Abstract
Aims
Patients with end‐stage heart failure (HF) often require surrogate decision making for end‐of‐life care owing to a lack of decision‐making capacity. However, the clinical characteristics of surrogate decision making for life‐sustaining treatments in Japan remain to be investigated.
Methods and results
Among 934 patients admitted to our hospital for HF from January 2004 to December 2015, we retrospectively reviewed the medical records of consecutive 106 patients who died in hospital (mean age 73 ± 13 years; male, 52.6%). During hospitalization, attending physicians conducted an average of 2.1 ± 1.4 end‐of‐life conversations with patients and/or their families. Only 4.7% of patients participated in the conversations and declared their preferences; surrogates made medical care decisions in 95.3% of cases. Most decisions by surrogates (98.1%) were made without the patient's advance directive. During initial end‐of‐life conversations, 49.4% of surrogates requested cardiopulmonary resuscitation (CPR). However, 72.0% of CPR preferences were changed to do not attempt resuscitation (DNAR) orders in the final conversation. Female surrogates were more likely to change the preference from CPR to DNAR than were male surrogates (47.1% vs. 25.0%, P = 0.023).
Conclusions
Compared with male surrogates, female surrogates wavered more often in their decisions regarding life‐sustaining treatments of Japanese patients with end‐stage HF.
Keywords: End‐stage heart failure, Surrogate decision making, Sex difference
Introduction
Heart failure (HF) is a major health concern worldwide, and it is an unpredictable, progressive, and incurable condition. In recent years, HF has become the leading cause of hospital admission. HF is associated with high morbidity and mortality, which imposes a large and costly burden on the health‐care system.1 Despite major advances in therapy, the prognosis of HF remains poor. In one large population‐based study, the 5 year survival following the first admission for HF was worse than that for many cancers.2
In recent guidelines, palliative care is recommended as a treatment option for patients with advanced HF whose symptoms and clinical status have progressed despite maximal medical therapy.3 Palliative care in non‐cancer patients has not been well established, and cardiologists and cardiac nurses are often unfamiliar with end‐of‐life care for HF.4 Advance care planning (ACP) involves preparations made to support surrogate decision making for medical care. ACP is useful for decisions regarding the treatment strategy for hospitalized elderly patients with advanced HF who frequently lack decision‐making capacity.5 However, ACP for end‐of‐life care is less frequently discussed at hospital admission for patients with HF than for those admitted with cancer.6 In addition, the concept of ACP is unfamiliar in Japan, and Japanese culture, which traditionally emphasizes familial harmony, engenders unique end‐of‐life communication styles that differ from those in other countries.7 A fatal diagnosis and prognosis are not often disclosed to the patient in Japan because family members wish to remove the burden of making decision from patients.8 Thus, Japanese are more likely to prefer family‐centred decision making for end‐of‐life care,7 and patients are less likely to make advance directive for medical care. Although previous studies in western countries investigated the patient's advance directive for life‐sustaining treatments in patients with HF,9, 10 the current status of surrogate decision making for end‐of‐life care in Japanese patients with HF remains to be investigated. The present study was performed to clarify the clinical characteristics of surrogate decision making for life‐sustaining treatments in Japanese patients with end‐stage HF.
Methods
We retrospectively reviewed the medical records of consecutive 934 patients who were hospitalized with acute decompensated HF (ADHF) other than acute coronary syndrome in Tottori University Hospital from January 2004 to December 2015 (the total number of hospitalizations was 1385 cases during the period). Of these, 818 patients who were discharged alive from our hospital were excluded. Thus, 116 patients who finally died in our hospital were analysed in the present study. We excluded 10 patients with no documented medical records indicating the patient's or surrogate's preference for end‐of‐life treatment. Finally, the data of 106 patients were analysed in this study. ADHF is defined as new‐onset decompensated HF or decompensation of chronic HF, and acute coronary syndrome was excluded. HF was defined according to the Framingham Criteria, as previously described.11 , 12
Patients' medical records were retrospectively reviewed to obtain information on demographics, medical history, co‐morbidities, laboratory data, echocardiographic findings, treatments, cause of death, and conversations about life‐sustaining treatments. The severity of HF was assessed using the Acute Decompensated Heart Failure National Registry (ADHERE) algorithm, as previously described.13 The cause of death was classified as cardiovascular and non‐cardiovascular cause, on the basis of the clinical information. Cardiovascular cause of death was defined as death owing to HF, sudden death, or a vascular event (myocardial infarction, stroke, or other vascular diseases). Other causes of death were defined as non‐cardiovascular cause of death.
