Abstract
Background:
The endothelium plays an important role in cardiovascular regulation, from blood flow to platelet aggregation, immune cell infiltration and demargination. A dysfunctional endo-thelium leads to the onset and progression of Cardiovascular Disease (CVD). The aging endothelium displays significant alterations in function, such as reduced vasomotor functions and reduced angio-genic capabilities. This could be partly due to elevated levels of oxidative stress and reduced endothe-lial cell turnover. Circulating angiogenic cells, such as Endothelial Progenitor Cells (EPCs) play a significant role in maintaining endothelial health and function, by supporting endothelial cell prolifera-tion, or via incorporation into the vasculature and differentiation into mature endothelial cells. Howev-er, these cells are reduced in number and function with age, which may contribute to the elevated CVD risk in this population. However, lifestyle factors, such as exercise, physical activity obesity, and dietary intake of omega-3 polyunsaturated fatty acids, nitrates, and antioxidants, significantly af-fect the number and function of these circulating angiogenic cells.
Conclusion:
This review will discuss the effects of advancing age on endothelial health and vascular regenerative capacity, as well as the influence of diet, exercise, and obesity on these cells, the mecha-nistic links and the subsequent impact on cardiovascular health
Keywords: Diet, obesity, endothelial regeneration, progenitor cells, angiogenesis, exercise
1. Introduction: The Aging Endothelium
The inner lining of all blood vessels consists of a single monolayer of endothelial cells. These cells play a key role in diffusion and transport of nutrients, gases from the blood to surrounding tissues, as well as being central to the control of blood flow via the endothelium’s ability to secrete vasoactive substances, such as Nitric Oxide (NO) and prostacyclin (PGI2). The endothelium also plays a role in our immune system, whereby it controls the adhesion, rolling and trans-endothelial migration of leukocytes to sites of tissue damage and/or infection. The maintenance of the endothelium is key for optimal health, and specifically cardiovascular health, as endothelial dysfunction often precedes Cardiovascular Disease (CVD).
Advancing age is associated with endothelial dysfunction [1-4], which is highly predictive of cardiovascular event risk and mortality [5, 6]. Aging is also associated with increased endothelial susceptibility to apoptosis [7]. These aging effects are potentially due to elevated levels of vascular tissue oxidative stress [8] which may contribute to uncoupling of endothelial NO Synthase (eNOS) [9], key for NO bioavailability via the conversion of L-arginine to NO. Elevated levels of pro-oxidant free radicals, such as superoxide, have been found in vascular tissue from aged compared to younger rats [8]. This elevated production of superoxide leads to the formation of peroxynitrite [10], which has been observed to stimulate the uncoupling of eNOS [9].
A recent meta-analysis also demonstrated that peripheral vascular resistance is also elevated in aging populations, with negative alterations in smooth muscle function in older compared with younger men and women [11]. Upon specific dilator administration, such as nitroglycerine or sodium nitroprusside, older adults display reduced dilator capacity, indicative of reduced smooth muscle function. This has been attributed to decreased expression of soluble guanylyl cyclase in smooth muscle cells [12], attenuating the cell’s ability to relax, subsequently leading to impaired vasodilation and peripheral blood flow. It is clear that the deleterious impact of aging on vascular resistance is due to, in part, alterations in endothelial NO release, as well as smooth muscle function, but there are also data to suggest that changes in vascular resistance due to age may be related to abnormal responses to the metaboreflex [13].
In addition, angiogenic capabilities are reduced with advancing age in both mice [14-17] and human studies [18], which may contribute to the increased CVD risk amongst the elderly [19, 20] due to insufficient repair or replacement of damaged endothelial cells. This is highlighted in animal models, with the ability to re-vascularisation in response to vascular trauma or occlusion is reduced with age [21, 22], suggestive of an impaired endothelial regenerative capacity.
2. Endothelial Regeneration and Advancing Age
It was previously thought that endothelial cell turnover was wholly maintained by the proliferation of vascular resident endothelial cells. However, in 1997, researchers discovered a circulating cell subset which had the ability to differentiate into mature endothelial cells in vitro [23], and these researchers termed these cells ‘endothelial progenitor cells’ (EPCs). These cells were human CD34+ cells, and after a period of 7 days in culture expressed mature endothelial cell markers (VEGFR2, CD31, E-selectin, eNOS). These cells could also form tubes on fibronectin-coated plates in vitro [23, 24]. A number of studies have shown that such EPCs have the ability to stimulate neovascularization in rodent [25, 26] and human models [21, 27]. However, the origin of these cells has been widely debated. Some studies show that these CD34+ vasculogenic progenitors are derived from the bone marrow using tracking models [25, 26], however, there is some evidence to suggest that progenitor cells within tumour vasculature did not derive from bone marrow [28].
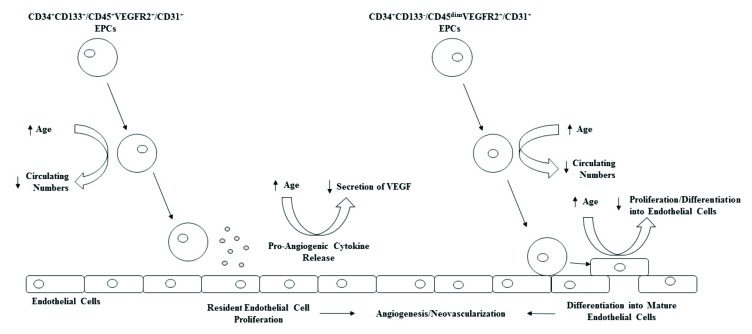
These cells may maintain endothelial integrity and health via differentiating into mature endothelial cells, therefore replacing damaged or apoptotic endothelial cells, or via paracrine means by secreting vasculogenic growth factors such as VEGF and IL-8 [29]. However, via cellular tracking, EPCs from humans transplanted into a mouse hindlimb ischemic model were found to stimulate neovascularisation and were later found incorporated in the injured vasculature [21], suggesting that the integrity of the endothelium may be partly dependent upon the reparative capacity of such EPCs [30]. It is now generally accepted that circulating EPCs that act in a paracrine manner, or as genuine endothelial precursors, are phenotypically distinct, with the former expressing CD34, CD133, being CD45bright as well as expressing an endothelial cell surface antigen, such as VEGFR2 or CD31 [29, 31]. Circulating CD34+ progenitors that have been shown to have potential to differentiate into mature endothelial cells express CD34, dimly express CD45 (CD45dim), lack CD133 expression whilst also expressing endothelial cell surface antigens [29, 31]. These two distinct phenotypes of EPCs have been termed ‘early’ and ‘late’ outgrowth endothelial cells because of the time of their appearance in culture. Early-outgrowth endothelial cells (EOC) appear early in culture, and function primarily via paracrine means, whereas late outgrowth endothelial cells (LOC) appear late in culture and have the ability to differentiate into endothelial cells in vitro [29]. Together, both EOC and LOC can be considered as contributing to the maintenance of endothelial cell integrity, just via differing means. For this review, EOC and LOC will be grouped together as ‘EPCs’. For a more in-depth review on EPC subsets and physiological functions, see review by Medina et al. [32].
Circulating EPCs are rare in peripheral blood, often making up to 0.05% of all mononuclear cells in humans [33], however, despite their small number, they remain, independent predictors of endothelial function [34, 35], and mortality in patient populations [36, 37], with lower numbers, often reflecting endothelial dysfunction and heightened cardiovascular mortality risk. Many studies have demonstrated lower circulating number and function of EPCs in vascular-related disease states (such as stroke, cerebrovascular disease, atherosclerosis) compared to age-matched healthy controls [30, 34, 35, 38-47]. The reduction in these cells in the circulation may be due to an exhaustion of the bone marrow progenitor cell pool due to an increased need for vascular repair [46], and increased apoptosis of these cells [43, 48].
Older adults display reduced number and function of circulating EPCs [21, 27, 49-54] which may play a role in the increased CV risk with advancing age [20]. Advancing age is linked with reduced vascular repair mechanisms, as observed by Torella et al. [22] who found that endothelial repair after balloon injury in a rat model was significantly reduced in older vs. younger rats. Our laboratory has shown that older adults display significantly reduced circulating angiogenic cells compared to younger counterparts, independent of several cardiometabolic risk factors (e.g. fasting glucose, triglycerides, LDL, HDL) [54]. Thijssen et al. [49] also observed reduced circulating CD34+VEGFR2+ EPCs in old (67-76 years) vs. younger men (19-28 years), but the reasons for these differences remain unclear.
EPC function appears to be affected also by advancing age. EPC migration, proliferation and tube forming capacity is reduced in older individuals [21, 27, 50-53, 55-57]. In an elegant study, Xia, Yang [21] took human EPCs from young and older adults, and investigated their re-endothelialization ability in a hindlimb ischemia model in mice, and found that transplanted EPCs from older adults did not stimulate endothelialization or recovery of perfusion to the same extent as transplanted EPCs from younger individuals. The underlying mechanisms explaining the age-related reduction in both EPC number and function are still unclear. It is highly likely that a combination of age-related increases in oxidative stress [58], bone marrow niche alterations [59], telomere shortening [56] and other circulating factors [60] may explain these observations.
Together, this data strongly suggests a deleterious effect of aging on EPC number and function (Table 1 and Fig. (1) for a summary of the effect of age on EPC number and function), and studies have investigated the effect of pharmacological interventions to improve EPC number and function in at-risk individuals [61-64]. However, as a preventative measure, lifestyle modifications may hold significant promise as these cells are significantly affected by lifestyle factors such as smoking [65, 66], physical activity/inactivity, and exercise [67-69].
Table 1.
