Abstract
Introduction:
Calcium (Ca) is the phenomenon intracellular molecule that regulate many cellular process in neurons physiologically. Calcium dysregulation may occur in neurons due to excessive synaptic release of glutamate or other reasons related with neurodegeneration. Astaxanthin is a carotenoid that has antioxidant effect in cell. The purpose of this study was to investigate whether astaxanthin affects NMDA subunits, calcium binding proteins and L Type voltage sensitive Ca-channels (LVSCC) in primary cortical neuron cultures in order to see its role in calcium metabolism.
Methods:
Primary cortical neurons were prepared from embryonic day 16-Sprague Dawley rat embryos. The cultures were treated with 10 nM and 20 nM astaxanthin on day 7. NMDA subunits, LVSCC-A1C and LVSCC-A1D, calbindinD28k and parvalbumin mRNA expression levels was determined by qRT-PCR at 4, 24 and 48 hours.
Results:
Our findings indicate that astaxanthin could have direct or indirect outcome on calcium homeostasis by regulating mRNA expression levels of NMDA subunits, LVSCC-A1C and LVSCC-A1D, calbindinD28k and parvalbumin by a dose and time dependent manner.
Conclusion:
Neuroprotective effects of astaxanthin as a Ca homeostasis regulator should be noted throughout neurodegenerative disorders, and neurosurgery applications.
Keywords: astaxanthin, calcium, NMDA subunits, calcium binding proteins, L-type Ca channels A1C and A1D
INTRODUCTION
Aggregation and deposition of misfolded proteins in the brain, calcium dysregulation, energy misbalance and oxidative stress are most common features in neurodegenerative diseases such as Alzheimer’s and Parkinson Disease (1).
Calcium is an important intracellular messenger, triggers many cellular signaling pathways in neurons associated with differentiation and growth, exocytosis, synaptic transmission and synaptic plasticity during learning and memory (2). While intracellular calcium concentration of neurons is about 50–100 nM at the resting state, it can rise transiently during electrical activity 10–100 times higher. In physiological conditions cytosolic calcium level is controlled by the balance between calcium influx and efflux by ion channels, ATP-dependent ion pumps, buffers such as calbindin-D28 k, parvalbumin (3, 4). However, dysregulation of Ca2+ in neurons provided abasis for the Ca2+ hypothesis of brain aging and dementia (5), by its effects on many downstream pathways involving the ones in AD pathogenesis such as synapse loss, amyloid beta (Aβ) production, abnormal tau phosphorylation, mitochondrial dysfunction, oxidative stress, and inflammation (4).
L-type voltage-sensitive calcium channels (LVSCC) plays important roles in neuro-excitability and neurotransmission, thus in neural plasticity, learning, and memory. Two subtypes of L-type calcium channels, L-type voltage-sensitive calcium channels A1C (LVSCC-A1C) and A1D (LVSCC-A1D) genes expressed by excitable cells in the brain. In neuronal cells, LVSCC-A1C and LVSCC-A1D are often found in the similar overall neuronal compartments, predominantly in dendrites (6). Besides, increased LVSCC activity leads to up-regulation of Ca2+ related biomarkers in the aging hippocampus (5).
Glutamate is the major excitatory neurotransmitter in mammalian central nervous system (CNS). Abnormal release of glutamate results in over activation of its receptor (N-methyl-D-aspartate-NMDA-, or α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-AMPA-) that can cause the dysregulation of Ca2+ homeostasis as a consequence of glutamate neurotoxicity (2), although glutamate excitotoxicity suggested as a complex event (7). N-Methyl-D-aspartate receptors (NMDA) receptors are tetrameric structures (8), consist of Grin1, Grin2 (A-D) and Grin3 (A-B) subunits (9), and mediates postsynaptic Ca2+ influx in dendritic spines (3). Ca2+ permeability of channel is controlled by an asparagine residue (N598) in the Grin1 subunit. Also, the Grin1 subunit is critical for the gathering of a functional NMDA receptor. It has been reported that Grin2 (A-D) subunits have temporal expression pattern during prenatal stage (2). On the other hand, physiological roles of Grin3 has been a matter of focus in recent studies. Grin3A has multiple role in synaptic development in early postnatal stages, participate in neuron-glia interaction and regulation of myelin formation (8).