The following detailed information about end‐of‐life conversations was obtained: (i) the timing of conversations in days, from the initial conversation to the time of death; (ii) the number of conversations; (iii) participants in each conversation (patient, family members, and medical staff); (iv) the decision maker for life‐sustaining treatments; (v) preference for life‐sustaining treatment—cardiopulmonary resuscitation (CPR) or do not attempt resuscitation (DNAR); and (vi) changes in the preference for life‐sustaining treatments during the hospital stay. The advance directive for life‐sustaining treatment was defined as the patient's written documents indicated their wishes for medical care (living will) or confirmed information recorded in the medical records during previous hospitalizations for HF.
This retrospective study conformed to the principles outlined in the Declaration of Helsinki and the guiding principles for epidemiologic studies established by the Ministry of Health, Labour and Welfare, Japan. This study was approved by the research ethics committee of Tottori University Hospital. We were permitted to collect and analyse data without obtaining written informed consent from each patient by agreeing to release research information regarding this study to the public.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation. Categorical variables are expressed as percentages. Differences in continuous variables between the two groups were compared using the t‐test. Categorical variables were compared using Fisher's exact test. Multivariate logistic regression analysis was used to assess the independent association among variables. A P value of <0.05 was considered statistically significant. All statistical analyses were conducted using IBM SPSS version 22.0 (IBM Corp., Armonk, NY, USA).
Results
Study patients
Patient characteristics are shown in Table 1. The mean age of the overall cohort was 76 ± 13 years, and 52.8% of patients (n = 56) were male. A previous history of HF hospitalization was found for 63.2% (n = 67) of patients, and the average number of hospitalizations was 3.0 ± 2.6. The prevalence of ischaemic heart disease, valvular heart disease, and non‐ischaemic cardiomyopathy was 33.0% (n = 35), 29.2% (n = 31), and 14.1% (n = 15), respectively. Approximately 70% of patients (n = 71) had intermediate or high in‐hospital mortality risk as assessed by ADHERE risk score on admission. The mean length of hospital stay was 50 ± 57 days. Patient cause of death was as follows: cardiac cause, 71.7% (n = 76); vascular cause, 5.7% (n = 6); and non‐cardiovascular cause, 22.6% (n = 24). CPR was attempted in 13.2% of patients (n = 14) just before death.
Table 1.
Patient characteristics
| Characteristics | n = 106 |
|---|---|
| Age (years) | 76 ± 13 |
| Male, n (%) | 56 (52.8) |
| Systolic blood pressure (mmHg) | 122 ± 32 |
| Prior heart failure admissions, n (%) | 67 (63.2) |
| LVEF (%)a | 47.0 ± 18.3 |
| ADHERE risk score (low/intermediate/high), n (%) | 35 (33.0)/67 (63.2)/4 (3.8) |
| Ischaemic heart disease, n (%) | 35 (33.0) |
| Co‐morbidities | |
| Hypertension, n (%) | 47 (44.3) |
| Diabetes, n (%) | 38 (35.8) |
| Atrial fibrillation, n (%) | 42 (39.6) |
| Cerebrovascular disease, n (%) | 19 (17.9) |
| Malignancy, n (%) | 20 (18.9) |
| Laboratory value on admission | |
| Sodium (mmol/L) | 136.9 ± 5.2 |
| BUN (mg/dL) | 51.7 ± 35.8 |
| Creatinine (mg/dL) | 2.4 ± 1.8 |
| BNP (pg/mL)b | 1466.6 ± 1332.4 |
| In‐hospital treatments | |
| ACE‐I/ARB, n (%) | 54 (50.9) |
| Beta‐blockers, n (%) | 45 (42.5) |
| Intravenous inotropy, n (%) | 72 (67.9) |
| Intubation and MV, n (%) | 33 (31.1) |
| IABP/PCPS, n (%) | 7 (6.6) |
| Renal replacement therapy, n (%) | 12 (11.3) |
| Cardiopulmonary resuscitation, n (%) | 14 (13.2) |
| End‐of‐life conversations during hospital stay | |
| The numbers of conversations (times) | 2.1 ± 1.4 |
| Days from initial conversation to death (days) | 25 ± 42 |
| Patient's participation in the conversations, n (%) | 5 (4.7) |
| Nurse's participation in the conversations, n (%) | 51 (48.1) |
| Hospital stay (days) | 50 ± 57 |
| Cardiac cause death, n (%) | 76 (71.7) |
ACE‐I, angiotensin‐converting enzyme inhibitor; ADHERE, Acute Decompensated Heart Failure National Registry; ARB, angiotensin II receptor blocker; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; IABP, intra‐aortic balloon pump; LVEF, left ventricular ejection fraction; MV, mechanical ventilation; PCPS, percutaneous cardiopulmonary support.