Influence of age on circulating endothelial progenitor cell number and function.
| References | Subjects | EPC Assay | Findings |
|---|---|---|---|
| Xia et al., 2012a [21] | 10 young, 10 older males. | Flow cytometry CD34+VEGFR2+ EPC migration and adhesion Human EPC re-endothelialization in mice |
Lower CD34+VEGFR2+ cells in elderly. Reduced migration, adhesion and re-endothelialization capacity in elderly vs. young males. |
| Xia et al., 2012b [27] | 25 young, 22 elderly males. Resting | Flow cytometry CD34+VEGFR2+/CD133+VEGFR2+ EPC migration and adhesion Human EPC re-endothelialization in mice |
Lower CD34+VEGFR2+/ CD133+VEGFR2+ cells in elderly. Reduced migration, adhesion and re-endothelialization capacity in elderly vs. young males. |
| Thijssen et al., 2006 [49] | 8 young, 8 older sedentary males. | Flow cytometry CD34+VEGFR2+ |
Lower CD34+VEGFR2+ EPCs in older vs. younger males |
| Thum et al., 2007 [50] | 10 young, 16 middle-aged, 12 older males. | Flow cytometry CD133+VEGFR2+ EPC migration and eNOS gene expression. |
Lower EPC number and migration in older vs. middle-aged and younger males. Lower EPC eNOS gene expression in older vs. younger adults. |
| Heiss et al., 2005 [51] | 20 young and 20 older male and female subjects. | Flow cytometry CD34+VEGFR2+/CD133+VEGFR2+ EPC survival, migration and proliferation assays. |
No difference in EPC number between young and older subjects. Lower survival, migration and proliferation of EPCs in older subjects. |
| Hoetzer et al., 2007 [52] | 10 young, 15 middle-aged, 21 older men. | EPC EC-CFU assay. EPC migration |
Lower EC-CFU in older and middle-aged adults compared to young subjects. Lower migration of EPCs from older subjects vs. middle-aged and younger adults. |
| Williamson et al. 2013 [53] | EPCs from 5 young, and 4 older subjects. | EPC apoptosis, migration, and tube formation assays | No difference in proliferation, apoptosis and tube formation of EPCs from young and older subjects. EPC migration lower in older subjects vs. younger subjects. |
| Ross et al., 2018 [54] | 107 males, aged 18-75yrs. | Flow cytometry CD34+CD45dimVEGFR2+ Cell surface expression of CXCR4 |
Age inversely associated with EPC number and cell surface CXCR4 expression. |
| Yang et al., 2013 [55] | 10 young, 10 older male subjects. | Flow cytometry: CD34+VEGFR2+ EPC migration and proliferative assays. |
Lower EPC number, migration and proliferation in older vs. younger subjects. |
| Kushner et al., 2009 [56] | 12 young, 12 middle-aged, and 16 older sedentary males. | EPC telomere length | Lower EPC telomere length in older vs. middle-aged and younger males. |
| Kushner et al., 2010 [57] | 17 young and 20 older males. | Stimulated release of EPC-derived pro-angiogenic cytokines and growth factors | Lower release of G-CSF from EPCs from older vs. younger subjects. |
EPC- Endothelial Progenitor Cells, eNOS- endothelial nitric oxide synthase, EC-CFU- Endothelial Cell Colony-Forming Units. CXCR4- C-X-C Chemokine Receptor 4.
Fig. (1).
Effect of aging on circulating endothelial progenitor cell number and vasculogenic function.
In this review we will cover the influence of various dietary factors on EPC number and function, and the potential negative impact obesity has on EPCs, finally reviewing the literature on dietary strategies to induce weight loss, and the subsequent impact this may have on circulating EPCs to promote cardiovascular health in an at-risk, aging population.
3. Nitric Oxide-Mediated Mobilization of EPCs: Potential for Dietary Nitrate
Recently, the therapeutic role of dietary nitrate (in the form of beetroot [as a root vegetable or in concentrated form], watercress, and spinach) in vascular health has been explored due to the potential to modulate NO bioavailability. Acute and chronic supplementation of inorganic dietary nitrate has been shown to improve arterial vasomotor function [70-72], reduce blood pressure in healthy young [73] and older subjects [72] and reduce arterial stiffness as measured via pulse wave velocity [72]. The potential mechanisms by which dietary nitrate may improve these vascular health markers include an increase in NO bioavailability. Once ingested, nitrate is reduced to nitrite in the mouth and gut [74], where it can be absorbed into the circulation. Elevations of plasma nitrate and nitrite are observed as quickly as within 2 hours of ingestion of a high concentrated nitrate dose (in the form of beetroot juice) which subsequently results in significant alterations in both systolic and diastolic blood pressure within this timeframe [75, 76].
Recent epidemiological evidence suggests that nitrate has a strong positive effect on human health. In 2017, researchers found that plasma nitrate was inversely associated with all-cause mortality in the Offspring cohort of the Framingham Heart Study [77]. Interestingly, there was no such association with incidence CVD mortality, with their data also suggesting that the effect of plasma nitrate on mortality was attenuated after controlling for glomerular filtration rate, suggestive of a protective effect on renal function. In mice, 3 months of nitrate deficient diet resulted in greater visceral adiposity, reduced glycaemic control and vascular function [78]. Levels of eNOS were downregulated in the mice fed with the nitrate-depleted diet which may contribute to the reduced vascular function in these mice.
In addition to having an impact on endothelial function via modulating NO bioavailability, dietary nitrate may also impact on circulating angiogenic cells. The mobilization of EPCs has been shown to be eNOS dependent [79], and additionally, NO itself can mobilise these cells via activation of bone marrow matrix metalloproteinase-9 [80] which itself cleaves membrane-bound Kit ligand from bone marrow stromal cells, leading to the extravasation of progenitor cells into the circulation [81]. This led researchers to investigate if dietary nitrate can influence progenitor cell number and function. Indeed, the ingestion of a single dose of nitrate-rich solution led to the mobilisation of CD34+VEGFR2+ and CD133+VEGFR2+ cells into the circulation within 1 hour in healthy humans, which was accompanied by increases in Stem Cell Factor (SCF) and stromal-derived factor-1α (SDF-1α) [82]. Within the same study, this effect was abolished with the co-infusion of a NO scavenger (cPTIO) in a mouse model. A chronic supplementation study in hypercholesterolemic rabbits found that supplementing with L-arginine, the precursor to NO synthesis led to a significantly greater number of circulating CD34+VEGFR2+ EPCs than control hypercholesterolemic rabbits [83]. This data has been supported elsewhere, with a diet supplemented with L-arginine, in combination with exercise training, resulted in elevations in EPCs in mice, compared to exercise alone which also resulted in increases in circulating EPCs [84].
Together, these data suggest a potential role for nitrate diets to potentially mobilize EPCs from the bone marrow to maintain or improve vascular health. However, there is a paucity of data in humans, and in clinical conditions whereby such an intervention may have greater health implications. Future research must also include data on the functionality of such EPC populations to determine potential cellular effects outside of the bone marrow mobilisation itself.
4. Omega-3
Omega-3 polyunsaturated fatty acids (PUFA) have recently emerged as potential vascular protective foods. Omega-3 fatty acids, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are primarily found in oily fish, but also found in plant sources, such as nuts and seeds. Epidemiological data suggested that the ingestion of omega-3 fatty acids may reduce CVD rates [85]. This study observed a 10x reduced risk of myocardial infarction amongst Greenland Inuits compared to a Danish population, which may be due to their vastly different intake of omega-3 fatty acids per day (14g vs. 3g) [86]. However, clinical trial data of the impact of omega-3 fatty acids on cardiovascular and all-cause mortality are mixed with regards to the efficacy of these fatty acids on health [87-90].
Omega-3 fatty acids may influence health through affecting plasma membrane phospholipid composition, which may impact cell signalling via altering membrane fluidity, lipid raft structure and substrate availability [91, 92]. DHA upregulates eNOS phosphorylation in human endothelial cells in vitro [93] and suppresses cytokine-induced endothelial adhesion molecule expression [94], suggestive of a potent vascular benefit. There is also evidence that both EPA and DHA can attenuate H2O2- induced DNA damage in human aortic endothelial cells via reductions in intracellular oxidative stress as a result of upregulated levels of heme oxygenase-1, thioredoxin reductase 1, and manganese superoxide dismutase [95]. However, the evidence for a strong effect on vascular endothelial function is absent in human studies [96].
Interestingly, omega-3 fatty acids may play a role in angiogenesis. Recent studies showed that in aged mice, a diet rich in omega-3 PUFA was associated with improved post-ischemic stroke angiogenesis and neurogenesis [97], and transgenic mice that overproduce n-3 PUFAs were protected against ischemic stroke, displayed enhanced post-ischemic angiogenesis and greater survival than control mice [98]. Potential contributions to the augmented revascularization may be due to enhanced VEGF signalling in resident endothelial cells [98] or via mobilization of angiogenic cells to the infarct zone and manipulation of their angiogenic functions. In an in vitro study, incubation with EPA or DHA significantly improved EPC colony forming units and tube formation of these regenerative cells in vitro [99]. However, migratory capacity of these cells, reflective if the ability to migrate to ischemic tissue in vivo, only improved upon co-incubation with both EPA+DHA [99]. These results were somewhat supported by Tikhonenko et al. [100] who found that supplementing with DHA in a type 2 diabetes mouse model rescues EPCs in blood and bone marrow, as well as displaying protective effect of DHA on EPC migration in vitro further suggestive of protective effect of omega-3 fatty acids on EPC number and angiogenic function [100, 101]. In only two human supplementation studies, an eight- and six- week fish oil supplementation period significantly increased the number of circulating CD34+VEGFR2+ cells [102, 103], and also significantly reduced markers of vascular damage and platelet aggregation [103]. These changes in EPCs were not accompanied by changes in circulating biochemical markers of vascular health, such as total cholesterol, LDL, HDL, triglycerides or fasting glucose [103], suggestive of a direct effect on cellular survival [104] and/or mobilization. These effects of omega-3 fatty acids on EPCs are not long-lasting, as six weeks after cessation of the omega-3 fatty acid-rich diet, circulating EPCs returned to pre-diet levels [102].
These data strongly suggest despite not having clear benefits on vascular function in humans, omega-3-rich diets may augment the number and function of circulating EPCs which may have clinical significance for endothelial repair and may be of interest to older adults who display such EPC dysfunction.
