Abstract
Background:
Redox signaling plays an important role in the lives of cells. This signaling not only becomes apparent in pathologies but is also thought to be involved in maintaining physiolog-ical homeostasis. Reactive Oxygen Species (ROS) can activate protein kinases: CaMKII, PKG, PKA, ERK, PI3K, Akt, PKC, PDK, JNK, p38. It is unclear whether it is a direct interaction of ROS with these kinases or whether their activation is a consequence of inhibition of phosphatases. ROS have a biphasic effect on the transport of Ca2+ in the cell: on one hand, they activate the sarcoplasmic reticu-lum Ca2+-ATPase, which can reduce the level of Ca2+ in the cell, and on the other hand, they can inac-tivate Ca2+-ATPase of the plasma membrane and open the cation channels TRPM2, which promote Ca2+-loading and subsequent apoptosis. ROS inhibit the enzyme PHD2, which leads to the stabiliza-tion of HIF-α and the formation of the active transcription factor HIF.
Conclusion:
Activation of STAT3 and STAT5, induced by cytokines or growth factors, may include activation of NADPH oxidase and enhancement of ROS production. Normal physiological produc-tion of ROS under the action of cytokines activates the JAK/STAT while excessive ROS production leads to their inhibition. ROS cause the activation of the transcription factor NF-κB. Physiological levels of ROS control cell proliferation and angiogenesis. ROS signaling is also involved in beneficial adaptations to survive ischemia and hypoxia, while further increases in ROS can trigger programmed cell death by the mechanism of apoptosis or autophagy. ROS formation in the myocardium can be re-duced by moderate exercise
Keywords: Reactive oxygen species, intracellular signaling molecules, heart, exercise, cardiovascular system, autophagy
1. Introduction
In the 1980s and 1990s, it was generally accepted that free radicals and Reactive Oxygen Species (ROS) play an extremely negative role in the body [1-5], causing organ and tissue damage in ischemia-reperfusion, stress, Alzheimer's disease, diabetes mellitus [2-5]. Indeed, both in experimental animals [6, 7] and in the course of clinical observations [8, 9] it was reported that antioxidants seemed to increase the resistance of the heart to the impact of ischemia-reperfusion. However, gradually the attitude towards free radicals began to change. Now we know that they can also act as intracellular signaling molecules which are involved in the homeostatic equilibrium and can participate in beneficial adaptation of organs and tissues to various stresses such as hypoxia or ischemia [10-17], or may be intracellular messengers of pathological processes [18-23].
2. Which reactive oxygen species act as messengers?
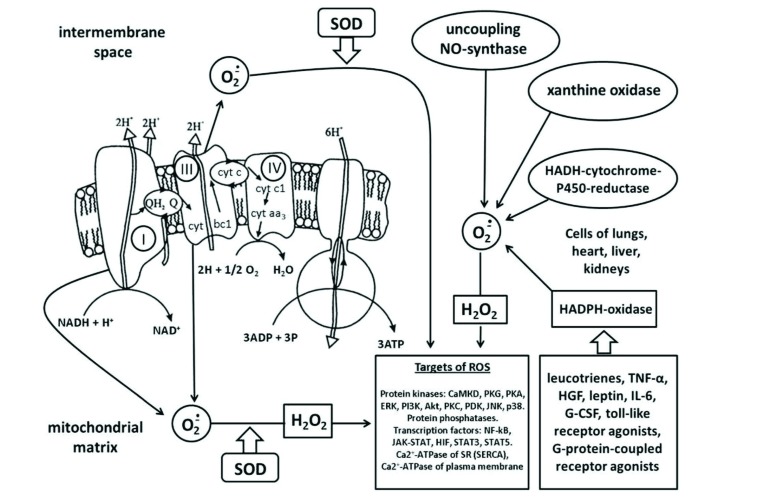
The family of ROS includes small molecules, characterized by high reactivity and biological activity. These include the free radicals: superoxide radical anion (O2•) and the hydroxyl radical (OH•). In addition, ROS include: hydrogen peroxide (H2O2), singlet oxygen (1O2), peroxynitrite (ONOO-), hypochlorous acid (HOCl) [17]. The half-life of O2• in tissues is brief, 10-6 -10-3 s, as a result of spontaneous dismutation or dismutation catalyzed by superoxide dismutase (SOD) [5, 24] making it an unlikely signaling molecule. O2• is converted to H2O2 [17] whose half-life in tissues is also brief: 10-9 to 10-6 s [5, 24, 25]. 1O2 in aqueous solutions is no better with a half life of 10-12 - 3 x 10-6 s [5, 26, 27]. The half-life of H2O2, however, is a few seconds [24]. In addition, H2O2 dissolves in lipids and, due to lack of charge, can easily pass through membranes [28]. Therefore hydrogen peroxide is the most likely candidate for an intercellular and intracellular signaling molecule. The superoxide radical, in our opinion, can only function as an intracellular messenger, acting on protein molecules near the site of its formation such as the inner mitochondrial membrane, NADPH oxidase, xanthine oxidase, or NO-synthase.
It has been a matter of debate as to whether O2• can cross the outer membrane of the mitochondria [24]. but a number of studies have shown the ability of intact mitochondria to spontaneously release this radical [29, 30]. Due to its short half-life, hydroxyl radical would seem to be an unlikely participant in intracellular signaling [25]. However, in the presence of Fe2+ OH• can be a intermediate between H2O2 and its sensor molecule due to the Haber-Weiss reaction: H2O2 + Fe2+ = Fe3+ + OH• + OH-. Peroxynitrite (ONOO-) is formed as a result of the interaction of O2• with NO• [17]. The half-life of ONOO- is ~1 s [31] and it can penetrate cell membranes [31] making it a possible intracellular messenger. Hypochlorite: H2O2 + Cl- → H2O + OCl- [17] is a bactericidal molecule produced in mammals by the myeloperoxidase enzyme, which is almost exclusively confined to neutrophils. Thus, the most likely candidates for the role of intracellular signaling molecules are: H2O2, O2•, and ONOO-.
3. Sources of reactive oxygen species in the cell
3.1. Mitochondria
The main source of superoxide radicals in some cells is mitochondria [24, 32]. It has been reported that as much as 4 - 5% of the oxygen consumed by mitochondria is diverted to O2• synthesis [33] while others estimate the leak as only 1 - 2% [34]. The main source of O2• is NADH dehydrogenase in complex I of the mitochondrial respiratory chain [33, 35]. Blockade of complex I with rotenone transfers electrons from complex I to ubiquinone and leads to a several-fold increase in O2• production [33]. The free radical of ubisemiquinone Q and complex III (cytochrome-bc1 complex) of the mitochondrial respiratory chain can also be a source of O2• [24, 32]. The superoxide anion formed in complex I enters the matrix, while O2•, formed in complex III, enters both the intermembrane space and the matrix [36, 37]. Mitochondria are the main source of O2• for those cells in which the mitochondrial content is high such as cardiomyocytes where the occupy 22 to 37% of the cell’s volume [38]. Mitochondria are also the main source of O2• in mitochondria-rich brain cells [39]. The opening of ATP-sensitive potassium channels in the mitochondrial inner membrane also generates ROS [40].
3.2. The Nox Family of NADPH oxidases (Nox)
In other cells such as endothelial cells, these organelles occupy only 2 - 5% of the cytoplasmic cell volume [41]. In these cells, the main source of O2• is NADPH oxidase, which catalyzes the reaction:
NADPH + 2O2 → NADP+ + H+ + 2O2• .
It was originally thought that these NADPH oxidases were exclusive to phagocytic cells, but then Nox homologs were found in the plasma membranes of many mammalian cells including kidney cells, epithelial cells, endothelial cells, smooth muscle cells, lymphoid cells, and macrophages [24]. Both Nox2 and Nox4 isoforms are expressed in cardiomyocytes [42].
The activity of Nox isoenzymes is enhanced by leukotrienes, tumor necrosis factor-α (TNF-α), toll-like receptor agonists, hepatocyte growth factor (HGF), leptin, interleukin-6, and granulocyte colony-stimulating factor (G-CSF) [43]. There is also evidence that Nox isoenzymes may be stimulated by certain G-protein-coupled receptor agonists such as angiotensin II and morphine [44, 45]. It is believed that protein kinase C (PKC) is involved in the signaling from the angiotensin II receptor to a Nox [43]. Morphine signals differently. In Nox1 knockout mice, the analgesic effect of morphine weakens and translocation (activation) of PKC to the cell membrane is disrupted [44]. The following chain of events is thought to describe the signaling:
morphine → OR → Nox1 → PKC → analgesia
OR is the opioid receptor. The fundamental difference between the angiotensin and morphine receptors is that the angiotensin receptors are coupled to Gq proteins, and the ORs interact with Gi/o proteins. Apparently this feature determines the nature of their interaction with Nox.
3.3. NO Synthase
In pathological conditions, the source of O2• can be from the so-called “uncoupling of NO synthase” [45], which, in the absence of its cofactor tetrahydrobiopterin, or as a result of lack of L-arginine substrate, synthesizes O2• instead of NO•.
3.4. Xanthine Oxidase
Xanthine oxidase can be a source of O2• in cells of the intestine, lungs, skin, brain, skeletal muscles, liver, pancreas, testes, kidneys [39]. During ischemia most cells release adenosine which is quickly converted to hypoxanthine. Then during reperfusion xanthine oxidase catalyzes the two-step reaction [17]:
hypoxanthine + H2O + O2 → xanthine + H+ + O2•
xanthine + H2O + O2 → uric acid + H+ + O2•
While xanthine oxidase is found in the hearts of many animals and is a source of ROS during ischemia-reperfusion, the enzyme is undetectable in human myocardium [46].
Flavoprotein NADPH-cytochrome-P450-reductase is yet another source of ROS (O2•, OH•, H2O2) [17]. The highest activity of NADPH-cytochrome-P450 reductase and NADH-cytochrome-P450 reductase is observed in cells of the lungs, liver, heart, kidneys [39]. A small contribution to the production of ROS is made by monoamine oxidases (heart, kidney, brain) or lipoxygenase and cyclooxygenases (brain, pancreas) [39].