Date are presented as mean ± standard deviation.
Available data for 80 patients.
Available data for 98 patients.
End‐of‐life conversations during hospital stay
Attending physicians conducted an average of 2.1 ± 1.4 end‐of‐life conversations with patients and/or their families (Table 1). The initial conversation took place an average 25 ± 42 days before the patient died. Only 4.7% of patients (n = 5) participated in the conversation; most medical care decisions were made by patient surrogates (95.3%, n = 101). The participation of medical staff other than doctors included only nurses, and they participated in less than half of conversations (48.1%, n = 51).
Figure 1 shows the primary decision makers for life‐sustaining treatments. An adult child (55.7%, n = 59) was the most common surrogate decision maker, followed by a spouse (32.1%, n = 34). Surrogate decision making most often took place without the patient's advance directive (98.1%, n = 99; Figure 2 ).
Figure 1.
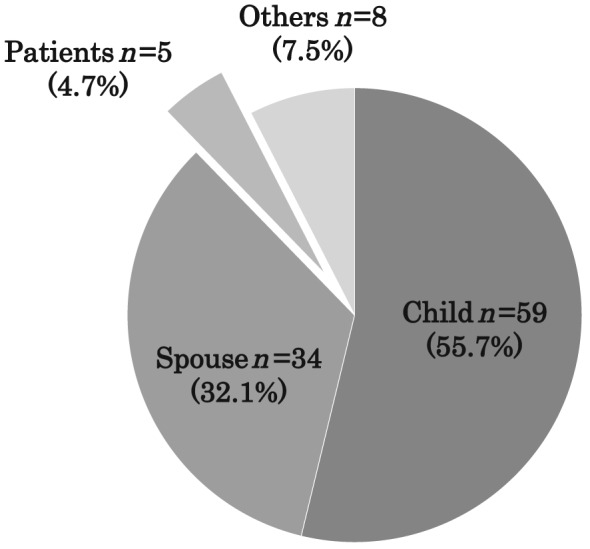
Decision makers for life‐sustaining treatment.
Figure 2.
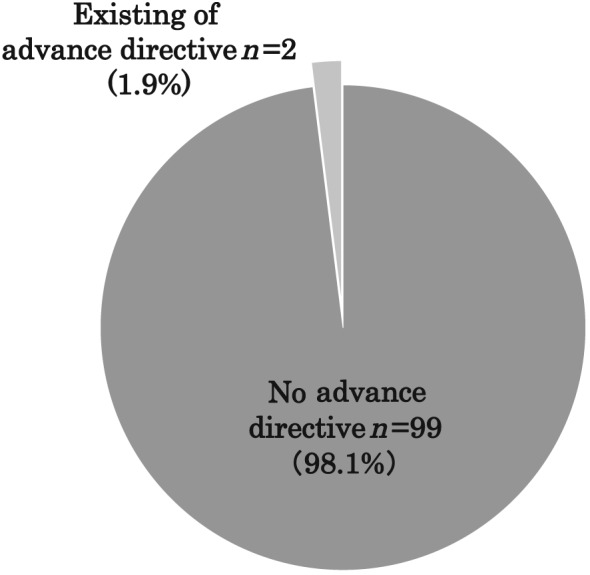
Rate of existing advance directives for life‐sustaining treatments.