Astaxanthin (ATX) is member of xanthophylls family. ATX is an antioxidant, that has ability to reduce oxygen free radicals and prevent oxidative stress (10). Many advantages of ATX has been suggested when compared to other antioxidants, including a particular one that it could bind with cell membrane from inside and outside (11).
In the present study, we aim to clarify that whether ATX has a neuroprotective effect by means of regulating calcium homeostasis via L type channels and calcium binding proteins and NMDA subunits in primary cortical neurons.
MATERIALS AND METHODS
Preparation of primary cortical neuron cultures
Primary cortical neurons were prepared from the cerebral cortex of embryonic day 16 (E16) Sprague-Dawley rat embryos, according to our previous studies. The glia ratio of the cultures was determined by immunofluorescent labelling with neuronal (Millipore MAB2300) and glial (Invitrogen AB5804) markers as previously described (5, 12–14). Cultures were defined as neuron rich cultures based on their 20% glia content.
The study was approved by the Animal Welfare and Ethics Committee of Istanbul University. The procedures involving experimentation on animal subjects were performed in accordance with both the guidelines of Istanbul University and with the National Research Council’s guidelines for the care and use of laboratory animals.
Astaxanthin preparation and treatment
Astaxanthin was reconstituted in DMSO at calculated concentrations of 10nM and 20nM and was administrated to primary cortical neurons for 4 h, 24 h and 48 h after 7 days in vitro.
Quantitative real time polymerase chain reaction (qRT-PCR)
RNA isolation from cultured neurons was performed with PureLink RNA Mini Kit (Thermo Fischer 12183018A). Fixed amount of RNA was set to 60 ng/µl for each sample during cDNA synthesis reaction with High Capacity RNA-to-cDNA Kit (Thermo Fischer 4387406). mRNA levels of NMDARs, LVSCC-A1C, LVSCC-A1D, calbindinD28 k and parvalbumin expressions in the cortical neurons were analyzed by quantitative real-time PCR with Universal Probe Library (UPL) probes and Lightcycler 480 Probe Master Mix kit (Roche 04707494001) with LIGHTCYCLER 480.
Primers and probes were as follows:
CalbindinD28k, NM_031984.1, UPL Probe #128 (Roche 04693647001); Parvalbumin, NM_022499.1, UPL Probe #49 (Roche 04688104001); Grin1 NM_001270602.1 UPL Probe #69 (Roche 04688686001); Grin2a NM_012573.3 UPL Probe #66 (Roche 04688651001); Grin2b NM_012574.1 UPL Probe # 29 (Roche 04687612001); Grin2c NM_012575.3 UPL Probe #106 (Roche 04692250001); Grin2d NM_022797.1 UPL Probe #4 (Roche 04685016001); Grin3a NM_001198583.1 UPL Probe #105 (Roche 04692241001) CACNA1C, NM_012517.2, UPL Probe #73 (Roche 04688961001); CACNA1D, NM_017298.1, UPL Probe #82 (Roche 04689054001).
GAPDH (GAPDH Gene Assay, Roche 05046203001), Actin beta (ACTB Gene Assay, Roche 05046203001) and HPRT (HPRT NM_012583.2 UPL Probe #95, Roche 04692128001) were used as endogenous reference genes for normalization in qRT-PCR, the data were also confirmed by multiplex assays including ACTB Gene Assay, and target gene simultaneously. Each PCR amplification was performed in triplicate, using the profile previously described. Five serial dilutions of control samples were used to calculate the PCR efficiency. Reaction mixture excluding cDNA template was utilized as a negative control (15).
Statistical analysis
Cycle threshold (Ct) values obtained from qRT-PCR were calculated as previously described for determining the relative expression levels of target genes.