4. Targets of reactive oxygen species
Cysteine Residues. ROS acts through post-translational modification of numerous proteins, such as receptors, kinases, phosphatases, ion channels and transcription factors. An extensive range of oxidation-reduction modifications can be involved in redox signaling, among which the most studied is the H2O2-mediated oxidation of the amino acid residues of cysteine in proteins. Cysteine is sensitive to oxidation and usually forms reversible intra- or intermolecular disulfide bonds [47, 48]. ROS-mediated oxidation of thiol groups can modulate the activity of proteins in which cysteine fragments are critical for substrate binding e.g. glyceraldehyde-3-phosphate dehydrogenase, p38 kinase, tropomyosin, and many of the tyrosine phosphatases [49-51]. Oxidation of cysteine can occur by binding with other chemicals such as NO (nitrosylation), nitroxyl (HNO) [52] or glutathione (glutathiolation) [47]. It is important to note that the effect of ROS is strongly modulated by the presence of antioxidants. Glutathione, which is the main cellular antioxidant, can remove disulfide bonds in oxidized proteins. The thioredoxin system may have a similar effect [17].
Ca2+/Calmodulin-dependent Kinase II (CaMKII) is a known target of ROS [42]. CaMKII is a supramolecular complex consisting of 12 monomers assembled as hexamers, each monomer having catalytic, autoinhibitory and Ca2+/calmodulin-binding domains. At rest, the auto-inhibitory domain binds to the catalytic site and blocks the kinase activity [53]. The binding of Ca2+ to the Ca2+/calmodulin domain induces autophosphorylation and removes the autoinhibitory domain to expose the catalytic site and commence its enzymatic function. Anderson’s laboratory found that after the initial Ca2+-dependent activation of CaMKII oxidation of methionine residues 281/282 in the regulatory domain further increases CaMKII activity [54]. Oxidation of methionines can be eliminated by methionine sulfoxide reductase A, which regulates the physiological role of this pathway. Thus, CaMKII through this mechanism combines two key signals, namely Ca2+ and ROS.
Studies in mice with modified genes have shown that the oxidation of CaMKII increases the death of cardiomyocytes and the development of heart failure following myocardial infarction or angiotensin II-induced stress [54]. In subsequent studies, the important role of increased oxidation of CaMKII in the pathogenesis of atrial fibrillation was also demonstrated [55]. It also has been shown that H2O2 can activate CaMKII in T-lymphocyte cultures [56]. All these facts indicate that CaMKII is a pathological target for ROS.
Protein Kinase G (PKG) is a cGMP-dependent protein kinase that is also a target for ROS [42]. PKG is a cytosolic protein that forms a homodimer of two identical subunits. This enzyme plays an important role in mediating the cardioprotective effect of NO [57]. The binding of cGMP to PKG’s regulatory domain activates its catalytic kinase domain. PKG type Iα has at least 5 Cys residues that can be oxidized. Oxidation of Cys42 leads to the formation of a disulfide bridge between the two monomers that activate the kinase independently of NO [58]. Eaton’s laboratory substituted PKGIα’s Cys42 with a serine and found that these animals developed a moderate degree of hypertension, which suggested that ROS-mediated vasodilation from PKG might be involved in the physiological regulation of blood pressure [59]. But Nakamura et al. demonstrated that PKGIα is oxidized both in patients with heart disease and in rodents with models of heart disease [60]. Moreover, this oxidation promoted unfavorable cardiac remodeling after prolonged pressure overload or stimulation of Gq-coupled receptors. Compared to the wild-type hearts and cardiomyocytes, the hearts and myocardial cells that express the PKGIα with the C42S substitution are better adapted to the functional stresses on the heart. Redox-dependent changes in PKG1α control intracellular translocation. While the activated, oxidized PKGIα was exclusively in the cytosol, the PKGIα C42S was translocated to the plasma membrane [60]. This observation suggests that oxidation of PKG with ROS can negatively affect myocardial response to increased loads. The source of the ROS oxidizing PKG has not yet been identified.
Protein Kinase A (PKA) is a cAMP-dependent protein kinase and is also activated by ROS [42]. PKA is a heterotetramer with a dimeric regulatory subunit and a dimeric catalytic subunit. It was found that the oxidation of PKA leads to the formation of a disulphide bond between the two regulatory subunits and that activates the enzyme independent of cAMP [61]. This modification of the PKA caused subcellular translocation of PKA to the myofilament components troponin I and myosin binding protein C in heart muscle which directly increased its contractility. The important role of PKA oxidation has recently been demonstrated using a model in mice, in which PKA became “redox dead” due to the mutation of C175S in the regulatory subunit [62]. The authors showed that this disrupts the PKA-dependent Vascular Endothelial Growth Factor (VEGF)/Extracellular Regulated Kinase (ERK) signal ratio. It was found that the functional effect of PKA oxidation is the enhancement of angiogenesis in models of ischemia in the hind limbs but, unfortunately, it also promotes angiogenesis in tumors [62].
ERK kinase is a member of the mitogen-activated protein kinase family which also includes JNK and P38 (see below). Erk is involved in cell division and survival and its activity is enhanced by activation of the EGF (epidermal growth factor) and PDGF (platelet-derived growth factor) receptors. These receptors can also be activated by ROS [63-65], which can lead to a phosphorylation-dependent activation of ERK. Activation of ERK by physiological concentrations of H2O2 was observed by Preston et al. [66]. Simultaneously, they noted an increase in cell proliferation. This activation of ERK by ROS was confirmed by other investigators [67-70]. Conversely, in 2013 Wang et al. [71] found that antioxidants could suppress the proliferation of HeLa cells along with a decrease in ERK activity. Adding hydrogen peroxide increased the phosphorylation and thus activation of ERK.
Phosphatase PTEN and PI3K/Akt. The phosphoinositide-3 kinase, PI3K, acts to phosphorylate inositol in its 3 position. The inositol-3-phosphate then causes a Phosphoinositide-dependent Kinase (PDK) to activate a kinase named Akt through phosphorylation. Akt was first identified as the oncogene for the retrovirus AKT8, hence the name. PTEN (Phosphatase and tensin homolog deleted on chromosome TEN) is a phosphatase that acts to remove phosphates in inositol’s 3 position which opposes Akt’s activation. In experiments on cell culture RAW264.7, it was shown that H2O2 causes phosphorylation of Akt and an increase in its activity [72]. Simultaneously, the inactivation of PTEN by oxidation was noted. The authors proposed that the oxidation of PTEN disrupts Akt’s dephosphorylation resulting in a net activation of Akt [72]. Analysis of various cysteine mutants showed that the main Cys124 residue in the active site of PTEN specifically forms a disulfide bond with Cys71 during oxidation with hydrogen peroxide (200 μM) [73]. Activation of Akt by ROS was also reported by other investigators [68].
Reduction of the level of H2O2-oxidized PTEN in cells appears to be mediated predominantly by thioredoxin because thioredoxin was more effective than glutaredoxin, glutathione, or a 14-kDa thioredoxin-like protein for the reduction of oxidized PTEN in vitro [73]. In experiments on H9c2 cell culture, it was shown that H2O2 (100 μM) can cause the formation of disulfide bonds between the Cys297 and Cys311 residues in the Akt molecule, which is accompanied by dephosphorylation and degradation of this enzyme [74]. Therefore, the ROS can not only activate, but also inactivate the PI3K/Akt tandem depending on its concentration. The final physiological effect apparently also depends on the type of cells studied. In experiments on cultured A549 lung cancer cells, it was shown that O2• and H2O2 promoted cell survival by activating the PI3K/Akt [75]. Akt along with ERK is known to play a prominent role in mediating the protective effect in the Ischemic Preconditioning (IPC) pathway (see below) [76].
Protein Kinase C (PKC). In the inactive state, PKC only weakly binds to membrane lipids and is located mainly in the cytosolic fraction, whereas activation of PKC by diacylglycerol or Ca2+ increases the affinity of the enzyme to the membrane lipids which stabilizes its association with the membranes. This translocation causes a conformational transition to its catalytically active form [77, 78]. Both regulatory and catalytic domains of PKC contain cysteine-rich regions, which makes PKC a very vulnerable target for oxidation-reduction regulation. Depending on the concentration, oxidants play a dual role in stimulating or inactivating PKC. IPC is a phenomenon whereby a brief transient ischemic insult causes the heart to become resistant to infarction from a subsequent extended period of ischemia. This cardioprotection is the result of protective signaling originating from the occupation of several Gi-coupled receptors during the conditioning ischemia and PKC plays a prominent role in this signaling [79].
The first evidence of ROS involvement in IPC was when combined SOD and catalase blunted the infarct-limiting effect of IPC in canine hearts [80]. Treating rabbits with SOD or N-2-mercaptopropionyl Glycine (2-MPG) did not affect the infarct size in rabbits without IPC but eliminated IPC’s infarct-reducing effect [81]. The involvement of ROS in the cardiac IPC signaling pathway was confirmed in later studies [82-84]. It has been established that ROS can act as triggers of the infarct-reducing effect of IPC [85-87]. PKCε is thought to be the target for ROS and inhibition of the Mitochondrial Permeability Transition Pore (MPTP) is thought to be the final effector for the cardioprotective effect of IPC [88-90].
The critical time for redox signaling is the brief period of reperfusion between the IPC coronary occlusion and the prolonged ischemic insult. Perfusing the heart with oxygen-free buffer during that period blocked the protection and the ROS scavenger only blocked IPC’s protection when it was present during that brief reperfusion period [91]. During the conditioning ischemia the signaling is initiated by Gi-coupled receptor (e.g. adenosine) occupation, but cannot proceed past the redox step until oxygen becomes available to fuel the signaling during a brief period of reperfusion. Ischemic postconditioning is observed when short occlusion/reperfusion cycles starting with the onset of reperfusion also protect against infarction. The occlusion cycles keep the pH of the tissue low by reducing coronary flow during the first minutes of reperfusion which inhibits MPTP opening. At the same time, enough oxygen is delivered to fuel ROS signaling as occurs in IPC and that keeps the MPTP closed thereafter [92].