Surrogate decision‐maker preferences for life‐sustaining treatments
Figure 3 shows details of the preferences of surrogate decision makers for life‐sustaining treatments (n = 101). At the initial end‐of‐life conversation, 49.5% of surrogates (n = 50) preferred CPR. However, after repeated conversations between physicians and surrogates during the patient's hospital stay, 72.0% of surrogates (n = 36) changed the preference from CPR to DNAR. In contrast, there was no change in preference from DNAR to CPR. To assess the factors contributing to the changes in preference for life‐sustaining treatments, we divided patients into two groups: those who changed (changed group, n = 36) and those who did not change (unchanged group, n = 63) the preference from CPR to DNAR. Two cases were excluded from the analysis, in which surrogates made the decision according to the patient's advance directive. As shown in Table 2, there were no significant differences in patient demographics, co‐morbidities, in‐hospital treatments, and disease severity, as assessed by ADHERE risk score, between the two groups. However, the prevalence of female surrogates was significantly higher in the changed group than in the unchanged group (66.7% vs. 42.9%, P < 0.05). End‐of‐life conversations were more frequently conducted in the changed group than in the unchanged group (2.9 ± 1.5 vs. 1.7 ± 1.2 times, P < 0.05), and the time from the initial conversation to death tended to be longer in the changed group than in the unchanged group (37.5 ± 57.7 vs. 19.0 ± 36.2 days, P = 0.075). On multivariate logistic regression analysis, female surrogates were significantly associated with a changed preference, independent of the number of end‐of‐life conversations and the duration from the initial conversation to death (female surrogates: odds ratio, 2.577; 95% confidence interval, 1.010–6.579, P < 0.05; the number of end‐of‐life conversations: odds ratio, 1.880, 95% confidence interval, 1.260–2.830, P < 0.05; the duration from the initial conversation to death: 1.000, 95% confidence interval, 0.989–1.010, P = 0.999). Figure 4 shows the association of sex and familial relationship with changes in surrogate decision‐maker preferences. Half of female surrogates (47.1%, n = 27) changed the preference from CPR to DNAR during the patient's hospital stay. In contrast, 75% of male surrogates (n = 36) did not change the preference (P < 0.05). Most female surrogates (n = 51) were the patient's wife (n = 25) or daughter (n = 21), and the remainder were the patient's mother (n = 3), sister (n = 1), or guardian (n = 1). There were no significant differences in the rate of changes in preference between patients' wives and daughters (P = 0.767; Figure 4 ).
Figure 3.
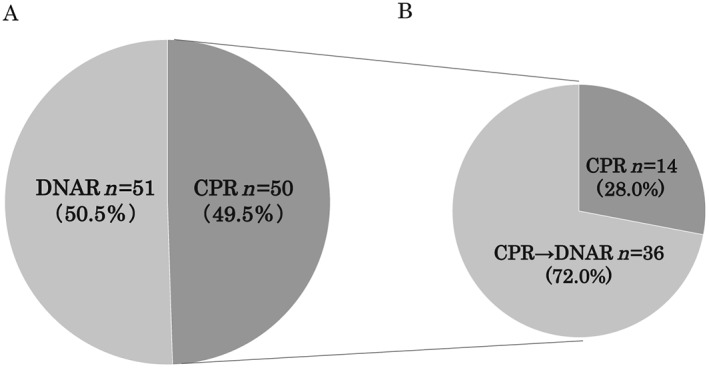
Changes in surrogate decision‐maker preferences for life‐sustaining treatments during patients' hospital stay. (A) Initial preference. (B) Final preference of surrogates who initially chose CPR. CPR, cardiopulmonary resuscitation; DNAR, do not attempt resuscitation.
Table 2.