Raw data for each group for each kind of experiments was analyzed using one-way ANOVA followed by Tukey-Kramer multiple comparisons test when data normally distributed or Kruskal-Wallis test followed by Dunn’s multiple comparisons test when data not normally distributed in GraphPad InStat DTCG 3.06. p<0.05 was considered to be statistically significant. All data shown is presented as means ± SD in the text and figure legends. The cell culture experiments were repeated at least four times (15).
RESULTS
To evaluate the effects of ATX on NMDA subunits, LVSCC-A1C, LVSCC-A1D, calbindinD28k and parvalbumin mRNA expression levels, primary cortical neurons were incubated with ATX at 10nM and 20nM concentration for different time stops such as 4 hours, 24 hours and 48 hours. Following that to determine the relative expression levels of target genes by measuring mRNA levels, quantitative real time polymerase chain reaction qRT-PCR was used.
Astaxanthin caused different mRNA expression patterns for each NMDA subunits
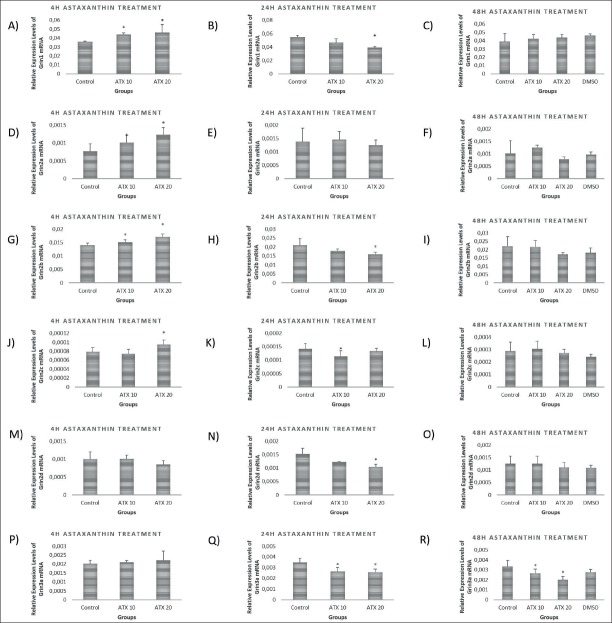
After 4 hours of treatment, Grin1, Grin2a, Grin2b mRNA levels were significantly increased at both 10nM (p<0.05) and 20nM (p<0.001) ATX concentration but Grin2 c mRNA levels were increased only at 20nM ATX treatment (p<0.05). On the other hand, Grin2 d and Grin3a mRNA levels did not change after 4 hours of treatment. At 24 hours of ATX treatment, while Grin1, Grin2b and Grin2 d mRNA levels were significantly decreased in the groups that received 20nM concentration (p<0.001), there was no alteration in Grin2a mRNA levels. Grin3a mRNA levels were significantly decreased at both 10 nM and 20 nM ATX concentrations (p<0.01). Only Grin3a mRNA expression levels were significantly decreased in both 10 nM and 20 nM ATX concentrations at 48 hours of treatment (p<0.01; p<0.001 respectively) (Figure 1).
Figure 1.