5. Mediterranean Diets
Mediterranean diets typically contain high levels of olive oil, fruits, nuts, vegetables and cereals, and often include moderate intake of fish and poultry, with low intake of red and processed meats. There is strong evidence that supports the use of a diet rich in olive oil, fruit, nuts, vegetables and low in red meats for the prevention of CV events and CVD [105, 106], with a meta-analysis indicating a reduced risk ratio for CV incidence or mortality, cancer incidence or mortality, and neurodegenerative disease incidence with for those adhering to such a diet [106]. The proposed mechanism for such effect on cardiovascular health may be due to specific effects on reducing atherosclerosis-associated inflammation [107], such as circulating high sensitivity C-reactive protein (CRP) and interleukin-6 (IL-6) [107, 108]. After 2 years of a Mediterranean diet, an improvement in endothelial function was observed, as well as a reduction in carotid intima-media thickness (cIMT) [107] and insulin sensitivity also improved significantly [107, 108].
One year of a ‘Mediterranean’ diet, rich in olive oil, fruit, vegetables, fish, legumes, and wholegrain foods improved vascular conductance in a group of older adults (mean age: 56 years) more so than a year-long exercise training intervention [109], indicating that the diet could be a beneficial strategy for preventing CV issues with aging, once again, potentially due to reductions in inflammatory biomarkers or improvement in antioxidant status [110, 111]. Considering also, the high omega-3 content of such a diet, potential vascular health benefits of a Mediterranean diet may be also due to the reductions in oxidative stress via the biological effects of EPA and DHA.
Several studies have investigated the impact of these types of diet on circulating EPCs in a variety of human populations (metabolic syndrome, type 2 diabetics, and the elderly), showing significant promise in modulating endothelial repair capacity. In those with type 2 diabetes mellitus, 4 years of the diet resulted in a significant increase in CD34+VEGFR2+ and CD34+CD133+VEGFR2+ EPCs at both year 2 and year 4 time-points [108]. There was an absence of any change in these markers of endothelial repair capacity in a parallel low-fat diet. The elevations in EPCs were concomitantly observed alongside reductions in inflammatory biomarkers CRP, and reductions in cIMT. Interestingly, the increases in EPCs were inversely associated with cIMT in the Mediterranean diet group [108]. After only 8 weeks, such a diet resulted in significant increases in CD34+VEGFR2+ EPCs in individuals with the metabolic syndrome, however, this increase was superseded by a combination of diet plus exercise intervention over the same duration [112].
In an aging population of both men and women (>65yrs), a 4 week dietary intervention resulted in >100% increases in circulating CD34+CD133+VEGFR2+ EPCs in participants undertaking a diet rich in olive oil, vegetables, and fish, as opposed to a low carbohydrate diet enriched with PUFA, and a significant reduction in endothelial microvesicles (indicative of endothelial damage and/or activation) [113]. Once again, these changes were irrespective of cardiometabolic risk factor changes. Cesari et al. [114] found that circulating number of EPCs (CD34+VEGFR2+/CD34+CD133+ VEGFR2+) were related to olive oil consumption, dietary vegetable servings and ‘Mediterranean diet score’ (a score of adherence to a Mediterranean diet devised by Panagiotakos et al. [115]) in a large population of nonagenarians. However, longer duration interventions in this aging population, as well as a functional assessment of endothelialization are lacking and thus are required to fully elucidate the impact of such diet on endothelial regeneration and repair. It must also be acknowledged that it is difficult to attribute the improvements in these vascular reparative cells to a certain aspect of the diet due to the wide variety of components of the diet.
6. Physical Activity and Exercise Effects on Endothelial Progenitor Cells
Exercise and physical activity have potent cardiovascular effects. These include the prevention or reversal of plaque formation in the vasculature [116, 117], improved endothelial function [118-121], and angiogenesis [122-124] in a variety of human populations. Single bouts of exercise have the remarkable ability to stimulate the mobilization of EPCs from peripheral tissues such as the bone marrow, into the circulation for up to 72 hours post-exercise [54, 68, 125-129]. However, some studies have failed to show any changes in circulating EPCs in the post-exercise recovery period [49, 130]. The response to exercise is not only duration and intensity-dependent [127], but also dependent on human population investigated, with the evidence showing those with CVD [131-133], and older adults [54] display an attenuated response. EPC mobilization in response to exercise is said to be due to changes in circulating chemoattractants, such as VEGF, G-CSF and SDF-1α [68, 129, 134], however, mechanistic studies in exercise and EPC mobilization are lacking.
It is not just single bouts of exercise which may have this profound effect on EPCs, but studies investigating the effect of regular exercise and physical activity on these cells generally report increase in EPC number and/or function [27, 52, 69, 122, 135-139], even in older adults [27, 52]. After a 3-month home-based aerobic exercise intervention, older men, who had displayed significantly reduced basal EPC number and migratory function, improved their EPC number and function nearly 2-fold [52]. Xia et al. [27] reported improvements in both in vitro and in vivo function of EPCs from older adults who had undergone a 12-week aerobic exercise program using a carotid artery injury mouse model. The researchers took EPCs from older adults before and after the exercise program and injected these into the left carotid of athymic nude mice after inducing carotid injury. Endothelial regeneration was evaluated by measuring the area of re-endothelialization in the denuded artery 3 days post-injection. The improvement observed in re-endothelialization due to EPCs from older individuals post-training was accompanied by improvements in intracellular CXCR4 signalling, which is key for EPC homing to sites of injury [41].
It is clear that single bouts and regular prolonged exercise can improve circulating number and function of these vasculogenic cells in humans. This improvement has been aligned with improvements in vascular function, and reduced arterial stiffness, offering a key mechanism by which exercise may benefit cardiovascular health in older populations. The potential effects of exercise and physical activity on EPCs in aging have been reviewed in depth elsewhere [67].
7. Obesity
Obesity is heavily linked with the development of key variables of the metabolic syndrome and type 2 diabetes mellitus (T2DM). The worldwide incidence of CVD and metabolic abnormalities, such as T2DM is increasing, and obesity is a significant risk factor. Data suggests that those who are overweight or obese are 50-75% more likely to develop CVD than those who are ‘normal weight’ [140]. This is likely to be driven by inflammatory pathways, including adipose tissue-derived tumour necrosis factor-α (TNF-α) [141], which may affect endothelial function specifically via activation of NADPH oxidase and subsequent production of superoxide [142]. Endothelial dysfunction with obesity precedes the development of atherosclerosis, with impaired vasodilator functions apparent [143], potentially as a direct result of impairments in the L-arginine-NO pathway. Therefore, obesity-induced endothelial dysfunction may be a primary cause of the increased CVD risk in this population.
Obesity may promote endothelial dysfunction via effects on endothelial regeneration and repair mechanisms, such as bone marrow-derived EPCs. Fadini et al. [144] observed a negative association between components of the metabolic syndrome, and CD34+ progenitor cell count, with accumulative scores of the metabolic syndrome strengthening this inverse relationship. Several studies report an inverse relationship between BMI and circulating total progenitor cells and EPC count [144, 145]. Furthermore, other studies have reported that obese men with metabolic syndrome had 40% fewer circulating EPCs than healthy age-matched controls [146]. Interestingly, EPC proliferative capacity reflected reductions in circulating EPCs in obese compared to lean individuals [147]. The same group showed that the in vitro pro-angiogenic function of EPCs was also impaired with obesity in 50+year-old individuals, with impaired stimulated the release of both VEGF and G-CSF, which may be linked to the finding that these EPCs displayed higher expression of caspase-3, a pro-apoptotic intracellular signal [148]. In a murine model of obesity, obese animals displayed impaired in vitro angiogenesis, suppressed EPC mobilization in response to limb ischemia, and reduced incorporation into aortic vessels after LPS-induced vascular damage [149], confirmed by other animal models also showing impaired recovery of blood flow after limb ischemia accompanying the reductions in ischemia-induced PC mobilization [150]. In humans, EPC adhesion, migration and angiogenesis in vitro were significantly lower than in lean individuals [151]. The ability of EPCs to home to sites of ischemia, adhere and migrate are key roles of EPCs in order for these cells to exert their vasculogenic function. These findings suggest that obesity suppresses the angiogenic potential of human EPCs to home to sites of vascular damage or tissue ischemia, and to promote blood vessel growth and repair.
There is clear evidence for obesity-mediated EPC dysfunction, which may be as a result of associated inflammation, impaired glucose tolerance and elevated oxidative stress. The resultant endothelial dysfunction and suppressed endothelial repair capacity increase the risk of atherosclerosis in this population. Interventions designed to stimulate weight loss may have significant health benefits by improving vascular endothelial health via modulating EPC number and functional capacity.
8. Calorie Restriction/Weight Loss Dietary Interventions to Combat Obesity-Mediated EPC Dysfunction
Recently, calorie restriction diets have been touted as a potential intervention to improve health and enhance longevity [152]. Recent reports suggest that calorie restriction may reduce CVD risk by modulating oxidative stress levels [153], and DNA damage [154]. Such diets have been proven to be beneficial for weight loss in overweight and obese individuals [155, 156] due to the stark effects on reducing oxidative stress [157] and improving the metabolic profile of obese and older humans [156, 158-160].
A 24-week low carbohydrate diet resulted in significant reductions in endothelial damage biomarkers in overweight post-menopausal women despite no changes in metabolic profiles [161], suggesting vascular benefit effect of such a diet is independent of metabolic changes. Added to this, there is a wealth of evidence showing vascular function benefits of calorie restriction/weight loss diets in obese and older individuals [162-168]. Mechanisms include reductions in NADPH oxidase activity, increased activation of sirtuin-1, a powerful intracellular antioxidant complex [169], increased antioxidant capacity (increased levels of manganese superoxide dismutase) and increasing tissue eNOS content and NO bioavailability [170]. Furthermore, improvements in vascular function with weight loss strategies may be preceded by improvements in endothelial regenerative capacity.