At high concentrations, ROS react with catalytically important cysteine residues and inactivate PKC. However, at low concentrations, oxidants induce activation of PKC [78]. In addition, it has been found that H2O2 stimulates the activation of tyrosine kinases and can indirectly regulate the phosphorylation of PKC-δ in tyrosine residues Tyr512 and Tyr523 [93]. The observation that MPG can block protection from IPC is an example of how beneficial physiological redox signaling in a microcompartment can be interfered with by systemic administration of a ROS scavenger
PDK1. Phospholipid-dependent kinase-1 is dependent on the phospholipid phosphatidylinositol-3-inositol and is the intermediate link between the kinases PI3K and Akt. PDK1 can also be phosphorylated and activated by PKC, which phosphorylates the residues of Ser744 and Ser748 [94]. It was found that PKCδ activates PDK1through the phosphorylation of Ser738 and Ser742 residues [95]. As we noted above, PKC can be activated by ROS. Therefore, the following chain of events can be built: ROS → PKC → PDK1 → Akt. Indeed PI3K and Akt are downstream of PKC in the protective signaling pathway of IPC [96]. It has also been reported that ROS can provide activation of PDK1 by phosphorylation of Tyr463 with tyrosine kinase Abl. Tyrosine kinase Abl, in turn, is activated by cytosolic tyrosine kinase Src [95]. According to Storz et al. ROS activates PKC and Src through the inhibition of protein phosphatase [95].
JNK (c-Jun N-terminal kinase). This kinase is known to promote transcription and thus protein synthesis. Preston et al. [66] showed physiological concentrations of H2O2 caused activation by phosphorylation of JNK in cultured fibroblasts. Simultaneously, they noted an increase in cell proliferation. In experiments with malignant cells of the large intestine, it was shown that H2O2 can stimulate cell proliferation by activating JNK [97]. In 2005, Kamata et al. [98] showed that oxidative stress leads to increased phosphorylation of JNK, not due to increased kinase activity but rather to the oxidation and inactivation of the phosphatases that target JNK.
p38 MAP kinase. This mitogen-activated kinase is activated by cell stress. Physiological concentrations of H2O2 increased the phosphorylation of p38 in cultured fibroblasts [66]. Simultaneously, they noted an increase in cell proliferation. In experiments with cultured A549 lung cancer cells, it was shown that O2• and H2O2 promoted cell survival through activation of p38 [75]. Pacing-induced heart failure increased ROS production and inhibition of p38 kinase by SB281832 counteracted it suggesting that p38 kinase activation was upstream rather than downstream of the ROS formation [51].
Protein phosphatases are a large diverse group of enzymes that remove the phosphate group from the phosphorylated amino acid residue of the substrate protein. Each only removes phosphate groups from a specific amino acid sequence. Phosphatases are often the target for inactivation by oxidation from ROS [56, 72, 95, 98, 99]. Inactivation of a phosphatase will have the effect of increasing the phosphorylation level of its target similar to activating the target’s kinase.
EGF and PDGF receptors are coupled to Nox. It was found that their activation leads to the production of ROS and increased cell proliferation [100].
Ca2+ transport system. During the contraction of cardiomyocytes, the initial influx of a small amount of Ca2+ through L-type sarcolemmal Ca2+ channels during an action potential triggers the opening of the ryanodine receptor (RyR) channels that are located in the Sarcoplasmic Reticulum (SR) where Ca2+ is stored. These high conductance channels allow the rapid release of Ca2+ into the cytoplasm (Ca2+-induced Ca2+ release) to cause contraction as it binds to the myofilament protein troponin C. During relaxation of the heart, Ca2+ is translocated back into the SR by the Ca2+-ATPase Sarco/Endoplasmic Reticulum Ca2+-ATPase, (SERCA) and out of the cytosol via the sarcolemmal Na+/Ca2+ exchanger. The affinity of SERCA to Ca2+ is regulated by its interaction with phospholamban [101]. Recently, indirect redox modulation of SERCA activity mediated by Nox2 has been observed [98]. In hearts overexpressing Nox2, SERCA activity and contractility increased as a result of increased phosphorylation of phospholamban. This increase in phospholamban phosphorylation was associated with a decrease in Ser/Thr Protein Phosphatase-1 (PP1) activity, which normally dephosphorylates phospholamban. Some of the proteins involved in the contraction, such as RyR and SERCA, are also direct targets of the ROS [42].
Recently, the structure of RyR has been clarified. It was found that this protein forms a tetrameric complex [102]. Each subunit has about 20 free Cys residues that can undergo various redox modifications. In addition, it was shown that mechanical stretching of cardiomyocytes induces cyclic activation of Nox2 in T-tubules, which leads to an increase in the release of Ca2+ from SR via RyR (Ca2+ spikes), thereby contributing to a fundamentally important physiological pathway for a stretch-induced increase in cardiac output known as “Starling’s law of the heart” [103, 104]. At the molecular level, this is proposed to be mediated by the reversible oxidation of the RyR. In pathological conditions, more extensive and often irreversible oxidation or S-nitrosylation of RyR contributes to the release of Ca2+ from the SR followed by rhythm and contractile dysfunction [105].
The exact source of the ROS that participates in the oxidation of the RyR remains unclear. Mitochondria and Nox2 are probably involved [103]. In skeletal muscle, it was reported that Nox4 can oxidize RyR1 and cause an increase in Ca2+ leakage during hypoxia [106]. Whether Nox4 influences similar effects in cardiomyocytes is unknown. The physiological redox regulation of SERCA in the heart remains poorly studied. The isoform SERCA2’s activity can be enhanced by HNO-induced phospholamban oligomerization [52]. Irreversible oxidation of cardiac SERCA in pathological conditions such as hemodynamic overload and chronic neurohumoral activation reduces the activity of SERCA and causes contractile dysfunction [107, 108].
All of the above apparently can be extrapolated to the Ca2+ transport system. In other cells, it has been established that H2O2 can oxidize and inactivate the purified Ca2+-ATPase from the plasma membrane [109]. In experiments on neuronal culture, it was shown that H2O2 can kill cells by the opening of the TRPM2 (transient receptor potential M2) channel with subsequent Ca2+ overload [110]. The ability of ROS to activate TRPM2 has been confirmed by others [111, 112]. The important role of TRPM2 in H2O2-induced apoptosis and cell death has also been corroborated by others [113, 114]. There is also the reverse situation where TRPV1 (transient receptor potential vanilloid) channel opening with capsaicin results in increased ROS production and autophagy [115].
Hypoxia Inducible Factor (HIF). Cells have developed complex mechanisms for adapting to hypoxia. One such mechanism is the activation of HIF [116]. When activated during hypoxia HIF binds to the hypoxia response elements. These promoters contain the sequence NCGTG to which HIF binds and control the expression of genes that cause a cellular adaptation to hypoxia. HIF proteins are heterodimers consisting of a HIF-α and a HIF-β subunit, each of which is constitutively expressed. At normal levels of O2, that is, with normoxia, HIF-α rapidly undergoes hydroxylation with Prolyl Hydroxylase 2 (PHD2) [117] and is subsequently degraded in the proteasomes [118]. Conversely, under hypoxic conditions, PHD2 becomes inactivated, which allows HIF-α to accumulate and bind to HIF-β to form active HIF. More than 70 genes are known to be activated by HIF-1 [119].
Under hypoxic conditions, mitochondria paradoxically increase their ROS production by complex III [42, 120]. The ROS formed in complex III are necessary for the stabilization of HIF-α as cells lacking the ability to generate ROS by complex III do not stabilize HIF-α during hypoxia [121-125]. Recent studies have shown that small molecules that selectively remove superoxide from complex III reduce hypoxic stabilization of the HIF-α protein [125]. Together, these data reveal that mitochondrial ROS, especially from complex III, plays a key role in this system. It has been shown that this ROS inhibits the activity of PHD2, but the exact mechanism by which they inactivate PHD2 is unknown [121]. There is indirect evidence that the signaling molecule between the complex III and PHD2 is H2O2 [122, 124]. Thus, the physiological adaptation appears to be affected by hypoxia-induced release of ROS (probably H2O2) that inhibits PHD2. That, in turn, leads to the stabilization of the HIF-α protein. However, there are also data that HIF-1α deficiency can abrogate mitochondrial ROS formation and prevent the oxidation of the phosphatase PTEN [126, 127]. It was proposed that HIF-1α is actually upstream of mitochondrial ROS formation and PTEN oxidation [126, 127].
Transcription factors STAT. The family of highly conserved signal transducer and activator of transcription (STAT) include STAT1-4, 5A, 5B and 6 [128, 129]. In unstimulated cells, inactivated STATs are localized in the cytoplasm together with Janus Kinase (JAK), and are induced by the binding of ligands to the receptors on the cell membrane which leads to phosphorylation of the corresponding proteins. STATs are activated by tyrosine phosphorylation followed by homo- or heterodimerization and then translocated to the nucleus where they directly bind to specific DNA recognition elements or interact with other transcription factors to regulate gene expression [130-132]. As an example, interleukin 6 and prolactin activate STAT3 and STAT5, respectively, to enhance the transcription of target genes in their target cell types [133, 134]. STAT3 and STAT5 become constitutively activated in human cancer cell lines obtained from the chest, head and neck, lungs, ovaries, pancreas, lymphoma, etc. [135]. STAT3 and, apparently, STAT5 also contribute to heart’s tolerance to ischemia-reperfusion during postconditioning and remote postconditioning [136, 137].
In the healthy individual ROS function as important signaling molecules to maintain normal tissue homeostasis. For example, cytokines rapidly increase the ROS level in resting hematopoietic cells, which is associated with an increase in tyrosine phosphorylation followed by activation of JAK2 and STAT5. This leads to induced expression of the early response gene c-FOS, and G1- to S-phase transition in the cell cycle [138, 139]. The use of antioxidants in assessing the effect of cytokines on cells or stimulating cells with hydrogen peroxide without cytokines demonstrates that ROS plays an important signaling role in the control of JAK, STAT3 and STAT5 [140]. Activation of STAT3 and STAT5 by ROS induced by cytokines or growth factors may also include activation of Nox [141, 142].
ROS interact with cysteine residues in the catalytic domain of JAK2, which, when oxidized, inhibit its kinase activity [143]. STAT3 can also interact directly with intracellular oxidants. In cells treated with hydrogen peroxide, STAT3 reversibly forms crosslinked monomers, dimers, trimers and tetramers through oligomerization associated with the formation of disulfide bonds in conserved cysteine residues. This inhibits STAT3 binding to DNA and transcriptional activity without affecting its activation by tyrosine phosphorylation [144, 145].