mRNA levels of NMDA subunits in astaxanthin treated primary cortical neurons at 4h, 24h and 48 h of treatments. A) Grin1 mRNA levels increased in 10 nM and 20 nM astaxanthin treated primary cortical neurons after 4 hours of treatment compared to the control group (p<0.05; p<0.05, respectively). B) Grin1 mRNA levels significantly decreased in 20 nM astaxanthin treated primary cortical neurons after 24 hours of treatment compared to the control and 10 nM astaxanthin treated groups (p<0.001). C) Grin1 mRNA levels did not change in both 10 nM astaxanthin and 20 nM astaxanthin treated primary cortical neurons after 48 hours of treatment compared to control and vehicle groups (p>0.05). D) Grin2a mRNA levels increased in 10 nM and 20 nM astaxanthin treated primary cortical neurons after 4 hours of treatment compared to the control group (p<0.05, p<0.001, respectively). E) Grin2a mRNA levels didn’t change in both 10 nM astaxanthin and 20 nM astaxanthin treated primary cortical neurons after 24 hours of treatment compared to control group (p>0.05). F) Grin2a mRNA levels didn’t change in both 10 nM astaxanthin and 20 nM astaxanthin treated primary cortical neurons after 48 hours of treatment compared to control and vehicle groups (p>0.05). G) Grin2b mRNA levels increased in both 10 nM and 20 nM astaxanthin treated primary cortical neurons after 4 hours of treatment compared to the control group (p<0.05; p<0.001, respectively). H) Grin2b mRNA levels decreased in 20 nM astaxanthin treated primary cortical neurons after 24 hours of treatment compared to the control and 10 nM astaxanthin treated groups (p<0.001). I) Grin2b mRNA levels didn’t change in both 10 nM astaxanthin and 20 nM astaxanthin treated primary cortical neurons after 48 hours of treatment compared to control and vehicle groups (p>0.05). J) Grin2c mRNA levels increased in 20 nM astaxanthin treated primary cortical neurons after 4 hours of treatment compared to the control group (p<0.05). K) Grin2c mRNA levels decreased in 10 nM astaxanthin treated primary cortical neurons after 24 hours of treatment compared to the control and 20 nM astaxanthin treated groups (p<0.01). L) Grin2c mRNA levels didn’t change in both 10 nM astaxanthin and 20 nM astaxanthin treated primary cortical neurons after 48 hours of treatment compared to control and vehicle groups (p>0.05). M) Grin2d mRNA levels didn’t change in both 10 nM astaxanthin and 20 nM astaxanthin treated primary cortical neurons after 4 hours of treatment compared to control group (p>0.05). N) Grin2d mRNA levels decreased in 20 nM astaxanthin treated primary cortical neurons after 24 hours of treatment compared to the control and 10 nM astaxanthin treated groups (p<0.001). O) Grin2d mRNA levels didn’t change in both 10 nM astaxanthin and 20 nM astaxanthin treated primary cortical neurons after 48 hours of treatment compared to control and vehicle groups (p>0.05). P) Grin3a mRNA levels didn’t change in both 10 nM astaxanthin and 20 nM astaxanthin treated primary cortical neurons after 4 hours of treatment compared to control group (p>0.05). Q) Grin3a mRNA levels decreased in both 10 nM and 20 nM astaxanthin treated primary cortical neurons after 24 hours of treatment compared to the control group p<0.01; (p<0.01). R) Grin3a mRNA levels decreased in both 10 nM and 20 nM astaxanthin treated primary cortical neurons after 48 hours of treatment compared to the control and vehicle groups (p<0.01; p<0.001, respectively).
L-type voltage-sensitive Ca channels
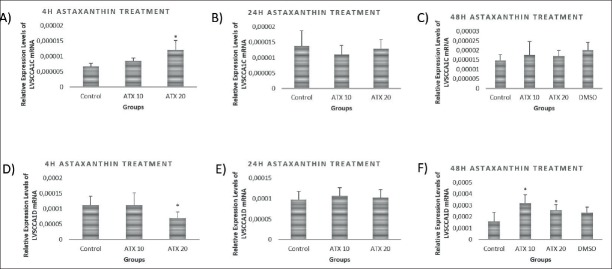
LVSCC-A1D mRNA expression levels were increased at 4 hours of treatment at 20nM astaxanthin concentration (p<0.001), but there were no differences at 24 hours and 48 hours of treatment. While LVSCC-A1C mRNA expression levels were decreased by 20 nM ATX treatment at 4 hours (p<0.05), it was significantly increased by 10 nM (p<0.001) and 20 nM (p<0.01) at 48 hours of treatment. There were no differences between any groups at 24 hours of treatments (Figure 2).