Indeed, preliminary data showed that weight loss strategies may be beneficial for improving EPC number [171]. The extent of reductions in body fat composition in response to a weight loss diet relates to the extent of EPC improvement in humans [172]. Xin et al. [173] exposed mice to prolonged fasting after cerebral ischemia. They observed significant upregulation of the antioxidant enzyme MnSOD, as well as eNOS in bone marrow-derived EPCs, increased capillary number in the infarct zone, and improved EPC migratory and tube formation capacity in the fasted mice compared to control mice. These observations were accompanied by reductions in the volume of infarct zone, which was also further improved by intravenous administration of EPCs from fasted mice compared to control mice [173], strongly suggesting a protective role of periodic fasting to improve EPC vascular regenerative capacity. Interestingly, exercise and diet may act synergistically to promote EPC number and function in obese populations [174]. An 8-week combined exercise and calorie restricted diet resulted in significant improvements in circulating EPCs, and EPC migratory capacity in obese populations [174]. However, the effect of combined strategies in older adults is yet to be investigated but may hold promise due to the already significant impact of exercise on EPC number and function [49, 54, 128].
9. Future Directions
Currently, large-scale cohort interventional studies in dietary influence on vascular regenerative capacity are lacking, especially in aging adults with or without CVD, and are thus warranted. In addition, other angiogenic cell populations, such as angiogenic T-cells [175, 176] and mesenchymal stem/progenitor cells [177] are being investigated for their influence on endothelial function and repair through their potent pro-angiogenic capacity and may be targeted for such therapeutic interventions, such as diet and/or exercise.
Additionally, the role of physical activity and exercise for cardiovascular benefit is clear, however, more studies are required to elucidate the benefit for older, and frail populations who are specifically at-risk of CVD and vascular-related disorders.
Summary & Conclusion
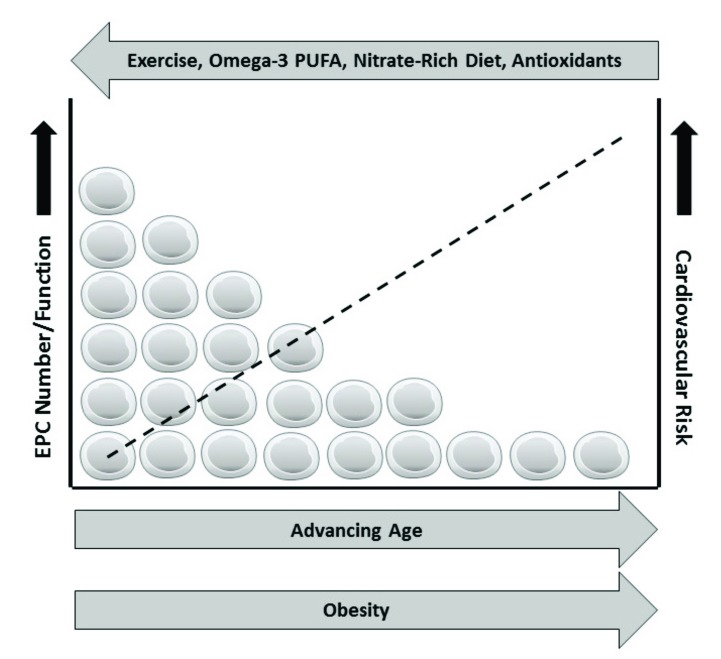
Age-related increased CVD risk is due to a plethora of factors. Reductions in endothelial repair capacity via alterations in both EPC number and functions may explain the aging impairments in endothelial function, thus promoting atherosclerotic disease risk. However, lifestyle factors such as diet, exercise and obesity (Fig. 2) can have a significant impact on these vascular regenerative cells, and thus older populations may be able to attenuate CVD risk through lifestyle modifications.
Fig. (2).
Possible effects of lifestyle factors on aging circulating endothelial progenitors and cardiovascular risk.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Black M.A., Cable N.T., Thijssen D.H., Green D.J. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am. J. Physiol. Heart Circ. Physiol. 2009;297(3):H1109–H1116. doi: 10.1152/ajpheart.00226.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taddei S., Virdis A., Ghiadoni L., et al. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38(2):274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 3.Muller-Delp J.M. Aging-induced adaptations of microvascular reactivity. Microcirculation. 2006;13(4):301–314. doi: 10.1080/10739680600619023. [DOI] [PubMed] [Google Scholar]
- 4.Soucy K.G., Ryoo S., Benjo A., et al. Impaired shear stress-induced nitric oxide production through decreased NOS phosphorylation contributes to age-related vascular stiffness. J. Appl. Physiol. 2006;101(6):1751–1759. doi: 10.1152/japplphysiol.00138.2006. [DOI] [PubMed] [Google Scholar]
- 5.Green D.J., Jones H., Thijssen D., Cable N.T., Atkinson G. Flow-mediated dilation and cardiovascular event prediction. Hypertension. 2011;57(3):363–369. doi: 10.1161/HYPERTENSIONAHA.110.167015. [DOI] [PubMed] [Google Scholar]
- 6.Shechter M., Matetzky S., Arad M., Feinberg M.S., Freimark D. Vascular endothelial function predicts mortality risk in patients with advanced ischaemic chronic heart failure. Eur. J. Heart Fail. 2009;11(6):588–593. doi: 10.1093/eurjhf/hfp053. [DOI] [PubMed] [Google Scholar]
- 7.Wang H., Listrat A., Meunier B., et al. Apoptosis in capillary endothelial cells in ageing skeletal muscle. Aging Cell. 2013;13(2):254–262. doi: 10.1111/acel.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilton C.A., Brosnan M.J., McIntyre M., Graham D., Dominiczak A.F. Superoxide excess in hypertension and aging: A common cause of endothelial dysfunction. Hypertension. 2001;37(2):529–534. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- 9.Csiszar A., Ungvari Z., Edwards J.G., et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ. Res. 2002;90(11):1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 10.van der Loo B., Labugger R., Skepper J.N., et al. Enhanced peroxynitrite formation is associated with vascular aging. J. Exp. Med. 2000;192(12):1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montero D., Pierce G.L., Stehouwer C.D.A., Padilla J., Thijssen D.H.J. The impact of age on vascular smooth muscle function in humans. J. Hypertens. 2015;33(3):445–453. doi: 10.1097/HJH.0000000000000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L., Daum G., Fischer J.W., et al. Loss of expression of the β subunit of soluble guanylyl cyclase prevents nitric oxide–mediated inhibition of DNA synthesis in smooth muscle cells of old rats. Circ. Res. 2000;86(5):520–525. doi: 10.1161/01.res.86.5.520. [DOI] [PubMed] [Google Scholar]
- 13.Milia R., Roberto S., Mulliri G., et al. Effect of aging on hemodynamic response to metaboreflex activation. Eur. J. Appl. Physiol. 2015;115(8):1693–1703. doi: 10.1007/s00421-015-3153-5. [DOI] [PubMed] [Google Scholar]
- 14.Rivard A., Fabre J-E., Silver M., et al. Age-dependent impairment of angiogenesis. Circulation. 1999;99(1):111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 15.Sadoun E., Reed M.J. Impaired angiogenesis in aging is associated with alterations in vessel density, matrix composition, inflammatory response, and growth factor expression. J. Histochem. Cytochem. 2003;51(9):1119–1130. doi: 10.1177/002215540305100902. [DOI] [PubMed] [Google Scholar]
- 16.Edelberg J.M., Tang L., Hattori K., Lyden D., Rafii S. Young adult bone marrow–derived endothelial precursor cells restore aging-impaired cardiac angiogenic function. Circ. Res. 2002;90(10):e89–e93. doi: 10.1161/01.res.0000020861.20064.7e. [DOI] [PubMed] [Google Scholar]
- 17.Wang J., Peng X., Lassance-Soares R., et al. Aging-induced collateral dysfunction: Impaired responsiveness of collaterals and susceptibility to apoptosis via dysfunctional eNOS signaling. J. Cardiovasc. Transl. Res. 2011;4(6):779–789. doi: 10.1007/s12265-011-9280-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunin A.G., Petrov V.V., Golubtzova N.N., Vasilieva O.V., Kornilova N.K. Age-related changes in angiogenesis in human dermis. Exp. Gerontol. 2014;55(1):143–151. doi: 10.1016/j.exger.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Lozano R., Naghavi M., Foreman K., et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kannel W.B., Gordan T. Evaluation of cardiovascular risk in the elderly: The Framingham study. Bull. N. Y. Acad. Med. 1978;54(6):573–591. [PMC free article] [PubMed] [Google Scholar]
- 21.Xia W.H., Yang Z., Xu S.Y., et al. Age-related decline in reendothelialization capacity of human endothelial progenitor cells is restored by shear stress. Hypertension. 2012;59(6):1225–1231. doi: 10.1161/HYPERTENSIONAHA.111.179820. [DOI] [PubMed] [Google Scholar]
- 22.Torella D., Leosco D., Indolfi C., et al. Aging exacerbates negative remodeling and impairs endothelial regeneration after balloon injury. Am. J. Physiol. Heart Circ. Physiol. 2004;287(6):H2850–H60. doi: 10.1152/ajpheart.01119.2003. [DOI] [PubMed] [Google Scholar]
- 23.Asahara T., Murohara T., Sullivan A., et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–966. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 24.Tasev D., Konijnenberg L.S., Amado-Azevedo J., van Wijhe M.H., Koolwijk P., van Hinsbergh V.W. CD34 expression modulates tube-forming capacity and barrier properties of peripheral blood-derived endothelial colony-forming cells (ECFCs). Angiogenesis. 2016;19(3):325–338. doi: 10.1007/s10456-016-9506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asahara T., Masuda H., Takahashi T., et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovasculari-zation. Circ. Res. 1999;85(3):221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 26.Reyes M., Dudek A., Jahagirdar B., Koodie L., Marker P.H., Verfaillie C.M. Origin of endothelial progenitors in human postnatal bone marrow. J. Clin. Invest. 2002;109(3):337–346. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia W.H., Li J., Su C., et al. Physical exercise attenuates age-associated reduction in endothelium-reparative capacity of endothelial progenitor cells by increasing CXCR4/JAK-2 signaling in healthy men. Aging Cell. 2012;11(1):111–119. doi: 10.1111/j.1474-9726.2011.00758.x. [DOI] [PubMed] [Google Scholar]