The function of oxidized STAT3 complexes still needs to be resolved. STAT3, in addition to its cytoplasmic and nuclear distribution, is also found in the mitochondria. One possibility is that oxidation of STAT3 by H2O2 in the mitochondria might cause it to modulate cellular respiration through its possible interaction with the electron transport chain [146]. Oxidation of STAT5 is observed in macrophages of aging mice. The oxidation blocks the phosphorylation of its tyrosine residues in response to the stimulation of cytokine receptors [147]. Thus, physiological ROS production activates the JAK/STAT tandem in response to cytokines while excessive ROS production leads to inhibition of JAK/STAT.
Transcription factors NF-κB (nuclear factor κ-light-chain-enhancer of activated B cells). The family of NF-κB transcription factors consists of 5 proteins: p65 (RelA), RelB (RelB), c-Rel (Rel), p105 / p50 (NFκB1) and p100 / p52 (NFκB2). NF-κB exhibits activity only in dimeric form (both hetero- and homodimers are possible) There are 15 known active combinations of these dimers [148]. The most common forms are the dimers of the subunits NF-κB1 or NF-κB2 with the p65 subunit. Members of the NF-κB family all have an N-terminal RHD (Rel-homology domain) which is necessary for homo- and heterodimerization, nuclear translocation, and association with IκB group proteins that inhibit NF-κB and prevent its binding to DNA. The NF-κB subunits can bind to a DNA sequence that contains the nucleotide sequence 5-GGGRNWYYCC-3 (wherein R is any purine, N is any nucleotide, W is adenine or thymine, and Y is any pyrimidine) [148]. In addition, p65, RelB and c-Rel contain the C-terminal TAD (transcription activation domain) which is necessary for the activation of transcription of target genes. In unstimulated cells, the NF-κB dimers are inactivated by being bound to IκBs which sequester NF-κB in the cytoplasm. There are 8 known IκBs: IκBα, IκBβ, IκBγ, IκBε, IκBζ, Bcl-3, p100 and p105 which bind to NF-κB via ankyrin repeats consisting of 33 amino acid residues [148]. Cytokines activate the NF-κB signaling pathway by activating IKK (IκB kinase). IKK phosphorylates the IκB marking it for proteolytic degradation by the proteosome. When NF-κB is released from its inhibiting complex it translocates into the nucleus and activates the transcription of its genetic program.
ROS can affect NF-κB’s activation by affecting the phosphorylation of IκB. Exogenous H2O2 causes phosphorylation of Tyr42 and other tyrosine residues in the IκB resulting in NF-κB activation [149, 150]. EL4 cell culture experiments have shown that H2O2 causes IκB phosphorylation, but these phosphorylated amino acid residues differ from those phosphorylated by cytokines. Their phosphorylation catalyzes casein kinase II rather than IKK [149]. Treatment of KBM-5 cells with H2O2 resulted in the activation of NF-κB [150], but without the enhancing IκB degradation. Hydrogen peroxide induced serine phosphorylation in the p65 subunit, followed by translocation of p65. In addition, it was found that H2O2 causes tyrosine phosphorylation of IκBα, which is necessary for the activation of NF-κB. It was also found that Syk tyrosine kinase is involved in the H2O2-induced activation of NF-κB as stimulation of NF-κB is observed only in those cells that express Syk tyrosine kinase [150]. Depending on the cell type, H2O2 induced NF-κB activation may occur with the aid of casein kinase II or Syk-tyrosine kinase. In experiments on MCF-7 cells, it was shown that H2O2 (1-10 μM) activates the NIK kinase (NF-κB-inducing kinase), which leads to phosphorylation (activation) of IKKα, followed by phosphorylation of IκB and activation of NF-κB [151]. Okadaic acid, which is a Ser/Thr phosphatase inhibitor, has an effect similar to that of H2O2, so the authors [151] proposed that H2O2 might be inhibiting a Ser/Thr-phosphatase, thereby promoting NIK phosphorylation. In 2008, Kim et al. [72] reported that H2O2 causes redox activation of the signaling pathways: PI3K/PTEN/Akt and NIK/IKK both of which contributed to the IKK-dependent activation of NF-κB.
5. EFFECT OF PHYSICAL EXERCISE ON ROS PRODUCTION IN THE HEART AND SKELETAL MUSCLES
Physical exercise is an important regulator of redox signaling in the heart [152]. In animal models, physical activity has been shown to increase the heart’s tolerance to ischemia-reperfusion [153, 154]. Knocking down Mn-SOD by pretreatment with an antisense oligodeoxyribonucleotide eliminated the exercise-induced decrease in experimental infarct size indicating that Mn-SOD induction was involved in the protection [153, 154]. Aerobic exercise prevents pressure overload-induced cardiac dysfunction and hypertrophy [155]. Aerobic exercise training also decreased the myocardial ROS and Malonyldialdehyde (MDA) content and increased myocardial SOD content [155]. Physical exercise is reported to reduce ROS production in aging heart by increasing the expression of antioxidant enzymes [156]. Physical exercise also attenuates aortic stenosis-induced heart failure [157]. Acute heavy exercise induces mitochondrial stress and enhanced ROS generation in cardiomyocytes [158]. Those ROS, apparently, play the role of an intracellular messenger and mediate the beneficial adaptation from physical training on the heart. It has been shown that tocopherol-enriched diet in trained rats prevents this increase in tissue content of mitochondrial proteins, and cytochrome c expression in the heart [159]. However, physical exertion not only induces the formation of ROS, but paradoxically it also combats oxidative stress in the heart. It has been found that swimming training attenuates isoproterenol-induced fibrosis and isoproterenol-induced ROS production in the myocardium [160]. At the same time, physical training’s reported to reduce OH• production of skeletal muscle mitochondria, but not that of the heart [161].
One month after experimental infarction in rat hearts, an increase in cardiac H2O2, other ROS, and MDA were observed. But after two months of subsequent exercise training, these indicators returned to their control values [162]. After exercise training signs of heart failure were also reduced in these rats. A reduction in skeletal muscle atrophy and skeletal muscle Nox activity were also reported [163]. Physical training increases the activity of myocardial Aldehyde Dehydrogenase 2 (ALDH2), which eliminates toxic aldehydes including MDA [164]. It is possible that the increase in ALDH2 contributes to the exercise-induced decrease in MDA formation [162].
Above we have written about the cardioprotective effects of physical training. However, it should be remembered that exhausting exercise itself can also cause heart damage. Intensive exercise in mice causes an increase in the cardiospecific marker of necrosis, creatine kinase MB and ROS production by cardiac mitochondria. The flavonoid quercetin, which has antioxidant properties, opposed those negative manifestations [165]. Thus, while moderate exercise exerts a positive effect on redox signaling in the heart that ameliorates cardiac injury, exhausting physical exercises can also cause oxidative stress.
Conclusion
In this review, we note only that the normal physiological level of ROS controls cell proliferation [66, 71, 100, 166, 167] and angiogenesis [58, 166-168]. It is believed that an elevated level of ROS exceeding physiological values triggers programmed cell death by apoptosis [169] or autophagy [169, 170], the occurrence of arrhythmias, and contractile dysfunction of the heart [101]. On the other hand, it may put the heart or other organs into a protected (conditioned) state.
We have reviewed a large number of reports in which ROS have the clear ability to affect signaling pathways. Most of these were discovered by exposing cells to exogenous ROS or scavengers. In a few cases, such as the control of HIF, a clear physiological signaling role has been revealed. In many cases, a biphasic response is seen: Low levels of ROS production, as might be generated by physiological signaling, seem to stimulate a pathway while higher levels capable of oxidizing amino acids within a signaling protein appear to inhibit it. But in most cases it is largely unknown, how extensively the body actually utilizes these signaling mechanisms in its normal physiology or whether they only come into play under pathological conditions.
Fig. (1).
An involvement of reactive oxygen species in intracellular signaling. ROS-mediated signaling pathway is involved kinases (CaMKII, PKG, PKA, ERK, PI3K, Akt, PKC, PDK, JNK, Kinase p38), phosphatases (PTEN, PP1, other protein phosphatases), transcription factors (HIF, STAT, NF-κB), Ca2+ transport system.