Figure 2.
mRNA levels of voltage sensitive calcium channels in astaxanthin treated primary cortical neurons at 4h, 24h and 48 h of treatments. A) LVSCCA1D mRNA levels significantly increased in 20 nM astaxanthin treated primary cortical neurons after 4 hours of treatment compared to the control and 10 nM astaxanthin treated groups (p<0.001). B) LVSCCA1D mRNA levels didn’t change in both 10 nM astaxanthin and 20 nM astaxanthin treated primary cortical neurons after 24 hours of treatment compared to control group (p>0.05). C) LVSCCA1D mRNA levels didn’t change in both 10 nM astaxanthin and 20 nM astaxanthin treated primary cortical neurons after 48 hours of treatment compared to control and vehicle groups (p>0.05). D) LVSCCA1C mRNA levels significantly decreased in 20 nM astaxanthin treated primary cortical neurons after 4 hours of treatment compared to the control and 10 nM astaxanthin treated groups (p<0.001). E) LVSCCA1C mRNA levels didn’t change in both 10 nM astaxanthin and 20 nM astaxanthin treated primary cortical neurons after 24 hours of treatment compared to control group (p>0.05). F) LVSCCA1C mRNA levels increased in both 10 nM and 20 nM astaxanthin treated primary cortical neurons after 48 hours of treatment compared to the control and vehicle groups (p<0.001, p<0.01, respectively)
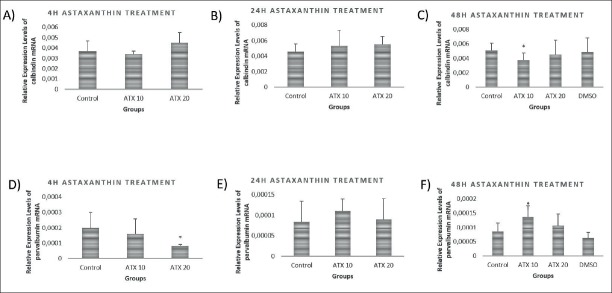
CalbindinD28 k and Parvalbumin
As shown in Figure 3A and B, calbindinD28k mRNA expression levels did not change in any concentration at either 4 hours or 24 hours of ATX treatment. Yet, calbindinD28k mRNA level was increased after 48 hours by 10 nM ATX treatment (p<0.05) (Figure. 3C). While parvalbumin mRNA expression levels were decreased by 20 nM ATX treatment after 4 hours (p<0.05) (Figure 3D), there was no significant difference between any groups at 24 hours of treatment (Figure 3E). Parvalbumin mRNA expression levels increased after 48 hours by 10 nM ATX treatment (p<0.05) (Figure 3F).
Figure 3.
mRNA levelsof calcium binding proteins in astaxanthin treated primary cortical neurons at 4h, 24h and 48 h of treatments. A) CalbindinD28k mRNA levels did not change in both 10 nM astaxanthin and 20 nM astaxanthin treated primary cortical neurons after 4 hours of astaxanthin treatment compared to the control group (p>0.05). B) CalbindinD28k mRNA levels didn’t change in both 10 nM astaxanthin and 20 nM astaxanthin treated primary cortical neurons after 24 hours of astaxanthin treatment compared to the control group (p>0.05). C) CalbindinD28k mRNA levels decreased in 10 nM astaxanthin treated primary cortical neurons after 48 hours of treatment compared to control, 20 nM astaxanthin treated and vehicle groups (p<0.05). D) Parvalbumin mRNA levels decreased in 20 nM astaxanthin treated primary cortical neurons after 4 hours of treatment compared to the control and 10 nM astaxanthin treated groups (p<0.05). E) Parvalbumin mRNA levels didn’t change in both 10 nM astaxanthin and 20 nM astaxanthin treated primary cortical neurons after 24 hours of treatment compared to control group (p>0.05). F) Parvalbumin mRNA levels increased in 10 nM astaxanthin treated primary cortical neurons after 48 hours of treatment compared to the control, 20 nM astaxanthin treated group and vehicle groups (p<0.05).