Characteristics of patients with changed and unchanged preferences for life‐sustaining treatments
| Characteristics | Unchanged group (n = 63) | Changed group (n = 36) | P value |
|---|---|---|---|
| Age (years) | 77 ± 12 | 73 ± 16 | 0.209 |
| Male, n (%) | 30 (47.6) | 22 (61.1) | 0.196 |
| Systolic blood pressure (mmHg) | 127 ± 35 | 117 ± 24 | 0.145 |
| Prior heart failure admissions, n (%) | 40 (63.5) | 22 (61.1) | 0.814 |
| LVEF (%)a | 48.5 ± 18.5 | 44.5 ± 18.2 | 0.375 |
| ADHERE risk score (low/intermediate/high), n (%) | 21/40/2 | 13/21/2 | 0.790 |
| (33.3)/(63.5)/(3.2) | (36.1)/(58.3)/(5.6) | ||
| Ischaemic heart disease, n (%) | 24 (38.1) | 11 (30.6) | 0.450 |
| Co‐morbidities | |||
| Hypertension, n (%) | 31 (49.2) | 15 (41.7) | 0.469 |
| Diabetes, n (%) | 20 (31.7) | 17 (47.2) | 0.126 |
| Atrial fibrillation, n (%) | 22 (34.9) | 16 (44.4) | 0.349 |
| Cerebrovascular disease, n (%) | 10 (15.9) | 8 (22.2) | 0.431 |
| Malignancy, n (%) | 9 (14.3) | 8 (22.2) | 0.314 |
| Laboratory value on admission | |||
| Sodium (mmol/L) | 137.4 ± 5.4 | 136.7 ± 4.8 | 0.554 |
| BUN (mg/dL) | 50.3 ± 30.0 | 55.4 ± 46.1 | 0.557 |
| Creatinine (mg/dL) | 2.2 ± 1.7 | 2.0 ± 1.6 | 0.467 |
| BNP (pg/mL)b | 1573 ± 1299 | 1301 ± 1454 | 0.363 |
| In‐hospital treatments | |||
| ACE‐I/ARB, n (%) | 30 (47.6) | 21 (58.3) | 0.305 |
| Beta‐blockers, n (%) | 31 (49.2) | 11 (30.6) | 0.071 |
| Intravenous inotropy, n (%) | 42 (66.7) | 26 (72.2) | 0.566 |
| Intubation and MV, n (%) | 21(33.3) | 12 (33.3) | 1.000 |
| IABP/PCPS, n (%) | 5 (7.9) | 2 (5.6) | 1.000 |
| Renal replacement therapy, n (%) | 6 (9.5) | 5 (13.9) | 0.506 |
| Cardiopulmonary resuscitation, n (%) | 14 (22.2) | 0 (0.0) | 0.002 |
| End‐of‐life conversations | |||
| The number of conversations (times) | 1.7 ± 1.2 | 2.9 ± 1.5 | <0.001 |
| Days from initial conversation to death (days) | 19 ± 36 | 38 ± 58 | 0.075 |
| Nurse's participation in the conversations, n (%) | 30 (47.6) | 16 (44.4) | 0.761 |
| Surrogate decision maker | |||
| Male, n (%) | 36 (57.1) | 12 (33.3) | 0.023 |
| Child, n (%) | 38 (60.3) | 20 (55.6) | 0.676 |
| Spouse, n (%) | 19 (30.2) | 15 (41.7) | 0.276 |
| Hospital stay (days) | 45 ± 50 | 58 ± 66 | 0.319 |
| Cardiac cause death, n (%) | 42 (66.7) | 29 (80.6) | 0.140 |
Date are presented as mean ± standard deviation. Abbreviations are given in Table 1.
Available data for 75 patients.
Available data for 91 patients.
Figure 4.
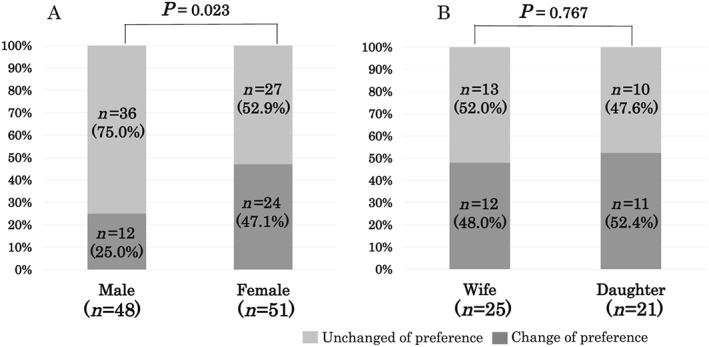
Association of sex and familial relationship with changes in surrogate decision makers' preference for life‐sustaining treatment. (A) Differences between male and female surrogates. (B) Differences between surrogates who were wives and daughters.
Discussion
The present study demonstrated that female surrogates more often changed their decision for life‐sustaining treatments of Japanese patients with end‐stage HF than did male surrogates.
