar.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 114.Zhao Y., Liu J., Li L., et al. Role of Ras/PKCzeta/MEK/ERK1/2 signaling pathway in angiotensin II-induced vascular smooth muscle cell proliferation. Regul. Pept. 2005;128(1):43–50. doi: 10.1016/j.regpep.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 115.Meloche S., Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26(22):3227–3239. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- 116.Zhou C.H., Qian Z.Y., Xiang M., et al. Involvement of Ca2+ in the inhibition by crocetin of angiotensin II-induced ERK1/2 activation in vascular smooth muscle cells. Eur. J. Pharmacol. 2007;554(2-3):85–91. doi: 10.1016/j.ejphar.2006.09.069. [DOI] [PubMed] [Google Scholar]
- 117.He S.Y., Qian Z.Y., Tang F.T. Effect of crocin on intracellular calcium concentration in cultured bovine aortic smooth muscle cells. Yao Xue Xue Bao. 2004;39(10):778–781. [PubMed] [Google Scholar]
- 118.Xu G.L., Qian Z.Y., Yu S.Q., et al. Evidence of crocin against endothelial injury induced by hydrogen peroxide in vitro. J. Asian Nat. Prod. Res. 2006;8(1-2):79–85. doi: 10.1080/10286020500044732. [DOI] [PubMed] [Google Scholar]
- 119.Xu G.L., Gong Z.N., Yu W.P., et al. Increased expression ratio of Bcl-2/Bax is associated with crocin-mediated apoptosis in bovine aortic endothelial cells. Basic Clin. Pharmacol. Toxicol. 2007;100(1):31–35. doi: 10.1111/j.1742-7843.2007.00001.x. [DOI] [PubMed] [Google Scholar]
- 120.Jose Bagur M., Alonso Salinas G.L., Jimenez-Monreal A.M., et al. Saffron: An old medicinal plant and a potential novel functional food. Molecules. 2017;23(1):E30. doi: 10.3390/molecules23010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kermani T., Kazemi T., Molki S., et al. The efficacy of crocin of saffron (Crocus sativus l.) on the components of metabolic syndrome: A randomized controlled clinical trial. J. Res. Pharm. Pract. 2017;6(4):228–232. doi: 10.4103/jrpp.JRPP_17_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kermani T., Zebarjadi M., Mehrad-Majd H., et al. Anti-inflammatory effect of Crocus sativus on serum cytokine levels in subjects with metabolic syndrome: A randomized, double-blind, placebo- controlled trial. Curr. Clin. Pharmacol. 2017;12(2):122–126. doi: 10.2174/1574884712666170622082737. [DOI] [PubMed] [Google Scholar]
- 123.Joukar S., Dehesh M.M. The safety assessment of saffron (Crocus sativus L.) on sympathovagal balance and heart rate variability; a comparison with amiodarone. Auton. Autacoid Pharmacol. 2015;35(4):46–50. doi: 10.1111/aap.12040. [DOI] [PubMed] [Google Scholar]
- 124.Chahine N., Hanna J., Makhlouf H., et al. Protective effect of saffron extract against doxorubicin cardiotoxicity in isolated rabbit heart. Pharm. Biol. 2013;51(12):1564–1571. doi: 10.3109/13880209.2013.802812. [DOI] [PubMed] [Google Scholar]
- 125.Razmaraii N., Babaei H., Mohajjel Nayebi A., et al. Crocin treatment prevents doxorubicin-induced cardiotoxicity in rats. Life Sci. 2016;157:145–151. doi: 10.1016/j.lfs.2016.06.012. [DOI] [PubMed] [Google Scholar]