ACKNOWLEDGEMENTS
The article was prepared with the support of the Russian Science Foundation. The authors express their gratitude to the professor M.V. Cohen for the discussion of the data presented in the article.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Jacobson M.D. Reactive oxygen species and programmed cell death. Trends Biochem. Sci. 1996;21(3):83–86. [PubMed] [Google Scholar]
- 2.Kuroda S., Siesjö B.K. Reperfusion damage following focal ischemia: pathophysiology and therapeutic windows. Clin. Neurosci. 1997;4(4):199–212. [PubMed] [Google Scholar]
- 3.Markesbery W.R. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic. Biol. Med. 1997;23(1):137–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 4.Meerson F.Z., Belkina L.M., Sazontova T.G., Saltykova V.A., Arkhipenko YuV. The role of lipid peroxidation in pathogenesis of arrhythmias and prevention of cardiac fibrillation with antioxidants. Basic Res. Cardiol. 1987;82(2):123–137. doi: 10.1007/BF01907060. [DOI] [PubMed] [Google Scholar]
- 5.Yu B.P. Cellular defenses against damage from reactive oxygen species. Physiol. Rev. 1994;74(1):139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 6.Pisarenko O.I., Studneva I.M., Lakomkin V.L., Timoshin A.A., Kapelko V.I. Human recombinant extracellular-superoxide dismutase type C improves cardioplegic protection against ischemia/reperfusion injury in isolated rat heart. J. Cardiovasc. Pharmacol. 1994;24(4):655–663. doi: 10.1097/00005344-199410000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Pisarenko O.I., Lakomkin V.L., Studneva I.M., et al. Allopurinol-enhanced postischemic recovery in the isolated rat heart involves repletion of high-energy phosphates. Biochem. Med. Metab. Biol. 1994;51(1):16–26. doi: 10.1006/bmmb.1994.1002. [DOI] [PubMed] [Google Scholar]
- 8.Cherniavskiĭ A.M., Maslov L.N., Ponomarenko I.V. Vecherskii YuYu, Lishmanov YuB, Karpov RS. Cardioprotective effect of emoxipin in the surgical reconstruction of the coronary arteries. Kardiologiya. 1996;36(8):35–38. [Google Scholar]
- 9.Lasukova T.V., Uskina E.V., Afanas’iev S.A., et al. Effects of emoxipine and histochrome on lipid peroxidation and activity of serum MB-creatine phosphokinase in patients with ischemic heart disease during aortocoronary shunting. Eksp. Klin. Farmakol. 1997;60(5):51–53. [PubMed] [Google Scholar]
- 10.Balkova P., Hlaváčková M., Milerová M., et al. N-acetylcysteine treatment prevents the up-regulation of MnSOD in chronically hypoxic rat hearts. Physiol. Res. 2011;60(3):467–474. doi: 10.33549/physiolres.932042. [DOI] [PubMed] [Google Scholar]
- 11.Cohen M.V., Downey J.M. Cardioprotection: Spotlight on PKG. Br. J. Pharmacol. 2007;152(6):833–834. doi: 10.1038/sj.bjp.0707453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen M.V., Downey J.M. Is it time to translate ischemic preconditioning’s mechanism of cardioprotection into clinical practice? J. Cardiovasc. Pharmacol. Ther. 2011;16(3-4):273–280. doi: 10.1177/1074248411407071. [DOI] [PubMed] [Google Scholar]
- 13.Heinzel F.R., Luo Y., Li X., et al. Impairment of diazoxide-induced formation of reactive oxygen species and loss of cardioprotection in connexin 43 deficient mice. Circ. Res. 2005;97(6):583–586. doi: 10.1161/01.RES.0000181171.65293.65. [DOI] [PubMed] [Google Scholar]
- 14.Heinzel F.R., Luo Y., Dodoni G., et al. Formation of reactive oxygen species at increased contraction frequency in rat cardiomyocytes. Cardiovasc. Res. 2006;71(2):374–382. doi: 10.1016/j.cardiores.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ. Res. 2015;116(4):674–699. doi: 10.1161/CIRCRESAHA.116.305348. [DOI] [PubMed] [Google Scholar]
- 16.Kolar F., Jezková J., Balková P., et al. Role of oxidative stress in PKC-δ upregulation and cardioprotection induced by chronic intermittent hypoxia. Am. J. Physiol. Heart Circ. Physiol. 2007;292(1):H224–H30. doi: 10.1152/ajpheart.00689.2006. [DOI] [PubMed] [Google Scholar]
- 17.Menshchikova E.B., Lankin V.Z., Zenkov N.K., Bondar I.A., Krugovykh N.F., Trufakin V.A. Oxidative stress. Prooxidants and antioxidants. Moscow: Slovo; 2006. p. 556. [Google Scholar]
- 18.Arcaro A., Pirozzi F., Angelini A., et al. Novel perspectives in redox biology and pathophysiology of failing myocytes: modulation of the intramyocardial redox milieu for therapeutic interventions - a review article from the working group of cardiac cell biology, Italian Society of Cardiology. Oxid. Med. Cell. Longev. 2016;2016:6353469. doi: 10.1155/2016/6353469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen G., Ni Y., Nagata N., Xu L., Ota T. Micronutrient antioxidants and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2016;17(9):e1379. doi: 10.3390/ijms17091379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen T., Galougahi K.K., Celermajer D., et al. Oxidative and nitrosative signalling in pulmonary arterial hypertension - Implications for development of novel therapies. Pharmacol. Ther. 2016;165:50–62. doi: 10.1016/j.pharmthera.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Peng J.J., Liu B., Xu J.Y., Peng J., Luo X.J. NADPH oxidase: its potential role in promotion of pulmonary arterial hypertension. Naunyn Schmiedebergs Arch. Pharmacol. 2017;390(4):331–338. doi: 10.1007/s00210-017-1359-2. [DOI] [PubMed] [Google Scholar]
- 22.Richter K., Kietzmann T. Reactive oxygen species and fibrosis: further evidence of a significant liaison. Cell Tissue Res. 2016;365(3):591–605. doi: 10.1007/s00441-016-2445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuo L., Chuang C.C., Hemmelgarn B.T., Best T.M. Heart failure with preserved ejection fraction: Defining the function of ROS and NO. J. Appl. Physiol. 2015;119(8):944–951. doi: 10.1152/japplphysiol.01149.2014. [DOI] [PubMed] [Google Scholar]
- 24.Hempel N, Trebak M. Crosstalk between calcium and reactive oxygen species signaling in cancer. 2017. [DOI] [PMC free article] [PubMed]
- 25.Roots R., Okada S. Estimation of life times and diffusion distances of radicals involved in X-ray induced DNA strand breaks or killing of mammalian cells. Radiat. Res. 1975;64(2):306–320. [PubMed] [Google Scholar]
- 26.Butorina D.N., Krasnovskiĭ A.A., Priezzhev A.V. Study of kinetic parameters of singlet molecular oxygen in aqueous porphyrin solutions. Effect of detergents and the quencher sodium azide. Biofizika. 2003;48(2):201–209. [PubMed] [Google Scholar]
- 27.Kanofsky J.R. Quenching of singlet oxygen by human red cell ghosts. Photochem. Photobiol. 1991;53(1):93–99. doi: 10.1111/j.1751-1097.1991.tb08472.x. [DOI] [PubMed] [Google Scholar]
- 28.Bienert G.P., Moller A.L., Kristiansen K.A., et al. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007;282(2):1183–1192. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- 29.Li K., Zhang W., Fang H., et al. Superoxide flashes reveal novel properties of mitochondrial reactive oxygen species excitability in cardiomyocytes. Biophys. J. 2012;102(5):1011–1021. doi: 10.1016/j.bpj.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W., Fang H., Groom L., et al. Superoxide flashes in single mitochondria. Cell. 2008;134(2):279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marla S.S., Lee J., Groves J.T. Peroxynitrite rapidly permeates phospholipid membranes. Proc. Natl. Acad. Sci. USA. 1997;94(26):14243–14248. doi: 10.1073/pnas.94.26.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y.R., Zweier J.L. Cardiac mitochondria and reactive oxygen species generation. Circ. Res. 2014;114(3):524–537. doi: 10.1161/CIRCRESAHA.114.300559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Genova M.L., Pich M.M., Bernacchia A., et al. The mitochondrial production of reactive oxygen species in relation to aging and pathology. Ann. N. Y. Acad. Sci. 2004;1011:86–100. doi: 10.1007/978-3-662-41088-2_10. [DOI] [PubMed] [Google Scholar]
- 34.Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 35.Genova M.L., Ventura B., Giuliano G., et al. The site of production of superoxide radical in mitochondrial Complex I is not a bound ubisemiquinone but presumably iron-sulfur cluster N2. FEBS Lett. 2001;505(3):364–368. doi: 10.1016/s0014-5793(01)02850-2. [DOI] [PubMed] [Google Scholar]
- 36.Brand M.D. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 2010;45(7-8):466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barth E., Stämmler G., Speiser B., Schaper J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J. Mol. Cell. Cardiol. 1992;24(7):669–681. doi: 10.1016/0022-2828(92)93381-s. [DOI] [PubMed] [Google Scholar]
- 39.Granger D.N., Kvietys P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015;6:524–551. doi: 10.1016/j.redox.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pain T., Yang X.M., Critz S.D., et al. Opening of mitochondrial KATP channels triggers the preconditioned state by generating free radicals. Circ. Res. 2000;87(6):460–466. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- 41.Oldendorf W.H., Cornford M.E., Brown W.J. The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann. Neurol. 1977;1(5):409–417. doi: 10.1002/ana.410010502. [DOI] [PubMed] [Google Scholar]
- 42.Santos C.X., Raza S., Shah A.M. Redox signaling in the cardiomyocyte: From physiology to failure. Int. J. Biochem. Cell Biol. 2016;74:145–151. doi: 10.1016/j.biocel.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Brandes R.P., Weissmann N., Schröder K. Nox family NADPH oxidases: Molecular mechanisms of activation. Free Radic. Biol. Med. 2014;76:208–226. doi: 10.1016/j.freeradbiomed.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 44.Ibi M., Matsuno K., Matsumoto M., et al. Involvement of NOX1/NADPH oxidase in morphine-induced analgesia and tolerance. J. Neurosci. 2011;31(49):18094–18103. doi: 10.1523/JNEUROSCI.4136-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo S., Lei H., Qin H., Xia Y. Molecular mechanisms of endothelial NO synthase uncoupling. Curr. Pharm. Des. 2014;20(22):3548–3553. doi: 10.2174/13816128113196660746. [DOI] [PubMed] [Google Scholar]