We found that during the final hospitalization in patients with end‐stage HF, only 4.7% of patients participated in an end‐of‐life conversation, and surrogates made medical care decisions for most patients. A potential reason for the high prevalence of surrogate decision making was the lack of decision‐making capacity on the part of the patient. A previous study showed that ~70% of elderly end‐stage patients lacked the ability to make their own care decisions.5 Although we could not assess the patient's decision‐making capacity from the medical records, elderly patients with HF often have depression, anxiety, dementia, and delirium,14, 15, 16 which likely affects their ability to make decisions. In addition, even if the patient's decision‐making capacity is preserved, attending physicians are more likely to avoid giving information to patients about their life expectancy in a critical situation in Japan.17, 18 In this study, few patients presented an advance directive for medical care before hospitalization (Figure 2 ). Previous studies have also reported a low incidence of advance directives in patients with HF,19 suggesting the difficulty of initiating ACP in patients with advanced‐stage HF. Compared with cancer patients, HF patients are less likely to have opportunities for ACP together with attending physicians. The following reasons might account for the low incidence of ACP in HF: (i) it is more difficult to assess the prognosis in HF patients than in cancer patients,20 (ii) cardiologists are inexperienced in end‐of‐life care and often avoid end‐of‐life conversations that might cause patients to lose hope,21 (iii) patients with HF often overestimate their expected survival,22 and (iv) patients with HF often hesitate to communicate their preferences for end‐of‐life care to their physicians and nurses.20, 21 Furthermore, Japanese patients frequently entrust their family with decision making for end‐of‐life care, and the family members play a central role in end‐of‐life decision making in Japan.23 These barriers may lead to delays in the timing of ACP with patients and their families in Japan.
In the present study, nearly half of the surrogates preferred CPR in the initial end‐of‐life conversation (Figure 3 ); however, 75% of patients changed their preference for CPR to a DNAR order in the final conversation. Previous studies have reported that patients with HF often change their preference for life‐sustaining treatment during the courses of their illness9, 10; however, this trend among surrogate decision makers has not been well studied. The present study revealed that female surrogates changed the preferences from CPR to DNAR more often than did male surrogates (Figure 4 ). Several previous studies have reported a relationship between the patient's sex and treatment preferences. Female patients admitted to the intensive care unit after surgery preferred full‐care treatment, including CPR, more than male patients did.24 Conversely, elderly female patients were less likely to prefer life‐sustaining treatments than were male patients.25 To our knowledge, this is the first study to demonstrate that the sex of surrogates potentially affects the decision making for end‐of‐life care in patients with HF.
Although female surrogates more often changed the preference than did male surrogates, there was no significant difference of the rate of the DNAR request at the final decision between male and female surrogates (male vs. female; 83.4% vs. 88.3%, P = 0.57). Therefore, both sexes reached the same conclusion even though female surrogates were more likely to need the time and repeated conversation to accept the patient's death than were male surrogates. This leads to the phenomenon of changed preferences from CPR to DNAR, which may reflect the sex difference for the process of accepting patient's death. In contrast, we found that there was no changed preference from DNAR to CPR in the current study. Surrogates who requested DNAR at first might already accept patient's death. Therefore, there was no changed preference from DNAR to CPR.
Medical decision making by surrogates includes several problems. First, there is the possibility that a surrogate's decisions do not reflect the patient's actual preferences. The patient's physician or family cannot necessarily know the patient's actual preferences.10, 26 Second, surrogate decision makers often feel stress, depression, and uneasiness. The changes in preferences among a third of the surrogates in this study (Figure 3 ) may reflect their uncertainty about their decisions as well as psychological distress. Surrogate decision makers may regret their decision after the patient's death. They have a risk of developing post‐traumatic stress disorder, and this is particularly true for female family members.27 More frequent changes of decisions by female surrogates (Figure 4 ) may be related to a higher incidence of psychological distress among female family members. Recent guidelines recommend the introduction of ACP to patients with advanced HF.3 ACP is the process of understanding and recording the patient's wishes, values, and preferences regarding their own future medical care. Experiences of psychological distress have been reported by bereaved family members when such decisions are made without knowing the patient's preferences.28 Several studies have reported that ACP improves satisfaction among patients and families by reducing stress, anxiety, and depression.28 ACP may be indispensable to improving end‐of‐life care in patients with HF in Japan.
Our study has several limitations. This was a retrospective, single‐centre study with a relatively small sample size, and we could not evaluate accurately the patient's decision‐making capacity, psychological status, and estimated preferences. Second, we could not assess the details of end‐of‐life conversation and the reasons for the changed preference for life‐sustaining treatments. In addition, unmeasured factors such as socio‐economic deprivation might affect the surrogate decision making. Furthermore, each patient's view of life and death is likely to differ according to race, region, and culture and social background. It is unknown whether the current results can be applied to other populations of patients with HF. Further investigation is needed among patients of various races and from different regions.