- 28.Purhonen S., Palm J., Rossi D., et al. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc. Natl. Acad. Sci. USA. 2008;105(18):6620–6625. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hur J., Yoon C.H., Kim H.S., et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler. Thromb. Vasc. Biol. 2004;24(2):288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 30.Hill J.M., Zalos G., Halcox J.P.J., et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 2003;348(7):593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 31.Case J., Mead L.E., Bessler W.K., et al. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp. Hematol. 2007;35(7):1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Medina R.J., Barber C.L., Sabatier F., et al. Endothelial progenitors: A consensus statement on nomenclature. Stem Cells Transl. Med. 2017;6(5):1316–1320. doi: 10.1002/sctm.16-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel R.S., Li Q., Ghasemzadeh N., et al. Circulating CD34+ progenitor cells and risk of mortality in a population with coronary artery disease. Circ. Res. 2015;116(2):289–297. doi: 10.1161/CIRCRESAHA.116.304187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruyndonckx L., Hoymans V.Y., Frederix G., et al. Endothelial progenitor cells and endothelial microparticles are independent predictors of endothelial function. J. Pediatr. 2014;165(2):300–305. doi: 10.1016/j.jpeds.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 35.Sibal L., Aldibbiat A., Agarwal S., et al. Circulating endothelial progenitor cells, endothelial function, carotid intima–media thickness and circulating markers of endothelial dysfunction in people with type 1 diabetes without macrovascular disease or microalbuminuria. Diabetologia. 2009;52(8):1464–1473. doi: 10.1007/s00125-009-1401-0. [DOI] [PubMed] [Google Scholar]
- 36.Samman Tahhan A., Hammadah M., Sandesara P.B., et al. Progenitor cells and clinical outcomes in patients with heart failure. Circ Heart Fail. 2017;10(8):e004106. doi: 10.1161/CIRCHEARTFAILURE.117.004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu C.L., Leu J.G., Liu W.C., et al. Endothelial progenitor cells predict long-term mortality in hemodialysis patients. Int. J. Med. Sci. 2016;13(3):240–247. doi: 10.7150/ijms.14209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fadini G.P., Miorin M., Facco M., et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. JAMA. 2005;45(9):1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 39.Fadini G.P., Coracina A., Baesso I., et al. Peripheral blood CD34+KDR+ endothelial progenitor cells are determinants of subclinical atherosclerosis in a middle-aged general population. Stroke. 2006;37(9):2277–2282. doi: 10.1161/01.STR.0000236064.19293.79. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt-Lucke C., Rossig L., Fichtlscherer S., et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111(22):2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 41.Walter D.H., Haendeler J., Reinhold J., et al. Impaired CXCR4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ. Res. 2005;97(11):1142–1151. doi: 10.1161/01.RES.0000193596.94936.2c. [DOI] [PubMed] [Google Scholar]
- 42.Xiao Q., Kiechl S., Patel S., et al. Endothelial progenitor cells, cardiovascular risk factors, cytokine levels and atherosclerosis – results from a large population-based study. PLoS One. 2007;2(10):e975. doi: 10.1371/journal.pone.0000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jung C., Rafnsson A., Shemyakin A., Böhm F., Pernow J. Different subpopulations of endothelial progenitor cells and circulating apoptotic progenitor cells in patients with vascular disease and diabetes. Int. J. Cardiol. 2010;143(3):368–372. doi: 10.1016/j.ijcard.2009.03.075. [DOI] [PubMed] [Google Scholar]
- 44.Rouhl R.P., Mertens A.E., van Oostenbrugge R.J., et al. Angiogenic T-cells and putative endothelial progenitor cells in hypertension-related cerebral small vessel disease. Stroke. 2012;43(1):256–258. doi: 10.1161/STROKEAHA.111.632208. [DOI] [PubMed] [Google Scholar]
- 45.Shantsila E., Wrigley B.J., Shantsila A., Tapp L.D., Gill P.S., Lip G.Y.H. Monocyte-derived and CD34+KDR+ endothelial progenitor cells in heart failure. J. Thromb. Haemost. 2012;10(7):1252–2161. doi: 10.1111/j.1538-7836.2012.04753.x. [DOI] [PubMed] [Google Scholar]
- 46.Teraa M., Sprengers R.W., Westerweel P.E., et al. Bone marrow alterations and lower endothelial progenitor cell numbers in critical limb ischemia patients. PLoS One. 2013;8(1):e55592. doi: 10.1371/journal.pone.0055592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vemparala K., Roy A., Bahl V., et al. Early accelerated senescence of circulating endothelial progenitor cells in premature coronary artery disease patients in a developing country- a case control study. BMC Cardiovasc. Disord. 2013;13(1):104. doi: 10.1186/1471-2261-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spinetti G., Cordella D., Fortunato O., et al. Global remodeling of the vascular stem cell niche in bone marrow of diabetic patients: Implication of the miR-155/FOXO3a signaling pathway. Circ. Res. 2013;112(3):510–522. doi: 10.1161/CIRCRESAHA.112.300598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thijssen D.H., Vos J.B., Verseyden C., et al. Haematopoietic stem cells and endothelial progenitor cells in healthy men: effect of aging and training. Aging Cell. 2006;5(6):495–503. doi: 10.1111/j.1474-9726.2006.00242.x. [DOI] [PubMed] [Google Scholar]
- 50.Thum T., Hoeber S., Froese S., et al. Age-dependent impairment of endothelial progenitor cells is corrected by growth hormone mediated increase of insulin-like growth factor-1. Circ. Res. 2007;100(3):434–443. doi: 10.1161/01.RES.0000257912.78915.af. [DOI] [PubMed] [Google Scholar]
- 51.Heiss C., Keymel S., Niesler U., Ziemann J., Kelm M., Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J. Am. Coll. Cardiol. 2005;45(9):1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 52.Hoetzer G.L., Van Guilder G.P., Irmiger H.M., Keith R.S., Stauffer B.L., DeSouza C.A. Aging, exercise, and endothelial progenitor cell clonogenic and migratory capacity in men. J. Appl. Physiol. 2007;102(3):847–852. doi: 10.1152/japplphysiol.01183.2006. [DOI] [PubMed] [Google Scholar]
- 53.Williamson K.A., Hamilton A., Reynolds J.A., et al. Age-related impairment of endothelial progenitor cell migration correlates with structural alterations of heparan sulfate proteoglycans. Aging Cell. 2013;12(1):139–147. doi: 10.1111/acel.12031. [DOI] [PubMed] [Google Scholar]
- 54.Ross M.D., Malone E.M., Simpson R., et al. Lower resting and exercise-induced circulating angiogenic progenitors and angiogenic T cells in older men. Am. J. Physiol. Heart Circ. Physiol. 2018;314(3):H392–H402. doi: 10.1152/ajpheart.00592.2017. [DOI] [PubMed] [Google Scholar]
- 55.Yang Z., Xia W.H., Su C., et al. Regular exercise-induced increased number and activity of circulating endothelial progenitor cells attenuates age-related decline in arterial elasticity in healthy men. Int. J. Cardiol. 2013;165(2):247–254. doi: 10.1016/j.ijcard.2011.08.055. [DOI] [PubMed] [Google Scholar]
- 56.Kushner E.J., Van Guilder G.P., MacEneaney O.J., Cech J.N., Stauffer B.L., DeSouza C.A. Aging and endothelial progenitor cell telomere length in healthy men. Clin. Chem. Lab. Med. 2009;47(1):47–50. doi: 10.1515/CCLM.2009.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kushner E., Van Guilder G., MacEneaney O., et al. Ageing and endothelial progenitor cell release of proangiogenic cytokines. Age Ageing. 2010;39(2):268–272. doi: 10.1093/ageing/afp244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mandraffino G., Sardo M.A., Riggio S., et al. Circulating progenitor cells and the elderly: A seven-year observational study. Exp. Gerontol. 2012;47(5):394–400. doi: 10.1016/j.exger.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 59.de Haan G., Van Zant G. Dynamic changes in mouse hematopoietic stem cell numbers during aging. Blood. 1999;93(10):3294–3301. [PubMed] [Google Scholar]
- 60.Conboy I.M., Conboy M.J., Wagers A.J., Girma E.R., Weissman I.L., Rando T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 61.Park A., Barrera-Ramirez J., Ranasinghe I., et al. Use of Statins to augment progenitor cell function in preclinical and clinical studies of regenerative therapy: A systematic review. Stem Cell Rev. 2016;12(3):327–339. doi: 10.1007/s12015-016-9647-7. [DOI] [PubMed] [Google Scholar]
- 62.Oikonomou E., Siasos G., Zaromitidou M., et al. Atorvastatin treatment improves endothelial function through endothelial progenitor cells mobilization in ischemic heart failure patients. Atherosclerosis. 2015;238(2):159–164. doi: 10.1016/j.atherosclerosis.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 63.Ye H., He F., Fei X., et al. High-dose atorvastatin reloading before percutaneous coronary intervention increased circulating endothelial progenitor cells and reduced inflammatory cytokine expression during the perioperative period. J. Cardiovasc. Pharmacol. Ther. 2014;19(3):290–295. doi: 10.1177/1074248413513500. [DOI] [PubMed] [Google Scholar]
- 64.Yu J.W., Deng Y.P., Han X., Ren G.F., Cai J., Jiang G.J. Metformin improves the angiogenic functions of endothelial progenitor cells via activating AMPK/eNOS pathway in diabetic mice. Cardiovasc. Diabetol. 2016;15(1):88. doi: 10.1186/s12933-016-0408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paschalaki K.E., Starke R.D., Hu Y., et al. Dysfunction of endothelial progenitor cells from smokers and chronic obstructive pulmonary disease patients due to increased DNA damage and senescence. Stem Cells. 2013;31(12):2813–2826. doi: 10.1002/stem.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lamirault G., Susen S., Forest V., et al. Difference in mobilization of progenitor cells after myocardial infarction in smoking versus non-smoking patients: Insights from the BONAMI trial. Stem Cell Res. Ther. 2013;4(6):152. doi: 10.1186/scrt382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ross M.D., Malone E., Florida-James G. Vascular ageing and exercise: Focus on cellular reparative processes. Oxid. Med. Cell. Longev. 2016;2016:3583956. doi: 10.1155/2016/3583956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ross M.D., Wekesa A.L., Phelan J.P., Harrison M. Resistance exercise increases endothelial progenitor cells and angiogenic factors. Med. Sci. Sports Exerc. 2014;46(1):16–23. doi: 10.1249/MSS.0b013e3182a142da. [DOI] [PubMed] [Google Scholar]