- 46.Eddy L.J., Stewart J.R., Jones H.P., Engerson T.D., McCord J.M., Downey J.M. Free radical-producing enzyme, xanthine oxidase, is undetectable in human hearts. Am. J. Physiol. 1987;253(3 Pt 2):H709–H11. doi: 10.1152/ajpheart.1987.253.3.H709. [DOI] [PubMed] [Google Scholar]
- 47.Rudyk O., Eaton P. Biochemical methods for monitoring protein thiol redox states in biological systems. Redox Biol. 2014;2:803–813. doi: 10.1016/j.redox.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wouters M.A., Iismaa S., Fan S.W., Haworth N.L. Thiol-based redox signalling: rust never sleeps. Int. J. Biochem. Cell Biol. 2011;43(8):1079–1085. doi: 10.1016/j.biocel.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 49.Finkel T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011;194(1):7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Canton M., Skyschally A., Menabò R., et al. Oxidative modification of tropomyosin and myocardial dysfunction following coronary microembolization. Eur. Heart J. 2006;27(7):875–881. doi: 10.1093/eurheartj/ehi751. [DOI] [PubMed] [Google Scholar]
- 51.Heusch P., Canton M., Aker S., et al. The contribution of reactive oxygen species and p38 mitogen-activated protein kinase to myofilament oxidation and progression of heart failure in rabbits. Br. J. Pharmacol. 2010;160(6):1408–1416. doi: 10.1111/j.1476-5381.2010.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sivakumaran V., Stanley B.A., Tocchetti C.G., et al. HNO enhances SERCA2a activity and cardiomyocyte function by promoting redox-dependent phospholamban oligomerization. Antioxid. Redox Signal. 2013;19(11):1185–1197. doi: 10.1089/ars.2012.5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luczak E.D., Anderson M.E. CaMKII oxidative activation and the pathogenesis of cardiac disease. J. Mol. Cell. Cardiol. 2014;73:112–116. doi: 10.1016/j.yjmcc.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erickson J.R., Pereira L., Wang L., et al. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013;502(7471):372–376. doi: 10.1038/nature12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Purohit A., Rokita A.G., Guan X., et al. Oxidized CaMKII triggers atrial fibrillation. Circulation. 2013;128(16):1748–1757. doi: 10.1161/CIRCULATIONAHA.113.003313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howe C.J., Lahair M.M., McCubrey J.A., Franklin R.A. Redox regulation of the calcium/calmodulin-dependent protein kinases. J. Biol. Chem. 2004;279(43):44573–44581. doi: 10.1074/jbc.M404175200. [DOI] [PubMed] [Google Scholar]
- 57.Frankenreiter S., Bednarczyk P., Kniess A., et al. cGMP-elevating compounds and ischemic conditioning provide cardioprotection against ischemia and reperfusion injury via cardiomyocyte-specific BK channels. Circulation. 2017;136(24):2337–2355. doi: 10.1161/CIRCULATIONAHA.117.028723. [DOI] [PubMed] [Google Scholar]
- 58.Burgoyne J.R., Madhani M., Cuello F., et al. Cysteine redox sensor in PKGIα enables oxidant-induced activation. Science. 2007;317(5843):1393–1397. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]
- 59.Prysyazhna O., Rudyk O., Eaton P. Single atom substitution in mouse protein kinase G eliminates oxidant sensing to cause hypertension. Nat. Med. 2012;18(2):286–290. doi: 10.1038/nm.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakamura T., Ranek M.J., Lee D.I., et al. Prevention of PKG1 oxidation augments cardioprotection in the stressed heart. J. Clin. Invest. 2015;125(6):2468–2472. doi: 10.1172/JCI80275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brennan J.P., Bardswell S.C., Burgoyne J.R., et al. Oxidant-induced activation of type I protein kinase A is mediated by RIsubunit interprotein disulfide bond formation. J. Biol. Chem. 2006;281(31):21827–21836. doi: 10.1074/jbc.M603952200. [DOI] [PubMed] [Google Scholar]
- 62.Burgoyne J.R., Rudyk O., Cho H.J., et al. Deficient angiogenesis in redox-dead Cys17Ser PKARI knock-in mice. Nat. Commun. 2015;10:7920–7928. doi: 10.1038/ncomms8920. [DOI] [PubMed] [Google Scholar]
- 63.Finch J.S., Tome M.E., Kwei K.A., Bowden G.T. Catalase reverses tumorigenicity in a malignant cell line by an epidermal growth factor receptor pathway. Free Radic. Biol. Med. 2006;40(5):863–875. doi: 10.1016/j.freeradbiomed.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 64.Lei H., Kazlauskas A. Growth factors outside of the platelet-derived growth factor (PDGF) family employ reactive oxygen species/Src family kinases to activate PDGF receptor α and thereby promote proliferation and survival of cells. J. Biol. Chem. 2009;284(10):6329–6336. doi: 10.1074/jbc.M808426200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leon-Buitimea A., Rodríguez-Fragoso L., Lauer F.T., Bowles H., Thompson T.A., Burchiel S.W. Ethanol-induced oxidative stress is associated with EGF receptor phosphorylation inMCF- 10A cells overexpressing CYP2E1. Toxicol. Lett. 2012;209(2):161–165. doi: 10.1016/j.toxlet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Preston T.J., Muller W.J., Singh G. Scavenging of extracellular H2O2 by catalase inhibits the proliferation of HER-2/Neu-transformed rat-1 fibroblasts through the induction of a stress response. J. Biol. Chem. 2001;276(12):9558–9564. doi: 10.1074/jbc.M004617200. [DOI] [PubMed] [Google Scholar]
- 67.Cheng C.W., Kuo C.Y., Fan C.C., et al. Overexpression of Lon contributes to survival and aggressive phenotype of cancer cells through mitochondrial complex I-mediated generation of reactive oxygen species. Cell Death Dis. 2013;4:e681. doi: 10.1038/cddis.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Q., Fu G.B., Zheng J.T., et al. NADPH oxidase subunit p22(phox)-mediated reactive oxygen species contribute to angiogenesis and tumor growth through AKT and ERK1/2 signaling pathways in prostate cancer. Biochim. Biophys. Acta. 2013;1833(12):3375–3385. doi: 10.1016/j.bbamcr.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 69.Lin X., Zheng W., Liu J., et al. Oxidative stress in malignant melanoma enhances tumor necrosis factor-α secretion of tumor-associated macrophages that promote cancer cell invasion. Antioxid. Redox Signal. 2013;19(12):1337–1355. doi: 10.1089/ars.2012.4617. [DOI] [PubMed] [Google Scholar]
- 70.Wang N., Zhan T., Ke T., et al. Increased expression of RRM2 by human papillomavirus E7 oncoprotein promotes angiogenesis in cervical cancer. Br. J. Cancer. 2014;110(4):1034–1044. doi: 10.1038/bjc.2013.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang J., Lin D., Peng H., Huang Y., Huang J., Gu J. Cancer-derived immunoglobulin G promotes tumor cell growth and proliferation through inducing production of reactive oxygen species. Cell Death Dis. 2013;4:e945. doi: 10.1038/cddis.2013.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim J.H., Na H.J., Kim C.K., et al. The non-provitamin A carotenoid, lutein, inhibits NF-κB-dependent gene expression through redox-based regulation of the phosphatidylinositol 3-kinase/PTEN/Akt and NF-κB-inducing kinase pathways: role of H2O2 in NF-κB activation. Free Radic. Biol. Med. 2008;45(6):885–896. doi: 10.1016/j.freeradbiomed.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 73.Lee S.R., Yang K.S., Kwon J., Lee C., Jeong W., Rhee S.G. Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 2002;277(23):20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 74.Murata H., Ihara Y., Nakamura H., Yodoi J., Sumikawa K., Kondo T. Glutaredoxin exerts an antiapoptotic effect by regulating the redox state of Akt. J. Biol. Chem. 2003;278(50):50226–50233. doi: 10.1074/jbc.M310171200. [DOI] [PubMed] [Google Scholar]
- 75.Liu Y., Shi Q.F., Ye Y.C., Tashiro S., Onodera S., Ikejima T. Activated O2(•−) and H2O2 mediated cell survival in SU11274-treated non-small-cell lung cancer A549 cells via c-Met-PI3K-Akt and c-Met-Grb2/SOSRas-p38 pathways. J. Pharmacol. Sci. 2012;119(2):150–159. doi: 10.1254/jphs.12048fp. [DOI] [PubMed] [Google Scholar]
- 76.Hausenloy D.J., Tsang A., Mocanu M.M., Yellon D.M. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2005;288(2):H971–H6. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 77.Mochly-Rosen D., Das K., Grimes K.V. Protein kinase C, an elusive therapeutic target? Nat. Rev. Drug Discov. 2012;11(12):937–957. doi: 10.1038/nrd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nitti M., Pronzato M.A., Marinari U.M., Domenicotti C. PKC signaling in oxidative hepatic damage. Mol. Aspects Med. 2008;29(1-2):36–42. doi: 10.1016/j.mam.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 79.Ytrehus K., Liu Y., Downey J.M. Preconditioning protects ischemic rabbit heart by protein kinase C activation. Am. J. Physiol. 1994;266(3 Pt 2):H1145–H52. doi: 10.1152/ajpheart.1994.266.3.H1145. [DOI] [PubMed] [Google Scholar]
- 80.Murry C.E., Richard V.J., Jennings R.B., Reimer K.A. Preconditioning with ischemia: Is the protective effect mediated by free radical-induced myocardial stunning? Circulation. 1988;78(Suppl. II):II-77. [Google Scholar]
- 81.Tanaka M., Fujiwara H., Yamasaki K., Sasayama S. Superoxide dismutase and N-2-mercaptopropionyl glycine attenuate infarct size limitation effect of ischaemic preconditioning in the rabbit. Cardiovasc. Res. 1994;28(7):980–986. doi: 10.1093/cvr/28.7.980. [DOI] [PubMed] [Google Scholar]
- 82.Chen W., Gabel S., Steenbergen C., Murphy E. A redox-based mechanism for cardioprotection induced by ischemic preconditioning in perfused rat heart. Circ. Res. 1995;77(2):424–429. doi: 10.1161/01.res.77.2.424. [DOI] [PubMed] [Google Scholar]
- 83.Das D.K., Maulik N., Sato M., Ray P.S. Reactive oxygen species function as second messenger during ischemic preconditioning of heart. Mol. Cell. Biochem. 1999;196(1-2):59–67. [PubMed] [Google Scholar]
- 84.Skyschally A., Schulz R., Gres P., Korth H.G., Heusch G. Attenuation of ischemic preconditioning in pigs by scavenging of free oxyradicals with ascorbic acid. Am. J. Physiol. Heart Circ. Physiol. 2003;284(2):H698–H703. doi: 10.1152/ajpheart.00693.2002. [DOI] [PubMed] [Google Scholar]
- 85.Baines C.P., Goto M., Downey J.M. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J. Mol. Cell. Cardiol. 1997;29(1):207–216. doi: 10.1006/jmcc.1996.0265. [DOI] [PubMed] [Google Scholar]