Conclusions
Surrogate decision making for the life‐sustaining treatment of patients with end‐stage HF is common in Japan. Such care decisions are often made without the patient having expressed their preferences because of a lack of ACP. Decisions about care are often changed, particularly by female surrogates. It is well known that bereaved family members may experience stress, anxiety, and depression when end‐of‐life care decisions are made without knowing the patient's wishes, beliefs, and values. ACP is needed to improve end‐of‐life care in patients with advanced HF, to benefit both the patients and their families in Japan.
Conflict of interest
None declared.
Acknowledgement
We thank Analisa Avila, ELS, of Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Nakamura, K. , Kinugasa, Y. , Sugihara, S. , Hirai, M. , Yanagihara, K. , Haruki, N. , Matsubara, K. , Kato, M. , and Yamamoto, K. (2018) Sex differences in surrogate decision‐maker preferences for life‐sustaining treatments of Japanese patients with heart failure. ESC Heart Failure, 5: 1165–1172. 10.1002/ehf2.12352.
References
- 1. Roger VL, Weston SA, Redfield MM, Hellermann‐Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community‐based population. JAMA 2004; 292: 344–350. [DOI] [PubMed] [Google Scholar]
- 2. Mamas MA, Sperrin M, Watson MC, Coutts A, Wilde K, Burton C, Kadam UT, Kwok CS, Clark AB, Murchie P, Buchan I, Hannaford PC, Myint PK. Do patients have worse outcomes in heart failure than in cancer? A primary care‐based cohort study with 10‐year follow‐up in Scotland. Eur J Heart Fail 2017; 19: 1095–1104. [DOI] [PubMed] [Google Scholar]
- 3. Allen LA, Stevenson LW, Grady KL, Goldstein NE, Matlock DD, Arnold RM, Cook NR, Felker GM, Francis GS, Hauptman PJ, Havranek EP, Krumholz HM, Mancini D, Riegel B, Spertus JA, on behalf of the American Heart Association Council on Quality of Care and Outcomes Research , Council on Cardiovascular Nursing , Council on Clinical Cardiology , Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia . Decision making in advanced heart failure: a scientific statement from the American Heart Association. Circulation 2012; 125: 1928–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sato Y. Multidisciplinary management of heart failure just beginning in Japan. J Cardiol 2015; 66: 181–188. [DOI] [PubMed] [Google Scholar]
- 5. Maria J, Scott Y, Kenneth M. Advance directive and outcomes of surrogate decision making before death. N Engl J Med 2010; 362: 1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tanvetyanon T, Leighton JC. Life‐sustaining treatments in patients who died of chronic congestive heart failure compared with metastatic cancer. Crit Care Med 2003; 31: 111–113. [DOI] [PubMed] [Google Scholar]
- 7. Long SO. Living poorly or dying well: cultural decisions about life supporting treatment for American and Japanese patients. J Clin Ethics 2000; 11: 236–250. [PubMed] [Google Scholar]
- 8. Kwak J, Haley WE. Current research finding on end‐of‐life decision making among racially or ethnically diverse groups. Gerontologist 2005; 45: 634–641. [DOI] [PubMed] [Google Scholar]
- 9. Janssen DJA, Spruit MA, Schols JMGA, Cox B, Nawrot TS, Curtis JR, Wouters EFM. Predicting changes in preferences for life‐sustaining treatment among patients with advanced chronic organ failure. Chest 2012; 141: 1251–1259. [DOI] [PubMed] [Google Scholar]
- 10. Krumholz HM, Phillips RS, Hamel MB, Teno JM, Bellamy P, Broste SK, Califf RM, Vidaillet H, Davis RB, Muhlbaier LH, Connors AF, Lynn J, Goldman L. Resuscitation preferences among patients with severe congestive heart failure Result from the SUPPORT Project. Circulation 1998; 98: 648–655. [DOI] [PubMed] [Google Scholar]
- 11. Kinugasa Y, Kato M, Sugihara S, Yanagihara K, Yamada K, Hirai M, Yamamoto K. Multidisciplinary intensive education in the hospital improves outcomes for hospitalized heart failure patients in a Japanese rural setting. BMC Health Serv Res 2014; 14: 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miyagi M, Kinugasa Y, Sota T, Yamada K, Ishisugi T, Hirai M, Yanagihara K, Haruki N, Matsubara K, Kato M, Yamamoto K. Diaphragm muscle dysfunction in patients with heart failure. J Card Fail 2018; 24: 209–216. [DOI] [PubMed] [Google Scholar]