- 69.Van Craenenbroeck E., Hoymans V., Beckers P., et al. Exercise training improves function of circulating angiogenic cells in patients with chronic heart failure. Basic Res. Cardiol. 2010;105(5):665–676. doi: 10.1007/s00395-010-0105-4. [DOI] [PubMed] [Google Scholar]
- 70.Bakker E., Engan H., Patrician A., et al. Acute dietary nitrate supplementation improves arterial endothelial function at high altitude: A double-blinded randomized controlled cross over study. Nitric Oxide. 2015;50:58–64. doi: 10.1016/j.niox.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 71.Casey D.P., Treichler D.P., Ganger C.T., Schneider A.C., Ueda K. Acute dietary nitrate supplementation enhances compensatory vasodilation during hypoxic exercise in older adults. J. Appl. Physiol. 2015;118(2):178–186. doi: 10.1152/japplphysiol.00662.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rammos C., Hendgen-Cotta U.B., Sobierajski J., Bernard A., Kelm M., Rassaf T. Dietary nitrate reverses vascular dysfunction in older adults with moderately increased cardiovascular risk. J. Am. Coll. Cardiol. 2014;63(15):1584–1585. doi: 10.1016/j.jacc.2013.08.691. [DOI] [PubMed] [Google Scholar]
- 73.Larsen F.J., Ekblom B., Sahlin K., Lundberg J.O., Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N. Engl. J. Med. 2006;355(26):2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 74.Govoni M., Jansson E.A., Weitzberg E., Lundberg J.O. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19(4):333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 75.McIlvenna L.C., Monaghan C., Liddle L., et al. Beetroot juice versus chard gel: A pharmacokinetic and pharmacodynamic comparison of nitrate bioavailability. Nitric Oxide. 2017;64:61–67. doi: 10.1016/j.niox.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 76.Hobbs D., Kaffa N., George T., Methvem L., Lovegrove J. Blood pressure-lowering effects of beetroot juice and novel beetroot-enriched bread products in normotensive male subjects. Br. J. Nutr. 2012;108(11):2066–2074. doi: 10.1017/S0007114512000190. [DOI] [PubMed] [Google Scholar]
- 77.Maas R., Xanthakis V., Göen T., et al. Plasma nitrate and incidence of cardiovascular disease and all‐cause mortality in the community: The Framingham offspring study. J. Am. Heart Assoc. 2017;6(11):e006224. doi: 10.1161/JAHA.117.006224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kina-Tanada M., Sakanashi M., Tanimoto A., et al. Long-term dietary nitrite and nitrate deficiency causes the metabolic syndrome, endothelial dysfunction and cardiovascular death in mice. Diabetologia. 2017;60(6):1138–1151. doi: 10.1007/s00125-017-4259-6. [DOI] [PubMed] [Google Scholar]
- 79.Aicher A., Heeschen C., Mildner-Rihm C., et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat. Med. 2003;9(11):1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 80.Balligand J-L., Feron O., Dessy C. eNOS activation by physical forces: From short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol. Rev. 2009;89(2):481–534. doi: 10.1152/physrev.00042.2007. [DOI] [PubMed] [Google Scholar]
- 81.Heissig B., Hattori K., Dias S., et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9mediated release of Kit-Ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heiss C., Meyer C., Totzeck M., et al. Dietary inorganic nitrate mobilizes circulating angiogenic cells. Free Radic. Biol. Med. 2012;52(9):1767–1772. doi: 10.1016/j.freeradbiomed.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 83.Javanmard S.H., Gheisari Y., Soleimani M., Nematbakhsh M., Monajemi A. Effect of L-arginine on circulating endothelial progenitor cells in hypercholesterolemic rabbits. Int. J. Cardiol. 2010;143(2):213–216. doi: 10.1016/j.ijcard.2008.11.203. [DOI] [PubMed] [Google Scholar]
- 84.Fiorito C., Balestrieri M.L., Crimi E., et al. Effect of l-arginine on circulating endothelial progenitor cells and VEGF after moderate physical training in mice. Int. J. Cardiol. 2008;126(3):421–423. doi: 10.1016/j.ijcard.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 85.Sinclair H. Deficiency of essential faty acids and atherosclerosis, etcetera. Lancet. 1956;267(6919):381–383. [PubMed] [Google Scholar]
- 86.Bang H.O., Dyerberg J., Sinclair H.M. The composition of the Eskimo food in north western Greenland. Am. J. Clin. Nutr. 1980;33(12):2657–2661. doi: 10.1093/ajcn/33.12.2657. [DOI] [PubMed] [Google Scholar]
- 87.Galan P., Kesse-Guyot E., Czernichow S., Briancon S., Blacher J., Hercberg S. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: A randomised placebo controlled trial. BMJ. 2010;341:c6273. doi: 10.1136/bmj.c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rauch B., Schiele R., Schneider S., et al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122(21):2152–2159. doi: 10.1161/CIRCULATIONAHA.110.948562. [DOI] [PubMed] [Google Scholar]
- 89.Yokoyama M., Origasa H., Matsuzaki M., et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet. 2007;369(9567):1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 90.Investigators G.H. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): A randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9645):1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 91.Jump D.B., Depner C.M., Tripathy S. Omega-3 fatty acid supplementation and cardiovascular disease: Thematic Review Series: New lipid and lipoprotein targets for the treatment of cardiometabolic diseases. J. Lipid Res. 2012;53(12):2525–2545. doi: 10.1194/jlr.R027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jump D.B. The biochemistry of n-3 polyunsaturated fatty acids. J. Biol. Chem. 2002;277(11):8755–8758. doi: 10.1074/jbc.R100062200. [DOI] [PubMed] [Google Scholar]
- 93.Stebbins C.L., Stice J.P., Hart C.M., Mbai F.N., Knowlton A.A. Effects of dietary decosahexaenoic acid (DHA) on eNOS in human coronary artery endothelial cells. J. Cardiovasc. Pharmacol. Ther. 2008;13(4):261–268. doi: 10.1177/1074248408322470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen W., Esselman W.J., Jump D.B., Busik J.V. Anti-inflammatory effect of docosahexaenoic acid on cytokine-induced adhesion molecule expression in human retinal vascular endothelial cells. Invest. Ophthalmol. Vis. Sci. 2005;46(11):4342–4347. doi: 10.1167/iovs.05-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sakai C., Ishida M., Ohba H., et al. Fish oil omega-3 polyunsaturated fatty acids attenuate oxidative stress-induced DNA damage in vascular endothelial cells. PLoS One. 2017;12(11):e0187934. doi: 10.1371/journal.pone.0187934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Egert S., Stehle P. Impact of n−3 fatty acids on endothelial function: Results from human interventions studies. Curr. Opin. Clin. Nutr. Metab. Care. 2011;14(2):121–131. doi: 10.1097/MCO.0b013e3283439622. [DOI] [PubMed] [Google Scholar]
- 97.Cai M., Zhang W., Weng Z., et al. Promoting neurovascular recovery in aged mice after ischemic stroke - prophylactic effect of omega-3 polyunsaturated fatty acids. Aging Dis. 2017;8(5):531–545. doi: 10.14336/AD.2017.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang J., Shi Y., Zhang L., et al. Omega-3 polyunsaturated fatty acids enhance cerebral angiogenesis and provide long-term protection after stroke. Neurobiol. Dis. 2014;68:91–103. doi: 10.1016/j.nbd.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Devaraj S., Chien A., Rao B., Chen X., Jialal I. Modulation of endothelial progenitor cell number and function with n-3 polyunsaturated fatty acids. Atherosclerosis. 2013;228(1):94–97. doi: 10.1016/j.atherosclerosis.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 100.Tikhonenko M., Lydic T.A., Opreanu M., et al. N-3 Polyunsaturated fatty acids prevent diabetic retinopathy by inhibition of retinal vascular damage and enhanced endothelial progenitor cell reparative function. PLoS One. 2013;8(1):e55177. doi: 10.1371/journal.pone.0055177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Turgeon J., Dussault S., Maingrette F., et al. Fish oil-enriched diet protects against ischemia by improving angiogenesis, endothelial progenitor cell function and postnatal neovascularization. Atherosclerosis. 2013;229(2):295–303. doi: 10.1016/j.atherosclerosis.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 102.Spigoni V., Lombardi C., Cito M., et al. N-3 PUFA increase bioavailability and function of endothelial progenitor cells. Food Funct. 2014;5(8):1881–1890. doi: 10.1039/c3fo60641d. [DOI] [PubMed] [Google Scholar]
- 103.Wu S.Y., Mayneris-Perxachs J., Lovegrove J.A., Todd S., Yaqoob P. Fish-oil supplementation alters numbers of circulating endothelial progenitor cells and microparticles independently of eNOS genotype. Am. J. Clin. Nutr. 2014;100(5):1232–1243. doi: 10.3945/ajcn.114.088880. [DOI] [PubMed] [Google Scholar]
- 104.Balakumar P., Taneja G. Fish oil and vascular endothelial protection: Bench to bedside. Free Radic. Biol. Med. 2012;53(2):271–279. doi: 10.1016/j.freeradbiomed.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 105.Estruch R., Ros E., Salas-Salvadó J., et al. Primary prevention of cardiovascular disease with a mediterranean diet. N. Engl. J. Med. 2013;368(14):1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 106.Sofi F., Abbate R., Gensini G.F., Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010;92(5):1189–1196. doi: 10.3945/ajcn.2010.29673. [DOI] [PubMed] [Google Scholar]