- 86.Penna C., Rastaldo R., Mancardi D., Raimondo S., Cappello S., Gattullo D., Losano G., Pagliaro P. Post-conditioning induced cardioprotection requires signaling through a redox-sensitive mechanism, mitochondrial ATP-sensitive K+ channel and protein kinase C activation. Basic Res. Cardiol. 2006;101(2):180–189. doi: 10.1007/s00395-006-0584-5. [DOI] [PubMed] [Google Scholar]
- 87.Tsutsumi Y.M., Yokoyama T., Horikawa Y., Roth D.M., Patel H.H. Reactive oxygen species trigger ischemic and pharmacological postconditioning: In vivo and in vitro characterization. Life Sci. 2007;81(15):1223–1227. doi: 10.1016/j.lfs.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Costa A.D., Jakob R., Costa C.L., Andrukhiv K., West I.C., Garlid K.D. The mechanism by which the mitochondrial ATP-sensitive K+ channel opening and H2O2 inhibit the mitochondrial permeability transition. J. Biol. Chem. 2006;281(30):20801–20808. doi: 10.1074/jbc.M600959200. [DOI] [PubMed] [Google Scholar]
- 89.Costa A.D., Garlid K.D. Intramitochondrial signaling: Interactions among mitoKATP, PKCε, ROS, and MPT. Am. J. Physiol. Heart Circ. Physiol. 2008;295(2):H874–H82. doi: 10.1152/ajpheart.01189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sharma A., Singh M. Protein kinase C activation and cardioprotective effect of preconditioning with oxidative stress in isolated rat heart. Mol. Cell. Biochem. 2001;219(1-2):1–6. doi: 10.1023/a:1011038531656. [DOI] [PubMed] [Google Scholar]
- 91.Dost T., Cohen M.V., Downey J.M. Redox signaling triggers protection during the reperfusion rather than the ischemic phase of preconditioning. Basic Res. Cardiol. 2008;103(4):378–384. doi: 10.1007/s00395-008-0718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cohen M.V., Yang X.M., Downey J.M. The pH hypothesis of postconditioning: staccato reperfusion reintroduces oxygen and perpetuates myocardial acidosis. Circulation. 2007;115(14):1895–1903. doi: 10.1161/CIRCULATIONAHA.106.675710. [DOI] [PubMed] [Google Scholar]
- 93.Konishi H., Tanaka M., Takemura Y., et al. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc. Natl. Acad. Sci. USA. 1997;94(21):11233–11237. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Q.J. PKD at the crossroads of DAG and PKC signaling. Trends Pharmacol. Sci. 2006;27(6):317–323. doi: 10.1016/j.tips.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 95.Storz P., Döppler H., Toker A. Activation loop phosphorylation controls protein kinase D-dependent activation of nuclear factor κB. Mol. Pharmacol. 2004;66(4):870–879. doi: 10.1124/mol.104.000687. [DOI] [PubMed] [Google Scholar]
- 96.Yang X., Cohen M.V., Downey J.M. Mechanism of cardioprotection by early ischemic preconditioning. Cardiovasc. Drugs Ther. 2010;24(3):225–234. doi: 10.1007/s10557-010-6236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim S.H., Kim K.H., Yoo B.C., Ku J.L. Induction of LGR5 by H2O2 treatment is associated with cell proliferation via the JNK signaling pathway in colon cancer cells. Int. J. Oncol. 2012;41(5):1744–1750. doi: 10.3892/ijo.2012.1596. [DOI] [PubMed] [Google Scholar]
- 98.Kamata H., Honda S., Maeda S., Chang L., Hirata H., Karin M. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120(5):649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 99.Zhang M., Prosser B.L., Bamboye M.A., et al. Contractile function during angiotensin-II activation: increased Nox2 activity modulates cardiac calcium handling via phospholamban phosphorylation. J. Am. Coll. Cardiol. 2015;66(3):261–272. doi: 10.1016/j.jacc.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mesquita F.S., Dyer S.N., Heinrich D.A., Bulun S.E., Marsh E.E., Nowak R.A. Reactive oxygen species mediate mitogenic growth factor signaling pathways in human leiomyoma smooth muscle cells. Biol. Reprod. 2010;82(2):341–351. doi: 10.1095/biolreprod.108.075887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kranias E.G., Hajjar R.J. Modulation of cardiac contractility by the phospholamban/SERCA2a regulatome. Circ. Res. 2012;110(12):1646–1660. doi: 10.1161/CIRCRESAHA.111.259754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zalk R., Clarke O.B., des Georges A., et al. Structure of a mammalian ryanodine receptor. Nature. 2015;517(7532):444–449. doi: 10.1038/nature13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Prosser B.L., Ward C.W., Lederer W.J. X-ROS signaling: Rapid mechano-chemo transduction in heart. Science. 2011;333(6048):1440–1445. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- 104.Prosser B.L., Khairallah R.J., Ziman A.P., Ward C.W., Lederer W.J. X-ROS signaling in the heart and skeletal muscle: stretch-dependent local ROS regulates [Ca2+]i. J. Mol. Cell. Cardiol. 2013;58:172–181. doi: 10.1016/j.yjmcc.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simon J.N., Duglan D., Casadei B., Carnicer R. Nitric oxide synthase regulation of cardiac excitation-contraction coupling in health and disease. J. Mol. Cell. Cardiol. 2014;73:80–91. doi: 10.1016/j.yjmcc.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 106.Sun Q.A., Hess D.T., Nogueira L., et al. Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel by NADPH oxidase 4. Proc. Natl. Acad. Sci. USA. 2011;108(38):16098–16103. doi: 10.1073/pnas.1109546108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lancel S., Qin F., Lennon S.L., et al. Oxidative posttranslational modifications mediate decreased SERCA activity and myocyte dysfunction in Gαq-overexpressing mice. Circ. Res. 2010;107(2):228–232. doi: 10.1161/CIRCRESAHA.110.217570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qin F., Siwik D., Pimentel D.R., et al. Cytosolic H2O2 mediates hypertrophy, apoptosis, and decreased SERCA activity in mice with chronic hemodynamic overload. Am. J. Physiol. Heart Circ. Physiol. 2014;306(10):H1453–H63. doi: 10.1152/ajpheart.00084.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zaidi A., Barŕon L., Sharov V.S., Schöneich C., Michaelis E.K., Michaelis M.L. Oxidative inactivation of purified plasma membrane Ca2 -ATPase by hydrogen peroxide and protection by calmodulin. Biochemistry. 2003;42(41):12001–12010. doi: 10.1021/bi034565u. [DOI] [PubMed] [Google Scholar]
- 110.Kaneko S., Kawakami S., Hara Y., et al. A critical role of TRPM2 in neuronal cell death by hydrogen peroxide. J. Pharmacol. Sci. 2006;101(1):66–76. doi: 10.1254/jphs.fp0060128. [DOI] [PubMed] [Google Scholar]
- 111.Kühn F.J., Heiner I., Lückhoff A. TRPM2: a calcium influx pathway regulated by oxidative stress and the novel second messenger ADP-ribose. Pflugers Arch. 2005;451(1):212–219. doi: 10.1007/s00424-005-1446-y. [DOI] [PubMed] [Google Scholar]
- 112.Naziroğlu M., Lückhoff A. A calcium influx pathway regulated separately by oxidative stress and ADP-ribose in TRPM2 channels: single channel events. Neurochem. Res. 2008;33(7):1256–1262. doi: 10.1007/s11064-007-9577-5. [DOI] [PubMed] [Google Scholar]
- 113.Ishii M., Oyama A., Hagiwara T., et al. Facilitation of H2O2-induced A172 human glioblastoma cell death by insertion of oxidative stress-sensitive TRPM2 channels. Anticancer Res. 2007;27(6B):3987–3992. [PubMed] [Google Scholar]
- 114.Sun L., Yau H.Y., Wong W.Y., Li R.A., Huang Y., Yao X. Role of TRPM2 in H2O2-induced cell apoptosis in endothelial cells. PLoS One. 2012;7(8):e43186. doi: 10.1371/journal.pone.0043186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Farfariello V., Amantini C., Santoni G. Transient receptor potential vanilloid 1 activation induces autophagy in thymocytes through ROS-regulated AMPK and Atg4C pathways. J. Leukoc. Biol. 2012;92(3):421–431. doi: 10.1189/jlb.0312123. [DOI] [PubMed] [Google Scholar]
- 116.Wang G.L., Jiang B.H., Rue E.A., Semenza G.L. Hypoxia-inducible factor 1 is a basic-helix-loophelix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA. 1995;92(12):5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Semenza G.L. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kaelin W.G., Ratcliffe P.J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell. 2008;30(4):393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 119.Semenza G.L. Hydroxylation of HIF-1: Oxygen sensing at the molecular level. Physiology (Bethesda) 2004;19:176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- 120.Waypa G.B., Marks J.D., Guzy R., et al. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ. Res. 2010;106(3):526–535. doi: 10.1161/CIRCRESAHA.109.206334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bell E.L., Klimova T.A., Eisenbart J., et al. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J. Cell Biol. 2007;177(6):1029–1036. doi: 10.1083/jcb.200609074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brunelle J.K., Bell E.L., Quesada N.M., et al. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1(6):409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 123.Guzy R.D., Hoyos B., Robin E., et al. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1(6):401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 124.Mansfield K.D., Guzy R.D., Pan Y., et al. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-α activation. Cell Metab. 2005;1(6):393–399. doi: 10.1016/j.cmet.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Orr A.L., Vargas L., Turk C.N., et al. Suppressors of superoxide production from mitochondrial complex III. Nat. Chem. Biol. 2015;11(11):834–836. doi: 10.1038/nchembio.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cai Z., Zhong H., Bosch-Marce M., Fox-Talbot K., et al. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1α. Cardiovasc. Res. 2008;77(3):463–470. doi: 10.1093/cvr/cvm035. [DOI] [PubMed] [Google Scholar]
- 127.Heusch G. HIF-1α and paradoxical phenomena in cardioprotection. Cardiovasc. Res. 2012;96(2):214–215. doi: 10.1093/cvr/cvs145. [DOI] [PubMed] [Google Scholar]
