We read with interest the report by Bocchi and colleagues of their post hoc analysis of the Systolic Heart failure treatment with the I f inhibitor ivabradine Trial (SHIFT), examining the effect of study drug in the 38 patients with chronic chagasic cardiomyopathy (CCC).1
The authors reported that study drug lowered heart rate and improved New York Heart Association class. The sample size was too small to allow estimation of the effect of treatment on mortality or hospitalization. However, this analysis did suggest that patients with CCC experienced high event rates, despite excellent background therapy.
We examined outcomes in patients with CCC in the Prospective comparison of Angiotensin Receptor Neprilysin Inhibitor with Angiotensin Converting Enzyme Inhibitor to Determine Impact on Global Mortality and morbidity in Heart Failure (PARADIGM‐HF) and the Aliskiren trial to Minimize OutcomeS in Patients with Heart failure (ATMOSPHERE).2, 3
These trials included 195 CCC patients from among a total of 2552 recruited in Latin America. Despite being younger and having less co‐morbidity, the CCC patients had higher hospitalization and mortality rates, compared with other aetiologies, despite similarly good treatment.4
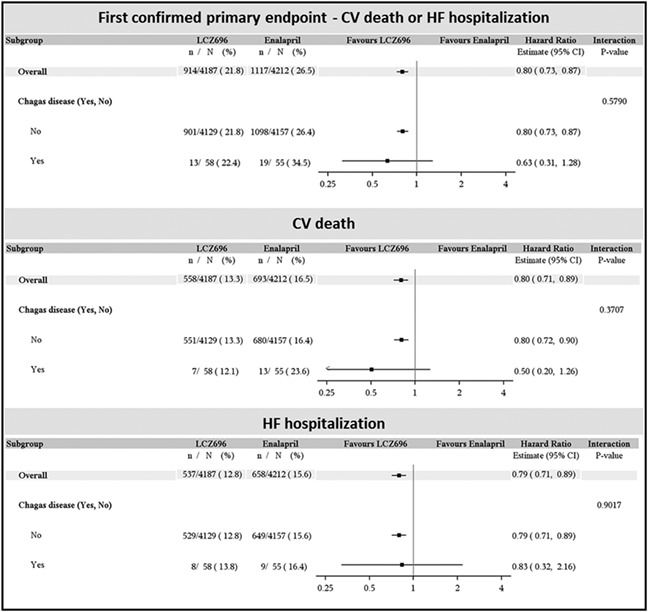
We also conducted an exploratory post hoc analysis of the effect of sacubitril/valsartan (formerly known as LCZ696) in CCC patients in PARADIGM‐HF. Of a total of 113 patients, 58 were randomized to sacubitril/valsartan and 55 to enalapril. The two treatment groups were similar in terms of demographics, co‐morbidity, and heart failure (HF) severity. Patients with CCC treated with sacubitril/valsartan, as compared with enalapril, had a lower risk of experiencing cardiovascular death or HF hospitalization, the primary composite endpoint, and each of its components (Figure). The point estimate for risk reduction was comparable with or greater than that seen with the drug vs. enalapril in the entire study population. This analysis is underpowered and should be interpreted with caution.
CCC is a major health issue in Latin America and is now recognized in the USA and Europe, reflecting contemporary migration patterns.5, 6, 7, 8 Indeed, a recent study from Brazil concluded that the population attributable mortality risk from CCC increased between 2002/2004 and 2012/2014.9 Future trials should consider recruiting larger numbers of patients with CCC to allow adequately powered subgroup analysis and even trials specifically in CCC would be justified, given the magnitude of this problem. Until that time, patients with CCC should be treated empirically with therapies recommended by guidelines, on the assumption that treatments for patients with reduced ejection fraction are effective, irrespective of aetiology of HF.
Ramires, F. J. A. , Martinez, F. , Gómez, E. A. , Demacq, C. , Gimpelewicz, C. R. , Rouleau, J. L. , Solomon, S. D. , Swedberg, K. , Zile, M. R. , Packer, M. , and McMurray, J. J. V. (2018) Post hoc analyses of SHIFT and PARADIGM‐HF highlight the importance of chronic Chagas' cardiomyopathy Comment on: “Safety profile and efficacy of ivabradine in heart failure due to Chagas heart disease: a post hoc analysis of the SHIFT trial” by Bocchi et al. . ESC Heart Failure, 5: 1069–1071. 10.1002/ehf2.12355.
References
- 1. Bocchi EA, Rassi S, Guimaraes GV, Argentina C, Brazil SI. Safety profile and efficacy of ivabradine in heart failure due to Chagas heart disease: a post hoc analysis of the SHIFT trial. ESC Heart Fail 2018; 5: 249‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krum H, McMurray JJ, Abraham WT, Dickstein K, Kober L, Desai AS, Solomon SD, Chiang Y, Gimpelewicz C, Reimund B, Ali MA, Tarnesby G, Massie BM, ATMOSPHERE Committees and Investigators . The Aliskiren Trial to Minimize OutcomeS in Patients with HEart failure trial (ATMOSPHERE): revised statistical analysis plan and baseline characteristics. Eur J Heart Fail 2015; 17: 1075–1083. [DOI] [PubMed] [Google Scholar]
- 3. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 4. Shen L, Ramires F, Martinez F, Bodanese LC, Echeverria LE, Gomez EA, Abraham WT, Dickstein K, Kober L, Packer M, Rouleau JL, Solomon SD, Swedberg K, Zile MR, Jhund PS, Gimpelewicz CR, McMurray JJV. Contemporary characteristics and outcomes in chagasic heart failure compared with other nonischemic and ischemic cardiomyopathy. Circ Heart Fail 2017; 10: e004361. [DOI] [PubMed] [Google Scholar]
- 5. Benziger CP, do Carmo GAL, Ribeiro ALP. Chagas cardiomyopathy: clinical presentation and management in the Americas. Cardiol Clin 2017; 35: 31–47. [DOI] [PubMed] [Google Scholar]
- 6. Bern C. Chagas' disease. N Engl J Med 2015; 373: 456–466. [DOI] [PubMed] [Google Scholar]
- 7. Antinori S, Galimberti L, Bianco R, Grande R, Galli M, Corbellino M. Chagas disease in Europe: a review for the internist in the globalized world. Eur J Intern Med 2017; 43: 6–15. [DOI] [PubMed] [Google Scholar]
- 8. Kalil‐Filho R. Globalization of Chagas disease burden and new treatment perspectives. J Am Coll Cardiol 2015; 66: 1190–1192. [DOI] [PubMed] [Google Scholar]
- 9. Nadruz W Jr, Gioli‐Pereira L, Bernardez‐Pereira S, Marcondes‐Braga FG, Fernandes‐Silva MM, Silvestre OM, Sposito AC, Ribeiro AL, Bacal F, Fernandes F, Krieger JE, Mansur AJ, Pereira AC. Temporal trends in the contribution of Chagas cardiomyopathy to mortality among patients with heart failure. Heart 2018; 104: 1522–1528. [DOI] [PubMed] [Google Scholar]