- 107.Esposito K., Marfella R., Ciotola M., et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA. 2004;292(12):1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 108.Maiorino M.I., Bellastella G., Petrizzo M., et al. Effect of a Mediterranean diet on endothelial progenitor cells and carotid intima-media thickness in type 2 diabetes: Follow-up of a randomized trial. Eur. J. Prev. Cardiol. 2017;24(4):399–408. doi: 10.1177/2047487316676133. [DOI] [PubMed] [Google Scholar]
- 109.Klonizakis M., Alkhatib A., Middleton G. Long-term effects of an exercise and Mediterranean diet intervention in the vascular function of an older, healthy population. Microvasc. Res. 2014;95:103–107. doi: 10.1016/j.mvr.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 110.Perona J.S., Cabello-Moruno R., Ruiz-Gutierrez V. The role of virgin olive oil components in the modulation of endothelial function. J. Nutr. Biochem. 2006;17(7):429–445. doi: 10.1016/j.jnutbio.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 111.Kolomvotsou A.I., Rallidis L.S., Mountzouris K.C., et al. Adherence to Mediterranean diet and close dietetic supervision increase total dietary antioxidant intake and plasma antioxidant capacity in subjects with abdominal obesity. Eur. J. Nutr. 2013;52(1):37–48. doi: 10.1007/s00394-011-0283-3. [DOI] [PubMed] [Google Scholar]
- 112.Fernández J., Rosado-Alvarez D., Da Silva-Grigoletto M., et al. Moderate-to-high intensity training and hypocaloric Mediterranean diet enhance endothelial progenitor cells and fitness in subjects with metabolic syndrome. Clin. Sci. (Lond.) 2012;123(6):361–373. doi: 10.1042/CS20110477. [DOI] [PubMed] [Google Scholar]
- 113.Marin C., Ramirez R., Delgado-Lista J., et al. Mediterranean diet reduces endothelial damage and improves the regenerative capacity of endothelium. Am. J. Clin. Nutr. 2011;93(2):267–274. doi: 10.3945/ajcn.110.006866. [DOI] [PubMed] [Google Scholar]
- 114.Cesari F., Sofi F., Molino Lova R., et al. Aging process, adherence to Mediterranean diet and nutritional status in a large cohort of nonagenarians: Effects on endothelial progenitor cells. Nutr. Metab. Cardiovasc. Dis. 2018;28(1):84–90. doi: 10.1016/j.numecd.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 115.Panagiotakos D.B., Pitsavos C., Arvaniti F., Stefanadis C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev. Med. 2007;44(4):335–340. doi: 10.1016/j.ypmed.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 116.Madssen E., Videm V., Moholdt T., Wisloff U., Hegbom K., Wiseth R. Predictors of beneficial coronary plaque changes after aerobic exercise. Med. Sci. Sports Exerc. 2015;47(11):2251–2256. doi: 10.1249/MSS.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 117.Szostak J., Laurant P. The forgotten face of regular physical exercise: A ‘natural’ anti-atherogenic activity. Clin. Sci. (Lond.) 2011;121(3):91–106. doi: 10.1042/CS20100520. [DOI] [PubMed] [Google Scholar]
- 118.Black M.A., Green D.J., Cable N.T. Exercise prevents age-related decline in nitric-oxide-mediated vasodilator function in cutaneous microvessels. J. Physiol. 2008;586(14):3511–3524. doi: 10.1113/jphysiol.2008.153742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Farsidfar F., Kasikcioglu E., Oflaz H., Kasikcioglu D., Meric M., Umman S. Effects of different intensities of acute exercise on flow-mediated dilatation in patients with coronary heart disease. Int. J. Cardiol. 2008;124(3):372–374. doi: 10.1016/j.ijcard.2006.11.243. [DOI] [PubMed] [Google Scholar]
- 120.Rakobowchuk M., Tanguay S., Burgomaster K.A., Howarth K.R., Gibala M.J., MacDonald M.J. Sprint interval and traditional endurance training induce similar improvements in peripheral arterial stiffness and flow-mediated dilation in healthy humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295(1):R236–R42. doi: 10.1152/ajpregu.00069.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Birk G.K., Dawson E.A., Atkinson C., et al. Brachial artery adaptation to lower limb exercise training: role of shear stress. J. Appl. Physiol. 2012;112(10):1653–1658. doi: 10.1152/japplphysiol.01489.2011. [DOI] [PubMed] [Google Scholar]
- 122.Laufs U., Werner N., Link A., et al. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109(2):220–226. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 123.Chinsomboon J., Ruas J., Gupta R.K., et al. The transcriptional coactivator PGC-1α mediates exercise-induced angiogenesis in skeletal muscle. Proc. Natl. Acad. Sci. USA. 2009;106(50):21401–21406. doi: 10.1073/pnas.0909131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Geng T., Li P., Okutsu M., et al. PGC-1α plays a functional role in exercise-induced mitochondrial biogenesis and angiogenesis but not fiber-type transformation in mouse skeletal muscle. Am. J. Physiol. Cell Physiol. 2010;298(3):C572–C9. doi: 10.1152/ajpcell.00481.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Adams V., Lenk K., Linke A., et al. Increase of circulating endothelial progenitor cells in patients with coronary artery disease after exercise-induced ischemia. Arterioscler. Thromb. Vasc. Biol. 2004;24(4):684–690. doi: 10.1161/01.ATV.0000124104.23702.a0. [DOI] [PubMed] [Google Scholar]
- 126.Rehman J., Li J., Parvathaneni L., et al. Exercise acutely increases circulating endothelial progenitor cells and monocyte-/macrophage-derived angiogenic cells. J. Am. Coll. Cardiol. 2004;43(12):2314–2318. doi: 10.1016/j.jacc.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 127.Laufs U., Urhausen A., Werner N., et al. Running exercise of different duration and intensity: Effect on endothelial progenitor cells in healthy subjects. Eur. J. Cardiovasc. Prev. Rehabil. 2005;12(4):407–414. doi: 10.1097/01.hjr.0000174823.87269.2e. [DOI] [PubMed] [Google Scholar]
- 128.Van Craenenbroeck E.M., Vrints C.J., Haine S.E., et al. A maximal exercise bout increases the number of circulating CD34+/KDR+ endothelial progenitor cells in healthy subjects. Relation with lipid profile. J. Appl. Physiol. 2008;104(4):1006–1013. doi: 10.1152/japplphysiol.01210.2007. [DOI] [PubMed] [Google Scholar]
- 129.Möbius-Winkler S., Hilberg T., Menzel K., et al. Time-dependent mobilization of circulating progenitor cells during strenuous exercise in healthy individuals. J. Appl. Physiol. 2009;107(6):1943–1950. doi: 10.1152/japplphysiol.00532.2009. [DOI] [PubMed] [Google Scholar]
- 130.Rummens J., Daniels A., Dendale P., et al. Suppressed increase in blood endothelial progenitor cell content as a result of single exhaustive exercise bout in male revascularised coronary artery disease patients. Acta Clin. Belg. 2012;67(4):262–269. doi: 10.2143/ACB.67.4.2062670. [DOI] [PubMed] [Google Scholar]
- 131.Sandri M., Beck E.B., Adams V., et al. Maximal exercise, limb ischemia, and endothelial progenitor cells. Eur. J. Cardiovasc. Prev. Rehabil. 2011;18(1):55–64. doi: 10.1097/HJR.0b013e32833ba654. [DOI] [PubMed] [Google Scholar]
- 132.Scalone G., De Caterina A., Leone A., et al. Effect of exercise on circulating endothelial progenitor cells in microvascular angina. Circ. J. 2013;77(7):1777–1782. doi: 10.1253/circj.cj-12-0996. [DOI] [PubMed] [Google Scholar]
- 133.Van Craenenbroeck E., Bruyndonckx L., Van Berckelaer C., Hoymans V., Vrints C., Conraads V. The effect of acute exercise on endothelial progenitor cells is attenuated in chronic heart failure. Eur. J. Appl. Physiol. 2011;111(9):2375–2379. doi: 10.1007/s00421-011-1843-1. [DOI] [PubMed] [Google Scholar]
- 134.Chang E., Paterno J., Duscher D., et al. Exercise induces stromal cell-derived factor-1alpha-mediated release of endothelial progenitor cells with increased vasculogenic function. Plast. Reconstr. Surg. 2015;135(2):340e–350e. doi: 10.1097/PRS.0000000000000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Steiner S., Niessner A., Ziegler S., et al. Endurance training increases the number of endothelial progenitor cells in patients with cardiovascular risk and coronary artery disease. Atherosclerosis. 2005;181(2):305–310. doi: 10.1016/j.atherosclerosis.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 136.Sarto P., Balducci E., Balconi G., et al. Effects of exercise training on endothelial progenitor cells in patients with chronic heart failure. J. Card. Fail. 2007;13(9):701–708. doi: 10.1016/j.cardfail.2007.06.722. [DOI] [PubMed] [Google Scholar]
- 137.Cesari F., Marcucci R., Gori A.M., et al. Impact of a cardiac rehabilitation program and inflammatory state on endothelial progenitor cells in acute coronary syndrome patients. Int. J. Cardiol. 2013;167(5):1854–1859. doi: 10.1016/j.ijcard.2012.04.157. [DOI] [PubMed] [Google Scholar]
- 138.Sonnenschein K., Horváth T., Mueller M., et al. Exercise training improves in vivo endothelial repair capacity of early endothelial progenitor cells in subjects with metabolic syndrome. Eur. J. Cardiovasc. Prev. Rehabil. 2011;18(3):406–414. doi: 10.1177/1741826710389373. [DOI] [PubMed] [Google Scholar]