- 128.Darnell J.E., Kerr I.M., Stark G.R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 129.Darnell J.E. STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 130.Ginsberg M., Czeko E., Müller P., Ren Z., Chen X., Darnell J.E. Amino acid residues required for physical and cooperative transcriptional interaction of STAT3 and AP-1 proteins c-Jun and c-Fos. Mol. Cell. Biol. 2007;27(18):6300–6308. doi: 10.1128/MCB.00613-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grivennikov S.I., Karin M. Dangerous liaisons: STAT3 and NF-κB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21(1):11–19. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Magne S., Caron S., Charon M., Rouyez M.C., Dusanter-Fourt I. STAT5 and Oct-1 form a stable complex that modulates Cyclin D1 expression. Mol. Cell. Biol. 2003;23(24):8934–8945. doi: 10.1128/MCB.23.24.8934-8945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.DaSilva L., Rui H., Erwin R.A., et al. Prolactin recruits STAT1, STAT3 and STAT5 independent of conserved receptor tyrosines TYR402, TYR479, TYR515 and TYR580. Mol. Cell. Endocrinol. 1996;117(2):131–140. doi: 10.1016/0303-7207(95)03738-1. [DOI] [PubMed] [Google Scholar]
- 134.Hirano T., Ishihara K., Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19(21):2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 135.Buettner R., Mora L.B., Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin. Cancer Res. 2002;8(4):945–954. [PubMed] [Google Scholar]
- 136.Heusch G., Musiolik J., Gedik N., Skyschally A. Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ. Res. 2011;109(11):1302–1308. doi: 10.1161/CIRCRESAHA.111.255604. [DOI] [PubMed] [Google Scholar]
- 137.Heusch G., Musiolik J., Kottenberg E., Peters J., Jakob H., Thielmann M. STAT5 activation and cardioprotection by remote ischemic preconditioning in humans: Short communication. Circ. Res. 2012;110(1):111–115. doi: 10.1161/CIRCRESAHA.111.259556. [DOI] [PubMed] [Google Scholar]
- 138.Iiyama M., Kakihana K., Kurosu T., Miura O. Reactive oxygen species generated by hematopoietic cytokines play roles in activation of receptor-mediated signaling and in cell cycle progression. Cell. Signal. 2006;18(2):174–182. doi: 10.1016/j.cellsig.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 139.Sattler M., Winkler T., Verma S., Byrne C.H., Shrikhande G., Salgia R., Griffin J.D. Hematopoietic growth factors signal through the formation of reactive oxygen species. Blood. 1999;93(9):2928–2935. [PubMed] [Google Scholar]
- 140.Simon A.R., Rai U., Fanburg B.L., Cochran B.H. Activation of the JAK-STAT pathway by reactive oxygen species. Am. J. Physiol. 1998;275(6 Pt 1):C1640–C52. doi: 10.1152/ajpcell.1998.275.6.C1640. [DOI] [PubMed] [Google Scholar]
- 141.Jay D.B., Papaharalambus C.A., Seidel-Rogol B., Dikalova A.E., Lassègue B., Griendling K.K. Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic. Biol. Med. 2008;45(3):329–335. doi: 10.1016/j.freeradbiomed.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yoon S., Woo S.U., Kang J.H., et al. STAT3 transcriptional factor activated by reactive oxygen species induces IL6 in starvation-induced autophagy of cancer cells. Autophagy. 2010;6(8):1125–1138. doi: 10.4161/auto.6.8.13547. [DOI] [PubMed] [Google Scholar]
- 143.Smith J.K., Patil C.N., Patlolla S., Gunter B.W., Booz G.W., Duhé R.J. Identification of a redox-sensitive switch within the JAK2 catalytic domain. Free Radic. Biol. Med. 2012;52(6):1101–1110. doi: 10.1016/j.freeradbiomed.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li L., Cheung S.H., Evans E.L., Shaw P.E. Modulation of gene expression and tumor cell growth by redox modification of STAT3. Cancer Res. 2010;70(20):8222–8232. doi: 10.1158/0008-5472.CAN-10-0894. [DOI] [PubMed] [Google Scholar]
- 145.Li L., Shaw P.E.A. STAT3 dimer formed by inter-chain disulphide bridging during oxidative stress. Biochem. Biophys. Res. Commun. 2004;322(3):1005–1011. doi: 10.1016/j.bbrc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 146.Shaw P.E. Could STAT3 provide a link between respiration and cell cycle progression? Cell Cycle. 2010;9(21):4294–4296. doi: 10.4161/cc.9.21.13677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sebastian C., Herrero C., Serra M., Lloberas J., Blasco M.A., Celada A. Telomere shortening and oxidative stress in aged macrophages results in impaired STAT5a phosphorylation. J. Immunol. 2009;183(4):2356–2364. doi: 10.4049/jimmunol.0901131. [DOI] [PubMed] [Google Scholar]
- 148.Van der Heiden K., Cuhlmann S. Luong le A, Zakkar M, Evans PC. Role of nuclear factor κB in cardiovascular health and disease. Clin. Sci. (Lond.) 2010;118(10):593–605. doi: 10.1042/CS20090557. [DOI] [PubMed] [Google Scholar]
- 149.Schoonbroodt S., Ferreira V., Best-Belpomme M., et al. Crucial role of the amino-terminal tyrosine residue 42 and the carboxylterminal PEST domain of of IκBα in NF-κB activation by an oxidative stress. J. Immunol. 2000;164(8):4292–4300. doi: 10.4049/jimmunol.164.8.4292. [DOI] [PubMed] [Google Scholar]
- 150.Takada Y., Mukhopadhyay A., Kundu G.C., Mahabeleshwar G.H., Singh S., Aggarwal B.B. Hydrogen peroxide activates NF-κB through tyrosine phosphorylation of IκBα and serine phosphorylation of p65. Evidence for the involvement of IκBα kinase and Syk protein-tyrosine kinase. J. Biol. Chem. 2003;278(26):24233–24441. doi: 10.1074/jbc.M212389200. [DOI] [PubMed] [Google Scholar]
- 151.Li Q., Engelhardt J.F. Interleukin-1β induction of NFκB is partially regulated by H2O2-mediated activation of NFκB-inducing kinase. J. Biol. Chem. 2006;281(3):1495–1505. doi: 10.1074/jbc.M511153200. [DOI] [PubMed] [Google Scholar]
- 152.Diebold L., Chandel N.S. Mitochondrial ROS regulation of proliferating cells. Free Radic. Biol. Med. 2016;100:86–93. doi: 10.1016/j.freeradbiomed.2016.04.198. [DOI] [PubMed] [Google Scholar]
- 153.Campos J.C., Gomes K.M., Ferreira J.C. Impact of exercise training on redox signaling in cardiovascular diseases. Food Chem. Toxicol. 2013;62:107–119. doi: 10.1016/j.fct.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 154.Yamashita N., Hoshida S., Otsu K., Asahi M., Kuzuya T., Hori M. Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. J. Exp. Med. 1999;189(11):1699–1706. doi: 10.1084/jem.189.11.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yamashita N., Hoshida S., Otsu K., Hori M. Repeated physiologic stresses provide persistent cardioprotection against ischemia-reperfusion injury in rats. J. Am. Coll. Cardiol. 2002;40(4):826–831. doi: 10.1016/s0735-1097(02)02001-6. [DOI] [PubMed] [Google Scholar]
- 156.Wang B., Xu M., Li W., Li X., Zheng Q., Niu X. Aerobic exercise protects against pressure overload-induced cardiac dysfunction and hypertrophy via β3-AR-nNOS-NO activation. PLoS One. 2017;12(6):e0179648. doi: 10.1371/journal.pone.0179648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Corbi G., Conti V., Russomanno G., et al. Is physical activity able to modify oxidative damage in cardiovascular aging? Oxid. Med. Cell. Longev. 2012;2012:728547. doi: 10.1155/2012/728547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gomes M.J., Martinez P.F., Campos D.H., et al. Beneficial effects of physical exercise on functional capacity and skeletal muscle oxidative stress in rats with aortic stenosis-induced heart failure. Oxid. Med. Cell. Longev. 2016;2016:8695716. doi: 10.1155/2016/8695716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li H., Miao W., Ma J., et al. Acute exercise-induced mitochondrial stress triggers an inflammatory response in the myocardium via NLRP3 inflammasome activation with mitophagy. Oxid. Med. Cell. Longev. 2016;2016:1987149. doi: 10.1155/2016/1987149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Venditti P., Napolitano G., Barone D., Pervito E., Di Meo S. Vitamin E-enriched diet reduces adaptive responses to training determining respiratory capacity and redox homeostasis in rat heart. Free Radic. Res. 2016;50(1):56–67. doi: 10.3109/10715762.2015.1106530. [DOI] [PubMed] [Google Scholar]
- 161.Ma X., Fu Y., Xiao H., et al. Cardiac fibrosis alleviated by exercise training is AMPK-dependent. PLoS One. 2015;10(6):e0129971. doi: 10.1371/journal.pone.0129971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Farhat F., Dupas J., Amérand A., et al. Effect of exercise training on oxidative stress and mitochondrial function in rat heart and gastrocnemius muscle. Redox Rep. 2015;20(2):60–68. doi: 10.1179/1351000214Y.0000000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Campos J.C., Queliconi B.B., Bozi L.H.M., et al. Exercise reestablishes autophagic flux and mitochondrial quality control in heart failure. Autophagy. 2017;13(8):1304–1317. doi: 10.1080/15548627.2017.1325062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cunha T.F., Bechara L.R., Bacurau A.V., et al. Exercise training decreases NADPH oxidase activity and restores skeletal muscle mass in heart failure rats. J. Appl. Physiol. 2017;122(4):817–827. doi: 10.1152/japplphysiol.00182.2016. [DOI] [PubMed] [Google Scholar]
- 165.Campos J.C., Fernandes T., Bechara L.R., et al. Increased clearance of reactive aldehydes and damaged proteins in hypertension-induced compensated cardiac hypertrophy: impact of exercise training. Oxid. Med. Cell. Longev. 2015;2015:464195. doi: 10.1155/2015/464195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gao C., Chen X., Li J., et al. Myocardial mitochondrial oxidative stress and dysfunction in intense exercise: regulatory effects of quercetin. Eur. J. Appl. Physiol. 2014;114(4):695–705. doi: 10.1007/s00421-013-2802-9. [DOI] [PubMed] [Google Scholar]
- 167.Prasad S., Gupta S.C., Tyagi A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017;387:95–105. doi: 10.1016/j.canlet.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 168.Watanabe Y., Cohen R.A., Matsui R. Redox regulation of ischemic angiogenesis - another aspect of reactive oxygen species. Circ. J. 2016;80(6):1278–1284. doi: 10.1253/circj.CJ-16-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang L., Wang K., Lei Y., Li Q., Nice E.C., Huang C. Redox signaling: Potential arbitrator of autophagy and apoptosis in therapeutic response. Free Radic. Biol. Med. 2015;89:452–465. doi: 10.1016/j.freeradbiomed.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 170.Rodney G.G., Pal R., Abo-Zahrah R. Redox regulation of autophagy in skeletal muscle. Free Radic. Biol. Med. 2016;98:103–112. doi: 10.1016/j.freeradbiomed.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]