- 13. Fonarow GC, Adams KF Jr, Abraham WT, Yancy CW, Boscardin WJ, ADHERE Scientific Advisory Committee, Study Group, and Investigators . Risk stratification for in‐hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 2005; 293: 572–580. [DOI] [PubMed] [Google Scholar]
- 14. Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KRR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure‐Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman D, O'Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits P Jr, Valvo WJ. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry 2005; 58: 175–189. [DOI] [PubMed] [Google Scholar]
- 15. Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease: a population‐based cohort study. Arch Intern Med 2006; 166: 1003–1008. [DOI] [PubMed] [Google Scholar]
- 16. Thomason JW, Shintani A, Peterson JF, Pun BT, Jackson JC, Ely EW. Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 261 non‐ventilated patients. Crit Care 2005; 9: R375–R381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Makino J, Fujitani S, Twohig B, Krasnica S, Oropello J. End‐of‐life considerations in the ICU in Japan: ethical and legal perspectives. J Intensive Care 2014; 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matsumura S, Bito S, Liu H, Kahn K, Fukuhara S, Kagawa‐Singer M, Wenger N. Acculturation of attitudes toward end‐of‐life care: a cross‐cultural survey of Japanese Americans and Japanese. J Gen Intern Med 2002; 17: 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Butler J, Binney Z, Kalogeropoulos A, Owen M, Clevenger C, Gunter D, Georgiopoulou V, Quest T. Advance directives among hospitalized patients with heart failure. JACC Heart Fail 2015; 3: 112–121. [DOI] [PubMed] [Google Scholar]
- 20. Meyers DE, Goodlin SJ. End‐of‐life decisions and palliative care in advanced heart failure. Can J Cardiol 2016. Sep; 32: 1148–1156. [DOI] [PubMed] [Google Scholar]
- 21. Dev S, Abernethy AP, Rogers JG, O'Connor CM. Preferences of people with advanced heart failure‐a structured narrative literature review to inform decision making in the palliative care setting. Am Heart J 2012; 164: 313–319. [DOI] [PubMed] [Google Scholar]
- 22. Barclay S, Momen N, Case‐Upton S, Kuhn I, Smith E. End‐of‐life care conversations with heart failure patients: a systematic literature review and narrative synthesis. Br J Gen Pract 2011; 61: e49–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruhnke GW, Wilson SR, Akamatsu T, Kinoue T, Takashima Y, Goldstein MK, Koenig BA, Hornberger JC, Raffin TA. Ethical decision making and patient autonomy: a comparison of physicians and patients in Japan and the United States. Chest 2000; 118: 1172–1182. [DOI] [PubMed] [Google Scholar]
- 24. Lissauer ME, Naranjo LS, Kirchoffner J, Scalea TM, Johnson SB. Patient characteristics associated with end‐of‐life decision making in critically ill surgical patients. J Am Coll Surg 2011; 213: 766–770. [DOI] [PubMed] [Google Scholar]
- 25. Bookwala J, Coppola KM, Fagerlin A, Ditto PH, Danks JH, Smucker WD. Gender differences in older adult's preferences for life‐sustaining medical treatments and end‐of‐life care. Death Stud 2001; 25: 127–149. [DOI] [PubMed] [Google Scholar]
- 26. Sulmasy DP, Terry PB, Weisman CS, Miller DJ, Stallings RY, Vettese MA, Haller KB. The accuracy of substituted judgment in patients with terminal diagnosis. Ann Intern Med 1998; 128: 621–629. [DOI] [PubMed] [Google Scholar]
- 27. Azoulay E, Pochard F, Kentish‐Barnes N, Chevret S, Aboab J, Adrie C, Annane D, Bleichner G, Bollaert PE, Darmon M, Fassier T, Galliot R, Garrouste‐Orgeas M, Goulenok C, Goldgran‐Toledano D, Hayon J, Jourdain M, Kaidoma M, Laplace C, Larché J, Liotier J, Papazian L, Poisson C, Reignier J, Saidi F, Schlemmer B, FAMIREA Study Group. Risk of post‐traumatic stress symptoms in family members of intensive care unit patients. Am J Respir Crit Care Med 2005; 171: 987–994. [DOI] [PubMed] [Google Scholar]
- 28. Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomized controlled trial. BMJ 2010; 340: c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]