- 139.Choi J., Moon K., Jung S., et al. Regular exercise training increases the number of endothelial progenitor cells and decreases homocysteine levels in healthy peripheral blood. Korean J. Physiol. Pharmacol. 2014;18(2):163–168. doi: 10.4196/kjpp.2014.18.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wilson P.F., D’Agostino R.B., Sullivan L., Parise H., Kannel W.B. Overweight and obesity as determinants of cardiovascular risk: The framingham experience. Arch. Intern. Med. 2002;162(16):1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 141.Tilg H., Moschen A.R. Inflammatory mechanisms in the regulation of insulin resistance. Mol. Med. 2008;14(3-4):222–231. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li J-M., Fan L.M., Christie M.R., Shah A.M. Acute Tumor necrosis factor alpha signaling via NADPH oxidase in microvascular endothelial cells: Role of p47(phox) phosphorylation and binding to TRAF4. Mol. Cell. Biol. 2005;25(6):2320–2330. doi: 10.1128/MCB.25.6.2320-2330.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Van Guilder G.P., Hoetzer G.L., Dengel D.R., Stauffer B.L., DeSouza C.A. Impaired endothelium-dependent vasodilation in normotensive and normoglycemic obese adult humans. J. Cardiovasc. Pharmacol. 2006;47(2):310–313. doi: 10.1097/01.fjc.0000205097.29946.d3. [DOI] [PubMed] [Google Scholar]
- 144.Fadini G.P., de Kreutzenberg S.V., Coracina A., et al. Circulating CD34+ cells, metabolic syndrome, and cardiovascular risk. Eur. Heart J. 2006;27(18):2247–2255. doi: 10.1093/eurheartj/ehl198. [DOI] [PubMed] [Google Scholar]
- 145.Müller-Ehmsen J., Braun D., Schneider T., et al. Decreased number of circulating progenitor cells in obesity: Beneficial effects of weight reduction. Eur. Heart J. 2008;29(12):1560–1568. doi: 10.1093/eurheartj/ehn213. [DOI] [PubMed] [Google Scholar]
- 146.Westerweel P.E., Visseren F.L.J., Hajer G.R., et al. Endothelial progenitor cell levels in obese men with the metabolic syndrome and the effect of simvastatin monotherapy vs. simvastatin/ ezetimibe combination therapy†. Eur. Heart J. 2008;29(22):2808–2817. doi: 10.1093/eurheartj/ehn431. [DOI] [PubMed] [Google Scholar]
- 147.MacEneaney O.J., Kushner E.J., Van Guilder G.P., Greiner J.J., Stauffer B.L., DeSouza C.A. Endothelial progenitor cell number and colony-forming capacity in overweight and obese adults. Int. J. Obes. 2009;33(2):219–225. doi: 10.1038/ijo.2008.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.MacEneaney O.J., Kushner E.J., Westby C.M., et al. Endothelial progenitor cell function, apoptosis, and telomere length in overweight/obese humans. Obesity (Silver Spring) 2010;18(9):1677–1682. doi: 10.1038/oby.2009.494. [DOI] [PubMed] [Google Scholar]
- 149.Tsai T.H., Chai H.T., Sun C.K., et al. Obesity suppresses circulating level and function of endothelial progenitor cells and heart function. J. Transl. Med. 2012;10:137. doi: 10.1186/1479-5876-10-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen Y.L., Chang C.L., Sun C.K., et al. Impact of obesity control on circulating level of endothelial progenitor cells and angiogenesis in response to ischemic stimulation. J. Transl. Med. 2012;10(1):86. doi: 10.1186/1479-5876-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Heida N.M., Müller J.P., Cheng I.F., et al. Effects of obesity and weight loss on the functional properties of early outgrowth endothelial progenitor cells. J. Am. Coll. Cardiol. 2010;55(4):357–367. doi: 10.1016/j.jacc.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 152.Lopez-Lluch G., Navas P. Calorie restriction as an intervention in ageing. J. Physiol. 2016;594(8):2043–2060. doi: 10.1113/JP270543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Minamiyama Y., Bito Y., Takemura S., et al. Calorie restriction improves cardiovascular risk factors via reduction of mitochondrial reactive oxygen species in type II diabetic rats. J. Pharmacol. Exp. Ther. 2007;320(2):535–543. doi: 10.1124/jpet.106.110460. [DOI] [PubMed] [Google Scholar]
- 154.Heilbronn L., de Jonge L., Frisard M., et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals. JAMA. 2006;295(13):1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Miyaki A., Maeda S., Yoshizawa M., et al. Effect of weight reduction with dietary intervention on arterial distensibility and endothelial function in obese men. Angiology. 2009;60(3):351–357. doi: 10.1177/0003319708325449. [DOI] [PubMed] [Google Scholar]
- 156.Lefevre M., Redman L.M., Heilbronn L.K., et al. Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis. 2009;203(1):206–213. doi: 10.1016/j.atherosclerosis.2008.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Meydani M., Das S., Band M., Epstein S., Roberts S. The effect of caloric restriction and glycemic load on measures of oxidative stress and antioxidants in humans: Results from the calerie trial of human caloric restriction. J. Nutr. Health Aging. 2011;15(6):456–460. doi: 10.1007/s12603-011-0002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Das S.K., Gilhooly C.H., Golden J.K., et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: A 1-y randomized controlled trial. Am. J. Clin. Nutr. 2007;85(4):1023–1030. doi: 10.1093/ajcn/85.4.1023. [DOI] [PubMed] [Google Scholar]
- 159.Fontana L., Villareal D., Weiss E., et al. Calorie restriction or exercise: Effects on coronary heart disease risk factors. A randomized, controlled trial. Am. J. Physiol. Endocrinol. Metab. 2007;293(1):E197–E202. doi: 10.1152/ajpendo.00102.2007. [DOI] [PubMed] [Google Scholar]
- 160.Fontana L., Meyer T.E., Klein S., Holloszy J.O. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc. Natl. Acad. Sci. USA. 2004;101(17):6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wekesa A.L., Doyle L.M., Fitzmaurice D., et al. Influence of a low-carbohydrate diet on endothelial microvesicles in overweight women. Appl. Physiol. Nutr. Metab. 2016;41(5):522–527. doi: 10.1139/apnm-2015-0507. [DOI] [PubMed] [Google Scholar]
- 162.Aversa A., Bruzziches R., Francomano D., et al. Weight loss by multidisciplinary intervention improves endothelial and sexual function in obese fertile women. J. Sex. Med. 2013;10(4):1024–1033. doi: 10.1111/jsm.12069. [DOI] [PubMed] [Google Scholar]
- 163.Yassine H.N., Marchetti C.M., Krishnan R.K., Vrobel T.R., Gonzalez F., Kirwan J.P. Effects of exercise and caloric restriction on insulin resistance and cardiometabolic risk factors in older obese adults—A randomized clinical trial. J Gerontol: Series A. 2009;64A(1):90–95. doi: 10.1093/gerona/gln032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Raitakari M., Ilvonen T., Ahotupa M., et al. Weight reduction with very-low-caloric diet and endothelial function in overweight adults: Role of plasma glucose. Arterioscler. Thromb. Vasc. Biol. 2004;24(1):124–128. doi: 10.1161/01.ATV.0000109749.11042.7c. [DOI] [PubMed] [Google Scholar]
- 165.Pierce G.L., Beske S.D., Lawson B.R., et al. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension. 2008;52(1):72–79. doi: 10.1161/HYPERTENSIONAHA.108.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bigornia S.J., Mott M.M., Hess D.T., et al. Long-term successful weight loss improves vascular endothelial function in severely obese individuals. Obesity (Silver Spring) 2010;18(4):754–759. doi: 10.1038/oby.2009.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Haspicova M., Milek D., Siklova-Vitkova M., et al. Post-prandial endothelial dysfunction is ameliorated following weight loss in obese premenopausal women. Med. Sci. Monit. 2011;17(11):CR634–CR639. doi: 10.12659/MSM.882048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Merino J., Megias-Rangil I., Ferré R., Plana N., et al. Body weight loss by very-low-calorie diet program improves small artery reactive hyperemia in severely obese patients. Obes. Surg. 2013;23(1):17–23. doi: 10.1007/s11695-012-0729-6. [DOI] [PubMed] [Google Scholar]
- 169.Zhang W., Huang Q., Zeng Z., Wu J., Zhang Y., Chen Z. Sirt1 inhibits oxidative stress in vascular endothelial cells. Oxid. Med. Cell. Longev. 2017;2017:7543973. doi: 10.1155/2017/7543973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rippe C., Lesniewski L., Connell M., LaRocca T., Donato A., Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010;9(3):304–312. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bruyndonckx L., Hoymans V.Y., De Guchtenaere A., et al. Diet, exercise, and endothelial function in obese adolescents. Pediatrics. 2015;135(3):e653–e661. doi: 10.1542/peds.2014-1577. [DOI] [PubMed] [Google Scholar]
- 172.Mikirova N., Casciari J., Hunninghake R., Beexley M. Effect of weight reduction on cardiovascular risk factors and CD34-positive cells in circulation. Int. J. Med. Sci. 2011;8(6):445–452. doi: 10.7150/ijms.8.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xin B., Liu C.L., Yang H., et al. Prolonged fasting improves endothelial progenitor cell-mediated ischemic angiogenesis in mice. Cell. Physiol. Biochem. 2016;40(3-4):693–706. doi: 10.1159/000452581. [DOI] [PubMed] [Google Scholar]
- 174.Huang J., Wang S., Xu F., et al. Exercise training with dietary restriction enhances circulating irisin level associated with increasing endothelial progenitor cell number in obese adults: An intervention study. PeerJ. 2017;5:e3669. doi: 10.7717/peerj.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hur J., Yang H.M., Yoon C.H., et al. Identification of a novel role of T cells in postnatal vasculogenesis: characterization of endothelial progenitor cell colonies. Circulation. 2007;116(15):1671–1682. doi: 10.1161/CIRCULATIONAHA.107.694778. [DOI] [PubMed] [Google Scholar]
- 176.Kushner E.J., MacEneaney O.J., Morgan R.G., Van Engelenburg A.M., Van Guilder G.P., DeSouza C.A. CD31+ T cells represent a functionally distinct vascular T cell phenotype. Blood Cells Mol. Dis. 2010;44(2):74–78. doi: 10.1016/j.bcmd.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 177.Kamprom W, Kheolamai P. Effects of mesenchymal stem cell-derived cytokines on the functional properties of endothelial progenitor cells. Eur. J. Cell Biol. 2016;95(3-5):153–163. doi: 10.1016/j.ejcb.2016.02.001. [DOI] [PubMed] [Google Scholar]