DISCUSSION
Recent studies indicated neuroprotective effect of astaxanthin in neurodegenerative diseases such as Parkinson, Alzheimer and Amyotrophic lateral sclerosis (10, 16). The effects of astaxanthin include reducing the level of matrix metalloproteinase-9, which plays a role in secondary brain damage, mitochondrial inhibition associated with apoptosis, lipid peroxidation and oxidative injury by crossing the blood brain barrier (17, 18). We know for a long time that many neural pathology and aging metabolism of the brain are associated with underlying mechanisms Ca+2 homeostasis (2, 4).
In addition to physiological aging of neural tissue, regardless of ischemia, bleeding, neural damage is also caused by factors such as direct effect of pathology, primary damage caused by chain reaction of subsequent metabolic events and secondary damages caused by the direct effect of pathology. In many neural cases, secondary damage has a more severe and destructive effects than primary damage. Protection from primary damage may be prevented, with certain extent by preventive medicine practices. However, a lot of studies are carried out on neuroprotective agents to minimize the effects of secondary damages or to prevent them.
In the aging brain, several physiological events such as protein folding, unfolded protein response, calcium regulation, antioxidant systems, regulation of energy metabolism get worst and finally collapse and result in neuronal loss. One of these enormous events is that calcium dysregulation (5). Glutamate dependent neurotoxicity via over-activated NMDA receptors as well, is a key pathogenic event that is a part of neurodegeneration (19).
In the present study, we investigated the role of astaxanthin on calcium homeostasis throughout calcium channels, calcium binding proteins and NMDA receptor subunits. Given that, we determined the effect of ATX treatment on mRNA levels of L-type calcium channels A1C and A1D, calbindinD28k, parvalbumin, and Grin (1-3) subunits in primary cortical neurons.
Glutamate release at synaptic region cause calcium influx by NMDA and L-type voltage-gated calcium channels (20). Glutamate is the most common excitatory neurotransmitter in the central nervous system. Each NMDA subunits which are glutamate receptors, shows different structural and functional features that might involve with its expression and localization patterns. Although all NDMA subunits share similar properties, Grin3a has distinctive feature with reduced Mg2+ sensitivity and Ca2+ permeability (21). According to our results Grin3a expression significantly reduced at 24 and 48 hours of ATX treatment. Thus, the effect of ATX on Grin3a expressions might have more significant impact than other subunits due its relevance on calcium pathway. On the other hand, as if nearly all subunits expression were increased at 4 hours of astaxanthin treatment, we analyzed reduced and then no affected expression pattern at 24 hours and 48 hours of astaxanthin treatment, respectively.
LVSCC-A1C and LVSCC-A1D channels are expressed in the soma and dendrites, are predominantly post-synaptic neuron. Downstream of the depolarization induced by Ca2+ through both types of LVSCCs, activates calcium-dependent non-specific cationic channels (CAN) that maintains the neuron in a depolarized state. On the other hand, calcium influx through LVSCCs also activates calcium-dependent potassium channels (KCa). Ca2+ entry through LVSCC-A1C regulates calcium-dependent signaling pathways and also activates transcription factors such as CREB (22). Other studies reported that LVSCC-A1C have double functions which are ion pore and transcription factor. C terminal of LVSCC-A1C reported to transfer to the nucleus and affects the transcription of many neuronal genes, including NMDA-receptor subunits (23). According to our result LVSCC-A1C mRNA expression levels first decreased at 4 hours of treatment but then significantly increased at 48 hours of ATX treatment and this results might be also relevant with the change in NMDA subunits expression levels at 48 hours of treatment which might be regulated by LVSCC-A1C transcriptionally.
Parvalbumin has been related with fast-firing and permit individual neurons to maintain a fast firing rate by reducing the Ca2+-dependent potassium outflow. This ion outflow is play a significant role in the after-hyperpolarization period (24). Our result we showed that mRNA expression level of NMDA subunits significant increased at 4 hours of treatment. In that case, astaxanthin might prevent neurons having a short after-hyperpolarization period by reducing parvalbumin mRNA expression levels at 4 hours.
A recent study showed that, astaxanthin has protective effects against H2O2-induced or serum deprivation-induced cell deaths in cultured retinal ganglion cells and also found that astaxanthin showed neuroprotective effects in NMDA-triggered retinal damage in vivo (10).
Consequently, our study indicated that the potential neuroprotective effects of astaxanthin may include the maintenance of calcium homeostasis since the molecule has regulatory effects on the mRNA expression levels of certain NMDA subunits, voltage sensitive calcium channels and calcium binding proteins.
Footnotes
Ethics Committee Approval: The study was approved by the Animal Welfare and Ethics Committee of Istanbul University. The procedures involving experimentation on animal subjects were performed in accordance with both the guidelines of Istanbul University and with the National Research Council’s guidelines for the care and use of laboratory
Peer-review: Externally peer-reviewed
Author Contributions: Concept – MEA; Design – ED; Supervision – DGA; Resources – ED; Materials – MEA; Data Collection and/or Processing – İLA; Analysis and/or Interpretation – MEA, DGA; Literature Search – EC; Writing Manuscript – MEA, İLA; Critical Review – İLA, DGA.
Conflict of Interest: Authors have declared that they have no conflict of interest.
Financial Disclosure: The present work was supported by the Research Fund of Istanbul University (Project No: 26298).
REFERENCES
- 1.Angelova PR, Abramov AY. Alpha-synuclein and beta-amyloid –different targets, same players:calcium, free radicals and mitochondria in the mechanism of neurodegeneration. Biochem Biophys Res Commun. 2017;483:1110–1115. doi: 10.1016/j.bbrc.2016.07.103. [DOI] [PubMed] [Google Scholar]
- 2.Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–337. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- 3.Grienberger C, Konnerth A. Imaging calcium in neurons. Neuron. 2012;73:862–885. doi: 10.1016/j.neuron.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Alzheimer's Association Calcium Hypothesis Workgroup. Calcium Hypothesis of Alzheimer's disease and brain aging:A framework for integrating new evidence into a comprehensive theory of pathogenesis. Alzheimer's Dement. 2017;13:178–182.e17. doi: 10.1016/j.jalz.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Gezen-Ak D, Dursun E, Yilmazer S. The effects of vitamin D receptor silencing on the expression of LVSCC-A1C and LVSCC-A1D and the release of NGF in cortical neurons. PLoS One. 2011;6:e17553. doi: 10.1371/journal.pone.0017553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vega-Vela NE, Osorio D, Avila-Rodriguez M, Gonzalez J, García-Segura LM, Echeverria V, Barreto GE. L-Type Calcium Channels Modulation by Estradiol. Mol Neurobiol. 2017;54:4996–5007. doi: 10.1007/s12035-016-0045-6. [DOI] [PubMed] [Google Scholar]
- 7.Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflügers Arch. 2010;460:525–542. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- 8.Pankratov Y, Lalo U. Calcium permeability of ligand-gated Ca2+channels. Eur J Pharmacol. 2014;739:60–73. doi: 10.1016/j.ejphar.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Wee KSL, Tan FCK, Cheong YP, Khanna S, Low CM. Ontogenic Profile and Synaptic Distribution of GluN3 Proteins in the Rat Brain and Hippocampal Neurons. Neurochem Res. 2016;41:290–297. doi: 10.1007/s11064-015-1794-8. [DOI] [PubMed] [Google Scholar]
- 10.Lin X, Zhao Y, Li S. Astaxanthin attenuates glutamate-induced apoptosis via inhibition of calcium influx and endoplasmic reticulum stress. Eur J Pharmacol. 2017;806:43–51. doi: 10.1016/j.ejphar.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Stephenson RC, Clarke S. Succinimide formation from aspartyl and asparaginyl peptides as a model for the spontaneous degradation of proteins. J Biol Chem. 1989;264:6164–6170. [PubMed] [Google Scholar]
- 12.Dursun E, Gezen-Ak D, Yilmazer S. A novel perspective for Alzheimer's disease:vitamin D receptor suppression by amyloid-beta and preventing the amyloid-beta induced alterations by vitamin D in cortical neurons. J Alzheimer's Dis. 2011;23:207–219. doi: 10.3233/JAD-2010-101377. [DOI] [PubMed] [Google Scholar]
- 13.Dursun E, Gezen-Ak D, Yilmazer S. A new mechanism for amyloid-beta induction of iNOS:vitamin D-VDR pathway disruption. J Alzheimer's Dis. 2013;36:459–474. doi: 10.3233/JAD-130416. [DOI] [PubMed] [Google Scholar]
- 14.Gezen-Ak D, Atasoy IL, Candaş E, Alaylioglu M, Yılmazer S, Dursun E. Vitamin D Receptor Regulates Amyloid Beta 1-42 Production with Protein Disulfide Isomerase A3. ACS Chem Neurosci. 2017;8:2335–2346. doi: 10.1021/acschemneuro.7b00245. [DOI] [PubMed] [Google Scholar]
- 15.Gezen-Ak D, Dursun E, Yilmazer S. Vitamin D inquiry in hippocampal neurons:consequences of vitamin D-VDR pathway disruption on calcium channel and the vitamin D requirement. Neurol Sci. 2013;34:1453–1458. doi: 10.1007/s10072-012-1268-6. [DOI] [PubMed] [Google Scholar]
- 16.Wu H, Niu H, Shao A, Wu C, Dixon B, Zhang J, Yang S, Wang Y. Astaxanthin as a Potential Neuroprotective Agent for Neurological Diseases. Mar Drugs. 2015;13:5750–5766. doi: 10.3390/md13095750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Y, Xie T, He XX, Mao ZF, Jia LJ, Wang WP, Zhen JL, Liu LM. Astaxanthin rescues neuron loss and attenuates oxidative stress induced by amygdala kindling in adult rat hippocampus. Neurosci Lett. 2015;597:49–53. doi: 10.1016/j.neulet.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 18.Zhang XS, Zhang X, Zhang QR, Wu Q, Li W, Jiang TW, Hang CH. Astaxanthin reduces matrix metalloproteinase-9 expression and activity in the brain after experimental subarachnoid hemorrhage in rats. Brain Res. 2015;1624:113–124. doi: 10.1016/j.brainres.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 19.Simoes AP, Silva CG, Marques JM, Pochmann D, Porciúncula LO, Ferreira S, Oses JP, Beleza RO, Real JI, Köfalvi A, Bahr BA, Lerma J, Cunha RA, Rodrigues RJ. Glutamate-induced and NMDA receptor-mediated neurodegeneration entails P2Y1 receptor activation. Cell Death Dis. 2018:9. doi: 10.1038/s41419-018-0351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyons MR, Chen LF, Deng JV, Finn C, Pfenning AR, Sabhlok A, Wilson KM, West AE. The transcription factor calcium-response factor limits NMDA receptor-dependent transcription in the developing brain. J Neurochem. 2016;137:164–176. doi: 10.1111/jnc.13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henson MA, Roberts AC, Pérez-Otaño I, Philpot BD. Influence of the NR3A subunit on NMDA receptor functions. Prog Neurobiol. 2010;91:23–37. doi: 10.1016/j.pneurobio.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roca-Lapirot O, Radwani H, Aby F, Nagy F, Landry M, Fossat P. Calcium signalling through L-type calcium channels:role in pathophysiology of spinal nociceptive transmission. Br J Pharmacol. 2017;175:2362–2374. doi: 10.1111/bph.13747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez-Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R. The C terminus of the L-type voltage-gated calcium channel Ca (V)1.2 encodes a transcription factor. Cell. 2006;127:591–606. doi: 10.1016/j.cell.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hontanilla B, Parent A, de las Heras S, Giménez-Amaya JM. Distribution of calbindin D-28k and parvalbumin neurons and fibers in the rat basal ganglia. Brain Res Bull. 1998;47:107–116. doi: 10.1016/s0361-9230(98)00035-5. [DOI] [PubMed] [Google Scholar]